Abstract
Background
South Africa (SA) is facing a heavy burden of non-communicable diseases (NCDs). Few studies address multimorbidity, control and treatment of NCDs in patients attending primary healthcare (PHC) clinics.
Objectives
To describe multimorbidity, related risk factors, disease severity and treatment status of patients with four important NCDs attending public sector PHC clinics in two districts in SA.
Methods
A cross-sectional sample of patients completed baseline data collection for a randomised controlled trial of a health systems intervention. The study population comprised adults attending PHC clinics in the Eden and Overberg districts of the Western Cape in 2011. Four subgroups of patients were identified: hypertension, diabetes, chronic respiratory disease and depression. A total of 4 393 participants enrolled from 38 clinics completed a baseline structured questionnaire and had measurements taken. Prescription data were recorded.
Results
Of participants with hypertension, diabetes, respiratory disease and depression, 80%, 92%, 88% and 80%, respectively, had at least one of the other three conditions. There were low levels of control and treatment: 59% of participants with hypertension had a blood pressure ≥140/90 mmHg, the mean haemoglobin A1c (HbA1c) value in participants with diabetes was 9%, 12% of participants in the depression group were prescribed an antidepressant at a therapeutic dose, and 48% of respiratory participants were prescribed a β2-agonist and 34% an inhaled corticosteroid.
Conclusion
Considerable multimorbidity and unmet treatment needs exist among patients with NCDs attending public sector PHC clinics. Improved strategies are required for diagnosing and managing NCDs in this sector.
South Africa (SA) faces a rise in non-communicable diseases (NCDs) in both rural and urban populations, driven by an increase in risk factors such as tobacco use, physical inactivity and unhealthy diets.[1] These place a heavy burden on public sector primary healthcare (PHC) services. A recent cross-sectional survey of reasons for consultations in PHC in four SA provinces confirmed that management of hypertension was the most common reason for attendance, with NCDs accounting for 14% of visits.[2,3]
PHC in the public sector is nurse-led with support from doctors, with nurses seeing over 85% of all patients.[2] However, at present nurses working at PHC clinics often do not have the necessary skills or capacity to deal adequately with NCDs.[1] Chronic diseases and risk factors are often undiagnosed and inadequately treated, resulting in high levels of poor control and morbidity.[1,4–6]
There is renewed focus on NCD care in SA. However, despite the magnitude of the NCD burden, there are few recent studies addressing multimorbidity, control and treatment of NCDs in this country. In particular, little is known about the multimorbidity of depression with other NCDs.
We present an analysis of the clinical characteristics, level of disease control, presence of multimorbidity and treatment of patients with hypertension, diabetes, chronic respiratory disease and symptoms of depression, identified in PHC clinics in the Eden and Overberg districts of the Western Cape Province, SA, as part of the Primary Care 101 trial described below.
Methods
This study describes the characteristics of patients participating in the Primary Care 101 trial at enrolment. The Primary Care 101 programme comprised a customised clinical practice guideline and training programme aimed at assisting healthcare providers, largely nurses, with the primary care management of adults. The pragmatic cluster randomised controlled trial evaluated the effects of the programme on the quality and outcomes of care for hypertension, diabetes, chronic respiratory disease and depression. Clinics were randomised either to receive the intervention or not. The main results of the trial will be published elsewhere.
The 33 largest clinics in the Eden district that provided NCD care, and a convenience sample of five clinics in the neighbouring Overberg district, were included in the study. Each clinic had at least 10 000 attendances per year and was staffed by nurse practitioners, doctors, and community health workers who manage clinic patients in the communities. These clinics are the main providers of PHC for local populations with high levels of unemployment and socioeconomic deprivation.
Eligible participants were clinic attenders aged 18 years or older, likely to reside in the area for the next year, and able to engage in an interviewer-administered questionnaire. Patients attending each clinic were interviewed to assess their eligibility for inclusion in the hypertension, diabetes, chronic respiratory and/or depression groups, until the sample size required for each group was reached. For the hypertension and diabetes groups, a self-reported history of medication use for these conditions was required. For the respiratory group, a self-report of prescription of medications for chronic respiratory disease or symptoms of chronic respiratory disease (cough or difficult breathing for >2 weeks),[7] and no current or recent treatment for tuberculosis, was required. The depression group comprised participants with a score of ≥10 on the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10).[8] Individuals could be included in more than one group. In this article ‘comorbidity’ refers to two coexisting conditions and ‘multimorbidity’ to three or more coexisting conditions.
