Abstract
Objective:
To examine the efficacy and safety of dual blockade of the renin-angiotensin-aldosterone system (RAAS) among patients with type 2 diabetic kidney disease.
Data Sources:
We searched the major literature repositories, including the Cochrane Central Register of Controlled Trials, MEDLINE and EMBASE, for randomized clinical trials published between January 1990 and October 2015 that compared the efficacy and safety of the use of dual blockade of the RAAS versus the use of monotherapy, without applying any language restrictions. Keywords for the searches included “diabetic nephropathy,” “chronic kidney disease,” “chronic renal insufficiency,” “diabetes mellitus,” “dual therapy,” “combined therapy,” “dual blockade,” “renin-angiotensin system,” “angiotensin-converting enzyme inhibitor,” “angiotensin-receptor blocker,” “aldosterone blockade,” “selective aldosterone blockade,” “renin inhibitor,” “direct renin inhibitor,” “mineralocorticoid receptor blocker,” etc.
Study Selection:
The selected articles were carefully reviewed. We excluded randomized clinical trials in which the kidney damage of patients was related to diseases other than diabetes mellitus.
Results:
Combination treatment with an angiotensin-converting enzyme inhibitor supplemented by an angiotensin II receptor blocking agent is expected to provide a more complete blockade of the RAAS and a better control of hypertension. However, existing literature has presented mixed results, in particular, related to patient safety. In view of this, we conducted a comprehensive literature review in order to explain the rationale for dual blockade of the RAAS, and to discuss the pros and cons.
Conclusions:
Despite the negative results of some recent large-scale studies, it may be immature to declare that the dual blockade is a failure because of the complex nature of the RAAS surrounding its diversified functions and utility. Further trials are warranted to study the combination therapy as an evidence-based practice.
Keywords: Diabetes Mellitus, Type 2, Diabetic Nephropathies, Dual Blockade, Renin-angiotensin-aldosterone System
INTRODUCTION
Diabetes mellitus is a serious global public health problem. In 2010, the worldwide diabetic population had reached 284 million and is expected to reach 438 million by 2030.[1] A study conducted in 2010 by the China Noncommunicable Disease Surveillance Group[2] estimated a national prevalence of diabetes of 11.6% with prediabetes occurring among 50.1% of the Chinese adults.
Approximately, 30–40% of diabetic patients will develop kidney disease, which might lead to end-stage renal disease (ESRD).[3] Proteinuria is a primary characteristic of type 2 diabetic kidney disease (DKD) and an independent risk factor for ESRD.[4,5,6] As demonstrated in recent studies, blockade of the renin-angiotensin-aldosterone system (RAAS) is effective in blood pressure (BP) control, as well as reducing proteinuria, slowing the progression of kidney disease[7,8,9] and helping to prevent cardiovascular disease (CVD).[10,11,12] Current guidelines have recommended RAAS blocker medications as a preferred agent for the treatment of kidney diseases,[13,14] as a reduction of proteinuria may further reduce the risk of disease progression.[15] Treatment with a combination of an angiotensin-converting-enzyme inhibitor (ACEI) and an angiotensin II (AII)-receptor blocker (ARB) has formed the basis for dual blockade of the RAAS.[16]
That said, recent trials of dual blockers have not rendered clear support for the use of dual blockers, given a variety of methodological concerns, conflicting results, and the occurrence of adverse events. Without a systematic review of existing studies, it is unclear whether these conflicting findings have nullified the combination theory or warranted further studies. This review describes the rationale for dual blockade of the RAAS, gives possible explanations to the conflicting results, and discusses the pros and cons of this treatment approach based on clinical research evidence.
RATIONALE FOR DUAL BLOCKADE OF THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
RAAS is a key in regulating BP [Figure 1]. The mechanism can be described as follows. First, the renin enzyme initiates a cascade reaction, followed by the production of protease in the juxtaglomerular apparatus of the kidney. Protease then converts the angiotensinogen to an inactive substance, angiotensin I (AI), which is further converted to the vasorelaxant substance, AII, by angiotensin-converting enzymes (ACE) produced mostly in the lungs. The AII promotes the release of aldosterone by binding to the receptor, which directly induces renal vasoconstriction, and increases myocardial contractility and water-sodium retention. All these reactions increase the circulating blood volume and maintain the stability of BP. Finally, inducing negative feedback among the juxtaglomerular cells, the sodium reabsorption completes the canonical pathways.
Figure 1.
