Abstract
Most physiological functions originate with the communication between organs. Mouse genetics has revived this holistic view of physiology through the identification of inter-organ communications that are unanticipated, functionally important and would have been difficult to uncover otherwise. This review highlights this point by showing how two tissues usually not seen as endocrine ones, bone and striated muscles, influence in a significant manner several physiological processes.
Introduction
The molecular biology revolution we have experienced over the last half-century has transformed our understanding of all biological processes, be they developmental or postnatal, that occur in complex organisms and especially vertebrates. This unprecedented impact of molecular biology had another consequence: It reshaped the way we think about biology, such that most biological disciplines redefined themselves in molecular terms. Yet, all disciplines of biology were not affected in the same manner by the molecular revolution. Physiology and endocrinology, for instance, are two disciplines that do not center solely on the molecular events occurring in one cell type; rather they are concerned with the communications that exist between different organs. Such communications occur through secreted molecules. An important contribution of molecular biology has been to identify new systemic cues that mediate these inter-organ communications. An equally important contribution has been providing the toolkit necessary to determine the functions and biological importance of these circulating molecules hormones and cytokines, in vivo. Specifically, it is the ability to inactivate single genes in single cell types and in a time-specific manner in the mouse that has led to the identification of many novel endocrine organs and hormones. In this way, mouse genetics has illustrated the value of a whole organism approach to the study of physiology, provided of course it is conducted one molecule at a time. This revival in whole organism approaches to physiology brings up the question of the biological relevance of some of these new findings. One could argue that as a rule, when the functions of these recently discovered inter-organ communications and hormones are observed in unchallenged animals they must be considered biologically relevant and may in fact be as important as the ones discovered in the early part of the 20th century. As a result, one can anticipate that some of these new endocrine regulatory loops have the potential to lead to adapted therapies for some degenerative diseases. Looking at the recent evolution of biology and at the importance of inter-organ communication as an emerging theme, it is also likely that many more functionally important inter-organ communications and new physiologies remain to be discovered.
The wealth of knowledge regarding inter-organ communications that has emerged in the last 15 years makes writing a review article on this topic a daunting task. Rather than superficially reviewing the many inter-organ communications revealed by mouse genetics, we focus here on two tissues, bone and muscle, for the following reasons. Neither tissue was suspected to perform endocrine functions, let alone to affect in a significant manner so many physiological processes. Moreover, the realization that these organs have endocrine functions occurred through rather different routes (Grueter et al., 2012; Lee et al., 2007). By focusing on the endocrine functions of bone and striated muscles, we propose a rationale for why these tissues would act in an endocrine manner on the physiological functions they influence. This rationale could then be used to reveal additional physiological processes bone or muscle may regulate.
Expanding bone biology
The realization that bone is an endocrine organ came from two contemporaneous and very different lines of investigation. As illustrated later in this review in the case of fibroblast growth factor 23 (FGF23), the endocrine nature of bone came from the investigation of phosphate metabolism, which is critical for proper bone mineralization (Murshed et al., 2005). In another case the realization imposed itself as the most plausible, if not only, interpretation of an experiment that sought and failed to answer a different question. The goal of this study was to provide a molecular explanation for why extracellular matrix (ECM) mineralization occurs in bones (and teeth) but in no other collagen-rich ECMs. Hypothesizing, wrongly as it turned out, that this process is driven by proteins secreted by osteoblasts in the bone ECM that would then trigger the deposition of mineral ions onto collagen fibers, our attention focused on one protein osteocalcin. There were excellent reasons for this, even in retrospect. Osteocalcin is an osteoblast–specific protein that is secreted in large amounts in the bone ECM, and the genes encoding osteocalcin starts to be expressed during development, around the time bone mineralization begins. Even more suggestive was the fact that through the gamma carboxylation of three glutamic acid residues (“Gla” residues) osteocalcin acquires a high affinity for mineral ions (Lian et al., 1978). And yet, neither the inactivation of Osteocalcin nor its overexpression in osteoblasts or other cell types had any effects on ECM mineralization in bones or other tissues (Ducy et al., 1996; Murshed et al., 2004). However, Osteocalcin-deficient (Osteocalcin−/−) mice had two obvious, albeit unexpected, phenotypes. The most overt phenotype was the dramatic increase in abdominal fat compared to wild-type littermates. A second more subjective observation was that Osteocalcin deficient mice started to breed later and became infertile sooner than wild-type littermates. Thus, the inactivation of an osteoblast-specific protein secreted in the bone ECM influenced two physiological processes that do not directly involve bones: fat accumulation and reproduction. The surprising nature of these observations brought to fore other features of osteocalcin that had been previously overlooked. First, like most peptide hormones, osteocalcin is produced as a pre-pro-molecule that is sequentially cleaved in osteoblasts so that only the mature protein is secreted. Second, like many hormones, osteocalcin is present in the general circulation in the ng/ml range in all species tested and its circulating levels follow a circadian rhythm in humans (Gundberg et al., 1985; Hauschka et al., 1989).
Thus, phenotypes of Osteocalcin deficient mice, and some biochemical characteristics of osteocalcin, suggested that it might be a peptide hormone secreted by osteoblasts. In fact, osteocalcin is not the only hormone synthesized by bone cells. FGF23 is also synthesized by osteoblasts, reaches the general circulation and acts in the kidney to favor phosphate elimination (Fukumoto and Shimizu, 2011). There is, however, an important difference between FGF23 and osteocalcin: FGF23 regulates phosphate metabolism, a process intimately linked to bone health itself (Murshed et al., 2004). In contrast, at least one of the functions of osteocalcin – the regulation of fat mass - had no known link to bone health. This set of observations also raised questions of greater significance: why would bone have any other function besides making bone and if it does, what might these functions be?
