Abstract
Background:
Postmastectomy pain syndrome (PMPS) is defined as a chronic (continuing for 3 or more months) neuropathic pain affecting the axilla, medial arm, breast, and chest wall after breast cancer surgery. The prevalence of PMPS has been reported to range from 20% to 68%. In this study, we aimed to determine the prevalence of PMPS among mastectomy patients, the severity of neuropathic pain in these patients, risk factors that contribute to pain becoming chronic, and the effect of PMPS on life quality.
Methods:
This cross-sectional study was approved by the Sakarya University, Medical Faculty Ethical Council and included 146 patients ranging in age from 18 to 85 years who visited the pain clinic, general surgery clinic, and oncology clinic and had breast surgery between 2012 and 2014. Patients were divided into two groups according to whether they met PMPS criteria: pain at axilla, arm, shoulder, chest wall, scar tissue, or breast at least 3 months after breast surgery. All patients gave informed consent prior to entry into the study. Patient medical records were collected, and pain and quality of life were evaluated by the visual analog scale (VAS) for pain, a short form of the McGill Pain Questionnaire (SF-MPQ), douleur neuropathique-4 (DN-4), and SF-36.
Results:
Patient mean age was 55.2 ± 11.8 years (33.0–83.0 years). PMPS prevalence was 36%. Mean scores on the VAS, SF-MPQ, and DN-4 in PMPS patients were 1.76 ± 2.38 (0–10), 1.73 ± 1.54 (0–5), and 1.64 ± 2.31 (0–8), respectively. Of these patients, 31 (23.7%) had neuropathic pain characteristics, and 12 (9.2%) had phantom pain according to the DN-4 survey. Patients who had modified radical mastectomy were significantly more likely to develop PMPS than patients who had breast-protective surgery (P = 0.028). Only 2 (2.4%) of PMPS patients had received proper treatment (anticonvulsants or opioids).
Conclusions:
PMPS seriously impacts patients’ emotional situation, daily activities, and social relationships and is a major economic burden for health systems. We conclude that the rate of PMPS among patients receiving breast cancer surgery in Turkey is 64.1% and that challenges to the proper treatment of these patients deserve further investigation.
Keywords: Breast Cancer, Mastectomy, Neuropathic Pain, Postmastectomy Pain, Surgery
INTRODUCTION
Breast cancer is currently the most common type of cancer in females; approximately, one million new cases are diagnosed every year, and this number is expected to increase in future decades. Current standard surgery options for invasive breast carcinoma are breast-protective surgery and modified radical mastectomy. Patients with residual tumors following surgery, T3–T4 stage tumors, metastasis to four or more axillar lymph nodes, or extracapsular disease at axillary lymph nodes are treated with adjuvant chemotherapy indications after surgery. The probabilities of disease-free recovery and general survival are further increased with postmastectomy radiotherapy in these cases.[1] The side effects of these treatment approaches, especially pain and life quality, deserve a thorough investigation. Postmastectomy pain syndrome (PMPS) consists of chronic neuropathic pain following breast surgery. The prevalence of PMPS has been reported to range from 20% to 68%.[2,3,4] PMPS is defined as pain of the axilla, medial arm, breast, or chest wall that continues for at least 3 months after breast surgery and is not caused by infection. Chronic pain seriously impacts patients’ emotional condition, daily activities, and social life and is a major economic burden for health systems.[5,6] Factors affecting the likelihood of progression of postsurgery pain to a chronic condition include adjuvant treatment, age, gender, psychosocial status, pain before surgery, surgery type, analgesic treatment, and genetic factors;[7,8] their relative impacts have not been determined.
The purpose of this cross-sectional study was to examine the prevalence of PMPS and the treatment-related and physiological factors associated with the syndrome in Turkey. The secondary goal was to clarify the severity of PMPS pain and its impact on daily life.
METHODS
This cross-sectional study was approved by the Sakarya University, Medical Faculty Ethical Council. Patients were recruited from those visiting the pain, general surgery, and oncology clinics of the Sakarya University Hospital between 2012 and 2014 and had breast surgery under general anesthesia during that period. A patient who had breast cancer surgery provided written informed consent.
Exclusion criteria included infection of the breast tissue (detected by pathology following surgery), American Society of Anesthesiologists score of III, secondary tumors or metastases. Only patients whose surgery took place at least 3 months prior and were 18–85 years old were included.
