Abstract
It is widely accepted that dietary fats and chronic inflammation are risk factors for developing colorectal cancer. Arachidonic acid is a major component of animal fats and the bioactive lipids produced from this substrate play critical roles in a variety of biologic processes, including cancer. Cyclooxygenase-derived prostaglandin E2 (PGE2) is a known pro-inflammatory lipid mediator and promotes tumor progression. Metabolism of arachidonic acid by the cyclooxygenase pathway provides one mechanism for the contribution of dietary fats and chronic inflammation to carcinogenesis. In this review, we highlight recent advances in our understanding of how pro-inflammatory mediator PGE2 promotes colorectal cancer immune evasion. These findings may provide a rationale for the development of new therapeutic approaches to subvert tumor-induced immunosuppression.
INTRODUCTION
Colorectal cancer (CRC) is the fourth most common malignant neoplasm and the second leading cause of cancer deaths in the USA. Currently, the most effective treatment for CRC, including various approaches using surgical resection, radiation, and/or chemotherapy, works best when the disease is detected at a very early stage. Although colonoscopy screening is an effective way to detect and prevent CRC by removing precancerous adenomas, even today over fifty percent of patients still present to their physician with advanced cancer when standard treatments are not as effective resulting in low 5-year survival rates. Thus, we have always known that a more effective approach to control cancer is to develop improved prevention and early detection measures. Chemoprevention and cancer vaccines have been considered plausible approaches for cancer prevention. A significant effort has been made in the development of novel drugs for both cancer prevention and treatment over the past two decades. One group of compounds found to have beneficial effects on reducing the risk of developing some solid tumors including the four most prevalent cancers worldwide (colorectal, breast, lung, and prostate cancer) is nonsteroidal anti-inflammatory drugs (NSAIDs), which primarily target the cyclooxygenase enzymes (COX-1 and COX-2). Epidemiologic and clinical studies have demonstrated that long-term use of NSAIDs reduces the relative risk of CRC by 40-50% (Smalley and DuBois, 1997). Unlike COX-2 selective inhibitors (COXIBs) and other nonselective NSAIDs, long-term daily aspirin use is beneficial for prevention of both CRC and cardiovascular diseases. Treatment of FAP patients with celecoxib significantly reduced the polyp burden (Steinbach et al., 2000), while daily use of aspirin significantly suppressed polyp growth in FAP patients (Burn et al., 2011a) and substantially reduced cancer incidence in patients with Lynch syndrome (Burn et al., 2011b). In sporadic CRC, four randomized controlled trials demonstrated that aspirin use reduced risk of adenoma recurrence in patients with a history of colorectal adenomas (Baron et al., 2003; Benamouzig et al., 2003; Logan et al., 2008; Sandler et al., 2003). More intriguingly, recent observational and clinical studies revealed that daily use of aspirin was associated with a reduced risk of metastatic spread (Algra and Rothwell, 2012) and inhibited the spread of primary tumor cells to other organs after the diagnosis of localized disease, in particular CRC (Rothwell et al., 2012), suggesting the potential therapeutic efficacy of NSAIDs in advanced CRC. Furthermore, epidemiologic studies showed that regular use of aspirin specifically reduced risk of the subgroup of patients whose colon tumors expressed COX-2 at higher levels (Chan et al., 2007) and its use after the diagnosis of CRC at stage I, II and III prolonged overall survival, especially among individuals whose tumors overexpress COX-2 (Chan et al., 2009). These results suggest that the preventive and inhibitory effects of aspirin on CRC might depend on the presence of COX-2. COX-2 expression is elevated in approximately 50% of colorectal adenomas and 85% of adenocarcinomas (Eberhart et al., 1994; Gupta and Dubois, 2001; Marnett and DuBois, 2002) and is associated with a worse survival among CRC patients (Ogino et al., 2008).
Cyclooxygenase enzymes catalyze the conversion of arachidonic acid into prostanoids, including prostaglandins (PGs) and thromboxane A2 (TxA2). These bioactive lipids exert their biological effects in autocrine and/or paracrine manner by binding to their cognate cell surface receptors that belong to the G protein-coupled receptors (Fig. 1). It has been hypothesized that some of the adverse cardiovascular effects related to NSAID use are associated with a global reduction in prostanoid production (Fitzgerald, 2004). One possible way to avoid these undesired effects is to only target COX-derived prostanoids that mediate the tumor-promoting effects. Among prostanoids, pro-inflammatory PGE2 plays a predominant role in promoting tumor growth in colorectal cancer. PGE2 is the most abundant PG found in various types of human malignancies including colon, lung, breast, head and neck cancer and is often associated with a poor prognosis (Hambek et al., 2007; McLemore et al., 1988; Rigas et al., 1993; Wang and Dubois, 2004). A urinary PGE2 metabolite (PGE-M) has been used as a promising biomarker for CRC (Cai et al., 2006; Johnson et al., 2006) and other cancer patients (Dong et al., 2009; Kekatpure et al., 2009; Kim et al., 2013; Morris et al., 2013). The steady-state accumulation of PGE2 in tumor tissues depends on the relative rates of COX-2/PGE synthase-dependent biosynthesis and 15-hydroxyprostaglandin dehydrogenase (15-PGDH)-dependent degradation (Fig. 1). 15-PGDH is highly expressed in normal tissues but is ubiquitously lost in many human cancers including colorectal cancer (Backlund et al., 2005; Yan et al., 2004), lung (Ding et al., 2005) and transitional bladder cancer (Gee et al., 2003). Loss of 15-PGDH in these tumor tissues results in increased endogenous PGE2 levels. More importantly, epidemiologic studies revealed that levels of urinary PGE-M in healthy humans (Murphey et al., 2004) and breast cancer patients (Kim et al., 2013; Morris et al., 2013) are suppressed significantly not only by treatment with nonselective NSAIDs, including aspirin, but also by COXIBs, suggesting that the majority of PGE2 formed in vivo may be derived from COX-2.
