Abstract
IMPORTANCE
The American College of Cardiology and the American Heart Association (ACC/AHA) cholesterol treatment guidelines have wide-scale implications for treating adults without history of atherosclerotic cardiovascular disease (ASCVD) with statins.
OBJECTIVE
To estimate the cost-effectiveness of various 10-year ASCVD risk thresholds that could be used in the ACC/AHA cholesterol treatment guidelines.
DESIGN, SETTING, AND PARTICIPANTS
Microsimulation model, including lifetime time horizon, US societal perspective, 3% discount rate for costs, and health outcomes. In the model, hypothetical individuals from a representative US population aged 40 to 75 years received statin treatment, experienced ASCVD events, and died from ASCVD-related or non-ASCVD–related causes based on ASCVD natural history and statin treatment parameters. Data sources for model parameters included National Health and Nutrition Examination Surveys, large clinical trials and meta-analyses for statin benefits and treatment, and other published sources.
MAIN OUTCOMES AND MEASURES
Estimated ASCVD events prevented and incremental costs per quality-adjusted life-year (QALY) gained.
RESULTS
In the base-case scenario, the current ASCVD threshold of 7.5% or higher, which was estimated to be associated with 48% of adults treated with statins, had an incremental cost-effectiveness ratio (ICER) of $37 000/QALY compared with a 10% or higher threshold. More lenient ASCVD thresholds of 4.0% or higher (61% of adults treated) and 3.0% or higher (67% of adults treated) had ICERs of $81 000/QALY and $140 000/QALY, respectively. Shifting from a 7.5% or higher ASCVD risk threshold to a 3.0% or higher ASCVD risk threshold was estimated to be associated with an additional 161 560 cardiovascular disease events averted. Cost-effectiveness results were sensitive to changes in the disutility associated with taking a pill daily, statin price, and the risk of statin-induced diabetes. In probabilistic sensitivity analysis, there was a higher than 93% chance that the optimal ASCVD threshold was 5.0% or lower using a cost-effectiveness threshold of $100 000/QALY.
CONCLUSIONS AND RELEVANCE
In this microsimulation model of US adults aged 45 to 75 years, the current 10-year ASCVD risk threshold (≥7.5% risk threshold) used in the ACC/AHA cholesterol treatment guidelines has an acceptable cost-effectiveness profile (ICER, $37 000/QALY), but more lenient ASCVD thresholds would be optimal using cost-effectiveness thresholds of $100 000/QALY (≥4.0% risk threshold) or $150 000/QALY (≥3.0% risk threshold). The optimal ASCVD threshold was sensitive to patient preferences for taking a pill daily, changes to statin price, and the risk of statin-induced diabetes.
In November 2013 the American College of Cardiology and the American Heart Association (ACC/AHA) released new recommendations to guide statin treatment initiation for the primary prevention of cardiovascular disease.1 These guidelines were a departure from previous recommendations,2 most notably for deemphasizing low-density lipoprotein (LDL) cholesterol thresholds to focus on total atherosclerotic cardiovascular disease (ASCVD) risk, which is defined by new Pooled Cohort Equations.3 The ACC/AHA guidelines established 4 categories for statin treatment eligibility for adults aged 40 to 75 years, including 10-year ASCVD risk of 7.5% or higher. Based on the new ASCVD risk threshold, Pencina et al4 estimated that 8.2 million additional adults in the United States would be recommended for statin treatment compared with previous recommendations.
The expansion of statin treatment eligibility under ACC/AHA guidelines has been controversial.5 Critics have argued that the Pooled Cohort Equations used in the guidelines substantially overestimate risk, and that, when taken in conjunction with more lenient treatment thresholds, millions of adults in the United States would be exposed to unnecessary statin treatment costs and risks.6,7 Although statins are generally well tolerated, recent evidence suggests that statin treatment could increase the risk of incident diabetes.8
On the other hand, many experts have supported expanded statin treatment under ACC/AHA guidelines, citing evidence that statins are effective for reducing risk regardless of LDL cholesterol or total risk levels.2,9,10 Previous studies have shown that relatively lenient total risk treatment thresholds (10-year coronary heart disease or cardiovascular risk, ≈ 5%-10%) could be cost-effective, although the use of the Pooled Cohort Equations and the risk of statin-induced diabetes have not yet been assessed in cost-effectiveness analyses.11 Therefore, our study objective was to perform a cost-effectiveness analysis of the ACC/AHA guidelines to find the optimal value for the 10-year ASCVD risk threshold (keeping all other elements of the guidelines unchanged).
Methods
CVD Microsimulation Model
We developed a CVD microsimulation model to project the lifetime health outcomes and CVD–related costs of 1 million hypothetical adults (starting age, 40–75 years) in the United States. The model was populated by weighted sampling (with replacement) of individuals from the fasting data samples of the 2005–2006, 2007–2008, and 2009–2010 waves of the nationally representative National Health and Nutrition Examination Surveys (NHANES).12 All other model parameters were estimated from published sources. Table 1 lists the base-case model inputs varied in sensitivity analyses. All other model inputs are shown in the Supplement (eTable 1 and eTable 2 in the Supplement).
Table 1.