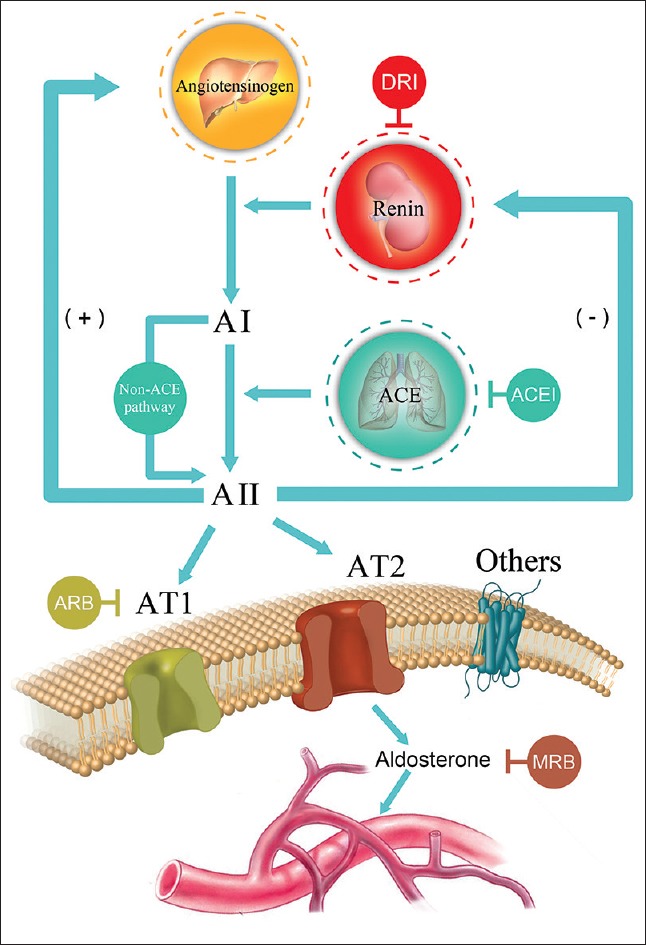
Renin-angiotensin-aldosterone system and the targets for therapeutic interventions in the renin-angiotensin-aldosterone system cascade. AI: Angiotensin I; AII: Angiotensin II; AT1: Type 1 angiotensin II receptor; AT2: Type 2 angiotensin II receptor; ACE: Angiotensin-converting enzyme; DRI: Direct renin inhibitor; ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin II receptor blocker; MRB: Mineralocorticoid receptor blocker.
On the other hand, ACEI competitively blocks the action of ACE and thus the conversion of AI to AII, thereby reducing circulating and the local levels of AII. It further decreases aldosterone and vasopressin secretion and sympathetic nerve activity. However, the ARB produces a selective, dose-dependent blockade of the AT1 receptor, independently of the different pathways of AII generation that do not reduce AII concentrations. These interventions can reduce glomerulosclerosis, tubulointerstitial fibrosis, albuminuria, and loss of glomerular filtration rate (GFR), as demonstrated in animal experimental models and/or human randomized clinical trials (RCTs).
However, the RAAS is complicated in that alongside the pathways mentioned above; the AII can be activated by autocrine and paracrine pathways in local organs. Because aldosterone is related to tissue and vascular remodeling, the RAAS induces kidney damage by proinflammatory and oxidative reactions. These non-ACE pathways may partly explain the aldosterone “escape” phenomenon, i.e., both the angiotensin and aldosterone levels that are initially lowered by ACE inhibition eventually return to the baseline level in the long run.
The benefits of monotherapy using an RAAS blocker have been demonstrated in the cardiovascular and renal systems, and in overall prognosis. However, there is value in the study of whether a combination treatment, or dual blockade of the RAAS, has the potential to yield additional benefits. The combination theory arises primarily for two reasons. Monotherapy can result in an incomplete blockade of AI or renin, and the “escape” phenomenon of AII or aldosterone.[17,18,19,20] Moreover, many DKD patients have a high residual level of proteinuria after using RAAS blockers; the use of an additional RAAS blocker may further reduce proteinuria to improve the long-term outcome.[21,22,23,24]
Ménard et al.[25] first proposed the potential additive or synergistic effects of ACEI and ARB through a mouse model. With the goal of maximizing the antagonistic effect on the escape phenomenon, supplementing ACEI with an ARB has been expected to provide a more complete blockade of the RAAS and enhance the BP control.
CLINICAL EVIDENCE FOR DUAL BLOCKADE OF THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
Effect of dual blockade of renin-angiotensin-aldosterone system on blood pressure
Current studies show that combination therapy for DKD patients with hypertension has a dose-response relationship in lowering BP. That is, larger doses of dual blockers may lead to larger effects in the BP control.
