Abstract
Background
Although spinal magnetic resonance imaging (MRI) findings of neuromyelitis optica (NMO) have been described, there is limited data available that help differentiate NMO from other causes of longitudinally extensive transverse myelitis (LETM).
Objective
To investigate the spinal MRI findings of LETM that help differentiate NMO at the acute stage from multiple sclerosis (MS) and other causes of LETM.
Methods
We enrolled 94 patients with LETM into our study. Bright spotty lesions (BSL), the lesion distribution and location were evaluated on axial T2-weighted images. Brainstem extension, cord expansion, T1 darkness and lesion enhancement were noted. We also reviewed the brain MRI of the patients during LETM.
Results
Patients with NMO had a greater amount of BSL and T1 dark lesions (p < 0.001 and 0.003, respectively). The lesions in NMO patients were more likely to involve greater than one-half of the spinal cord’s cross-sectional area; to enhance and be centrally-located, or both centrally- and peripherally-located in the cord. Of the 62 available brain MRIs, 14 of the 27 whom were NMO patients had findings that may be specific to NMO.
Conclusions
Certain spinal cord MRI features are more commonly seen in NMO patients and so obtaining brain MRI during LETM may support diagnosis.
Keywords: Brain, diagnostic imaging, lesions, magnetic resonance imaging, neuroimaging, neuromyelitis optica, spinal cord, transverse myelitis
Introduction
Longitudinally extensive transverse myelitis (LETM) is defined as a hyperintense spinal cord lesion extending over three or more vertebral levels on sagittal T2-weighted (T2W) spinal magnetic resonance imaging (MRI).1 Most patients with LETM encountered in routine radiology practice have limited available clinical information at the time of their spinal MRI interpretation, especially those presenting with myelopathy for the first time. Although LETM is accepted as one of the supportive diagnostic criteria2 of neuromyelitis optica (NMO), there are other causes of LETM; such as infections, rheumatic disorders, sarcoidosis, dural arteriovenous fistula and multiple sclerosis (MS), to name a few that have diagnostic and treatment management different from NMO.3 Differentiating NMO from other etiologies is clinically important, to provide early accurate treatment, and may prevent future attacks and avoid subsequent severe disability.4–6
Brain MRI may help in the differential diagnosis of LETM. The brain MRI of a NMO patient is historically thought to be normal or non-specific, especially at the onset of the disease; however, the presence of certain lesions described as specific to NMO may differentiate NMO from other causes of LETM.7–9 Thus, we retrospectively reviewed the spinal MRI of patients presenting with LETM, to investigate the most sensitive and specific findings to discriminate NMO at the acute stage from MS and other causes of LETM. We also evaluated the added value of brain MRI acquired during acute LETM.
Materials and methods
Patients
We collected the clinical data reviewed for this retrospective study from institutional review board (IRB)-approved databases; additional permission was granted for publication. We retrospectively searched the radiology department’s database of MRI spine studies conducted between 2002 and 2012, using the keyword ‘myelitis’. A radiology resident reviewed the MRI reports and identified those patients with ‘transverse myelitis longer than three vertebral segments’ or with ‘hyperintense spinal cord lesion extending over three or more vertebral levels’ on their sagittal T2W spinal MRI. For the NMO patients, only spinal MRIs that showed transverse myelitis longer than three vertebral segments (thus, LETM) and that were obtained within 30 days after the clinical onset of their NMO were enrolled into the study. When available, we also reviewed any brain MRI obtained with the spinal MRI.
We excluded the following patients from our study: A set of 23 with the diagnosis of idiopathic transverse myelitis whom were without follow-up MRIs after 5 years and/or not tested for NMO-IgG, 13 patients whom were seronegative for NMO-IgG and were eventually diagnosed with NMO, six NMO patients with spinal cord MRIs obtained 30 or more days after clinical onset, 13 patients with artifacts that prevented evaluating the axial and/or sagittal spinal cord images (nine MS, three NMO and one bacterial meningitis patient), and one patient with compressive myelopathy whom was not tested for NMO-IgG.
The criteria for NMO diagnosis was based on the revised diagnostic criteria proposed by Wingerchuk et al.2 in 2006. Only the patients whom were positive for serum NMO-IgG (either with the Mayo Clinic test, using an indirect immunofluorescence assay, or the Quest enzyme-linked immunosorbent assay (ELISA)) were included.
The diagnostic criteria for MS were based on the McDonald criteria10; all patients fulfilled the 2010 McDonald criteria and carried a clinical diagnosis of MS. The specific diagnoses of other patients with LETM were made by using relevant diagnostic criteria and these diagnoses were verified with follow-up clinical documents and/or seronegativity for NMO-IgG. The diagnosis of vascular myelopathy was confirmed by spinal angiogram and included dural arteriovenous fistulas, spinal cord strokes and myelopathic venous hypertension. Rheumatologic diseases were diagnosed by recent clinical criteria and supported by negative serologic testing for NMO. Other infectious and granulomatous diseases were diagnosed with the appropriate laboratory tests and supported by seronegativity for NMO. The diagnoses of paraneoplastic myelitis and radiation myelitis were made by clinical history, laboratory tests and seron-egativity for NMO.
Spinal MRI
At our institution, the MRI examinations were performed using either a 1.5T or 3T magnet from different manufacturers: Philips (Philips Medical Systems, Best, the Netherlands), GE (GE Medical Systems, Milwaukee, WI, USA), and Siemens (Siemens AG, Erlangen, Germany). A routine spinal MRI included a sagittal T1-weighted (T1W) sequence, sagittal and axial T2W sequences and a sagittal short tau inversion recovery (STIR) sequence. In this study, 88 patients underwent contrast imaging after intravenous administration of gadolinium-based contrast media.
All images were retrieved from our picture archives and communication system, and were retrospectively reviewed by two neuroradiologists. The two readers did not interpret the original studies and were blinded to the patients’ clinical data, laboratory findings and the final diagnosis.
