Abstract
Objectives:
Bee venom (BV) is a complex mixture of proteins and contains proteins such as phospholipase and melittin, which have an effect on blood clotting and blood clots. The mechanism of action of honey bee venom (HBV, Apis mellifera) on human plasma proteins and its anti-thrombotic effect were studied. The purpose of this study was to investigate the anti-coagulation effect of BV and its effects on blood coagulation and purification.
Methods:
Crude venom obtained from Apis mellifera was selected. The anti-coagulation factor of the crude venom from this species was purified by using gel filtration chromatography (sephadex G-50), and the molecular weights of the anti-coagulants in this venom estimated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Blood samples were obtained from 10 rabbits, and the prothrombin time (PT) and the partial thromboplastin time (PTT) tests were conducted. The approximate lethal dose (LD) values of BV were determined.
Results:
Crude BV increased the blood clotting time. For BV concentrations from 1 to 4 mg/mL, clotting was not observed even at more than 300 seconds, standard deviations (SDs) = ± 0.71; however, clotting was observed in the control group 13.8 s, SDs = ± 0.52. Thus, BV can be considered as containing anti-coagulation factors. Crude BV is composed 4 protein bands with molecular weights of 3, 15, 20 and 41 kilodalton (kDa), respectively. The LD50 of the crude BV was found to be 177.8 μg/mouse.
Conclusion:
BV contains anti-coagulation factors. The fraction extracted from the Iranian bees contains proteins that are similar to anti-coagulation proteins, such as phospholipase A2 (PLA2) and melittin, and that can increase the blood clotting times in vitro.
Keywords: anti-coagulants, bee venom, chromatography, LD50
1. Introduction
Honey bee venom (HBV, Apis mellifera) is a bitter, colorless liquid, and its active portion contains a mixture of proteins that, cause local inflammation and act as anti-coagulants. HBV is a complex mixture of enzymes and polypeptides with low molecular weights, and it has been reported to contain the enzymes phospholipase A2 (PLA2), hyaluronidase, phosphomonoesterase acid esterase, α-D-glucosidase, lysopphospholipase, α-galactosidase, α-acetylamino-deosiglucosidase and arylamidase. The compositions of fresh and dried bee venom (BV) differ mainly in regards to the volatile components; however, the overall biological activities are similar [1].
Enzymes are proteins catalyzing specific reactions, and contains five enzymes. Polypeptides are smaller in molecular weight than enzymes and are made of two or more amino acids. BV also has numerous polypeptides, the main one being melittin, which is also the main component of BV and has many positive biological effects and a relatively low toxicity. Melittin has a molecular weight of 2,840 Daltons, but it can reach 12,500 Daltons because it can also be in a tetrameric form [2, 3].
BV also contains smaller quantities of low molecular compounds that are different in nature: amino acids, catecholamines, sugars and minerals. Sugars have been identified in some BV preparations, but if BV is collected with a collector to prevent the contamination by pollen and nectar, it does not contain carbohydrates [4].
The mast cell degranulating (MCD) peptide and PLA2 are the two most toxic components in BV. Individual BV components can be used to achieve definite biological effects, and due to its having different effects on the central and the peripheral nervous systems (PNS), BV can be used to treat patients with different heart conditions. Reports have been published on the use of BV to treat patients with different degenerative diseases of the nervous system, such as multiple sclerosis (MS), Alzheimer’s disease and Parkinson’s disease [5-7].
BV has also been reported to act against different types of cancers in cell and animal experiments [8]. However, no differences between the cancer incidences in normal humans and beekeepers have been reported [9]. Thus, melittin, a powerful anti-cancer peptide, might be a better choice than whole BV for treating various diseases. On the other hand, BV acupuncture and melittin have been used to control neuropathy caused by cancer chemotherapy [10, 11].
Because, most human deaths resulting from one or a few bee stings are due to allergic reactions, heart failure, or suffocation from swelling around the neck or the mouth, as compared with other human diseases, accidents and other unusual cases, deaths dues to bee stings are rare, indicating that BV is very safe for treating diseases in humans [12].
