Abstract
Background:
Despite the rapid growth in the incidence of acute myocardial infarction (AMI) in China, there is limited information about patients’ experiences after AMI hospitalization, especially on long-term adverse events and patient-reported outcomes (PROs).
Methods:
The China Patient-centered Evaluative Assessment of Cardiac Events (PEACE)-Prospective AMI Study will enroll 4000 consecutive AMI patients from 53 diverse hospitals across China and follow them longitudinally for 12 months to document their treatment, recovery, and outcomes. Details of patients’ medical history, treatment, and in-hospital outcomes are abstracted from medical charts. Comprehensive baseline interviews are being conducted to characterize patient demographics, risk factors, presentation, and healthcare utilization. As part of these interviews, validated instruments are administered to measure PROs, including quality of life, symptoms, mood, cognition, and sexual activity. Follow-up interviews, measuring PROs, medication adherence, risk factor control, and collecting hospitalization events are conducted at 1, 6, and 12 months after discharge. Supporting documents for potential outcomes are collected for adjudication by clinicians at the National Coordinating Center. Blood and urine samples are also obtained at baseline, 1- and 12-month follow-up. In addition, we are conducting a survey of participating hospitals to characterize their organizational characteristics.
Conclusion:
The China PEACE-Prospective AMI study will be uniquely positioned to generate new information regarding patient's experiences and outcomes after AMI in China and serve as a foundation for quality improvement activities.
Keywords: Acute Myocardial Infarction, Outcomes Research, Patient-reported Outcome Measures, Prospective Cohort
INTRODUCTION
Understanding major adverse events and patient-reported outcomes (PROs) after acute myocardial infarction (AMI) is fundamental to improving the quality and effectiveness of health care.[1] With the emergence of validated instruments to measure health status, physical function, and other PROs, several observational studies in Western countries have explored these outcomes after AMI, providing an opportunity for driving both cost-effectiveness and clinical quality improvement.[2,3,4,5] However, due to substantial differences in health care systems, treatment patterns, lifestyle, and environment, data from Western countries may not necessarily be extrapolated elsewhere.
China, home to one-fifth of the world's population, has experienced an epidemiological transition, marked by a shift from a predominance of infectious to noncommunicable diseases in a much shorter time span than many other countries.[6,7] The number of patients hospitalized with ST-segment elevation myocardial infarction in China has quadrupled over the last decade.[8] These epidemiological shifts have created an imperative to understand a broad range of outcomes in longitudinal follow-up for high-impact noncommunicable conditions as AMI.
Despite the increasing prevalence of AMI in China, little is known about the experience of patients after the acute event, including mortality, major adverse vascular events, and PROs. Previous studies in China have found that patients with acute coronary syndromes have worse in-hospital outcomes compared with patients in Western countries, and the use and maintenance of appropriate combinations of evidence-based treatment are suboptimal over both the short- and long-term.[9,10] Existing studies, however, have been either limited to in-hospital mortality and in-hospital clinical events[10] or lacking the comprehensive evaluation of a broad range of outcomes, including PROs.[11] No information is currently available on longitudinal outcomes, risk factors, risk prediction tools, and in-hospital and long-term management of a national sample of patients following AMI in China. Such information would provide a perspective on the current results that are achieved and identify areas for improvement. To understand the experience of patients after AMI and guide quality improvement initiatives for post-AMI care in China, we designed and implemented the China Patient-centered Evaluative Assessment of Cardiac Events Prospective Study of AMI (China PEACE-Prospective AMI study), which is the first longitudinal study on clinical outcomes and PROs among AMI patients who are discharged alive in China. It aims to (1) examine major vascular events and a broad range of PROs (i.e., health status, depression, social support, cognitive function, and sexual health) in patients with AMI in the short- (i.e., within 1 month) and long-term (i.e., within 12 months); (2) determine risk factors associated with these patient outcomes, including patient characteristics (e.g., demographic, clinical, psychosocial, and behavioral factors), in-hospital care, and hospital attributes; (3) generate tools for risk prediction among Chinese patients with AMI; and (4) evaluate the control of risk factors for subsequent events after AMI hospitalization.
METHODS
Study overview
The China PEACE-Prospective AMI study is based on the China PEACE platform, a collaborative effort among China National Center for Cardiovascular Diseases (NCCD), the Yale-New Haven Hospital, Center for Outcomes Research and Evaluation, the Chinese government, and 208 Chinese hospitals to improve cardiovascular disease outcomes in China [Figure 1].[12,13] The China PEACE-Prospective AMI study recruited 4000 patients treated for AMI in 53 hospitals (35 tertiary and 18 secondary hospitals) located in 21 of the 31 provinces in China (Supplementary material 1 (95.6KB, pdf) ) and followed them prospectively for 12 months. The first patient was enrolled in December 2012; follow-up finished in June 2015. Data were collected at baseline (i.e., index hospitalization for AMI), as well as at 1, 6, and 12 months following hospital discharge.
Figure 1.
