Abstract
Background:
G-protein β-polypeptide 3 (GNB3) is a β subunit isoform of G-protein that plays important role in signal transduction of membrane G-protein coupled receptors (GPCRs). The GNB3 splice variant C825T (rs5443) is associated with risk for essential hypertension (EH) and efficacy of therapeutic drugs targeting GPCRs. It is unknown whether the polymorphism is associated with blood pressure (BP) response to telmisartan or amlodipine, two widely prescribed antihypertensive drugs.
Methods:
A total of 93 subjects initially diagnosed as EH were recruited and underwent a 4-week treatment with telmisartan (42 patients) or amlodipine (51 patients) monotherapy. Both baseline and after-treatment BP were measured. GNB3 C825T polymorphism was genotyped by polymerase chain reaction-restriction fragment length polymorphism.
Results:
Baseline systolic BP (SBP) and diastolic BP (DBP) were comparable among C825T genotypes in both telmisartan and amlodipine treatment groups. Patients with the CT or TT genotypes showed significantly lower body mass index (BMI) as compared with CC homozygotes in both groups (P < 0.05, respectively). GNB3 825TT homozygotes showed significantly higher after-treatment DBP and mean arterial pressure (MAP) than those carrying at least one 825C allele (P < 0.01) in the telmisartan treatment group. No difference in after-treatment SBP, DBP, and MAP levels among C825T genotypes was observed in the amlodipine treatment group. No significant difference in absolute changes in BP levels was observed among the genotypes in either treatment group.
Conclusion:
The GNB3 C825T splice variant is associated with the DBP-lowering effect of telmisartan but not amlodipine in Chinese EH patients.
Keywords: Amlodipine, Chinese Population, G-protein, β-polypeptide 3, Single Nucleotide Polymorphism, Telmisartan
INTRODUCTION
Hypertension affects approximately one-third of adults and causes more than 10 million deaths worldwide annually.[1] High blood pressure (BP) becomes the major risk factor for cardiovascular disease, stroke, and kidney disease.[2] Variety kinds of antihypertensive drugs are being prescribed for essential hypertension (EH) patients to reduce morbidity and mortality of cardiovascular and renal diseases.[3] Amlodipine, one of long-acting dihydropyridine calcium channel blockers, acts through binding to the transmembrane site on L-type calcium channels in cardiac and vascular smooth muscle cells, thereby decreasing the intracellular availability of Ca2+ and directly inhibiting their contractility.[4,5] The effectiveness of amlodipine has been demonstrated in several clinical trials.[6,7] Telmisartan is a long-lasting and nonpeptide angiotensin II (Ang II) receptor blocker that is highly selective to angiotensin II type 1 receptors (AT1), a G-protein coupled receptor (GPCR).[8] The favorite effects on lipid and glucose profile through activation of peroxisome proliferator-activated receptor-γ make the drug very popular than other AT1 blockers in the clinic.[9,10] However, clinic data has shown that BP response rate of amlodipine and telmisartan is 62–78% and 52–56%, respectively.[11]
Recent genetic studies have shown that genetic polymorphisms associated with risk for EH may also influence therapeutic responsiveness to these antihypertensive drugs.[12,13] One commonly studied candidate gene for EH is G-protein β-polypeptide 3 gene (GNB3). Heterotrimeric G-proteins are composed of α, β, and γ subunit and play a vital role in the intracellular signal transduction processes via interaction with GPCRs such as AT1 and voltage-dependent Ca2+ channels.[14,15,16] A single nucleotide polymorphism C825T (rs5443) resulting in the formation of a splice variant of Gβ3, termed Gβ3s, which is biologically active and can even enhance signal transduction.[17] The C825T polymorphism was initially reported to be associated with risk for EH by Siffert et al.[17] Since then, much research has been focused on the GNB3 C825T polymorphism and risk for EH in different races.[18,19,20,21] It is also assumed that C825T polymorphism is a promising pharmacogenetics marker for the treatment of hypertension. The aim of this study was to determine whether the GNB3 C825T polymorphism can influence antihypertensive response to amlodipine and telmisartan monotherapy in Chinese EH patients.
METHODS
Subjects
Patients were initially diagnosed as EH in outpatient medical clinics of Xiangya Hospital, Central South University between November 2013 and November 2014 in Changsha, Hunan Province, China. Patients aged 40–75 years and with average sitting systolic BP (SBP) of 140–180 mmHg and/or average sitting diastolic BP (DBP) of 90–110 mmHg were recruited. Patients showed evidence by medical record review and history of secondary hypertension, white coat hypertension, renal or liver dysfunction, and serious heart disease were excluded from the study. Moreover, subjects with tumors, drug addiction, mental disorder, and pregnant and breastfeeding women were also excluded. This study was approved by the Ethics Committee of the Institute of Clinical Pharmacology of Central South University. All subjects were residents in Hunan Province and were informed of the study. All participants gave written informed consents.