We evaluated the lesion localization, presence of brainstem extension and cord expansion on sagittal T2W images. On the axial T2W images, special attention was given to the bright spotty lesions (BSLs), similar or higher in signal intensity than cerebrospinal fluid (CSF), which were previously described by Yonezu et al.11 (Figure 1). We also evaluated for preserved peripheral T2 hypointensity, the lesion distribution and lesion location on axial imaging. Lesion distribution was classified as: ‘peripherally-located’, ‘centrally-located’, or ‘both centrally- and peripherally-located’. Lesions that were ≥ 50% of the spinal cord area (transversally extensive myelitis)11 were noted (Figure 2, Figure 3).
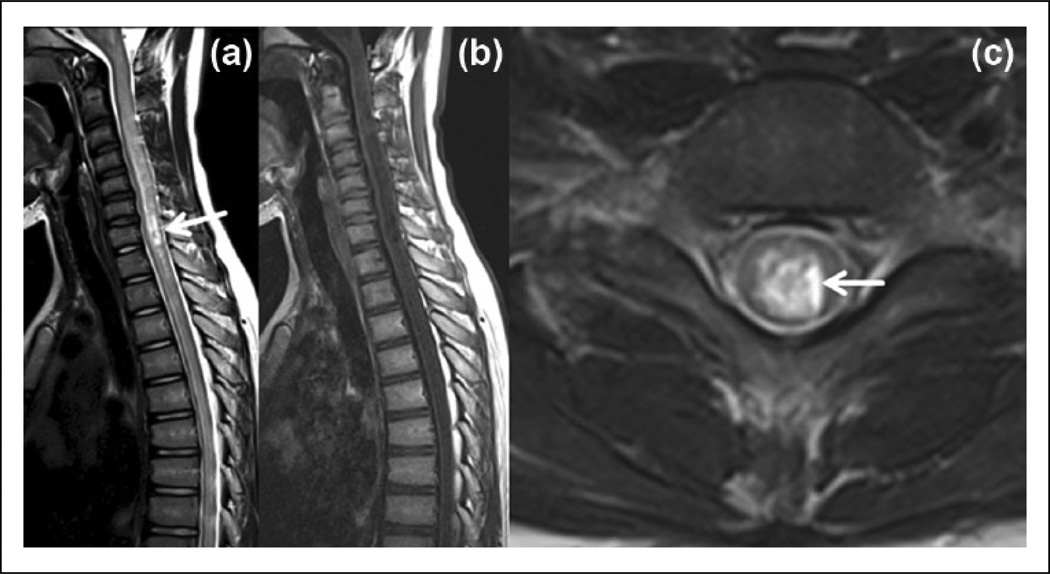
Figure 1.
Spinal MRI of a 12-year-old female with seropositive NMO shows: (a) A typical longitudinally extensive transverse myelitis extending into the brainstem with ‘bright spotty lesions’ (arrow) on a T2-weighted sagittal image. (b) The lesion is a very hypointense, ‘T1 dark’ on a T1-weighted image. (c) On a T2-weighted axial image, the lesion is both centrally- and peripherally-located and peripheral T2 hypointensity is preserved. Arrow demonstrates the ‘bright spotty lesion’.
MRI: magnetic resonance imaging; NMO: neuromyelitis optica.
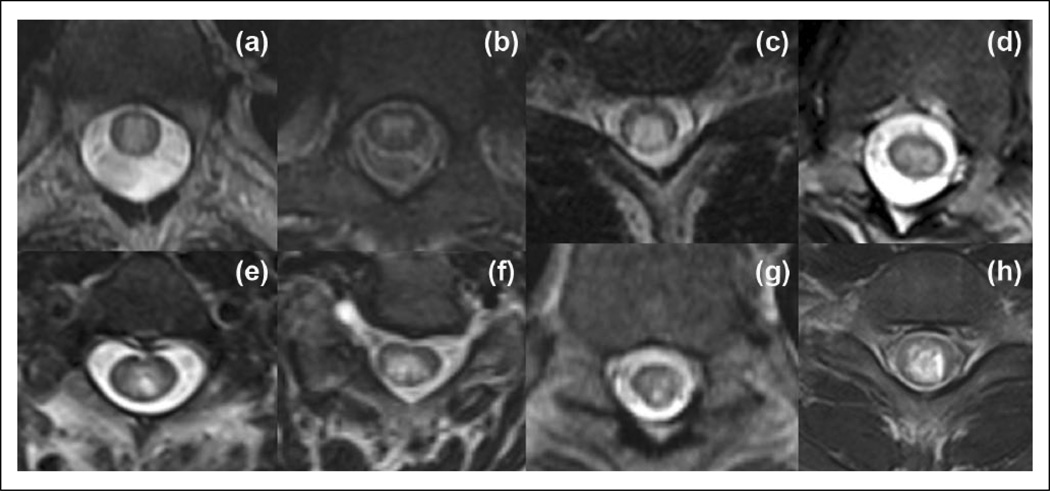
Figure 2.
T2-weighted axial images in different patients with NMO. (a)–(d) Images show centrally-located (in (b)) and both centrally- and peripherally-located lesions (in (a), (c), (d)) and lesions occupy about one-half or more than one-half of the cord area (in (a), (c) and (d)). (e)–(h) Images demonstrate punctate (in (e), (f), (g) or larger (in (h)) ‘bright spotty lesions’.
NMO: neuromyelitis optica.
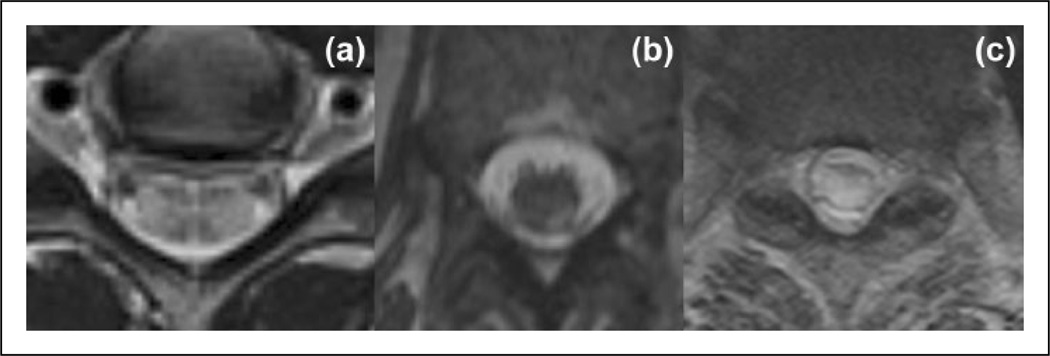
Figure 3.