Collection of medical records
Demographic data and information on risk factors related to the development of chronic pain were collected from the hospital information system. Specifically, age, weight, height, marital status, diabetes mellitus and hypertension diagnoses, type of surgery, the number of removed sentinel lymph and axillar nodes, estrogen and progesterone receptor status, and adjuvant treatment protocol (neoadjuvant chemotherapy, radiotherapy, chemotherapy, and hormone therapy) were recorded.
Study procedure
Patients were informed whether they met study criteria, and informed consents were obtained from patients who agreed to participate. All patients were assessed using the short form of the McGill Pain Questionnaire (SF-MPQ),[9] SF-36.[10] and douleur neuropathique-4 [DN-4, Table 1][11] Pain scores on the visual analog scale (VAS) and all requested medical documents were collected. Patients were assigned to two groups according to whether they met the PMPS diagnostic criteria.
Table 1.
DN-4 questionnaire, Turkish version[10]
| Questions | Symptoms | Answers |
|---|---|---|
| 1: Does the pain has one or more of the following characteristics? | Burning | Yes (1)/no (0) |
| Painful cold | Yes (1)/no (0) | |
| Electric shocks | Yes (1)/no (0) | |
| 2: Is the pain associated with one or more of the following symptoms in the same area? | Tingling | Yes (1)/no (0) |
| Pins and needles | Yes (1)/no (0) | |
| Itching | Yes (1)/no (0) | |
| Examination of patient | Yes (1)/no (0) | |
| 3: Does the pain located in the area where the physical examination was done reveal one or more of the following characteristics? | Hypoesthesia to touch | Yes (1)/no (0) |
| Hypoesthesia to pinprick | Yes (1)/no (0) | |
| 4: In the painful area, can the pain be caused or increased by brushing? | Yes (1)/no (0) |
Total score = 10. If the patient score is ≥4, neuropathic pain is diagnosed. DN-4: Douleur neuropathique-4.
Surgical technique
Patients were treated with breast-protective surgery or modified radical mastectomy according to standard recommendations. In patients treated with breast-protective surgery, axillar lymph nodes were either resected (level I–II) if palpable prior to surgery or biopsied during the procedure. Sentinel lymph nodes were identified by isosulfan blue dye mapping. If malignancy was confirmed histologically in the sentinel lymph node, axillar nodes were similarly resected; surgery was limited to the breast in cases without lymph node malignancy. Modified radical mastectomy consisted of resection of all breast tissues and level I–II axillary lymph nodes.
Design of survey form
Questionnaires consisted of three parts. The first evaluated pain severity according to the VAS (0: No pain, to 10: Worst imaginable pain). The second part evaluated pain characteristics using the SF-MPQ. The SF-MPQ describes the temporal characteristics of pain, recognizing the time of appearance after surgery, and the timing of occurrence. Patients identify pain location on body charts and describe it as dull, pins and needles, burning, stabbing, or ache. The third part assessed the quality of life using the SF-36. The SF-36 assesses eight scales: Physical function (PF), role limitations due to physical problems (role physical), body pain, general health, vitality, social function, role limitations due to emotional problems (role emotional), and mental health (MH). The raw scores of each subscale were converted to range from 0 to 100: Higher scores indicated better levels of functioning or well-being. Both questionnaires have previously been used in breast cancer surgery research.[5]
The probability of developing chronic neuropathic pain was assessed during a regular postoperative examination in the pain clinic using the DN-4.
Statistical analysis
Categorical data were shown as counts (n) and percentages (%). A Chi-squared test was used to compare categorical data. Continuous data were presented as means ± standard deviation (SD). Normality of continuous data was evaluated using the Kolmogorov-Smirnov test. Two independent-samples t-tests were used to compare parametric continuous data between groups. One-way analysis of variance (ANOVA) was used to compare parametric continuous data among groups (for multiple post-hoc comparisons, ANOVA Tukey or Tamhane tests were used). Pearson's correlations coefficients were calculated to determine correlations among VAS, DN-4, McGill, and SF-36 scores. P < 0.05 were considered to indicate statistical significance. Analyses were performed using the SPSS statistics software (version 22.0, IBM Corp., Armonk, NY, USA).