For trial purposes the sample sizes for each disease group were calculated separately, and were 27 participants per clinic for the chronic respiratory disease group and 60 per clinic for each of the other three groups. These target sample sizes were attained or exceeded for all disease groups except the diabetes group, which reached 48 participants per clinic.
Fieldworkers invited patients attending the trial clinics to be screened for inclusion in the study using a structured questionnaire. Eligible patients who provided informed consent were enrolled and completed another, baseline, questionnaire. Between March and October 2011, 4 904 patients were screened, of whom 4 393 were enrolled. The questionnaire was available in Afrikaans, isiXhosa and English. It included questions relating to demographic characteristics, socioeconomic factors, medical history and smoking status. Three blood pressure (BP) readings were taken at least 2 minutes apart with an Omron automatic monitor. The first reading was excluded and the values from the second and third readings were averaged.[9] Height, weight and waist circumference were measured using standardised techniques. Height and weight were measured with participants barefoot and wearing light clothing. A flexible tape was used to measure waist circumference, 2.5 cm above the umbilicus. Haemoglobin A1c (HbA1c) was measured only in 20 randomly selected clinics because of cost and logistical constraints. Diabetic participants in the 20 clinics were referred to a clinic nurse at the end of the interview for an HbA1c test, which was processed by the National Health Laboratory Service.
The severity of respiratory disease was assessed with the Symptom and Activity domains of the St Georges Respiratory Questionnaire (SGRQ)[10] in participants enrolled in the respiratory disease group. Scores are expressed as percentages, with 100% representing worst and 0% best possible health status. The presence of symptoms of depression was assessed with the CESD-10 scale, which was administered to all participants enrolled in the study.[8] The items were scored from 0 (rarely or none of the time) to 3 (most of the time).
Treatment for depression was defined as having received counselling or been referred to psychiatric services within the past year, or currently receiving an antidepressant. Counselling was defined as a consultation that intended to seek solutions to problems or provide emotional support, and not simply give advice on medication use. Participants who reported receiving counselling from a mental health nurse, clinic counsellor, social worker, psychiatrist or psychologist were considered to have received counselling, and those who reported receiving counselling from a mental health nurse, psychiatrist or psychologist were considered to have been referred to psychiatric services.
Chronic medication prescribed to participants at the time of their interview for depression, hypertension, diabetes and respiratory disease was recorded. Fieldworkers photocopied all available prescription charts for the year preceding the interview. The trial manager (NF) examined the prescription charts of each participant to identify medications prescribed at the time of their interview. A data capturer entered the prescription data (drug names, doses and frequencies) into an access database, and a total daily dose for each drug was calculated. Prescription charts were available for 99.3% of participants.
Quality control measures included supervision of fieldworkers, electronic alert messages for fieldworkers who entered unusually high or low values, and regular monitoring of the data to identify unusual values or trends. Quality control checks performed on the capturing of prescription data included double entry and checking all unusually high or low doses and frequencies.
The trial was registered with Current Controlled Trials (ISRCTN20283604) and the Office for Human Research Protections Database. Ethical approval was obtained from the University of Cape Town Human Research Ethics Committee and the Western Cape Provincial Department of Health. Participants provided written informed consent for their interview and prescription data to be collected and analysed. All data were anonymised for analysis, and participant identities were revealed only to the fieldworker and a limited number of researchers who received and prepared the data for analysis.
Results
Thirty-eight clinics were included in the study. The median number of nurses per clinic was four. Forty per cent of clinics had daily doctor support, the remainder had sessional support from doctors, and 42% of clinics had an on-site pharmacy.
A total of 4 393 participants were enrolled into the study, of whom 73% were women. The median age was 52 years, and 73% had hypertension, 42% diabetes, 26% chronic respiratory disease and 56% a CESD-10 score of ≥10 and so could be considered to be at risk of depression.
The majority of the participants (84%) were Afrikaans speaking, 75% were unemployed, 7% had never attended school, and 42% had achieved high-school education. Fifty-eight per cent reported receiving a social welfare grant, including 44% of participants under the age of 60 years. The median income in the month prior to the interview date was ZAR1 140, including personal non-grant income plus any household grant that benefited the participant, such as a disability or child grant.