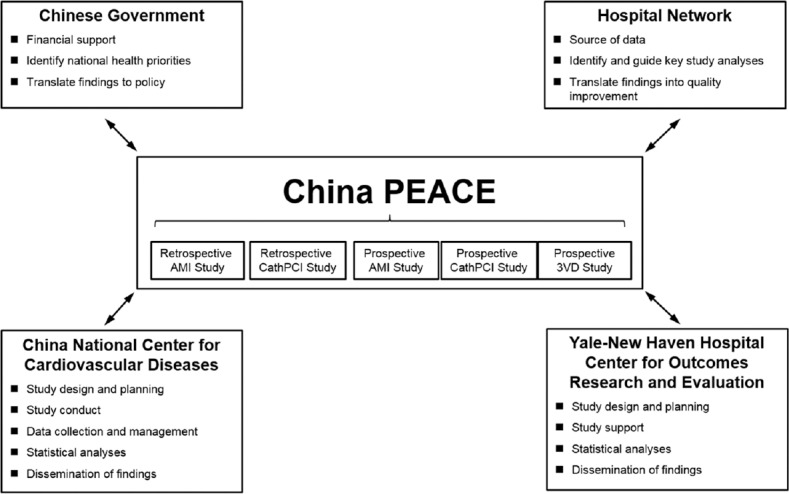
The China Patient-centered Evaluative Assessment of Cardiac Events Initiative.[12,13] Key partners include the Chinese government, collaborating hospitals, the China National Center for Cardiovascular Disease, and the Yale-New Haven Hospital, Center for Outcomes Research and Evaluation. AMI: Acute myocardial infarction; PCI: Percutaneous coronary intervention; 3VD: Revascularization in patients with triple-vessel disease.
SITE INVESTIGATORS BY HOSPITAL
The National Coordinating Center (NCC) of this study is based at the NCCD/Fuwai Hospital in Beijing, and works in close collaboration with 10 regional coordinating centers in various regions of China. The NCCD/Fuwai Hospital ethics committee approved this study and, where required, individual hospitals received approval from their local ethics committee. The Chinese government, which provides financial support for the study, has no role in the design or conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or approval of the article or any of the articles that will be derived from the study. This study was registered on www.clinicaltrials.gov (Registration No. NCT01624909).
Site network
We selected geographically matched hospitals, consisting of one secondary and at least one tertiary hospital in each geographic region (typically a province). This design was implemented to examine the treatment outcomes and associated risk factors of patients following AMI, under different treatment circumstances and patterns with the ability to compare tertiary hospitals (with the capacity of performing primary percutaneous coronary intervention [PCI]) with secondary hospitals (without the capacity of performing primary PCI). Although selecting pairs of secondary and tertiary hospitals from the same city was a priority, when this was not feasible, we selected secondary and tertiary hospitals from geographically proximal cities.
Study population
Eligible patients included those age 18 years or older admitted to a participating hospital for AMI within 24 h of symptom onset. The diagnosis of AMI was defined as an increase in cardiac biomarker levels (troponin or creatine kinase [CK]-MB) with at least one value above the 99th percentile of the upper reference limit, and clinical evidence of acute myocardial ischemia (i.e., symptoms of ischemia, or electrocardiogram changes indicative of new ischemia). These criteria are consistent with other published studies.[3,4] Patients were excluded if they have any of the following: AMI caused by physical trauma, elevated cardiac biomarkers as a complication of elective coronary revascularization. The diagnosis confirmed by local physicians is adjudicated centrally in the NCC based on patient's medical charts.
All eligible patients were registered and invited to participate in the study by trained/certified investigators at each site. The consecutiveness of patient registration was checked against patient databases of local hospitals. Hospitals with enrollment rates that were lower than expected were provided assistance from the NCC, which shares the best practices and strategies used by more successfully enrolling hospitals. Eligible patients who signed informed consent were enrolled and interviewed [Figure 2]. We established the study cohort for follow-up, which included all enrolled AMI patients who were alive at discharge. In order to evaluate potential selection bias by comparing enrolled versus nonenrolled patients, we collected information on baseline characteristics, in-hospital care, and in-hospital outcomes for all eligible patients through medical chart abstraction. Reasons for nonenrollment within eligible patients were also collected.
Figure 2.