T2-weighted axial images of longitudinally extensive transverse myelitis in patients with MS and spinal cord stroke. (a) and (b) Peripherally-located lesions in different patients with MS. (c) T2-weighted axial image of the patient with spinal cord stroke. Whole spinal cord cross-sectional area is expanded and hyperintense.
MS: multiple sclerosis.
We also recorded the T1 signal intensity of the lesion. Lesions were defined as ‘T1 dark’ when the signal intensity of the lesion approached that of CSF on the T1W images (Figure 1(b)). We noted if the spinal cord lesions showed enhancement after the administration of intravenous gadolinium contrast material. Enhancement was classified as either ‘present’, which was subdivided as ‘well-defined and homogeneous’, ‘ill-defined and heterogeneous’, or ‘not present’ on the T1 post-contrast images.
Brain MRI
The brain MRI examinations were performed at either 1.5T or 3T magnet strength on scanners from different manufacturers. The MRI protocol included diffusion-weighted images with calculated apparent diffusion coefficient (ADC) maps, T1W images, fast spine-cho T2W images, fluid-attenuated inversion recovery (FLAIR) images and contrast-enhanced T1W images. We classified brain MRI findings as: ‘ normal’, ‘non-specific’ and ‘specific’. ‘Specific’ brain MRI findings reflect a specific illness and were classified as: ‘MS-like’, ‘NMO-like’ and ‘other’. ‘Other’ was used to define brain MRI findings that were seen with an illness, such as ischemia and infection.
Lesions on the brain MRI were ‘MS-like’ when they fulfilled the 2010 McDonald MRI criteria (Figure 4).10 Lesions were ‘NMO-like’ when they were:
Located in the hypothalamus, around the third and the fourth ventricle, or the cerebral aqueduct;
Located in the dorsal medulla, area postrema (may be linear and continuous with cervical LETM);
Located in the corticospinal tract; and
When ‘Dawson’s finger’-type periventricular lesions and juxtacortical lesions were not present.
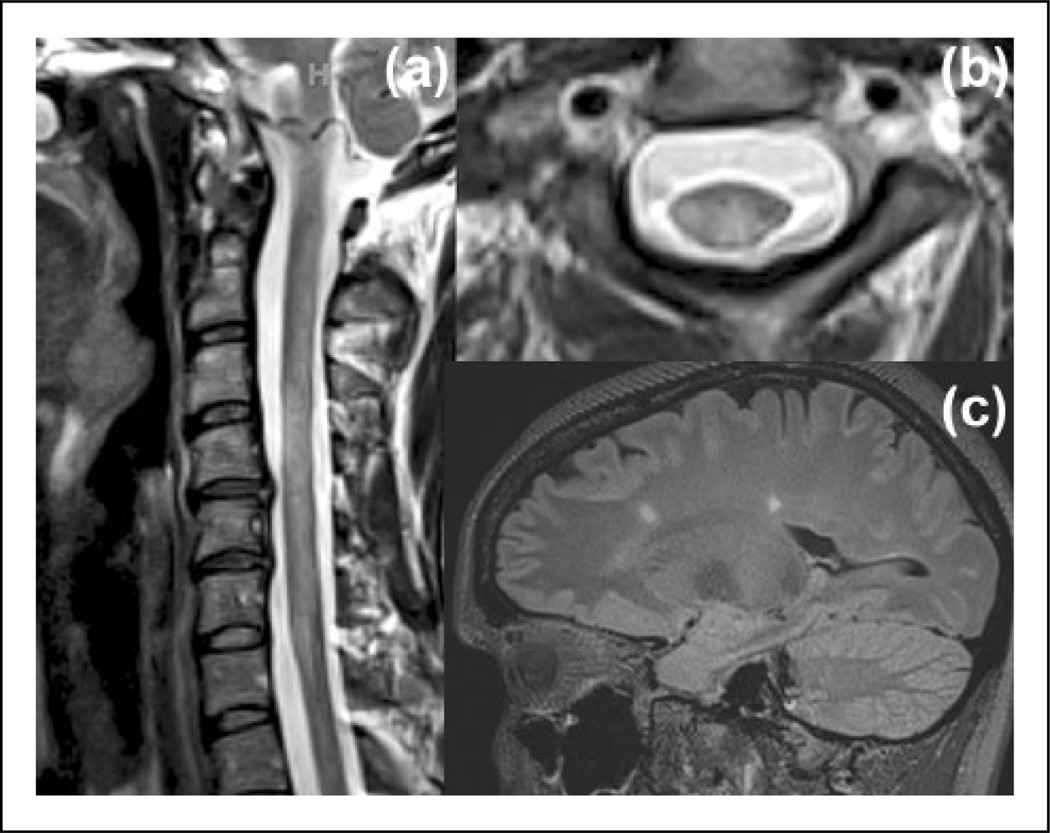
Figure 4.
Brain and spinal MRI findings of a MS patient with LETM. The spinal MRI was obtained about 3 days after clinical onset. (a) T2-weighted sagittal image shows LETM. (b) On T2-weighted axial image the lesion is both centrally- and peripherally-located, and occupies more than one-half of the cord area. (c) FLAIR sagittal image shows vertically-oriented periventricular lesions (Dawson’s fingers) and juxtacortical lesions (not shown).
FLAIR: fluid-attenuated inversion recovery; LETM: longitudinally extensive transverse myelitis; MRI: magnetic resonance imaging; MS: multiple sclerosis.