Bone as an endocrine organ
The view of bones as an assembly of calcified tubes allowing animals living on land to stand, walk, and run is deeply entrenched in our biomedical culture. So much so that there is a need to explain why of all tissues, bone should be suspected a priori, to be an endocrine organ and, as such, what physiological functions it would be expected to regulate. For that purpose one needs to describe a cell biological feature that is unique to bone, its implications, and how clinical observations confirm the suspicion this feature of bone biology generates.
Bone is the only tissue in our body that contains a cell type, the osteoclast, whose main function is to destroy the tissue in which it resides (Teitelbaum, 2000). This destruction, or resorption, of bone must be energetically demanding since it occurs daily in multiple locations in one of the largest organs in the body. Bone resorption is only one aspect of a biphasic physiological process called bone modeling during childhood and remodeling during adulthood, in which bone formation invariably follows bone resorption. Since bone formation requires that osteoblasts synthesize and secrete daily, large amounts of proteins to form the ECM, it is also likely to be energetically expensive. This constant alternation of bone destruction and bone formation fulfills biological functions of fundamental importance: bone modeling allows the longitudinal growth of the skeleton during childhood and therefore the ability to stand, walk and run; as of bone remodeling it was originally intended to repair micro- and macro-damages, i.e., fractures. The hypothesis that bone (re)modeling is an energetically costly physiological process becomes a reality when looking at clinical situations. Most clearly, when access to food, i.e., energy, is limited bone growth stops in children and bone mass decreases in adults.
The powerful influence of food/energy intake on bone (re)modeling revealed by clinical observations raises two questions. If energy intake is so important for bone, does bone in turn regulate energy metabolism, or is bone an endocrine organ regulating energy metabolism? The second and more ambitious question is to rationalize why evolution came up with an invention as energetically costly as bone precisely at a time when food/energy was so scarce? A possible answer to this question could be that the evolutionary advantage conferred by bone to animals living on land by the ability to stand, walk and run justified its invention. Another possible answer to this question, that is not mutually exclusive, is that bone fulfills important aspects of energy metabolism and maybe other physiological functions. One other function possibly regulated by osteocalcin is suggested by another clinical observation that resonates with the suspected decrease in fertility of the Osteocalcin deficient mice. This clinical observation is that bone mass declines in both sexes, when gonadal functions end. One could then argue that in a feedback loop bone may also regulate fertility in one or both sexes.
When confronting the phenotypes of Osteocalcin deficient mice and the cell biological and clinical observations mentioned above we proposed that there may be a coordinated regulation, endocrine in nature, of bone mass, energy metabolism and reproduction. Although we verified other tenets of it (Ducy et al., 2000; Takeda et al., 2002; Yadav et al., 2009), the most novel implication of this hypothesis is that bone should be an endocrine organ.
The anticipated functions of osteocalcin
The difficulty in testing whether osteoblasts affect energy metabolism through osteocalcin is that energy metabolism includes a loosely defined aggregate of processes ranging from food intake to the utilization of nutrients in peripheral tissues. The approach used to circumvent this difficulty was to co-culture osteoblasts with other cell types or organ explants that secrete hormones regulating key aspects of energy metabolism. The two structures tested in this experimental scheme were pancreatic islets, given the number of aspects of energy metabolism regulated by insulin, and adipocytes since leptin indirectly regulates osteoblast function (Ducy et al., 2000; Elefteriou et al., 2005; Takeda et al., 2002; Yadav et al., 2009).
Supernatants of wild-type osteoblasts enhance the expression of Insulin in pancreatic islets and of Adiponectin in adipocytes. Several criteria of specificity strengthened these findings. For instance, the supernatant of fibroblasts, the cell type that is the most closely related to osteoblasts, fails to stimulate Insulin or Adiponectin expression. A second criterion of specificity is that among all the hormones expressed by pancreatic islets and adipocytes, only Insulin and Adiponectin expression is affected by the supernatant of osteoblast cultures. That none of these changes in gene expression are observed when using the supernatants of Osteocalcin deficient osteoblasts identified osteocalcin as an osteoblast-derived hormone that affects Insulin and Adiponectin expression. These studies also identified the active form of osteocalcin since addition of uncarboxylated but not carboxylated osteocalcin increased Insulin and Adiponectin expression in islets and adipocytes, respectively (Lee et al., 2007).
The in vivo analysis of the endocrine functions of osteocalcin has greatly benefited from the existence of mouse models of global (Osteocalcin deficient) and osteoblast-specific loss-(Osteocalcinosb-deficient mice) and gain-of-function of osteocalcin (Esp-deficient and Esposb-deficient mice), each model serving as a mirror image control for the other. Consistent with the results of cell-based assays, insulin secretion and β-cell proliferation/mass are both decreased in Osteocalcin deficient mice. These mutant mice are also glucose-intolerant and insulin-resistant when fed normal chow, whereas the opposite is true in Esposb−/− mice. It remains to be determined if what appears as an insulin resistance is instead indicative of the fact that osteocalcin may favor glucose uptake, independently of insulin. Explaining in part their changes in fat mass, energy expenditure is significantly decreased in Osteocalcin deficient and increased in Esposb−/− mice (Lee et al., 2007). These observations have ben recently extended to human pancreatic islets, in which the addition of uncarboxylated osteocalcin increases the expression of the Insulin and of Cyclind2 and Cdk4 genes, two genes needed for β-cell proliferation (Sabek et al., 2015). Moreover, circulating osteocalcin levels are inversely correlated in adults with fasting glucose circulating insulin levels, body mass index and body fat (Fernandez-Real et al., 2009).
These results demonstrate that osteocalcin is a bone-derived hormone that regulates β-cell proliferation, Insulin expression and insulin secretion in mice and in humans (Figure 1). They also suggest that the hormonally active form of osteocalcin is the decarboxylated one. This has been subsequently confirmed through genetic means (Ferron et al., 2015).