Although 31% of participants were current smokers, their median pack-year history was only 7.5. Twenty-five per cent of participants provided a history of cardiovascular disease (heart attack, angina or stroke), 11% had a history of tuberculosis (TB), 2% reported being on medication for TB at the time of the interview, and 2% reported being on antiretroviral drugs.
The NCD-related health characteristics of participants in each of the four disease groups are presented in Table 1.
Table 1.
Characteristics of study participants
| Hypertension group |
All participants (N=3 227) |
| Systolic BP (mmHg), mean (SD) (n=3 220) | 139 (23.6) |
| Diastolic BP (mmHg), mean (SD) (n=3 220) | 90 (13.2) |
| BP ≥140/90 mmHg, n (%) | 1 917 (59.4) |
| BP ≥180/110 mmHg, n (%) | 334 (10.4) |
| BMI (kg/m2), mean (SD) (n=3 066) | 31.1 (7.5) |
| BMI, proportion obese (BMI ≥30), n (%) | 1 628 (50.5) |
| Waist circumference (cm), mean (SD) (n=3 194) | 100.5 (15.6) |
| Waist circumference (cm), proportion greater than ideal,* n (%) | 2 293 (71.1) |
| Current smokers, n (%) | 885 (27.4) |
| Pack-year history for current smokers, median (IQR) (n=756) | 8 (4.8 – 13.8) |
| Cardiovascular disease,† n (%) | 849 (26.3) |
| Diabetes group |
All participants (N=1 842) |
| HbA1c (%),‡ mean (SD) (n=704) | 9.1 (2.5) |
| Proportion HbA1c ≥7%, n (%) | 544/704 (77.3) |
| SBP (mmHg), mean (SD) (n=1 840) | 137 (23.2) |
| DBP (mmHg), mean (SD) (n=1 840) | 88 (12.4) |
| BP ≥140/80 mmHg, n (%) | 1 414 (76.8) |
| BP ≥180/110 mmHg, n (%) | 139 (7.5) |
| BMI, mean (SD) (n=1 742) | 32.0 (7.3) |
| BMI, proportion obese (BMI ≥30), n (%) | 1 011 (54.9) |
| Waist circumference, mean (SD) (n=1 822) | 103.6 (14.8) |
| Waist circumference, proportion greater than ideal,* n (%) | 1 436/1 842 (78.0) |
| Current smokers, n (%) | 415 (22.5) |
| Pack-year history for current smokers, median (IQR) (n=353) | 8.25 (4.8 – 16.0) |
| Cardiovascular disease,† n (%) | 423 (23.0) |
| Chronic respiratory disease group |
All participants (N=1 157) |
| Self-reported CRD on respiratory medication, n (%) | 699 (60.4) |
| Self-reported CRD symptoms and not on respiratory medication, n (%) | 458 (39.6) |
| SGRQ symptom domain (% maximum weight), median (IQR) (n=833) | 59.50 (36.4 – 74.8) |
| SGRQ activity domain (% maximum weight), median (IQR) (n=1 054) | 73.71 (53.6 – 92.5) |
| Current smokers, n (%) | 454 (39.2) |
| Pack-year history for current smokers, median (IQR) | 7.8 (4.4 – 13.5) |
| Previous tuberculosis, n (%) | 211 (18.2) |
| Depression group |
All participants (N=2 466) |
| CESD-10 score, mean (SD); median (IQR) | 15.3 (4.3); 14 (12 – 18) |
| Current smokers, n (%) | 860 (34.9) |
| Pack-year history for current smokers, median (IQR) (n=754) | 7.2 (3.9 – 13.5) |
SD = standard deviation.
≥88 cm for women, ≥102 cm for men.
History of heart attack, angina or stroke.
HbA1c measured in a preplanned subgroup of 20 randomly selected clinics.
In the hypertension group (3 227 participants), 59% had a BP ≥140/90 mmHg and 10% had a BP ≥180/110 mmHg, indicating poor control. Their mean body mass index (BMI, kg/m2) was 31, 27% were current smokers, and 26% reported a history of cardiovascular disease. Of the 1 166 participants not in the hypertension group, 25% had a BP ≥140/90 mmHg and were not on medication for hypertension.