In a 2005 meta-analysis, Doulton et al. concluded that use of dual blockers in hypertensive patients reduced BP by 4.7/3.0 mm Hg (1 mm Hg = 0.133 kPa), contrasting with a 3.8/2.9 mm Hg reduction achieved with ACEI/ARB monotherapy treatment. Further subgroup analysis showed an even more effective reduction – by 6.8/4.7 mm Hg – in the patients with diabetes.[26]
As shown in a more recent meta-analysis, combination therapy outperforms monotherapy in reducing the systolic BP (SBP), diastolic BP, and mean arterial pressure, or in controlling the rate of BP in chronic kidney disease (CKD).[27] Some studies have reached similar conclusions.[28,29]
Some other trials, however, have differed. Fernandez Juarez et al.[30] randomly assigned patients with type 2 diabetic nephropathy to treatment with lisinopril, irbesartan, or a combination of the two (1/2 dose of each). After a median follow-up of 32 months, they failed to find benefits of the combination vis-a-vis monotherapy in the BP control. This conclusion was concurred in a study of Asian type 2 patients with nephropathy.[31]
In a recent trial on 1448 patients with type 2 diabetes, all participants initially received losartan and were then randomly assigned to two groups, to additionally receive lisinopril or placebo.[32] The combination therapy group received losartan and lisinopril at a dose of 100 mg/d and 10–40 mg/d, respectively, while the monotherapy group received only losartan at the same dose and placebo. After a median follow-up of 2.2 years, the combination therapy group had a slightly lower BP than the monotherapy group (within 2 mm Hg).
It is noted that most of these studies drew their conclusions based on short-term observations; long-term evidence remains elusive. Furthermore, researchers have not yet determined the optimal dose of dual blockers – the antihypertensive effect is similar when both agents are prescribed at half of their routine mono use dosage.[30,31] It seems necessary to study titration to the highest or the next-highest dose to maximize the dual effect.
Effect of reduction in proteinuria using dual blockade of the renin-angiotensin-aldosterone system
The effect of reducing proteinuria by using ACEI or ARB alone has been established among the type 2 DKD patients;[7,8,9] at the highest tolerable dosage, the effect is even more pronounced among the patients whose level of proteinuria is at least 300 mg/d, independent of BP control.[6] ACEI or ARB agents are thus the first-line choices for DKD patients with hypertension.
Some early studies[28,31,33,34,35,36,37,38,39,40,41] suggested that ACEI combined with ARB could further reduce proteinuria and result in about 20% reduction in albuminuria.[16,42,43,44] However, many of these studies were inadequately powered with small sample sizes or with insufficient length of follow-up. A meta-analysis showed that as compared with ACEI or ARB alone, combination therapy results in 20–30% additional reduction in proteinuria.[16,45,46]
The “Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint” trial (ONTARGET) involved the largest cohort of patients to date (n = 25,620).[47] Patients were assigned to receive telmisartan alone, ramipril alone, or a combination of both. After 56-month follow-up, it was reported that the risks of development and progression of microalbuminuria and macroalbuminuria were lower for those receiving combination therapy (hazard ratio [HR] = 0.88, P = 0.003 and HR = 0.76, P = 0.019, respectively), compared to the ramipril alone treatment.
Safety and prognosis of dual blockade of the renin-angiotensin-aldosterone system
Due attention must be paid to the evaluation of the risks and prognosis of using dual blockade of the RAAS under contested conditions. Current hypertension treatment guidelines[48] do not recommend a therapy that combines ACEI and ARB, in consideration of increased risks of adverse events such as hyperkalemia and hypotension.
Among the DKD patients, however, proteinuria is usually comorbid with hypertension. The effects of reduction of proteinuria theoretically correlate with renal function and CVD. Thus, control of proteinuria can result in parallel benefits to all conditions. These potential benefits cannot be sought without consideration of the associated treatment risks, however.
A meta-analysis of dual blockade among the DKD patients found an only slightly increased incidence of hyperkalemia; the increase in the level of potassium was limited to 0.2 mmol/L, and the decrease in renal function to 3 ml/min.[29] Unfortunately, most similar studies used either proteinuria or albuminuria alone as the end point, with follow-up <4 months, and were not powered to assess potential long-term effects.
The ONTARGET study did not find additional benefits of the combination therapy in reducing the mortality rate and risk of cardiovascular events[47] but rather a significantly higher incidence of hyperkalemia, hypotensive symptoms, and over declined estimated GFR (eGFR) than treatment with ramipril or telmisartan alone. Moreover, the decline of eGFR and dialysis (especially acute dialysis) was higher than that of monotherapy group.[47]
The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) clinical trial assessed the safety and efficacy of the combination of losartan with lisinopril in diabetes patients with progression of kidney disease.[32] This study was suspended for safety concerns: Among 148 patients with a median follow-up of 2.2 years, combination therapy increased the risk of hyperkalemia and acute kidney injury.