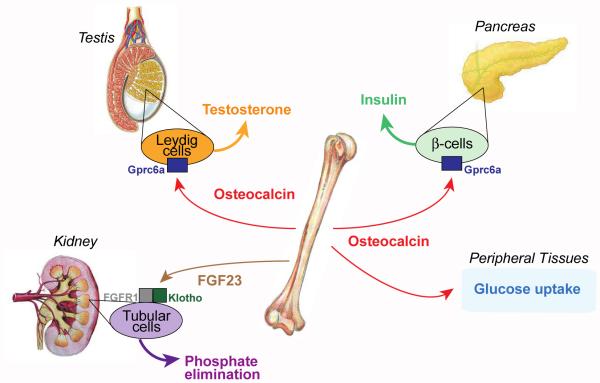
Figure 1. Endocrine Roles for Osteocalcin.
Schematic representation of the main organs whose functions are affected by osteocalcin (pancreas and testes) and FGF23 (kidney), the two osteoblast-derived hormones. Gprc6a is the receptor for osteocalcin in pancreatic β-cells and Leydig cells of the testes; FGFR1 and Klotho mediates FGF23 signal in tubular cells of the kidney.
The same co-culture assay has been used to test whether osteoblasts and presumably osteocalcin favor reproductive functions. Surprisingly given that osteoporosis is primarily a disease of post-menopausal women, neither the supernatant of osteoblasts nor uncarboxylated osteocalcin influence the secretion of estrogen by ovary or follicular cells. In contrast, recombinant osteocalcin and supernatants of wild-type but not of Osteocalcin deficient osteoblast cultures induces the secretion of testosterone by Leydig cells of the testes. Here again the experiment is controlled in several ways; supernatants of no other mesenchymal cell types could increase testosterone secretion by Leydig cells of the testes and osteocalcin does not increase the secretion of estradiol, a derivative of testosterone, by Leydig cells. Extending these cell-based assays, the analysis of cell-specific loss- and gain-of-function mouse models of osteocalcin function as well as the treatment of Leydig cells with osteocalcin show that uncarboxylated osteocalcin signals directly to Leydig cells to favor the expression of all genes encoding the enzymes necessary for testosterone synthesis, but does not affect the expression of Cyp19 that is needed for the aromatization of testosterone into estradiol. As a result, male mice lacking osteocalcin show typical features of hypo-testosteronemia e.g., low sperm count and lower weight of the epididymis and testes (Figure 1). In contrast, the circulating levels of luteinizing hormone that favor testosterone secretion were increased in Osteocalcin deficient mice (Oury et al., 2011).
The other side of osteocalcin
Once the predicted endocrine functions of osteocalcin were demonstrated, a systematic search for other target organs for this hormone began. In this regard, Osteocalcin deficient mice, regardless of gender, are markedly more docile than their wild-type littermates. Initially this docility was ascribed to the low circulating level of sex steroid hormones that was suspected to exist in Osteocalcin deficient mice of both sexes. However, once it was shown that osteocalcin regulates sex steroid hormones synthesis only in male mice, this phenotype took another dimension and was analyzed rigorously. The docility of female Osteocalcin deficient mice could be traced to a decrease in the synthesis of all monoamine neurotransmitters and to an increase in GABA. In agreement with the extent of these abnormalities and the biological importance of these neurotransmitters, these mice displayed severe behavioral phenotypes such as increased anxiety and a profound deficit in spatial learning and memory. These abnormalities are due to a lack of signaling of osteocalcin in the brain, since delivery of the hormone in the brain through intracerebroventicular infusion corrects them. Osteocalcin affects neurotransmitter synthesis because it crosses the blood brain barrier and binds specifically to serotonergic neurons of the raphe nuclei in the brainstem, to neurons of the CA3 region of the hippocampus and of the dopaminergic nucleus of ventral tegmental area in the midbrain. Hence, these experiments demonstrate a significant influence of bone on neurotransmitter synthesis in the brain and on cognition (Oury et al., 2013b).
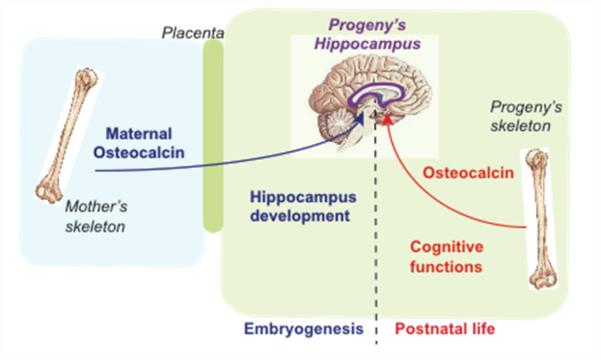
As unexpected as these findings were, another observation was even more surprising. A histological analysis showed that the hippocampi of Osteocalcin deficient mice are hypoplastic compared to those of WT littermates. This developmental defect is puzzling because Osteocalcin is not expressed during mouse embryogenesis before the last two days of gestation (Oury et al., 2013b). Studying this observation reveals that maternally-derived osteocalcin crosses the placenta and favors hippocampus development by preventing neuronal apoptosis. As a result Osteocalcin deficient mice exhibited a more severe cognitive deficit when their mothers were also lacking Osteocalcin. Conversely, providing osteocalcin once a day to pregnant Osteocalcin deficient mothers normalized the development of the hippocampus and partly rescued the deficit in memory in their Osteocalcin deficient progeny. The observations that maternal osteocalcin contributes to the development of the brain in the embryo, identifies osteocalcin as a molecule responsible for the beneficial influence of the mother’s health on the development of the brain and cognitive functions of the offspring (Figure 2) (Oury et al., 2013b). The notion that maternal osteocalcin is present in the embryo before expression of Osteocalcin also suggests that osteocalcin may have a broader than anticipated influence on the health of the offspring.
Figure 2. Functions of osteocalcin in the brain.