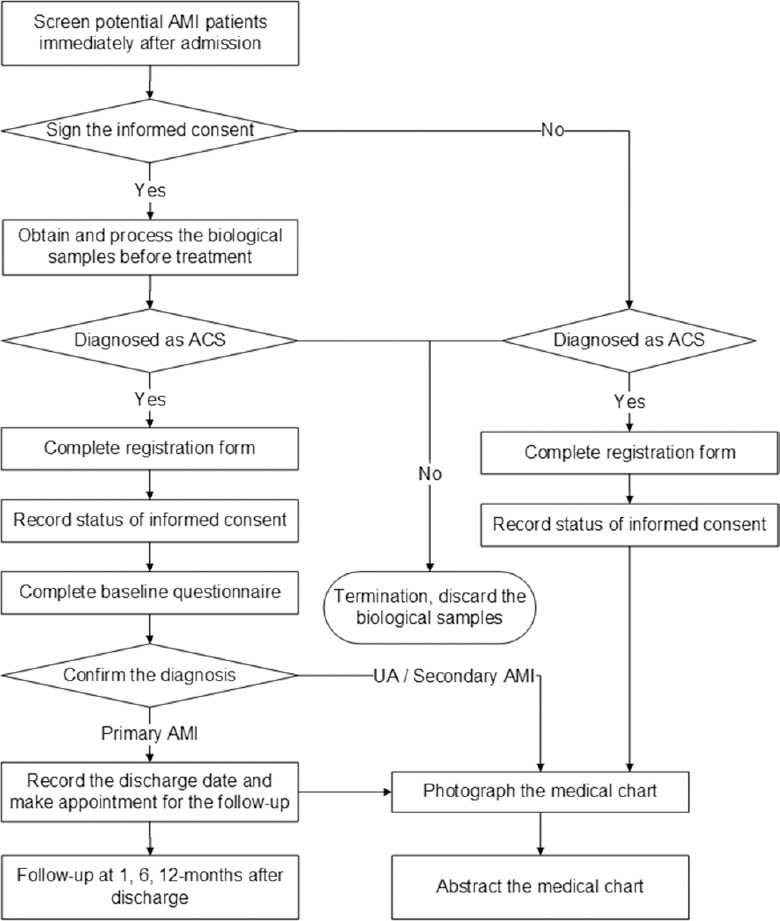
The China Patient-centered Evaluative Assessment of Cardiac Events Prospective Study of Acute Myocardial Infarction study flow chart. AMI: Acute myocardial infarction; ACS: Acute coronary syndrome; UA: Unstable angina.
Data collection
Data are collected via central medical chart abstraction, interviews, and physical examinations by site investigators, local lab tests, and central lab analysis [Table 1]. We used standardized instruments that have been previously validated for the Chinese population. This allows for comparability of results with prior longitudinal studies of patients with AMI.[2,3,4] All data are treated as protected health information and are securely stored in an encrypted and password-protected database at the NCC with frequent local and offsite backup on secure servers.
Table 1.
Data collected during the AMI index hospitalization and follow-up
| Domain | Assessment | ||||
|---|---|---|---|---|---|
| Scale | Baseline | 1-month | 6-month | 12-month | |
| Medical charts abstraction | √ | ||||
| Medical history/risk factors | √ | ||||
| Clinical characteristics | √ | ||||
| Pre-AMI care | √ | ||||
| Diagnostic tests | √ | ||||
| Treatments/procedures | √ | ||||
| Discharge medications | √ | ||||
| In-hospital outcomes | √ | ||||
| Patient interviews | |||||
| CVD functional status | SAQ[14] | √ | √ | √ | √ |
| Health-related quality of life | EQ-5D[15] | √ | √ | √ | √ |
| Depression | PHQ-8[16] | √ | √ | √ | √ |
| Stress | PSS[17] | √ | √ | √ | √ |
| Social support | ESSI[18] | √ | √ | ||
| Obstructive sleep apnea | MBQ[19] | √ | |||
| Cognitive function* | MMSE[20] | √ | √ | √ | |
| Sexual activity* | Lindau[21] | √ | √ | √ | |
| Major vascular events | √ | √ | √ | √ | |
| Any hospitalization | √ | √ | √ | √ | |
| Onset of symptoms | |||||
| Seeking care for symptoms | √ | ||||
| Health care service | √ | √ | |||
| TCM clinic/therapies | √ | √ | |||
| Health care insurance | √ | √ | |||
| Medical expenses | √ | √ | |||
| Socioeconomic status | √ | √ | |||
| Education | √ | ||||
| Work status | √ | √ | |||
| Marital/living status | √ | √ | |||
| Household income | √ | √ | |||
| Health knowledge | √ | ||||
| Risk factors | |||||
| Blood pressure | √ | √ | √ | √ | |
| Family history | √ | ||||
| Smoking status | √ | ||||
| Lifestyle factors | √ | √ | |||
| Physical activity | √ | ||||
| Alcohol consumption | √ | ||||
| Preventive medications | √ | √ | √ | ||
| BMI/hip circumference | √ | √ | √ | √ | |
| Local lab tests | |||||
| Blood cell count | √ | √ | |||
| Urine analysis | √ | √ | |||
| Alanine transaminase | √ | √ | √ | ||
| Creatinine/BUN | √ | √ | √ | ||
| Blood glucose | √ | √ | |||
| Electrocardiogram | √ | √ | √ | ||
| Central lab analysis | |||||
| Blood lipid profile | √ | √ | |||
| HbA1c | √ | √ | |||
| hs-CRP | √ | √ | |||
| Bio-samples for long-term storage | |||||
| Plasma/serum | à | à | à | ||
| DNA | √ | ||||
| RNA from periphery blood | √ | √ | |||
| Urine | à | à | à | ||
*Only performed in 6 hospitals (3 tertiary and 3 secondary hospitals, from 3 provinces); †Only collected in secondary hospitals. AMI: Acute myocardial infarction; CVD: Cardiovascular disease; SAQ: Seattle angina questionnaire; EQ-5D: EuroQol group 5-dimension self-report questionnaire; PHQ-8: Patient health questionnaire 8-item depression scale; PSS: Perceived stress scale; ESSI: Enhancing recovery in coronary heart disease (ENRICHD) social support inventory; MBQ: Modified Berlin questionnaire; MMSE: Mini mental state examination; TCM: Traditional Chinese medicine; BUN: Blood urea nitrogen; HbA1c: Hemoglobin A1c; hs-CRP: High-sensitivity C-reactive protein; DNA: Deoxyribonucleic acid; RNA: Ribonucleic acid; BMI: Body mass index.
