Abstract
Background:
Vascular endothelial growth factor (VEGF) in the thymus was mainly produced by the thymic epithelial cells (TECs), the predominant component of the thymic microenvironment. The progression of TECs and the roles of VEGF in the neonatal thymus during sepsis have not been reported. This study aimed to explore the alterations of TECs and VEGF level in the neonatal thymus involution and to explore the possible mechanisms at the cellular level.
Methods:
By establishing a model of clinical sepsis, the changes of TECs were measured by hematoxylin-eosin staining, confocal microscopy, and flow cytometry. Moreover, the levels of VEGF in serum and thymus were assessed based on enzyme-linked immunosorbent assay and Western blotting.
Results:
The number of thymocytes and TECs was significantly decreased 24 h after lipopolysaccharide (LPS) challenge, (2.40 ± 0.46)×107 vs. (3.93 ± 0.66)×107 and (1.16 ± 0.14)×105 vs. (2.20 ± 0.19)×105, P < 0.05, respectively. Cortical TECs and medullary TECs in the LPS-treated mice were decreased 1.5-fold and 3.9-fold, P < 0.05, respectively, lower than those in the controls. The number of thymic epithelial progenitors was also decreased. VEGF expression in TECs was down-regulated in a time-dependent manner.
Conclusion:
VEGF in thymic cells subsets might contribute to the development of TECs in neonatal sepsis.
Keywords: Lipopolysaccharide, Thymic Epithelial Cells, Thymus Involution, Vascular Endothelial Growth Factor
INTRODUCTION
Neonates are at high risk of developing sepsis due to quantitative and qualitative insufficiencies of innate immunity. The thymus is the major site for regulatory T-cell development and plays a crucial role in the development of the immune system. The thymus is virtually fully developed at birth,[1] but it is highly susceptible to acute involution induced by a wide array of stresses, such as bacterial infection.[2,3] It was reported that the pro-inflammatory cytokine could lead to thymocyte apoptosis and thereby causing thymus atrophy in adult sepsis.[2,3,4,5,6,7] Thymus atrophy was found to reduce the output of naive T-cells and their repertoire, thereby rendering the host potentially vulnerable to infection.[4] Therefore, it is of great significance to develop strategies of promoting the postinfection functional recovery of thymus for the treatment thymus atrophy.
As we know it, the positive and negative selections of thymocytes are mediated by thymic epithelial cells (TECs), the predominant component in the thymic microenvironment. On the other hand, the development and survival of TECs depend on the presence of thymocytes.[8] On the basis of the discussion, question will naturally arise as to what happens in the response of the neonate to infection and whether the progression of TECs is associated with the events.
Lipopolysaccharide (LPS) is an endotoxin released by Gram-negative bacteria that represent the major pathogenic bacteria of sepsis in the developing countries. LPS stimulation contributes to vascular remodeling and the vascular endothelial growth factor (VEGF) in one of the important factors that promotes the migration, growth, and survival of endothelial cells.[9] A recent study examined the role VEGF in the postnatal thymus and found that VEGF was produced by immature thymocytes and TECs in the neonatal but not in the adult thymus,[10] and functioned as an immune-regulatory factor in the normal thymus.[11] VEGF does not induce thymocyte apoptosis, but, instead, rapidly decreases the number of the earliest observable progenitors in the thymic tumor.[12] The roles of VEGF in the neonatal thymus during sepsis and the underlying mechanisms have not reported. In this study, we examined time-dependent alterations of VEGF levels in neonatal thymus involution and explored the possible mechanisms at cellular levels.
METHODS
Animals
C57BL/6 mice were provided by the Animal Center of Huazhong University of Science and Technology, Wuhan, China (Certificate No. SCXK 2010-0009). Neonatal mice (aged 5–7 days)[13,14] were randomly assigned to experimental groups and kept with their mother until completion of the experiments. The effort was made to minimize cannibalization by employing previously reported methods.[13] The animals were maintained at 25 ± 1°C and in 60 ± 10% humidity under a 12 h light-dark cycle during the experiments. All animals were handled in strict accordance with the protocols formulated by the Animal Welfare and Research Ethics Committee of the Institute of Chinese Academy of Sciences, China.
