Abstract
Sterile syringe access is an important means to reduce HIV risk, but many injection drug users (IDU) who obtain syringes from sterile sources continue to share syringes. We examined the factors associated with continuing syringe sharing in New York City. We recruited 500 active IDU in 2005 through respondent-driven sampling. In multiple logistic regression, not obtaining all syringes in the past year exclusively from sterile sources was associated with increased syringe sharing. Ensuring adequate syringe availability as well as engaging and retaining nonusers and inconsistent users in sterile syringe services may increase sterile syringe access and decrease syringe sharing.
Keywords: injection drug use, HIV, risk behavior, syringe exchange, syringe sharing
BACKGROUND
Injection drug use creates an HIV transmission risk when discordant injectors share syringes and other injection equipments and when they engage in unprotected sex (Des Jarlais, Hagan, et al., 2007c; Des Jarlais, Kamyar, et al., 2007d; Santibanez et al., 2006). The decline in syringe sharing over the past two decades has been a major factor responsible for the corresponding decline in HIV incidence among injection drug users (IDU) (Des Jarlais, Braine, Huso, & Turner, 2007b; Des Jarlais et al., 1998, 2005; Metsch et al., 2007). Many IDU who do continue to share syringes, moreover, have lowered their overall HIV risk by serosorting with and reducing the number of sharing partners (Des Jarlais et al., 2004). However, syringe sharing is a persistent HIV risk in high-prevalence injection networks and among IDU who do not otherwise reduce risks (Des Jarlais, Braine, & Friedmann, 2007; Golub et al., 2007; Thiede et al., 2007).
Syringe exchange programs (SEPs) and other legal syringe sources, such as pharmacies and medical providers, prevent HIV by increasing sterile syringe availability and providing risk reduction education (Bluthenthal, Kral, Gee, Erringer, & Edlin, 2000; Coffin, 2000; Ksobiech, 2003). New York City (NYC), with the largest and most diverse IDU population in the United States, provides IDU with a variety of sterile syringe options (Friedman et al., 2004; Rockwell, Deren, Goldstein, Friedman, & Des Jarlais, 2002). New York policymakers approved a pilot of syringe exchange in 1988 and formally endorsed SEPs in 1992 (Vlahov et al., 2001). Currently, 13 organizations operate SEPs in NYC. Additionally, the New York Expanded Syringe Access Program (ESAP) legislation, enacted in 2001, allows licensed pharmacies and medical providers to sell or distribute syringes without a prescription (Deren et al., 2006). New York laws criminalizing syringe possession have also been amended, although their interpretation remains ambiguous.
Research has demonstrated reductions in syringe sharing as a result of SEP participation (Bluthenthal et al., 2004; Huo & Ouellet, 2007), and while the results for pharmacy syringe access have not been as conclusive (Cotten-Oldenburg, Carr, DeBoer, Collison, & Novotny, 2001; Fuller et al., 2007; Pouget et al., 2005), pharmacies increase the options for syringes above potentially unsterile sources, such as friends or street sources. Ultimately, it remains unclear what impact sterile sources collectively have on sharing in an environment with this variety of options. Furthermore, many IDU access sterile syringe sources but do so inconsistently. The effect of consistency on syringe sharing merits further inquiry (Ksobiech, 2004).
In this study, we investigated the association between consistent sterile syringe access and syringe sharing among NYC IDU. We described the demographics and drug use in this population and examined factors associated with syringe sharing. Our hypothesis was that obtaining syringes exclusively from sterile syringe sources would be associated with a reduced likelihood of syringe sharing.
METHODS
Sampling and Eligibility
Data were collected as part of the National HIV Behavioral Surveillance (NHBS) study conducted in NYC. NHBS is a cross-sectional study funded by the Centers for Disease Control and Prevention to characterize HIV risk and prevalence among HIV risk groups. The overall NHBS design has been described in detail elsewhere (Gallagher, Sullivan, Lansky, & Onorato, 2007; Lansky, Sullivan, Gallagher, & Fleming, 2007; Sanchez et al., 2006). The NHBS-IDU study cycle employed respondent-driven sampling (RDS) to recruit participants (Abdul-Quader et al., 2006; Lansky et al., 2007). RDS is a variation on snowball sampling that uses mathematical modeling to weight data for theoretically unbiased population estimates (Heckathorn, 1997, 2002).
On the basis of RDS methods, we recruited an initial group of eligible IDU (“seeds”) from four NYC neighborhoods identified through ethnographic research as having dense populations of IDU. SEPs within these neighborhoods were chosen as interview locations. Study seeds took the survey and were then offered the opportunity to recruit up to three more participants. The seeds’ eligible recruits were then also offered the opportunity to recruit up to three others; this continued until we met our sample size. Participants who agreed to recruit were given numbered coupons, which allowed for tracking the recruitment chains linking recruiters and recruits. Incentives were provided for taking the survey and also for recruiting eligible participants to ensure that recruitment chains would penetrate social networks (Magnani, Sabin, Saidel, & Heckathorn, 2005). Trained interviewers administered the survey and collected data on handheld computers between July 2005 and December 2005.
The eligibility criteria for the study were as follows: (1) injection drug use in the past 12 months (confirmed by interviewers observing visible injection marks and assessing injection knowledge), (2) adulthood (≥18 years old), (3) residency in the NYC Metropolitan Statistical Area, (4) ability to speak English or Spanish, and (5) possession of a valid recruitment coupon from a member of their social network who had previously participated. Following established RDS methods, seeds were removed from the data analysis (Heckathorn, 2007).
Measures
The survey included questions on sociodemographics, drug use, injection-related and sexual HIV risk behaviors, and exposure to HIV testing and prevention services. Our main outcome for this analysis was receptive syringe sharing in the past year, defined as injecting drugs with a syringe after another person had injected with it. We also examined distributive syringe sharing (sharing a syringe with another person after the participant had used it) and sharing other injection equipments such as “cookers” or water (used to prepare drugs) and “cottons” (used to filter drugs while filling syringes). Independent factors for this analysis included sociodemographics, drug use patterns, and syringe sources in the past year. We dichotomized age at the median. Homelessness was defined as living on the streets, in a shelter, or single-room occupancy hotel at any time during the past year.