Cardiovascular Disease Simulation Model Variables Examined in Sensitivity Analyses
| Variable | Base-Case Valuea | Distributionb | Source |
|---|---|---|---|
| Statin Effectiveness and Risks | |||
| Relative risk, mean (95% CI) | |||
| CHD events | 0.75 (0.71–0.78) | Log normal | Baigent et al,13 2005 |
| Stroke events | 0.83 (0.76–0.87) | Log normal | Baigent et al,13 2005 |
| Patients with adverse event, mean %c | |||
| Mild | 4.7 | β | Zhang et al,14 2013 |
| Major | 0.006 | β | Zhang et al,14 2013 |
| Probability major adverse event is fatal, mean %c |
0.09 | β | Alsheikh-Ali et al,15 2005 |
| Statin-induced diabetes, OR (95% CI) | 1.09 (1.02–1.17) | Log normal | Sattar et al,8 2010 |
| Annual statin costs, 2013 US $ | |||
| Simvastatin, 20 mg | 11 | NA | Redbook et al,16 2014 |
| Market share, base-case value (alternative scenario analysis), %d |
38.5 (42.3) | NA | Jackevicius et al,17 2012 |
| Atorvastin, 20 mg | 110 | NA | Redbook et al,16 2014 |
| Market share, base-case value (alternative scenario analysis), %d |
52.5 (57.7) | NA | Jackevicius et al,17 2014 |
| Rosuvastatin, 20 mg | 2277 | NA | Redbook et al,16 2014 |
| Market share, base-case value (alternative scenario analysis), %d |
9.0 (0.0) | NA | Jackevicius et al,17 2014 |
| CVD costs (range), 2013 US $ | |||
| General practitioner screening visit | 75 (52–98) | γ | RBRVS18 |
| Cholesterol laboratory test | 35 (17–52) | γ | RBRVS18 |
| Acutec | |||
| Cardiac arrest | 19 271 | γ | O’Sullivan et al,19 2011 |
| Fatal myocardial infarction | 17 259 | γ | O’Sullivan et al,19 2011 |
| Nonfatal myocardial infarction | 62 200 | γ | O’Sullivan et al,19 2011 |
| Angina | 29 139 | γ | O’Sullivan et al,19 2011 |
| Fatal stroke | 10 647 | γ | O’Sullivan et al,19 2011 |
| Nonfatal stroke | 20 509 | γ | O’Sullivan et al,19 2011 |
| Coronary artery bypass grafting | 36 872 | γ | O’Sullivan et al,19 2011 |
| Percutaneous transluminal coronary angioplasty |
34 742 | γ | O’Sullivan et al,19 2011 |
| Post–first-year annual cost, $ | |||
| CHD | 3201 (2134–5336) | γ | Lee et al,20 2010 |
| Stroke | 2115 (1058–3173) | γ | Pignone et al,21 2006 |
| Non–Health Care Costs | |||
| Time per outpatient visit, minc | |||
| Travel | 35 | γ | Russell et al,22 2008 |
| Waiting | 42 | γ | Russell et al,22 2008 |
| Wage for adults aged >45 y (per hour), 2013 US $c |
14.40 | γ | Bureau of Labor Statistics,23 2013 |
| Utility Weights | |||
| Disease free | |||
| No statin treatment | 1.0 | β | Assumption |
| On statin treatment | 0.998 (0.991–1.0) | β | Gage et al,24 1996 Hutchins et al,25 2015 |
| Cardiac arrestc | 0.808 | β | Sullivan et al,26 2006 |
| Myocardial infarctionc | 0.778 | β | Sullivan et al,26 2006 |
| Anginac | 0.768 | β | Sullivan et al,26 2006 |
| Strokec | 0.768 | β | Sullivan et al,26 2006 |
| Statin-induced diabetesc | 0.800 | β | Sullivan et al,26 2006 |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; NA, not applicable; OR, odds ratio; RBRVS, resource-based relative value scale.
Upper and lower bounds used in sensitivity analyses were based on 95% CIs unless noted otherwise in the Methods section.
β is a continuous statistical distribution bounded between 0 and 1. γ is a continuous statistical distribution with a lower bound value of 0.
A base-case value of ±15% was used to create upper and lower bounds used in sensitivity analyses because 95% CIs or other values were not available from source data.
Market share is defined as the percentage of statin users assumed to be taking the specified drug.
This structure was based on a previously published CVD model27–29 (eMethods, eFigure 1A, and eFigure 1B in the Supplement). Model input parameters were estimated based on extensive literature reviews and model calibration methods.30 Individuals without history of CVD started in the “disease-free” health state. In this health state, individuals were assumed to receive cardiovascular risk assessment as recommended by the ACC/AHA–every 5 years at a routine general physician visit until they were characterized as eligible for statin treatment, experienced a coronary heart disease or stroke event, or died. The ACC/AHA guidelines use an ASCVD risk threshold of 7.5% or higher 10-year risk as part of their statin eligibility criteria; we varied this threshold in our cost-effectiveness analysis, keeping the other components of the statin treatment algorithm unchanged from how they were out-lined in the ACC/AHA guidelines.
Transitions from the disease-free state (with or without treatment) to either coronary heart disease or stroke events were based on total risk equations derived from the Framingham Study.31,32 We chose to use calibrated Framing-ham functions, and not the Pooled Cohort Equations, to guide underlying risk in the model so that we could separately predict coronary heart disease and stroke, which have different implications for mortality, quality of life, and costs. Framingham risk factors (age, smoking status, diabetes, systolic blood pressure, and total and high-density lipoprotein cholesterol) update every year for each individual in the model; these updates were based on regressions derived from historical NHANES data.33 Non-CVD–based mortality was informed by age- and sex-specific US life tables.34 Acute (ie, within 1 year of experiencing the event) and post–first-year (ie, all years beyond the first year of the event) mortality were estimated separately.35 Repeat and subsequent coronary heart disease and stroke events were tracked for each individual and affected mortality, quality of life, and costs accordingly. We decreased acute CVD mortality by 20% in sensitivity analyses to explore the statistical effect that continued declines in CVD mortality could have on our cost-effectiveness results.36
Model Calibration and Validation
We calibrated our model-generated CVD incidence results to observed rates from large US cohort studies. Specifically, we calibrated our model to 16 age- and sex-specific coronary heart disease and stroke incidence targets from the Framingham Offspring Study (observation years, 1980–2003) and Atherosclerosis Risk in Communities (ARIC; observation years, 1987–2001) cohorts.37–39 We defined an incidence target as being met if the model-based incidence fell within the interval between the estimates from these 2 cohorts, which had more-inclusive (Framingham Offspring Study) and less-inclusive (ARIC) definitions of composite CVD outcomes compared with our model. We validated our calibrated model by comparing model-predicted overall and CVD mortality against observed and predicted mortality data from the NHANES III population (baseline data collected, 1988–1994; cause-specific mortality data available through 2006).40 Model-predicted CVD mortality was defined by the range of acute postevent mortality (ie, any death within the first year of the event) and any postevent death (ie, any time after the event) due to the separate estimation of acute and post–first-year mortality in CVD health states in the model. The calibration and validation methods are described in more detail in the eMethods in the Supplement.