In terms of kidney disease prognosis, the existing studies have provided mixed results on reduction of proteinuria. The Olmesartan Reducing Incidence of ESRD in Diabetic Nephropathy Trial enrolled type 2 diabetic patients who were receiving ACEI and ARB. The evaluation indicators consisted of reduced BP, proteinuria, and the rate of decline of the serum creatinine reciprocal (1/sCr). Post-hoc analysis found no significant difference between the combination therapy group and monotherapy group in cardiovascular and renal outcomes.[49]
However, the newest network meta-analysis[50] evaluated the prognosis of dual blockade of the RAAS, the researchers did a network analysis of randomized trials from around the world comparing BP lowering agents in adults with DKD and found that ESRD was significantly less likely after dual treatment with ACEI plus ARB (odds ratio = 0.62, 95% confidence interval = 0.43–0.90). These results fully confirm the possibility of improving prognosis when using combination therapy.
Makani et al.[51] analyzed all RCTs between January 1990 and August 2012 to compare treatment with dual blockers of RAAS to monotherapy. The authors examined data of studies measuring long-term efficacy for at least 1 year, and with a sample size of at least 50 participants. Although dual therapy was associated with an 18% reduction in hospitalization for heart failure as compared with monotherapy, it was also associated with a 55%, 66%, and 41% increase in the risks of hyperkalemia, hypotension, and renal failure, respectively. These outcomes introduce the caveat that dual RAAS blockade treatment should be used with abundant caution in clinical practice.
Use of dual blockade for selected populations
Most of the existing large-scale studies limited enrollment to those patients with BP <160/100 mm Hg, while in clinical practice the BP of DKD patients is frequently higher than this cut-off value. However, because of the related health risks such as CVD and increased mortality, the strategy of combination antihypertensive treatment is already widely adopted for this population.
Wong et al.[52] designed a case-control study that assessed the prognosis of the dual blockade in patients with BP below and above 160/100 mm Hg. After a median follow-up of more than 3.7 years, among patients with BP lower than 160/100 mm Hg, the incidence of a decline of more than 20 ml/min in eGFR was higher than that in the monotherapy group; among patients with BP higher than 160/100 mm Hg, however, the opposite was observed. While this finding does not necessarily nullify the risks associated with ACEI/ARB combination reported in recent studies such as ONTARGET, it merits further exploration.
OTHER ADVANCES IN DUAL BLOCKADE OF THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
New RAAS blockers are being developed and introduced into clinical practice. Apart from the traditional ACEI/ARB combination therapy, combination treatment of mineralocorticoid receptor blockers (MRB) or direct renin inhibitors (DRI) with ACEI or ARB therapy has emerged recently. For example, among the 200,000 patients who were treated with dual blockade of the RAAS, more than 8% of them used ACEI or ARB in combination with a DRI.[53] However, the long-term efficacy and safety of such treatment has not been well supported so far.
Mineralocorticoid receptor blockers and angiotensin-converting-enzyme inhibitor or angiotensin-receptor blocker combination
Mineralocorticoid receptor blocking agents reduce the sodium and water reabsorption along epithelial sites, as well as cardiovascular and renal fibrotic injury at nonepithelial sites by inhibiting binding of aldosterone to mineralocorticoid receptors.[54]
Krum et al. found that adding eplerenone (50–100 mg daily) to ACEI or ARB in patients with poor BP control may contribute to a better SBP control with no significant difference in adverse events as compared to eplerenone alone.[55] Meanwhile, Epstein et al. concluded that eplerenone in combination with enalapril reduced albuminuria more than enalapril alone in patients with diabetes and did not result in any significant increase in serum potassium levels.[37]
Although an early review of MRB combined with ACEI or ARB showed that these pairings can significantly reduce proteinuria without increasing the incidence of hyperkalemia or impairing renal function, the authors acknowledged that the results reported were insufficient to conduct a meta-analysis because of the impaired reliability. This study did not constitute a reasonable basis for endorsing the combination of MRB with ACEI or ARB as a conventional treatment strategy.[56]
Recently, Bakris et al. evaluated the safety and efficacy of the novel nonsteroidal MRB finerenone for 90 days among patients with diabetes and high or very high albuminuria who received an ACEI or an ARB.[57] It was found that the addition of finerenone compared with placebo resulted in some improvement in albumin-to-creatinine rate (ACR). However, the short duration of the study did not allow assessment of any long-term effects of finerenone.
The most recent Cochrane analysis on this topic concluded that MRB may reduce proteinuria and BP among patients with mild to moderate CKD, who were treated with ACEI or ARB, but may also increase the risks of hyperkalemia and gynecomastia. It is unclear, however, how this may further influence the risks of CVD and ESRD.[58]
Direct renin inhibitors and angiotensin-converting-enzyme inhibitor or angiotensin-receptor blocker combination
DRI agents can antagonize the ACEI-, ARB-, or even MRB-induced renin increase by blocking renin activities.[59,60] The result of using DRI and other RAAS blockers in combination has been reported in a few small-scale studies,[61] which suggested that efficacy does not significantly differ between DRI and ACEI or ARB alone though a combination treatment may have additional benefits of reducing proteinuria.
