Abstract
Neurocardiovascular instability (NCVI) refers to abnormal neural control of the cardiovascular system affecting blood pressure and heart rate behavior. Autonomic dysfunction and impaired cerebral autoregulation in aging contribute to this phenomenon characterized by hypotension and bradyarrhythmia. Ultimately, this increases the risk of falls and syncope in older people. NCVI is common in patients with neurodegenerative disorders including dementia. This review discusses the various syndromes that characterize NCVI icluding hypotension, carotid sinus hypersensitivity, postprandial hypotension and vasovagal syncope and how they may contribute to the aetiology of cognitive decline. Conversely, they may also be a consequence of a common neurodegenerative process. Regardless, recognition of their association is paramount in optimizing management of these patients.
Keywords: neurocardiovascular instability, autonomic dysfunction, cognitive impairment, dementia, hypotension, orthostatic hypotension, carotid sinus syndrome, postprandial hypotension, syncope
Introduction
Neurocardiovascular instability (NCVI) represents abnormal neural control of the cardiovascular system [1]. This affects blood pressure (BP) and heart rate behavior, predominantly resulting in hypotension and bradyarrhythmia with abnormal baroreflex function manifesting as supine hypertension and exaggerated BP variability. Older adults are more susceptible to NCVI because of age-related physiological changes in the cardiovascular system, autonomic nervous system, cerebral blood flow, and humoral system.
Clinically, NCVI manifests as fatigue, falls, presyncope and syncope. These presentations are complicated and exacerbated by comorbidity and polypharmacy. Dysautonomic syndromes such as orthostatic hypotension (OH), carotid sinus hypersensitivity (CSH), postprandial hypotension (PPH), and vasovagal syncope (VVS) characterize NCVI. It is common in patients with neurodegenerative disorders including established dementias. OH and CSH occur predominantly, with a combined frequency of 40 percent in patients with Alzheimer’s dementia and 50 percent in patients suffering from dementia with Lewy bodies [2].
Understanding the mechanism of NCVI and its possible causative association with cognitive decline and dementia may help us target prevention and therapeutic strategies for a poorly understood, but well-established and burdensome disease. Similarly, recognition and management of NCVI in dementia patients can direct us in falls prevention, and thus reduce morbidity, institutionalization, and mortality in this physiologically vulnerable cohort.
Pathophysiology
The autonomic nervous system (ANS) is responsible for control of the body’s visceral functions, maintenance of homeostasis, and adaptation to changing conditions. The baroreceptor reflex is the key regulatory mechanism for short-term control of systemic BP. Arterial baroreceptors are stretch receptors in the walls of the carotid sinuses and aortic arch. Afferent sensory input from these receptors travel via cranial nerves IX and X, and the carotid sinus nerve to the brainstem. This information is subsequently relayed to the hypothalamus, cerebellum, substantia nigra, and cerebral hemispheres [3].
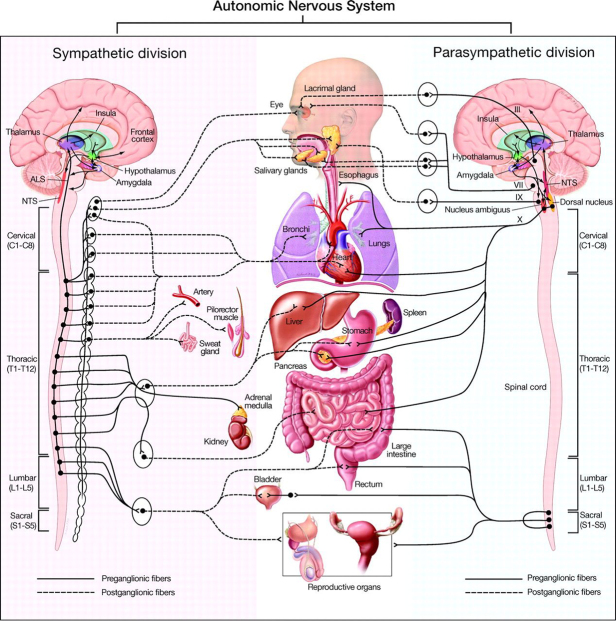
Efferent limbs of the ANS consist of sympathetic and parasympathetic fibers to the heart, as well as sympathetic fibers to the smooth muscles in the peripheral blood vessels (Figure 1). Pre-ganglionic sympathetic nerves release acetylcholine (ACh) and post-ganglionic nerves release norepinephrine (NE), with the exception of sweat glands. ACh is the primary neurotransmitter of pre- and post-ganglionic para-sympathetic nerves.
Figure 1.
Figure 1: Schematic representation of the autonomic nervous system divisions and the systemic anatomical structures each innervate [150]. Roman numerals III, VII, IX and X (vagus) represent cranial nerves.
ALS = anterolateral system
NTS = nucleus tractus solitarius
In healthy individuals, sympathetic activation is initiated in response to low BP, resulting in increased heart rate, cardiac contractility, and vasomotor tone to restore BP and maintain an adequate cardiac output for systemic and cerebral perfusion. Autonomic dysfunction occurs in all common dementias, but is especially prominent in Parkinson’s disease dementia (PDD) [4].
With age, there is dysregulation of a number of neurohumoral systems contributing to NCVI: decreased baroreflex sensitivity; diminished heart rate responses to orthostatic change and other stress responses; impaired α1-adrenergic vasoconstriction — particularly in the splanchnic system, which is responsible for one-third of venous pooling during standing; decreased parasympathetic tone, resulting in less cardioacceleration and vagal withdrawal on standing; proneness to dehydration due to impaired thirst response; inability of the kidney to conserve salt and water during times of relative dehydration; reduction in plasma renin; angiotensin and aldosterone levels; elevation in natriuretic peptides; an increase in cardiac “stiffness” with impaired diastolic filling; and reduced stroke volume resulting in decreased venous return during standing.
Most of the symptomatic consequences of NCVI are due to these neurohumoral changes and usually present during orthostatic change and mobilization.
Cerebral blood flow (CBF) is regulated by the effect of blood gases and neuronal metabolism, the ANS, and cerebral autoregulation [5]. Brain perfusion is highly sensitive to changes in PaCO2, and increased PaCO2 produces smooth muscle relaxation and increased flow. Cerebral autoregulation (CA) refers to the delicate process of maintaining stable cerebral perfusion against changes in systemic BP [6].
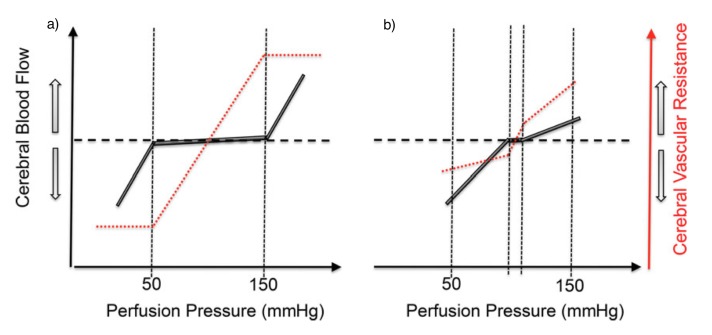
Lassen [7] constructed a plot of average BP and total blood flow from seven studies involving 11 different BP levels and this revealed a plateau wherein CBF remains relatively unchanged within the mean arterial BP range of 60 to 150 mmHg. Recent data [8] shows a smaller plateau indicating a more pressure-passive CBF than conventionally believed, with evidence that the brain defends more effectively against acute hypertension (HTN) than hypotension (Figure 2) [5].
Figure 2.