Models of neonatal sepsis
Neonatal mice were injected intraperitoneally with either phosphate buffer saline (PBS) or 10 mg LPS (Escherichia coli 055: B5, Sigma-Aldrich, USA).[15] Mice were sacrificed at different time points (6 h, 12 h, and 24 h) after LPS injection and their tissues were subjected to further processing as described below. The blood samples were harvested by cardiac puncture.
Hematoxylin-eosin staining for morphological examination
For the morphologic study, 5-μm sections of paraffin-embedded thymi were hematoxylin-eosin stained, and sections were observed under a microscope equipped with a Spot Insight QE camera (Olympus Corporation, Tokyo, Japan).
Confocal microscopy
Frozen thymic sections were stained with rabbit anti-mouse keratin (K) 5 and rat anti-mouse K8 monoclonal antibody (Covance Research Products, USA), and then with Alexa Fluor-488-, and 546-conjugated goat anti-rat immunoglobulin G (IgG), and goat anti-rabbit IgG (Invitrogen, USA). The sections were then observed under a Nikon A1R Spectral Confocal Microscope (Nikon, Kanagawa, Japan).
Thymic epithelial cell isolation
The thymi were incubated at 37°C in 0.01 (w/v) liberase (Roche, USA) and 0.02% (w/v) DNase I (Roche, USA) with gentle agitation as previously described.[16] The supernatant fractions were collected into a fresh tube placed on ice and containing PBS buffer with 0.02% (v/v) bovine serum albumin (Sigma, USA) and 5 mmol/L ethylenediaminetetraacetic acid to terminate the digestion. The thymic fragments were incubated in a fresh enzyme mix, and the incubation/digestion cycle was repeated 3 times. Cells were washed with and resuspended in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal calf serum (Hyclone, USA).
Flow cytometric analysis
Single-cell suspensions of thymocytes and TECs cells were stained with one or more of the following fluorochrome-conjugated antibodies: Allophycocyanin (APC)-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-CD8, PerCP/Cy5.5-conjugated anti-epithelial cellular adhesion molecule (EpCAM) 1, fluorescein isothiocyanate-conjugated anti-major histocompatibility complex (MHC) II, PE-conjugated anti-ulex europeus lectin (UEA) and APC-conjugated anti-CD45.1 (BioLegend, BD Biosciences, USA). The samples were analyzed on a FACSCalibur flow cytometer (Becton and Dickinson, USA). Data analysis was conducted using FlowJo software package (TreeStar, Inc., Ashland, USA).
Enzyme-linked immunosorbent assay for vascular endothelial growth factor levels
VEGF levels (R and D Systems, MN, USA) in the serum and the TECs were determined by the enzyme-linked immunosorbent assay (ELISA) method, and the ELISA was performed by following the manufacturer's instructions.
Western blot analysis
Proteins were extracted from TECs by using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, USA). Equal amount of proteins was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride filter (PVDF) membranes, and then incubated with antibodies. The membranes were developed with SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, USA). In brief, samples were boiled in SDS sample buffer for 7 min at 80°C, loaded onto a 10% SDS-PAGE, subjected to electrophoresis for 60 min, and electrophoretically transferred to PVDF membranes. To reduce nonspecific binding, the membrane was blocked with 5% nonfat milk in PBS containing 0.1% Tween 20, incubated overnight at 4°C with primary antibodies in PBS-Tween buffer, washed 3 times with PBS-Tween buffer, and then incubated with a suitable secondary antibody for 60 min at room temperature. The following antibodies were used: β-actin (Cell Signaling Technology Inc., USA), rabbit anti-human VEGF, antibody against phospho-p38 (p-p38) and total p38 (Santa Cruz Biotechnology, Inc., USA), and horseradish peroxidase-conjugated secondary antibody.
Statistical analysis
All experiments were repeated at least 3 times. Data were presented as a mean ± standard error (SE). Statistical analysis was performed using a one-way analysis of variance, followed by Tukey honest significant difference test for multiple comparisons. The differences were considered statistically significant when P < 0.05.