Statin Treatment Parameters and Assumptions
If patients in the disease-free state received statin treatment, their coronary heart disease and stroke risks were multiplied by relative risk estimates of 0.75 and 0.81, respectively.13 We also used an alternative statin effectiveness scenario in which the relative risks for coronary heart disease and stroke were based on an individual’s LDL level; this sensitivity analysis is described in more detail in the eMethods in the Supplement.41 Mild (myalgia or myopathy) and major (rhabdomyolysis) adverse event rates (4.7% and 0.006%, respectively) were based on a large retrospective cohort study of statin discontinuation.14 Statins have been found to cause a small but significant increased risk of incident type 2 diabetes (odds ratio, 1.09).8 Because the short-term effect of statin-induced diabetes on CVD is already reflected in the hazard ratios for statins on CVD events, we only included the quality of life (utility decrement, −0.20)26 and cost implications (additional annual costs, $4445)42 of statin-induced diabetes in our simulation model. Individuals with statin-induced diabetes in the disease-free state would also be at higher long-term risk for CVD because presence of diabetes contributes to the underlying coronary heart disease and stroke risk equations used in the model.31,32 Statin compliance was also derived from published estimates, with real-world treatment rates of 67% in the first year of statin initiation, 53% in the second year, and 50% in the third and all subsequent years.43,44 Treatment effectiveness, adverse event rates, and costs were both reduced proportionately with compliance rates; these adjustments are described in more detail in the eMethods in the Supplement.
Costs and Utilities
Like postevent mortality, CVD event costs were estimated separately for acute and post–first-year events. Base-case event-based cost values were estimated from a recent analysis of a large, managed care population in the United States, and Medicare reimbursement rates were used in sensitivity analyses.19,45 The base-case statin cost ($267/y) was estimated as the weighted average of the lowest Red Book wholesale acquisition costs for generic 20-mg simvastatin ($11/y, 38.5% market share), generic 20-mg atorvastatin ($110/y, 52.5% market share), and branded rosuvastatin ($2277/y, 9.0% market share).16,17 A generic price scenario was performed using market shares for simvastatin (42.3%) and atorvastatin (57.7%) only, which resulted in a weighted statin cost of $68 per year. Primary CVD screening and statin-related, adverse event costs were composed of general practitioner visits or laboratory fees. Non–health care costs included travel and wait times for outpatient visits.22 Quality-of-life (ie, utility) decrements were applied to each year spent in CVD-event states and were based on EuroQOL 5 Dimensions (EQ-5D) questionnaire estimates from the Medical Expenditure Panel Survey.26 Event-specific utilities were multiplied by time spent in each state to calculate quality-adjusted life-years (QALYs). We applied a small annual utility decrement for each year spent receiving statin therapy based on the median standard gamble value from stroke patients for taking aspirin therapy (0.002).24 In sensitivity analyses, we used the mean (0.009) and median (no disutility) standard gamble values from a cross-sectional Internet survey on taking pills for CVD prevention.25 Costs were considered from the societal perspective in 2013 US dollars.
Base-Case Cost-effectiveness Analysis
We projected the lifetime discounted CVD–related health care costs and QALYs accrued under 12 variations of the ACC/AHA guidelines, which did not change except for the ASCVD risk treatment threshold. Specifically, we evaluated the following ASCVD risk treatment thresholds: ≥30%, ≥20%, ≥15%, ≥10%, ≥7.5%, ≥5%, ≥4%, ≥3%, ≥2%, and ≥1%, in addition to treating all patients and no ASCVD risk–based treatment strategies. Incremental cost-effectiveness ratios (ICERs) were calculated per conventional cost-effectiveness analysis [CEA] rules; inefficient strategies were ruled out by strong dominance (higher incremental costs and lower incremental QALYs) or weak dominance (lower QALYs but larger ICER than a more expensive option).46 Costs and QALYs were each discounted at 3% as recommended by the US Panel on Cost-Effectiveness in Health and Medicine.47 We used cost-effectiveness thresholds of $50 000 per QALY, $100 000 per QALY, and $150 000 per QALY to determine the optimal screening strategy in base-case and sensitivity analyses. The $150 000 per QALY threshold has been justified in part by empirical evidence on surveys on willingness to pay for health, revealed preference studies, and general increases on health care spending, in addition to the World Health Organization recognizing 3 times the gross domestic product per capita as an upper threshold.48
Sensitivity Analyses
We varied values for all variables (or groups of related variables) through plausible ranges, or used alternative values, to assess the robustness of our CEA results to changes in these input parameters. In addition to acute CVD mortality, statin effectiveness, and the disutility with the act of taking a pill daily, we paid particular attention to the effect of statin price and statin-induced diabetes on the optimal ASCVD treatment threshold. Overall model uncertainty was assessed in a probabilistic sensitivity analysis (PSA). In the PSA, 1000 random values for key model parameters were drawn from prespecified probability distributions (Table 1). Distributions were assigned based on data characteristics and ability to account for distribution skewness. The microsimulation model was programmed in Visual C++ 2005 (Microsoft).