Figure 2: Stylized representation of the (a) classical and (b) contemporary relationships between mean arterial pressure and cerebral blood flow — i.e. autoregulation [5].
a) Classical view of cerebral autoregulation by Lassen et al. [7].
b) Contemporary view of cerebral autoregulation by Tan et al. [8].
With aging, there is a progressive reshaping of the CA curve from a sigmoid curve to a straight line, implying that any abrupt change in BP will result in an appreciable change in CBF [9]. Sub-threshold BP may lead to cerebral hypoperfusion and consequently ischemia, resulting in cerebral damage and vascular cognitive impairment [10].
Cognitive impairment and dementia
The majority of adults experience some decline in cognitive function over the course of their lifetime. Mild cognitive impairment (MCI) is defined as cognitive function worse than normative data for a set age and educational level, yet not severe enough to meet the criteria defining dementia [11]. Functional ability remains preserved, thus differentiating it from dementia, in which there is functional impairment.
Although patients with MCI have a greater risk of developing dementia compared with the general population, studies report substantial variability [12]. Reported annual rates of MCI conversion to dementia span from less than 5 percent [13] to 12 to 20 percent [14], depending on the country and population studied.
Our studies have shown that patients with MCI have a higher prevalence of NCVI and autonomic dysfunction than controls, and that the presence of NCVI at least doubles conversion rates to dementia [15]. The two most-common forms of dementia are Alzheimer’s dementia (AD) and vascular dementia (VaD).
AD is classified as a progressive neurodegenerative disorder with histopathological hallmarks of amyloid plaques, neurofibrillary tangles of hyperphosphorylated tau, and cerebral amyloid angiopathy [16]. Other mechanisms might contribute to sporadic AD, such as those suggested by the vascular hypothesis that states that cardiovascular diseases are important causal or contributing factors in AD, with HTN regarded as the most powerful vascular risk factor for AD [17,18]. Cognitive features of early AD include anterograde memory loss, visuo-spatial dysfunction and mild anomic aphasia.
VaD occurs as a result of cerebrovascular insults in cortical and subcortical areas responsible for memory and executive function. However, there are no neuropathological criteria to indicate the extent of vascular changes on imaging required to make a diagnosis [16]. Cerebrovascular ischemic abnormalities are often found in conjunction with pathological changes of AD [16]. This co-occurrence of the two disorders is commonly referred to as mixed dementia [19,20].
Certain polymorphisms of the Apolipoprotein E (APOE) gene are associated with both dementia subtypes. Its presence increases the incidence of VaD in post-stroke patients and is a risk factor for cerebral amyloid angiopathy in AD [21].
Dementia with Lewy bodies (DLB) is a progressive, degenerative dementia characterized by the deposition of Lewy bodies in the cerebral cortex. It is characterized by deficits in attention and visuospatial function, fluctuating cognition, recurrent visual hallucinations, and motor features of Parkinsonism. PDD occurs in the setting of well-established Parkinsonism and shares clinical and histopathological features with DLB.
Common to the major types of dementia is a deficit in cholinergic activity in the basal ganglia and neocortex, secondary to loss of cholinergic neurons. This is the focus of conventional pharmacotherapy, which aims to increase the levels of available ACh by inhibiting acetylcholinesterase.
A recent systematic review showed that cholinesterase inhibitors (ChEIs) produced small, short-lived improvements in cognitive function in mild to moderate AD and DLB, but no significant benefit in VaD [22]. ChEIs can exacerbate NCVI as they augment parasympathetic activity causing cardioinhibition and bradyarrhythmia [23]. They also increase the risk of syncope [24].
Hypertension and cognition
The association of BP with cognitive decline and dementia is complex, and systematic reviews have suggested that this association varies considerably with age and duration of follow-up [25,26]. Overall, the association is more evident in studies with a long follow-up and with BP measured in midlife [27]. Several longitudinal studies have reported an adverse effect of midlife HTN on late-life-related cognitive impairment and dementia [28-35]. In contrast, studies with a short follow-up or cross-sectional studies on BP measured in late life are less consistent [27].
In these studies, HTN does not seem to be associated with an increased risk of dementia and some studies reported a protective effect of hypertension [34,36-42]. It is of note, however, associations with late-life BP measurements may be modified by survival bias due to the premature death of individuals exposed to high BP levels [27]. Other studies report a “U-shaped” relationship between systolic blood pressure (SBP) and cognitive function, such that both low (SBP < 130 mmHg) and high SBP (SBP > 160 mmHg) are associated with cognitive impairment and dementia [43-46].
HTN is an important risk factor for progression of cerebral small vessel disease, markers of which include white matter lesions (WMLs), covert brain infarcts, microbleeds, and dilated perivascular spaces [27]. According to a meta-analysis in 2010 [47], increasing WML burden is associated with a two- to three-fold increased risk of stroke and dementia (mainly VaD), and also predicts an increased risk of cognitive decline and MCI. Cerebral small vessel disease ultimately results in cerebral hypoperfusion, and hypoxia driven pathways may potentiate cerebral amyloid accumulation and tau protein phosphorylation [48].
Chronic HTN also causes vascular remodeling and hypertrophy with loss of arterial elasticity and compliance. This results in increased myogenic tone affecting cerebral autoregulation and necessitating higher perfusion pressures to maintain the same level of CBF [49]. This diminished autoregulatory capacity means the brain is more vulnerable to ischemic insults when SBP dips below a critical threshold for maintaining perfusion [10].
Given the association between midlife HTN and cognitive decline, it is a reasonable hypothesis that the use of antihypertensive drugs would reduce this risk. Certain anti-hypertensives, including calcium channel blockers, have neuroprotective properties that may even reduce the risk of AD, independent of their effects on BP [50]. The target BP in later life that ensures adequate perfusion while preventing cognitive decline is less clear [51].
Recent research has suggested considerably lower goals in target BP than previously recommended [52]. However, there is evidence that antihypertensive drug therapy needs to be individualized to each person and that not only chronological age, but also biological age, needs to be taken into account, as well as the existing degree of systemic and cerebrovascular damage [53].
Excessive BP lowering in older patients with cognitive impairment and dementia may even be harmful as shown by Mossello et al. [54], who found that low daytime SBP was independently associated with greater progression of cognitive decline in these patients on antihypertensive drugs. Tinetti et al. additionally showed that antihypertensive drugs are associated with an increased risk of serious fall injuries [55].
Hypotension and cognition
Chronically low BP in elderly people can result in fatigue, dizziness, and falls. Essential hypotension refers to a chronic condition of inappropriately reduced BP independent of the presence of any other pathological factors [56], and may be secondary to low fluid intake or low body weight. Hypotension contributing to cerebral hypoperfusion may also be secondary to reduced cardiac output mediated by left ventricular dysfunction, arrhythmias, or valvulopathies [57].
As mentioned previously, CA does not necessarily protect the brain from chronic low BP in aging. Highly metabolically active neurons in the hippocampus are particularly vulnerable to impaired CBF not meeting energy demands and undergo oxidative and endoplasmic reticulum stress, resulting in decreased adenosine triphosphate (ATP). This compromises brain cell survival and results in progressive cognitive decline [57].
Numerous studies [38,43,45,54,58-60] have provided convincing evidence for the association of hypotension with cognitive impairment (Table 1), especially with regards to memory and attention [56].