Treatment
The patients were treated with 40 mg telmisartan (once daily with two 20 mg tablets; Hainan Sailike Medicine Co., Hainan, China) or 5 mg amlodipine (benzene sulfonic acid amlodipine tablet, once daily with one 5 mg tablet; Shanghai Heini Pharmaceutical Co., Shanghai, China) once daily for 4 weeks after recruitment, and were interviewed every 2 weeks by the clinician for BP monitoring. Compliance was assessed by pill counts. BP were measured in the seated position after quite resting for at least 15 min by using a sphygmomanometer by the same clinician between 8 am and 10 am about 24 h after the last amlodipine and telmisartan dosage. Values for SBP and DBP were recorded in accordance with the Joint National Committee 7 report.[22] Each patient was measured in triplicate and averages were used in the analyses. Peripheral venous blood samples of 2 ml were obtained from each subject with ethylenediaminetetraacetic acid used as the anticoagulant.
Determination of G-protein β-polypeptide 3 C825T genotypes
Genomic DNA was extracted from peripheral blood leukocytes using an E.Z.N.A® Blood DNA Midi Kit (Omega Bio-Tek Inc., Norcross, GA, USA) according to the manufacturers’ instructions. Genotyping of the GNB3 C825T polymorphism was performed using method of polymerase chain reaction-restriction fragment length polymorphism (PCR). PCR was performed with primers 5’- ctcagttcttccccaatgga-3’ (forward) and 5’- ttacccacacgctcagacttc-3’ (reverse). The PCR cycling profile was denatured at 94°C for 5 min, followed by 35 cycles of at 94°C for 45 s, 62°C for 45 s, 72°C for 30 s, and a final incubation at 72°C for 5 min. PCR products were restricted with BsaJI endonuclease (New England Biolabs, Beverly, CA, USA), separated on 3% agarose gel electrophoresis and visualized by GelRed staining. The unrestricted (TT genotypes) PCR product has a size of 226 bp and complete restricted (CC genotypes) shows bands of 130 bp and 96 bp. The genotyping of GNB3 C825T was verified by direct sequencing.
Statistical analysis
Statistical analyses were performed by the SPSS 13.0 software for Windows (SPSS Inc., Chicago, IL, USA). Data were shown as mean ± standard deviation. Genotype and allele frequencies were determined by direct gene counting. Fitness to Hardy–Weinberg equilibrium was analyzed by Chi-square test. Differences in mean BP levels among genotypes were analyzed using one-way analysis of variance or two-tailed t-test. Stepwise multiple linear regression analysis was performed to investigate the possible influence of GNB3 C825T polymorphism on posttreatment BP and BP response after adjustment for age, body mass index (BMI), heart rate (HR), baseline BP, gender (where 0 indicates female and 1 indicates male), the GNB3 C825T genotypes (where 0 indicates CC genotype, 1 indicates CT and 2 indicates TT). The associations found in multivariate analyses are presented as multiple regression coefficients (B) ± standard error of the mean (SEM) and standardized regression coefficients (β). P < 0.05 was considered to be statistically significant. Pulse pressure (PP) was calculated as PP = (SBP − DBP); mean arterial pressure (MAP) was calculated as MAP = (2 × DBP + SBP)/3.
RESULTS
Characteristics of patients and genotype distribution of the G-protein β-polypeptide 3 C825T polymorphism
Forty-two patients were allocated into the telmisartan treatment group, and 51 patients were in the amlodipine treatment group, respectively. Baseline characteristics of the patients were shown in Table 1. The prevalence of GNB3 C825T genotype and allele frequencies were shown in Table 2. Genotype frequencies for CC, CT and TT, were 40.5%, 45.2%, and 14.3%, respectively, in the telmisartan treatment group. Genotype frequencies for the CC, CT, and TT, were 19.6%, 52.9%, and 27.5%, respectively, in the amlodipine treatment group. Genotype distribution of the polymorphism was in accordance with Hardy–Weinberg equilibrium in our study (P > 0.05).
Table 1.