Participants were asked about obtaining syringes from five syringe source categories: SEPs, pharmacies, medical providers, friends (including relatives and sex partners), and street sources (including shooting galleries, street syringe sales, and other informal trading). Questions were asked about each source category in general and not about specific providers within each category. We classified the first three sources as sterile sources and the last two as potentially unsterile sources. Respondents who obtained syringes only from sterile sources in the past year were considered to have consistent sterile syringe access.
Data Analysis
Data analysis was conducted through the RDS Analysis Tool (RDSAT) 5.6 (Heckathorn, Cornell University, Ithaca, NY) and SAS 9.1 (SAS Institutes, Cary, NC). RD-SAT produces variable weights that control for differences in participant network size and preference for in-group recruitment, both of which may bias population estimates from peer-referral sampling methods (Salganik, 2006). RDSAT weights were generated for each statistic; the multiple logistic model was weighted by the univariate weight for the outcome variable (Heckathorn, 2007). RDSAT weights imported into SAS were used to generate weighted statistics. Two SAS procedures (PROC SUR-VEYFREQ and PROC SURVEYLOGISTIC) designed for analyzing weighted survey data with a complex sampling design were used to generate frequencies, Rao-Scott chi-square tests, and multiple logistic regression models (SAS Institute Inc., 2005).
We constructed a multiple logistic regression model incorporating factors significantly associated (p ≤ .05) with syringe sharing at the univariate level. To eliminate collinear variables and address our main hypothesis, we used aggregate measures for polydrug use (injection of at least three drug types in the past year) and consistent sterile syringe access. We ran tests for interaction on all significant factors. The final model retained all measured variables to control for potentially confounding factors. Values of p, adjusted odds ratios (AOR), and 95% confidence intervals (95% CI) were generated with maximum likelihood statistics (Rothman & Greenland, 1998).
RESULTS
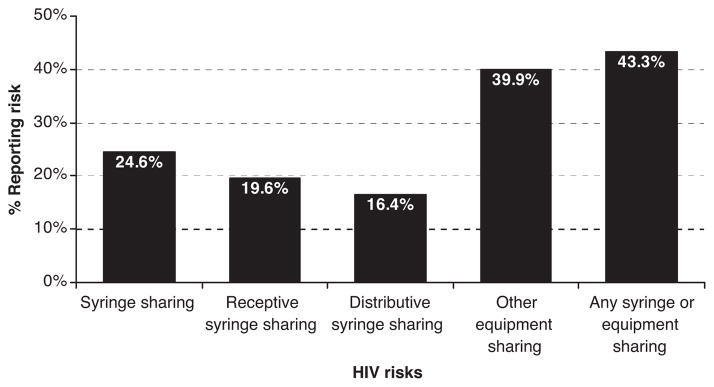
Five hundred nonseed IDU completed the NHBS survey. Injection-related HIV risk was common among sampled IDU (Figure 1). Nearly one quarter of IDU were engaged in either receptive or distributive syringe sharing in the past year. For our main outcome, 19.6% were engaged in receptive syringe sharing (hereafter, syringe sharing). In addition, 39.9% of IDU shared other injection equipment, and 43.3% shared syringes, other equipment, or both in the past year.
FIGURE 1.
Injection-related HIV risks among NYC IDU, 2005.
Males and Hispanics comprised a majority of IDU. There were no significant associations between gender or race/ethnicity and syringe sharing (Table 1). Age group was significantly associated with syringe sharing: Those below the median age (42.5 years) were more likely than those above it to share syringes. A large proportion of IDU were born in Puerto Rico or a foreign county, and most had lower income and education. None of these characteristics were associated with increased syringe sharing. However, homelessness and history of arrest in the past year were both common among IDU and were both significantly associated with increased likelihood of sharing. The self-reported HIV prevalence among IDU was 22.0%. Syringe sharing did not vary by self-reported HIV serostatus.
TABLE 1.
Sociodemographics of NYC IDU and associations with syringe sharing, 2005
| Characteristic | Total (%) | Shared syringe (%) | p |
|---|---|---|---|
| Gender | .98 | ||
| Female | 26.9 | 20.2 | |
| Male | 73.1 | 20.0 | |
| Race/ethnicity | .12 | ||
| Black | 29.4 | 13.3 | |
| Hispanic | 57.4 | 19.7 | |
| White | 12.1 | 23.2 | |
| Age (median = 42.5) | <.01 | ||
| 18–42 | 49.5 | 26.5 | |
| 43+ | 50.5 | 11.2 | |
| Sexual orientation | .09 | ||
| Heterosexual | 87.2 | 17.7 | |
| Gay/bisexual | 12.9 | 33.2 | |
| Birthplace | .90 | ||
| Puerto Rico | 35.2 | 18.4 | |
| United States | 60.2 | 19.5 | |
| Foreign | 4.7 | 24.9 | |
| Homeless in past year | .01 | ||
| No | 58.4 | 12.9 | |
| Yes | 41.6 | 27.7 | |
| Income | .22 | ||
| $0–10,000 | 69.6 | 21.4 | |
| >10,000 | 30.3 | 15.1 | |
| Education | .59 | ||
| ≤Some high school | 55.0 | 20.8 | |
| ≥High school graduate | 45.0 | 17.9 | |
| Arrested in past year | <.01 | ||
| No | 38.6 | 13.7 | |
| Yes | 61.4 | 30.1 | |
| Self-reported HIV status | .77 | ||
| HIV+ | 22.0 | 17.7 | |
| HIV−/unknown | 78.0 | 20.3 |
Heroin was the most commonly injected drug, with 85.9% of IDU reporting heroin injection (Table 2). Many IDU also injected “speedballs” (a mixture of heroin and cocaine) by 58.4%, cocaine alone by 46.1%, and other drugs (such as crack, Oxycontin, and methamphetamine) by 9.8% of IDU. IDU who injected cocaine or other drugs were more likely to share syringes. The 39.6% of IDU who injected at least three types of drugs in the past year were significantly more likely to share syringes. Most IDU injected at least once a day (64.3%) and many had attended a shooting gallery (26.6%) in the past year. Syringe sharing did not vary significantly by these two factors.
TABLE 2.