Table 1. Table 1: Studies investigating hypotension and cognitive impairment.
| Author and study setting | Participants and follow-up | Main conclusions |
| Mossello et al. [54] 2015 | 172 memory clinic patients of mean age 79 followed up for nine months | Patients with low daytime SBP treated with antihypertensive drugs had greater progression of cognitive decline. |
| Waldstein et al. [38] 2005 Baltimore Longitudinal Study of Aging | 847 people of mean age 70 followed up for 11 years | Both high and low DBP was associated with poorer performance on tests of executive function and confrontation naming. |
| Kahonen-Vare et al. [58] 2004 Helsinki Aging Study | 650 people aged 75 to 85 followed up for 10 years | Low MMSE scores were associated with low BP at baseline. |
| Pandav et al. [59] 2003 The Indo-US Cross National Dementia Epidemiology Study | 4,810 people aged >55 | In both Indian and the United States’ samples, lower DBP was inversely related to cognitive impairment, although not significantly in the latter. |
| Bohannon et al. [45] 2002 Duke Established Populations for Epidemiological Studies of the Elderly | 2,260 African-American and 1,876 white people aged 65 to 105 followed up for three years | Decline in cognitive function was associated with extremes of SBP (<110 mmHg and >165 mmHg) in older white people. |
| Zhu et al. [60] 1998 Kungsholmen Project | 924 people aged ≥75 followed up for three years | There was a correlation between SBP reduction and cognitive decline in women, which was not accounted for by other factors. |
| Guo et al. [43] 1997 Kungsholmen Project | 1,736 people ages 75 to 101 followed up for three years | Individuals with a baseline SBP <130 mmHg had odds ratio 1.88 for cognitive impairment (MMSE <24) compared with those with SBP 130-159 mmHg. |
Several longitudinal population studies [61-72] have identified that lower BP values may be a risk factor for later-life dementia (Table 2). A systematic review and meta-analysis of population studies examining this relationship concluded that the pooled estimates from included studies demonstrated an increased risk for AD with lower diastolic blood pressure (DBP) values in later life [73].
Table 2. Table 2: Longitudinal studies investigating hypotension and dementia.
| Author and study setting | Participants and follow-up | Main conclusions |
| Joas et al. 2012 [61] Prospective Population Study of Women in Gothenburg | 1,462 women of mean age 45 followed up for 37 years | A decline in SBP during later part of study was observed in those who developed dementia regardless of antihypertensive drugs. |
| Qiu et al. 2009 [62] Kungsholmen Project, Sweden | 422 people aged >75 followed for nine years | Low DBP (<70 mmHg) was associated with a multiadjusted hazard ratio of 2.13 for dementia and 2.16 for AD. BP declined substantially over about three years before dementia became evident. |
| Stewart et al. 2009 [63] Honolulu Heart Program/Honolulu-Asia Aging Study | 1,890 Japanese-American men followed up for 32 years of mean age 83 at end of study | Men who developed dementia had a greater increase, followed by a greater decrease in SBP. Both were modified by antihypertensive drugs. |
| Razay et al. 2009 [64] The OPTIMA Longitudinal Study | 477 people followed up for five years | High (110 mmHg) and low (60 mmHg) DBP levels were related to faster cognitive decline in AD patients. |
| Nilsson et al. 2007 [65] OCTO-Twin Study, Sweden | 599 people aged ≥80 followed up for four years | Low SBP and DBP were associated with a higher incidence of AD. |
| Qiu et al. 2003 [66] Kungsholmen Project, Sweden | 1,270 people aged >75 followed up for six years | Both very low DBP (≤65 mmHg) and very high SBP are associated with an increased risk of AD and dementia. |
| Qiu et al. 2003 [67]Kungsholmen Project, Sweden | 966 people aged >75 years followed up for six years | APOE-4 allele, high SBP (>140 mm Hg), and low DBP (<70 mmHg) were associated with an increased risk of AD. |
| Verghese et al. 2003 [68] Bronx Aging Study, US | 488 community volunteers aged ≥75 followed up to 21 years with a mean follow-up of 6.7 years | Individuals with a DBP (< 70 mmHg) were twice as likely to develop AD when compared to those with a DBP (>90 mmHg). |
| Ruitenberg et al. 2001 [69] Rotterdam Study and Gothenberg H-70 Study | 6,668 people aged ≥55 from Rotterdam Study and 382 people aged 85 from Gothenburg H-7 Study followed up for an average of 2.1 years | There was an inverse association between BP and dementia risk in elderly persons on antihypertensive drugs. Persons demented at baseline had stronger BP decline during follow-up than those who were not demented. |
| Morris et al. 2001 [70] East Boston Study, US | 378 people aged ≥65 followed up for four years | DBP (<70 mmHg) was associated with an increased risk of AD. |
| Guo et al. 1999 [71] Kungsholmen Project | 304 people aged 75 to 96 followed up for three years | Those with SBP (≤140 mmHg) had a significantly higher risk of dementia and AD. |
| Skoog et al. 1999 [72] | 382 people aged 70 to 75 followed for 15 years | BP declined in the years preceding onset of dementia and was similar to, or lower than, that in non-demented patients. |
While low DBP is a risk factor for dementia, overall low BP may also occur as a secondary phenomenon to disease progression. In the French Research Programme on AD (REAL-FR), Hanon et al. [74], noted a significant decrease in both SBP and DBP from baseline to follow-up a year later, and patients with the worst cognitive impairment showed a larger decrease in BP. The retrospective study by Burke et al. [75] on autopsy-proven patients with AD found sustained BP reduction beginning in the third to fourth year after diagnosis. The authors postulate that BP is altered in AD as a consequence of neuronal degeneration. It may also be related to diet changes and weight loss associated with dementia, or a deficit in central neurotransmitters that regulate BP [76].
Orthostatic hypotension and cognitive impairment
Classical OH is defined by consensus statement as a sustained reduction in SBP of at least 20 mmHg and/or DBP of 10 mmHg within 3 minutes of standing [77]. Prevalence of OH varies widely depending on the population characteristics (e.g. age range, institution, use of concomitant medications) and the methodology used. It has a prevalence of 16.2 percent in those older than age 65 living in the community [78], and its incidence increases exponentially with age, most commonly affecting men [79,80] and patients in institutions such as nursing homes.
The above definition is based on auscultatory and oscillometric BP measurement, but recently more accurate phasic beat-to-beat measurement using digital photo-plethysmography has been used. This allows the further identification and possible classification of initial OH, a transient BP decrease (>40 mmHg SBP and/or >20 mmHg DBP) within 15 seconds of standing, with symptoms of cerebral hypoperfusion [81].
In contrast to initial OH, delayed OH — symptomatic OH that occurs 3 minutes after standing — is characterized by a late drop in BP [82]. Symptoms of OH include lightheadedness, weakness, presyncope, visual and hearing disturbances, neck pain, chest pain, falls, and syncope. Orthostatic intolerance (OI), or subclinical OH, is defined as a systolic drop less than 20 mmHg, or a diastolic drop less than 10 mmHg, with a variety of orthostatic symptoms such as those named above [78].
Neurogenic, hypovolemic, and drug-induced OH comprise the major subtypes of OH. Neurogenic OH is caused by primary dysautonomia or can be secondary to hereditary, inflammatory, infectious, or metabolic diseases. OH is associated with a significant increased risk for overall mortality [83]. Patient education regarding aggravating factors, increased fluid and salt intake and physical counter maneuvers are imperative in the management of OH. Failing this, pharmacological options include midodrine, pyridostigmine, droxidopa, and fludrocortisone [84].