2. Materials and Methods
Lyophilized BV was prepared at the Venomous Animals Department, Razi Vaccine and Serum Institute, and was kept at — 20˚C. Sephadex G-50 was purchased from Pharmacia (Sweden). Calcium chloride (CaCl2) and protrombin time (PT) and partial thromboplastin time (PTT) Kit products were purchased from Fisher Diagnostics (Germany). The other reagents and chemicals of analytical grade were purchased from Fluka and Merck.
Blood samples were extracted from 10 healthy young rabbits of both sexes with no history of blood disease. Blood samples from these rabbits were centrifuged for 15 minutes at 3,000 rpm. The obtained plasma was separated and was used for the PT and PTT testing.
Tissue thromboplastin was reconstituted according to instructions and was labeled with the time, date and initials of the technician. The thromboplastin reagent was stabile for seven days after reconstitution. The sample was allowed to sit 10 — 15 minutes and was than inverted gently several times. The reagents were mixed well prior to pipetting any of them reagent at any step in this procedure.
One to two mls of the tissue thromboplastin- CaCl2 reagent (PT reagent) was pipetted into a test tube, and the test tube placed into an incubator at 37˚C. The level of the thromboplastin was not allowed to exceed the height of the heat block. Normal rabbit plasm, 100 μL was poured into the test tubes and at least one minute was allowed for the plasma to reach 37˚C. Then, 200 μL of the PT reagent was poured into the tube containing the rabbit plasma, and simultaneously stop watch was started.
The solution in the tube was mixed and left in the heat block for a minimum of 7 — 8 seconds. It was then removed, and its exterior was wiped. The tube was then tilted back and forth gently until a visible clot formed. As the clot formed, the mixture began to gelatinize and turn cloudy. The stop watch was immediately stopped when the clot began to form, and the time in seconds was recorded [13].
A sufficient quantity of CaCl2 reagent was heated to 37˚C for the tests to be performed. Normal rabbit plasma, 100 μL, was poured into a labeled test tube, and 100 μL of partial thromboplastin reagent was added. The plasma/partial thromboplastin mixture was incubated at 37˚C for a minimum of three minutes, and CaCl2 100 μL, was forcefully added into the plasma/partial thromboplastin mixture; a stop watch was started immediately.
The mixture in the tube was stirred once after the calcium reagent had been added, and the tube was allowed to remain in the heat block for approximately 20 seconds while being stirred occasionally. After 20 seconds, the tube was removed from the water bath/heat block, and its outside was dried. The tube was then gently tilted back and forth until a visible clot formed, at which time the stop watch was immediately stopped and the time in seconds was recorded [13].
The concentrations of the proteins in the BV were measured by using the method of Lowry with bovine serum albumin (BSA) as the standard [14]. Standard curves were constructed by measuring the concentration of the BSA solution; then, the amounts of the proteins in the crude BV were obtained. In addition, 10 mg of BV were used to prepare samples with concentrations of 1 to 4 mg/mL. The samples with these concentrations were the used to assess the effect of BV on clotting with PT.
The lethal dose 50 (LD50) of the Iranian crude BV was determined in mice (18 to 20 g National Institutes of Health (NIH) strain; NIH mouse are albino outbred mice which were derived from a nucleus colony obtained from the NIH). The sample at each dose was prepared in physiological sterile serum and was injected into the mice. For the selection of an appropriate dose, several different doses were injected into two mice per dose up to lethality. The doses were then chosen so that for the first dose, no mice died, and for last dose, all animals died. When different doses were prepared for each sample, 2 mL of each dose (0.5 mL for each mouse) were intravenously into four mice. Then, the mortality of the mice was recorded for 24 hours. The LD50 values of each sample were calculated by using the Spearman-Karber Finney statistics [15].