The Aliskiren in the Evaluation of Proteinuria in Diabetes trial was one of the largest studies that evaluated the value of the DRI and aliskiren among DKD patients.[42] In this study, 599 type 2 DKD patients who received losartan (100 mg/d) were assigned to either the 6-month aliskiren treatment group (150 mg/d for the first 3 months, then 300 mg/d) or the placebo group. At 3 months posttreatment, the aliskiren group experienced an 11% decrease of ACR as compared with placebo group, and an additional 20% decrease when the dose of aliskiren was increased to 300 mg/d. No difference in the rates of adverse events were found, nor was a delay in progression of renal failure.
The Aliskiren Trial in Type 2 Diabetes Using Cardio-renal Endpoints study investigated whether the use of aliskiren would reduce cardiovascular and renal events among patients with type 2 diabetes and CKD.[62] A total of 8561 patients were randomly assigned to the aliskiren (300 mg/d) or placebo group, in addition to receiving an ACEI or ARB. Unfortunately, the trial was stopped after a median follow-up of about 32.9 months following the results of the second efficacy analysis. They suggested that although there was a greater ACR decrease in the combination therapy, the incidence of hypotension and hyperkalemia events was higher than that in the placebo group. Therefore, the author recommended against the use of this combination therapy among type 2 patients with CKD or CVD.
ANALYTICAL ISSUES WITH STUDIES OF DUAL BLOCKADE OF THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
Even most small-scale clinical studies that used proteinuria as the end point demonstrated the additional effect of the dual blockade on reducing proteinuria and decreasing BP in DKD patients, findings from more recent large-scale studies, however, have not confirmed such results.
The most authoritative source of evidence, the ONTARGET study, does not exhibit ideal reliability. Participants in the ONTARGET study had near normal levels of albuminuria or microalbuminuria, and insignificant increases in BP. The benefits of dual blockade therapy, however, have occurred only in patients with overt proteinuria,[7,8,9,63] thus the populations are not comparable. In the post-hoc analysis of ONTARGET, the deleterious effects were only experienced by patients with nondiabetes and normal BP, who had no indications for ACEI or ARB treatment.[64] Additionally, the selection of endpoints in the study was very debatable. Specifically, the researchers found that the incidence rate of dialysis (including acute dialysis) could not fully reflect long-term treatment effects. After excluding the rate of acute dialysis events, the actual incidence of dialysis was low, exhibiting no significant differences. In addition, the declines in eGFR (ml·min–1·year–1) were 0.27 (ramipril), 0.44 (telmisartan), and 0.53 (combination) where age bias could not be eliminated (the age-related decline was 0.6-1.1).[65] Had the trial continued, the possibility of additional risk of adverse events could have been avoided, as the incidence of hyperkalemia was low with combination therapy.
The results of the VA NEPHRON-D study have further discredited the combination therapy. However, there were several issues with this study. First, the average age of the study population was over 64 years, and the average SBP was <140 mm Hg (no subgroup analyses were conducted). These patients failed to meet the condition of intensive treatment of BP due to much increased risks of adverse events, irrespective of the type of the antihypertension drugs. Subsequent addition of ACEI to ARB may result in a higher incidence of adverse events.[66] Although combination therapy may increase the risk of hyperkalemia, the level of potassium can be reduced via nondialysis methods before resorting to acute dialysis, and the potential benefit of reducing the risk of the progression of ESRD (HR = 0.66, P = 0.07) was the more exciting finding in this study.
Finally, the proper dosage of the dual blockade is still debatable. Almost, all the early studies suggested that in monotherapy a supramaximal dose might idealize the reduction of albuminuria.[67,68,69,70,71] As shown in the existing clinical studies, however, the choice between the maximal dose and half-maximal dose in combination therapy represents a trade-off between the effect of reducing proteinuria and the risk of adverse events. Thus, the best balance between benefits and safety is the key issue for future studies.
In summary, based on our reviews of some large trials such as ONTARGET, ALTITUDE, or VA NEPHRON-D we have found that these trials have some obvious flaws in study designs that should be factored in when forming clinical medication guidelines. We tend to believe that age, the grade of hypertension, the clinical stage, and the quantity of proteinuria are the important considerations in selecting drugs which previous studies precisely lack. The use of dual blockade of the RAAS may not be a contraindication in DKD patients and may be suitable for the early DKD patients with younger and higher stress levels.