While OH is common in patients with a diagnosis of dementia, the association between OH and cognitive decline and impairment is less clear and their causal relationship remains controversial [85]. It is not clear whether OH and cognitive impairment coexist owing to underlying neurodegeneration at multiple neural domains, or whether cardiovascular dysautonomia exerts negative effects on cognitive outcome through hypotensive and hypertensive peaks, which in turn increase the risk of brain ischemia [86].
A number of studies [15,87-98] (Table 3) have investigated the association, although results have been mixed, which may be because of small sample size [15,90,91,95,97] variable age range of sample [91,93], lack of adjustment for potential confounding factors [91,92], or use of cognitive tests with low sensitivity and specificity [87,90,94-96].
Table 3. Table 3: Studies investigating orthostatic hypotension and cognitive impairment.
| Author | Study design | Number of participants | Main conclusions |
| Hayakawa et al. [15] 2015 | LS | 225 | OH is more common and more prolonged in MCI. People with MCI and a SBP deficit >30 percent 30 seconds after standing are twiceas likely to convert to dementia. |
| Elmstahl et al. [87] 2014 | Cohort | 1,480 | OH and OI are risk factors for cognitive decline. |
| Frewen et al. [89]2014 | CS | 5,936 | OH is associated with poorer global cognitive function and memory independent of potential confounders in women. |
| Frewen et al. [88] 2014 | CS | 4,690 | OH coupled with supine HTN is associated with lower cognitive performance. |
| Schoon et al. [90] 2013 | Retrospective cohort | 184 | Cognitive impairment was not more prevalent in patients with hypotensive syndromes. |
| Czajkowska et al. [91] 2010 | CS | 74 | Poor BP regulation in response to orthostasis was associated with decreased verbal memory and decreased concentration. |
| Mehrabian et al [92] 2010 | CS | 495 | Subjects with OH showed poorer cognitive function. |
| Rose et al. [93] 2010 | LS | 12,702 | OH was associated with less-favorable cognitive function, but this was largely attributable to demographic and cardiovascular risk factors. |
| Yap et al. [94] 2008 | CS and LS | 2,321 | There was no significant association of OH with cognitive impairment. Hypotensive people with OH were more likely to have cognitive impairment. |
| Bendini et al. [95] 2007 | CS | 36 | There was no strong causal relationship between OH and cognitive impairment. |
| Viramo et al. [96] 1999 | LS | 1,159 | OH did not predict cognitive decline during a two-year follow-up. |
| Elmstahl et al. [97] 1997 | LS | 33 women | OH is a risk factor for cognitive decline. Elderly women who developed dementia tended to have a larger drop in BP. |
| Matsubayashi et al. [98] 1997 | CS | 334 | OH was associated with poorer scores on neurobehavioral function tests and more advanced leukoaraiosis on MRI. |
a) CS = Cross sectional study
b) LS = Longitudinal study
OH can be associated with supine HTN [99], representing extremes of BP variability and baroreceptor dysfunction. In a longitudinal study of aging in Ireland (TILDA), we have shown that OH, coupled with supine HTN, is associated with poorer performance on cognitive tasks [88]. Nocturnal hypertension refers to reversal of physiological BP circadian rhythm [86]. It is common in PD patients [100], especially those with OH, and may be secondary to sympathovagal disruption [101].
Collins et al. [102] demonstrated that patients with MCI had significant autonomic dysfunction compared with controls. This was predominantly parasympathetic asevidenced by deficits in heart rate responses to cardiovascular reflex tests and reduced high frequency on heart rate variability (HRV). Sympathetic activity was also somewhat reduced and patients with MCI demonstrated a significantly greater orthostatic fall in SBP than controls.
HRV was independently associated with poor global cognitive performance in a large representative population sample of community-dwelling, non-demented persons older than 50 [103]. This further strengthens the relationship between autonomic dysfunction and cognitive disorders. The concept of orthostatic HTN, an elevation in BP on standing [104], has not been studied so well. It has, however, been associated with greater leukoariosis and silent infarction on MRI brain imaging [98,105].
Orthostatic hypotension and dementia
Autonomic dysfunction is common in all of the dementia subtypes [106,107], but particularly in PDD and DLB [106,108]. It is the second-most common dysautonomic feature after urinary dysfunction [109,110].
In AD, it has been hypothesized that the deficit in cholinergic function could lead to autonomic dysfunction [111]. There is evidence that cortical perivascular cholinergic nerve terminals are largely lost resulting in impaired vasodilation and reduced CBF; the cholinergic-vascular hypothesis [112,113]. Also, various central nervous system structures affected in AD are implicated in ANS regulation, such as the hypothalamus, locus coeruleus, cerebral neocortex, insular cortex, and brainstem [114].
Braak [114] speculated that these structures may be affected by neurodegeneration in a preclinical stage and that autonomic dysfunction may be present before the onset of clinical symptoms of AD. It has also been suggested that the presence of autonomic cardiac dysfunction in AD patients might be due to a cholinergic deficit in the peripheral ANS, as HRV showed a significant correlation with blood levels of acetylcholinesterase activity [115].
A meta-analysis in 2011 reported a prevalence of OH in 30 percent of Parkinson’s disease (PD) patients [116], and it has been reported in up to 50 percent of DLB patients [117]. Most epidemiological studies probably underestimate the prevalence of cardiovascular autonomic dysfunction as they are based on clinical symptoms of OH, rather than more accurate measurements of SBP or DBP change with standing [86].
Involvement of the sympathetic nervous system in PDD and DLB is likely related to both CNS damage as well as postganglionic sympathetic lesions [118]. Lewy body deposits have neurotoxic effects on high metabolic rate neurons [119]. This dual involvement could account for the abnormal failure to release appropriate amounts of norepinephrine on standing, resulting in OH due to pooling of blood in the lower limbs and splanchnic circulation [118].
Sympathetic dysfunction may precede cognitive decline in DLB [120,121] and there appears to be a more significant and prolonged drop of SBP in DLB patients, compared to AD and controls [122].
In general, studies on the relation between cognitive impairment and OH in PD patients reported no differences in global function, but significant differences in single tasks, especially executive tasks. As reported for elderly and demented patients, PD patients with OH were generally older than patients without, and this may contribute to a decline in cognitive function. Despite different disease duration and motor disability, the Mini-Mental State Exam (MMSE) scores were similar in PD patients with and without OH [85].
WMLs in alpha-synucleinopathies (PD, Multi-System Atrophy (MSA) and DLB) have been correlated with more severe NCVI. This raises the question of whether WML are the consequence of chronic alternate hypotensive and hypertensive stress or, alternatively, whether WML involving autonomic networks may cause future development and/or worsening of cardiovascular autonomic failure [86].
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is diagnosed when carotid sinus massage (CSM) of 10 seconds produces either a ventricular pause lasting >3 seconds (cardioinhibitory CSH) or a decrease in SBP of >50 mmHg (vasodepressor CSH) [123], or a combination of both (mixed CSH). It is caused by an abnormal baroreceptor reflex involving cardioinhibition via the vagus nerve and vasodepression secondary to sympathetic withdrawal [124].
CSH may present clinically as falls, presyncope, and syncope in older people, more commonly males [125]. Precipitating factors may include sudden movement of the head and neck [126], e.g. when shaving or compression of the carotid sinus by a tight collar, or less frequently by a neck tumor.
Symptomatic CSH is believed to be secondary to impaired cerebral autoregulation [127]. When CSH is associated with syncope it is known as carotid sinus syndrome (CSS). Syncope in CSS typically has little or no prodrome [124], and is associated with appreciable morbidity [1]. It frequently coexists with other dysautonomic hypotensive disorders such as OH and VVS, making its diagnosis elusive and impeding appropriate intervention.