The diabetes group comprised 1 842 participants, of whom 704 had their HbA1c measured. The mean HbA1c value was 9.1% and 77% had an HbA1c above the target of 7%, indicating poor glycaemic control. The mean BMI for all participants with diabetes was 32, 23% were current smokers, and 23% reported a history of cardiovascular disease. An elevated BP (≥140/80 mmHg)[11] was present in 77%, and 8% had a BP ≥180/110 mmHg.
The chronic respiratory disease group comprised 1 157 participants, of whom 50% reported being on medication for respiratory disease and 50% were identified by symptoms alone. Eighteen per cent had a previous history of TB and 39% were current smokers. Their median pack-year history was 7.5. The median symptom and activity domain scores of the SGRQ were 60 and 74, respectively.
The depression group comprised a total of 2 466 participants. Their median CESD-10 score was 14 (interquartile range (IQR) 12 – 18).
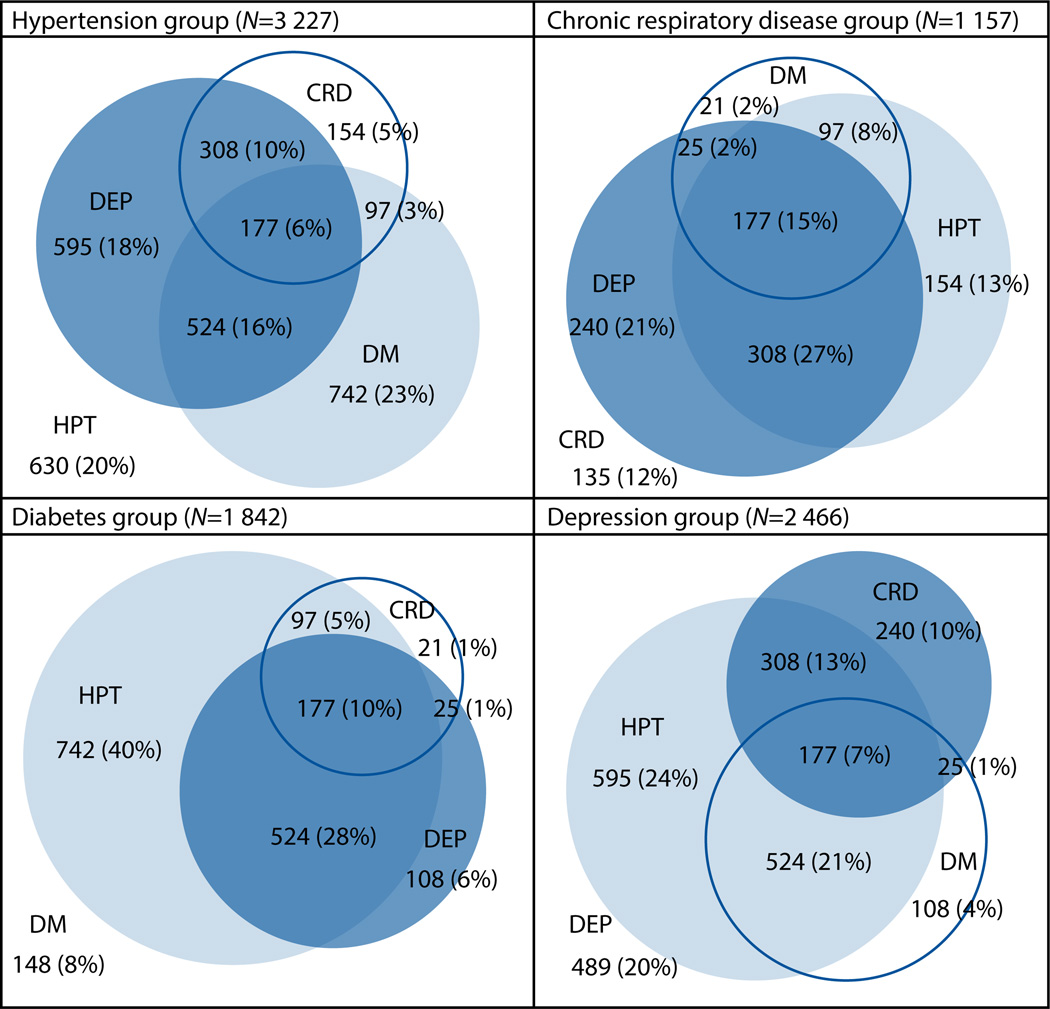
Of participants with hypertension, diabetes, respiratory disease and depression, 80%, 92%, 88% and 80%, respectively, had at least one of the other three conditions, and 34%, 45%, 53% and 42% had at least two other conditions (Fig. 1). Hypertension was the commonest comorbidity in participants with other categories of chronic disease, followed by depression, diabetes and chronic respiratory disease. Forty-seven per cent of participants in the hypertension group also had diabetes, 84% of participants with diabetes also had hypertension, 22% of participants with hypertension or diabetes also had chronic respiratory disease, and 51% of participants with hypertension, diabetes or respiratory disease had a CESD-10 score of ≥10.