Results
Model Calibration and Validation
Prior to model calibration, model results for CVD incidence fell within 8 of the 16 age- and sex-specific incidence observed ranges. After model calibration, 13 of the 16 incidence targets were met. In model validation analyses, model-predicted total mortality results were with in 3% for 5 of 6 targets with the exception of 10-year total mortality compared with the NHANES III, which had a 7.1% deviation (model predicted 10-year death for 8.1% of the population compared with 7.6% in the NHANES III population). CVD mortality observed in the NHANES III cohort fell within the model-predicted ranges at 5 and 10 years. Life expectancy for the NHANES III population was 77.8 years; model-predicted life expectancy for the same population was 77.7 years. The Supplement shows model calibration and validation results in more detail (eFigures 2–5 and eTable 3 in the Supplement).
Clinical Results
Under an ACC/AHA cholesterol treatment strategy that does not include an ASCVD risk–based criterion, an estimated 8% of adults would still be eligible for statins through other criteria (history of CVD or diabetes or elevated LDL cholesterol); the recommended ASCVD threshold of 7.5% or higher was associated with an estimate that 48% of adults would be eligible for statin treatment. Strategies with higher ASCVD thresholds were associated with estimates of fewer statin-induced diabetes cases but more CVD events. When applied to the115.4 million adults aged 40 to 75 years, shifting from the 7.5% or higher ASCVD threshold to the 3.0% or higher ASCVD threshold was associated with an estimated additional 161 560 CVD events averted. QALY swere maximized using an ASCVD thresh-old of 2.0% or higher even though the 1.0% or higher threshold and treat all patients strategies were projected to avert more CVD events. This can be explained as the net effect of statins reducing cardiovascular risk but increasing adverse event risks and the disutility of daily medication use. Table 2 shows the base-case clinical and cost-effectiveness results.
Table 2.
Individual Lifetime Clinical Outcomes, QALYs, Costs, and ICERs for Base-Case Analysis
| ACC/AHA ASCVD Risk Threshold, % |
Adults Statin Eligible, % |
Statin-Induced Diabetes Casesa |
CVD Eventsa,b | Life Expectancy, y | QALYsc | Costs, 2013 US $c |
ICER (US $/QALY) |
|---|---|---|---|---|---|---|---|
| No ASCVD threshold |
8 | 0.0019 | 0.4493 | 81.237 | 17.276 | 21 310 | 1 [Reference] |
| ≥30.0 | 34 | 0.0030 | 0.4437 | 81.265 | 17.287 | 21 649 | Extended dominanced |
| ≥20.0 | 36 | 0.0039 | 0.4405 | 81.293 | 17.299 | 21 898 | Extended dominanced |
| ≥15.0 | 39 | 0.0045 | 0.4384 | 81.315 | 17.309 | 22 109 | 24 000/QALY |
| ≥10.0 | 44 | 0.0055 | 0.4365 | 81.341 | 17.320 | 22 455 | 30 000/QALY |
| ≥7.5e | 48 | 0.0062 | 0.4353 | 81.356 | 17.327 | 22 696 | 37 000/QALY |
| ≥5.0 | 57 | 0.0072 | 0.4344 | 81.371 | 17.333 | 23 039 | 57 000/QALY |
| ≥4.0 | 61 | 0.0076 | 0.4340 | 81.377 | 17.335 | 23 200 | 81 000/QALY |
| ≥3.0 | 67 | 0.0080 | 0.4337 | 81.382 | 17.336 | 23 406 | 140 000/QALY |
| ≥2.0 | 75 | 0.0085 | 0.4334 | 81.386 | 17.337 | 23 656 | 830 000/QALY |
| ≥1.0 | 87 | 0.0091 | 0.4333 | 81.389 | 17.336 | 23 952 | Strong dominancef |
| Treat all adults with statins |
100 | 0.0097 | 0.4332 | 81.391 | 17.334 | 24 225 | Strong dominancef |
Abbreviations: ACC/AHA, American College of Cardiology and American Heart Association; ASCVD, atherosclerotic cardiovascular disease; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years.
Values indicate proportion of all adults that experience this outcome at some point in their lifetime. Results for statin-induced diabetes cases and CVD events correspond to the average lifetime risk of experiencing each of these outcomes for individuals in the model population.
Defined as nonfatal or fatal: myocardial infarction, angina, cardiac arrest, or stroke.
Discounted at 3%.
Extended dominance: other, more effective strategies have lower cost-effectiveness ratios than this strategy.
ASCVD risk threshold used in 2013 ACC/AHA guidelines.
Dominated: other strategies are less costly and more effective than this strategy. Based on recommendations, strategies that are dominated by either mechanism (strong dominance or extended dominance) are eliminated from further consideration in a cost-effectiveness analysis.
Cost-effectiveness Analysis
The base-case ICER for the recommended ASCVD treatment threshold of 7.5% or higher was $37 000 per QALY (compared with the ≥ 10% ASCVD risk threshold), which is below the commonly used cost-effectiveness thresholds of $50 000 to $150 000 per QALY.48 Per conventional incremental CEA rules, more lenient ASCVD thresholds of 4.0% or higher (61% of adults treated; ICER, $81 000/QALY) or 3.0% or higher (67% of adults treated; ICER, $140 000/QALY) would be considered optimal using cost-effectiveness thresholds $100 000/QALY or $150 000/QALY, respectively.46,47
Table 3 shows the cost-effectiveness results using generic statin prices ($68/y). ASCVD thresholds of 4.0% or higher and 3.0% or higher were optimal, using cost-effectiveness thresholds $50 000/QALY or $100 000/QALY, respectively. The 2% or higher strategy had ICERs that would not be considered cost-effective (ICERs: $830 000/QALY for blended statin prices and $460 000/QALY for generic statin prices). Figure 1 and eTable 4 in the Supplement show the optimal ASCVD thresholds as a function of statin price using cost-effectiveness thresholds of $50 000 per QALY, $100 000 per QALY, and $150 000 per QALY (eTable 4 in the Supplement). The disutility associated with taking a pill daily also affected the optimal ASCVD threshold. Applying a disutility of 0.009 was projected to result in optimal ASCVD thresholds ranging from 7.5% or higher to 15.0% or higher for cost-effectiveness thresholds of $50 000 to $150,000 per QALY; assuming no disutility had corresponding optimal ASCVD thresholds ranging from 3.0% or higher to 5.0% or higher. An odds ratio of statin-induced diabetes of 1.50 or higher was projected to result in an optimal ASCVD threshold of 15% or higher (ie, 39% or less of adults receiving statins) for a cost-effectiveness threshold of $100 000 per QALY (eTable 5 in the Supplement). The upper and lower 95% CI bounds for this odds ratio (1.17 for the upper bound and 1.02 for the lower bound) resulted in ICERs of $58 000 per QALY and $25 000 per QALY for the 7.5% or higher ASCVD threshold, respectively. Sensitivity analysis results for reduced acute CVD mortality and statin effectiveness assumptions were similar to base-case findings (eTable 6 in the Supplement).