Medical chart abstraction
For all registered patients, we abstracted detailed information from medical chart of the index hospitalization (case report form shown in Supplementary material 2 (323.1KB, pdf) and data dictionary shown in Supplementary material 3 (332.8KB, pdf) ). Site investigators photograph complete hospital medical charts of all eligible patients, with identity information concealed (name, national ID, and contact information). Electronic copies of the charts are sent to the NCC via encrypted flash drives for central abstraction. Following receipt of each chart, research staff at the NCC checks the hospitalization ID and date of hospital admission to verify the correct patient identity. The data are evaluated to ensure completeness, quality, and concealment of personal identifiers. Incomplete or poorly scanned records are rescanned and retransmitted. Per the China PEACE-Retrospective AMI study methodology,[12] two contracted vendors abstract details of each patients’ hospitalization using their medical charts. One vendor abstracts the part that can be abstracted verbatim without need for interpretation (face sheet, laboratory test results, and physician orders) of all medical charts via double entry by separate abstractors to ensure the accuracy. The other vendor abstracts the other part (the admission record, discharge record, daily record, and procedure reports) of all medical charts. To ensure the accuracy of interpretation, this part is abstracted by certified abstractors and checked by senior abstractors. Research staff at the NCC randomly audits 5% of all charts on an ongoing basis. If the charts are not abstracted with at least 98% accuracy, all medical charts in the audited batch will be considered unqualified and will be re-reviewed.
CASE REPORT FORM
DATA DICTIONARY
Participant interviews
Participants were interviewed at baseline (i.e., during the index hospitalization for AMI), and at 1, 6, and 12 months following hospital discharge (questionnaires shown in Supplementary material 4 (513.7KB, pdf) ). During follow-up period, participants were instructed to return to the hospital for interviews by site investigators. The telephone interviews are conducted only when in-person interviews are not feasible.
BASELINE AND FOLLOW-UP QUESTIONNAIRES
At baseline, we collect detailed information on demographics, socioeconomic status, cardiovascular risk factors, medical history, sleep apnea,[19,22] generic[15,23] and disease-specific health status,[14,24] depression,[16] and stress [Table 1].[17,25] We collected patient outcomes within 12 months after the index AMI, including clinical events and PROs. We collected all hospitalizations during follow-up with the medical record as supporting documents. Clinicians at NCC adjudicate all major vascular events (defined as cardiac death, nonfatal AMI, coronary revascularization, or ischemic stroke) using standard criteria employed in clinical trials. To provide a complete picture of patients’ health status, we collect both generic and disease-specific measures, as well as estimates of patients’ psychosocial status, and sexual functioning. The study used the EuroQol group 5-dimension instrument as a measure of generic health-related quality of life,[15,23] which also enables the estimation of utilities, and the Seattle Angina Questionnaire[14,24] to assess condition-specific functioning and quality of life. Psychosocial status was assessed for depressive symptoms (8-item patient health questionnaire),[16] stress (4-item perceived stress scale),[17,25] cognitive function (mini-mental state examination),[20] and sexual activity/function.[4,21] During follow-up, we also asked questions regarding adherence to secondary prevention and assessed the management of cardiovascular risk factors.
To assess sexual health and cognitive functioning after AMI, we conducted a sub-study among 6 of the participating hospitals (3 tertiary and 3 secondary hospitals, from 3 provinces). Sexual activity/function and cognitive function were assessed at baseline, 1, and 12 months after the index AMI [Table 1] by trained physicians. Sexual activity/function was assessed using instruments from previous large-scale studies of adult sexuality following AMI.[4,21,26] This includes information on partner status, sexual activity (active or not, frequency), attitudes (importance, motivation), function, and physician counseling about sexual activity. Cognitive function was assessed using a validated instrument.[20]
Site investigators complete the questionnaires electronically on a tablet computer, which allows real-time logic checks to ensure the accuracy and completeness of data. Site investigators photographed signed consent forms with the tablet camera and upload them to the central server. The data were transferred to the server for synchronization into the central database at NCC via a customized virtual private network. The NCC coordinators checked consents of all participants, reviewed missing data and made data queries, and requested reasons for missed follow-up appointments. In addition, an audio record was automatically made during the patient interview and uploaded to the server along with questionnaire data. Coordinators at NCC listen to a sample of these audio records for quality assurance.