Fig. 1.
Venn diagram of multimorbidity associated with hypertension, diabetes, chronic respiratory disease and depression. (HPT = hypertension; DM = diabetes; CRD = chronic respiratory disease; DEP = depression.)
Treatment received by participants in the hypertension group at the time of their interview is presented in Table 2. Four per cent of participants in the hypertension group had no evidence of having received antihypertensive medication, 14% were on one antihypertensive agent, and 15% were on three or more antihypertensive drugs at optimal dosages. Fifty-five per cent of participants with hypertension were prescribed aspirin and 34% a statin.
Table 2.
Treatment, hypertension group
| Medications prescribed | All participants (N=3 227) n (%) |
Controlled BP*† (N=1 303) n (%) |
Uncontrolled BP‡† (N=1 917) n (%) |
|---|---|---|---|
| Hypertension medications | |||
| No hypertension medication | 129 (4.0) | 80 (62.0) | 47 (36.4) |
| 1 hypertension medication | 436 (13.5) | 207 (47.5) | 229 (52.5) |
| ≥2 hypertension medications | 2 638 (81.7) | 1 003 (38.0) | 1 631 (61.8) |
| ≥3 antihypertensive medications at optimal dosage | 482 (14.9) | 140 (29.0) | 341 (70.8) |
| Missing information | 24 (0.7) | ||
| Aspirin | 1 760 (54.5) | 711 (40.4) | 1 047 (59.5) |
| Statin | 1 093 (33.9) | 445 (40.7) | 647 (59.2) |
BP <140/90 mmHg.
Frequencies and row percentages exclude 7 participants with missing BP readings.
BP ≥140/90 mmHg.
Treatment received by participants in the diabetes group is presented in Table 3. Sixty-one per cent of participants with diabetes had been prescribed an oral hypoglycaemic agent (without insulin) and 32% were prescribed insulin (with or without oral hypoglycaemic agents). Aspirin had been prescribed for 63% in the diabetes group, and statins for 51%. Seven per cent of participants with diabetes had had no hypoglycaemic agents prescribed. In both the hypertension and diabetes groups, we observed higher medication use in participants with poorer control.
Table 3.
Treatment, diabetes group
| Medications prescribed |
All participants (N=1 842) n (%) |
Subgroup with HbA1c test (N=704) n (%) |
Controlled* (N=160) n (%) |
Uncontrolled† (N=544) n (%) |
|---|---|---|---|---|
| Hypoglycaemic medications | ||||
| No diabetes medications | 119 (6.5) | 33 (4.7) | 23 (69.7) | 10 (30.3) |
| Metformin and/or sulfonylurea (but not insulin) | 1 126 (61.1) | 438 (62.2) | 112 (25.6) | 326 (74.4) |
| Insulin (with/without oral agents) | 588 (31.9) | 230 (32.7) | 25 (10.9) | 205 (89.1) |
| Missing information | 9 (0.5) | |||
| Aspirin | 1 155 (62.7) | 477 (67.8) | 101 (21.2) | 376 (78.8) |
| Statin | 931 (50.5) | 407 (57.8) | 82 (20.1) | 325 (79.9) |
| ACE inhibitor | 1 215 (66.0) | 475 (67.5) | 108 (22.7) | 367 (77.3) |
ACE = angiotensin-converting enzyme.
HbA1c <7%.