Table 3.
Individual Lifetime Clinical Outcomes, QALYs, Costs, and ICERs for Generic Statin Cost ($68/y) Scenario
| ACC/AHA ASCVD Risk Threshold, % |
Adults Statin Eligible, % |
Statin-Induced Diabetes Casesa |
CVD Eventsa,b | Life Expectancy, y | QALYsc | Costs, 2013 US $c |
ICER (US $/QALY) |
|---|---|---|---|---|---|---|---|
| No ASCVD threshold |
8 | 0.0019 | 0.4493 | 81.237 | 17.276 | 20 695 | 1 [Reference] |
| ≥30.0 | 34 | 0.0030 | 0.4437 | 81.265 | 17.287 | 20 808 | Extended dominanced |
| ≥20.0 | 36 | 0.0039 | 0.4405 | 81.293 | 17.299 | 20 878 | Extended dominanced |
| ≥15.0 | 39 | 0.0045 | 0.4384 | 81.315 | 17.309 | 20 942 | 7400/QALY |
| ≥10.0 | 44 | 0.0055 | 0.4365 | 81.341 | 17.320 | 21 073 | 12 000/QALY |
| ≥7.5e | 48 | 0.0062 | 0.4353 | 81.356 | 17.327 | 21 169 | 15 000/QALY |
| ≥5.0 | 57 | 0.0072 | 0.4344 | 81.371 | 17.333 | 21 330 | 27 000/QALY |
| ≥4.0 | 61 | 0.0076 | 0.4340 | 81.377 | 17.335 | 21 406 | 38 000/QALY |
| ≥3.0 | 67 | 0.0080 | 0.4337 | 81.382 | 17.336 | 21 514 | 72 000/QALY |
| ≥2.0 | 75 | 0.0085 | 0.4334 | 81.386 | 17.337 | 21 651 | 460 000/QALY |
| ≥1.0 | 87 | 0.0091 | 0.4333 | 81.389 | 17.336 | 21 819 | Strong dominancef |
| Treat all adults with statins |
100 | 0.0097 | 0.4332 | 81.391 | 17.334 | 21 986 | Strong dominancef |
Abbreviations: ACC/AHA, The American College of Cardiology and the American Heart Association; ASCVD, atherosclerotic cardiovascular disease; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years.
Values indicate proportion of all adults that experience this outcome at some point in their lifetime. Results for statin-induced diabetes cases and CVD events correspond to the average lifetime risk of experiencing each of these outcomes for individuals in the model population.
Defined as nonfatal or fatal: myocardial infarction, angina, cardiac arrest, or stroke.
Discounted at 3%.
Extended dominance: other, more effective strategies have lower cost-effectiveness ratios than this strategy.
ASCVD risk threshold used in 2013 ACC/AHA guidelines.
Dominated: other strategies are less costly and more effective than this strategy. Based on recommendations, strategies that are dominated by either mechanism (strong dominance or extended dominance) are eliminated from further consideration in a cost-effectiveness analysis.
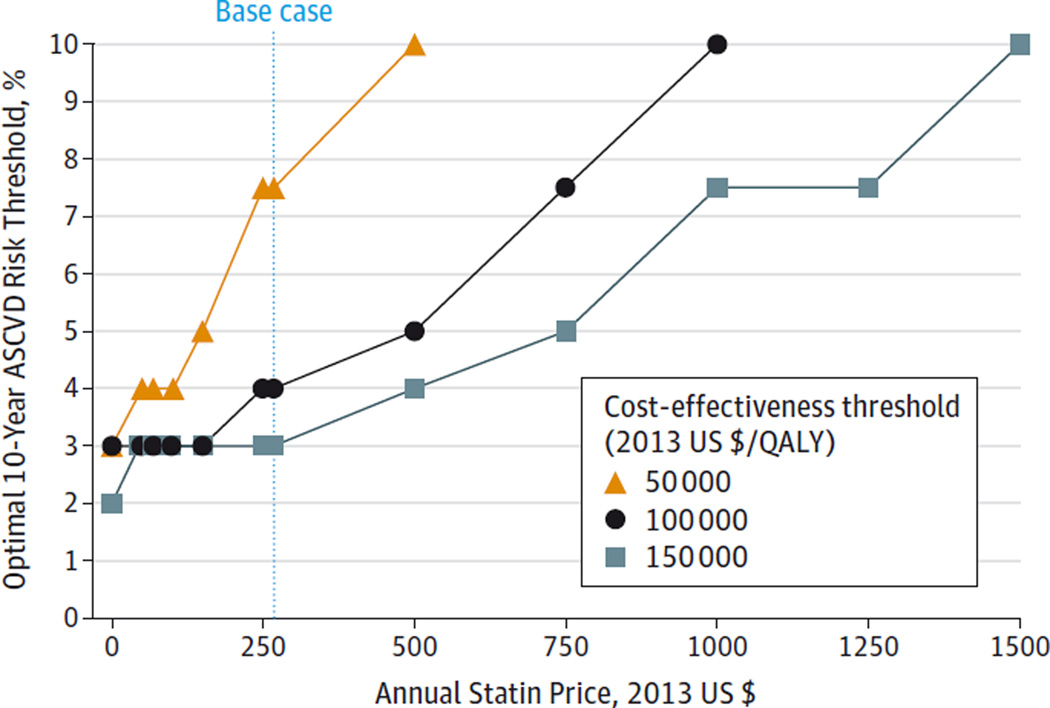
Figure 1. One-Way Sensitivity Analysis Showing the Optimal ASCVD Threshold as a function of Statin Price.