Baseline characteristics of the subjects enrolled
| Variables | Telmisartan group (n = 42) | Amlodipine group (n = 51) |
|---|---|---|
| Male, n (%) | 20 (47.6) | 26 (50.1) |
| Age (years) | 65 ± 10 | 58 ± 7 |
| BMI (kg/m2) | 27.0 ± 4.0 | 25.0 ± 3.3 |
| SBP (mmHg) | 158 ± 13 | 150 ± 9 |
| DBP (mmHg) | 91 ± 10 | 92 ± 7 |
| PP (mmHg) | 68 ± 15 | 59 ± 10 |
| MAP (mmHg) | 113 ± 9 | 112 ± 6 |
| HR (bpm) | 77 ± 9 | 74 ± 8 |
| Cigarette smoking, n (%) | 10 (23.8) | 13 (25.5) |
| Alcohol drinking, n (%) | 11 (26.2) | 10 (19.6) |
| FBG (mmol/L) | 5.5 ± 1.9 | 5.2 ± 1.3 |
| TG (mmol/L) | 3.2 ± 18.6 | 2.9 ± 7.2 |
| TC (mmol/L) | 5.2 ± 1.4 | 5.1 ± 1.1 |
| HDL (µmol/L) | 1.4 ± 0.3 | 1.3 ± 0.3 |
| LDL (µmol/L) | 3.3 ± 1.3 | 3.3 ± 1.0 |
| ALT (µmol/L) | 33.8 ± 20.0 | 29.9 ± 15.4 |
| BUN (mmol/L) | 5.7 ± 6.0 | 5.0 ± 5.2 |
| UCr (mmol/L) | 79.8 ± 15.5 | 73.1 ± 16.2 |
| UA (mmol/L) | 324.6 ± 86.3 | 309.7 ± 76.4 |
Quantitative data are shown as mean ± standard deviation, whereas numbers and percentage are provided for the qualitative data. BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; PP: Pulse pressure; MAP: Mean arterial pressure; HR: Heart rate; FBG: Fasting blood glucose; TG: Triglyceride; TC: Total cholesterol; HDL: High-density lipoprotein; LDL: Low density lipoprotein; ALT: Alanine aminotransferase; BUN: Blood urea nitrogen; UCr: Urine creatinine; UA: Uric acid; BMI = Weight/height2.
Table 2.
Genotype and allele frequencies of GNB3 C825T in the study population
| Group | Genotype, n (%) | Allele (%) | P* | ||||
|---|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | |||
| Overall cases | 27 (29.0) | 46 (49.5) | 20 (21.5) | 46.2 | 53.8 | 0.96 | |
| Telmisartan group | 17 (40.5) | 19 (45.2) | 6 (14.3) | 63.1 | 36.9 | 0.85 | |
| Amlodipine group | 10 (19.6) | 27 (52.9) | 14 (27.5) | 46.1 | 53.9 | 0.64 | |
*P value for test of fitness to HWE. HWE: Hardy–Weinberg equilibrium; GNB3: G-protein β-polypeptide 3.
Covariate traits
No significant differences in baseline DBP, SBP, PP, MAP levels, baseline HR or age was observed between the three genotypes in both treatment groups [Table 3]. However, patients with the CT or TT genotypes showed significantly lower BMI as compared with patients with the CC genotype in telmisartan treatment group, amlodipine treatment group, and the overall patients [Table 3]. BMI values were comparable between the TT and CT genotype groups [Table 3].
Table 3.
Comparison of the main covariate traits among GNB3 C825T genotypes in EH patients at baseline
| Covariate factors | Genotype of telmisartan group (n = 42) | P | Genotype of amlodipine group (n = 51) | P | ||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 17) | CT (n = 19) | TT (n = 6) | CC (n = 10) | CT (n = 27) | TT (n = 14) | |||
| Age (years) | 65 ± 9 | 68 ± 11 | 56 ± 8† | 0.056 | 57 ± 8 | 58 ± 7 | 56 ± 7 | 0.604 |
| SBP (mmHg) | 157 ± 15 | 159 ± 11 | 158 ± 11 | 0.841 | 149 ± 7 | 153 ± 10 | 148 ± 9 | 0.207 |
| DBP (mmHg) | 91 ± 11 | 89 ± 9 | 96 ± 10 | 0.340 | 92 ± 7 | 92 ± 8 | 92 ± 7 | 0.980 |
| PP (mmHg) | 66 ± 15 | 70 ± 17 | 62 ± 5 | 0.466 | 57 ± 7 | 61 ± 10 | 56 ± 11 | 0.196 |
| MAP (mmHg) | 113 ± 11 | 113 ± 6 | 117 ± 10 | 0.554 | 112 ± 7 | 112 ± 7 | 110 ± 6 | 0.739 |
| HR (bpm) | 78 ± 11 | 76 ± 9 | 76 ± 9 | 0.813 | 72 ± 9 | 75 ± 9 | 75 ± 7 | 0.738 |
| BMI (kg/m2) | 29.5 ± 4.1 | 25.3 ± 3.1† | 25.2 ± 3.0† | 0.002* | 28.2 ± 3.5 | 24.0 ± 2.7† | 24.8 ± 3.0† | 0.003* |
Data are shown as mean ± standard deviation. *P value for contrast of covariate factors between different genotypes; †P<0.05, as compared with the CC genotype. DBP: Diastolic blood pressure; SBP: Systolic blood pressure; PP: Pulse pressure; MAP: Mean arterial pressure; HR: Heart rate; EH: Essential hypertension; GNB3: G-protein β-polypeptide 3; BMI: Body mass index.