Periependymal linear or patchy enhancement with blurred margins was accepted as typical to NMO.6,7,12–16 Having two of the first three lesions and absence of ‘Dawson’s finger’ type periventricular lesions and absence of juxtacortical lesions with/without associated typical enhancement were accepted as a ‘NMO-like’ finding (Figure 5). Lesions that could be seen in NMO patients, but also in normal elderly patients (chronic small vessel ischemic changes), in MS patients (nonspecific corpus callosum lesions) and in ADEM patients (extensive cerebral lesions) were categorized as ‘nonspecific’.17–19
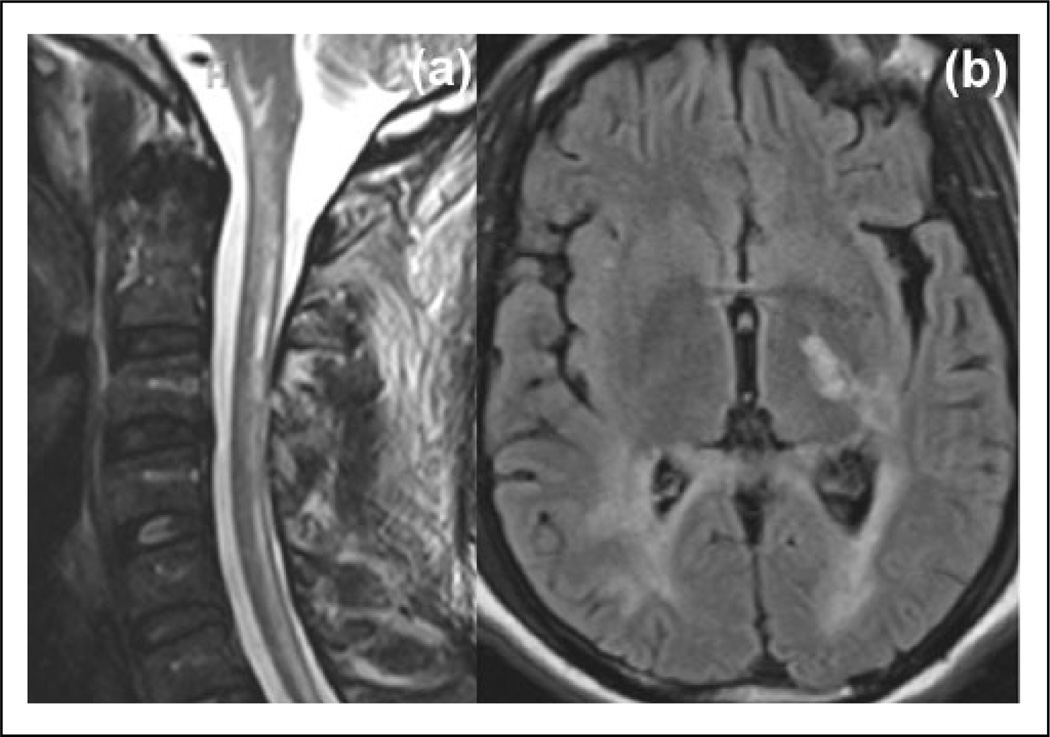
Figure 5.
Brain MRI findings of a NMO patient with acute LETM. (a) Spinal cord MRI displays LETM extending into the brainstem. (b) FLAIR axial image shows lesions in the left corticospinal tract and periventricular white matter that extend to the corpus callosum (with no ‘Dawson’s finger’ lesions).
FLAIR: fluid-attenuated inversion recovery; LETM: longitudinally extensive transverse myelitis; NMO: neuromyelitis optica; MRI: magnetic resonance imaging; MS: multiple sclerosis.
Statistical analysis
Fisher’s exact test was performed to compare spinal cord MRI findings between NMO patients presenting with acute LETM, MS and other causes of LETM. The Kruskal-Wallis test was used to compare age at presentation of LETM on spinal MRI among the three groups. Results with p-values ⩽ 0.05 were considered to be statistically significant. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each spinal MRI feature for distinguishing NMO from all other causes of LETM, including MS, were calculated. All statistical analyses were performed using STATA 13.1 (STATA Corp, College Station, TX, USA).
Results
Patient characteristics
Initial database inquiry of radiology reports yielded 818 spinal MRI scans with ‘myelitis’; 160 patients fulfilled the criteria for LETM, i.e. either ‘transverse myelitis longer than three vertebral segments’ or ‘hyperintense spinal cord lesion extending over three or more vertebral levels’ on sagittal T2W spinal MRI. Of the 160 patients who were diagnosed with LETM, 94 patients were enrolled in the study after meeting the inclusion criteria: 68 patients were female and 26 patients were male, and their mean age at spinal MRI was 44.5 years (age range: 5–89 years).
The causes of the LETM were demonstrated in Table 1.
Table 1.
Causes of the LETM in our study population.a.
| Cause | Patients n (%) |
|---|---|
| Neuromyelitis opticab | 48 (51%) |
| MS | 22 (23%) |
| Spinal vascular causes | |
| Spinal cord stroke | 6 (6%) |
| SDAVF | 4 (4%) |
| Infectious and parainfectious | |
| HIV | 2 (2%) |
| VZV | 1 (1%) |
| Lyme disease | 1 (1%) |
| HSV | 1 (1%) |
| Bacterial meningitis | 1 (1%) |
| Neurosarcoidosis | 3 (3%) |
| Paraneoplastic | 3 (3%) |
| SLE | 1 (1%) |
| Radiation | 1 (1%) |
After exclusion.
NMO-IgG positive patients.
HIV: Human Immunodeficiency Virus; HSV: herpes simplex virus; IgG: immunoglobulin G (G type antibodies); LETM: longitudinally extensive transverse myelitis; MS: multiple sclerosis; NMO: neuromyelitis optica; SDAVF: Spinal dural arteriovenous fistula; SLE: systemic lupus erythematosus; VZV: varicella-zoster virus.
All NMO patients were NMO-IgG positive. Only five patients with MS and six patients with vascular causes were tested for NMO-IgG and were negative. Because of the retrospective study design, not all patients were tested for NMO-IgG, including MS patients with typical clinical/radiological findings and follow-up, and patients with vascular lesions that were proved by further imaging (angiography and diffusion weighted imaging). All other patients were tested and found negative for NMO-IgG.
The mean age of the patients at the time of spinal MRI was similar, but the number of female patients and African American patients were significantly higher in the NMO group who presented with acute LETM, when compared with MS and other causes of LETM (p < 0.001).
Spinal MRI findings
Distinctive spinal MRI findings of the NMO, when compared with MS and other causes of LETM, are outlined in Table 2.
