Abstract
Background:
Trichophyton rubrum represents the most common infectious fungus responsible for dermatophytosis in human, but the mechanism involved is still not completely understood. An appropriate model constructed to simulate host infection is the prerequisite to study the pathogenesis of dermatophytosis caused by T. rubrum. In this study, we intended to develop a new T. rubrum infection model in vitro, using the three-dimensional reconstructed epidermis - EpiSkin®, and to pave the way for further investigation of the mechanisms involved in T. rubrum infection.
Methods:
The reconstructed human epidermis (RHE) was infected by inoculating low-dose (400 conidia) and high-dose (4000 conidia) T. rubrum conidia to optimize the infection dose. During the various periods after infection, the samples were processed for pathological examination and scanning electron microscopy (SEM) observation.
Results:
The histological analysis of RHE revealed a fully differentiated epidermis with a functional stratum corneum, which was analogous to the normal human epidermis. The results of hematoxylin and eosin staining and the periodic acid-Schiff staining showed that the infection dose of 400 conidia was in accord with the pathological characteristics of host dermatophytosis caused by T. rubrum. SEM observations further exhibited the process of T. rubrum infection in an intuitionistic way.
Conclusions:
We established the T. rubrum infection model on RHE in vitro successfully. It is a promising model for further investigation of the mechanisms involved in T. rubrum infection.
Keywords: EpiSkin, Infection Model, Trichophyton Rubrum
INTRODUCTION
Trichophyton rubrum represents the most common infectious fungus responsible for dermatophytosis in human.[1,2] A typical characteristic of dermatophytes is keratinophilic and always invades keratinized structures such as the skin stratum corneum, hairs, and nails. The infection caused by T. rubrum, except for diffusing into the deeper parts of the body in some immunosuppressed patients,[3,4] is normally limited to the superficial skin and tends to be chronic and recurrent,[5] in which the pathogenic mechanism involved is still not completely understood. Various models have been explored in vitro and ex vivo to investigate the mechanism of dermatophyte infection such as the animal model,[6] stripped sheets of the stratum corneum,[7,8] nail plates, or monolayer cell culture model.[9,10] However, due to the anthropophilic feature of T. rubrum and the spontaneous healing of animals after the T. rubrum infection,[11] animal model is not the optimal choice. The stripped sheets of stratum corneum or nail plates from healthy volunteers could not response to the dermatophyte infection on account of missing living cells and no immune function, which is obviously different from the actual situation in vivo. Our previous studies have already demonstrated that human keratinocytes can recognize T. rubrum and induce native immune responses against T. rubrum infection.[12,13,14] Nevertheless, different from the infection localized to the skin cornified layers, T. rubrum submerged under the culture medium and contacted with keratinocytes directly in the monolayer cell culture model, which led to the immunogenicity and pathogenicity of T. rubrum distinct from in vivo.[15] Taken together, all the above models have certain limitations to imitate the human T. rubrum infection, respectively.
In recent years, several reconstructed human skin models have been developed. These reconstructed human skin equivalents demonstrated with fully differentiated epidermis could closely resemble native human epidermis,[16,17] therefore providing a morphologically relevant means to assess skin irritation and to research the skin-related disease in vitro. In this study, we intended to establish a T. rubrum infection model in vitro, based on the three-dimensional reconstructed human epidermis (RHE) - EpiSkin®. It will pave the way for further investigation of the mechanisms involved in T. rubrum infection in the future.
METHODS
Reconstructed human epidermis EpiSkin®
Commercial epidermal tissues EpiSkin (L’Oreal Research and Innovation Center, Shanghai, China) is an in vitro RHE from normal human keratinocytes cultured on a collagen matrix at the air-liquid interface. This RHE is histologically similar to the in vivo human epidermis. Immediately upon arrival, the EpiSkin models were removed from the nutrient agar and transferred to 12-well plates filled with medium without antifungals provided by the manufacturer. After equilibration overnight in an incubator (37°C, 5.0% CO2), the medium was changed, and the tissues were used in the following experiments.
Fungal strain and conidia collection
T. rubrum strain T1a used in the present experiment was obtained from the China Medical Microbiological Culture Collection Center and was confirmed as T. rubrum by morphological identification and sequencing of internal transcribed spacer regions and the D1–D2 domain of the large-subunit rRNA gene.