RESULTS
Acute thymic atrophy induced by lipopolysaccharide challenge
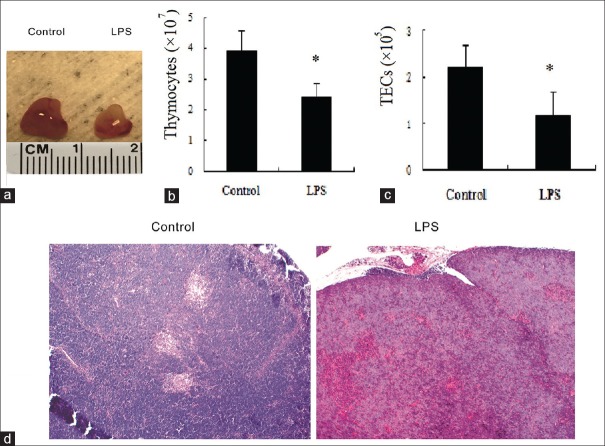
The chest was opened to harvest neonatal thymus after LPS challenge. Twenty-four hour after the treatment, thymus size was found to be significantly smaller in LPS-exposed mice than in shame-treated mice (only PBS was given). Compared with shame-treated mice, the thymus of LPS-treated mice looked paler [Figure 1a]. The number of thymocytes and TECs was significantly decreased at 24 h post-LPS challenge, (2.40 ± 0.46)×107 vs. (3.93 ± 0.66)×107 and (1.16 ± 0.14)×105 vs. (2.20 ± 0.19)×105, P < 0.05, respectively [Figure 1b and 1c]. Normal neonatal thymus predominantly consisted of the cortex, with a very small medullary area. The neonatal murine thymus showed congestion and the medullary area of the thymus in LPS-treated mice began to shrink 24 h after exposure to LPS [Figure 1d].
Figure 1.
Lipopolysaccharide-induced acute thymic atrophy. Mice were administered either phosphate buffer saline or lipopolysaccharide. (a) The size and appearance of thymus. (b) Total number of thymocytes. (c) Total number of thymic epithelial cells. (d) Representative hematoxylin-eosin stains (original magnification, ×10) of thymus for controls and 24-h lipopolysaccharide-exposed animals. Values are mean ± standard error (n = 6). *P < 0.05 with respect to the control mice. LPS: Lipopolysaccharide.
Reduced thymic epithelial cells and thymocytes in response to lipopolysaccharide
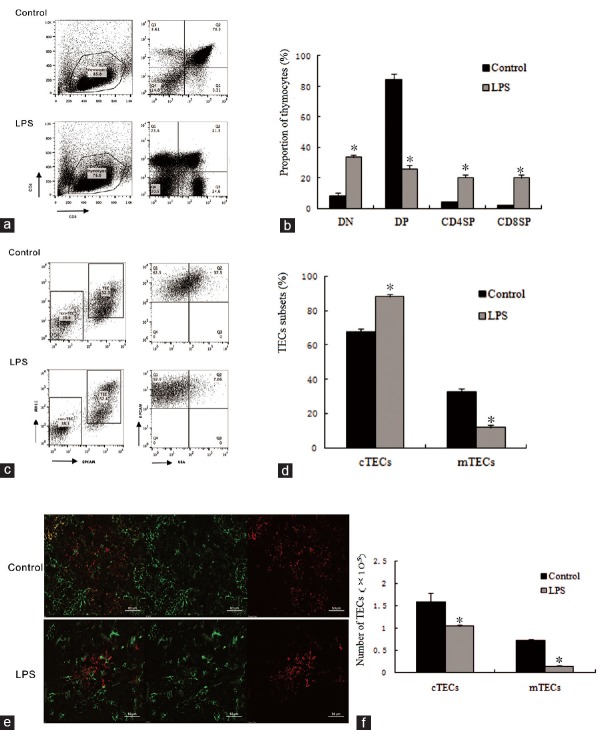
FACS analysis showed that the thymocyte subsets include CD4 and CD8 double-negative (CD4− CD8− DN) cells, double-positive (CD4+CD8+DP) cells, and CD4+CD8− or CD4− CD8+single-positive (CD4+CD8− SP or CD4− CD8+SP) cells. The immature SP cells represent the transitional state between the CD4− CD8− DN and CD4+CD8+DP cells. TECs can be broadly divided into cortical TECs (cTECs, CD45− EpCAM1+MHCII+UEA−) and medullary TECs (mTECs, CD45− EpCAM1+MHCII+UEA+). Immunohistochemical staining showed that the TECs subsets contained K5− K8+cortical epithelial cells (green-stained), or cTECs, and K5+K8− medullary epithelial cells (red-stained), or mTECs. Thymocytes subset distribution pattern was significantly altered 24 h after LPS treatment. The late-stage cells of thymopoiesis (DP cells) exhibited a dramatically decreased proportion [Figure 2a and 2b]. The total number of thymocytes was decreased since the populations of both DN immature T-cell precursors and DP cells were reduced. Although the proportion of SP was changed, the change was not statistically significant. The proportion of TECs subsets also underwent alternation [Figure 2c and 2d]. Specifically, the number of cTECs and mTECs in the LPS-treated mice were decreased 1.5-fold and 3.9-fold, P < 0.05, respectively, lower than in their controls [Figure 2f]. The number of K5+K8+cells (yellow-stained), representing thymic epithelial progenitors (TEPs), was also decreased [Figure 2e].