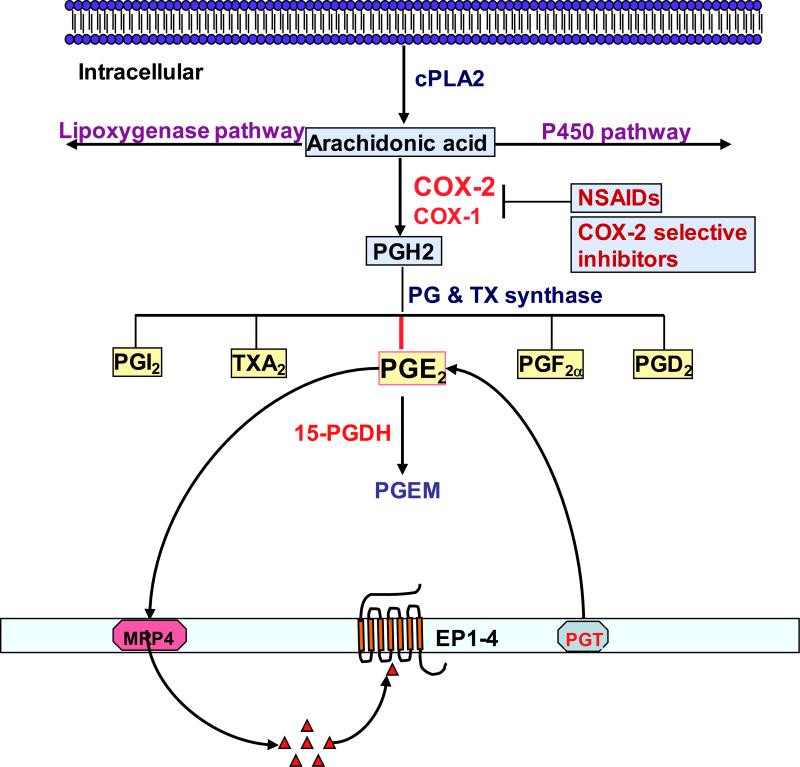
Figure 1. An overview of prostanoid synthesis pathways.
Arachidonic acid (AA) is a polyunsaturated fatty acid that constitutes the phospholipid domain of most cell membranes and is liberated from the cellular membranes by cytoplasmic phospholipase A2 (cPLA2). Free AA can be metabolized to eicosanoids through three major pathways: the cyclooxygenase (COX), the lipoxygenase (LOX), and the cytochrome P-450 monooxygenase pathway. In the COX pathway, the key step is the enzymatic conversion of the AA to intermediate PGG2, which is then reduced to an intermediate PGH2 by the peroxidase activity of COX. PGH2 is sequentially metabolized to prostanoids, including prostaglandins (PGs) and thromboxanes (TXs) via specific PG and TX synthases. The MRP gene family is comprised of efflux transporters for both PGs while PGT is an influx transporter for PGs. 15-PGDH mainly metabolizes the intracellular PGE2 and PGF2α to a stable 13,14-dihydro-15-keto-PGA2 (PGEM) and 13,14-dihydro-15-keto-PGF2α.
Direct evidence that PGE2 promotes tumor growth came from our previous work and other studies showing that PGE2 treatment dramatically increased both small and large intestinal adenoma burden in ApcMin/+ mice and significantly enhanced AOM-induced colon tumor incidence and multiplicity (Kawamori et al., 2003; Wang et al., 2004). Furthermore, elevated endogenous PGE2 by genetic deletion of 15-Pgdh promotes colon tumor growth in ApcMin/+ and AOM mouse models (Myung et al., 2006). In contrast, inhibition of endogenous PGE2 by genetic deletion of PGE2 synthase (mPges-1) suppresses intestinal tumor formation and growth in ApcMin/+ and AOM models (Nakanishi et al., 2008). The central role of PGE2 in colorectal tumorigenesis has been further confirmed by evaluating mice with a homozygous deletion of individual PGE2 receptors (Mutoh et al., 2002; Sonoshita et al., 2001; Watanabe et al., 1999). PGE2 has been shown to promote tumor formation, growth, and metastasis through 1) directly inducing tumor epithelial cell proliferation, survival, and migration/invasion as well as epigenetic changes and 2) switching the tumor microenvironment from “normal” to one supporting tumor growth and metastatic spread by inhibiting immunosurveillance and inducing angiogenesis (Wang and DuBois, 2010; Xia et al., 2012). Given that PGE2 appears to play a dominant role in carcinogenesis, more selective pharmacological inhibition of PGE2 production and signaling may be efficacious and may avoid some of the cardiovascular side effects associated with NSAIDs and COXIBs.
The roles of PGE2 in colon tumor epithelial cells and tumor-associated angiogenesis have been summarized in recent reviews (Wang and Dubois, 2006; Wang and DuBois, 2010). In this review, we highlight recent breakthroughs in our understanding of the role of PGE2 in tumor-induced immunosuppression.
PGE2 AND IMMUNE CELLS
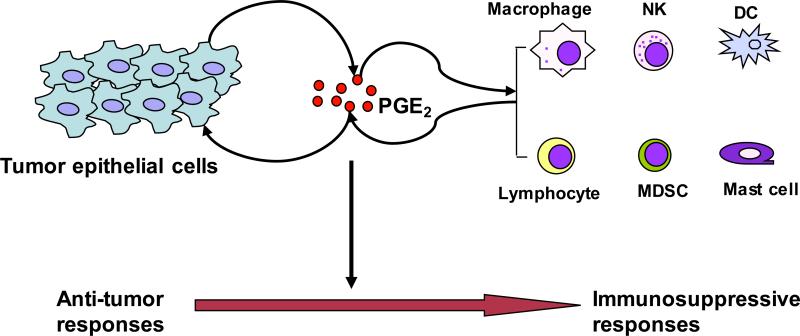
CRC formation and progression depends on the escape from the host immuno-surveillance. Cross talk between transformed epithelial cells and their surrounding stromal cells switch a normal tissue microenvironment to tumor microenvironment that supports tumor growth and spread by inducing angiogenesis and/or immune evasion. Similar to other solid tumors, the CRC immune evasion involves a shift from Th1 to Th2 immune responses, a defective antigen-presenting cell (APC) function, impaired cytotoxic activity of CD8+ T cells and nature killer (NK) cell activity, and enhancement of immunosuppressive cells such as regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Emerging evidence supports the concept that PGE2 signaling is one of essential pathways that govern tumor-mediated immune dysfunction. Overall, PGE2 contributes to a shift in the tumor microenvironment from anti-tumor responses to immunosuppressive responses (Fig. 2).
Figure 2. Models of pro-inflammatory PGE2 in switching tumor microenvironment from anti-tumor to immunosuppressive responses.