CONCLUSION
Despite the negative results of some recent large-scale studies, it is unclear that the concepts of the dual blockade are a failure. The complexity of the RAAS determines the diversity of its blockers, influencing the utility of dual blockade. Further trials are needed to study the combination therapy as an evidence-based practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Prof. Yi Li from the University of Michigan for many helpful suggestions that have substantially improved this manuscript.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 3.Shrishrimal K, Hart P, Michota F. Managing diabetes in hemodialysis patients: Observations and recommendations. Cleve Clin J Med. 2009;76:649–55. doi: 10.3949/ccjm.76a.09054. doi: 10.3949/ccjm.76a.09054. [DOI] [PubMed] [Google Scholar]
- 4.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–19. doi: 10.1038/ki.1997.260. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Ellis SG, Tuzcu M, Whitlow PL, Franco I, Raymond RE, et al. The importance of proteinuria as a determinant of mortality following percutaneous coronary revascularization in diabetics. J Am Coll Cardiol. 1999;33:1269–77. doi: 10.1016/s0735-1097(99)00035-2. doi: 10.1016/S0735-1097(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 6.De Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int. 2004;65:2309–20. doi: 10.1111/j.1523-1755.2004.00653.x. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–290. doi: 10.1053/j.ajkd.2004.03.003. [PubMed] [Google Scholar]
- 11.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 12.Fagard R. Reappraisal of the European guidelines on hypertension management: The European Society of Hypertension Task Force document: A short review. Pol Arch Med Wewn. 2010;120:31–5. [PubMed] [Google Scholar]
- 13.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. doi: 10.1053/j.ajkd.2005.01.019. [PubMed] [Google Scholar]
- 14.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–38. doi: 10.1097/HJH.0b013e328364ca4c. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 15.De Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment? Kidney Int Suppl. 2004;66:S2–6. doi: 10.1111/j.1523-1755.2004.09201.x. doi: 10.1111/j.1523-1755.2004.09201.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: Effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 17.Nussberger J, Brunner DB, Waeber B, Brunner HR. Plasma angiotensins under sustained converting enzyme inhibition with enalapril in normal humans. J Hypertens Suppl. 1985;3:S269–70. [PubMed] [Google Scholar]
- 18.Van den Meiracker AH, Man in ‘t Veld AJ, Admiraal PJ, Ritsema van Eck HJ, Boomsma F, Derkx FH, et al. Partial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: Does it exist and does it affect the antihypertensive response? J Hypertens. 1992;10:803–12. doi: 10.1097/00004872-199208000-00015. [PubMed] [Google Scholar]
- 19.Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: Evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–92. doi: 10.1161/01.hyp.32.3.387. doi: 10.1161/01.HYP.32.3.387. [DOI] [PubMed] [Google Scholar]
- 20.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–92. doi: 10.1038/ncpneph0575. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 21.Borch-Johnsen K, Kreiner S. Proteinuria: Value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294:1651–4. doi: 10.1136/bmj.294.6588.1651. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–6. doi: 10.1001/jama.286.4.421. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 23.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 24.De Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care. 2009;32:1833–8. doi: 10.2337/dc09-0191. doi: 10.2337/dc09-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ménard J, Campbell DJ, Azizi M, Gonzales MF. Synergistic effects of ACE inhibition and Ang II antagonism on blood pressure, cardiac weight, and renin in spontaneously hypertensive rats. Circulation. 1997;96:3072–8. doi: 10.1161/01.cir.96.9.3072. doi: 10.1161/01.CIR.96.9.3072. [DOI] [PubMed] [Google Scholar]
- 26.Doulton TW, He FJ, MacGregor GA. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension. 2005;45:880–6. doi: 10.1161/01.HYP.0000161880.59963.da. doi: 10.1161/01.HYP.0000161880.59963.da. [DOI] [PubMed] [Google Scholar]