Figure 2.
Lipopolysaccharide-induced acute thymic atrophy and the cells subsets of thymus after a 24 h lipopolysaccharide treatment. (a) Representative flow cytometry plots of thymocyte subsets (CD4, CD8 double-negative [DN], double-positive [DP], or single-positive [SP]) were determined. (b) Proportion of thymocytes subsets. Percentage of thymocytes subsets were calculated based on the fluorescence-activated cell sorting profiles in the control and lipopolysaccharide treatment groups. (c) Representative flow cytometry plots of medullary thymic epithelial cells and cortical thymic epithelial cells. Cell subsets were determined based on the fluorescence-activated cell sorting profiles gated from CD45−EpCAM1+MHCII+cells, cortical thymic epithelial cells (CD45−EpCAM1+MHCII+UEA−) and medullary thymic epithelial cells (CD45−EpCAM1+MHCII+UEA+). (d) Frequency of thymic epithelial cells subsets were quantified by the fluorescence-activated cell sorting after a 24 h lipopolysaccharide challenge. (e) Immunohistochemical staining showed the thymic epithelial cell subsets, K5−K8+ cells (green-stained) represents cortical thymic epithelial cells, K5+K8− cells (red-stained) represents medullary thymic epithelial cells, and K5+K8+ cells (yellow-stained) represents thymic epithelial progenitors. Scar bar is 50 μm. (f) Total number of medullary thymic epithelial cells and cortical thymic epithelial cells determined by the fluorescence-activated cell sorting. Values are mean ± standard error (n = 5). *P < 0.05 with respect to the control mice. EpCAM: Epithelial cellular adhesion molecule; MHC: Major histocompatibility complex; UEA: Ulex europeus lectin; K: Keratin. LPS: Lipopolysaccharide; TECs: thymic epithelial cells; cTECs: cortical thymic epithelial cells; mTECs: medullary thymic epithelial cells.
Levels of vascular endothelial growth factor in serum and in thymus
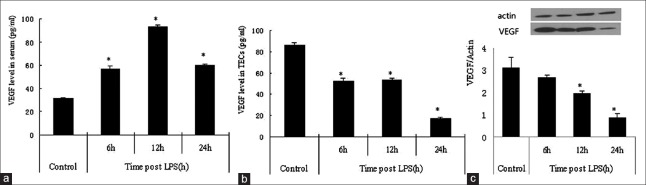
Serum VEGF level was elevated at 6 h and 12 h after LPS administration in the sepsis mice model, but was decreased in 24 h [Figure 3a]. Local VEGF productions varied with different in tissues after LPS stimulated. In the neonatal thymus, VEGF is locally produced by TECs and few immature thymocyte subsets.[10] After LPS challenge, VEGF expression in TECs was down-regulated in a time-dependent manner [Figure 3b and 3c]. Moreover, our study showed that TECs numbers and VEGF level was decreased simultaneously.
Figure 3.
The expression of vascular endothelial growth factor in the control and lipopolysaccharide-treated mice. (a) The vascular endothelial growth factor level in the serum; the vascular endothelial growth factor protein expression levels of vascular endothelial growth factor in the thymic epithelial cells were analyzed by enzyme-linked immunosorbent assay (b) and Western blot analysis. (c) Values are mean ± standard error (n = 5). *P < 0.05 with respect to the control mice. VEGF: vascular endothelial growth factor; LPS: Lipopolysaccharide; TECs: thymic epithelial cells.
The p38 pathway in thymic epithelial cells by lipopolysaccharide challenge
TECs were isolated from the thymi of neonatal mice after they were exposed to LPS for 24 h. Western blotting showed that exposure to LPS for 24 h decreased the protein expression of p-p38 in TECs after the LPS treatment as compared with that of the shame-treated mice [Figure 4].