Following the initiation of epithelial tumors, the reciprocal interactions between transformed epithelial and immune cells play a key role in facilitating cancer progression. Pro-inflammatory PGE2 produced by tumor epithelial cells and infiltrating immune cells is a key mediator in this cross talk and can accelerate tumor growth and metastasis through evading attack by the immune system.
Antigen-presenting cells
Antigen-presenting cells (APCs) include dendritic cells (DCs), monocytes/macrophages and B cells.
Dendritic cells
Dendritic cells (DCs), including myeloid dendritic cells (MDCs) and plasmacytoid dendritic cells (PDCs), are thought to participate in tumor immunity by inducing primary and secondary T cell responses as well as immune tolerance (Banchereau and Steinman, 1998). Reduced DC numbers and activity affects the prognosis of patients with CRC (Della Porta et al., 2005; Huang et al., 2003). Mature DCs interact with antigen-specific T cells that initiate a Th1-type immune response. For example, mature DCs pulsed with tumor cell lysate can induce tumor-specific cytotoxic T cell (CTL) activity that inhibits colon tumor growth both in vitro and in mice implanted subcutaneously with CT26 colon tumor cells (Wu et al., 2010). Immature dendritic cells (iDCs) exhibit reduced phagocytic ability and reduced antigen presentation to T cells (Pockaj et al., 2004) and promote tumor angiogenesis by secreting proangiogenic cytokines (Curiel et al., 2004). In breast cancer patients, PGE2 levels are positively correlated with inhibition of DC maturation (Pockaj et al., 2004).
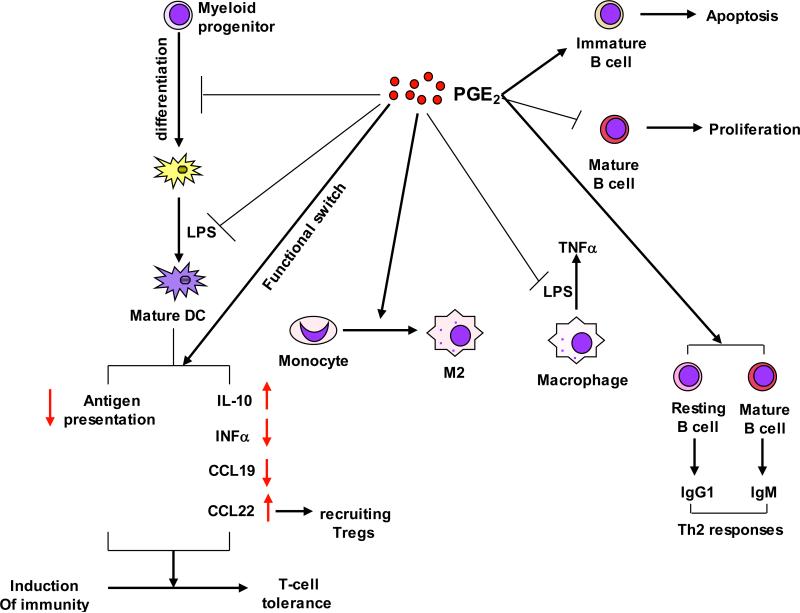
PGE2 modulates the activities of DCs by altering their differentiation, maturation, and their ability to secrete cytokines (Fig. 3). PGE2 has been shown to induce intestinal immune tolerance via DCs in vivo (Chinen et al., 2011). In tumor implantation models of colon and lung cancers, PGE2 promoted tumor growth by suppressing differentiation of DCs from bone marrow progenitors (Yang et al., 2003). A recent study revealed that PGE2 inhibited DC differentiation via downregulation of retinal dehydrogenases (RALDH) in vitro and in vivo (Stock et al., 2011). RALDH is the enzyme responsible for synthesis of retinotic acid (RA) that is required to modulate intestinal DC function. Another study reported that PGE2 inhibited LPS-induced DC maturation via blocking LPS-induced indoleamine 2,3-dioxygenase (IDO), IL-12 p70, and TNFα expression as well as the mature markers such as CD80, CD86 and MHC class I in murine bone marrow-derived DCs (BM-DCs) (Jung et al., 2010). Multiple in vitro studies elucidated the mechanisms underlying PGE2 inhibition of DC function. PGE2 switches the function of DCs from induction of immunity to T-cell tolerance via upregulation of CD25 and IDO (von Bergwelt-Baildon et al., 2006). PGE2 inhibits the antigen presentation ability of bone marrow-derived DC (BM-DC) by up-regulation of IL-10 (Harizi et al., 2002). Furthermore, PGE2 shifts the IL-12/IL-23 balance in DCs via EP2 and EP4 receptors in favor of IL-23, which in turn increases the number of Th17 cells in vitro (Sheibanie et al., 2007). IL-12 promotes Th1 responses and suppresses Th17 development and function whereas IL-23 is essential for Th17 expansion and survival. Similarly, PGE2 inhibits secretion of IFNα by Toll-like receptor (TLR)-activated PDCs via EP2/4, which results in reduction of Th1 cytokine secretion and induction of Th2 cytokine secretion by T cells (Fabricius et al., 2010). PGE2 also inhibits the ability of DCs to produce CCL19 that attracts CCR7-expressing naïve CD4+ T cells (Muthuswamy et al., 2010). More interestingly, PGE2 has been recently showed to redirect the differentiation of human DCs into monocytic MDSCs (Obermajer et al., 2011a). This body of work strongly supports the notion that PGE2 represents a crucial signaling pathway regulating DC differentiation, maturation, and function.
Figure 3. PGE2 provides coordinated regulation of tumor immunosuppression.
Pro-inflammatory PGE2 produced by tumor epithelial cells and/or their surrounding stromal cells induces immunosuppression through 1) inhibiting DC differentiation, and switching the function of DCs from induction of immunity to T-cell tolerance; 2) inducing monocytes to M2 macrophages and inhibiting LPS-induced TNFα in macrophages; and 3) inhibition of mature B cell proliferation and induction of immature B cell apoptosis as well as stimulation of B cell Th2 responses.