While dual chamber pacing is indicated for cardioinhibitory CSS, treatment for vasodepressor CSS is less satisfactory. Similar to OH, first-line management includes reduction in antihypertensive drugs and advice on increased fluid intake. Failing this, pharmacological treatment may be considered.
CSH is associated with neurodegenerative dementias including AD, DLB and PDD [128-131]. The prevalence of cardioinhibitory CSH is particularly high in DLB — up to 32 percent compared with 11.1 percent in AD, and 3.2 percent in case controls. In addition, patients with DLB have greater heart rate slowing (>2 seconds) and decreases in SBP (>20 mmHg) than those with AD or controls during CSM [129].
Miller et al. [132] found increased tau accumulation in baroreflex associated medullary nuclei in CSH patients, and suggested that this accumulation may be involved in the pathogenesis of CSH and precipitation of CSH symptoms. This is of particular interest as the selective degeneration of medullary autonomic nuclei is implicated in the pathogenesis of PDD, DLB, and AD. Alpha-synuclein accumulates in autonomic nuclei in early asymptomatic stages of PD and DLB [133], while medullary autonomic and reticular formation nuclei show a selective vulnerability to tau and amyloid plaques in AD [134,135].
Also of note, the density of deep WMLs on Magnetic-Resonance Imaging (MRI) correlate with the degree of hypotension induced during CSM in patients with DLB, supporting a causal link between episodic hypotension and cerebral small vessel damage [136].
CSS is an important diagnosis in unexplained falls in the elderly, especially in patients with cognitive impairment, when frequently the history is unreliable, or there is retrograde amnesia for the loss of consciousness and there is lack of a witness [137].
Postprandial hypotension
Classically, PPH has been defined as a decrease in SBP of ≥20 mmHg or a decrease below 90 mmHg from a pressure of ≥100 mmHg within two hours after a meal [138]. Studies have shown the prevalence in institutionalized elders is about 25 to 38 percent [139-141]. PPH in older nursing home residents was associated at long-term follow-up with a higher incidence of falls, syncope, new coronary events, new stroke, and total mortality [142]. It is also associated with asymptomatic cerebrovascular damage in hypertensive patients [143], and is a risk factor for asymptomatic lacunar infarction [144].
Risk factors include polypharmacy, diuretics, carbohydrate-rich meals, hot meals, diabetes, PD, and HTN [145]. PPH appears to result from an inadequate sympathetic response to the normal physiologic post-meal decrease in BP rather than to an exaggerated amount of splanchnic pooling [145]. Vasodilator effects of insulin and other gut peptides, namely neurotensin and VIP, contribute to postprandial BP drops [146].
Preliminary cross-sectional studies reported PPH in 48 percent of dysautonomic PD patients [147], but Idiaquez et al. [148] reported no consistent association between OH or PPH and cognitive deficits in PD. In another small study by the same author, seven out of 10 patients with AD were found to have PPH, compared to six out of 23 controls [149].
Conflicting evidence regarding episodic hypotension and cognitive impairment was reported by Schoon [90], who investigated the association of OH, PPH, and CSH with cognitive impairment in 184 elderly patients presenting with falls.
It was found that patients with one or more hypotensive syndromes were not likely to have cognitive impairment, thus contradicting current data that hypotension is associated with cognitive decline.
Vasovagal syncope
Syncope is defined as a sudden loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous complete recovery [123]. As mentioned above, OH, PPH, and CSH may cause syncope if cerebral blood flow is sufficiently compromised.
VVS, also known as the “common faint,” is a type of reflex syncope that is usually triggered by emotion or by orthostatic stress. In the presence of hypertension and atherosclerotic cerebrovascular disease, excessive loss of baroreflex sensitivity leads to dysautonomic responses during prolonged orthostasis (in which blood pressure and heart rate decline steadily over time), and patients become susceptible to VVS [1]. It is the cause of syncope in 20 to 30 percent of older patients with single or recurrent presyncope or syncope [150].
Similar to CSH, the responses in VVS can be inhibitory or vasodepressor or both. It is diagnosed using a head-up tilt test with continuous blood pressure and heart rate monitoring.
The European Society of Cardiology has recommended that syncope be considered causal in patients with unexplained falls [123], a frequent presentation in elderly people causing major mortality and morbidity [151]. Syncope and unexplained falls were shown to be independently associated with poorer cognitive performance among individuals aged ≥50 in the TILDA cohort [152].
Conclusion
There is a discernible and intricate association between NCVI, the aging brain and cognition. The direction of causality is less apparent. Given the above evidence, it is possible that NCVI contributes to cognitive decline. Equally, NCVI may be a consequence of a neurodegenerative process; indeed they may even be parallel age-related pathological processes. Future research should focus on a deeper understanding of phenotypes to better guide treatment of HTN and other clinical components of NCVI in patients with cognitive impairment and dementia.
Regardless of the direction of causality, recognition of their coexistence is important particularly in relation to prescribing in older adults with dementia. The use of ChEIs should be carefully considered and initiated in the appropriate patient group, while concomitantly minimizing risk of syncope. Similar caution should be adopted with antihypertensive drugs, tailoring suitability, and avoiding excessive BP lowering, which contributes to falls risk and exacerbates cognitive decline.
In summary, enhanced understanding of the link between NCVI and cognition will improve overall management of these patients with disrupted homeostatic adaptive capacity and vulnerability to physiological stress.