Figure 4.
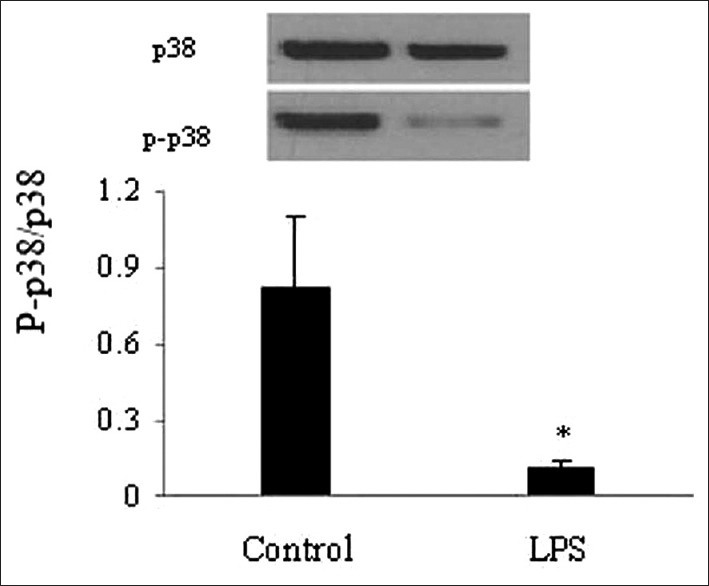
Lipopolysaccharide block the phosphorylated of p38 signaling pathway in the thymic epithelial cells of neonatal mice. The level of p38 and phospho-p38 were measured by Western blot. Values are mean ± standard error (n = 4). *P < 0.05 with respect to the control mice. LPS: Lipopolysaccharide.
DISCUSSION
LPS induces acute involution of the adult and fetal thymus[2,3,17,18] and, as a consequence impairs immune functions. It is of great importance to understand, in general, the alternation of the neonatal thymus in sepsis, and in specific, differences in cell subsets between the neonatal and adult thymusin the event.[10] In this study, we demonstrated that the specific loss of DP thymocytes and TECs in sepsis substantially reduced the output of naive T-cells and the VEGF expression in TECs was down-regulated in a time-dependent fashion.
T-cell development, which is critical for neonatal immune responses, takes place primarily in the thymus, and is fundamentally dependent on the thymic microenvironment. TECs are the principal source of immune-regulatory molecules and perform a number of irreplaceable functions that are essential for thymocyte growth and maturation. Our data demonstrated that not only thymocytes but also TECs contribute to thymus involution in the neonatal sepsis. Moreover, we showed that the number of TEPs was also decreased during the thymus involution, suggesting that both mature TECs and immature TECs, including TEPs, are responsible for thymus involution. After endotoxin challenge, both systemic and intrathymic pro-inflammatory cytokine cascade are activated. As a result, the thymic cellularity was diminished and thymocyte distribution profile was changed.[6] Moreover, our study also showed that LPS exerted a strong impact on the immature thymocytes and mTECs in our neonatal sepsis model, which was found to have a higher proportion of immature thymocytes.[10] Furthermore, the neonatal TEC dominance was rapidly changed to an adult pattern, which is characterized by, among others, alternation from cTEC dominance to mTEC dominance.[10]
Recent studies demonstrated that plasma VEGF levels were increased during severe sepsis.[19,20,21,22,23] However, with the condition progressing to septic shock, the VEGF levels dropped, and the reduced VEGF levels were found to be associated with poor outcome.[19,21] Our data, consistent with these findings, showed that the plasma VEGF levels were increased in the first 12 h, and then quickly went down in neonatal sepsis. A previous study showed that the prolonged (>21 days) and high-dose (>100 ng/ml) systemic administration of VEGF in adult cancer mice could block thymocyte precursors from leaving the bone marrow and traveling to the thymus, resulting in thymic atrophy.[12] However, our study found that the level of systemic VEGF was transiently increased and lower than 100 ng/ml. Thus, it is most likely that serum VEGF did not work on TECs in at the early stage of sepsis. VEGF expression is organ-specific during sepsis, and it was significantly elevated in the heart[22] and hepatic tissues,[24] but was decreased in the lung[23] and the renal tissue.[25] Different from adult thymus, VEGF message was found to be highest in neonatal TECs, but whether local VEGF expression had an effect on thymus atrophy in neonatal sepsis remains unknown. In this study, we demonstrated that local VEGF of the thymus was decreased time-dependently because TECs number was reduced. Moreover, we found that DN immature T-cell precursors and DP cells were reduced with the disease progression. Of note, there were some critical differences in thymocytes between neonatal and adult thymus in terms of stages of thymic development and the expressions of local VEGF and VEGF-receptor 2, both being found to have significantly higher expression in neonatal TECs than in adult thymus.[10] It is possible that, at the early phase of thymic development, the thymus tends to be specifically affected by elevated local VEGF. According to a previous study, VEGF rapidly decreased the number of the earliest observable progenitors in the thymus.[12] The local production of VEGF supports and/or maintains thymic endothelia, which, in turn, influence thymopoiesis.[10]