- 27.Susantitaphong P, Sewaralthahab K, Balk EM, Eiam-ong S, Madias NE, Jaber BL. Efficacy and safety of combined vs. single renin-angiotensin-aldosterone system blockade in chronic kidney disease: A meta-analysis. Am J Hypertens. 2013;26:424–41. doi: 10.1093/ajh/hps038. doi: 10.1093/ajh/hps038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: A systematic review of the efficacy and safety data. Am J Kidney Dis. 2006;48:8–20. doi: 10.1053/j.ajkd.2006.04.077. doi: 10.1053/j.ajkd.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 29.Jennings DL, Kalus JS, Coleman CI, Manierski C, Yee J. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: A meta-analysis. Diabet Med. 2007;24:486–93. doi: 10.1111/j.1464-5491.2007.02097.x. doi: 10.1111/j.1464-5491.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez Juarez G, Luño J, Barrio V, de Vinuesa SG, Praga M, Goicoechea M, et al. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: A randomized trial. Am J Kidney Dis. 2013;61:211–8. doi: 10.1053/j.ajkd.2012.07.011. doi: 10.1053/j.ajkd.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Tan F, Mukherjee JJ, Lee KO, Lim P, Liew CF. Dual blockade of the renin-angiotensin-aldosterone system is safe and effective in reducing albuminuria in Asian type 2 diabetic patients with nephropathy. Singapore Med J. 2010;51:151–6. [PubMed] [Google Scholar]
- 32.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903. doi: 10.1056/NEJMoa1303154. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 33.Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: The candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440–4. doi: 10.1136/bmj.321.7274.1440. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63:1874–80. doi: 10.1046/j.1523-1755.2003.00940.x. doi: 10.1046/j.1523-1755.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 35.Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Tarnow L, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68:2829–36. doi: 10.1111/j.1523-1755.2005.00756.x. doi: 10.1111/j.1523-1755.2005.00756.x. [DOI] [PubMed] [Google Scholar]
- 36.Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: A randomized, double-masked, cross-over study. Diabetes Care. 2005;28:2106–12. doi: 10.2337/diacare.28.9.2106. doi: 10.2337/diacare.28.9.2106. [DOI] [PubMed] [Google Scholar]
- 37.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–51. doi: 10.2215/CJN.00240106. doi: 10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 38.Bakris GL, Ruilope L, Locatelli F, Ptaszynska A, Pieske B, de Champlain J, et al. Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: Results of the IMPROVE trial. Kidney Int. 2007;72:879–85. doi: 10.1038/sj.ki.5002455. doi: 10.1038/sj.ki.5002455. [DOI] [PubMed] [Google Scholar]
- 39.Menne J, Farsang C, Deák L, Klebs S, Meier M, Handrock R, et al. Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: The VALERIA trial. J Hypertens. 2008;26:1860–7. doi: 10.1097/HJH.0b013e32830508aa. doi: 10.1097/HJH.0b013e32830508aa. [DOI] [PubMed] [Google Scholar]
- 40.Catapano F, Chiodini P, De Nicola L, Minutolo R, Zamboli P, Gallo C, et al. Antiproteinuric response to dual blockade of the renin-angiotensin system in primary glomerulonephritis: Meta-analysis and metaregression. Am J Kidney Dis. 2008;52:475–85. doi: 10.1053/j.ajkd.2008.03.008. doi: 10.1053/j.ajkd.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–50. doi: 10.1681/ASN.2009070737. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–46. doi: 10.1056/NEJMoa0708379. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 43.Kalaitzidis RG, Bakris GL. The current state of RAAS blockade in the treatment of hypertension and proteinuria. Curr Cardiol Rep. 2009;11:436–42. doi: 10.1007/s11886-009-0063-3. doi: 10.1007/s11886-009-0063-3. [DOI] [PubMed] [Google Scholar]
- 44.Bomback AS, Toto R. Dual blockade of the renin-angiotensin-aldosterone system: Beyond the ACE inhibitor and angiotensin-II receptor blocker combination. Am J Hypertens. 2009;22:1032–40. doi: 10.1038/ajh.2009.138. doi: 10.1038/ajh.2009.138. [DOI] [PubMed] [Google Scholar]
- 45.Forclaz A, Maillard M, Nussberger J, Brunner HR, Burnier M. Angiotensin II receptor blockade: Is there truly a benefit of adding an ACE inhibitor? Hypertension. 2003;41:31–6. doi: 10.1161/01.hyp.0000047512.58862.a9. doi: 10.1161/01.HYP.0000047512.58862.A9. [DOI] [PubMed] [Google Scholar]
- 46.Cohen DL, Townsend RR. Is there added value to adding ARB to ACE inhibitors in the management of CKD? J Am Soc Nephrol. 2009;20:1666–8. doi: 10.1681/ASN.2008040381. doi: 10.1681/ASN.2008040381. [DOI] [PubMed] [Google Scholar]