Past year drug use, related behaviors, and syringe sources for NYC IDU and associations with syringe sharing, 2005
| Characteristic | Total (%) | Shared syringe (%) | p |
|---|---|---|---|
| Injected heroin | .08 | ||
| No | 14.1 | 8.6 | |
| Yes | 85.9 | 21.3 | |
| Injected speedballs | .14 | ||
| No | 41.6 | 15.4 | |
| Yes | 58.4 | 23.3 | |
| Injected cocaine | .02 | ||
| No | 53.9 | 14.1 | |
| Yes | 46.1 | 26.2 | |
| Injected other | <.01 | ||
| No | 90.2 | 17.2 | |
| Yes | 9.8 | 41.8 | |
| Number of drugs injected | <.01 | ||
| 1–2 | 60.4 | 13.4 | |
| 3+ | 39.6 | 29.3 | |
| Frequency of injection | .07 | ||
| At least one time/day | 64.3 | 22.9 | |
| Less than one time/day | 35.7 | 13.4 | |
| Attended shooting gallery | .35 | ||
| No | 73.4 | 18.2 | |
| Yes | 26.6 | 23.3 | |
| Syringes from exchange program | .60 | ||
| No | 34.8 | 17.6 | |
| Yes | 65.2 | 20.5 | |
| Syringes from pharmacy | .52 | ||
| No | 50.0 | 21.6 | |
| Yes | 50.0 | 18.1 | |
| Syringes from medical provider | .52 | ||
| No | 89.4 | 18.9 | |
| Yes | 10.6 | 26.2 | |
| Syringes from friend/partner/relative | .02 | ||
| No | 47.2 | 12.2 | |
| Yes | 52.8 | 26.3 | |
| Syringes from street sources | <.01 | ||
| No | 75.8 | 15.2 | |
| Yes | 24.2 | 35.8 | |
| All syringes from sterile sources | .02 | ||
| No | 57.4 | 26.5 | |
| Yes | 42.6 | 10.4 |
Most IDU had obtained some syringes from a legal sterile source in the past year, including SEPs (65.2%), pharmacies (50.0%), or medical providers (10.6%). Overall, 84.0% of IDU obtained some syringes from at least one sterile source in the past year. Many had obtained syringes from potentially unsterile sources, including a friend, partner, or relative (52.8%), or from street sources (24.2%). We found no significant differences in likelihood of syringe sharing by obtaining syringes from any of the three sterile sources. However, there were significant differences in sharing by obtaining syringes from either of the two potentially unsterile sources: IDU who obtained syringes from friends/partners and IDU who obtained syringes from street sources were more likely to report syringe sharing compared to IDU who did not obtain syringes from friends/partners or street sources, respectively. For our aggregate measure of consistent sterile syringe access, fewer than half of IDU consistently accessed sterile syringes (42.6%), but those who did were significantly less likely to share syringes. Whereas 26.5% of IDU who had inconsistent or nonuse of sterile syringe sources shared syringes, only 10.4% of IDU who had consistent sterile syringe access reported sharing.
The final regression model is shown in Table 3. Two demographics associated with syringe sharing at the univariate level, age group and history of arrest in the past year, remained significantly associated with sharing in the regression model. IDU who were below the median age were more than twice as likely as those above it to share syringes (AOR = 2.62, 95% CI = 1.06–6.48). IDU who had been arrested were almost three times more likely to share than those who had not (AOR = 2.74, 95% CI = 1.34–5.61). IDU who had injected at least three drug types in the past year were twice as likely to share as IDU who injected one or two drugs (AOR = 2.08, 95% CI = 1.02–4.27). Finally, IDU who had not obtained all syringes from sterile sources were 4.5 times more likely (AOR = 4.43, 95% CI = 2.14–9.17) to share than those who consistently obtained all syringes from sterile sources.
TABLE 3.
Multivariate logistic regression modela of syringe sharing for NYC IDU, 2005
| Characteristic | AOR | 95% CI | p |
|---|---|---|---|
| Age | .04 | ||
| 18–42 | 2.62 | 1.06–6.48 | |
| 43+ | 1.00 | ||
| Arrested in past year | <.01 | ||
| No | 1.00 | ||
| Yes | 2.74 | 1.34–5.61 | |
| Number of drugs injected | .05 | ||
| 1–2 | 1.00 | ||
| 3+ | 2.08 | 1.02–4.27 | |
| All syringes from sterile sources | <.01 | ||
| No | 4.43 | 2.14–9.17 | |
| Yes | 1.00 |
Controls for gender, race/ethnicity, sexual orientation, birthplace, income, education, self-reported HIV status, frequency of injection, and shooting gallery attendance.
DISCUSSION
Syringe Source and Syringe Sharing
New York policymakers have recognized the important role of sterile syringe coverage in preventing HIV transmission and have thus permitted and partially funded the distribution of sterile syringes by SEPs, pharmacies, and medical providers (Des Jarlais, McKnight, & Friedmann, 2002). Although federal funds have not been used to support SEPs, state and local SEP funding has grown nationally, as research has continued to demonstrate the public health benefits of access to sterile syringes (Centers for Disease Control and Prevention, 2007; Tempalski et al., 2007). In addition, many states now permit pharmacies to sell syringes without a prescription, and pharmacists are generally receptive to selling syringes to IDU (Reich et al., 2002; Taussig, Weinstein, Burris, & Jones, 2000). With the increasing availability of sterile syringes, the proportion of IDU who obtain syringes from these sources has risen over the past two decades (Friedman et al., 2005). This increase has been associated with decreases in HIV risk behaviors and HIV incidence among IDU (Des Jarlais et al., 2000).
However, there is still cause for concern. We found that many IDU inconsistently or never accessed syringes from sterile sources (57.4%), shared syringes (19.6%), or reported as HIV-positive (22.0%). These related factors may continue to drive the HIV epidemic among NYC IDU. Furthermore, mounting evidence of sexual HIV transmission from IDU to non-IDU signals ongoing HIV risk that is not directly connected to syringe sharing (Des Jarlais, Kamyar, et al., 2007; Somlai, Kelly, McAuliffe, Ksobiech, & Hackl, 2003).