Table 2.
Distinctive clinical features and spinal MRI findings of patients with LETM.
| Characteristics | NMO |
MS |
Othera |
p-value |
|---|---|---|---|---|
| n = 48 | n = 22 | n = 24 | ||
| Female, n (%) | 44 (64.7%) | 17 (25%) | 7 (10.3%) | < 0.001 |
| African American, n (%) | 33 (76.8%) | 5 (11.6%) | 5 (11.6%) | < 0.001 |
| Age at MRI study, years | 44.4 ± 16.4 | 41.6 ± 13.0 | 45.7 ± 23.7 | 0.655 |
| T2 sagittal, lesion localization | ||||
| Cervical, n (%) | 16 (51.6%) | 11 (35.5%) | 4 (12.9%) | 0.007 |
| Cervicodorsal, n (%) | 13 (65%) | 2 (10%) | 5 (25%) | |
| Dorsal, n (%) | 16 (55.2%) | 5 (17.2%) | 8 (27.6%) | |
| Dorsolumbar, n (%) | 0 (0%) | 0 (100%) | 5 (100%) | |
| CDL (holocord), n (%) | 3 (33.3%) | 4 (44.5%) | 2 (22.2%) | |
| Brainstem extension, n (%) | 18 (64.3%) | 7 (25%) | 3 (10.7%) | 0.082 |
| Cord expansion, n (%) | 36 (54.5%) | 11 (16.7%) | 19 (28.8%) | 0.369 |
| T2 axial lesion | ||||
| > 1/2 of the cord area, n (%) | 45 (58.4%) | 11 (14.3%) | 21 (27.3%) | < 0.001 |
| T2 axial lesion distribution | ||||
| Centrally-located, n (%) | 15 (57.7%) | 1 (3.8%) | 10 (38.5%) | < 0.001 |
| Both areas, n (%) | 32 (56.1%) | 13 (22.8%) | 12 (21.1%) | |
| Peripherally-located, n (%) | 1 (9.1%) | 8 (72.7%) | 2 (18.2%) | |
| T2 axial, | 24 (58.5%) | 3 (7.3%) | 14 (34.2%) | 0.003 |
| preserved peripheral dark rim, n (%) | ||||
| T2 axial, | 31 (86.1%) | 2 (5.6%) | 3 (8.3%) | < 0.001 |
| bright spotty lesions, n (%) | ||||
| T1 dark, n (%) | 26 (70.3%) | 5 (13.5%) | 6 (16.2%) | 0.012 |
| Contrast enhancement, n (%)b | 28 (65.1%) | 6 (14%) | 9 (20.9%) | 0.083 |
| Enhancement pattern | 0.714 | |||
| Well-defined, homogeneous, n (%) | 8 (57.1%) | 2 (14.3%) | 4 (38.6%) | |
| ill-defined, heterogeneous, n (%) | 20 (69%) | 4 (13.8%) | 5 (17.2%) |
Other etiologies of LETM.
We had 88 patients whom had contrast enhanced images.
CDL: Cervicodorsolumbar; LETM: longitudinally extensive transverse myelitis; MRI: magnetic resonance imaging; MS: multiple sclerosis; NMO: neuromyelitis optica.
Bright spotty lesions on axial T2W images were the most distinctive finding of NMO in spinal MRI (p < 0.001). The presence of ‘T1 dark’ signal was also significantly higher in NMO patients. Lesions that involved ≥ 50% of the spinal cord axial cross-sectional area and central localization were more frequently found in NMO patients. NMO patients with acute LETM more frequently had enhancement than all the other causes of LETM, including MS (p = 0.004); however, when we considered MS as separate group and compared the presence of enhancement among NMO, MS and other causes, the statistical analyses showed no significant difference. Enhancement patterns showed no significant differences. The sensitivity, specificity, PPV and NPV of the significant spinal MRI features for distinguishing NMO from other etiologies, including MS, are displayed in Table 3.
Table 3.
Sensitivity, specificity, PPV and NPV of the spinal cord MRI features of LETM for distinguishing NMO from other etiologies including MS.
| Centrally- located lesion |
Lesion >1/2 of the cord area |
T2 bright, spotty lesions |
Contrast enhancement |
T1 dark | |
|---|---|---|---|---|---|
| Sensitivity, % | 97.9 | 93.8 | 64.6 | 64.6 | 54.2 |
| Specificity, % | 21.7 | 30.4 | 89.1 | 60.9 | 76.1 |
| PPV, % | 56.6 | 58.4 | 86.1 | 63.3 | 70.3 |
| NPV, % | 90.9 | 82.4 | 70.7 | 62.2 | 61.4 |
LETM: Longitudinally extensive transverse myelitis; MRI: magnetic resonance imaging; MS: multiple sclerosis; NMO: neuromyelitis optica; NPV: negative predictive value; PPV: positive predictive value.
Preserved peripheral T2 hypointensity of the spinal cord was statistically higher in NMO at the acute stage (p = 0.003), when we considered MS as a separate group, and compared preserved peripheral T2 hypointensity among NMO, MS and other causes of LETM. Lesion location (cervical, thoracic, etc.) on the sagittal T2W images was found to be distinctive, when we compared among NMO, MS and other causes of LETM. Brainstem extension and cord expansion were not significantly different between NMO patients and other etiologies of acute LETM.
Brain MRI findings
Of the 94 patients with LETM, 62 patients had a brain MRI on the same day or within 1 week of spinal MRI. Patients’ brain MRI findings during LETM are summarized in Table 4. Brain MRI findings specific to NMO, the so-called ‘NMO-like’ lesions, were found in 14 of 27 NMO patients (51.8%); 17 of 21 patients (81%) with MS had findings that fulfilled MS criteria with the so-called ‘MS-like’ lesions on their MRIs. One patient showed diffuse leptomeningeal enhancement and eventually was diagnosed with myelitis secondary to bacterial meningitis. One patient had findings of hypoxic ischemic injury with hyperintensity of the basal ganglia and cortex on FLAIR, hyperintensity on T1W images, and restricted diffusion. In this particular patient, the LETM was diagnosed as secondary to a spinal cord infarction.