HbA1c ≥7%.
In the respiratory group, 48% of participants had been prescribed a β2-agonist metered dose inhaler (MDI) or nebuliser, 34% an inhaled corticosteroid (any dose), 8% an ipratropium bromide MDI or nebuliser, and 11% slow-release theophylline as maintenance treatment (Table 4).
Table 4.
Treatment, chronic respiratory disease group
| Medications prescribed | All participants (N=1 157) n (%) |
SGRQ symptom domain score Median (IQR) |
SGRQ activity domain score Median (IQR) |
|---|---|---|---|
| No chronic respiratory disease medications | 567 (49.0) | 52.6 (29.8 – 68.5) | 67.2 (47.7 – 85.8) |
| Selective β2-agonist | 558 (48.2) | 64.6 (44.9 – 78.9) | 79.8 (59.5 – 92.5) |
| Inhaled corticosteroids (any dose) | 388 (33.5) | 64.6 (42.8 – 79.3) | 80.3 (60.3 – 92.5) |
| Inhaled corticosteroid (≥800 µg/d) | 346 (29.9) | 64.9 (41.8 – 80.2) | 80.4 (60.3 – 92.5) |
| Theophylline | 121 (10.5) | 70.4 (51.8 – 83.0) | 79.8 (60.4 – 92.5) |
| Ipratropium bromide | 91 (7.9) | 69.7 (52.2 – 81.6) | 85.8 (60.4 – 93.2) |
In the depression group, 12% of participants had been prescribed a therapeutic dose of antidepressant and 10% a subtherapeutic dose (<50 mg/d) of amitriptyline or imipramine (Table 5). Twenty-five per cent were receiving treatment for depression, defined as receiving counselling, referral to psychiatric services or being on an antidepressant at a therapeutic dose. Forty-five per cent were not receiving treatment, and it could not be established whether the remaining 30% were receiving treatment for depression, either because prescription charts were not available (14 participants) or because the participant failed to answer the question regarding counselling and psychiatric referral (729 participants).
Table 5.
Treatment, depression group
| Medications prescribed or other management | All participants (N=2 466) n (%) |
CESD-10 score Median (IQR) |
|---|---|---|
| Antidepressant medications | ||
| No antidepressant medications | 1 902 (77.1) | 14 (12 – 17) |
| Antidepressant at therapeutic dose | 294 (11.9) | 19 (14 – 22) |
| Antidepressant at subtherapeutic dose | 250 (10.1) | 15 (12 – 19) |
| Missing information | 20 (0.8) | |
| Received counselling in past year | 402 (16.3) | 15 (12 – 20) |
| Psychiatric referral in past year | 175 (7.1) | 17 (13 – 21) |
| Antidepressant at therapeutic dose or counselling in past year or psychiatric referral in past year | 614 (24.9) | 16 (12 – 20) |
Discussion
This study described the clinical profile, disease control, multimorbidity and treatment received by patients with the target conditions attending PHC clinics in two districts in SA. The results indicated poor disease control, high levels of multimorbidity and unmet treatment needs in the public sector in these districts.
These findings confirm previous reports of poor control and treatment of NCDs, and demonstrate little improvement in NCD control since these earlier studies were conducted. A study of 1 089 patients in 18 community health centres in 1999 demonstrated poor levels of control of hypertension and diabetes in community health centres in the Cape Peninsula, with 67% of hypertensive patients recording a BP ≥140/90 mmHg and a mean HbA1c of 8.8% in diabetic patients.[4] A study of goldminers in Gauteng Province, SA, in 2009/2010 found that only 42% of patients diagnosed with hypertension received antihypertensive medication, while 69% of patients on antihypertensive medication were poorly controlled.[12] The South African Stress and Health Study, a community survey of 4 351 adult South Africans between 2002 and 2004, found that only one-quarter of patients with depression, anxiety and substance use disorders received treatment.[6] Comorbidity of hypertension and diabetes was found to be high in a cross-sectional study in Cape Town townships in 2008/2009, with 21% of participants with hypertension also having diabetes, compared with 7% of non-hypertensive patients having diabetes.[9] The cross-sectional study in the Cape Peninsula in 1999 found 31% of participants with hypertension to have diabetes and 64% of participants with diabetes to have hypertension.[4] A more recent survey of SA PHC found that 18% of patients with hypertension also had diabetes, and 63% of patients with diabetes also had hypertension.[3] Our study demonstrated higher levels of comorbidity than these studies, with 47% of participants with hypertension also having diabetes, and 84% of participants with diabetes also having hypertension. A study of urban SA women demonstrated high rates of comorbid psychological distress with physical disease,[13] consistent with our finding of 51% of participants with hypertension, diabetes or chronic respiratory disease also having symptoms of depression. However, the high rates of multimorbidity in our study, particularly in the reporting of diabetes in the hypertension group, may partly be due to the sampling strategy, as explained in ‘Study limitations’ below.