T. rubrum was cultured for 2 weeks at 27°C on potato glucose agar (OXOID, UK) to produce conidia. A mixed suspension of conidia and hyphae fragments was obtained by covering the fungal colonies with sterile saline (0.85%) and gently rubbing the colonies with the inoculation loop. Next, the suspension was filtered with a Whatman filter paper model 1 (pore size, 11 μm; Whatman, UK) to remove the hyphae,[18] and then centrifuged at 4000 r/min for 10 min to collect the conidia. These conidia were washed twice by agitation in sterile saline. The concentration of conidia was adjusted with sterile saline to a final concentration of 8 × 104 CFU/ml and 8 × 105 colony-forming unit (CFU)/ml by hemacytometer counts.
Infection EpiSkin® with Trichophyton rubrum conidia
For the pilot experiment, two concentrations of conidia suspensions (8 × 104 CFU/ml and 8 × 105 CFU/ml) were used to infect RHE. A 5 μl inoculum of T. rubrum conidia suspensions (400 conidia and 4000 conidia) was added to the center of each RHE surface-cultured in 12-well plates. The identical volume of sterile saline was added as negative controls. Then, the 12-well plates were transferred to an incubator (37°C, 5.0% CO2), and the maintenance medium was changed every other day. The epidermis tissues were processed after infection for 2 days, 4 days, and 10 days.
Pathological Examination: Hematoxylin and eosin and periodic acid-Schiff staining
After rinsing 3 times with 0.01 mol/L phosphate-buffered saline (PBS), the specimens were fixed overnight in 4% paraformaldehyde at 4°C. Afterward, cut the epidermis from the insert with a surgical scalpel and immersed in 0.01 mol/L PBS for 5 min, and then the tissues were processed for dehydration using graded ethanol, vitrification by xylene, immersion, and embedding in paraffin. Slices with 5 μm thicknesses were cut from the embedded tissues and dealt with hematoxylin and eosin (H and E) staining and periodic acid-Schiff (PAS) staining for light microscopic examination. The normal prepuce tissues harvested from clinical circumcision surgery as normal human skin were processed with H and E staining at the same time for comparison.
Scanning electron microscopy
The specimens were washed 3 times with PBS and fixed with glutaraldehyde at 4°C. Then, samples were dehydrated with an increasing gradient of ethanol (50–100%), dried using the critical point method with CO2 in liquid state, and coated with gold. After that, the interaction between the T. rubrum and the RHE was viewed under a FEI Quanta 200 scanning electron microscope (SEM, USA).
RESULTS
Structure of the reconstructed human epidermis was similar to human epidermis
The results of H and E staining [Figure 1a and 1c] showed that the structure of the reconstructed epidermis was composed of stratum corneum, keratinocyte layer, and organic support layer, which was similar to human normal epidermis from prepuce tissue [Figure 1b and 1d].
Figure 1.
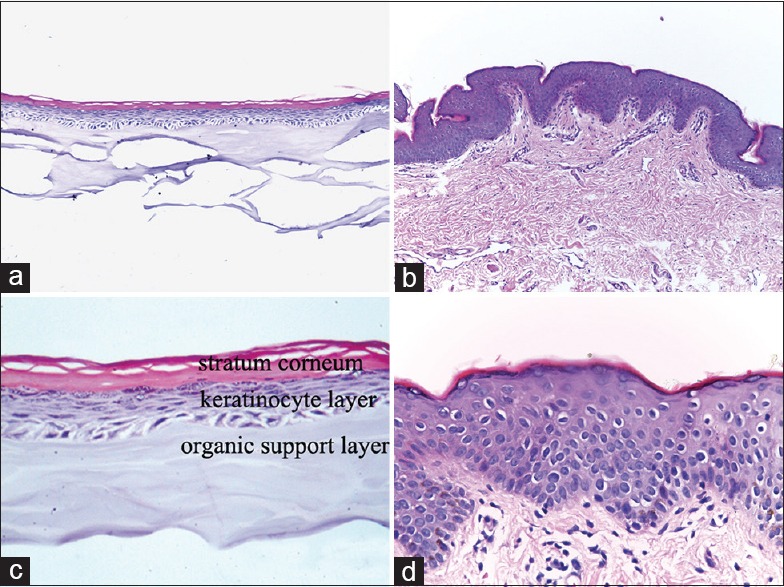
Morphology of the reconstructed human epidermis EpiSkin®, compared with the normal human skin derived from prepuce tissue through H and E staining. (a and b) original magnification, ×100; (c and d) original magnification, ×400.