Influence of G-protein β-polypeptide 3 C825T polymorphism on antihypertensive response by telmisartan or amlodipine therapy
After telmisartan or amlodipine therapy for 4 weeks, mean DBP, SBP, PP, and MAP levels in both study groups were decreased remarkably [Table 4]. Significant difference in mean DBP and MAP levels among GNB3 C825T genotypes was observed after telmisartan therapy (P = 0.001, P = 0.014, respectively) [Table 5], and patients carrying the 825TT (n = 6) genotype showed significantly higher DBP and MAP than those carrying at least one 825C allele (CC + CT, n = 25, P = 0.009, P = 0.003, respectively), [Table 5]. No significant difference in DBP, SBP, PP, or MAP was observed among GNB3 C825T genotypes after 4-week amlodipine therapy [Table 6].
Table 4.
Comparison of BP at baseline and after 4-week antihypertensive therapy with telmisartan or amlodipine
| Parameters | Telmisartan group (n = 42) | P | Amlodipine group (n = 51) | P | ||
|---|---|---|---|---|---|---|
| Baseline | Posttreatment | Baseline | Posttreatment | |||
| SBP (mmHg) | 158 ± 13 | 139 ± 15 | <0.001 | 151 ± 10 | 133 ± 10 | <0.001 |
| DBP (mmHg) | 91 ± 10 | 85 ± 8 | 0.007 | 92 ± 8 | 84 ± 7 | <0.001 |
| PP (mmHg) | 68 ± 15 | 54 ± 15 | <0.001 | 59 ± 10 | 49 ± 11 | <0.001 |
| MAP (mmHg) | 113 ± 15 | 103 ± 8 | <0.001 | 112 ± 7 | 101 ± 6 | <0.001 |
| HR (bpm) | 77 ± 9 | 76 ± 8 | 0.588 | 74 ± 8 | 73 ± 8 | 0.430 |
Data are shown as mean ± standard deviation. DBP: Diastolic blood pressure; SBP: Systolic blood pressure; PP: Pulse pressure; MAP: Mean arterial pressure; HR: Heart rate; BP: Blood pressure.
Table 5.
Comparison of BP after telmisartan therapy among GNB3 C825T genotypes in EH patients
| Parameters | Genotype groups | P* | P† | P‡ | ||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 17) | CT (n = 19) | TT (n = 6) | CT + TT (n = 25) | CC + CT (n = 36) | ||||
| DBP (mmHg) | 85 ± 7 | 82 ± 6 | 96 ± 8 | 85 ± 9 | 83 ± 7 | 0.001 | 0.847 | 0.009 |
| SBP (mmHg) | 135 ± 15 | 142 ± 16 | 144 ± 9 | 142 ± 15 | 138 ± 15 | 0.245 | 0.101 | 0.365 |
| PP (mmHg) | 50 ± 14 | 60 ± 16 | 49 ± 12 | 57 ± 15 | 55 ± 16 | 0.098 | 0.134 | 0.356 |
| MAP (mmHg) | 102 ± 8 | 102 ± 7 | 112 ± 6 | 104 ± 8 | 102 ± 7 | 0.014 | 0.269 | 0.003 |
Data are shown as mean ± standard deviation. *P value for comparison among three genotypes; †P value analyzed under dominant model (CC vs. CT + TT); ‡P value analyzed under recessive model (TT vs. CC + CT). DBP: Diastolic blood pressure; SBP: Systolic blood pressure; PP: Pulse pressure; MAP: Mean arterial pressure; GNB3: G-protein β-polypeptide 3; EH: Essential hypertension.
Table 6.