Physical examinations and local laboratory tests
Blood pressure, height, weight, and waist circumference are measured at baseline as well as each follow-up visit. At the 1- and 12-month follow-up visits, lipid profiles, glucose, renal function, liver function, blood counts, and urinalysis were analyzed locally, which are consistent with the routine clinical practice. The results were also recorded electronically.
Central laboratory tests and long-term storage of blood and urine samples
For the purpose of central lab analysis, separate blood and urine samples are obtained during the index hospitalization (primarily prior to acute treatments, particularly reperfusion therapies), as well as at 1-month (only in the 18 secondary hospitals) and 12-month follow-up visits. This will facilitate the assessment of AMI biomarkers and long-term management of risk factors (Supplementary material 5 (89.2KB, pdf) ). Samples were processed within 24 h following collection, stored at −40°C or −80°C, transported with dry ice from local sites within 6 months, and then stored in liquid nitrogen at China NCCD for central analysis and long-term storage. The following will be analyzed at a central laboratory: Blood lipid profiles (total cholesterol, high-density lipid-cholesterol, and low-density lipid-cholesterol) and metabolic markers (i.e., alanine aminotransferase, creatinine, CK, high-sensitivity C-reactive protein, glucose, and hemoglobin A1c). All blood/urine samples are stored for future genetic and metabolic analysis.
BLOOD AND URINE COLLECTION
Hospital survey
To help investigate the effects of health care system and institutional factors on the treatment and outcomes of patients with AMI, participating hospitals are surveyed about their treatment capacity and processes, as well as their organizational learning behaviors and cultures (Supplementary material 6 (136KB, pdf) ). Questions about organizational learning were drawn from previously developed instruments.[27,28,29,30] We invite two respondents from each hospital to complete the survey: One is the local principal investigator of the China PEACE-Prospective AMI study at each hospital (usually the director of the Cardiology Department or Internal Medicine Department) and the other is the coordinator of this study at each hospital (usually a physician involved in routine clinical practice).
HOSPITAL SURVEY QUESTIONNAIRE
Statistical analysis
Our strategy of data collection permits a broad range of analysis and analytic approaches, based upon the primary research question (Supplementary material 7 (92KB, pdf) ). We will calculate summary statistics for major vascular events, as well as PROs within 1, 6, and 12 months after AMI. We will report summary statistics for patient demographic, clinical, psychosocial, and behavioral characteristics, use of diagnostic tests, treatments received, and control of cardiovascular risk factors. To help identify factors associated with the major vascular events, we will use standard parametric and nonparametric tests for bivariate analyses, including t-test, Chi-square test, Fisher's exact test, and Wilcoxon rank sum tests. In addition, appropriate multivariable regression analyses, such as linear, logistic, Cox proportional hazard, and Poisson models, will be conducted to determine a factor's association with the outcome measures while adjusting for potential confounders. As patients are clustered within hospitals and there are repeated observations clustered within patients, our analyses will account for clustering in data (e.g., generalized estimating equations or random effects models). While all efforts are made to obtain high response rates in follow-up PROs, some missing data are inevitable. We will carefully evaluate any potential selection biases introduced by missing data and conduct inverse probability weighting when appropriate, based upon a propensity model for participation in the follow-up assessments, to preferentially weight the experiences of patients who were most like those who did not participate in follow-up.
ANALYTIC METHODS AND PLAN
The current study is primarily a descriptive one. We sought a large enough study to provide some precision to the estimates. A sample size of 4000 is determined based on both feasibility and consideration of adequate statistical precision for describing 12-month major vascular events and PROs in the overall sample, and secondary or tertiary hospitals separately (Supplementary material 8 (107KB, pdf) ).
SAMPLE SIZE CACULATION
DISCUSSION
The China PEACE-Prospective AMI study is the first national longitudinal study on both clinical events and PROs among patients with AMI in China. The goal of the study is to assess a broad range of outcomes of patients recovering from AMI to directly address an important knowledge gap and serve as a foundation with which to develop novel interventions to improve the health outcomes of AMI patients in China. Several unique features of this study, including the consecutive enrollment of patients, the validation processes to ensure accurate capture of source data, the collection of patient-centered data elements through serial interviews, and the detailed longitudinal follow-up, augment the value of this study.
PRO measures will provide critically important and currently unknown information to support quality improvement initiatives for AMI patients in China. Traditional clinical methods of measuring health and the effects of treatment are increasingly accompanied by PROs, which enable the patients’ perspectives to be taken into account in key aspects of healthcare.[31,32] During the past decade, the treatment of AMI has advanced considerably, resulting in significantly decreased mortality and underscoring the importance of capturing the health outcomes of survivors. Physicians need to take into account the impact of treatments on patients’ health status including psychosocial outcomes that may influence the patient recovery process. However, previous large-scale international or national studies lack information about PROs.[33,34,35] In recent years, several observational studies have broadened the range of outcomes among patients with AMI to include these outcomes.[2,3,4,5] However, these studies were conducted in Western countries and in-hospitals with the capacity for angiography. Previous nationwide cohorts of Chinese patients with AMI did not collect PROs.[11,36,37] Findings from the China PEACE-Prospective AMI study will provide information about the long-term outcomes of patients with AMI, in settings both with and without advanced technical capability, such as PCI, and thus inform generalizable strategies to improve care and reduce disparities with a focus on a broad range of outcomes including those that reflect the patient's experience.