ASCVD indicates atherosclerotic cardiovascular disease; QALY, quality-adjusted life-years. The optimal treatment threshold (using a cost-effectiveness threshold of $100 000/QALY) changes from ≥3.0% to ≥10.0% as statin price increases from $150/y to $1000/y (base-case value is $268/y). Optimal strategies are also shown for cost-effectiveness thresholds of $50 000/QALY and $150 000/QALY. No ASCVD treatment threshold was cost-effective for statin prices greater than $500/y using a cost-effectiveness threshold of $50 000/QALY. No ASCVD treatment threshold was cost-effective for statin prices greater than $1000/y using a cost-effectiveness threshold of $100 000/QALY.
The PSA results are shown in cost-effectiveness acceptability curves (Figure 2), which show the probabilities that each ASCVD threshold would be considered cost-effective from a societal standpoint for various willingness-to-pay (ie, cost-effectiveness) thresholds. Panel A shows that the current ASCVD threshold used in the ACC/AHA guidelines (≥7.5%) was optimal in 52% of PSA iterations using a cost-effectiveness threshold of $50 000 per QALY. In other words, the 7.5% or higher ASCVD threshold was projected to be optimal (assuming a societal willingness-to-pay value of $50 000/QALY) in 520 of the 1000 PSA iterations. Panel B shows that there was a higher than 99% chance that the optimal ASCVD threshold would be 7.5% or lower (ie, at least 48% of adults receiving statins) at the commonly used $100 000 per QALY cost-effectiveness threshold; more than 86% of PSA iterations had an optimal ASCVD threshold of 7.5% or lower using a cost-effectiveness threshold of $50 000 per QALY. Panel B also shows that 93% of PSA iterations had optimal ASCVD thresholds of 5% or lower at $100 000 per QALY; 33% and 61% of PSA iterations had optimal ASCVD thresholds of 3% or lower at $100 000 per QALY and $150 000 per QALY, respectively.
Figure 2. Cost-effectiveness Acceptability Curves for the Probabilistic Sensitivity Analysis.
ASCVD indicates atherosclerotic cardiovascular disease. The y-axis shows the likelihood that strategies would be considered cost-effective for a given cost-effectiveness (willingness-to-pay) threshold. Panel A shows the probability of a given ASCVD threshold being optimal; the ASCVD treatment threshold of ≥30% and the treat all patients with statins strategies were not optimal in any probabilistic sensitivity analysis iterations. Panel B shows the probability that ASCVD thresholds of 7.5% or lower, 5.0% or lower, and 3.0% or lower were optimal.
Discussion
Our model-based analyses suggest that the health benefits associated with the 10-year ASCVD risk threshold of 7.5% or higher used in the ACC-AHA guidelines are worth the additional costs required to achieve these health gains (with an ICER lower than the conservative $50 000/QALY threshold), and importantly, that more lenient ASCVD risk thresholds of 4.0% or higher or 3.0% or higher represent cost-effective options based on commonly used cost-effectiveness thresholds of $100 000 per QALY and 150 000 per QALY, respectively. Shifting from the 7.5% or higher threshold to 3.0% or higher to 4.0% or higher was associated with an estimated additional 125 000 to 160 000 CVD events averted.
The decision to initiate statin treatment for adults without CVD should ultimately be informed by both evidence-based policies and patient preferences.9Individuals might have legitimate personal reasons to avoid taking statin medications despite meeting ASCVD risk thresholds.49 Our sensitivity analysis around the disutility from taking a pill daily supports this intuition. Although median values for this disutility (0.998–1.0) were estimated to result in lenient projected optimal thresholds (≥3%-≥7.5%), the mean standard gamble value (0.991) from the survey by Hutchins et al25 was associated with stricter projected optimal thresholds (≥7.5%-≥15.0%). In general, we found that the projected optimal treatment threshold did change based on the cost-effectiveness threshold used (ranging from 3.0%-7.5% in the base-case analysis), but the optimal ASCVD threshold in this sensitivity analysis only exceeded 7.5% with the most conservative (and arguably outdated48) threshold of $50 000 per QALY coupled with the lower bound for utility while taking statin treatment (0.991).
The cost-effectiveness of treating such a large proportion of the population with statins was also estimated to be sensitive to statin price. In our base-case analyses we used a blend of prices for generic and branded (market share, 9.0%), assuming that patients using branded drugs did so for medical reasons. Assuming generic statin prices only, we projected that it would be cost-effective to treat up to 61% to 67% of adults with statins. Previous cost-effectiveness studies have found that the price of statins is a key driver of cost-effectiveness results.11 Specifically, Prosser et al50 used the Coronary Heart Disease Policy model to find that statin treatment was not cost-effective for primary prevention in the year 2000 with branded pravastatin prices; later, Pletcher et al51 and Lazar et al52 used versions of the same model to find statin treatment would be cost-effective in primary prevention with fairly lenient risk thresholds (10-year coronary heart disease [CHD] risks, 0%-10% [depending on presence of other risk factors]) assuming generic statin prices. Our model results are consistent with these findings. Future guidelines should make treatment recommendations with drug prices in mind.