Abbreviations
- NCVI
neurocardiovascular instability
- BP
blood pressure
- OH
orthostatic hypotension
- CSH
carotid sinus hypersensitivity
- PPH
postprandial hypotension
- VVS
vasovagal syncope
- ANS
autonomic nervous system
- ACh
acetylcholine
- NE
norepinephrine
- PPD
Parkinson’s disease dementia
- CBF
cerebral blood flow
- CA
cerebral autoregulation
- HTN
hypertension
- MCI
mild cognitive impairment
- AD
Alzheimer’s dementia
- VaD
vascular dementia
- APOE
Aplipoprotein E
- DLB
dementia with Lewy bodies
- ChEI
Cholinesterase Inhibitor
- SBP
systolic blood pressure
- WML
white matter lesions
- ATP
Adenosine Triphosphate
- DBP
diastolic blood pressure
- REAL-FR
French Research Programme on AD
- OI
orthostatic intolerance
- TILDA
The Irish Longitudinal Study on Aging
- HRV
heart rate variability
- PD
Parkinson’s Disease
- MMSE
Mini-Mental State Exam
- MSA
Multi-System Atrophy
- CSM
carotid sinus massage
- CSS
carotid sinus syndrome
- MRI
Magnetic-Resonance Imaging
References
- Kenny RA, Kalaria R, Ballard C. Neurocardiovascular instability in cognitive impairment and dementia. Ann N Y Acad Sci. 2002;977:183–195. doi: 10.1111/j.1749-6632.2002.tb04816.x. [DOI] [PubMed] [Google Scholar]
- Ballard C, Shaw F, McKeith L, Kenny R. High prevalence of neurovascular instability in neurodegenerative dementias. Neurol. 1998;51(6):1760–1762. doi: 10.1212/wnl.51.6.1760. [DOI] [PubMed] [Google Scholar]
- Perlmuter LC, Sarda G, Casavant V, O’Hara K, Hindes M, Knott PT. et al. A review of orthostatic blood pressure regulation and its association with mood and cognition. Clin Auton Res. 2012;22(2):99–107. doi: 10.1007/s10286-011-0145-3. [DOI] [PubMed] [Google Scholar]
- Marigold JRG, Arias M, Vassallo M, Allen SC, Kwan JS. Autonomic dysfunction in older people. Rev Clin Gerontol. 2011;21(1):28–44. [Google Scholar]
- Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592(Pt 5):841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YC, Ainslie PN. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol. 2014;114(3):545–559. doi: 10.1007/s00421-013-2667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Tan CO. Defining the characteristic relationship between arterial pressure and cerebral flow. J Appl Physiol (Bethesda, Md: 1985) 2012;113(8):1194–1200. doi: 10.1152/japplphysiol.00783.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4(2):395–402. doi: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33(4):1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia (Barcelona, Spain) 2000;15(3):93–101. [PubMed] [Google Scholar]
- Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB. et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, McGarrigle CA, Coen RF, Soraghan CJ, Foran T, Lawlor BA. et al. Orthostatic Blood Pressure Behavior in People with Mild Cognitive Impairment Predicts Conversion to Dementia. J Am Geriatr Soc. 2015;63(9):1868–1873. doi: 10.1111/jgs.13596. [DOI] [PubMed] [Google Scholar]
- Love S. Neuropathological investigation of dementia: a guide for neurologists. J Neurol Neurosurg Psychiatry. 2005;(76 Suppl 5):v8–v14. doi: 10.1136/jnnp.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA. Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab. 2013;33(11):1696–1706. doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CL, Araujo L, Guerrier R, Henry KA. “Mixed dementia”: adequate or antiquated? A critical review. Am J Geriatr Psychiatry. 1997;5(4):279–283. doi: 10.1097/00019442-199700540-00002. [DOI] [PubMed] [Google Scholar]
- Kaye JA. Diagnostic challenges in dementia. Neurol. 1998;51(1 Suppl 1):S45–S52. doi: 10.1212/wnl.51.1_suppl_1.s45. discussion S65-7. [DOI] [PubMed] [Google Scholar]
- Giau VV, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015;11:1723–1737. doi: 10.2147/NDT.S84266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JS, Salpeter SR. A Risk-Benefit Assessment of Dementia Medications: Systematic Review of the Evidence. Drugs Aging. 2015;32(6):453–467. doi: 10.1007/s40266-015-0266-9. [DOI] [PubMed] [Google Scholar]
- Masuda Y. Cardiac effect of cholinesterase inhibitors used in Alzheimer’s disease — from basic research to bedside. Curr Alzheimer Res. 2014;1(4):315–321. doi: 10.2174/1567205043332009. [DOI] [PubMed] [Google Scholar]
- Kim DH, Brown RT, Ding EL, Kiel DP, Berry SD. Dementia medications and risk of falls, syncope, and related adverse events: meta-analysis of randomized controlled trials. J Am Geriatr Soc. 2011;59(6):1019–1031. doi: 10.1111/j.1532-5415.2011.03450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Williams JW Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension. 2014;63(5):894–903. doi: 10.1161/HYPERTENSIONAHA.113.00147. [DOI] [PubMed] [Google Scholar]
- Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138(6):353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274(23):1846–1851. [PubMed] [Google Scholar]
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31(3):780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM. et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR. et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K. et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ (Clinical research ed) 2001;322(7300):1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27(2):260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Piguet O, Grayson DA, Creasey H, Bennett HP, Brooks WS, Waite LM. et al. Vascular risk factors, cognition and dementia incidence over 6 years in the Sydney Older Persons Study. Neuroepidemiology. 2003;22(3):165–171. doi: 10.1159/000069886. [DOI] [PubMed] [Google Scholar]
- Reinprecht F, Elmstahl S, Janzon L, Andre-Petersson L. Hypertension and changes of cognitive function in 81-year-old men: a 13-year follow-up of the population study “Men born in 1914”, Sweden. J Hypertens. 2003;21(1):57–66. doi: 10.1097/00004872-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45(3):374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Li G, Rhew IC, Shofer JB, Kukull WA, Breitner JC, Peskind E. et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc. 2007;55(8):1161–1167. doi: 10.1111/j.1532-5415.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- Rastas S, Pirttila T, Mattila K, Verkkoniemi A, Juva K, Niinisto L. et al. Vascular risk factors and dementia in the general population aged >85 years: prospective population-based study. Neurobiol Aging. 2010;31(1):1–7. doi: 10.1016/j.neurobiolaging.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G. et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20(2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- Sabayan B, Oleksik R]AM, Maier AB, van Buchem MA, Poortvliet RK, de Ruijter W. et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc. 2012;60(11):2014–2019. doi: 10.1111/j.1532-5415.2012.04203.x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood pressure and performance on the Mini-Mental State Examination in the very old. Cross-sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol. 1997;145(12):1106–1113. doi: 10.1093/oxfordjournals.aje.a009073. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. JAMA. 1999;281(5):438–445. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- Bohannon AD, Fillenbaum GG, Pieper CF, Hanlon JT, Blazer DG. Relationship of race/ethnicity and blood pressure to change in cognitive function. J Am Geriatr Soc. 2002;50(3):424–429. doi: 10.1046/j.1532-5415.2002.50104.x. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bennett DA, Bienias JL, Wilson RS, Morris MC. et al. Blood pressure and late-life cognitive function change: a biracial longitudinal population study. Neurology. 2004;62(11):2021–2024. doi: 10.1212/01.wnl.0000129258.93137.4b. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly S, Collins O. Walking the cognitive ‘minefield’ between high and low blood pressure. J Alzheimers Dis. 2012;32(3):609–621. doi: 10.3233/JAD-2012-120748. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7(6):476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia — a comprehensive review. Ther Adv Neurol Disord. 2009;2(4):241–260. doi: 10.1177/1756285609103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7(12):686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV. et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery LH, Allen M, Fleming I, Kelly K, Bowles S, Duncan J. et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med. 2014;81(7):427–437. doi: 10.3949/ccjm.81a.13110. [DOI] [PubMed] [Google Scholar]