The collection of detailed data describing patient characteristics (e.g., demographic, clinical, psychosocial, behavioral factors, and hospital care received) and hospital attributes will enable investigators to define the prognostic importance of both patient- and hospital-level variables on both short- and long-term outcomes. This information will serve to improve the care for Chinese AMI patients.
Risk prediction tools developed in the Chinese population can facilitate understanding of patient prognosis, stratification, and personalized treatment according to individual risk.[38] Currently used risk scores (e.g., GRACE, TIMI, GUSTO) were developed based on non-Chinese patients, and the performance of these models in Chinese populations may differ from that in Western patients.[39] Accordingly, a risk model tailored to identify high-risk patients with AMI in China may be more effective in predicting poor prognosis including major adverse vascular events and/or poor quality of life.
Data from this study will be used to assess patient- and hospital-level factors influencing continuation of medications and the control of risk factors to optimize secondary prevention following AMI, as well as the association between secondary prevention and patient outcomes. Such studies will address a substantial gap in knowledge regarding long-term adherence to preventive medications, particularly in low- and middle-income countries (LMICs). As most prior studies focused only on in-hospital care and discharge medications, there is a continued need to understand the barriers in adherence to secondary prevention, as well as associated patient characteristics and institutional factors. For example, identifying patient- or system-level factors associated with nonadherence can support novel strategies for improving treatment after discharge and serve as a foundation for strategies to improve medication adherence and risk factor control in AMI patients.
Besides the clinical and psychosocial patterns examined for optimizing the treatment and prognosis of patients with AMI, the blood and urine samples collected in our study will serve the need of basic science, translational, or clinical research to advance the diagnosis, prevention, or treatment of AMI. The large sample of well-characterized patients will be stored long-term as a bio-repository, enabling identification of novel biomarkers, detecting the association between genetic and phenotype factors, and exploring potential mechanisms of AMI onset and recovery.
The strengths of conducting a longitudinal study on clinical outcomes and PROs among patients with AMI have been well-recognized both in China and other LMICs, which are all in need of a better understanding of where the greatest opportunities lie in optimizing patient outcomes in the face of an increasing burden of cardiovascular disease. In addition, the established organizational infrastructure of our research team, based on partnership with the Chinese government, collaboration with international experts in observational studies, and the involvement of local hospitals, ensures a rigorous study design and rapid dissemination of findings. Furthermore, the implementation of the high-quality and cost-effective study is supported by a broad research network as well as a robust management system. During the past decade, China NCCD has collaborated with world's leading academic institutions to conduct several of the largest clinical trials in China.[40,41,42,43] Continuous efforts have been made in establishing the research network through in-depth training and rigorous quality control procedures. We have also established a management system for large multi-center studies, which has internationally-accepted quality standards and accounts for the diverse health care settings in China. The collaborative research and performance improvement network created by the China PEACE platform will ultimately improve patient outcomes for a broad range of conditions and may present a model for research and quality improvement in other international settings. It should be noted that only patients who consented can be enrolled and followed-up. These patients may differ from those who were eligible but did not consent to participate. In addition, missing data, particularly follow-up PRO data, can introduce biased estimates of outcomes and will require extreme diligence in collecting all follow-up PRO data and advanced statistical methods for the planned analyses to account for missing data.
In conclusion, the China PEACE-Prospective AMI study is uniquely positioned to help improve the quality of care and patient outcomes for China and similar LMICs by generating novel, high-quality, and comprehensive data about patients’ experience following hospitalizations for AMI. The partnership among the Chinese government, an expert clinical trial group, a large network of hospitals with geographic and capability diversity, and international experts in outcomes research, will be leveraged to create a platform for research on cardiovascular diseases which will facilitate policy-making and inform the development of novel quality improvement tools.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by the Research Special Fund for Public Welfare Industry of Health (No. 201202025) from National Health and Family Planning Commission of China. HMK is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The sponsors had no role in the conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or approval of the manuscript.
Conflicts of interest
Dr. Krumholz works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures, is chair of a cardiac scientific advisory board for UnitedHealth, and is the recipient of research grants from Medtronic, Johnson and Johnson (Janssen) through Yale University, to develop methods of clinical trial data sharing. JAS owns the copyright to the Seattle Angina Questionnaire. Other authors declare no relevant conflicts of interest.