Comparison of BP after amlodipine therapy among GNB3 C825T genotypes in EH patients
| Parameters | Genotype groups | P* | P† | P‡ | ||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 10) | CT (n = 27) | TT (n = 14) | CT + TT (n = 41) | CC + CT (n = 37) | ||||
| SBP (mmHg) | 133 ± 13 | 134 ± 10 | 133 ± 8 | 134 ± 9 | 134 ± 11 | 0.844 | 0.873 | 0.688 |
| DBP (mmHg) | 85 ± 7 | 84 ± 7 | 84 ± 8 | 84 ± 7 | 85 ± 7 | 0.823 | 0.601 | 0.627 |
| PP (mmHg) | 48 ± 15 | 50 ± 10 | 49 ± 11 | 50 ± 10 | 49 ± 11 | 0.876 | 0.635 | 0.961 |
| MAP (mmHg) | 101 ± 6 | 101 ± 6 | 100 ± 6 | 101 ± 6 | 101 ± 6 | 0.831 | 0.772 | 0.548 |
Data are shown as mean ± standard deviation.*P value for comparison among three genotypes; †P value analyzed under dominant model (CC vs. CT + TT); ‡P value analyzed under recessive model (TT vs. CC + CT). DBP: Diastolic blood pressure; SBP: Systolic blood pressure; PP: Pulse pressure; MAP: Mean arterial pressure; GNB3: G-protein β-polypeptide 3; EH: Essential hypertension.
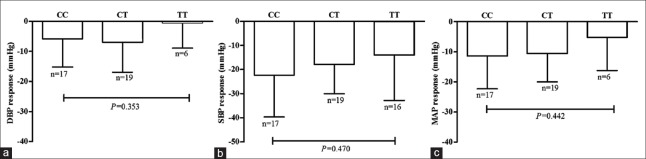
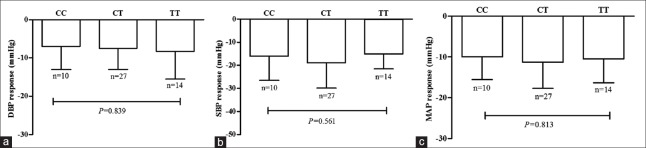
Figures 1 and 2 show the absolute changes in SBP, DBP, and MAP after 4-week therapy by telmisartan or amlodipine. Mean absolute decreases in SBP and DBP were 22 ± 17 mmHg and 6 ± 9 mmHg, respectively, in 825CC homozygotes, 18 ± 12 mmHg and 7 ± 10 mmHg, respectively, in 825CT heterozygotes, and 14 ± 19 mmHg and 1 ± 8 mmHg, respectively, in 825TT homozygotes by telmisartan therapy. Mean absolute decreases in SBP and DBP were 16 ± 10 mmHg and 7 ± 6 mmHg, respectively, in 825CC homozygotes, 19 ± 11 mmHg and 7 ± 6 mmHg, respectively, in 825CT heterozygotes, 15 ± 7 and 8 ± 7 mmHg, respectively, in 825CC homozygotes by amlodipine therapy. No difference in absolute changes in SBP, DPB or MAP among GNB3 C825T genotypes was observed after either telmisartan or amlodipine therapy.
Figure 1.
Association G-protein β-polypeptide 3 C825T genetic polymorphism with blood pressure response to telmisartan. Decrease in diastolic blood pressure, (a) systolic blood pressure, (b) and mean arterial pressure (c) in essential hypertension patients with different G-protein β-polypeptide 3 C825T genotype after telmisartan therapy for 4 weeks.
Figure 2.
Association G-protein β-polypeptide 3 C825T genetic polymorphism with blood pressure response to amlodipine. Decline in diastolic blood pressure, (a) systolic blood pressure, (b) and mean arterial pressure (c) in essential hypertension patients carrying G-protein β-polypeptide 3 825CC, CT, and TT genotype after-treatment with amlodipine for 4 weeks.
Stepwise multiple linear regression analysis showed that the significant predictors of posttreatment DBP after 4 weeks telmisartan treatment were GBN3 825TT genotype (B ± SEM = 10.733 ± 2.748, β =0.472, P < 0.001) and baseline MAP (B ± SEM = 0.377 ± 0.114, β =0.401, P = 0.002). In addition to 825TT genotype (B ± SEM = 10.049 ± 2.961, β = 0.442, P = 0.002), baseline SBP (B ± SEM = 0.238 ± 0.082, β = 0.379, P = 0.006) also remained significant predictors of posttreatment MAP. However, the C825T polymorphism showed no significant effect on posttreatment SBP and BP reduction after 4 weeks telmisartan treatment and had no contribution to the prediction of both posttreatment BP and BP reduction after 4 weeks amlodipine treatment.