RESULTS
This study included 146 breast cancer surgery patients. Of these, 15 were excluded because they chose not to provide all necessary information. Of the 131 patients that remained, 47 without surgery-related pain were classified as the non-PMPS group, while 84 patients had pain and were considered affected by PMPS.
Demographic characteristics
No demographic differences between the groups were found [P > 0.05, Table 2]. Specifically, PMPS and non-PMPS patients did not differ according to education status; 75 (57.3%) patients were educated only through primary school. The majority of patients (115, 87.5%) were homemakers and smokers (99, 75.6%) while none reported alcohol consumption.
Table 2.
Demographic data
| Characteristics | Non-PMPS (n = 47) | PMPS (n = 84) | Total (n = 131) | P |
|---|---|---|---|---|
| Age (years) | 57.1 ± 11.9 | 54.2 ± 11.7 | 55.2 ± 11.8 | 0.173 |
| Weight (kg) | 75.57 ± 15.73 | 77.07 ± 15.60 | 76.53 ± 15.60 | 0.600 |
| Height (cm) | 161.09 ± 8.26 | 159.43 ± 7.47 | 160.02 ± 7.77 | 0.243 |
| BMI (kg/m2) | 29.10 ± 5.79 | 30.29 ± 5.61 | 29.86 ± 5.68 | 0.251 |
| Marital status, n (%) | ||||
| Married | 42 (89.4) | 80 (95.2) | 122 (93.1) | 0.281 |
| Single | 5 (10.6) | 4 (4.8) | 9 (6.9) | |
| Number of children | 2.7 ± 1.6 | 2.6 ± 1.3 | 2.6 ± 1.4 | 0.647 |
| Education status, n (%) | ||||
| No literacy | 11 (23.4) | 16 (19) | 27 (20.6) | 0.820 |
| Primary school | 24 (51.1) | 51 (60.7) | 75 (57.3) | |
| Middle school | 4 (8.5) | 4 (4.8) | 8 (6.1) | |
| High school | 4 (8.5) | 7 (8.3) | 11 (8.4) | |
| University | 4 (8.5) | 6 (7.1) | 10 (7.6) | |
| Occupation, n (%) | ||||
| Housewife | 44 (93.6) | 71 (84.5) | 115 (87.8) | 0.217 |
| Laborer | 0 (0) | 7 (8.3) | 7 (5.3) | |
| Teacher | 1 (2.1) | 3 (3.6) | 4 (3.1) | |
| Officer | 2 (4.3) | 3 (3.6) | 5 (3.8) |
Data are represented as mean ± SD and n (%). SD: Standard deviation; PMPS: Postmastectomy pain syndrome; BMI: Body mass index.
Risk factors
We identified two key risk factors for PMPS among breast cancer surgery patients: Tumor localization in the upper lateral quarter and radiotherapy. Risk factors not related to PMPS risk included age, surgery type, tumor diameter, tumor grade, estrogen and progesterone receptor status, amount of removed sentinel node, the amount of removed lymph node, lymphedema, preoperative neoadjuvant chemotherapy, postoperative chemotherapy, and postoperative hormone therapy [Table 3].
Table 3.