Study limitations and strengths
This study had several limitations. It did not consider other potential comorbid conditions such as osteoarthritis which are likely in such patient populations, so multimorbidity and comorbidity were probably underestimated. The study was not intended to provide estimates of the prevalence of NCDs or depression symptoms, but its inclusion criteria may have influenced the interpretation of results. Because the inclusion criteria for each condition involved self-reporting, there was misclassification; some participants’ reported diseases were not confirmed, while others were found to be receiving medications for a disease that they had not reported. For example, of 1 166 participants not enrolled in the hypertension group (denying receiving medication for this diagnosis), 13% had received a prescription for antihypertensive medication and 30% had a BP ≥140/90 mmHg. Further, of 3 227 participants who reported being on medication for hypertension, 5% had no evidence of a prescription for hypertension. The inclusion criterion for the diabetes group was self-reported diabetes medication. Patients with diabetes on dietary control alone were therefore not included in the study. The study’s chronic respiratory disease definition was probably more inclusive than usual clinical practice and so may have overestimated disease prevalence. Spirometry was not used to diagnose respiratory disease. We did not distinguish between asthma, chronic obstructive pulmonary disease or other symptomatic chronic lung diseases, and severity was not assessed by lung function tests. For these reasons, the appropriateness of treatments prescribed could not be assessed for individual participants. The study’s definition of possible depression indicates, but does not confirm, clinical depression. In addition, the percentage receiving counselling or referral is an underestimate owing to an error resulting in this question not being administered to all participants. Finally, the sampling strategy may have led to over-representation of reported comorbidities. For the randomised controlled trial we estimated that 60 patients were needed per clinic for each disease group except for the respiratory group, which required 27 patients per clinic. Owing to the high prevalence of hypertension in this clinic population, targets were easily met for the hypertension group, although it was more difficult to do the same for the diabetes group. Fieldworkers were asked to continue recruitment until targets were met for all four groups, with the result that targets were exceeded for all groups except diabetes, where recruitment fell short (81% of target). Since the majority of patients with diabetes also had hypertension, extended recruitment of this group may have led to an over-representation of the proportion of those with hypertension who also had diabetes.
The study had a number of strengths. The sample size was large, data were collected for four disease groups, and prescription data were collected for 99% of participants. There are few other recent studies addressing multimorbidity, control and treatment of NCDs in public sector PHC clinics in SA.
Conclusion
The rising prevalence of NCDs is a major challenge facing healthcare systems worldwide, with multimorbidity becoming the norm for people with chronic diseases. Despite this, health systems tend to be configured for individual diseases.[14] The high levels of multimorbidity demonstrated in this study stress the need for PHC services to provide better-integrated NCD care. Clinicians need to consider potential coexistence of, and interactions between, diseases. Training of clinicians to manage multimorbidity is essential, and should address both appropriateness of prescribing and adherence to medication. Management of NCDs and multimorbidity need to be addressed at a health systems level and factored into clinical training. The Integrated Chronic Disease Management[15] and Primary Care 101[16] programmes are important current initiatives aimed at integrating chronic disease care and addressing multimorbidity. After a decade of focusing on scaling up antiretroviral therapy programmes, management of NCDs in PHC needs to be prioritised and requires similar investment in order to improve outcomes and limit the impact on morbidity and mortality. With limited time and resources in the PHC setting, careful consideration of how to prioritise care is required.
Understanding the causes of poor NCD control will assist in prioritising care and resources. Further research is required into the development and evaluation of interventions to address the burden and unmet treatment needs of NCDs, including mental health.