Reconstructed human epidermis infected with Trichophyton rubrum conidia
To better mimic host dermatophyte infection, we optimized the infection dose by inoculating low-dose (400 conidia) and high-dose (4000 conidia) on the RHE. After 2 days of infection, the results of H and E and PAS staining did not show any conidia or hyphae in the horny layer in either group (data not shown). After T. rubrum was inoculated 4 days, conidia and hyphae fragments were found in the stratum corneum [Figures 2a, 2b and 3a, 3b]; moreover, infection with 4000 conidia showed more conidia and hyphae fragments compared with infection with 400 conidia. On the 10th day of co-culture, the histopathology feature exhibited the great difference between the two groups. The group infected by 400 conidia showed that the invasion limited to the cornified layer without penetrating through the stratum corneum to keratinocytes layer [Figure 2c and 2d], which is in accordance with the pathological characteristics of superficial dermatophytosis caused by T. rubrum in vivo. However, the group infected with 4000 conidia displayed enormous destruction–encroached almost the full epidermis and presented obvious necrosis of keratinocytes [Figure 3c and 3d]. Hence, the inoculum of 400 conidia was used to imitate host dermatophyte infection.
Figure 2.
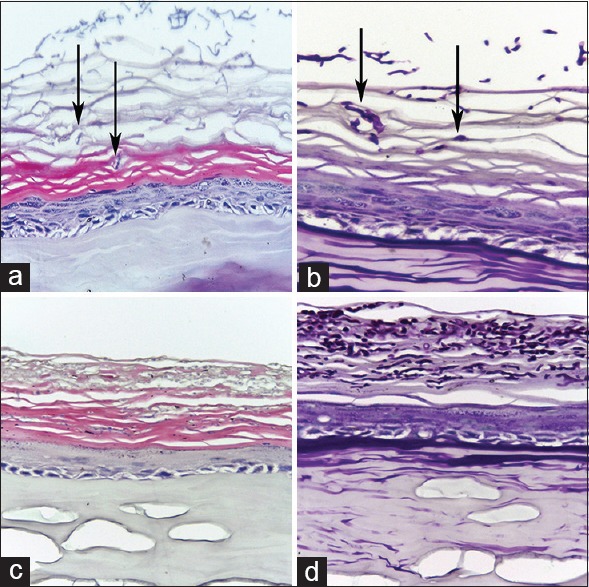
The H and E and periodic acid-Schiff staining of reconstructed human epidermis infected with Trichophyton rubrum conidia of 400. (a and b) Stained by H and E and periodic acid-Schiff after 4 days of infection (original magnification, ×400). (c and d) Stained by H and E and periodic acid-Schiff after 10 days of infection (original magnification, ×400). After infected by Trichophyton rubrum conidia, conidia and hyphae were found in the stratum corneum (black arrow).
Figure 3.
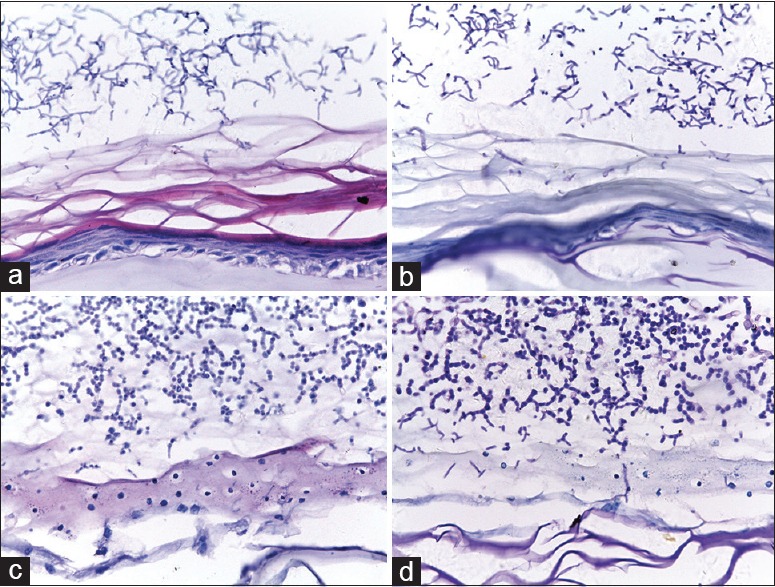
The reconstructed human epidermis infected with Trichophyton rubrum conidia of 4000. (a and b) Stained by H and E and periodic acid-Schiff after 4 days of infection (original magnification, ×400). (c and d) Stained by H and E and periodic acid-Schiff after 10 days of infection (original magnification, ×400). After infection for 4 days, abundance of conidia and hyphae was presented in the stratum corneum. At the 10th day of infection, the infection extended almost the full epidermis and the epidermis displayed enormous destruction.