Macrophages
Macrophages are highly plastic and can be activated to either M1 (Th1 response) or M2 (Th2 response) polarization states depending on the microenvironment stimuli. The M1 designation is given for classically activated macrophages characterized by elevated expression of IL-12, TNFα, IL-23, and MHCII. Also, M1 macrophages typically generate reactive nitrogen (NO) and oxygen intermediates (ROI) to counteract pathogens and tumor cells. In contrast, the M2 designation is for alternatively activated macrophages expressing high levels of IL-10, IL-4, and IL-13. In most cancers, tumor associated macrophages (TAMs) resemble an M2-like phenotype (Mantovani et al., 2002) and are a major component of the leukocytic tumor infiltrate. TAMs are recognized as a poor prognostic sign in various tumors, including CRC (Bacman et al., 2007; Qian and Pollard, 2010). It has be well established that TAMs promote cancer progression and metastasis through supporting tumor-associated angiogenesis, enhancing tumor cell migration, invasion, and intravasation, and suppressing immuno-surveillance (Qian and Pollard, 2010).
Limited data are available on the regulation of macrophage function by PGE2. In a colon tumor implantation model, overexpression of 15-PGDH in tumor tissue is sufficient to redirect the differentiation of intratumoral CD11b cells from immunosuppressive M2-oriented TAMs to M1-oriented macrophages (Eruslanov et al., 2009), suggesting that PGE2 can alter the differentiation of monocytes favoring the development of M2-type TAMs at the tumor sites. In a spontaneous murine model of gastric cancer, PGE2 and bacterial infection promote tumor growth by cooperatively recruiting macrophages into tumors via up-regulation of CCL2 expression (Oshima et al., 2010). An in vitro study showed that treatment of LPS-activated macrophages with PGE2 diminished LPS-induced TNFα expression, suggesting that PGE2 can downregulate cell-mediated immune responses (Th1 response) (Kunkel et al., 1988). These studies suggest that PGE2 may be able to recruit monocytes/macrophages to tumor sites and shift them into M2-type macrophages (Fig. 3). Moreover, PGE2 is able to inhibit alveolar macrophage phagocytosis via multiple signaling pathways such as EP2-cAMP (Aronoff et al., 2004), PTEN (Canetti et al., 2007), and IL-1R-associated Kinase-M (Hubbard et al., 2010). However, there is no direct evidence demonstrating that PGE2 regulates macrophage function during tumor initiation and progression.
B cells
B cells not only function to produce antibodies against antigens but also as important APCs for CD4 and CD8 T cells. At present, little is known about the role of B cells in human CRC and other solid tumors. In tumor implantation mouse models of CRC, B cell-deficient mice exhibit spontaneous regression of tumor growth as compared to WT mice (Shah et al., 2005), suggesting that B cells promote tumor growth.
PGE2 is able to inhibit B cell development in vivo (Shimozato and Kincade, 1999). In vitro studies further reveal that PGE2 promotes B cell receptor (BCR)-induced apoptosis in immature B cells and inhibits BCR-stimulated proliferation of mature B cells via EP4 (Brown et al., 1992; Murn et al., 2008; Prijatelj et al., 2011) and B cell activation (Roper and Phipps, 1992). In addition, PGE2 promotes the production of switched isotypes in B cells. For example, PGE2 significantly induces IgG1 production in resting B cells and uncommitted B cells expressing high levels of surface IgM (Roper et al., 2002). PGE2 also induces activated B cell Ig isotype switching to IgE (Roper et al., 1995). The PGE2 induction of IgG1 and IgE supports the notion that PGE2 acts predominantly to induce Th2 responses (Fig. 3). In the context of the colonic tumor microenvironment, however, the precise role of PGE2 in B cells has not been investigated.
Natural killer cells
Although NK cells are functionally similar to cytotoxic T cells, NK cells are able to kill transformed or virus-infected cells but spare normal cells without prior sensitization. Suppressed NK cell activity has been found in human CRC and is an important prognostic factor for the development of distant metastases (Espi et al., 1996; Kondo et al., 2003).
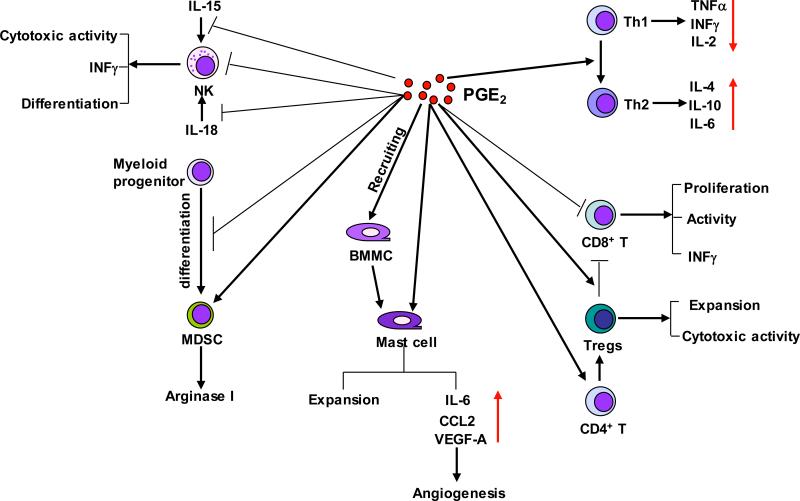
Multiple lines of studies have investigated the effects of PGE2 on NK cells (Fig. 4). In vivo studies showed that treatment of rats with PGE2 inhibited NK cell activity and enhanced lung metastases (Yakar et al., 2003) and reversed the effects of a NSAID on enhancing NK cell activity (Voth et al., 1986). A recent report revealed that EP4 receptor mediated the effects of PGE2 on suppression of NK cell function and promoted breast cancer metastasis in a syngeneic murine model of metastatic breast cancer (Ma et al., 2013). Several in vitro studies have demonstrated that PGE2 suppresses NK cell function (Bankhurst, 1982; Goto et al., 1983). PGE2 not only directly inhibits NK cells to produce INFγ, which is essential for NK cell functions, but also attenuates IL-12-induced or IL-18-induced INFγ expression in NK cells via EP2 receptor (Mailliard et al., 2005; Walker and Rotondo, 2004). Moreover, PGE2 inhibits IL-15-activated human NK cell function through downregulation of common gamma-chain (Joshi et al., 2001) and hepatic NK cell activities (Liu et al., 2000). PGE2 reduces the cytotoxic activities of NK cells by inhibiting NK receptors (NKR) including NKG2D, CD16 and natural cytotoxicity receptors (NCR: NKp30, NKp44, NKp46) (Martinet et al., 2010).
Figure 4. PGE2 provides coordinated regulation of tumor immunosuppression.