Our statin-induced diabetes sensitivity analysis demonstrates the potential importance of accurately measuring the adverse effects of statin treatment. Findings from the JUPITER trial53 suggested that the risk of rosuvastatin-induced diabetes was only statistically significant for individuals already at high risk for diabetes; if additional studies for atorvastatin and simvastatin can further identify and quantify individual-specific factors that influence this risk, then individualized statin treatment guidelines can alter treatment recommendations accordingly.
There have been conflicting reports on how well the Pooled Cohort Equations used in the ACC/AHA guidelines predict CVD risk.6,54 We projected that using ASCVD thresholds would result in cost-effective cardiovascular prevention strategies; whether this was in spite of or because of the Pooled Cohort Equations’ ability to discriminate CVD remains an open question. Our analysis focused on evaluating the Pooled Cohort Equations as a screening tool only. We used calibrated CHD- and stroke-specific risk functions derived from the Framingham Study to predict underlying CVD risk in our simulation model, and found that our model performed well on internal and external validity assessments. Calibration and validation are important criteria for assessing the quality and usefulness of disease models,55 but few CHD policy models have been calibrated or validated.56
There are several limitations of our study that should be noted. First, our calibration and validation data sources did not go beyond the year 2006, and acute CVD mortality has been declining over the past several decades.36,57 We explored the statistical effect that decreased acute CVD mortality would have in a sensitivity analysis and found that our cost-effectiveness results were robust to this change, although the true values for these inputs in future years cannot be known with certainty in the present. Second, we extrapolated statin benefit for patients receiving treatment over the course of their lifetime, which is a commonly used assumption in CVD modeling studies.11 We did, however, assume long-term compliance rates of 50% and reduced treatment effects accordingly. Third, we did not evaluate age-or sex-specific treatment guidelines, even though a previous modeling study has found that optimal treatment thresholds vary according to these characteristics.43 Finally, we evaluated 12 treatment thresholds, although it is probable that there is a true optimal threshold that lies somewhere between the strategies included in our study. We decided to limit our analysis in these ways to reduce the complexity of having age- and sex-specific thresholds with more precision.
Conclusions
In this microsimulation model of US adults aged 45 to 75 years, the current 10-year ASCVD risk threshold (≥7.5% risk threshold) used in the ACC/AHA cholesterol treatment guidelines has an acceptable cost-effectiveness profile (ICER, $37 000/QALY), but more lenient ASCVD thresholds would be optimal using cost-effectiveness thresholds of $100 000/QALY (≥4.0% risk threshold) or $150 000/QALY (≥3.0% risk threshold). The optimal ASCVD threshold was sensitive to patient preferences for taking a pill daily, changes to statin price, and the risk of statin-induced diabetes.
Supplementary Material
Acknowledgments
Funding/Support: This work is supported by grant 5R01HL104284-03 to the Harvard School of Public Health from the National Heart, Lung, and Blood Institute (Dr Gaziano).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Dr Pandya reports receiving grant funding from the National Institutes of Health (NIH) and the American Heart Association. Dr Weinstein reports receiving grant funding from the NIH and personal fees from Optuminsight. Dr Gaziano reports receiving grant funding from the NIH.
Glossary
- ASCVD
atherosclerotic cardiovascular disease
- CEA
cost-effectiveness analysis
- ICER
incremental cost-effectiveness ratio
- LDL
low-density lipoprotein
- PSA
probabilistic sensitivity analysis
- QALY
quality-adjusted life-years
Footnotes
Supplemental content at jama.com
Author Contributions: Drs Pandya and Gaziano had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pandya, Weinstein, Gaziano.
Acquisition, analysis, or interpretation of data: Pandya, Sy, Cho, Weinstein, Gaziano.
Drafting of the manuscript: Pandya, Cho, Gaziano.
Critical revision of the manuscript for important intellectual content: Pandya, Sy, Weinstein, Gaziano.
Statistical analysis: Pandya, Sy, Weinstein, Gaziano.
Obtained funding: Gaziano.
Administrative, technical, or material support: Cho, Weinstein, Gaziano.
Study supervision: Pandya, Gaziano.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
Correction: This article was corrected for incorrect data in the Abstract and Conclusion on August 10, 2015.
REFERENCES
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Weiss NS. 2013 ACC/AHA guideline on the treatment of blood cholesterol: a fresh interpretation of old evidence. JAMA. 2014;311(5):461–462. doi: 10.1001/jama.2013.284203. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pencina MJ, Navar-Boggan AM, D’Agostino RBSr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 5.Guallar E, Laine C. Controversy over clinical guidelines: listen to the evidence, not the noise. Ann Intern Med. 2014;160(5):361–362. doi: 10.7326/M14-0112. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382(9907):1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 7.Abramson JD, Redberg RF. [Accessed June 9, 2015];Don't give more patients statins. http://www.nytimes.com/2013/11/14/opinion/dont-give-more-patients-statins.html?_r=0.