Table 4.
Brain MRI findings of the patients during LETM.
| Diagnosis by brain MRI (n patients) |
Brain MRI findings | ||||
|---|---|---|---|---|---|
| Normal n (%) | Nonspecific n (%) | Maybe specific |
|||
| MS-like n (%) | NMO-like n (%) | Other n (%) | |||
| NMO (27) | 3 (11.1%) | 10 (37%) | 0 | 14 (51.8%) | 0 |
| MS (21) | 1 (4.8%) | 3 (14.3%) | 17 (81%) | 0 | 0 |
| Vascular causes | |||||
| Spinal cord stroke (3) | 2 (66.6%) | 0 | 0 | 0 | 1(33.3%)a |
| SDAVF (3) | 1 (33.3%) | 2 (66.6%) | 0 | 0 | 0 |
| Infectious, parainfectious | |||||
| HIV myelopathy (2) | 1 (50%) | 1 (50%) | 0 | 0 | 0 |
| VZV myelopathy (1) | 1 (100%) | 0 | 0 | 0 | 0 |
| HHV6 (1) | 0 | 1 (100%) | 0 | 0 | 0 |
| Bacterial (1) | 0 | 0 | 0 | 0 | 1(100%)b |
| Lyme (1) | 0 | 1 (100%) | 0 | 0 | 0 |
| Paraneoplastic (1) | 0 | 1 (100%) | 0 | 0 | 0 |
| Sarcoidosis (1) | 0 | 1 (100%) | 0 | 0 | 0 |
Findings compatible with hypoxic ischemic encephalopathy.
Diffuse leptomeningeal enhancement.
HHV6: human herpesvirus 6; HIV: human immunodeficiency virus; LETM: longitudinally extensive transverse myelitis; MRI: magnetic resonance imaging; MS: multiple sclerosis; NMO: neuromyelitis optica; SDAVF: spinal dural arteriovenous fistula; SLE: systemic lupus erythematosus; VZV: varicella-zoster virus.
Discussion
We retrospectively reviewed the spinal MRI of patients with LETM and found important distinctive features of NMO. The most useful MRI findings were: The presence of BSLs (either punctuate or as larger cavities) and T1 dark lesions, centrally-located or both centrally- and peripherally-located lesions, and a lesion involving ≥ 50% of the cord area. The BSLs were recently described as the most striking NMO spinal MRI finding, when compared to MS (sensitivity: 88%; specificity: 97%).11 According to our study, it was also found to be a specific finding (sensitivity: 64.6%; specificity: 89.1%) for not only differentiating NMO from MS, but also for differentiating NMO from all other etiologies of LETM. Our investigation compared NMO patients not only with MS, but also with other causes of LETM. An earlier diagnosis of NMO may be possible, with careful attention to the presence of BSLs and other above-mentioned features. Making an earlier diagnosis of NMO with the aid of distinctive MRI features may help prevent further disability, by allowing for early immunosuppressive treatment.20,21
T1 signal intensity of the lesion approaching that of CSF, ‘T1 dark’, was a relatively specific finding in our study and may be used in conjunction with the BSLs. Both these features probably reflect the same pathology: Early necrotic and cavitary changes, due to severe intrinsic cord damage in NMO.11,22 T1 hypointensity was described in previous studies as being preferentially centrally located23 and higher in prevalence in seropositive patients.24 Because of the previously detected poor interobserver agreement in defining the T1 hypointensity in LETM patients,24 we used the term ‘T1 dark’ to clarify the terminology regarding T1 signal intensity. We also noted some BSLs show corresponding T1 isointense signal, which might relate to a less severe cord injury.
Although NMO spinal lesions are known to be centrally-located and preferentially involve gray matter,23,25 we found the NMO lesions to be either centrally-located or both centrally- and peripherally-located, in our study. The peripherally-located lesions may be a useful finding to discriminate the MS lesions from NMO.
Lesions occupying ≥ 50% of the spinal cord axial cross-sectional area, the so called ‘transversally-extensive lesion’, have been reported to have a higher incidence in NMO patients than in MS.11 This was also a distinctive spinal MRI finding in our study and it was highly sensitive in differentiating NMO from all other causes of LETM (93.8%), but it did not reach an acceptable specificity (30.4%).
Previous studies show contrast enhancement of the spinal cord lesions in both seronegative and seropositive NMO patients, during acute myelitis attacks, 26 with no significant difference between the NMO and MS patients.11 Contrast enhancement, either well-defined and homogeneous or ill-defined heterogeneous, was significantly higher in NMO patients than all other causes of LETM, including MS, in our study. This may be due to acquisitions of images in the acute stage of the NMO, in our study. It has been described that the timing of the MRI acquisition influences the imaging findings27; but when we compared the presence of contrast enhancement among NMO, MS, each as a separate group, and the other causes of LETM, we found no significant difference. T2 hyperintensity of the central cord with a preserved peripheral T2 hypointensity is reported more commonly in NMO, when compared to MS, as we observed in our study25; but it can also be seen in other pathologies, and is not specific to NMO.28
In our study, most of the NMO lesions were localized in the cervical, dorsal or cervicodorsal spinal levels. Almost none of the NMO and MS patients had LETM that was located only at the dorsolumbar level. Additionally, brainstem extension and cord expansion, which is thought to be specific to NMO3,29and reportedly useful in distinguishing NMO from MS, was not found to be statistically significant. This likely reflected our inclusion criteria, as we enrolled not only MS patients, but also other causes of LETM, within 30 days after their clinical findings began.
Brain MRI revealed 14 additional NMO patients (51.8%) with LETM who had brain findings that might be specific to NMO, which is higher than has previously been reported.5,30 A previous study demonstrated brain MRI findings of three cases with NMO during acute relapses that were different from MS, and those lesions disappeared with time.31 Given the importance of the brain MRI findings in differential diagnosis and potential reversibility of some lesions,31,32 obtaining brain MRI during LETM might give valuable information to diagnose NMO.