The anti-coagulation factors from BV were purified by using chromatographic techniques. To separate the coagulation factor, we dissolved 300 mg of the crude BV in 4 mL of ammonium acetate buffer, which was then centrifuged for 15 minutes at 14,000 rpm. The impurities were removed by using a micro filter 0.45. The gel chromatography column was equilibrated by using 20 mM ammonium acetate buffer (pH 4.75). After the column had been equilibrated, the protein solution was applied on sephadex G-50 (3 × 200 cm); then, the column was eluted with the same buffer. The absorption of each fraction was read at 280 nm. All the fractions were dialyzed for 24 hours against distilled water and were concentrated at 4˚C. After concentration, the intensities of peaks were accurately measured. The PT test was done for peaks (fractions) with the same concentration and fractions that showed anti-coagulation activity were detected [16, 17].
To confirm the presence of coagulation and anti-coagulation activities, we performed PT tests with the same concentrations, 20 μg/mL, for the fractions obtained by using gel chromatography. For determining the molecular weight and the purity of the crude BV and the fractions obtained by using the gel chromatography technique, we performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli [18].
Analyses to determine the means, standard deviations (SDs) and P-values of the test data were performed using the software mini tab. P-value was calculated, and a P-value less than 0.05 was meaningful.
3. Results
Comparing the coagulation time of normal plasma (without venom) with those obtained for different concentrations of BV, crude BV was found to increase the plasma clotting time. In other words, the venom prolonged the blood coagulation time, making this venom an anti-coagulant (Table 1). The amount of protein in crude BV solution was 3.6 mg/mL.
Table. 1. PT values for different concentration of Apis mellifera crude venom.
| Concentration of venom and fractions | *Average PT (sec) | Description |
|---|---|---|
| 1 mg/mL | 0.71 ± 187 | Clots were formed as thin filaments |
| 2 mg/mL | More than 300 | No clots were formed |
| 3 mg/mL | More than 300 | No clots were formed |
| 4 mg/mL | More than 300 | No clots were formed |
| Normal PT (No venom) | 0.52 ± 13/8 | Clots were formed completely |
| Normal PTT (No venom) | 0.4 ± 33.45 | Clots were formed completely |
P-value < 0.05, *n = 6. PT, protrombin time; PTT, partial thromboplastin time.
The values of the LD50 in all mice were determined for all samples. The samples were prepared at different doses. Each dose was injected intravenously into 40 mice. The numbers of dead mice within 24 hours were recorded for each dose. After the registration of deaths, the LD50 of each dose was determined by using the Spearman-Karber statistics method, which is as follows: M = × 100 ± d/n (Σr-n/2), d = log 1.25 = 0.097, d/n = 0.024 M = log LD50, × 100 = log 244 = 2.38 n = 4, Σr = 5, M = 2.38 + 0.121 = 2.25 LD50 = Antilog 2.25 = 177.8, LD50 = 177.8 μg