Potential risk factors for PMPS
| Items | Non-PMPS (n = 47) | PMPS (n = 84) | Total (n = 131) | P |
|---|---|---|---|---|
| Surgery type, n (%) | ||||
| MRM - ALND | 34 (72.3) | 63 (75) | 97 (74.0) | 0.900 |
| BCS - ALND | 13 (27.7) | 21 (25) | 34 (26.0) | |
| Tumor diameter (cm) | 3.32 ± 1.82 | 2.93 ± 1.22 | 3.07 ± 1.47 | 0.145 |
| Grade, n (%) | ||||
| 1 | 5 (10.6) | 10 (11.9) | 15 (11.5) | 0.379 |
| 2 | 23 (48.9) | 50 (59.5) | 73 (55.7) | |
| 3 | 19 (40.4) | 24 (28.6) | 43 (32.8) | |
| 4 | 0 | 0 | 0 | |
| Estrogen receptor involvement, n (%) | 34 (72.3) | 57 (68.7) | 91 (70) | 0.811 |
| Progesterone receptor involvement, n (%) | 28 (59.6) | 51 (61.4) | 79 (60.8) | 0.982 |
| Sentinel LR, n (%) | 20 (42.6) | 45 (53.6) | 65 (49.6) | 0.304 |
| Number of removed sentinel nodes | 3.40 ± 4.27 | 3.22 ± 2.85 | 3.28 ± 3.32 | 0.593 |
| Number of removed axillar nodes | 14.09 ± 8.22 | 12.43 ± 7.07 | 13.03 ± 7.52 | 0.844 |
| Tumor localization within breast, n (%) | ||||
| Upper lateral quarter | 31 (66.0) | 49 (59.8) | 80 (62.0) | 0.483 |
| Upper interior quarter | 5 (10.6) | 14 (17.1) | 19 (14.7) | |
| Lower lateral quarter | 10 (21.3) | 13 (15.9) | 23 (17.8) | |
| Lower interior quarter | 0 (0) | 3 (3.7) | 3 (2.3) | |
| Retroareolar | 1 (2.1) | 3 (3.7) | 4 (3.1) | |
| Lymphedema, n (%) | 8 (17.0) | 12 (14.3) | 20 (15.3) | 0.869 |
| Neoadjuvant chemotherapy, n (%) | 1 (2.2) | 7 (8.3) | 8 (6.2) | 0.259 |
| Postoperative radiotherapy, n (%) | 27 (58.7) | 66 (78.6) | 93 (71.5) | 0.028* |
| Postoperative chemotherapy, n (%) | 43 (93.5) | 77 (91.7) | 120 (92.3) | 1.000 |
| Postoperative hormone therapy, n (%) | 30 (65.2) | 51 (60.7) | 81 (62.3) | 0.751 |
Data are shown as mean ± SD and n (%). *Significant difference: P<0.05. MRM: Modified radical mastectomy; BCS: Breast-conserving surgery; ALND: Axillar lymph node dissection; SD: Standard deviation; PMPS: Postmastectomy pain syndrome; LR: Lymph node.
Pain localization and characteristics
Among PMPS patients, pain generally affected more than one region; most frequently the arm and armpit (60 patients). Phantom pain was rare, affecting 12 patients [Figure 1]. Pain and quality of life, as indicated by VAS, McGill, and DN-4 values differed significantly between PMPS patients and other patients who had undergone surgery to remove breast cancer [P < 0.001, Table 3].
Figure 1.
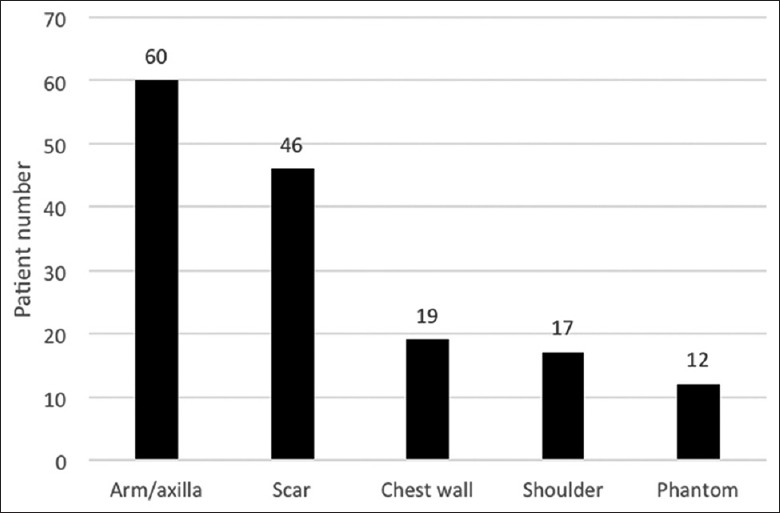
Localization of postmastectomy pain syndrome symptoms.
Of the 131 breast cancer patients treated with surgery, 84 (64.1%) had PMPS symptoms. Within this group, the number of patients reporting low pain levels (VAS 0–3) was 66 (78.6%), while 18 reported moderate pain (VAS 4–7, 21.4%). No patients reported VAS >7. The pain was determined to have neuropathic character (4 or more points according to the DN-4 survey) in 31 patients; probable neuropathy correlated with VAS.