DISCUSSION
In this study, we investigated the effect of GNB3 C825T polymorphism on antihypertensive effects of telmisartan and amlodipine in Chinese EH patients. Though we observed no significant difference in BP-lowering effects of both drugs among GNB3 C825T genotypes as indicated by an absolute decrease in BP, we found that 825TT homozygotes showed significantly higher posttreatment DBP and MAP levels than carriers of the 825C allele after 4-week telmisartan therapy. In addition, we found that carriers of the 825T allele showed significantly lower baseline BMI than patients with the 825CC homozygotes.
Gβ3 is an important G-protein subunit and play a role in GPCRs signal transduction and regulation of Ca2+ channels. GNB3 C825T polymorphism is a splice variant that results in gain-of-function.[21] There is increasing interest in pharmacogenetics of this polymorphism in therapeutic drugs targeting GPCRs such as hydralazine, isosorbide dinitrate, sildenafil, and bofutsushosan.[23,24,25] We are the first to investigate the association of C825T polymorphism with drug response to telmisartan and amlodipine. The significantly higher posttreatment DBP and MAP levels in 825TT homozygotes by telmisartan therapy while the baseline DBP and MAP levels were comparable among genotypes, our findings indicate that the polymorphism is associated with decreased telmisartan response. Our findings are in contrast to the findings of better drug responses in TT homozygotes or carriers of the 835T allele for hydralazine, isosorbide dinitrate, sildenafil, and bofutsushosan as reported previously.[23,24,25] The 825TT genotype is associated with greater therapeutic effects with isosorbide dinitrate and hydralazine combined therapy in heart failure patients[23] and better improvement in posttreatment parameters and longer time to clinical worsening in pulmonary hypertension patients treated with sildenafil,[24] and better anti-obesity response to bofutsushosan, a traditional herbal medicine.[25]
Potential causes for the different direction of the association
The difference in the direction of association for the pharmacogenetics of C825T polymorphism between our findings and the literature remains unknown. Of note, previous association studies are based on the idea that Gβ3s is a gain-of-function variant. While in a recent study, Sun et al. have demonstrated that Gβ3s is unstable, unable to interact with Gγ and Gα subunits, and cannot activate diverse Gβγ effectors.[26] And also, earlier functional studies for C825T polymorphism neglected the influence of the polymorphism on Gβ3s expression and stability. What's more, the effects of GNB3 C825T polymorphism are likely to be tissue and/or stimuli specific.[26] Therefore, we can assume that the significantly higher posttreatment DBP and MAP levels in 825TT homozygotes by telmisartan therapy may be due to decreased Gβ3s expression in these patients. Of course, further studies are warranted to explore the exact mechanism.
Association between G-protein β-polypeptide 3 C825T polymorphism and body mass index
Importantly, we found that patients with the CT or TT genotypes had significantly lower BMI as compared with 825CC homozygotes. In line with our observation, a previous study by Khamidullaeva et al. showed the EH patients with CC genotype exhibit higher BMI in Uzbek hypertensive men.[27] BMI is a standard evaluation of overweight and obesity. Several studies have observed an association of the GNB3 825T polymorphism with obesity in the different population though controversial reports are also available. The C825T variant also seems to affect BMI and body fat in the German population,[28] and is associated with obesity-related metabolic traits including triglyceride and total cholesterol levels in Chinese in Taiwan, China.[29] Transgenic mice model of GNB3 duplication including 825T variant recapitulates obesity phenotype that implicates GNB3 duplication and overexpression in increasing weight or obesity risk.[30] Several studies have also suggested that GNB3 C825T polymorphism may influence the efficacy of anti-obesity drugs.[25,31,32] Obese patients with TT or TC genotype have the stronger effect of sibutramine, a noradrenergic, and serotonergic reuptake inhibitor approved for the long term treatment of obesity, on weight reduction, and body fat loss.[31,32] Carriers of the 825T allele showed improvement in several obesity-related parameters after-treatment with bofutsushosan.[25] Association of this polymorphism with risk for obesity and anti-obesity therapy deserves further study.
Limitations
No significant association between GNB3 C825T polymorphism and telmisartan therapy was found when an absolute decrease in BP levels was analyzed. The small number of cases recruited may explain, at least partially, for this negative observation in our study. And also, GNB3 C825T is associated with risk for salt sensitive hypertension and can serve as a molecular switch for salt sensitivity in the Chinese population,[33] while information on salt consumption is not considered in our study.