Maternally derived osteocalcin crosses the placenta, reaches the developing brain and favors hippocampal development. In adult animals osteocalcin crosses the blood brain barrier regulates the synthesis of various neurotransmitters, prevents anxiety and favors spatial learning and memory. The receptor for osteocalcin in the brain has not been identified yet.
Questions raised by the functions of osteocalcin
The nature, extent and number of the endocrine functions of osteocalcin raises interesting new questions. Key among them is the identity of the receptor for osteocalcin and whether the biological relevance of these findings extend beyond genetically-modified mice. In other words are the endocrine functions of osteocalcin observed in mice are conserved in humans? These questions have been addressed through the study of the reproductive functions of osteocalcin.
Osteocalcin’s stimulation of testosterone secretion by Leydig cells follows a bell-shaped curve reminiscent of what is observed when a ligand binds to a G protein coupled receptor (GPCR). Further supporting this notion, treating Leydig cells with osteocalcin induces production of cAMP while it fails to induce tyrosine phosphorylation, ERK activation or intracellular calcium accumulation. These data justified a search for a GPCR that would be expressed in Leydig cells of the testes but not in follicular cells of the ovary and would transduce the osteocalcin signal. This screen identified a single candidate, Gprc6a (Oury et al., 2011). Of note, mice lacking Gprc6a display metabolic and male reproductive abnormalities similar to those seen in Osteocalcin deficient mice mice (Pi et al., 2008). Analysis of cell-specific gene deletion and compound mutant mice lacking one allele of Osteocalcin and one allele of Gprc6a in Leydig cells or β-cells verifies that Gprc6a mediates the osteocalcin signal in these cell types (Oury et al., 2011; Wei et al., 2014b).
Remarkably, Gprc6a is not expressed in any of the brain structures to which osteocalcin binds, Gprc6a deficient mice have normal neurotransmitter accumulation in the brain, normal hippocampal development and normal cognitive functions. These findings rule out that the cognitive defects seen in mice lacking Osteocalcin are secondary to their metabolic or endocrine abnormalities since these abnormalities are equally severe in Osteocalcin and Gprc6a-deficient mice; on the other hand they raise the question of the identity of the receptor(s) of osteocalcin in the brain.
Providing evidence that the endocrine functions of osteocalcin also exist in humans is helped by the fact that the reproductive phenotype of Osteocalcin deficient mice, low testosterone levels and high circulating levels of luteinizing hormone, bears resemblance to peripheral testicular insufficiency in humans. Sequencing all exons of Osteocalcin and Gprc6a in 59 of these patients identified two unrelated individuals harboring the same dominant negative mutation in a conserved residue of the GPRC6A extracellular domain, a mutation that could not be found in control individuals. Of note, glucose tolerance was also abnormal in both patients (Oury et al., 2013a). Although more studies are needed, these data suggest that signaling through GPRC6A, presumably by osteocalcin, is required for testosterone synthesis by Leydig cells in humans.
This work also raises the question of the potential influence of osteoblasts on insulin and adiponectin, the two hormones whose expression is regulated by osteocalcin. Addressing the relationship between bone and pancreas from the viewpoint of insulin helps explain how osteocalcin, a constituent of the bone ECM, becomes a hormone. Through its signaling in osteoblasts, insulin inhibits the expression of Osteoprotegerin, an inhibitor of osteoclast differentiation, thus insulin signaling in osteoblasts favors bone resorption. Since the only known means to decarboxylate a protein once outside the cell is a low pH such as the one reigning within the resorption lacunae, insulin signaling in osteoblasts allows the carboxylated form of osteocalcin present in the bone ECM to be decarboxylated on one particular Gla residue and released in the general circulation (Ferron et al., 2010). These results identified bone as a more significant insulin target organ than previously thought and led to the demonstration that bone is a site of insulin resistance in diabetic mice (Wei et al., 2014a). The demonstration that osteocalcin also regulates adiponectin expression led to the realization that in unchallenged mice adiponectin is a powerful regulator of bone formation (Kajimura et al., 2013).
More topical questions remain to be addressed. For instance, we do not know how osteocalcin favors energy expenditure or glucose uptake in peripheral tissues. Likewise, the influence of maternal osteocalcin on glucose homeostasis in the offspring has not been accounted for. The fact that another hormone besides luteinizing hormone, be it osteocalcin made in bone, favors sex steroid synthesis in the male but not female gonads raises the question of whether such a hormone exists in females and, if so, which tissue synthesizes it. As for the cognitive functions of osteocalcin, not only do we need to identify its receptor in the brain but we also need to determine whether osteocalcin can improve cognition in wild-type animals, young and old. Given the progressive aging of the general population and the paucity of drugs to treat or prevent the age-related decline in cognition this is a question of critical importance. There is an important aspect of bone biology that is worth mentioning in that context. Bone mass remains relatively constant for the first 3 decades of life and begins to decrease thereafter. This decrease is greatly accelerated at the time of menopause in women but in both sexes there is a marked decrease in bone mass with aging. This observation raises the question of whether bone in its endocrine capacity may in fact delay the age-related decrease in some physiological functions and may prevent the appearance of some aging manifestations. We now have the tools to address to test this hypothesis in vivo.
But again the question of broader significance looming behind the apparently disparate nature of the physiological functions regulated by osteocalcin is to understand what were the evolutionary advantages of the endocrine function of bone. Addressing this question in full will require identifying all functions of osteocalcin and possibly the ones of other hormones made by bone cells. Yet, one could use features shared by the known functions of osteocalcin to try to get at the logic of bone as an endocrine organ. It is obvious that male fertility is needed for the survival of any species. To a certain extent, the same can be said of the ability to utilize glucose in peripheral tissues and to remember where food sources and predators are located, for animals living in a hostile environment such as the ones in which bony vertebrates lived early on. Under this light, the endocrine functions of osteocalcin and the classical functions of bone suggest that this tissue may have conferred evolutionary advantages of two types. By allowing walking and running, bone enabled animals to escape danger and to find food. At the same time, through its endocrine functions bone may have provided a means of survival in hostile environments. This view of the endocrine functions of bone is mainly a tool to search for additional physiological processes it may regulate. One would expect that not all endocrine functions of bone will be fulfilled by osteocalcin or FGF 23 and that other bone-derived hormones will be characterized in the future.