The expression pattern of local VEGF in the signal pathway of neonatal thymus had not been well-understood. As we know it, mitogen-activated protein kinase signaling is a quite unique proliferation-initiating process. The p38 was found to play a critical role in the regulation of VEGF production in neonatal stem cells.[26] LPS-induced VEGF production depends on the p38 activation in macrophages.[27] Our study showed that the p-p38 was blocked in the thymus of LPS-challenged mice, suggesting that TECs respond directly to LPS and p-p38 inactivation is involved in the further differentiation of thymocytes from immature to maturity.[28,29] The p-p38 was present in the entire Hassall's corpuscles,[30] VEGF is significantly highly expressed in neonatal TECs.[10] Hence, we are led to speculate that, unlike in adult thymus, elevated VEGF, might be due to the difference in p-p38 signaling pathway in the neonatal thymus.[10] In this study, we only demonstrated that specific roles of p38 signaling pathway in the thymic involution after bacterial infection. Nonetheless, it remains unclear which of these processes is the initial event and whether other signal pathways are involved.
In conclusion, we, by establishing a model of clinical sepsis, showed that VEGF in thymic cells subsets might contribute to the development of TECs in neonatal sepsis. Further investigation is warranted to explore the strategies of promoting the functional recovery of thymus by elevating VEGF levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–6. doi: 10.1023/a:1006611518223. doi: 10.1023/A:1006611518223. [DOI] [PubMed] [Google Scholar]
- 2.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–21. [PubMed] [Google Scholar]
- 3.Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PLoS One. 2011;6:e17940. doi: 10.1371/journal.pone.0017940. doi: 10.1371/journal.pone.0017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberholzer C, Oberholzer A, Bahjat FR, Minter RM, Tannahill CL, Abouhamze A, et al. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc Natl Acad Sci U S A. 2001;98:11503–8. doi: 10.1073/pnas.181338198. doi: 10.1073/pnas.181338198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–23. doi: 10.1189/jlb.0108025. doi: 10.1189/jlb. 0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177:169–76. doi: 10.4049/jimmunol.177.1.169. doi: 10.4049/jimmunol.1771169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crompton T, Outram SV, Hager-Theodorides AL. Sonic hedgehog signalling in T-cell development and activation. Nat Rev Immunol. 2007;7:726–35. doi: 10.1038/nri2151. doi: 10.1038/nri2151. [DOI] [PubMed] [Google Scholar]
- 8.van Ewijk W, Holländer G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–91. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 9.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 10.Cuddihy AR, Ge S, Zhu J, Jang J, Chidgey A, Thurston G, et al. VEGF-mediated cross-talk within the neonatal murine thymus. Blood. 2009;113:2723–31. doi: 10.1182/blood-2008-06-162040. doi: 10.1182/blood-2008-06-162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cimpean AM, Raica M, Encica S, Cornea R, Bocan V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat. 2008;190:238–45. doi: 10.1016/j.aanat.2007.05.003. doi: 10.1016/j.aanat.200705003. [DOI] [PubMed] [Google Scholar]
- 12.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–86. doi: 10.1182/blood-2002-07-1956. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 13.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–683. doi: 10.1097/SHK.0b013e3180556d09. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 14.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 15.Sepehr R, Audi SH, Maleki S, Staniszewski K, Eis AL, Konduri GG, et al. Optical imaging of lipopolysaccharide-induced oxidative stress in acute lung injury from hyperoxia and sepsis. J Innov Opt Health Sci. 2013;6:1350017. doi: 10.1142/S179354581350017X. doi: 10.1142/S179354581350017X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seach N, Wong K, Hammett M, Boyd RL, Chidgey AP. Purified enzymes improve isolation and characterization of the adult thymic epithelium. J Immunol Methods. 