Acknowledgments
Funding. This project has been funded in part with Federal funds by the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900030C. Funding was also received from United Health, USA; the Department of Health of the Provincial Government of the Western Cape; the Department of Medicine, University of Cape Town; the UK Department for International Development; and the University of Cape Town Lung Institute. The study sponsors did not contribute to the design of the study, to the collection, analysis and interpretation of data, or to the writing of this article or the decision to submit it for publication. The researchers were independent from funders and sponsors, and researchers involved in the collection, analysis and interpretation of the data had access to all the data.
The authors thank all clinic nurses, doctors, managers, pharmacists and pharmacy assistants at participating study facilities; the Department of Health of the Provincial Government of the Western Cape; the Eden and Overberg district management; Primary Care 101 trainers and fieldworkers; and the National Health Laboratory Service.
References
- 1.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374(9693):934–947. doi: 10.1016/S0140-6736(09)61087-4. [ http://dx.doi.org/10.1016/S01406736(09)61087-4] [DOI] [PubMed] [Google Scholar]
- 2.Mash B, Fairall L, Adejayan O, et al. A morbidity survey of South African primary care. PLoS One. 2012;7(3):e32358. doi: 10.1371/journal.pone.0032358. [ http://dx.doi.org/10.1371/journal.pone.0032358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalkhen H, Mash R. Comorbidity and multimorbidity in non-communicable diseases in South African primary healthcare. S Afr Med J. 2015;105(2):134–138. doi: 10.7196/samj.8696. [ http://dx.doi.org/10.7196/SAMJ.8696] [DOI] [PubMed] [Google Scholar]
- 4.Steyn K, Levitt NS, Patel M, et al. Hypertension and diabetes: Poor care for patients at community health centres. S Afr Med J. 2008;98(8):618–622. [PubMed] [Google Scholar]
- 5.Poyser MA, Nelson H, Ehrlich RI, et al. Socioeconomic deprivation and asthma prevalence and severity in young adolescents. Eur Respir J. 2002;19(5):892–898. doi: 10.1183/09031936.02.00238402. [ http://dx.doi.org/10.1183/0903193 6.02.00238402] [DOI] [PubMed] [Google Scholar]
- 6.Williams DR, Herman A, Stein DJ, et al. Twelve-month mental disorders in South Africa: Prevalence, service use and demographic correlates in the population-based South African Stress and Health Study. Psychol Med. 2008;38(2):211–220. doi: 10.1017/S0033291707001420. [ http://dx.doi.org/10.1017/S0033291707001420] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English RG, Bateman ED, Zwarenstein MF, et al. Development of a South African Integrated Syndromic Respiratory Disease Guideline for Primary Care. Prim Care Respir J. 2008;17(3):156–163. doi: 10.3132/pcrj.2008.00044. [ http://dx.doi.org/10.3132/pcrj.2008.00044] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 9.Peer N, Steyn K, Lombard C, Gwebushe N, Levitt N. A high burden of hypertension in the urban black population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) Study. PLoS One. 2013;8(11):e78567. doi: 10.1371/journal.pone.0078567. [ http://dx.doi.org/10.1371/journal.pone.0078567] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [ http://dx.doi.org/10.1016/S0954-6111(06)80166-6] [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary: Standards of Medical Care in Diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S4–S10. doi: 10.2337/dc13-S004. [ http://dx.doi.org/10.2337/dc13-S004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maepe LM, Outhoff K. Hypertension in goldminers. S Afr Med J. 2012;102(1):30–33. [PubMed] [Google Scholar]
- 13.Mendenhall E, Richter LM, Stein A, Norris SA. Psychological and physical co-morbidity among urban South African women. PLoS One. 2013;8(10):e78803. doi: 10.1371/journal.pone.0078803. [ http://dx.doi.org/10.1371/journal.pone.0078803] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [ http://dx.doi.org/10.1016/S0140-6736(12)60240-2] [DOI] [PubMed] [Google Scholar]
- 15.Department of Health, Republic of South Africa. [accessed 27 June 2014];Department of Health Annual Report 2011/2012. http://www.health.gov.za/
- 16.Department of Health, Republic of South Africa. [accessed 4 November 2014];Primary Care. 101 http://www.health.gov.za/docs/Policies/2013/Low_res_PC_101_Guideline_v2.pdf. [Google Scholar]