Sensory-discriminative descriptors were chosen more frequently than motivational-affective, cognitive-evaluative, or miscellaneous terms on the SF-MPQ pain survey. The most frequently selected SF-MPQ terms were hot-burning (n = 31), annoying (n = 24), aching (n = 19), shooting (n = 17), gnawing (n = 15), stabbing (n = 14), and throbbing (n = 8). Terms selected most often on the DN-4 survey were pins and needles (n = 45), burning (n = 31), and tingling (n = 17). The longest reported onset duration of PMPS symptoms was 77 months after surgery, and the average onset duration among all breast surgery patients was 6.1 ± 8.5 months. Among non-PMPS and PMPS patients, the onset duration of symptoms averaged 2.0 ± 1.4 and 6.2 ± 8.6 months, respectively (P = 0.495).
Effect of pain on daily life and pain treatment
The effect of pain on daily life was lesser in patients who had axillar dissected modified radical mastectomy than in those treated by breast-conserving surgery according to responses on the SF-36 [P < 0.001, Table 4].
Table 4.
Comparison of pain and quality of life between PMPS and non-PMPS patients
| Items | Non-PMPS (n = 47) | PMPS (n = 84) | Total (n = 131) | P |
|---|---|---|---|---|
| SF-MPQ | 0.02 ± 0.15 | 2.74 ± 2.48 | 1.76 ± 2.38 | <0.001 |
| DN-4 | 0.11 ± 0.43 | 2.5 ± 2.48 | 1.64 ± 2.31 | <0.001 |
| DN-4, n (%) | ||||
| <4 | 47 (35.9) | 53 (40.5) | 100 (76.3) | <0.001 |
| ≥4 | 0 (0) | 31 (23.7) | 31 (23.7) | |
| SF-36 | ||||
| Physical functioning | 86.51 ± 1.89 | 79.87 ± 5.67 | 82.27 ± 5.65 | <0.001 |
| Role-physical functioning | 89.51 ± 2.77 | 81.67 ± 7.14 | 84.51 ± 7.03 | <0.001 |
| Bodily pain | 87.51 ± 4.89 | 80.05 ± 7.31 | 82.75 ± 7.45 | <0.001 |
| General health perceptions | 76.45 ± 4.14 | 69.64 ± 6.32 | 72.10 ± 6.50 | <0.001 |
| Vitality | 70.96 ± 3.50 | 64.31 ± 5.51 | 66.72 ± 5.83 | <0.001 |
| Social functioning | 94.43 ± 3.60 | 88.46 ± 60 | 90.62 ± 5.98 | <0.001 |
| Role-emotional | 91.32 ± 6.52 | 88.70 ± 6.36 | 91.32 ± 6.52 | <0.001 |
| Mental health | 72.95 ± 6.25 | 70.41 ± 5.82 | 72.95 ± 6.25 | <0.001 |
Data are shown as mean ± SD and n (%). SD: Standard deviation; SF-MPQ: Short form of the McGill Pain Questionnaire; DN-4: Douleur neuropathique-4; SF-36: Short form-36; PMPS: Postmastectomy pain syndrome; SD: Standard deviation.
Among patients experiencing pain after breast cancer surgery, 32 (38.1%) never took any pain medication, including simple analgesics, and 50 (59.5%) used only simple analgesics and/or anti-inflammatory drugs. Only 2 (2.4%) patients were treated with opioid or anticonvulsant drugs.
DISCUSSION
In this cross-sectional study, we found that PMPS developed in 64.1% of breast cancer surgery patients and that the key risk factors for PMPS are upper lateral tumor localization and secondary treatment with radiotherapy. An additional 23.6% had PMPS-like symptoms as indicated by responses on the DN-4 survey. Further, we observed that PMPS developed in patients treated by either breast-conserving surgery or modified radical mastectomy when axillary lymph nodes were also removed. Almost no patients had no complaints after breast cancer surgery. While conversion of postoperative pain to a chronic condition has been reported to take 12 years after these types of surgeries,[4] the latest onset of PMPS symptoms in this study was 77 months after breast cancer surgery.