Acknowledgments
We appreciate the multiple contributions made by study teams at the China Oxford Centre for International Health Research and the Yale-New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operation, particularly the data collection by Jia-Peng Lu, Xue-Kun Wu, Yuan Yu, Ying Sun, Qian Xiao, Qiu-Lan Xie, Si-Ying Niu, Hao Yang, Si-Ming Wang, Cai-Hong Zhao and Jia-Min Liu. We appreciate the advice from Yong-Fei Wang, Nihar R. Desai, Joseph S. Ross, Khurram Nasir, and Hai-Qun Lin. We are grateful for the support provided by the Chinese government.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Rahimi K, Malhotra A, Banning AP, Jenkinson C. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: Systematic review. BMJ. 2010;341:c5707. doi: 10.1136/bmj.c5707. doi: 10.1136/bmj.c5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. Cardiovascular Outcomes Research Consortium. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) – Evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–97. doi: 10.1016/j.ahj.2005.05.026. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–76. doi: 10.1161/CIRCOUTCOMES.110.960468. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtman JH, Lorenze NP, D’Onofrio G, Spertus JA, Lindau ST, Morgan TM, et al. Variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–93. doi: 10.1161/CIRCOUTCOMES.109.928713. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilote L, Karp I. GENESIS-PRAXY (GENdEr and Sex determInantS of cardiovascular disease: From bench to beyond-Premature Acute Coronary SYndrome) Am Heart J. 2012;163:741$–6. doi: 10.1016/j.ahj.2012.01.022. doi: 10.1016/j.ahj.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Kong L, Zhao W, Wan X, Zhai Y, Chen LC, et al. Emergence of chronic non-communicable diseases in China. Lancet. 2008;372:1697–705. doi: 10.1016/S0140-6736(08)61366-5. doi: 10.1016/S0140-6736(08)61366-5. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): A retrospective analysis of hospital data. Lancet. 2015;385:441–51. doi: 10.1016/S0140-6736(14)60921-1. doi: 10.1016/S0140-6736(14)60921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi Y, Gao R, Patel A, Su S, Gao W, Hu D, et al. Evidence-based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: Results from the clinical pathways for acute coronary syndromes in China (CPACS) study. Am Heart J. 2009;157:509$–16. doi: 10.1016/j.ahj.2008.09.026. doi: 10.1016/j.ahj.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Zhao D, Liu J, Wang W, Liu J; Bridging the Gap on Coronary Heart Disease Secondary Prevention in China. Current clinical practice patterns and outcome for acute coronary syndromes in China: Results of BRIG project (in Chinese) Chin J Cardiovasc Dis. 2009;37:213–7. [PubMed] [Google Scholar]
- 11.Gao R, Patel A, Gao W, Hu D, Huang D, Kong L, et al. Prospective observational study of acute coronary syndromes in China: Practice patterns and outcomes. Heart. 2008;94:554–60. doi: 10.1136/hrt.2007.119750. doi: 10.1136/hrt.2007.119750. [DOI] [PubMed] [Google Scholar]
- 12.Dharmarajan K, Li J, Li X, Lin Z, Krumholz HM, Jiang L China PEACE Collaborative Group. The China Patient-centered Evaluative Assessment of Cardiac Events (China PEACE) retrospective study of acute myocardial infarction: Study design. Circ Cardiovasc Qual Outcomes. 2013;6:732–40. doi: 10.1161/CIRCOUTCOMES.113.000441. doi: 10.1161/CIRCOUTCOMES.113.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Dharmarajan K, Li X, Lin Z, Normand SL, Krumholz HM, et al. Protocol for the China PEACE (Patient-centered Evaluative Assessment of Cardiac Events) retrospective study of coronary catheterisation and percutaneous coronary intervention. BMJ Open. 2014;4:e004595. doi: 10.1136/bmjopen-2013-004595. doi: 10.1136/bmjopen-2013-004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 15.EuroQol Group. EuroQol – A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 18.Enhancing recovery in coronary heart disease patients (ENRICHD): Study design and methods. The ENRICHD investigators. Am Heart J. 2000;139(1 Pt 1):1–9. doi: 10.1016/s0002-8703(00)90301-6. doi: 10.1016/S0002-8703(00)90301-6. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124:281–90. [PubMed] [Google Scholar]
- 20.Tang G, Chen Z, Li B. Comparison of cognitive functin scale in Chinese (in Chinese) Chin J Clin Rehabil. 2004;8:3882–4. [Google Scholar]
- 21.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–74. doi: 10.1056/NEJMoa067423. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Luo N. Application of EQ-5D Chinese version (in Chinese) China J Pharm Econ. 2009;1:49–57. [Google Scholar]
- 24.Li J, Chang G. Assessement of SAQ for measuring quality of life among patients with coronary heart disease (in Chinese) Chin J Public Helath. 2004;20:594. [Google Scholar]
- 25.Yang Y, Huang H. An epidemiological study on stress among urban residentsin social transition period (in Chinese) Chin J Epidemiol. 2003;24:760–4. [PubMed] [Google Scholar]