- Mossello E, Pieraccioli M, Nesti N, Bulgaresi M, Lorenzi C, Caleri V. et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015;175(4):578–585. doi: 10.1001/jamainternmed.2014.8164. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP. et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res. 2007;17(2):69–76. doi: 10.1007/s10286-006-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahonen-Vare M, Brunni-Hakala S, Lindroos M, Pitkala K, Strandberg T, Tilvis R. Left ventricular hypertrophy and blood pressure as predictors of cognitive decline in old age. Aging Clin Exp Res. 2004;16(2):147–152. doi: 10.1007/BF03324544. [DOI] [PubMed] [Google Scholar]
- Pandav R, Dodge HH, DeKosky ST, Ganguli M. Blood pressure and cognitive impairment in India and the United States: a cross-national epidemiological study. Arch Neurol. 2003;60(8):1123–1128. doi: 10.1001/archneur.60.8.1123. [DOI] [PubMed] [Google Scholar]
- Zhu L, Viitanen M, Guo Z, Winblad B, Fratiglioni L. Blood pressure reduction, cardiovascular diseases, and cognitive decline in the mini-mental state examination in a community population of normal very old people: a three-year follow-up. J Clin Epidemiol. 1998;51(5):385–391. doi: 10.1016/s0895-4356(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Joas E, Backman K, Gustafson D, Ostling S, Waern M, Guo X. et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59(4):796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. Low diastolic pressure and risk of dementia in very old people: a longitudinal study. Dement Geriatr Cogn Disord. 2009;28(3):213–219. doi: 10.1159/000236913. [DOI] [PubMed] [Google Scholar]
- Stewart R, Xue QL, Masaki K, Petrovitch H, Ross GW, White LR. et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54(2):233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razay G, Williams J, King E, Smith AD, Wilcock G. Blood pressure, dementia and Alzheimer’s disease: the OPTIMA longitudinal study. Dement Geriatr Cogn Disord. 2009;28(1):70–74. doi: 10.1159/000230877. [DOI] [PubMed] [Google Scholar]
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res. 2007;19(1):41–47. doi: 10.1007/BF03325209. [DOI] [PubMed] [Google Scholar]
- Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223–228. doi: 10.1001/archneur.60.2.223. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fastbom J, Fratiglioni L. Combined effects of APOE genotype, blood pressure, and antihypertensive drug use on incident AD. Neurology. 2003;61(5):655–660. doi: 10.1212/wnl.61.5.655. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61(12):1667–1672. doi: 10.1212/01.wnl.0000098934.18300.be. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JC, Lernfelt B. et al. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. 2001;12(1):33–39. doi: 10.1159/000051233. [DOI] [PubMed] [Google Scholar]
- Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. 2001;58(10):1640–1646. doi: 10.1001/archneur.58.10.1640. [DOI] [PubMed] [Google Scholar]
- Guo Z, Viitanen M, Winblad B, Fratiglioni L. Low blood pressure and incidence of dementia in a very old sample: dependent on initial cognition. J Am Geriatr Soc. 1999;47(6):723–726. doi: 10.1111/j.1532-5415.1999.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L. et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology (Cambridge, MA) 2011;22(5):646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon O, Latour F, Seux ML, Lenoir H, Forette F, Rigaud AS. Evolution of blood pressure in patients with Alzheimer's disease: a one year survey of a French Cohort (REAL.FR) J Nutr Health Aging. 2005;9(2):106–111. [PubMed] [Google Scholar]
- Burke J, Knight RG, Partridge FM. Priming deficits in patients with dementia of the Alzheimer type. Psychol Med. 1994;24(4):987–993. doi: 10.1017/s003329170002907x. [DOI] [PubMed] [Google Scholar]
- Maule S, Caserta M, Bertello C, Verhovez A, Naso D, Bisbocci D. et al. Cognitive decline and low blood pressure: the other side of the coin. Clin Exp Hypertens (New York, NY: 1993) 2008;30(8):711–719. doi: 10.1080/10641960802573344. [DOI] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I. et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci: basic & clinical. 2011;161(1-2):46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- Masaki KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D. et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98(21):2290–2295. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120(11):975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (London: 1979) 2007;112(3):157–165. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67(1):28–32. doi: 10.1212/01.wnl.0000223828.28215.0b. [DOI] [PubMed] [Google Scholar]
- Angelousi A, Girerd N, Benetos A, Frimat L, Gautier S, Weryha G. et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32(8):1562–1571. doi: 10.1097/HJH.0000000000000235. discussion 71. [DOI] [PubMed] [Google Scholar]
- Low PA, Tomalia VA. Orthostatic Hypotension: Mechanisms, Causes, Management. J Clin Neurol (Seoul, Korea) 2015;11(3):220–226. doi: 10.3988/jcn.2015.11.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambati L, Calandra-Buonaura G, Poda R, Guaraldi P, Cortelli P. Orthostatic hypotension and cognitive impairment: a dangerous association? Neurol Sci. 2014;35(6):951–957. doi: 10.1007/s10072-014-1686-8. [DOI] [PubMed] [Google Scholar]
- Fanciulli A, Strano S, Colosimo C, Caltagirone C, Spalletta G, Pontieri FE. The potential prognostic role of cardiovascular autonomic failure in α-synucleinopathies. Eur J Neurol. 2013;20(2):231–235. doi: 10.1111/j.1468-1331.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- Elmstahl S, Widerstrom E. Orthostatic intolerance predicts mild cognitive impairment: incidence of mild cognitive impairment and dementia from the Swedish general population cohort Good Aging in Skane. Clin Interv Aging. 2014;9:1993–2002. doi: 10.2147/CIA.S72316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen J, Finucane C, Savva GM, Boyle G, Kenny RA. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol A Biol Sci Med Sci. 2014;69(7):878–885. doi: 10.1093/gerona/glt171. [DOI] [PubMed] [Google Scholar]
- Frewen J, Savva GM, Boyle G, Finucane C, Kenny RA. Cognitive performance in orthostatic hypotension: Findings from a nationally representative sample. J Am Geriatr Soc. 2014;62(1):117–122. doi: 10.1111/jgs.12592. [DOI] [PubMed] [Google Scholar]
- Schoon Y, Lagro J, Verhoeven Y, Rikkert MO, Claassen J. Hypotensive syndromes are not associated with cognitive impairment in geriatric patients. Am J Alzheimers Dis Other Demen. 2013;28(1):47–53. doi: 10.1177/1533317512466692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowska J, Ozhog S, Smith E, Perlmuter LC. Cognition and hopelessness in association with subsyndromal orthostatic hypotension. J Gerontol A Biol Sci Med Sci. 2010;65(8):873–879. doi: 10.1093/gerona/glq068. [DOI] [PubMed] [Google Scholar]
- Mehrabian S, Duron E, Labouree F, Rollot F, Bune A, Traykov L. et al. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci. 2010;299(1-2):45–48. doi: 10.1016/j.jns.2010.08.056. [DOI] [PubMed] [Google Scholar]
- Rose KM, Couper D, Eigenbrodt ML, Mosley TH, Sharrett AR, Gottesman RF. Orthostatic hypotension and cognitive function: the Atherosclerosis Risk in Communities Study. Neuroepidemiology. 2010;34(1):1–7. doi: 10.1159/000255459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap PL, Niti M, Yap KB, Ng TP. Orthostatic hypotension, hypotension and cognitive status: early comorbid markers of primary dementia? Dement Geriatr Cogn Disord. 2008;26(3):239–246. doi: 10.1159/000160955. [DOI] [PubMed] [Google Scholar]
- Bendini C, Angelini A, Salsi F, Finelli ME, Martini E, Neviani F. et al. Relation of neurocardiovascular instability to cognitive, emotional and functional domains. Arch Gerontol Geriatr. 2007;44(SUPPL.):69–74. doi: 10.1016/j.archger.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Viramo P, Luukinen H, Koski K, Laippala P, Sulkava R, Kivela SL. Orthostatic hypotension and cognitive decline in older people. J Am Geriatr Soc. 1999;47(5):600–604. doi: 10.1111/j.1532-5415.1999.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Elmstahl S, Rosen I. Postural hypotension and EEG variables predict cognitive decline: results from a 5-year follow-up of healthy elderly women. Dement Geriatr Cogn Disord. 1997;8(3):180–187. doi: 10.1159/000106629. [DOI] [PubMed] [Google Scholar]