Reliable diagnosis and identification of neuropathic pain, defined by the International Association for the Study of Pain as pain caused by lesioned or dysfunctional nerves, remains challenging. Four types of pain after breast cancer surgery were identified by a recent review of neuropathic pain in this population: Intercostabrachial neuralgia, neuroma pain, phantom pain and pain caused by other neuronal damage. Intercostabrachial neuralgia pain accompanies sensorial changes in the region of intercostabrachial nerve distribution. Neuroma pain stems from scar tissue in the breast, chest, or arm that is triggered by percussion.[5,12,13]
While pain-identifying words have been used to distinguish neuropathic from nonneuropathic pain, especially in population-based studies, specific identification words have been shown to further characterize pain. The Neuropathic Pain Questionnaire developed by Krause and Backonja[14] in 2003 converted a 32-item survey into a 12-item survey form and replaced including the term “dull, aching pain” with the more specific terms tingling, numbness, and increased pain due to contact.[15] In contrast, Galer and Jensen's[16] Neuropathy Pain Scale includes dull and aching pain terms. As categorization of these pain types is nearly impossible without detailed neurologic examination, we used three assessment forms to characterize pain as thoroughly as possible. The SF-MPQ (15 items) and DN-4 (10 items) surveys allow identification of neuropathic pain, while the VAS defines severity (9–10). In this study, patients defined their pain in terms more consistent with the DN-4 survey, which includes the “pins and needles” classification (not included in the SF-MPQ), the most frequent type of pain experienced by this sample of Turkish breast cancer surgery patients (n = 45). According to DN-4 survey results, the number of patients experiencing neuropathic pain was 47, including some patients who rated their pain as 0 on the VAS. In fact, 31 patients categorized their pain as hot-burning on the SF-MPQ; the two surveys yielded conflicting results (P < 0.05). We conclude that shortened survey forms cannot give adequate and reliable results in all societies. Further, some of the patients we considered to have PMPS-like symptoms or who had sensorial changes scored <4 points on the DN-4 survey and rated their pain as 0 on the VAS. Similarly, in a study of 225 postmastectomy patients, Meijuan et al.[12] diagnosed 47 (32.6%) as having PMPS, while another 97 (67.4%) were reported to have “sensory disturbance” without PMPS diagnosis. In another study, 10% of postmastectomy patients were described as having “PMPS-like” sensorial changes.[2]
In this study, survey forms were completed in person by physicians blinded to the study design, as the literacy rate among our sample was low. Thus, we adapted the use of surveys according to the population's education level and culture of individuals, phrasing questions so that each patient could understand them and define their pain. Survey studies that allow patients to complete forms without considering their educational level may be disputable.
Neuropathic pain affects the quality of daily life, including physical, social, and psychological functions. In this study, we examined the quality of life of patients experiencing neuropathic pain after breast cancer surgery and diagnosed with PMPS or PMPS-like symptoms. Physical health is known to decrease to a greater degree than MH in females as they age, but the quality of life, especially PFs, recovers with time after surgery.[5] The SF-36 is a valid assessment of the quality of life, as validated in chronic lumbar pain[17] and complex regional pain syndrome type 1[18] patients, and has been used in many studies among Turkish populations. As we did not include normal healthy individuals, we cannot conclude whether daily life quality is restricted after breast surgery. Nevertheless, average SF-36 scores of PMPS patients have been reported to be markedly lower than those among the normal population, although statistical significance was not assessed.[5]
Risk for PMPS did not differ according to surgery type (breast-conserving surgery or modified radical mastectomy). The two key factors we identified as influencing risks for PMPS development were tumor localization at the upper lateral quadrant and secondary treatment with radiotherapy. Tumor localization was not found as a risk factor for PMPS in previous studies. We hypothesize that the reason for the association of PMPS with this tumor locate is the potential for secondary damage to axillary nerves.
In a study of 219 breast cancer patients, Vilholm et al.[2] reported that tumor localization in the upper lateral quarter is a risk factor for PMPS development, in agreement with our study. Similarly, many studies have reported radiotherapy as a risk factor for chronic pain.[5,12,13] None of this study mention any information about the radiation dose and application area. However, delivering radiotherapy to the supraclavicular glands and axillar region has been thought to cause more pain in the breast, shoulder, and arm.
The limitations of this study are its consideration of patients only at a single center, a relatively small sample size, nonrandomized design, and the lack of a control group and preoperative pain and quality of life data. In addition, evaluation of neuropathic pain did not rely on a neuropathy-specific survey.