Sterile syringe sources reduce HIV risk through two underlying mechanisms. First, adequate provision of sterile syringes reduces the need to share syringes (Bluthenthal et al., 2007; Coffin, 2000; Lurie, Jones, & Foley, 1998). Ideally, providing IDU with new and sterile syringes for each injection would eliminate any reason to share. Realistically, when sterile syringes are more easily obtained, IDU may be less likely to share. Further research on the factors that inhibit consistent access to sterile syringes is needed, and the impact of expanded geographic and demographic coverage of SEP-distributed syringes to IDU without adequate syringe access and additional methods to increase sterile syringe provision outside of SEP distribution should be a focus. Pharmacy sales and medical provider distribution of sterile syringes fill a critical gap in syringe provision to IDU who live far away from SEPs or who may be uncomfortable with their semipublic nature (Deren et al., 2006; Masson et al., 2007). Although providing syringes does not ensure their one-time use, our finding that consistent access to sterile syringes was associated with lower syringe sharing may suggest that sterile sources reduce syringe scarcity enough to influence sharing behaviors.
Second, sterile syringe sources, particularly SEPs, reduce risk through structured or informal interventions to effect positive behavioral change (Kellerman, Drake, Lansky, & Klevens, 2006; Porter, Metzger, & Scotti, 2002). Obtaining syringes from SEPs is frequently supplemented with education in safer injection and other risk reduction practices and with referrals for support services that prevent risk (Coyle, Needle, & Normand, 1998; Metzger & Navaline, 2003). Unsterile syringe sources, such as friends or street sources, may not offer the same risk reduction benefits as sterile syringe sources (Coyle et al., 1998; Tortu, McMahon, Hamid, & Neaigus, 2003). While the secondary distribution of sterile syringes from legal sources may reduce risk by providing sterile syringes to those in need, the risk reduction benefits from interventions are lost (Lorvick et al., 2006).
Consistency of sterile syringe access is also a key factor when assessing the impact of syringe access on HIV risk (Brogly, Bruneau, Vincelette, Lamothe, & Franco, 2000). Our study shows that access to sterile syringes was inconsistent: Most IDU had obtained syringes from at least one sterile source in the past year (84.0%), but most also did not obtain syringes exclusively from sterile sources (57.4%). On average, risk reduction outcomes may be proportional to the frequency of sterile syringe access (Golub et al., 2007). Merely obtaining sterile syringes once or twice a year does not appear to provide the same risk reduction benefits as consistently obtaining all syringes only from sterile sources. The importance of consistent sterile syringe access was demonstrated in an over fourfold increase in likelihood of sharing among inconsistent or nonusers of sterile sources. Efforts to target, engage, and retain inconsistent users in sterile syringe sources may reduce HIV risk further.
Other Factors in Syringe Sharing
The three other significant predictors of syringe sharing in our regression model highlight vulnerable subpopulations of IDU. Previous literature has shown that young IDU may be at high HIV risk through drug-related behaviors (Fuller et al., 2003; Golub et al., 2007; Thiede et al., 2007). Younger IDU may be more likely to share for similar reasons that younger men who have sex with men (MSM) continue to engage in risky unprotected anal sex: Younger IDU may have not seen firsthand the negative health effects associated with HIV/AIDS as older IDU have (Clatts, Goldsamt, Neaigus, & Welle, 2003; Kershaw, 2008; Osmond, Pollack, Paul, & Catania, 2007). Younger IDU may also share more frequently because they are less experienced with injecting or are less likely to attend an SEP for fear of being identified as a drug user (Novelli, Sherman, Havens, Strathdee, & Sapun, 2005; O’Connell et al., 2005). Previous research has shown that polydrug use has also been found to put IDU at greater risk for HIV (De, Cox, Boivin, Platt, & Jolly, 2007), and, more generally, polydrug users are at higher risk for drug overdose and thereby represent a very high-risk group in general for intervention (van Ameijden et al., 1999). Finally, incarceration has been historically associated with increased HIV risk among IDU (Friedman et al., 2006; Rhodes et al., 2004). History or fear of arrest has been shown to discourage SEP use among IDU and thereby limits their access to sterile syringes (Bluthenthal, Kral, Lorvick, & Watters, 1997; Rhodes et al., 2003; Rich, Strong, Towe, & McKenzie, 1999). Police crack-downs on IDU for syringe possession occur even when local law permits syringe possession because of ambiguity over what constitutes legal possession (Cooper, Moore, Gruskin, & Krieger, 2005; Davis, Burris, Kraut-Becher, Lynch, & Metzger, 2005; Fuller et al., 2004). Also, injection behaviors resulting in incarceration (e.g., street injection) may also be factors related to syringe sharing (Kang et al., 2005). Overall, these three factors associated with sharing may distinguish IDU who engage in disordered patterns of drug use leading to risky unplanned injections.
Limitations
One potential limitation to this study is that the elimination of recruitment biases associated with RDS peer recruitment depends on a methodological assumption that respondents recruit randomly among their social networks (Heckathorn, 2002). RDSAT may not produce valid population estimates when nonrandom recruitment occurs. This assumption may be unreasonable given the economic dynamics inherent in peer-referral methods, which favor selection of readily available and trusted peers (Heimer, 2005).
A second potential limitation is that variables for obtaining syringes from unsterile sources and sharing syringes may measure, in effect, the same behavior, which might explain the association between syringe source and syringe sharing. However, our data provide evidence to the contrary: Most IDU who obtained syringes from unsterile sources did not share syringes (and many IDU who obtained syringes from sterile sources did share syringes). This study explored relationship between the variables, but the correlation was far from perfect, and therefore, the variables did not capture the same behavior. One reason may be that we used long-term measures (syringe sources and sharing behaviors in the past year) rather than event-specific measures (e.g., source of last syringe and sharing of that syringe) where the time between the two acts (obtaining syringes and using them) is shorter and thus naturally connected. Longer-term patterns of sharing are a product of syringe scarcity that relates to syringe sources (sterile sources reduce scarcity) but are ultimately independent of sources. In the regression model, our measures were also broad in terms of consistency of access and aggregated sources categories, which further mitigate the effects of this potential limitation.
A third limitation to the study is its cross-sectional design. We cannot infer causality between access to sterile syringe sources and reduction of risk behavior. Fourth, the definition of our main predictor variable (inconsistently obtaining syringes from sterile sources) may not be an accurate measure for use of unsterile syringes. IDU may obtain sterile syringes from potentially unsterile sources, as in the case of secondary syringe exchange, because the sterility of the syringes obtained from potentially unsterile sources is ultimately unknown. Finally, participants may misreport syringe sources and sharing behaviors because of social desirability, recall bias, or other reasons.