The regulation of phosphate metabolism by bone
A key aspect of bone physiology is mineralization of the bone ECM, a process in which phosphate plays a critical role (Murshed et al., 2004). There is no better example of inter-organ communication than the regulation of phosphate balance in the body since it involves the kidneys, intestines, bones and parathyroid glands.
The notion that phosphate metabolism is regulated by a secreted factor came from the observation that transplanting a healthy kidney in a patient with a phosphate wasting disease could not correct the disease; this implied that the cause of phosphate wasting was not in kidney itself but originated from another organ (Morgan et al., 1974). The humoral factor with phosphaturic activity whose existence was suggested by this observation was later identified as FGF23, a protein synthesized by osteoblasts and osteocytes (Fukumoto and Shimizu, 2011; Razzaque and Lanske, 2007). FGF23 acts in the renal proximal and distal tubules where it inhibits phosphate reabsorption. In addition, FGF23 suppresses the production of 1, 25(OH)2 Vitamin D3 by inhibiting the 1α-hydroxylase. This latter function also contributes to the phosphate wasting effect of FGF23 that, in pathological circumstances, leads to poor mineralization of the skeleton (rickets in children, osteomalacia in adults). To regulate phosphate reabsorption, FGF23 binds to a complex made of FGFR1 and the co-receptor Klotho (Figure 1) (Urakawa et al., 2006). It is still an open question whether FGF23 has additional physiological functions apart from its inhibition of phosphate reabsorption and 1, 25(OH)2 Vitamin D3 biosynthesis.
Cardiac and skeletal muscle as endocrine tissues
Mouse genetics has identified several other organs besides bone for which an endocrine signaling function was not intuitively obvious. For instance, while cardiac and skeletal muscle participates in some expected endocrine dialogs with other tissues, other endocrine functions of these striated muscles, such as the control of chondrogenesis, were unexpected (Baskin et al., 2015; Doroudgar and Glembotski, 2011; Pedersen and Febbraio, 2012; Shimano et al., 2012). Inter-tissue signaling from muscle often occurs in response to contractile activity and, in many cases, serves to modulate energy storage or consumption in distal tissues. Communication between muscle, liver, adipose tissues and the brain is especially important for balancing metabolism across the body. The importance of muscle as a gatekeeper of energy metabolism is best illustrated by the variety and severity of the disorders such as metabolic syndrome, obesity, and type 2 diabetes that are secondary to perturbations of inter-tissue metabolic communication to and from skeletal muscle. Aging, which is associated with the loss of muscle mass and function, also perturbs signaling from muscle to distal tissues. While the role of muscle in modulation of metabolism of distal tissues is the best understood aspect of muscle endocrine signaling at present, it seems likely that additional endocrine influences of muscle will also be uncovered, as suggested by recent studies demonstrating an influence of the heart and skeletal muscle on the skeleton (Dankbar et al., 2015; Kondo et al., 2015; Tagliaferri et al., 2015).
Systemic metabolic control by the heart
Although representing a relatively minor contribution to body mass, the heart consumes a disproportionate amount of energy, estimated to account for ~10% of whole body energy consumption. In addition to its obvious role in maintaining blood flow, it has become increasingly apparent that the heart participates in endocrine signaling between tissues to regulate energy consumption and metabolism (Fig. 3). Indeed, given the importance of uninterrupted cardiac function for life, and the fact that the heart stores only enough energy for the ensuing few heart beats, it makes sense that the heart is intimately linked to the body’s energy sources. Thus, under conditions of compromised cardiac function, the heart may signal to distal organs to enhance the release of energy substrates and/or to diminish energy consumption. In addition to these metabolic connections, the heart has been shown to modulate other unexpected functions in distal tissues, such as the control of sodium balance and endochondral bone growth and remodeling. The heart is comprised of numerous cell types, including atrial and ventricular cardiomyocytes, endocardial cells, and epicardial cells, each of which may exert short- and long-range endocrine actions.
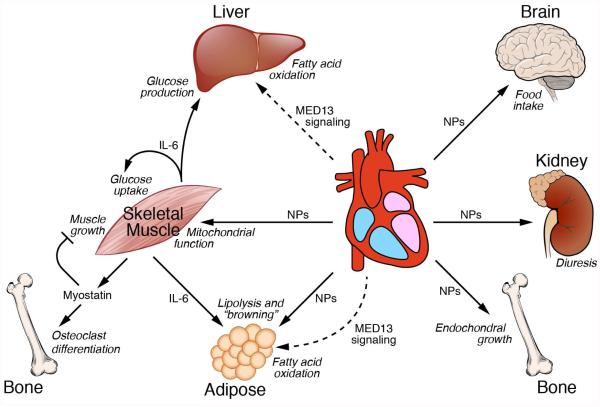
Figure 3. Endocrine signaling from striated muscles to distal tissues.
Myostatin and IL-6 signal from skeletal muscle to distal tissues and also exert autocrine functions to suppress muscle growth and enhance glucose uptake, respectively. Natriuretic peptides (NPs) released from the heart regulate diverse processes in distal tissues. The Mediator subunit MED13 acts in the heart to enhance fatty acid oxidation in liver and white adipose tissue. The factor(s) that mediate(s) signaling from cardiac MED13 to distal tissues have not been identified.