We conclude that PMPS is a common complication of breast cancer surgery that significantly affects daily life activities. Its prevalence becomes especially high (87.6%) if patients experiencing PMPS-like symptoms are included. The main risk factors for PMPS in this population were tumor localization in the upper lateral quadrant and secondary treatment with radiotherapy. Our survey strategy was modified to fit our study population, combining multiple surveys and accounting for education levels of by allowing verbal completion of surveys. Confirming these risk factors and identifying alterations in treatment strategies that reduce the incidence of PMPS will require prospective randomized controlled studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Unsal D, Aksu A, Bagriacik U, Akmansu M. The fatigue rate and association with serum cytokine and leptin levels in breast cancer patients undergoing postmastectomy radiotherapy: A prospective evaluation. Turk J Oncol. 2007;22:1–12. [Google Scholar]
- 2.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. Effect of levetiracetam on the postmastectomy pain syndrome. Eur J Neurol. 2008;15:851–7. doi: 10.1111/j.1468-1331.2008.02206.x. doi: 10.1111/j.1468-1331.2008.02206.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith WC, Bourne D, Squair J, Phillips DO, Chambers WA. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91–5. doi: 10.1016/s0304-3959(99)00076-7. doi: 10.1016/S0304-3959(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 4.Peintinger F, Reitsamer R, Stranzl H, Ralph G. Comparison of quality of life and arm complaints after axillary lymph node dissection vs sentinel lymph node biopsy in breast cancer patients. Br J Cancer. 2003;89:648–52. doi: 10.1038/sj.bjc.6601150. doi: 10.1038/sj.bjc.6601150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92:225–30. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Møller S, et al. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain. 2009;13:478–85. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Mejdahl MK, Andersen KG, Gärtner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: Six year nationwide follow-up study. BMJ. 2013;346:f1865. doi: 10.1136/bmj.f1865. doi: 10.1136/bmj.f1865. [DOI] [PubMed] [Google Scholar]
- 8.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–46. doi: 10.1016/j.jpain.2010.12.005. doi: 10.1016/j.jpain201012005. [DOI] [PubMed] [Google Scholar]
- 9.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Unal-Cevik I, Sarioglu-Ay S, Evcik D. A comparison of the DN4 and LANSS questionnaires in the assessment of neuropathic pain: Validity and reliability of the Turkish version of DN4. J Pain. 2010;11:1129–35. doi: 10.1016/j.jpain.2010.02.003. doi: 10.1016/j.jpain.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Meijuan Y, Zhiyou P, Yuwen T, Ying F, Xinzhong C. A retrospective study of postmastectomy pain syndrome: Incidence, characteristics, risk factors, and influence on quality of life. ScientificWorldJournal 2013. 2013 doi: 10.1155/2013/159732. 159732. doi:10.1155/2013/159732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker C, Pham DN, Assouad J, Badia A, Foucault C, Riquet M. Postmastectomy neuropathic pain: results of microsurgical lymph nodes transplantation. Breast. 2008;17:472–6. doi: 10.1016/j.breast.2007.12.007. doi: 10.1016/j.breast.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14. doi: 10.1097/00002508-200309000-00004. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Backonja MM, Krause SJ. Neuropathic pain questionnaire - Short form. Clin J Pain. 2003;19:315–6. doi: 10.1097/00002508-200309000-00005. doi: 10.1097/00002508-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: The neuropathic pain scale. Neurology. 1997;48:332–8. doi: 10.1212/wnl.48.2.332. doi: 10.1212/WNL.48.2.332. [DOI] [PubMed] [Google Scholar]
- 17.Sezgin M, Hasanefendioglu EZ, Sungur MA, Incel NA, Çimen ÖB, Kanik A, et al. Sleep quality in patients with chronic low back pain: A cross-sectional study assesing its relations with pain, functional status and quality of life. J Back Musculoskelet Rehabil. 2015;28:433–41. doi: 10.3233/BMR-140537. doi: 10.3233/BMR-140537. [DOI] [PubMed] [Google Scholar]
- 18.Van Velzen GA, Perez RS, van Gestel MA, Huygen FJ, van Kleef M, van Eijs F, et al. Health-related quality of life in 975 patients with complex regional pain syndrome type 1. Pain. 2014;155:629–34. doi: 10.1016/j.pain.2013.12.017. doi: 10.1016/j.pain. 2013.12.017. [DOI] [PubMed] [Google Scholar]