CONCLUSIONS
We found a strong association between consistently accessing sterile syringes and reduced syringe sharing. Among the IDU in our study with largely inconsistent sterile syringe access and high HIV prevalence, continued sharing indicates continuing injection-related HIV risk. Our main findings could be used to support HIV prevention outreach and intervention with the goal of encouraging IDU to exclusively obtain syringes from sterile sources and not share those syringes with others. Ensuring adequate syringe coverage to IDU will be necessary to reduce ongoing HIV risk and encourage long-term risk reduction.
Highest-risk IDU are hard to find, engage, and retain in HIV prevention services (Masson et al., 2007). Many IDU at highest risk may be a younger, underground population of IDU (Hagan et al., 2007). Considering the factors influencing syringe sharing, increased engagement of IDU with inconsistent use of sterile syringe sources may be a starting point. SEP staff could examine their records to identify occasional SEP users for outreach. Retention of occasional users in consistent sterile syringe access services may improve as a result of integrating medical and social services into SEPs (Cunningham, Sohler, Berg, Shapiro, & Heller, 2006; Hennessy, Weisfuse, & Schlanger, 2007; Rothman et al., 2007). Finally, strategies that lower the barriers to accessing sterile syringes, such as increasing geographic coverage and demographic targeting of sterile syringes, as well as clarifying the ambiguities in syringe possession laws may also be important methods to drive sterile syringe access up and syringe sharing down.
Acknowledgments
This work was funded by a cooperative agreement between the New York City Department of Health and Mental Hygiene and the Centers for Disease Control and Prevention (grant no. U62/CCU223595–03-1) and supported by a contract to the National Development and Research Institutes from the New York City Department of Health and Mental Hygiene (contract no. 816–20070005237). The authors would like to acknowledge the valuable contributions of the National HIV Behavioral Surveillance (NHBS) field and data management staff (Alix Conde, Libertad Guerra, Shavvy Raj-Singh, Noel Trejo, and Aundrea Woodall). We also thank Elizabeth Begier, Thomas Farley, and Lucia Torian for their helpful comments on earlier drafts of this manuscript.
Biographies

Samuel M. Jenness, M.P.H., is an epidemiologist with the HIV Epidemiology Program at the New York City Department of Health and Mental Hygiene. In this position, he is the Project Director and data analyst for two national studies concerning HIV behavioral risk and patterns of HIV medical care underutilization. Prior to this position, he was a public health policy analyst at the Massachusetts Department of Public Health. He received his M.P.H. from the Boston University School of Public Health.

Holly Hagan, Ph.D., is a Senior Research Scientist at the New York University College of Nursing. Dr. Hagan holds a Ph.D. in epidemiology from the University of Washington. She has led a number of longitudinal cohort studies examining blood-borne viral transmission in injection drug users, and, in recent years, her research has sought to characterize the etiology, epidemiology, and prevention of hepatitis C virus infections in drug users. She is currently Principal Investigator of a study of sexual health among drug users in Harlem.
Kai-Lih Liu, Ph.D., M.P.H., is currently Associated Director of the Strategic Information Unit at Family Health International Cambodia office in Phnom Penh. During this study, he was a Research Scientist in the HIV Epidemiology Program of the New York City Department of Health and Mental Hygiene. He completed postdoctoral training in behavioral sciences research in HIV infection at the Columbia University, New York, his doctorate in epidemiology at the Yale University, and M.P.H. in epidemiology at the National Taiwan University, Taipei.

Travis Wendel, J.D., has been an Ethnographer working with New York City drug users and distributors since 1996. He is Principal Investigator of a study of the role of social networks in methamphetamine distribution in New York City and Co-Investigator/Ethnographer of the New York City National HIV Behavioral Survey. His research interests center around social networks and the social organization of the distribution and consumption of illegal commodities. Prior to entering upon a research career, he worked as a bicycle messenger, cook, and nightclub bouncer.

Christopher Murrill, Ph.D., was at the time of this study the Director of the HIV Epidemiology Program’s Research Unit at the New York City Department of Health and Mental Hygiene. As the Director of this unit, Dr. Murrill serves as the Principal Investigator on a variety of HIV epidemiologic activities conducted among populations at risk for HIV within New York City. Prior to this position, Dr. Murrill was with the Centers for Disease Control and Prevention’s Global AIDS Program. He provided technical expertise and guidance for conducting HIV surveillance in a variety of developing countries throughout Africa, Southeast Asia, and the Caribbean. Dr. Murrill received his doctorate in epidemiology at the Tulane University and has worked extensively both domestically and internationally in the field of HIV surveillance and epidemiology.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Abdul-Quader AS, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Sabin K, et al. Effectiveness of respondent-driven sampling for recruiting drug users in New York City: Findings from a pilot study. Journal of Urban Health. 2006;83(3):459–476. doi: 10.1007/s11524-006-9052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal R, Malik MR, Grau LE, Singer M, Marshall P, Heimer R. Sterile syringe access conditions and variations in HIV risk among drug injectors in three cities. Addiction. 2004;99(9):1136–1146. doi: 10.1111/j.1360-0443.2004.00694.x. [DOI] [PubMed] [Google Scholar]