In conclusion, our results demonstrated that GNB3 C825T polymorphism is associated with the DBP-lowering effect of telmisartan but not amlodipine in Chinese EH patients.
Financial support and sponsorship
This project was supported by grants from the National Natural Science Foundation of China (No. 81373489, No. 81422052), Hunan Provincial Natural Science Foundation of China (No. 13JJ1010).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Na JO, Seo HS, Choi CU, Lim HE, Kim JW, Kim EJ, et al. Results of a 14-week, multicenter, prospective, randomized, open-label, noninferiority clinical trial comparing the antihypertensive effect and edema incidence of lacidipine and amlodipine in older Korean patients with mild-to-moderate hypertension. Curr Ther Res Clin Exp. 2013;74:54–61. doi: 10.1016/j.curtheres.2013.02.001. doi: 10.1016/j.curtheres.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messerli FH. Evolution of calcium antagonists: Past, present, and future. Clin Cardiol. 2003;26(2 Suppl 2):II12–6. doi: 10.1002/clc.4960261405. doi: 10.1002/clc.496026140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 7.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 8.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 9.Nagel JM, Tietz AB, Göke B, Parhofer KG. The effect of telmisartan on glucose and lipid metabolism in nondiabetic, insulin-resistant subjects. Metabolism. 2006;55:1149–54. doi: 10.1016/j.metabol.2006.04.011. doi: 10.1016/j.metabol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Yin SN, Liu M, Jing DQ, Mu YM, Lu JM, Pan CY. Telmisartan increases lipoprotein lipase expression via peroxisome proliferator-activated receptor-alpha in HepG2 cells. Endocr Res. 2014;39:66–72. doi: 10.3109/07435800.2013.828741. doi: 10.3109/07435800.2013.828741. [DOI] [PubMed] [Google Scholar]
- 11.Giles TD, Bakris GL, Smith DH, Davidai G, Weber MA. Defining the antihypertensive properties of the angiotensin receptor blocker telmisartan by a practice-based clinical trial. Am J Hypertens. 2003;16:460–6. doi: 10.1016/s0895-7061(03)00848-3. doi: 10.1016/S0895-7061(03)00848-3. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira-Paula GH, Lacchini R, Coeli-Lacchini FB, Junior HM, Tanus-Santos JE. Inducible nitric oxide synthase haplotype associated with hypertension and responsiveness to antihypertensive drug therapy. Gene. 2013;515:391–5. doi: 10.1016/j.gene.2012.12.059. doi: 10.1016/j.gene.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava K, Chandra S, Bhatia J, Narang R, Saluja D. Association of angiotensinogen (M235T) gene polymorphism with blood pressure lowering response to angiotensin converting enzyme inhibitor (Enalapril) J Pharm Pharm Sci. 2012;15:399–406. doi: 10.18433/j3kw3b. [DOI] [PubMed] [Google Scholar]
- 14.Maguire JJ, Davenport AP. Regulation of vascular reactivity by established and emerging GPCRs. Trends Pharmacol Sci. 2005;26:448–54. doi: 10.1016/j.tips.2005.07.007. doi: 10.1016/j.tips.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Pietruck F, Moritz A, Montemurro M, Sell A, Busch S, Rosskopf D, et al. Selectively enhanced cellular signaling by Gi proteins in essential hypertension. G alpha i2, G alpha i3, G beta 1, and G beta 2 are not mutated. Circ Res. 1996;79:974–83. doi: 10.1161/01.res.79.5.974. doi: 10.1161/01.RES.79.5.974. [DOI] [PubMed] [Google Scholar]
- 16.Kleuss C, Scherübl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–6. doi: 10.1038/358424a0. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 17.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998;18:45–8. doi: 10.1038/ng0198-45. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 18.Beige J, Hohenbleicher H, Distler A, Sharma AM. G-Protein beta3 subunit C825T variant and ambulatory blood pressure in essential hypertension. Hypertension. 1999;33:1049–51. doi: 10.1161/01.hyp.33.4.1049. doi: 10.1161/01.HYP.33.4.1049. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Zhu H, Sagnella GA, Carter ND, Cook DG, Cappuccio FP. Association between the C825T polymorphism of the G protein beta3-subunit gene and hypertension in blacks. Hypertension. 1999;34:1193–6. doi: 10.1161/01.hyp.34.6.1193. doi: 10.1161/01.HYP.34.6.1193. [DOI] [PubMed] [Google Scholar]