Scanning electron microscopy showed the infection process of Trichophyton rubrum conidia in reconstructed human epidermis
After 2 days of infection (400 conidia), SEM revealed the germination of the conidia, an early germ tube originated from the adhered conidia [Figure 4a], as well as the elongation of the germ tubes [Figure 4b]. With further inoculation (4 days), the distribution of hyphae was observed on the surface in netlike manner [Figure 4c]. We can also find the invasion of the hyphae extended horizontally and entered into the RHE [Figure 4d], which indicated that the infecting of the stratum corneum was achieved.
Figure 4.
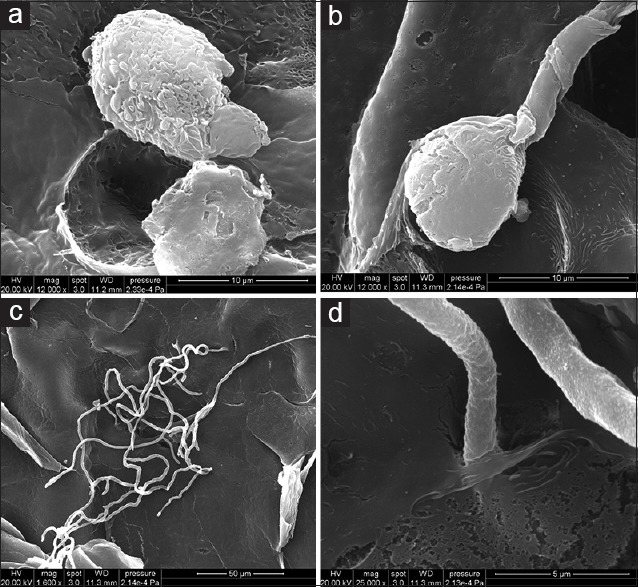
Scanning electron microscopy observation is the process of reconstructed human epidermis infected with Trichophyton rubrum conidia. (a and b) Revealing the germination of the conidia and elongation of the germ tubes 2 days after infection. (c and d) Showing the invasion of the hyphae spreading along the reconstructed human epidermis surface and penetrating through the outer surface layer after infection for 4 days. (a and b) Scale bar = 10 μm, (c) Scale bar = 50 μm, and (d) Scale bar = 5 μm.
DISCUSSION
An appropriate model to simulate host dermatophyte infection is the prerequisite to study the pathogenesis of dermatophytosis. There is still some distance of the models made in vitro and ex vivo from the actual situation of dermatophyte infection in vivo because of the defects described earlier in this study. Recently, reconstructed human skin as an alternative has developed quickly and gradually applied to study skin-related disease. In 1995, Rashid et al. used the living skin equivalent to study the effects of antifungal drugs on dermatophyte (Trichophyton mentagrophytes) for the 1st time.[19] Liu's laboratory also constructed the fungal (including T. mentagrophytes and T. rubrum)-infected, tissue-engineered skin in vitro.[20] However, both of their models presented obvious necrosis of keratinocytes, along with the dermatophytes not only invaded the stratum corneum but also penetrated the full thickness epidermis to dermal component in a short time. These were conflicted with the actual situation in vivo that dermatophytes were restricted to the outer stratum corneum of the epidermis. Recently, Achterman et al. used EpiDerm reconstructed epidermis to mimic human dermatophytosis caused by various dermatophytes (including T. rubrum);[21] in their research, the release of cytoplasmic lactate dehydrogenase was used to indicate the progression of infection; however, this method could not certify if the infection was limited to the stratum corneum, thus could not affirm that the results they got were in accord with the real situation in vivo. In this study, we adopted the commercial RHE EpiSkin® as a platform, successfully elaborated the T. rubrum infection epidermis model in vitro. Morphological structure of the RHE confirmed that it was analogous to the normal human skin from prepuce tissue.
Preliminary experiments were designed to determine the optimal inoculum amount so as to more resemble the actual situation of host T. rubrum dermatophytosis. The H and E staining and the PAS staining exhibited that no matter on the 4th day or the 10th day after infection of 400 conidia; the T. rubrum just limited to the stratum corneum, which is in accord with the real situation of T. rubrum dermatophytosis that always invades cornified layer. Moreover, SEM observations were adopted to further exhibit the process of adhesion and invasion stratum corneum in more intuitionistic way after infecting with 400 conidia. During the infection, T. rubrum conidia first adhered to the RHE surface, followed by germination, then invaded keratinized-structure, which is consistent with the reported general process of dermatophytes infection of skin.[22,23] Therefore, this model was more closely resembling that in vivo T. rubrum infection. Taken together, the inoculum of 400 conidia can be used to mimic host T. rubrum infection and after the infection of at least 10 days, the pathological features conformed to the in vivo infection, during which this model can be used to research the mechanisms involved in T. rubrum infection.