Pro-inflammatory PGE2 produced by tumor epithelial cells and/or their surrounding stromal cells induces immunosuppression through 1) suppressing NK cell function; 2) inducing MDSC differentiation and production of arginase I; 3) recruiting BMMCs and stimulating pro-angiogenic factors; 4) downregulation of antitumor Th1 cytokines and upregulation of immunosuppressive Th2 cytokines; 5) inhibiting CD8+ T cell proliferation and activity, and 6) inhibition of CD8+ T cell anti-tumor functions through Tregs.
Myeloid-derived suppressor cells
MDSCs are a heterogenous population of immature myeloid cell that suppress T and NK cell functions. The levels of MDSC in the blood are positive correlated with clinical cancer stage and metastatic tumor burden in mice and patients including colon cancers (Diaz-Montero et al., 2009; Mandruzzato et al., 2009). It is widely accepted that myeloid-derived suppressor cells (MDSCs) contribute to cancer immune evasion via suppressing functions of T and natural killer (NK) cells (Gabrilovich and Nagaraj, 2009). Emerging evidence demonstrates that MDSCs are also able to promote the development of Foxp3 positive Treg cells (Huang et al., 2006).
It has been reported that PGE2 promoted tumor progression via inducing the differentiation of MDSCs from bone marrow myeloid progenitor cells, whereas inhibition of PGE2 signaling by deletion of EP2 or its antagonists blocked this differentiation in mice with implanted 4T1 mammary carcinoma (Sinha et al., 2007) (Fig. 4). Similarly, treatment of tumor-bearing mice with a COX-2 selective inhibitor prevented the local and systemic expansion of MDSCs in vivo (Veltman et al., 2010). In vitro studies showed that PGE2 induced arginase I expression via EP4 in MDSCs (Rodriguez et al., 2005). Arginase I is involved in MDSC-mediated immune suppression by blocking effector T cell function. PGE2 also mediates the Fas ligation-induced MDSC recruitment in 3LL Lewis lung carcinoma (Zhang et al., 2009) and is required for CXCL4-induced MDSC chemotaxis via induction of CXCR4 expression on MDSCs (Obermajer et al., 2011b). However, the mechanism(s) by which PGE2 regulates MDSC differentiation, expansion, and suppressive functions in cancers remains unclear.
T cells
T helper cells
The progression of human CRC is associated with imbalance in Th1/Th2 responses with reduced production of cytokines from Th1 cells and normal or even elevated levels of cytokines from Th2 cells (O'Hara et al., 1998; Pellegrini et al., 1996; Shibata et al., 2002). PGE2 contributes to this imbalance by switching anti-tumor Th1 responses to immunosuppressive Th2 responses via downregulation of Th1 cytokines (TNFα, IFNγ, and IL-2) and upregulation of Th2 cytokines (IL-4, IL-10, and IL-6) in T helper cells (Hilkens et al., 1995; Snijdewint et al., 1993). In addition, PGE2 exacerbates inflammation and disease severity through elevating infiltration of IL-17-producing T helper (Th17) cells to the colonic tissue in a murine model of IBD (Sheibanie et al., 2007). In mammary gland-bearing mice, PGE2 secreted from tumor induces IL-23, which results in Th17 cell expansion in the tumor microenvironment (Qian et al., 2013). In addition, PGE2 can facilitate IL-23-induced Th17 expansion from peripheral blood mononuclear cells and naive T cells in vitro (Boniface et al., 2009; Chizzolini et al., 2008). PGE2 also directly promotes differentiation of memory CD4+ T cells to Th17 cells by induction of IL-17 expression and reduction of INFγ expression (Napolitani et al., 2009). PGE2 induces Th1 cell differentiation and Th17 cell expansion in vitro and treatment with an EP4 antagonist reduces accumulation of both Th1 and Th17 cells in regional lymph nodes and suppresses the disease progression in an animal model of chronic inflammation (Yao et al., 2009). However, relative little is known about the impact of PGE2 on the imbalance of Th1/Th2 response and Th17 cells in tumor microenvironment.
CD8+ T cells
The massive infiltration of CD8+ T cells at the site of tumors is significantly associated with a better survival of CRC patients (Naito et al., 1998). Although much less is known about directly suppressive effects of PGE2 on CD8+ T cells, a few reports indicated that PGE2 can directly inhibit CD8+ T cell proliferation (Hendricks et al., 2000) and the cytotoxic activity of CD8+ T cells via upregulation of CD94 and the NKG2A complex in vitro (Zeddou et al., 2005). PGE2 also attenuates TCR-induced IFNγ release from CD8+ T cells (Ganapathy et al., 2000). Reduction of IFNγ production favors type-2 responses in general. In addition to the direct effects of PGE2 on CD8+ T cells, PGE2 produced by cancer cells and their surrounding stromal cells can also indirectly abolish the anti-tumor effects of cytotoxic T cells in vivo and in vitro through downregulation of both direct antigen presentation by tumor cells and cross-presentation by APCs such as DCs (Ahmadi et al., 2008).
Regulatory T cells
Regulatory T cells (Tregs) are essential for suppressing immune responses and maintaining self-tolerance by regulating the activity of other immune cells. The frequency and suppressor function of Tregs are elevated in the peripheral blood and at the tumor sites of cancer patients including CRC (Strauss et al., 2007; Wolf et al., 2003). There is a positive correlation between PGE2 levels and the numbers of Foxp3+ Tregs in peripheral blood, tumor tissues and draining lymph nodes of CRC patients (Yaqub et al., 2008).