- 47.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. doi: 10.1016/S0140-6736(08)61236-2. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 48.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 49.Imai E, Haneda M, Yamasaki T, Kobayashi F, Harada A, Ito S, et al. Effects of dual blockade of the renin-angiotensin system on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy and hypertension in the ORIENT: A post-hoc analysis (ORIENT-Hypertension) Hypertens Res. 2013;36:1051–9. doi: 10.1038/hr.2013.86. doi: 10.1038/hr.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: A network meta-analysis. Lancet. 2015;385:2047–56. doi: 10.1016/S0140-6736(14)62459-4. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 51.Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: Meta-analysis of randomised trials. BMJ. 2013;346:f360. doi: 10.1136/bmj.f360. doi: 10.1136/bmj.f360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK. Beyond ONTARGET: Angiotensin-converting enzyme inhibition and angiotensin II receptor blockade in combination, a lesser evil in some? Diabetes Obes Metab. 2010;12:1072–8. doi: 10.1111/j.1463-1326.2010.01298.x. doi: 10.1111/j.1463.1326.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- 53.Messerli FH, Staessen JA, Zannad F. Of fads, fashion, surrogate endpoints and dual RAS blockade. Eur Heart J. 2010;31:2205–8. doi: 10.1093/eurheartj/ehq255. doi: 10.1093/eurheartj/ehq255. [DOI] [PubMed] [Google Scholar]
- 54.Delyani JA. Mineralocorticoid receptor antagonists: The evolution of utility and pharmacology. Kidney Int. 2000;57:1408–11. doi: 10.1046/j.1523-1755.2000.00983.x. doi: 10.1046/j.1523-1755.2000.00983.x. [DOI] [PubMed] [Google Scholar]
- 55.Krum H, Nolly H, Workman D, He W, Roniker B, Krause S, et al. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension. 2002;40:117–23. doi: 10.1161/01.hyp.0000025146.19104.fe. doi: 10.1161/01.HYP.0000025146.19104. [DOI] [PubMed] [Google Scholar]
- 56.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: A systematic review. Am J Kidney Dis. 2008;51:199–211. doi: 10.1053/j.ajkd.2007.10.040. doi: 10.1053/j.ajkd.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA. 2015;314:884–94. doi: 10.1001/jama.2015.10081. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 58.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004. doi: 10.1002/14651858.CD007004.pub3. doi: 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 59.Gradman AH, Kad R. Renin inhibition in hypertension. J Am Coll Cardiol. 2008;51:519–28. doi: 10.1016/j.jacc.2007.10.027. doi: 10.1016/j.jacc.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Peixoto AJ, Orias M. Is there a role for direct renin inhibitors in chronic kidney disease? Curr Opin Nephrol Hypertens. 2009;18:397–403. doi: 10.1097/MNH.0b013e32832e3183. doi: 10.1097/MNH.0b013e32832e3183. [DOI] [PubMed] [Google Scholar]
- 61.Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, et al. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care. 2009;32:1873–9. doi: 10.2337/dc09-0168. doi: 10.2337/dc09-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13. doi: 10.1056/NEJMoa1208799. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 63.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–64. doi: 10.1016/S0140-6736(98)10363-X. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 64.Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Rydén L, et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: Results of the ONTARGET trial. J Hypertens. 2013;31:414–21. doi: 10.1097/HJH.0b013e32835bf7b0. doi: 10.1097/HJH.0b013e32835bf7b0. [DOI] [PubMed] [Google Scholar]
- 65.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85. doi: 10.1111/j.1532-5415.1985.tb07117.x. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 66.Izumi Y, Kawahara K, Nonoguchi H. Combined angiotensin inhibition in diabetic nephropathy. N Engl J Med. 2014;370:777–8. doi: 10.1056/NEJMc1315504. doi: 10.1056/NEJMc1315504#SA2. [DOI] [PubMed] [Google Scholar]
- 67.Rossing K, Schjoedt KJ, Jensen BR, Boomsma F, Parving HH. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int. 2005;68:1190–8. doi: 10.1111/j.1523-1755.2005.00511.x. doi: 10.1111/j.1523-1755.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- 68.Schmieder RE, Klingbeil AU, Fleischmann EH, Veelken R, Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: A double-blind, randomized, prospective study. J Am Soc Nephrol. 2005;16:3038–45. doi: 10.1681/ASN.2005020138. doi: 10.1681/ASN.2005020138. [DOI] [PubMed] [Google Scholar]
- 69.Burgess E, Muirhead N, Rene de Cotret P, Chiu A, Pichette V, Tobe S. SMART (Supra Maximal Atacand Renal Trial) Investigators. Supramaximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20:893–900. doi: 10.1681/ASN.2008040416. doi: 10.1681/ASN.2008040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, et al. Optimal antiproteinuric dose of aliskiren in type 2 diabetes mellitus: A randomised crossover trial. Diabetologia. 2010;53:1576–80. doi: 10.1007/s00125-010-1789-6. doi: 10.1007/s00125-010-1789-6. [DOI] [PubMed] [Google Scholar]
- 71.Weir MR, Hollenberg NK, Zappe DH, Meng X, Parving HH, Viberti G, et al. Antihypertensive effects of double the maximum dose of valsartan in African-American patients with type 2 diabetes mellitus and albuminuria. J Hypertens. 2010;28:186–93. doi: 10.1097/HJH.0b013e328332bd61. doi: 10.1097/HJH.0b013e328332bd61. [DOI] [PubMed] [Google Scholar]