- 26.Lindau ST, Abramsohn EM, Bueno H, D’Onofrio G, Lichtman JH, Lorenze NP, et al. Sexual activity and counseling in the first month after acute myocardial infarction among younger adults in the United States and Spain: A prospective, observational study. Circulation. 2014;130:2302–9. doi: 10.1161/CIRCULATIONAHA.114.012709. doi: 10.1161/CIRCULATIONAHA.114.012709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer SJ, Moore SC, Meterko M, Williams S. Development of a short-form learning organization survey: The LOS-27. Med Care Res Rev. 2012;69:432–59. doi: 10.1177/1077558712448135. doi: 10.1177/1077558712448135. [DOI] [PubMed] [Google Scholar]
- 28.Nembhard IM, Tucker AL. Deliberate learning to improve performance in dynamic service settings: Evidence from hospital intensive care units. Organ Sci. 2011;22:907–22. doi: 10.1287/orsc.1100.0570. [Google Scholar]
- 29.Bradley EH, Curry LA, Spatz ES, Herrin J, Cherlin EJ, Curtis JP, et al. Hospital strategies for reducing risk-standardized mortality rates in acute myocardial infarction. Ann Intern Med. 2012;156:618–26. doi: 10.1059/0003-4819-156-9-201205010-00003. doi: 10.7326/0003-4819-156-9-201205010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krumholz HM, Curry LA, Bradley EH. Survival after acute myocardial infarction (SAMI) study: The design and implementation of a positive deviance study. Am Heart J. 2011;162:981–7.e9. doi: 10.1016/j.ahj.2011.09.004. doi: 10.1016/j.ahj.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meadows KA. Patient-reported outcome measures: An overview. Br J Community Nurs. 2011;16:146–51. doi: 10.12968/bjcn.2011.16.3.146. doi:10.12968/bjcn.2011.16.3.146. [DOI] [PubMed] [Google Scholar]
- 32.Krumholz HM. Outcomes research: Generating evidence for best practice and policies. Circulation. 2008;118:309–18. doi: 10.1161/CIRCULATIONAHA.107.690917. doi: 10.1161/CIRCULATIONAHA.107.690917. [DOI] [PubMed] [Google Scholar]
- 33.Hanssen M, Cottin Y, Khalife K, Hammer L, Goldstein P, Puymirat E, et al. French registry on acute ST-elevation and non ST-elevation myocardial infarction 2010.FAST-MI 2010. Heart. 2012;98:699–705. doi: 10.1136/heartjnl-2012-301700. doi: 10.1136/heartjnl-2012-301700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson ED, Roe MT, Chen AY, Fonarow GC, Lytle BL, Cannon CP, et al. The NCDR ACTION registry-GWTG: Transforming contemporary acute myocardial infarction clinical care. Heart. 2010;96:1798–802. doi: 10.1136/hrt.2010.200261. doi:10.1136/hrt.2010.200261. [DOI] [PubMed] [Google Scholar]
- 35.GRACE Investigators. Rationale and design of the GRACE (Global Registry of Acute Coronary Events) project: A multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141:190–9. doi: 10.1067/mhj.2001.112404. doi:10.1067/mhj.2001.112404. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Zhao D, Liu J. Rationale and design of baseline survey of BRIG (Bridging the gap on CAD secondary prevention) project: A multi-provincial implementation study on CAD patients’ management in China. J Cardiovasc Pulm Dis. 2008;27:37–41. [Google Scholar]
- 37.Xu H, Li W, Yang J, Wiviott SD, Sabatine MS, Peterson ED, et al. The China acute myocardial infarction (CAMI) registry: A national long-term registry-research-education integrated platform for exploring acute myocardial infarction in China. Am Heart J. doi: 10.1016/j.ahj.2015.04.014. Epub before printed. doi: 10.1016/j.ahj.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Arnold SV, Masoudi FA, Rumsfeld JS, Li Y, Jones PG, Spertus JA. Derivation and validation of a risk standardization model for benchmarking hospital performance for health-related quality of life outcomes after acute myocardial infarction. Circulation. 2014;129:313–20. doi: 10.1161/CIRCULATIONAHA.113.001773. doi: 10.1161/CIRCULATIONAHA.113.001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan MY, Shah BR, Gao F, Sim LL, Chua T, Tan HC, et al. Recalibration of the global registry of acute coronary events risk score in a multiethnic Asian population. Am Heart J. 2011;162:291–9. doi: 10.1016/j.ahj.2011.05.016. doi: 10.1016/j.ahj.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet. 2005;366:1607–21. doi: 10.1016/S0140-6736(05)67660-X. doi: 10.1016/s0140-6736(05)67660-x. [DOI] [PubMed] [Google Scholar]
- 41.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet. 2011;377:2181–92. doi: 10.1016/S0140-6736(11)60739-3. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.HPS-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91. doi: 10.1093/eurheartj/eht055. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, et al. HPS-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. doi: 10.1056/NEJMoa1300955. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SITE INVESTIGATORS BY HOSPITAL
CASE REPORT FORM
DATA DICTIONARY
BASELINE AND FOLLOW-UP QUESTIONNAIRES
BLOOD AND URINE COLLECTION
HOSPITAL SURVEY QUESTIONNAIRE
ANALYTIC METHODS AND PLAN
SAMPLE SIZE CACULATION