In the polyps of ApcΔ468 mice, Tregs shift from a protective anti-inflammatory (CD4+CD45RBlowCD25high) to a cancer-promoting pro-inflammatory phenotype (CD4+CD25+Foxp3+) (Gounaris et al., 2009). In a mouse model, deletion of mPges-1 gene suppresses AOM-induced colon carcinogenesis accompanied with reduced frequency of CD4+Foxp3+ Tregs in the draining mesenteric lymph nodes and serum PGE2 levels (Nakanishi et al., 2011), indicating that PGE2 may enhance tumor growth via expansion of Tregs. Moreover, PGE2 can directly enhance the differentiation of naïve CD4+ T cells into FOXP3-positive Tregs in vitro and induce FOXP3 expression and Treg activities in lung cancer in vivo (Baratelli et al., 2005; Sharma et al., 2005). PGE2 has also been reported to indirectly attract FOXP3+ Tregs via induction of CCL22 in mature DCs (Muthuswamy et al., 2008) (Fig. 3). Interestingly, CD4+CD25+Foxp3+ Tregs produce PGE2 and suppress effector T cell responses in a PGE2-dependent manner (Mahic et al., 2006). In addition, treatment with an EP4 antagonist resulted in a decreased number of Tregs in LNs and the skin after UV irradiation (Soontrapa et al., 2011), suggesting that EP4 mediates the effect of PGE2 on Treg expansion. An in vitro study indicated that PGE2 secreted from breast cancer cells induced Treg cell migration (Karavitis et al., 2012). Collectively, PGE2-enhanced Treg expansion, migration, and activities provide another mechanism for contribution of PGE2 to promotion of an immunosuppressive microenvironment (Fig. 4).
Mast cells
Although mast cells (MCs) are key effector cells in allergic diseases, it has become apparent that they also contribute to other pathologies, including cancers. Recent studies revealed that lower numbers of mast cells (MCs) are associated with hypovascularity and better survival in CRC patients (Gulubova and Vlaykova, 2009). In contrast, higher MC infiltration is associated with poor clinical outcome with increased vascularity, tumor growth and invasion in the many of human cancers including CRC (Groot Kormelink et al., 2009; Kashiwase et al., 2008). In ApcΔ468 mice, MCs have been demonstrated to be an essential component for polyp growth (Gounaris et al., 2007). These findings suggest that the infiltration of MCs into tumor contributes the tumor growth.
PGE2 functions as a chemotactic factor for immature and mature bone marrow-derived mast cells (BMMCs) in vitro and in vivo (Weller et al., 2007). The PI3K-mTOR signaling pathway mediates the effects of PGE2 on attracting BMMCs (Kuehn et al., 2011). Moreover, PGE2 has been shown to enhance mast cell induction from murine spleen mononuclear cells and BMMCs as well as human umbilical cord blood mononuclear cells (Gomi et al., 2000; Hu et al., 1995; Saito et al., 1996). In addition, PGE2 promotes release of pro-inflammatory cytokines and chemokines as well as proangiogenic factors such as IL-6, CCL2, and VEGF-A from BMMCs, human umbilical cord blood mononuclear cell-derived MCs, and spleen-derived MC (Abdel-Majid and Marshall, 2004; Gomi et al., 2000; Nakayama et al., 2006). These results indicate that PGE2 promotes colon tumor growth via enhancing recruiting MCs, MC maturation, stimulating MCs to produce proinflammatory cytokines and chemokines as well as proangiogenic factors (Fig. 4).
Conclusions
PGE2 promotes cancer progression via several mechanisms. PGE2 can directly bind to its cell surface receptors on tumor epithelial cells to regulate cell proliferation, apoptosis, migration and invasion as well as to induce tumor epithelial cells to secrete growth factors, pro-inflammatory mediators, and angiogenic factors that stimulate angiogenesis and local inflammation. In addition to the direct effects of PGE2 on tumor cells, PGE2 also serves as an immunomodulator that shifts the tumor microenvironment from anti-tumor to immunosuppressive responses, resulting in escape of tumor cells from effective immunosurveillance.
Most cancer immunotherapies have poor clinical efficiency. The observations that use of selective COX-2 inhibitors enhances the efficacy of certain cancer vaccines (DeLong et al., 2003; Hahn et al., 2006; Zeytin et al., 2004) support a hypothesis that a major barrier to successful immunotherapies against cancer is the immune modulators secreted by cancer cells and their surrounding stromal cells, such as PGE2. Therefore, understanding the diverse functions of PGE2 in the tumor microenvironment is crucial for the development of novel combinational therapies aimed at targeting PGE2 production and signaling along with immune intervention for prevention and amelioration of CRC with decreased cardiovascular side effects associated with selective COX-2 inhibitors and enhanced efficacy of immunotherapies.
ACKNOWLEDGEMENTS
This work is supported, in part, from the National Institutes of Health Grants P01-CA-77839 and R37-DK47297. RND (R37-DK47297) is recipient of an NIH MERIT award. We also thank the National Colorectal Cancer Research Alliance (NCCRA) for its generous support (RND).
Footnotes
Conflict of Interests
The authors declare no conflict of interests.
References
- Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Emery DC, Morgan DJ. Prevention of both direct and cross-priming of antitumor CD8+ T-cell responses following overproduction of prostaglandin E2 by tumor cells in vivo. Cancer Res. 2008;68:7520–7529. doi: 10.1158/0008-5472.CAN-08-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The lancet oncology. 2012 doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, Milne GL, Katkuri S, DuBois RN. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bankhurst AD. The modulation of human natural killer cell activity by prostaglandins. Journal of clinical & laboratory immunology. 1982;7:85–91. [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Warner GL, Ales-Martinez JE, Scott DW, Phipps RP. Prostaglandin E2 induces apoptosis in immature normal and malignant B lymphocytes. Clin Immunol Immunopathol. 1992;63:221–229. doi: 10.1016/0090-1229(92)90226-e. [DOI] [PubMed] [Google Scholar]
- Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, Eccles D, Ellis A, Evans DG, Fodde R, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011a;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011b;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, Wen W, Rothman N, Li HL, Morrow JD, Zheng W. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol. 2006;24:5010–5016. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- Canetti C, Serezani CH, Atrasz RG, White ES, Aronoff DM, Peters-Golden M. Activation of phosphatase and tensin homolog on chromosome 10 mediates the inhibition of FcgammaR phagocytosis by prostaglandin E2 in alveolar macrophages. J Immunol. 2007;179:8350–8356. doi: 10.4049/jimmunol.179.12.8350. [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen T, Komai K, Muto G, Morita R, Inoue N, Yoshida H, Sekiya T, Yoshida R, Nakamura K, Takayanagi R, Yoshimura A. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat Commun. 2011;2:190. doi: 10.1038/ncomms1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G. Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology. 2005;68:276–284. doi: 10.1159/000086784. [DOI] [PubMed] [Google Scholar]