- 20.Benjafield AV, Jeyasingam CL, Nyholt DR, Griffiths LR, Morris BJ. G-protein beta3 subunit gene (GNB3) variant in causation of essential hypertension. Hypertension. 1998;32:1094–7. doi: 10.1161/01.hyp.32.6.1094. doi: 10.1161/01.HYP.32.6.1094. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Xu H, Cui B, Xie N, Wang Z, Luo M. Association between polymorphism of the G-protein ß3 subunit C825T and essential hypertension: An updated meta-analysis involving 36,802 subjects. Biol Res. 2013;46:265–73. doi: 10.4067/S0716-97602013000300007. doi: 10.4067/S0716-97602013000300007. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.McNamara DM, Taylor AL, Tam SW, Worcel M, Yancy CW, Hanley-Yanez K, et al. G-protein beta-3 subunit genotype predicts enhanced benefit of fixed-dose isosorbide dinitrate and hydralazine: Results of A-HeFT. JACC Heart Fail. 2014;2:551–7. doi: 10.1016/j.jchf.2014.04.016. doi: 10.1016/j.jchf.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Sekine A, Tanabe N, Sugiura T, Shigeta A, Jujo T, Nishimura R, et al. Polymorphism of the G protein ß3 subunit gene influences the efficacy of sildenafil in patients with pulmonary hypertension. Intern Med. 2014;53:291–7. doi: 10.2169/internalmedicine.53.0658. doi: 10.2169/internalmedicine.53.0658. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Bose S, Hong SW, Lee DK, Yoo JW, Lim CY, et al. Impact of GNB3-C825T, ADRB3-Trp64Arg, UCP2-3’UTR 45 bp del/ins, and PPARγ-Pro12Ala polymorphisms on Bofutsushosan response in obese subjects: A randomized, double-blind, placebo-controlled trial. J Med Food. 2014;17:558–70. doi: 10.1089/jmf.2013.2836. doi: 10.1089/jmf.2013.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z, Runne C, Tang X, Lin F, Chen S. The Gß3 splice variant associated with the C825T gene polymorphism is an unstable and functionally inactive protein. Cell Signal. 2012;24:2349–59. doi: 10.1016/j.cellsig.2012.08.011. doi: 10.1016/j.cellsig.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamidullaeva GA, Eliseyeva MR, Nagay AV, Abdullaeva GJ. C825T polymorphism of the G-protein ß3 subunit and its association with essential hypertension in Uzbek males. Turk Kardiyol Dern Ars. 2011;39:198–204. doi: 10.5543/tkda.2011.01103. doi: 10.5543/tkda.2011.01103. [DOI] [PubMed] [Google Scholar]
- 28.Stefan N, Stumvoll M, Machicao F, Koch M, Häring HU, Fritsche A. C825T polymorphism of the G protein beta3 subunit is associated with obesity but not with insulin sensitivity. Obes Res. 2004;12:679–83. doi: 10.1038/oby.2004.78. doi: 10.1038/oby.2004.78. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao TJ, Hwang Y, Liu CH, Chang HM, Lin E. Association of the C825T polymorphism in the GNB3 gene with obesity and metabolic phenotypes in a Taiwanese population. Genes Nutr. 2013;8:137–44. doi: 10.1007/s12263-012-0304-8. doi: 10.1007/s12263-012-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldlust IS, Hermetz KE, Catalano LM, Barfield RT, Cozad R, Wynn G, et al. Mouse model implicates GNB3 duplication in a childhood obesity syndrome. Proc Natl Acad Sci U S A. 2013;110:14990–4. doi: 10.1073/pnas.1305999110. doi: 10.1073/pnas.1305999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao DJ, Wu LS, Huang SY, Lin E. Weight loss and body fat reduction under sibutramine therapy in obesity with the C825T polymorphism in the GNB3 gene. Pharmacogenet Genomics. 2009;19:730–3. doi: 10.1097/FPC.0b013e3283307cf1. doi: 10.1097/FPC.0b013e3283307cf1. [DOI] [PubMed] [Google Scholar]
- 32.Grudell AB, Sweetser S, Camilleri M, Eckert DJ, Vazquez-Roque MI, Carlson PJ, et al. A controlled pharmacogenetic trial of sibutramine on weight loss and body composition in obese or overweight adults. Gastroenterology. 2008;135:1142–54. doi: 10.1053/j.gastro.2008.07.009. doi: 10.1053/j.gastro.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu W, Qi Y. Association of α-adducin and G-protein ß3 genetic polymorphisms with hypertension: A meta-analysis of Chinese populations. PLoS One. 2011;6:e17052. doi: 10.1371/journal.pone.0017052. doi: 10.1371/journal.pone.0017052. [DOI] [PMC free article] [PubMed] [Google Scholar]