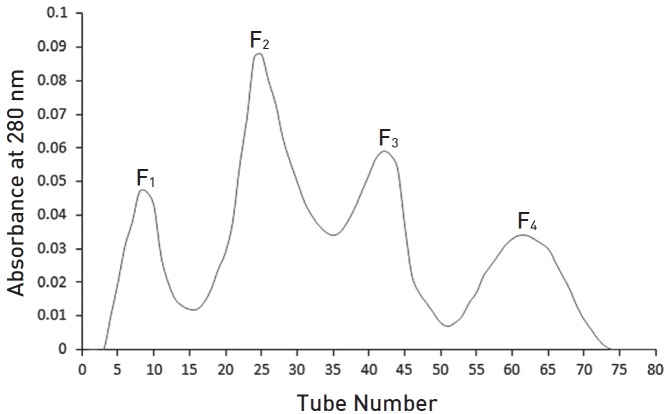
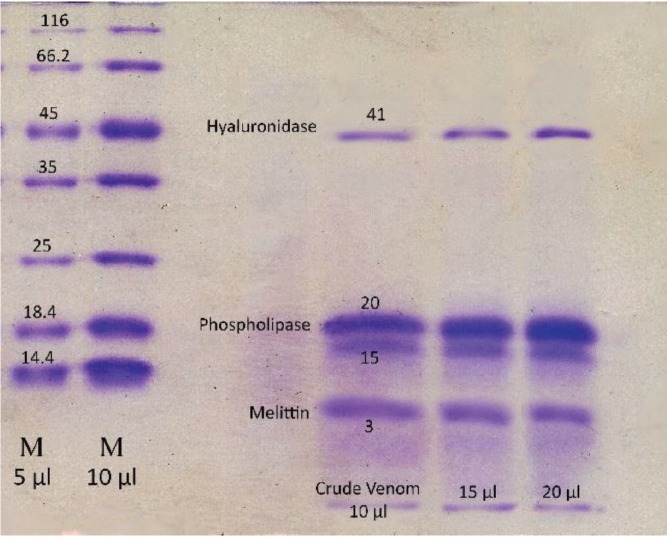
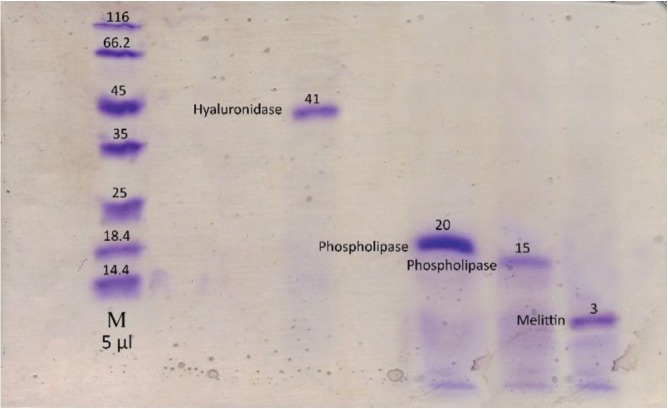
Using gel filtration chromatography, four fractions were obtained. These fractions were labeled F1 to F4. According to (Fig. 1), fractions F1 to F4 containing proteins from high to low molecular weight were extracted from the gel chromatography column. It seems that the fraction F1 contain hyaluronidase, F2 and F3 contains phospholipase and F4 contain melittin. Fraction F2 containing the highest amount of protein had the greatest absorbance (Fig. 1). With regard to the determination of the standard molecular weights of the protein bands, the exact locations of the crude BV proteins and their fractions were identified (Figs. 2,3). Crude venom has four pro-tein bands with molecular weights of 3, 15, 20 and 41 kilodalton (kDa). After chromatography, each fraction showed a single band.
Fig. 1. Sephadex G-50 chromatography of Apis mellifera venom.
Fig. 2. SDS-PAGE of Apis mellifera crude venom.
SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Fig. 3. SDS-PAGE of Apis mellifera fractions.
SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
The anti-coagulant property was determined by using PT tests on fractions F1 to F4. The coagulation times were obtained by adding fractions from the gel chromatography to the plasma of three healthy rabbits of each sex. The results are gathered in (Table 2). Although fractions F2, F3 and F4 had more anti-coagulation activity than F1, F1 also had some anti-coagulation activity. According to this method, if the time until coagulation occurs is increased compared to the control sample, then the anti-coagulation activity is increased.
Table. 2. PT value for fractions of Apis mellifera crude venom.
| PT (sec) | Sample |
|---|---|
| 240 | Fraction F1 |
| More than 300 | Fraction F2 |
| More than 300 | Fraction F3 |
| More than 300 | Fraction F4 |
PT, protrombin time.
4. Discussion
This research offers an effective and repeatable method for separating the anti-coagulation factors in BV. Venom from bees in the family Apidae like Apis mellifera, is a rich source of new compounds that have applications in medicine and biochemistry [19], and BV from Iranian bees seen to be effective in delaying the clotting of blood. Coagulation tests, including PT tests, have shown that BV, in general, has anti-coagulation properties.
From the results of tests carried out on six samples, we can be concluded that all were normal, as the time for each was in the normal range when tested by using PT (10 — 14 seconds) and PTT (30 — 40 seconds).
To evaluate the anti-thrombotic properties of BV, we performed PT tests on plasma samples from four groups of 6 animals each (24 animals total), and the 4 groups received injections of BV with doses of 1, 2, 3 and 4 mg/mL, respectively. The average duration of the PT test in this case also highlights the anti-coagulation properties of the Iranian crude BV (PT = more than 300 seconds). Thus, the anti-coagulation factor BV has been proven.