We acknowledge several limitations in our study. First, we excluded patients with acute LETM whom were seronegative to NMO-IgG and eventually diagnosed with NMOSD. Recently, antibodies to myelin oligodendrocyte glycoprotein (MOG) were found in NMO-IgG seronegative patients with clinically-definite and high-risk NMOSD.33 In addition, with the increased sensitivity and specificity of the Mayo cell-based assay, more patients have proved to be NMO-IgG positive, when compared to other assays. Our goal was to include patients with definite diagnoses into our retrospective study, so that we could test for distinctive MRI criteria. Additionally, patients who were seropositive to NMO-IgG have been shown to be at high risk for relapse.34 We also excluded patients with the diagnosis of idiopathic LETM whom had no clinical nor radiologic follow-up longer than 5 years and whom were not tested for NMO-IgG. It is difficult to be completely certain whether the LETM is monophasic without any follow-up; and furthermore, NMO can remit for years, and rarely, even for decades.35 It seems unlikely to definitely differentiate idiopathic LETM from NMO, based on only MRI findings.
Second, our investigation was a retrospective study and its images were obtained with different scanner magnet strengths, which could affect the sensitivity. Third, due to the retrospective design of our study, most of the MS patients with LETM were not tested for NMO-IgG, but all of these patients had a follow-up, and over time, their clinical and radiological phenotypes were consistent with MS. Only five MS patients presented with acute LETM, while the others had slowly progressing flares; however, when they presented with their complaints and a spinal MRI was performed, multiple MS cord lesions had resulted in a longitudinally extensive diffuse increase in the cord signal on the T2-weighted images. MS patients diagnosed for more than 5–7 years tend to have more multiple, but diffuse, cord involvement that might look like LETM. Lastly, although we retrospectively evaluated almost all patients that had ‘transverse myelitis longer than three vertebral segments’ or ‘hyperintense spinal cord lesion, extending over three or more vertebral levels’ on the sagittal T2W spinal MRI, we still could miss some patients with LETM.
In conclusion, it is clinically important to differentiate LETM in patients with an acute stage of NMO from other etiologies, in order to initiate early immunosuppression therapy and prevent future attacks. BSL is a highly-specific finding, for not only differentiating NMO from MS, but also for differentiating NMO from all the other etiologies of LETM. Obtaining a brain MRI during LETM may help diagnosis and potentially help to guide an earlier initiation of therapy.
Acknowledgments
Funding statement
We would like to acknowledge support for the statistical analysis from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
Maureen A Mealy received honoraria from the International Organization of Multiple Sclerosis Nurses and from EMD Serono. Scott Newsome has received consultant fees from Biogen-Idec, Genzyme and Novartis; and research support from Biogen-Idec and Novartis. Carlos A Pardo Villamizar, has received support from the Bart McLean Fund for Neuroimmunology Research and from Project Restore. Michael Levy receives research support from the US National Institute of Health (NIH), the Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem and Genentech; and he serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline and Medimmune. Peter A Calabresi has received personal compensation for consulting or serving on scientific advisory boards of Merck, Vertex, Vaccinex and Abbvie; and has received research funding from Biogen-IDEC, Novartis and Medimmune. Izlem Izbudak received research support from Bayer for reading magnetic resonance angiography images.
Footnotes
Conflict of interest
Yeliz Pekcevik, Charles H Mitchell, Gunes Orman, In H Lee and Carol B Thompson declare that they have no conflicts of interest.
There are no direct financial nor other conflicts of interest in relation to the current publication.
Contributor Information
Yeliz Pekcevik, Russell H Morgan, Department of Radiology and Radiological Science, Division of Neuroradiology, Johns Hopkins Hospital, Baltimore, MD, USA.
Charles H Mitchell, Russell H Morgan, Department of Radiology and Radiological Science, Division of Neuroradiology, Johns Hopkins Hospital, Baltimore, MD, USA.
Maureen A Mealy, Johns Hopkins Transverse Myelitis and Multiple Sclerosis Centers, Baltimore, MD, USA.
Gunes Orman, Russell H Morgan, Department of Radiology and Radiological Science, Division of Neuroradiology, Johns Hopkins Hospital, Baltimore, MD, USA.
In H Lee, Russell H Morgan, Department of Radiology and Radiological Science, Division of Neuroradiology, Johns Hopkins Hospital, Baltimore, MD, USA/Department of Radiology, Chungnam National University Hospital, Daejeon, Korea.
Scott D Newsome, Division of Neuroimmunology and Neuroinfectious Diseases, Johns Hopkins Hospital, Baltimore, MD, USA.
Carol B Thompson, Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins, Baltimore, MD, USA.
Carlos A Pardo, Division of Neuroimmunology and Neuroinfectious Diseases, Johns Hopkins Hospital, Baltimore, MD, USA.
Peter A Calabresi, Department of Neurology, Johns Hopkins Hospital, Baltimore, MD, USA.
Michael Levy, Department of Neurology, Johns Hopkins Hospital, Neuromyelitis Optica Clinic Baltimore, MD, USA.
Izlem Izbudak, Russell H Morgan, Department of Radiology and Radiological Science, Division of Neuroradiology, Johns Hopkins Hospital, Baltimore, MD, USA.