- Bluthenthal R, Ridgeway G, Schell T, Anderson R, Flynn N, Kral A. Examination of the association between syringe exchange program (SEP) dispensation policy and SEP client-level syringe coverage among injection drug users. Addiction. 2007;102(4):638–646. doi: 10.1111/j.1360-0443.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Kral AH, Gee L, Erringer EA, Edlin BR. The effect of syringe exchange use on high-risk injection drug users: A cohort study. AIDS. 2000;14(5):605–611. doi: 10.1097/00002030-200003310-00015. [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Kral AH, Lorvick J, Watters JK. Impact of law enforcement on syringe exchange programs: A look at Oakland and San Francisco. Medical Anthropology. 1997;18(1):61–83. doi: 10.1080/01459740.1997.9966150. [DOI] [PubMed] [Google Scholar]
- Brogly SB, Bruneau J, Vincelette J, Lamothe F, Franco EL. Risk behaviour change and HIV infection among injection drug users in Montreal. AIDS. 2000;14(16):2575–2582. doi: 10.1097/00002030-200011100-00021. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Syringe exchange programs—United States, 2005. MMWR. Morbidity and Mortality Weekly Report. 2007;56(44):1164–1167. [PubMed] [Google Scholar]
- Clatts MC, Goldsamt L, Neaigus A, Welle DL. The social course of drug injection and sexual activity among YMSM and other high-risk youth: An agenda for future research. Journal of Urban Health. 2003;80(4 Suppl 3):iii26–iii39. doi: 10.1093/jurban/jtg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin P. Syringe availability as HIV prevention: A review of modalities. Journal of Urban Health. 2000;77(3):306–330. doi: 10.1007/BF02386743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H, Moore L, Gruskin S, Krieger N. The impact of a police drug crackdown on drug injectors’ ability to practice harm reduction: A qualitative study. Social Science & Medicine. 2005;61(3):673–684. doi: 10.1016/j.socscimed.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cotten-Oldenburg NU, Carr P, DeBoer JM, Collison EK, Novotny G. Impact of pharmacy-based syringe access on injection practices among injecting drug users in Minnesota, 1998 to 1999. Journal of Acquired Immune Deficiency Syndromes. 2001;27(2):183–192. doi: 10.1097/00126334-200106010-00014. [DOI] [PubMed] [Google Scholar]
- Coyle SL, Needle RH, Normand J. Outreach-based HIV prevention for injecting drug users: A review of published outcome data. Public Health Reports. 1998;113(Suppl 1):19–30. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CO, Sohler NL, Berg KM, Shapiro S, Heller D. Type of substance use and access to HIV-related health care. AIDS Patient Care and STDs. 2006;20(6):399–407. doi: 10.1089/apc.2006.20.399. [DOI] [PubMed] [Google Scholar]
- Davis CS, Burris S, Kraut-Becher J, Lynch KG, Metzger D. Effects of an intensive street-level police intervention on syringe exchange program use in Philadelphia, PA. American Journal of Public Health. 2005;95(2):233–236. doi: 10.2105/AJPH.2003.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De P, Cox J, Boivin JF, Platt RW, Jolly AM. Rethinking approaches to risk reduction for injection drug users: Differences in drug type affect risk for HIV and hepatitis C virus infection through drug-injecting networks. Journal of Acquired Immune Deficiency Syndromes. 2007;46(3):355–361. doi: 10.1097/QAI.0b013e3181565dde. [DOI] [PubMed] [Google Scholar]
- Deren S, Cleland CM, Fuller CM, Kang SY, Des Jarlais DC, Vlahov D. The impact of syringe deregulation on sources of syringes for injection drug users: Preliminary findings. AIDS and Behavior. 2006;10(6):717–721. doi: 10.1007/s10461-006-9096-4. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Braine N, Friedmann P. Unstable housing as a factor for increased injection risk behavior at US syringe exchange programs. AIDS and Behavior. 2007;11(6 Suppl):78–84. doi: 10.1007/s10461-007-9227-6. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Braine N, Huso Y, Turner C. Residual injection risk behavior, HIV infection, and the evaluation of syringe exchange programs. AIDS Education and Prevention. 2007b;19(2):111–123. doi: 10.1521/aeap.2007.19.2.111. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Hagan H, Arasteh K, McKnight C, Perlman D, Friedman SR. Herpes simplex virus-2 and HIV among noninjecting drug users in New York city. Sexually Transmitted Diseases. 2007c;34(11):923–927. doi: 10.1097/OLQ.0b013e3180ca9647. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Kamyar A, Perlis T, Hagan H, Abdul-Quader A, Heckathorn D, et al. Convergence of HIV sero-prevalence among injecting and non-injecting drug users in New York City. AIDS. 2007d;21:231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Friedmann P. Legal syringe purchases by injection drug users, Brooklyn and Queens, New York City, 2000–2001. Journal of the American Pharmaceutical Association (Washington, DC) 2002;42(6 Suppl 2):S73–S76. doi: 10.1331/1086-5802.42.0.s73.de. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Arasteh K, Hagan H, Milliken J, Braine N, et al. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. Journal of Acquired Immune Deficiency Syndromes. 2004;35(2):158–166. doi: 10.1097/00126334-200402010-00010. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Beatrice S, Milliken J, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: Use of serologic test algorithm to assess expansion of HIV prevention services. American Journal of Public Health. 2005;95(8):1439–1444. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Friedman SR, Chapman T, Kwok J, Rockwell R, et al. Behavioral risk reduction in a declining HIV epidemic: Injection drug users in New York City, 1990–1997. American Journal of Public Health. 2000;90(7):1112–1116. doi: 10.2105/ajph.90.7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Friedman SR, Deren S, Chapman T, Sotheran JL, et al. Declining seroprevalence in a very large HIV epidemic: Injecting drug users in New York City, 1991 to 1996. American Journal of Public Health. 1998;88(12):1801–1806. doi: 10.2105/ajph.88.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Cooper HL, Tempalski B, Keem M, Friedman R, Flom PL, et al. Relationships of deterrence and law enforcement to drug-related harms among drug injectors in US metropolitan areas. AIDS. 2006;20(1):93–99. doi: 10.1097/01.aids.0000196176.65551.a3. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Lieb S, Tempalski B, Cooper H, Keem M, Friedman R, et al. HIV among injection drug users in large US metropolitan areas, 1998. Journal of Urban Health. 2005;82(3):434–445. doi: 10.1093/jurban/jti088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Tempalski B, Cooper H, Perlis T, Keem M, Friedman R, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. Journal of Urban Health. 2004;81(3):377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CM, Galea S, Blaney S, Ompad DC, Deren S, Des Jarlais DC, et al. Explaining the relationship between race/ethnicity and pharmacy purchased syringes among injection drug users in New York City. Ethnicity & Disease. 2004;14(4):589–596. [PubMed] [Google Scholar]