The venoms of most stinging insects, including honey bees, consist of enzymes, protein, peptides, and a variety of smaller molecules. The pharmacological and biochemical activities of venoms from various stinging insects are remarkably convergent. Most venoms induce immediate pain, contain phosphlipases, hyaluronidase, and other enzymes, and are capable of destroying red blood cells [20]. The phospholipases derived from honey bees, yellow jackets, and fire ants have very different molecular weights: 16,000 for bees [21], about 31,000 for yellow jackets [22, 23], and 28,000 in the form of tow roughly equal subunits for fire ants [24].
Melittin is the principal active component of apitoxin (BV) and is a powerful stimulator of PLA2. So far, melittin is the best characterized peptide. It inhibits protein kinase C, Ca2+/calmodulin-dependent protein kinase II, has alkaline characteristics similar to those of other venom compounds, and seems to be the major compound responsible for intense local pain [25, 26]. It corresponds to 40% — 50% of the venom’s dry weight, although this varies during aging [27]. Extensive work with melittin has shown that it has multiple effects, probably, as a result of its interaction with negatively-charged phospholipids.
Venoms from other Apis species are similar, but even the venoms from the various types within each species are slightly different from each other. The toxicity of Apis cerana venom has been reported to be twice as high as that of Apis mellifera [28].
5. Conclusion
BV contains pharmacologically important constituents. The important constituents present in BV are melittin, apamin, hyaluronidase, phospholipase and histamine. Melittin is considered to be the major constituent of BV.
The present study analyzed the venom of Apis mellifera with regard to anti-coagulation activities. Its anti-coagulation proteins were isolated and evaluated by using chromatographic methods. The fraction extracted from the Iranian bee contained protein similar to anti-coagulation proteins such as PLA2 and melittin, which are able to increase the blood clotting time in vitro.
In summary, bee products are very interesting and can either be developed further into medicinal products when they offer new and better treatment alternatives, or form the basis for the identification of new drugs that can be used according to the principles of pharmacology. In either case, much effort will be necessary in order to establish their position in modern medicine.
Footnotes
Conflict of interest The authors declare that there are no conflict of interest.
Contributor Information
Hossein Zolfagharian, Email: zolfagharianh@yahoo.com.
Mohammad Mohajeri, Email: mmrpentium@yahoo.com.
Mahdi Babaie, Email: m.babaie47@yahoo.com.
References
- 1.Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthritis, pain-releasing and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115(2):246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Habermann E, Jentsch J. Über die struktur des toxischen bienengiftpeptids melittin und deren beziehung zur pharmakologischen wirkung. Naunyn Schmiedebergs Arch Pharmacol. 1966;253(1):40–41. [Google Scholar]
- 3.Habermann E, Zeuner G. Comparative studies of native and synthetic melittins. Naunyn Schmiedebergs Arch Pharmakol. 1971;270(1):1–9. doi: 10.1007/BF00997294. [DOI] [PubMed] [Google Scholar]
- 4.Zemizdat; Bulgaria: 1983. Pcelni produkti, the bee products (in Bulgarian) ; pp. 1–238. [Google Scholar]
- 5.Kim JI, Yang EJ, Lee MS, Kim YS, Huh Y, Cho IH et al. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int J Neurosci. 2011;121(4):209–217. doi: 10.3109/00207454.2010.548613. [DOI] [PubMed] [Google Scholar]
- 6.Münstedt K, Bogdanov S. Bee products and their potential use in modern medicine. JAAS. 2009;1(3):57–63. [Google Scholar]