In summary, we established the T. rubrum infection reconstructed epidermis model in vitro successfully. Nevertheless, there are a few unavoidable limitations in the model. First, compared with the normal human epidermis, the RHE is absent of skin appendages such as hair follicles, sweat gland, lack of cutaneous microflora, and no Langerhans cell or merkel cell. Second, the maintenance time of the RHE cultured in vitro is limited. Third, compared with the natural human dermatophyte infection, 400 conidia are still a relative more exposure than human contaminated in their daily life. Considering the time limitation of the RHE, more conidia can also accelerate the course of infection, which facilitates the infection research in the laboratory. Although the reconstructed epidermis cannot behave exactly the same as native human epidermis, the RHE displays many advantages. First, the RHE can avoid ethical risk of acquiring tissues from the dermatophytosis patients since there is no need of proceeding pathological examination in the routine diagnosis of superficial fungal infection. Moreover, the RHE is a promising animal alternative model, which is in accordance with the animal welfare principle. Second, comparing with the animal model, the RHE can avoid the outcome bias caused by species gap because of the anthropophilic feature of T. rubrum. Third, the condition for preparing RHE is stable and standardized, which is beneficial to enhance the repeatability of the experiment. In short, the RHE, composed by fully differentiated keratinocytes with a functional stratum corneum, is a promising model to mimic host dermatophyte infection compared with other models in vitro to research the mechanisms involved in natural human T. rubrum infections.
Acknowledgments
We sincerely thank the L’Oreal Research and Innovation Center (Shanghai, China) for their relevant technical assistance on the EpiSkin kits.
Financial support and sponsorship
This study was supported by the grants from Guangdong Natural Science Foundation (No. 030313137) and the Special Fund for Young Scientists of Third Affiliated Hospital of Sun Yat-sen University (No. A1286).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–52. doi: 10.1007/s11046-008-9100-9. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 2.Nenoff P, Krüger C, Ginter-Hanselmayer G, Tietz HJ. Mycology – An update. Part 1: Dermatomycoses: Causative agents, epidemiology and pathogenesis. J Dtsch Dermatol Ges. 2014;12:188–209. doi: 10.1111/ddg.12245. doi: 10.1111/ddg.12245. [DOI] [PubMed] [Google Scholar]
- 3.Wu LC, Sun PL, Chang YT. Extensive deep dermatophytosis cause by Trichophyton rubrum in a patient with liver cirrhosis and chronic renal failure. Mycopathologia. 2013;176:457–62. doi: 10.1007/s11046-013-9696-2. doi: 10.1007/s11046-013-9696-2. [DOI] [PubMed] [Google Scholar]
- 4.Gönül M, Saraçli MA, Demiriz M, Gül U. Deep Trichophyton rubrum infection presenting with umbilicated papulonodules in a cardiac transplant recipient. Mycoses. 2013;56:361–4. doi: 10.1111/myc.12001. doi: 10.1111/myc12001. [DOI] [PubMed] [Google Scholar]
- 5.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8:240–59. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XJ, Shen YN, Lü GX, Liu WD. Establishing an experimental guinea pig model of dermatophytosis Using Trichophyton rubrum (in Chinese) Acta Acad Med Sin. 2008;30:599–602. doi: 10.3881/jissn1000-503X.2008.05.019. [PubMed] [Google Scholar]
- 7.Aljabre SH, Richardson MD, Scott EM, Shankland GS. Germination of Trichophyton mentagrophytes on human stratum corneum in vitro . J Med Vet Mycol. 1992;30:145–52. [PubMed] [Google Scholar]
- 8.Piérard GE, Arrese JE, De Doncker P. Antifungal activity of itraconazole and terbinafine in human stratum corneum: A comparative study. J Am Acad Dermatol. 1995;32:429–35. doi: 10.1016/0190-9622(95)90064-0. doi: 10.1016/0190-9622(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 9.Rashid A, Scott E, Richardson MD. Early events in the invasion of the human nail plate by Trichophyton mentagrophytes. Br J Dermatol. 1995;133:932–40. doi: 10.1111/j.1365-2133.1995.tb06929.x. doi: 10.1111/j.1365-2133.1995.tb06929.x. [DOI] [PubMed] [Google Scholar]