- 8.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 9.Krumholz HM. The new cholesterol and blood pressure guidelines: perspective on the path forward. JAMA. 2014;311(14):1403–1405. doi: 10.1001/jama.2014.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson JG. Accumulating evidence for statins in primary prevention. JAMA. 2013;310(22):2405–2406. doi: 10.1001/jama.2013.281355. [DOI] [PubMed] [Google Scholar]
- 11.Deaño RC, Pandya A, Jones EC, Borden WB. A look at statin cost-effectiveness in view of the 2013 ACC/AHA cholesterol management guidelines. Curr Atheroscler Rep. 2014;16(9):438. doi: 10.1007/s11883-014-0438-9. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 13.Baigent C, Keech A, Kearney PM Cholesterol Treatment Trialists’ (CTT) Collaborators et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051–3057. doi: 10.1161/CIRCULATIONAHA.105.555482. [DOI] [PubMed] [Google Scholar]
- 16.Thomson Healthcare. Red Book. Montvale, NJ: Thomson PDR; 2014. [Google Scholar]
- 17.Jackevicius CA, Chou MM, Ross JS, Shah ND, Krumholz HM. Generic atorvastatin and health care costs. N Engl J Med. 2012;366(3):201–204. doi: 10.1056/NEJMp1113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SL, Fischoff R, Klemp T American Medical Association. Medicare RBRVS: The Physicians’ Guide, 2011. Chicago, IL: American Medical Association; 2011. [Google Scholar]
- 19.O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the United States. Pharmacoeconomics. 2011;29(8):693–704. doi: 10.2165/11584620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Lee KK, Cipriano LE, Owens DK, Go AS, Hlatky MA. Cost-effectiveness of using high-sensitivity C-reactive protein to identify intermediate- and low-cardiovascular–risk individuals for statin therapy. Circulation. 2010;122(15):1478–1487. doi: 10.1161/CIRCULATIONAHA.110.947960. [DOI] [PubMed] [Google Scholar]
- 21.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. 2006;144(5):326–336. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 22.Russell LB, Ibuka Y, Carr D. How much time do patients spend on outpatient visits?: the American time use survey. Patient. 2008;1(3):211–222. doi: 10.2165/1312067-200801030-00008. [DOI] [PubMed] [Google Scholar]
- 23.US Bureau of Labor Statistics. [Accessed June 9, 2015];Highlights of women’s earnings in 2013. http://www.bls.gov/opub/reports/cps/highlights-of-womens-earnings-in-2013.pdf.
- 24.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–1836. [PubMed] [Google Scholar]
- 25.Hutchins R, Viera AJ, Sheridan SL, Pignone MP. Quantifying the utility of taking pills for cardiovascular prevention. Circ Cardiovasc Qual Outcomes. 2015;8(2):155–163. doi: 10.1161/CIRCOUTCOMES.114.001240. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandya A, Weinstein MC, Salomon JA, Cutler D, Gaziano TA. Who needs laboratories and who needs statins?: comparative and cost-effectiveness analyses of nonlaboratory-based, laboratory-based, and staged primary cardiovascular disease screening guidelines. Circ Cardiovasc Qual Outcomes. 2014;7(1):25–32. doi: 10.1161/CIRCOUTCOMES.113.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368(9536):679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaziano TA, Steyn K, Cohen DJ, Weinstein MC, Opie LH. Cost-effectiveness analysis of hypertension guidelines in South Africa: absolute risk vs blood pressure level. Circulation. 2005;112(23):3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 30.Vanni T, Karnon J, Madan J, et al. Calibrating models in economic evaluation: a 7-step approach. Pharmacoeconomics. 2011;29(1):35–49. doi: 10.2165/11584600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 pt 2):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 32.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 33.Pandya A, Gaziano TA, Weinstein MC, Cutler D. More Americans living longer with cardiovascular disease will increase costs while lowering quality of life. Health Aff (Millwood) 2013;32(10):1706–1714. doi: 10.1377/hlthaff.2013.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heron M. Deaths: Leading Causes for 2006. National Vital Statistics Reports. Vol. 58. Hyattsville, MD: National Center for Health Statistics; 2010. [Google Scholar]
- 35.Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–540. doi: 10.1161/CIRCOUTCOMES.111.964700. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Roger VL, Dunlay SM, Go AS, Rosamond WD. Challenges of ascertaining national trends in the incidence of coronary heart disease in the United States. J Am Heart Assoc. 2014;3(6):e001097. doi: 10.1161/JAHA.114.001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial 2 years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 38.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: Design and preliminary data. Prev Med. 1975;4(4):518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 39.National Heart Lung and Blood Institute (NHLBI) [Accessed June 9, 2015];Incidence and prevalence: 2006 chart book on cardiovascular and lung diseases. http://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/06_ip_chtbk.pdf.
- 40.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 41.van Kempen BJ, Ferket BS, Hofman A, Spronk S, Steyerberg E, Hunink MG. Do different methods of modeling statin treatment effectiveness influence the optimal decision? Med Decis Making. 2012;32(3):507–516. doi: 10.1177/0272989X12439754. [DOI] [PubMed] [Google Scholar]
- 42.Agency for Healthcare Research and Quality (AHRQ) [Accessed January 23, 2015];Medical expenditure panel survey. http://meps.ahrq.gov/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-036. [PubMed]
- 43.Greving JP, Visseren FL, de Wit GA, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? cost-effectiveness analysis. BMJ. 2011;342:d1672. doi: 10.1136/bmj.d1672. [DOI] [PubMed] [Google Scholar]
- 44.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 45.Optum. DRG Expert: A Comprehensive Guidebook to the DRG Classification System. Salt Lake City, UT: OPTUMInsight; 2014. [Google Scholar]
- 46.Hunink MM, Weinstein MC, Wittenberg E, et al. Decision Making in Health and Medicine: Integrating Evidence and Values. 2nd. Cambridge, UK: Cambridge University Press; 2014. [Google Scholar]
- 47.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 48.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50 000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum L. Beyond belief–how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183–187. doi: 10.1056/NEJMms1409015. [DOI] [PubMed] [Google Scholar]
- 50.Prosser LA, Stinnett AA, Goldman PA, et al. Cost-effectiveness of cholesterol-lowering therapies according to selected patient characteristics. Ann Intern Med. 2000;132(10):769–779. doi: 10.7326/0003-4819-132-10-200005160-00002. [DOI] [PubMed] [Google Scholar]
- 51.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150(4):243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 52.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124(2):146–153. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM. The JUPITER trial: results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes. 2009;2(3):279–285. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 54.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 56.Unal B, Capewell S, Critchley JA. Coronary heart disease policy models: a systematic review. BMC Public Health. 2006;6:213. doi: 10.1186/1471-2458-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coles AH, Fisher KA, Darling C, et al. Recent trends in postdischarge mortality among patients with an initial acute myocardial infarction. Am J Cardiol. 2012;110(8):1073–1077. doi: 10.1016/j.amjcard.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.