Several endocrine-like peptides originate from the heart. The most extensively studied of these are the natriuretic peptides (NPs), atrial NP (ANP) and B-type NP (BNP). These peptides are normally expressed by atrial cardiomyocytes and are induced in ventricular myocytes in response to mechanical, hemodynamic, ischemic and neurohumoral stress and signal to the kidneys where they promote diuresis and natriuresis, and induce vasodilation, protecting the heart from volume and pressure overload. Recently, unexpected functions of NPs in metabolic control have been uncovered (Collins, 2014; Collins and Bordicchia, 2013; Whittle and Vidal-Puig, 2012). For example, NPs are now known to act on skeletal muscle to enhance expression of genes involved in mitochondrial oxidation (Engeli et al., 2012). In addition, they act on white adipose tissues (WAT) to promote lipolysis and “browning” of adipocytes by activating expression of genes involved in energy expenditure and thermogenesis (Bordicchia et al., 2012). ANP also stimulates adipocytes to produce adiponectin, a peptide that controls glucose and fatty acid metabolism and food intake (Birkenfeld et al., 2012). NPs also inhibit leptin release by adipocytes and appear to act on the arcuate nucleus in the hypothalamus to control food intake (Yamada-Goto et al., 2013). Insulin up-regulates the expression of NP receptor C (NPRC) in human adipocytes, pointing to further endocrine interactions between the heart and its endocrine target tissues (Pivovarova et al., 2012). Circulating NP levels correlate inversely with visceral and liver adiposity and reduced NP levels have been linked to the development of type 2 diabetes. Finally, and perhaps least expected is the finding that NPs act on chondrocytes to stimulate endochondral bone growth (Kondo et al., 2015; Nakao et al., 2015). Accordingly, a loss-of-function of the NP receptor B (NPRB) is shown to cause acromesomelic dysplasia, Maroteaux type (AMDM) and plasma levels of CNP are elevated in other forms of chondrodysplasia (Olney et al., 2015).
A transcriptional basis of cardiac endocrine signaling.
The unraveling the endocrinology of heart and skeletal muscle is still in its infancy. Advances in this field are being made through the identification of new hormones or novel functions for known hormones, but they are also been made through a priori unrelated types of studies such as some transcriptional analyses. In a recent example, an unexpected endocrine influence of the heart was uncovered on the basis of metabolic phenotypes in mice in which MED13, a component of the mediator complex is genetically manipulated. The mediator complex coordinates transcription by linking signal-dependent and cell type-specific transcription factors with the basal transcription machinery (Baskin et al., 2015; Chen and Roeder, 2011). Analysis of the functions of the Mediator subunit MED13 in the heart reveals an unexpected signaling axis between the heart and the liver and WAT depots (Grueter et al., 2012) (Fig. 3). Cardiac over-expression of MED13 in transgenic mice confers a lean phenotype and resistance to obesity in response to high fat diet, whereas cardiac-specific deletion of MED13 sensitizes mice to weight gain on a high fat diet. Cardiac MED13 signaling promotes the systemic clearance of lipid from the blood and enhances expression of many genes involved in fatty acid oxidation and the Krebs cycle in WAT and liver (Baskin et al., 2014a). Parabiosis experiments in which the circulation of a mouse over-expressing MED13 in the heart is coupled to that of a wild-type mouse results in the acquisition of a lean phenotype by the wild-type parabiot (Baskin et al., 2014b).
Overall, these findings point to the existence of a secreted factor emanating from the heart that acts on distal energy reserves to promote energy consumption. The factor(s) responsible for this effect have not yet been defined. In principle, such factors could be peptides or metabolites.
Endocrine signaling by skeletal muscle
As the largest tissue in the body, accounting for ~40% of body mass of lean individuals, skeletal muscle plays a dominant role in the control of systemic energy metabolism and insulin sensitivity. The majority of diet-derived glucose is taken up by skeletal muscle in response to insulin signaling with excess glucose stored as muscle glycogen. In response to exercise, skeletal muscle emits signals to distal tissues involved in energy storage and production to fine tune energy utilization by the body (Fig. 4). Perturbation of inter-tissue metabolic communication to and from skeletal muscle is associated with a variety of disorders, including metabolic syndrome, obesity, and type 2 diabetes. Signaling from muscle to distal tissues to modulate systemic energy homeostasis appears to represent an ancient system for inter-tissue communication, as similar endocrine signaling mechanisms have been uncovered in Drosophila (Lee et al., 2014).
While the importance of skeletal muscle in the regulation of glucose homeostasis has long been appreciated, the realization that this tissue also functions in an endocrine manner by secretion of biologically active peptides that act at close and long range was not recognized until much later. In recent years, proteomics studies have identified hundreds of proteins secreted from mouse and human muscle, referred to as myokines that act in autocrine, paracrine and endocrine manners. In addition to their roles in the governance of metabolism, many of these proteins have been implicated in the modulation of inflammation, angiogenesis and myogenesis and are regulated by diverse stimuli, including exercise/activity, insulin resistance and aging. A few of many examples are discussed below.
Myostatin
The first myokine discovered was Myostatin (also referred to as growth and differentiation factor (GDF)-8, a muscle-specific member of the transforming growth factor-β (TGF-β) superfamily (McPherron et al., 1997). Myostatin acts in an autocrine manner to suppress skeletal muscle growth. Loss-of-function mutations in Myostatin in mice, as well as in sheep, cattle, dogs and humans, result in extreme skeletal muscle hypertrophy, reflecting the loss of the inhibitory influence of myostatin on muscle growth. The dramatic enhancement of muscle growth upon blockade of myostatin signaling reflects an increase in the number of myofibers formed during embryogenesis and an increase in size of myofibers postnatally. Myostatin suppresses muscle growth by binding either of the type II activin receptors (IIa or IIb), which couple to the type I receptors ALK4 and ALK5, stimulating the phosphorylation and activation of SMAD transcription factors (Sartori et al., 2014). Various myostatin binding proteins, such as follistatin, also serve to suppress myostatin action, thereby promoting muscle growth. In addition to the autocrine control of muscle size, myostatin acts distally to modulate metabolism (Buehring and Binkley, 2013; Feldman et al., 2006; Singh et al., 2014). Thus, mice lacking myostatin display reduced fat mass and improved insulin sensitivity (McPherron and Lee, 2002). Conversely, myostatin levels are elevated in obese individuals, although the potential significance of this observation remains to be established (Buehring and Binkley, 2013). Myostatin appears to act as an hormone and for instance can directly modulate bone remodeling by stimulating osteoclast differentiation (Dankbar et al., 2015).