- 7.Lee SM, Yang EJ, Choi SM, Kim SH, Baek MG, Jiang JH. Effects of bee venom on glutamate-induced toxicity in neuronal and glial cells. Evid Based Complement Alternat Med. 2012;2012:ID368196. doi: 10.1155/2012/368196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orslic N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31(1-2):173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 9.Mcdonald JA, Li FP, Mehta CR. Cancer mortality among beekeepers. J Occup Med. 1979;21(12):811–813. [PubMed] [Google Scholar]
- 10.Kim JM, Jeon HJ, Kim HJ, Cho CK, Yoo HS. Bee venom pharmacopuncture: an effective treatment for complex regional pain syndrome. J Pharmacopuncture. 2014;17(4):66–69. doi: 10.3831/KPI.2014.17.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JW, Jeon JH, Yoon J, Jung TY, Kwon KR, Cho CK et al. Effects of sweet bee venom pharmacopuncture treatment for chemotherapy-induced peripheral neuropathy: a case series. Integr Cancer Ther. 2012;11(2):166–171. doi: 10.1177/1534735411413265. [DOI] [PubMed] [Google Scholar]
- 12.Abdu Al-Samie., MA Studies on bee venom and its medical uses. IJOART. 2012;1(2):1–15. [Google Scholar]
- 13.Rizzo F, Papasouliotis K, Crawford E, Dodkin S, Cue S. Measurement of prothrombin time (PT) and activated partial thromboplastin time (APTT) on canine citrated plasma samples following different storage conditions. Res Vet Sci. 2008;85(1):166–170. doi: 10.1016/j.rvsc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 15.Meier J, Theakston RGD. Approximate LD50 determination of snake venoms using eight to ten experimental animals. Toxicon. 1986;24(4):395–401. doi: 10.1016/0041-0101(86)90199-6. [DOI] [PubMed] [Google Scholar]
- 16.Maulet Y, Brodbeck U, Fulpius BW. Purification from bee venom of melittin devoid of phospholipase A2 contamination. Anal Biochem. 1982;127(1):61–67. doi: 10.1016/0003-2697(82)90144-0. [DOI] [PubMed] [Google Scholar]
- 17.Babaie M, Zolfagharian H, Salmanizadeh H, Mirakabadi AZ, Alizadeh H. Isolation and partial purification of anti-coagulant fractions from the venom of the Iranian snake Echis carinatus. Acta Biochim Pol. 2013;60(1):17–20. [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Moreno M, Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins. 2015;7(4):1126–1150. doi: 10.3390/toxins7041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt JO, Blum MS, Overal WL. Comparative enzymology of venoms from stinging hymenoptera. Toxicon. 1986;24(9):907–921. doi: 10.1016/0041-0101(86)90091-7. [DOI] [PubMed] [Google Scholar]
- 21.Shipolini RA, Callewaert GL, Cottrell RG, Vernon CA. The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur J Biochem. 1974;48(2):465–476. doi: 10.1111/j.1432-1033.1974.tb03787.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman DR, Wood CL. Allergens in hymenoptera venom XI: isolation of protein allergens from Vespula maculifrons (yellow jacket) venom. J Allergy Clin Immunol. 1984;74(1):93–103. doi: 10.1016/0091-6749(84)90094-0. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman DR. Allergens in hymenoptera venom XIII: Isolation and purification of protein components from three species of vespid venoms. J Allergy Clin Immunol. 1985;75(5):599–605. doi: 10.1016/0091-6749(85)90036-3. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman DR, Dove DE, Jacobson RS. Allergens in hymenoptera venom XX: isolation of four allergens from imported fire ant (Solenopsis invicta) venom. J Allergy Clin Immunol. 1988;82(5):818–827. doi: 10.1016/0091-6749(88)90084-X. [DOI] [PubMed] [Google Scholar]
- 25.Edstrom A. Krieger Publishing Company; Malabar: 1992. Venomous and poisonous animals; pp. 210–20. [Google Scholar]
- 26.Habermann E. Bee and wasp venoms. Science. 1972;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 27.Owen MD, Pfaff LA. Melittin synthesis in the venom system of the honey bee (Apis mellifera L.) Toxicon. 1995;33(9):1181–1188. doi: 10.1016/0041-0101(95)00054-P. [DOI] [PubMed] [Google Scholar]
- 28.Benton AW, Morse RA. Venom toxicity and proteins of the genus Apis. J Api Res. 1968;7(3):113–118. [Google Scholar]