- 10.Firat YH, Simanski M, Rademacher F, Schröder L, Brasch J, Harder J. Infection of keratinocytes with Trichophytum rubrum induces epidermal growth factor-dependent RNase 7 and human beta-defensin-3 expression. PLoS One. 2014;9:e93941. doi: 10.1371/journal.pone.0093941. doi: 10.1371/journal.pone.0093941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimamura T, Kubota N, Shibuya K. Animal model of dermatophytosis. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/125384. 125384. doi: 10.1155/2012/125384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Chen J, Wan MJ, Lai W, Zheng Y, Li MR, et al. The immune response of human keratinocytes to Trichophyton rubrum conidia is partially mediated by toll-like receptor-2, 4, dectin-1 and cytokines (in Chinese) J Southern Med Univ. 2011;31:678–81. [PubMed] [Google Scholar]
- 13.Huang XQ, Yi JL, Yin SC, Chen RZ, Li MR, Gong ZJ, et al. Exposure to heat-inactivated Trichophyton rubrum resulting in a limited immune response of human keratinocytes. Chin Med J. 2013;126:215–9. doi: 10.3760/cma.j.issn.0366.6999.20122562. [PubMed] [Google Scholar]
- 14.Huang X, Yi J, Yin S, Li M, Ye C, Lai W, et al. Trichophyton rubrum conidia modulate the expression and transport of Toll-like receptor 2 in HaCaT cell. Microb Pathog. 2015;83-84:1–5. doi: 10.1016/j.micpath.2015.04.002. doi: 10.1016/j.micpath.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Yi J, Liu L, Yin S, Chen R, Li M, et al. Substrate adaptation of Trichophyton rubrum secreted endoproteases. Microb Pathog. 2010;48:57–61. doi: 10.1016/j.micpath.2009.12.001. doi: 10.1016/j.micpath.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Qiu J, Zhong L, Zhou M, Chen D, Huang X, Chen J, et al. Establishment and characterization of a reconstructed Chinese human epidermis model. Int J Cosmet Sci. 2015;83:1705–14. doi: 10.1111/ics.12249. doi: 10.1111/ics. 12249. [DOI] [PubMed] [Google Scholar]
- 17.Ponec M, Boelsma E, Gibbs S, Mommaas M. Characterization of reconstructed skin models. Skin Pharmacol Appl Skin Physiol. 2002;15(Suppl 1):4–17. doi: 10.1159/000066682. doi: 10.1159/000066682. [DOI] [PubMed] [Google Scholar]
- 18.Tani K, Adachi M, Nakamura Y, Kano R, Makimura K, Hasegawa A, et al. The effect of dermatophytes on cytokine production by human keratinocytes. Arch Dermatol Res. 2007;299:381–7. doi: 10.1007/s00403-007-0780-7. doi: 10.1007/s00403-007-0780-7. [DOI] [PubMed] [Google Scholar]
- 19.Rashid A, Edward M, Richardson MD. Activity of terbinafine on Trichophyton mentagrophytes in a human living skin equivalent model. J Med Vet Mycol. 1995;33:229–33. [PubMed] [Google Scholar]
- 20.Cao YP. Beijing: Institute of Dermatology Peking Union Medical College; 2011. Construction of in vitro sensitization testing model for chemicals and fungal infected tissue-engineered. [Google Scholar]
- 21.Achterman RR, Moyes DL, Thavaraj S, Smith AR, Blair KM, White TC, et al. Dermatophytes activate skin keratinocytes via mitogen-activated protein kinase signaling and induce immune responses. Infect Immun. 2015;83:1705–14. doi: 10.1128/IAI.02776-14. doi: 10.1128/IAI.02776-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldo A, Monod M, Mathy A, Cambier L, Bagut ET, Defaweux V, et al. Mechanisms of skin adherence and invasion by dermatophytes. Mycoses. 2012;55:218–23. doi: 10.1111/j.1439-0507.2011.02081.x. doi: 10.1111/j. 1439-0507.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 23.Duek L, Kaufman G, Ulman Y, Berdicevsky I. The pathogenesis of dermatophyte infections in human skin sections. J Infect. 2004;48:175–80. doi: 10.1016/j.jinf.2003.09.008. doi: 10.1016/j.jinf.2003.09.008. [DOI] [PubMed] [Google Scholar]