Involvement of myokines in energy metabolism
Interleukin-6 (IL-6), one of the first myokines identified, displays a marked increase in the circulation during exercise, however since Il-6 is not muscle-specific it is not known which tissue is responsible for this increase nor do we know what triggers it. This is an important question to address in order to understand the entire molecular cascade accounting for adaptation to exercise. IL-6 fulfills several functions that together favor exercise. On the one hand, IL-6 signals in the liver to promote glucose by up-regulating gluconeogenic genes and in white adipose tissue to enhance lipolysis; in so doing IL-6 favors the production of the nutrients needed by muscle during exercise. On the other hand IL-6 acts in an autocrine manner in skeletal muscle to stimulate glucose uptake during exercise (reviewed in (Pedersen and Febbraio, 2012)). IL-6 also stimulates fatty acid oxidation in adipocytes and enhances the production of anti-inflammatory cytokines.
For a comprehensive discussion of other myokines, the reader is directed to several excellent reviews (Shimano et al., 2012; Benatti and Pedersen, 2015; Rai and Demontis, 2015).
Summary
In the context of this issue focused on communication, this review uses two tissues traditionally not presented in this light, the skeleton and striated muscle, to illustrate how a molecular genetic approach has rediscovered that physiology is first a science of inter-organ communication. This re-learning of physiology through the prism of mouse genetics over the course of the last twenty years has established physiology as a new frontier in biology. That the osteoblast and the myoblast have become full-fledged endocrine cells could not have been anticipated twenty years ago. The discovery of their endocrine roles marks a dawn of a new era in physiology as it is likely that several other cell types in other organs will be found to play such a function and therefore that many novel inter-organ communications will be discovered. If we look at the unique physiological functions of the musculoskeletal systems, several lessons can already be learned. A first one is that, of course, the interplay between bone or striated muscles and various aspects of energy metabolism is a central feature of this novel physiology. This certainly can be rationalized given the high metabolic demands of these organs. A second lesson is that all physiological functions were and remain important to assure the survival of all vertebrates and therefore there is really no reason to think that the musculoskeletal system or any organ would regulate only energy metabolism. The extent to which male fertility and cognition are regulated by bone and the role of heart-derived natriuretic protein in the control of chondrogenesis are clear examples of that (Woods et al 2007). This is important to keep in mind, as the entire palette of physiological functions regulated by the musculoskeletal system is not even known. And again it should be emphasized that the musculoskeletal system is only one example. What we have learned through different means about its involvement in whole-organism physiology should be viewed as a promise that many other organs will be shown to regulate physiological functions not classically associated with them.
Lastly, since physiology is so closely linked to the development of acquired diseases, it is now possible to think of developing adapted therapies for degenerative diseases, whose prevalence is steadily growing along with the progressive aging of the population, for which there is still little understanding at the organismal level.
Acknowledgements
We thank, P. Ducy, M. Ferron, S. Kousteni, for critical reading of the manuscript. G. Karsenty is a Senior Scholar in Aging from the Lawrence Ellison Foundation. Work in the Karsenty lab is supported by grants form the NIA and NIDDK and NIAMS, work in the Olson Lab is supported by grants from the NIH (HL-077439, HL-111665, HL-093039, DK-099653 and U01-HL-100401), Fondation Leducq Networks of Excellence, and the Robert A. Welch Foundation (grant 1-0025 to E.N.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baskin KK, Bookout AL, Olson EN. The heart-liver metabolic axis: defective communication exacerbates disease. EMBO Mol Med. 2014a;6:436–438. doi: 10.1002/emmm.201303800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin KK, Grueter CE, Kusminski CM, Holland WL, Bookout AL, Satapati S, Kong YM, Burgess SC, Malloy CR, Scherer PE, et al. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol Med. 2014b;6:1610–1621. doi: 10.15252/emmm.201404218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin KK, Winders BR, Olson EN. Muscle as a "mediator" of systemic metabolism. Cell metabolism. 2015;21:237–248. doi: 10.1016/j.cmet.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases - myokine regulation. Nature Rev Rheumatology. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- Birkenfeld AL, Boschmann M, Engeli S, Moro C, Arafat AM, Luft FC, Jordan J. Atrial natriuretic peptide and adiponectin interactions in man. PloS one. 2012;7:e43238. doi: 10.1371/journal.pone.0043238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. The Journal of clinical investigation. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehring B, Binkley N. Myostatin--the holy grail for muscle, bone, and fat? Current osteoporosis reports. 2013;11:407–414. doi: 10.1007/s11914-013-0160-5. [DOI] [PubMed] [Google Scholar]
- Chen W, Roeder RG. Mediator-dependent nuclear receptor function. Semin Cell Dev Biol. 2011;22:749–758. doi: 10.1016/j.semcdb.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nature reviews Endocrinology. 2014;10:157–163. doi: 10.1038/nrendo.2013.234. [DOI] [PubMed] [Google Scholar]
- Collins S, Bordicchia M. Heart hormones fueling a fire in fat. Adipocyte. 2013;2:104–108. doi: 10.4161/adip.22515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, Beckmann D, Paruzel P, Bertrand J, Redlich K, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nature medicine. 2015;21:1085–1090. doi: 10.1038/nm.3917. [DOI] [PubMed] [Google Scholar]