References
- 1.Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 3.Kitley JL, Leite MI, George JS, et al. The differential diagnosis of longitudinally extensive transverse myelitis. Mult Scler. 2012;18:271–285. doi: 10.1177/1352458511406165. [DOI] [PubMed] [Google Scholar]
- 4.Jiao Y, Fryer JP, Lennon VA, et al. Aquaporin 4 IgG serostatus and outcome in recurrent longitudinally extensive transverse myelitis. J Am Med Ass Neurol. 2014;71:48–54. doi: 10.1001/jamaneurol.2013.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett Y, Sutton IJ, Ghadiri M, et al. Conventional and advanced imaging in neuromyelitis optica. Am J Neuroradiol. 2014;35:1458–1466. doi: 10.3174/ajnr.A3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura M, Miyazawa I, Fujihara K, et al. Preferential spinal central gray matter involvement in neuromyelitis optica A MRI study. J Neurol. 2008;255:163–170. doi: 10.1007/s00415-008-0545-z. [DOI] [PubMed] [Google Scholar]
- 7.Pittock SJ, Lennon VA, Krecke K, et al. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63:390–396. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 8.Matthews L, Marasco R, Jenkinson M, et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology. 2013;80:1330–1337. doi: 10.1212/WNL.0b013e3182887957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera-Gomez JA, Kister I. Conventional brain MRI in neuromyelitis optica. Eur J Neurol. 2012;19:812–819. doi: 10.1111/j.1468-1331.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonezu T, Ito S, Mori M, et al. ‘Bright spotty lesions’ on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult Scler. 2014;20:331–337. doi: 10.1177/1352458513495581. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Park MS, Lee SH, et al. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Mult Scler. 2010;16:1229–1236. doi: 10.1177/1352458510376640. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Kim SH, Lee SH, et al. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2011;17:1107–1112. doi: 10.1177/1352458511404917. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wu A, Zhang B, et al. Comparison of deep gray matter lesions on magnetic resonance imaging among adults with acute disseminated encephalomyelitis, multiple sclerosis and neuromyelitis optica. Mult Scler. 2014;20:418–423. doi: 10.1177/1352458513499420. [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Mori M, Makino T, et al. ‘Cloud-like enhancement’ is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol. 2009;66:425–428. doi: 10.1002/ana.21753. [DOI] [PubMed] [Google Scholar]
- 16.Banker P, Sonni S, Kister I, et al. Pencil-thin ependymal enhancement in neuromyelitis optica spectrum disorders. Mult Scler. 2012;18:1050–1053. doi: 10.1177/1352458511431730. [DOI] [PubMed] [Google Scholar]
- 17.Neema M, Guss ZD, Stankiewicz JM, et al. Normal findings on brain fluid-attenuated inversion recovery MR images at 3T. Am J Neuroradiol. 2009;30:911–916. doi: 10.3174/ajnr.A1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita T, Isobe N, Matsuoka T, et al. Extensive vasogenic edema of anti-aquaporin-4 antibody-related brain lesions. Mult Scler. 2009;15:1113–1117. doi: 10.1177/1352458509106613. [DOI] [PubMed] [Google Scholar]
- 19.Makino T, Ito S, Mori M, et al. Diffuse and heterogeneous T2-hyperintense lesions in the splenium are characteristic of neuromyelitis optica. Mult Scler. 2013;19:308–315. doi: 10.1177/1352458512454772. [DOI] [PubMed] [Google Scholar]
- 20.Kimbrough DJ, Fujihara K, Jacob A, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord. 2012;1:180–187. doi: 10.1016/j.msard.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murchison AG, Kitley J, George J, et al. Spinal cord MRI features in neuromyelitis optica (NMO): Characteristics and relationship with severity and recovery in longitudinally extensive transverse myelitis (LETM) attacks. Mult Scler. 2013;19:S329. [Google Scholar]
- 22.Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: Distinction from multiple sclerosis. Brain. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Miyazawa I, Fujihara K, et al. Preferential spinal central gray matter involvement in neuromyelitis optica: A MRI study. J Neurol. 2008;255:163–170. doi: 10.1007/s00415-008-0545-z. [DOI] [PubMed] [Google Scholar]
- 24.Downer JJ, Leite MI, Carter R, et al. Diagnosis of neuromyelitis optica (NMO) spectrum disorders: Is MRI obsolete? Neuroradiology. 2012;54:279–285. doi: 10.1007/s00234-011-0875-x. [DOI] [PubMed] [Google Scholar]
- 25.Krampla W, Aboul-Enein F, Jecel J, et al. Spinal cord lesions in patients with neuromyelitis optica: A retrospective long-term MRI follow-up study. Eur Radiol. 2009;19:2535–2543. doi: 10.1007/s00330-009-1425-3. [DOI] [PubMed] [Google Scholar]
- 26.Kıyat-Atamer A, Ekizoğlu E, Tüzün E, et al. Long-term MRI findings in neuromyelitis optica: Seropositive versus seronegative patients. Eur J Neurol. 2013;20:781–787. doi: 10.1111/ene.12058. [DOI] [PubMed] [Google Scholar]
- 27.Tackley G, Kuker W, Palace J. Magnetic resonance imaging in neuromyelitis optica. Mult Scler. 2014 doi: 10.1177/1352458514531087. pii: 1352458514531087. [DOI] [PubMed] [Google Scholar]
- 28.Hurst RW, Grossman RI. Peripheral spinal cord hypointensity on T2-weighted MR images: A reliable imaging sign of venous hypertensive myelopathy. Am J Neuroradiol. 2000;21:781–786. [PMC free article] [PubMed] [Google Scholar]
- 29.Cassinotto C, Deramond H, Olindo S, et al. MRI of the spinal cord in neuromyelitis optica and recurrent longitudinal extensive myelitis. J Neuroradiol. 2009;36:199–205. doi: 10.1016/j.neurad.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Huh SY, Min JH, Kim W, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Scler. 2014;20:695–704. doi: 10.1177/1352458513506953. [DOI] [PubMed] [Google Scholar]
- 31.Cabrera-Gómez J, Saiz-Hinarejos A, Graus F, et al. Brain magnetic resonance imaging findings in acute relapses of neuromyelitis optica spectrum disorders. Mult Scler. 2008;14:248–251. doi: 10.1177/1352458507082617. [DOI] [PubMed] [Google Scholar]
- 32.Magaña SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72:712–717. doi: 10.1212/01.wnl.0000343001.36493.ae. [DOI] [PubMed] [Google Scholar]
- 33.Rostásy K, Mader S, Hennes EM, et al. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody-negative pediatric neuromyelitis optica. Mult Scler. 2013;19:1052–1059. doi: 10.1177/1352458512470310. [DOI] [PubMed] [Google Scholar]
- 34.Marignier R, De Seze J, Vukusic S, et al. NMO-IgG and Devic’s neuromyelitis optica: A French experience. Mult Scler. 2008;14:440–445. doi: 10.1177/1352458507084595. [DOI] [PubMed] [Google Scholar]
- 35.Wingerchuk DM, Weinshenker BG. Neuromyelitis optica (Devic’s syndrome) Handb Clin Neurol. 2014;122:581–599. doi: 10.1016/B978-0-444-52001-2.00025-X. [DOI] [PubMed] [Google Scholar]