- DeLong P, Tanaka T, Kruklitis R, Henry AC, Kapoor V, Kaiser LR, Sterman DH, Albelda SM. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 2003;63:7845–7852. [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- Dong LM, Shu XO, Gao YT, Milne G, Ji BT, Yang G, Li HL, Rothman N, Zheng W, Chow WH, Abnet CC. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women's health study. Cancer Epidemiol Biomarkers Prev. 2009;18:3075–3078. doi: 10.1158/1055-9965.EPI-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Eruslanov E, Kaliberov S, Daurkin I, Kaliberova L, Buchsbaum D, Vieweg J, Kusmartsev S. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 2009;182:7548–7557. doi: 10.4049/jimmunol.0802358. [DOI] [PubMed] [Google Scholar]
- Espi A, Arenas J, Garcia-Granero E, Marti E, Lledo S. Relationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum. 1996;39:429–434. doi: 10.1007/BF02054059. [DOI] [PubMed] [Google Scholar]
- Fabricius D, Neubauer M, Mandel B, Schutz C, Viardot A, Vollmer A, Jahrsdorfer B, Debatin KM. Prostaglandin E2 inhibits IFN-alpha secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J Immunol. 2010;184:677–684. doi: 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Gurlo T, Jarstadmarken HO, von Grafenstein H. Regulation of TCR-induced IFN-gamma release from islet-reactive non-obese diabetic CD8(+) T cells by prostaglandin E(2) receptor signaling. Int Immunol. 2000;12:851–860. doi: 10.1093/intimm/12.6.851. [DOI] [PubMed] [Google Scholar]
- Gee JR, Montoya RG, Khaled HM, Sabichi AL, Grossman HB. Cytokeratin 20, AN43, PGDH, and COX-2 expression in transitional and squamous cell carcinoma of the bladder. Urol Oncol. 2003;21:266–270. doi: 10.1016/s1078-1439(02)00271-5. [DOI] [PubMed] [Google Scholar]
- Gomi K, Zhu FG, Marshall JS. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J Immunol. 2000;165:6545–6552. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- Goto T, Herberman RB, Maluish A, Strong DM. Cyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activity. J Immunol. 1983;130:1350–1355. [PubMed] [Google Scholar]
- Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot Kormelink T, Abudukelimu A, Redegeld FA. Mast cells as target in cancer therapy. Curr Pharm Des. 2009;15:1868–1878. doi: 10.2174/138161209788453284. [DOI] [PubMed] [Google Scholar]
- Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2009;24:1265–1275. doi: 10.1111/j.1440-1746.2007.05009.x. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- Hahn T, Alvarez I, Kobie JJ, Ramanathapuram L, Dial S, Fulton A, Besselsen D, Walker E, Akporiaye ET. Short-term dietary administration of celecoxib enhances the efficacy of tumor lysate-pulsed dendritic cell vaccines in treating murine breast cancer. Int J Cancer. 2006;118:2220–2231. doi: 10.1002/ijc.21616. [DOI] [PubMed] [Google Scholar]
- Hambek M, Baghi M, Wagenblast J, Schmitt J, Baumann H, Knecht R. Inverse correlation between serum PGE2 and T classification in head and neck cancer. Head Neck. 2007;29:244–248. doi: 10.1002/hed.20503. [DOI] [PubMed] [Google Scholar]
- Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- Hendricks A, Leibold W, Kaever V, Schuberth HJ. Prostaglandin E2 is variably induced by bacterial superantigens in bovine mononuclear cells and has a regulatory role for the T cell proliferative response. Immunobiology. 2000;201:493–505. doi: 10.1016/S0171-2985(00)80069-8. [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- Hu ZQ, Asano K, Seki H, Shimamura T. An essential role of prostaglandin E on mouse mast cell induction. J Immunol. 1995;155:2134–2142. [PubMed] [Google Scholar]
- Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134:270–278. doi: 10.1046/j.1365-2249.2003.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Hubbard LL, Ballinger MN, Thomas PE, Wilke CA, Standiford TJ, Kobayashi KS, Flavell RA, Moore BB. A role for IL-1 receptor-associated kinase-M in prostaglandin E2-induced immunosuppression post-bone marrow transplantation. J Immunol. 2010;184:6299–6308. doi: 10.4049/jimmunol.0902828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JC, Schmidt CR, Shrubsole MJ, Billheimer DD, Joshi PR, Morrow JD, Heslin MJ, Washington MK, Ness RM, Zheng W, et al. Urine PGE-M: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2006;4:1358–1365. doi: 10.1016/j.cgh.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J Immunol. 2001;166:885–891. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- Jung ID, Jeong YI, Lee CM, Noh KT, Jeong SK, Chun SH, Choi OH, Park WS, Han J, Shin YK, et al. COX-2 and PGE2 signaling is essential for the regulation of IDO expression by curcumin in murine bone marrow-derived dendritic cells. Int Immunopharmacol. 2010;10:760–768. doi: 10.1016/j.intimp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PloS one. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwase Y, Inamura H, Morioka J, Igarashi Y, Kawai-Kowase K, Kurosawa M. Quantitative analysis of mast cells in benign and malignant colonic lesions: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Allergol Immunopathol (Madr) 2008;36:271–276. doi: 10.1016/s0301-0546(08)75222-4. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- Kekatpure VD, Boyle JO, Zhou XK, Duffield-Lillico AJ, Gross ND, Lee NY, Subbaramaiah K, Morrow JD, Milne G, Lippman SM, Dannenberg AJ. Elevated levels of urinary prostaglandin e metabolite indicate a poor prognosis in ever smoker head and neck squamous cell carcinoma patients. Cancer Prev Res (Phila) 2009;2:957–965. doi: 10.1158/1940-6207.CAPR-09-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Taylor JA, Milne G, Sandler DP. Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev Res (Phila) 2013 doi: 10.1158/1940-6207.CAPR-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E, Koda K, Takiguchi N, Oda K, Seike K, Ishizuka M, Miyazaki M. Preoperative natural killer cell activity as a prognostic factor for distant metastasis following surgery for colon cancer. Dig Surg. 2003;20:445–451. doi: 10.1159/000072714. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Jung MY, Beaven MA, Metcalfe DD, Gilfillan AM. Prostaglandin E2 activates and utilizes mTORC2 as a central signaling locus for the regulation of mast cell chemotaxis and mediator release. J Biol Chem. 2011;286:391–402. doi: 10.1074/jbc.M110.164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel SL, Spengler M, May MA, Spengler R, Larrick J, Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988;263:5380–5384. [PubMed] [Google Scholar]
- Liu H, Cunnick JE, Hendrich S. Opposing effects of prostaglandin E(2)and F(2 alpha) on rat liver-associated natural killer cell activity in vitro. Prostaglandins Leukot Essent Fatty Acids. 2000;63:153–158. doi: 10.1054/plef.2000.0173. [DOI] [PubMed] [Google Scholar]
- Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, Fulton AM. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2:e22647. doi: 10.4161/onci.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- Martinet L, Jean C, Dietrich G, Fournie JJ, Poupot R. PGE2 inhibits natural killer and gamma delta T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- McLemore TL, Hubbard WC, Litterst CL, Liu MC, Miller S, McMahon NA, Eggleston JC, Boyd MR. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res. 1988;48:3140–3147. [PubMed] [Google Scholar]
- Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, Modi S, Fornier MN, Hudis CA, Dannenberg AJ. Increased Levels of Urinary PGE-M, a Biomarker of Inflammation, Occur in Association with Obesity, Aging, and Lung Metastases in Patients with Breast Cancer. Cancer Prev Res (Phila) 2013;6:428–436. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murn J, Alibert O, Wu N, Tendil S, Gidrol X. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205:3091–3103. doi: 10.1084/jem.20081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Analytical biochemistry. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, Kalinski P. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116:1454–1459. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, Tani K, Kobayashi M, Maruyama T, Kobayashi K, et al. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res. 2002;62:28–32. [PubMed] [Google Scholar]
- Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, Vella AT, Rosenberg DW. Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prev Res (Phila) 2011;4:1198–1208. doi: 10.1158/1940-6207.CAPR-11-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, Xu D, Rosenberg DW. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J Leukoc Biol. 2006;79:95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- O'Hara RJ, Greenman J, MacDonald AW, Gaskell KM, Topping KP, Duthie GS, Kerin MJ, Lee PW, Monson JR. Advanced colorectal cancer is associated with impaired interleukin 12 and enhanced interleukin 10 production. Clin Cancer Res. 1998;4:1943–1948. [PubMed] [Google Scholar]
- Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011a;118:5498–5505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011b;71:7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, Kirkner GJ, Nosho K, Irahara N, Kure S, Shima K, Hazra A, Chan AT, Dehari R, Giovannucci EL, Fuchs CS. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14:8221–8227. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Hioki K, Popivanova BK, Oguma K, Van Rooijen N, Ishikawa TO, Oshima M. Prostaglandin E signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2010;140:596–607. e597. doi: 10.1053/j.gastro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol. 2004;11:328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Prijatelj M, Celhar T, Mlinaric-Rascan I. Prostaglandin EP4 receptor enhances BCR-induced apoptosis of immature B cells. Prostaglandins Other Lipid Mediat. 2011 doi: 10.1016/j.prostaglandins.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Gu L, Ning H, Zhang Y, Hsueh EC, Fu M, Hu X, Wei L, Hoft DF, Liu J. Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2. J Immunol. 2013;190:5894–5902. doi: 10.4049/jimmunol.1203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RL, Brown DM, Phipps RP. Prostaglandin E2 promotes B lymphocyte Ig isotype switching to IgE. J Immunol. 1995;154:162–170. [PubMed] [Google Scholar]
- Roper RL, Graf B, Phipps RP. Prostaglandin E2 and cAMP promote B lymphocyte class switching to IgG1. Immunol Lett. 2002;84:191–198. doi: 10.1016/s0165-2478(02)00185-2. [DOI] [PubMed] [Google Scholar]
- Roper RL, Phipps RP. Prostaglandin E2 and cAMP inhibit B lymphocyte activation and simultaneously promote IgE and IgG1 synthesis. J Immunol. 1992;149:2984–2991. [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012 doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, Iikura Y, Awaji T, Tsujimoto G, Yanagida M, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, Rosenblatt JD. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, Fukuzawa M. Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol. 2002;34:416–420. doi: 10.1097/00004836-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Shimozato T, Kincade PW. Prostaglandin E(2) and stem cell factor can deliver opposing signals to B lymphocyte precursors. Cell Immunol. 1999;198:21–29. doi: 10.1006/cimm.1999.1575. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- Soontrapa K, Honda T, Sakata D, Yao C, Hirata T, Hori S, Matsuoka T, Kita Y, Shimizu T, Kabashima K, Narumiya S. Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc Natl Acad Sci U S A. 2011;108:6668–6673. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Stock A, Booth S, Cerundolo V. Prostaglandin E2 suppresses the differentiation of retinoic acid-producing dendritic cells in mice and humans. J Exp Med. 2011;208:761–773. doi: 10.1084/jem.20101967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, Fiore F, Roth U, Beyer M, Debey S, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- Voth R, Chmielarczyk W, Storch E, Kirchner H. Induction of natural killer cell activity in mice by injection of indomethacin. Nat Immun Cell Growth Regul. 1986;5:317–324. [PubMed] [Google Scholar]
- Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol. 2004;31:64–73. doi: 10.1053/j.seminoncol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, Das SK, Dey SK, DuBois RN. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Ushikubi F, Narumiya S, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, Wilson MS, Taylor GW, Jose PJ, Williams TJ. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc Natl Acad Sci U S A. 2007;104:11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- Wu YG, Wu GZ, Wang L, Zhang YY, Li Z, Li DC. Tumor cell lysate-pulsed dendritic cells induce a T cell response against colon cancer in vitro and in vivo. Med Oncol. 2010;27:736–742. doi: 10.1007/s12032-009-9277-x. [DOI] [PubMed] [Google Scholar]
- Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18:224–226. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10:469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, Lawrence E, Lutterbaugh J, Lu S, Willson JK, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, Tasken K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813–821. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeddou M, Greimers R, de Valensart N, Nayjib B, Tasken K, Boniver J, Moutschen M, Rahmouni S. Prostaglandin E2 induces the expression of functional inhibitory CD94/NKG2A receptors in human CD8+ T lymphocytes by a cAMP-dependent protein kinase A type I pathway. Biochem Pharmacol. 2005;70:714–724. doi: 10.1016/j.bcp.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Zeytin HE, Patel AC, Rogers CJ, Canter D, Hursting SD, Schlom J, Greiner JW. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice. Cancer Res. 2004;64:3668–3678. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Q, Zhang M, Yu Y, Liu X, Cao X. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol. 2009;182:3801–3808. doi: 10.4049/jimmunol.0801548. [DOI] [PubMed] [Google Scholar]