- Doroudgar S, Glembotski CC. The cardiokine story unfolds: ischemic stress-induced protein secretion in the heart. Trends Mol Med. 2011;17:207–214. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. The Journal of clinical investigation. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Streeper RS, Farese RV, Jr., Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A. 2006;103:15675–15680. doi: 10.1073/pnas.0607501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gomez-Ambrosi J, Moreno-Navarrete JM, Fruhbeck G, Martinez C, Idoate F, Salvador J, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. The Journal of clinical endocrinology and metabolism. 2009;94:237–245. doi: 10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- Ferron M, Lacombe J, Germain A, Oury F, Karsenty G. GGCX and VKORC1 inhibit osteocalcin endocrine functions. The Journal of cell biology. 2015;208:761–776. doi: 10.1083/jcb.201409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Shimizu Y. Fibroblast growth factor 23 as a phosphotropic hormone and beyond. J Bone Miner Metab. 2011;29:507–514. doi: 10.1007/s00774-011-0298-0. [DOI] [PubMed] [Google Scholar]
- Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149:671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. The Journal of clinical endocrinology and metabolism. 1985;60:736–739. doi: 10.1210/jcem-60-4-736. [DOI] [PubMed] [Google Scholar]
- Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo EX, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell metabolism. 2013;17:901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E, Yasoda A, Fujii T, Nakao K, Yamashita Y, Ueda-Sakane Y, Kanamoto N, Miura M, Arai H, Mukoyama M, et al. Increased Bone Turnover and Possible Accelerated Fracture Healing in a Murine Model With an Increased Circulating C-Type Natriuretic Peptide. Endocrinology. 2015;156:2518–2529. doi: 10.1210/en.2014-1801. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bassel-Duby R, Olson EN. Heart- and muscle-derived signaling system dependent on MED13 and Wingless controls obesity in Drosophila. Proc Natl Acad Sci U S A. 2014;111:9491–9496. doi: 10.1073/pnas.1409427111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Hauschka PV, Gallop PM. Properties and biosynthesis of a vitamin K-dependent calcium binding protein in bone. Federation proceedings. 1978;37:2615–2620. [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. The Journal of clinical investigation. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JM, Hawley WL, Chenoweth AI, Retan WJ, Diethelm AG. Renal transplantation in hypophosphatemia with vitamin D-resistant rickets. Arch Intern Med. 1974;134:549–552. [PubMed] [Google Scholar]
- Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes & development. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. The Journal of cell biology. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Osawa K, Yasoda A, Yamanaka S, Fujii T, Kondo E, Koyama N, Kanamoto N, Miura M, Kuwahara K, et al. The Local CNP/GC-B system in growth plate is responsible for physiological endochondral bone growth. Sci Rep. 2015;5:10554. doi: 10.1038/srep10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RC, Prickett TC, Espiner EA, Mackenzie WG, Duker AL, Ditro C, Zabel B, Hasegawa T, Kitoh H, Aylsworth AS, et al. C-type natriuretic peptide plasma levels are elevated in subjects with achondroplasia, hypochondroplasia, and thanatophoric dysplasia. The Journal of clinical endocrinology and metabolism. 2015;100:E355–359. doi: 10.1210/jc.2014-2814. [DOI] [PubMed] [Google Scholar]
- Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, Srinivas P, Chamouni A, Lugani F, Lejeune H, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. The Journal of clinical investigation. 2013a;123:2421–2433. doi: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, Huang YY, Lee H, Srinivas P, Gao XB, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013b;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature Reviews Endocrinology. 2012a;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature reviews Endocrinology. 2012b;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PloS one. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovarova O, Gogebakan O, Kloting N, Sparwasser A, Weickert MO, Haddad I, Nikiforova VJ, Bergmann A, Kruse M, Seltmann AC, et al. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? The Journal of clinical endocrinology and metabolism. 2012;97:E731–739. doi: 10.1210/jc.2011-2839. [DOI] [PubMed] [Google Scholar]
- Rai M, Demontis F. Systemic nutrient and stress signaling via myokines and myometabolites. Ann. Rev Physiol. 2016;78:6.1–6.23. doi: 10.1146/annurev-physiol-021115-105305. [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. The Journal of endocrinology. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabek OM, Nishimoto SK, Fraga D, Tejpal N, Ricordi C, Gaber AO. Osteocalcin Effect on Human beta-Cells Mass and Function. Endocrinology. 2015;156:3137–3146. doi: 10.1210/EN.2015-1143. [DOI] [PubMed] [Google Scholar]
- Sartori R, Gregorevic P, Sandri M. TGFbeta and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends in endocrinology and metabolism: TEM. 2014;25:464–471. doi: 10.1016/j.tem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Shimano M, Ouchi N, Walsh K. Cardiokines: recent progress in elucidating the cardiac secretome. Circulation. 2012;126:e327–332. doi: 10.1161/CIRCULATIONAHA.112.150656. [DOI] [PubMed] [Google Scholar]
- Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60. doi: 10.3389/fcell.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, Karsenty G. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. The Journal of clinical investigation. 2014a;124:1–13. doi: 10.1172/JCI72323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014b;63:1021–1031. doi: 10.2337/db13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Vidal-Puig A. NPs -- heart hormones that regulate brown fat? The Journal of clinical investigation. 2012;122:804–807. doi: 10.1172/JCI62595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Goto N, Katsuura G, Ebihara K, Inuzuka M, Ochi Y, Yamashita Y, Kusakabe T, Yasoda A, Satoh-Asahara N, Ariyasu H, et al. Intracerebroventricular administration of C-type natriuretic peptide suppresses food intake via activation of the melanocortin system in mice. Diabetes. 2013;62:1500–1504. doi: 10.2337/db12-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]