- Fuller CM, Galea S, Caceres W, Blaney S, Sisco S, Vlahov D. Multilevel community-based intervention to increase access to sterile syringes among injection drug users through pharmacy sales in New York City. American Journal of Public Health. 2007;97(1):117–124. doi: 10.2105/AJPH.2005.069591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CM, Vlahov D, Latkin CA, Ompad DC, Celentano DD, Strathdee SA. Social circumstances of initiation of injection drug use and early shooting gallery attendance: Implications for HIV intervention among adolescent and young adult injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2003;32(1):86–93. doi: 10.1097/00126334-200301010-00013. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the U.S.: The National HIV Behavioral Surveillance System. Public Health Reports. 2007;122(Suppl 1):32–38. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub ET, Strathdee SA, Bailey SL, Hagan H, Latka MH, Hudson SM, et al. Distributive syringe sharing among young adult injection drug users in five U.S. cities. Drug and Alcohol Dependence. 2007;91(Suppl 1):S30–S38. doi: 10.1016/j.drugalcdep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hagan H, Campbell JV, Thiede H, Strathdee SA, Ouellet LJ, Latka MH, et al. Injecting alone among young adult IDUs in five US cities: Evidence of low rates of injection risk behavior. Drug and Alcohol Dependence. 2007;91(Suppl 1):S48–S55. doi: 10.1016/j.drugalcdep.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn D. Respondent-driven sampling: A new approach to the study of hidden populations. Social Problems. 1997;44(2):174–199. [Google Scholar]
- Heckathorn D. Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Social Problems. 2002;49(1):11–34. [Google Scholar]
- Heckathorn D. Extensions of respondent-driven sampling: Analyzing continuous variables and controlling for differential recruitment. Sociological Methodology. 2007;37(1):151–207. [Google Scholar]
- Heimer R. Critical issues and further questions about respondent-driven sampling: Comment on Ramirez-Valles, et al. (2005) AIDS and Behavior. 2005;9(4):403–408. doi: 10.1007/s10461-005-9030-1. discussion 409–413. [DOI] [PubMed] [Google Scholar]
- Hennessy RR, Weisfuse IB, Schlanger K. Does integrating viral hepatitis services into a public STD clinic attract injection drug users for care? Public Health Reports. 2007;122(Suppl 2):31–35. doi: 10.1177/00333549071220S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo D, Ouellet LJ. Needle exchange and injection-related risk behaviors in Chicago: A longitudinal study. Journal of Acquired Immune Deficiency Syndromes. 2007;45(1):108–114. doi: 10.1097/QAI.0b013e318050d260. [DOI] [PubMed] [Google Scholar]
- Kang SY, Deren S, Andia J, Colon HM, Robles R, Oliver-Velez D. HIV transmission behaviors in jail/prison among Puerto Rican drug injectors in New York and Puerto Rico. AIDS and Behavior. 2005;9(3):377–386. doi: 10.1007/s10461-005-9011-4. [DOI] [PubMed] [Google Scholar]
- Kellerman SE, Drake A, Lansky A, Klevens RM. Use of and exposure to HIV prevention programs and services by persons at high risk for HIV. AIDS Patient Care and STDs. 2006;20(6):391–398. doi: 10.1089/apc.2006.20.391. [DOI] [PubMed] [Google Scholar]
- Kershaw S. New HIV cases drop but rise in young gay men. New York Times. 2008:A24. [Google Scholar]
- Ksobiech K. A meta-analysis of needle sharing, lending, and borrowing behaviors of needle exchange program attenders. AIDS Education and Prevention. 2003;15(3):257–268. doi: 10.1521/aeap.15.4.257.23828. [DOI] [PubMed] [Google Scholar]
- Ksobiech K. Assessing and improving needle exchange programs: Gaps and problems in the literature. Harm Reduction Journal. 2004;1(4):1–7. doi: 10.1186/1477-7517-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky A, Abdul-Quader AS, Cribbin M, Hall T, Finlayson TJ, Garfein RS, et al. Developing an HIV behavioral surveillance system for injecting drug users: The National HIV Behavioral Surveillance System. Public Health Reports. 2007;122(Suppl 1):48–55. doi: 10.1177/00333549071220S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky A, Sullivan PS, Gallagher KM, Fleming PL. HIV behavioral surveillance in the U.S.: A conceptual framework. Public Health Reports. 2007;122(Suppl 1):16–23. doi: 10.1177/00333549071220S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorvick J, Bluthenthal RN, Scott A, Gilbert ML, Riehman KS, Anderson RL, et al. Secondary syringe exchange among users of 23 California syringe exchange programs. Substance Use & Misuse. 2006;41(6–7):865–882. doi: 10.1080/10826080600669041. [DOI] [PubMed] [Google Scholar]
- Lurie P, Jones TS, Foley J. A sterile syringe for every drug user injection: How many injections take place annually, and how might pharmacists contribute to syringe distribution? Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;18(Suppl 1):S45–S51. doi: 10.1097/00042560-199802001-00009. [DOI] [PubMed] [Google Scholar]
- Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(Suppl 2):S67–S72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- Masson C, Sorensen J, Perlman D, Shopshire M, Delucchi K, TeChieh C, et al. Hospital- versus community-based syringe exchange: A randomized controlled trial. AIDS Education and Prevention. 2007;19(2):97–110. doi: 10.1521/aeap.2007.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Pereyra M, Purcell DW, Latkin CA, Malow R, Gomez CA, et al. Correlates of lending needles/syringes among HIV-seropositive injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2007;46(Suppl 2):S72–S79. doi: 10.1097/QAI.0b013e3181576818. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Navaline H. HIV prevention among injection drug users: The need for integrated models. Journal of Urban Health. 2003;4(Suppl 3):iii59–iii66. doi: 10.1093/jurban/jtg083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli LA, Sherman SG, Havens JR, Strathdee SA, Sapun M. Circumstances surrounding the first injection experience and their association with future syringe sharing behaviors in young urban injection drug users. Drug and Alcohol Dependence. 2005;77(3):303–309. doi: 10.1016/j.drugalcdep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- O’Connell JM, Kerr T, Li K, Tyndall MW, Hogg RS, Montaner JS, et al. Requiring help injecting independently predicts incident HIV infection among injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2005;40(1):83–88. doi: 10.1097/01.qai.0000157006.28535.ml. [DOI] [PubMed] [Google Scholar]