2012;385:23–34. doi: 10.1016/j.jim.2012.07.023. doi: 10.1016/j.jim. 2012.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Kuypers E, Collins JJ, Jellema RK, Wolfs TG, Kemp MW, Nitsos I, et al. Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One. 2012;7:e38257. doi: 10.1371/journal.pone.0038257. doi: 10.1371/journal.pone.0038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuypers E, Wolfs TG, Collins JJ, Jellema RK, Newnham JP, Kemp MW, et al. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod Sci. 2013;20:946–56. doi: 10.1177/1933719112472742. doi: 10.1177/1933719112472742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–8. doi: 10.1097/01.shk.0000150728.91155.41. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 20.Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, et al. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39:427–32. doi: 10.1097/SHK.0b013e3182903f0d. doi: 10.1097/SHK.0b013e3182903f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoilescu I, Teleman S, Cojocaru E, Mihaila D, Plamadeala P. Vascular endothelial growth factor (VEGF) expression in the lung in toxic septic shock. Rom J Morphol Embryol. 2011;52(1 Suppl):309–13. [PubMed] [Google Scholar]
- 22.Oki M, Jesmin S, Islam MM, Mowa CN, Khatun T, Shimojo N, et al. Dual blockade of endothelin action exacerbates up-regulated VEGF angiogenic signaling in the heart of lipopolysaccharide-induced endotoxemic rat model. Life Sci. 2014;118:364–9. doi: 10.1016/j.lfs.2014.02.008. doi: 10.1016/j.lfs. 2014.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Jesmin S, Zaedi S, Islam AM, Sultana SN, Iwashima Y, Wada T, et al. Time-dependent alterations of VEGF and its signaling molecules in acute lung injury in a rat model of sepsis. Inflammation. 2012;35:484–500. doi: 10.1007/s10753-011-9337-1. doi: 10.1007/s10753-011-9337-1. [DOI] [PubMed] [Google Scholar]
- 24.Zaedi S, Jesmin S, Yamaguchi N, Shimojo N, Maeda S, Gando S, et al. Altered expression of endothelin, vascular endothelial growth factor, and its receptor in hepatic tissue in endotoxemic rat. Exp Biol Med (Maywood) 2006;231:1182–6. [PubMed] [Google Scholar]
- 25.Xu C, Chang A, Hack BK, Eadon MT, Alper SL, Cunningham PN. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. 2014;85:72–81. doi: 10.1038/ki.2013.286. doi: 10.1038/ki.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markel TA, Wang M, Crisostomo PR, Manukyan MC, Poynter JA, Meldrum DR. Neonatal stem cells exhibit specific characteristics in function, proliferation, and cellular signaling that distinguish them from their adult counterparts. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1491–7. doi: 10.1152/ajpregu.00031.2008. doi: 10.1152/ajpregu.000312008. [DOI] [PubMed] [Google Scholar]
- 27.Koide N, Odkhuu E, Naiki Y, Tsolmongyn B, Ito K, Komatsu T, et al. Augmentation of LPS-induced vascular endothelial cell growth factor production in macrophages by transforming growth factor-ß1. Innate Immun. 2014;20:816–25. doi: 10.1177/1753425913509291. doi: 10.1177/1753425913509291. [DOI] [PubMed] [Google Scholar]
- 28.Fernández E. Thymocyte development past the CD4(+) CD8(+) stage requires an active p38 mitogen-activated protein kinase. Blood. 2000;95:1356–61. [PubMed] [Google Scholar]
- 29.Diehl NL, Enslen H, Fortner KA, Merritt C, Stetson N, Charland C, et al. Activation of the p38 mitogen-activated protein kinase pathway arrests cell cycle progression and differentiation of immature thymocytes in vivo. J Exp Med. 2000;191:321–34. doi: 10.1084/jem.191.2.321. doi: 10.1084/jem. 191.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishio H, Matsui K, Tsuji H, Tamura A, Suzuki K. Immunolocalization of the mitogen-activated protein kinase signaling pathway in Hassall's corpuscles of the human thymus. Acta Histochem. 2001;103:89–98. doi: 10.1078/0065-1281-00581. doi: 10.1078/0065-1281-00581. [DOI] [PubMed] [Google Scholar]