- Osmond DH, Pollack LM, Paul JP, Catania JA. Changes in prevalence of HIV infection and sexual risk behavior in men who have sex with men in San Francisco: 1997–2002. American Journal of Public Health. 2007;97(9):1677–1683. doi: 10.2105/AJPH.2005.062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Metzger D, Scotti R. Bridge to services: Drug injectors’ awareness and utilization of drug user treatment and social service referrals, medical care, and HIV testing provided by needle exchange programs. Substance Use & Misuse. 2002;37(11):1305–1330. doi: 10.1081/ja-120014080. [DOI] [PubMed] [Google Scholar]
- Pouget ER, Deren S, Fuller CM, Blaney S, McMahon JM, Kang SY, et al. Receptive syringe sharing among injection drug users in Harlem and the Bronx during the New York State Expanded Syringe Access Demonstration Program. Journal of Acquired Immune Deficiency Syndromes. 2005;39(4):471–477. doi: 10.1097/01.qai.0000152395.82885.c0. [DOI] [PubMed] [Google Scholar]
- Reich W, Compton WM, Horton JC, Cottler LB, Cunningham-Williams RM, Booth R, et al. Injection drug users report good access to pharmacy sale of syringes. Journal of the American Pharmaceutical Association (Washington, DC) 2002;42(6 Suppl 2):S68–S72. doi: 10.1331/1086-5802.42.0.s68.reich. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Judd A, Mikhailova L, Sarang A, Khutorskoy M, Platt L, et al. Injecting equipment sharing among injecting drug users in Togliatti City, Russian Federation: Maximizing the protective effects of syringe distribution. Journal of Acquired Immune Deficiency Syndromes. 2004;35(3):293–300. doi: 10.1097/00126334-200403010-00011. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Mikhailova L, Sarang A, Lowndes CM, Rylkov A, Khutorskoy M, et al. Situational factors influencing drug injecting, risk reduction and syringe exchange in Togliatti City, Russian Federation: A qualitative study of micro risk environment. Social Science & Medicine. 2003;57(1):39–54. doi: 10.1016/s0277-9536(02)00521-x. [DOI] [PubMed] [Google Scholar]
- Rich JD, Strong L, Towe CW, McKenzie M. Obstacles to needle exchange participation in Rhode Island. Journal of Acquired Immune Deficiency Syndromes. 1999;21(5):396–400. [PubMed] [Google Scholar]
- Rockwell R, Deren S, Goldstein MF, Friedman SR, Des Jarlais DC. Trends in the AIDS epidemic among New York City’s injection drug users: Localized or citywide? Journal of Urban Health. 2002;79(1):136–146. doi: 10.1093/jurban/79.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J, Rudnick D, Slifer M, Agins B, Heiner K, Birkhead G. Co-located substance use treatment and HIV prevention and primary care services, New York State, 1990–2002: A model for effective service delivery to a high-risk population. Journal of Urban Health. 2007;84(2):226–242. doi: 10.1007/s11524-006-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern epidemiology. Philadelphia: Lippincott Williams; 1998. [Google Scholar]
- Salganik MJ. Variance estimation, design effects, and sample size calculations for respondent-driven sampling. Journal of Urban Health. 2006;83(6 Suppl):98–112. doi: 10.1007/s11524-006-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Finlayson T, Drake A, Behel S, Cribbin M, Dinenno E, et al. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors—United States, National HIV Behavioral Surveillance System: Men who have sex with men, November 2003–April 2005. MMWR Surveillance Summaries. 2006;55(6):1–16. [PubMed] [Google Scholar]
- Santibanez SS, Garfein RS, Swartzendruber A, Purcell DW, Paxton LA, Greenberg AE. Update and overview of practical epidemiologic aspects of HIV/AIDS among injection drug users in the United States. Journal of Urban Health. 2006;83(1):86–100. doi: 10.1007/s11524-005-9009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, Inc. Design and analysis of probability surveys. Cary, NC: SAS Institute; 2005. [Google Scholar]
- Somlai AM, Kelly JA, McAuliffe TL, Ksobiech K, Hackl KL. Predictors of HIV sexual risk behaviors in a community sample of injection drug-using men and women. AIDS and Behavior. 2003;7(4):383–393. doi: 10.1023/b:aibe.0000004730.62934.ed. [DOI] [PubMed] [Google Scholar]
- Taussig JA, Weinstein B, Burris S, Jones TS. Syringe laws and pharmacy regulations are structural constraints on HIV prevention in the US. AIDS. 2000;14(Suppl 1):S47–S51. doi: 10.1097/00002030-200006001-00007. [DOI] [PubMed] [Google Scholar]
- Tempalski B, Flom P, Friedman SR, Des Jarlais DC, Friedman J, McKnight C, et al. Social and political factors predicting the presence of syringe exchange programs in 96 U.S. metropolitan areas. American Journal of Public Health. 2007;97(3):437–447. doi: 10.2105/AJPH.2005.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede H, Hagan H, Campbell JV, Strathdee SA, Bailey SL, Hudson SM, et al. Prevalence and correlates of indirect sharing practices among young adult injection drug users in five U.S. cities. Drug and Alcohol Dependence. 2007;91(Suppl 1):S39–S47. doi: 10.1016/j.drugalcdep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Tortu S, McMahon JM, Hamid R, Neaigus A. Women’s drug injection practices in East Harlem: An event analysis in a high-risk community. AIDS and Behavior. 2003;7(3):317–328. doi: 10.1023/a:1025452021307. [DOI] [PubMed] [Google Scholar]
- van Ameijden EJ, Krol A, Vlahov D, Flynn C, van Haastrecht HJ, Coutinho RA. Pre-AIDS mortality and morbidity among injection drug users in Amsterdam and Baltimore: An ecological comparison. Substance Use & Misuse. 1999;34(6):845–865. doi: 10.3109/10826089909037245. [DOI] [PubMed] [Google Scholar]
- Vlahov D, Des Jarlais DC, Goosby E, Hollinger PC, Lurie PG, Shriver MD, et al. Needle exchange programs for the prevention of human immunodeficiency virus infection: Epidemiology and policy. American Journal of Epidemiology. 2001;154(12 Suppl):S70–S77. doi: 10.1093/aje/154.12.s70. [DOI] [PubMed] [Google Scholar]