- Matsubayashi K, Okumiya K, Wada T, Osaki Y, Fujisawa M, Doi Y. et al. Postural dysregulation in systolic blood pressure is associated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke. 1997;28(11):2169–2173. doi: 10.1161/01.str.28.11.2169. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42(2):136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Berg D, Prieur S, Junghanns S, Schweitzer K, Globas C. et al. Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Mov Disord: official journal of the Movement Disorder Society. 2009;24(14):2136–2142. doi: 10.1002/mds.22767. [DOI] [PubMed] [Google Scholar]
- Frongillo D, Stocchi F, Buccolini P, Stecconi P, Viselli F, Ruggieri S. et al. Ambulatory blood pressure monitoring and cardiovascular function tests in multiple system atrophy. Fundam Clin Pharmacol. 1995;9(2):187–196. doi: 10.1111/j.1472-8206.1995.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging. 2012;33(10):2324–2333. doi: 10.1016/j.neurobiolaging.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Frewen J, Finucane C, Savva GM, Boyle G, Coen RF, Kenny RA. Cognitive function is associated with impaired heart rate variability in ageing adults: the Irish longitudinal study on ageing wave one results. Clin Auton Res: official journal of the Clinical Autonomic Research Society. 2013;23(6):313–323. doi: 10.1007/s10286-013-0214-x. [DOI] [PubMed] [Google Scholar]
- Ni Bhuachalla B, McGarrigle CA, Kenny RA. Neurocardiovascular instability may modulate end-organ damage: A review of this hypothesis investigating the eye and manifestations of NCVI. Med Hypotheses. 2015;85(5):594–602. doi: 10.1016/j.mehy.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T. et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol. 2002;40(1):133–141. doi: 10.1016/s0735-1097(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG. et al. Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatry. 2007;78(7):671–677. doi: 10.1136/jnnp.2006.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passant U, Warkentin S, Gustafson L. Orthostatic hypotension and low blood pressure in organic dementia: a study of prevalence and related clinical characteristics. Int J Geriatr Psychiatry. 1997;12(3):395–403. [PubMed] [Google Scholar]
- Sonnesyn H, Nilsen DW, Rongve A, Nore S, Ballard C, Tysnes OB. et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307–313. doi: 10.1159/000247586. [DOI] [PubMed] [Google Scholar]
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP. et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord: official journal of the Movement Disorder Society. 2009;24(11):1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- Colosimo C, Morgante L, Antonini A, Barone P, Avarello TP, Bottacchi E. et al. Non-motor symptoms in atypical and secondary parkinsonism: the PRIAMO study. J Neurol. 2010;257(1):5–14. doi: 10.1007/s00415-009-5255-7. [DOI] [PubMed] [Google Scholar]
- Femminella GD, Rengo G, Komici K, Iacotucci P, Petraglia L, Pagano G. et al. Autonomic dysfunction in Alzheimer’s disease: tools for assessment and review of the literature. J Alzheimers Dis: JAD. 2014;42(2):369–377. doi: 10.3233/JAD-140513. [DOI] [PubMed] [Google Scholar]
- Van Beek AH, Claassen JA. The cerebrovascular role of the cholinergic neural system in Alzheimer’s disease. Behav Brain Res. 2011;221(2):537–542. doi: 10.1016/j.bbr.2009.12.047. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Jansen RW. Cholinergically mediated augmentation of cerebral perfusion in Alzheimer’s disease and related cognitive disorders: the cholinergic-vascular hypothesis. J Gerontol A Biol Sci Med Sci. 2006;61(3):267–271. doi: 10.1093/gerona/61.3.267. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 8-84. [DOI] [PubMed] [Google Scholar]
- Giubilei F, Strano S, Imbimbo BP, Tisei P, Calcagnini G, Lino S. et al. Cardiac autonomic dysfunction in patients with Alzheimer disease: possible pathogenetic mechanisms. Alzheimer Dis Assoc Disord. 1998;12(4):356–361. doi: 10.1097/00002093-199812000-00017. [DOI] [PubMed] [Google Scholar]
- Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17(10):724–729. doi: 10.1016/j.parkreldis.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaisetthawatkul P, Boeve BF, Benarroch EE, Sandroni P, Ferman TJ, Petersen R. et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology. 2004;62(10):1804–1809. doi: 10.1212/01.wnl.0000125192.69777.6d. [DOI] [PubMed] [Google Scholar]
- Idiaquez J, Roman GC. Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci. 2011;305(1-2):22–27. doi: 10.1016/j.jns.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Mochizuki H. The regulatory role of alpha-synuclein and parkin in neuronal cell apoptosis; possible implications for the pathogenesis of Parkinson’s disease. Apoptosis: an international journal on programmed cell death. 2010;15(11):1312–1321. doi: 10.1007/s10495-010-0486-8. [DOI] [PubMed] [Google Scholar]
- Larner AJ, Mathias CJ, Rossor MN. Autonomic failure preceding dementia with Lewy bodies. J Neurol. 2000;247(3):229–231. doi: 10.1007/s004150050572. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63(6):1093–1095. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- Andersson M, Hansson O, Minthon L, Ballard CG, Londos E. The period of hypotension following orthostatic challenge is prolonged in dementia with Lewy bodies. Int J Geriatr Psychiatry. 2008;23(2):192–198. doi: 10.1002/gps.1861. [DOI] [PubMed] [Google Scholar]
- Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB. et al. Guidelines for the diagnosis and management of syncope. Eur Heart J. 2009;30(21):2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. Carotid sinus syndrome: Progress in understanding and management. Glob Cardiol Sci Pract. 2014;2014(2):1–8. doi: 10.5339/gcsp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda Gde C, Pedrosa RC, Lacerda RC, Santos MC, Perez Mde A, Teixeira AB. et al. Cardioinhibitory carotid sinus hypersensitivity: prevalence and predictors in 502 outpatients. Arquivos brasileiros de cardiologia. 2008;90(3):148–155. doi: 10.1590/s0066-782x2008000300002. [DOI] [PubMed] [Google Scholar]
- Schoon Y, Olde Rikkert MG, Rongen S, Lagro J, Schalk B, Claassen JA. et al. Head turning-induced hypotension in elderly people. PLoS One. 2013;8(8):e72837. doi: 10.1371/journal.pone.0072837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MP, Chadwick TJ, Kerr SR, Parry SW. Symptomatic presentation of carotid sinus hypersensitivity is associated with impaired cerebral autoregulation. J Am Heart Assoc. 2014;3(3) doi: 10.1161/JAHA.113.000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Shaw F, McKeith I, Kenny R. High prevalence of neurovascular instability in neurodegenerative dementias. Neurology. 1998;51(6):1760–1762. doi: 10.1212/wnl.51.6.1760. [DOI] [PubMed] [Google Scholar]
- Kenny RA, Shaw FE, O’Brien JT, Scheltens PH, Kalaria R, Ballard C. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966–971. doi: 10.1136/jnnp.2003.023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier P, Colsy M, Robert F, Bourenane G. Prevalence of positive carotid sinus massage and related risk of syncope in patients with Alzheimer’s disease. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2007;9(9):829–834. doi: 10.1093/europace/eum148. [DOI] [PubMed] [Google Scholar]
- Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72(6):721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Kenny RA, Slade JY, Oakley AE, Kalaria RN. Medullary autonomic pathology in carotid sinus hypersensitivity. Neuropathol Appl Neurobiol. 2008;34(4):403–411. doi: 10.1111/j.1365-2990.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Research. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The autonomic higher order processing nuclei of the lower brain stem are among the early targets of the Alzheimer’s disease-related cytoskeletal pathology. Acta neuropathologica. 2001;101(6):555–564. doi: 10.1007/s004010000320. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol. 2001;49(1):53–66. doi: 10.1002/1531-8249(200101)49:1<53::aid-ana30>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ballard C, O’Brien J, Barber B, Scheltens P, Shaw F, McKeit I. et al. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann N Y Acad Sci. 2000:442–445. doi: 10.1111/j.1749-6632.2000.tb06396.x. [DOI] [PubMed] [Google Scholar]
- Parry SW, Steen IN, Baptist M, Kenny RA. Amnesia for loss of consciousness in carotid sinus syndrome: implications for presentation with falls. J Am Coll Cardiol. 2005;45(11):1840–1843. doi: 10.1016/j.jacc.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Jansen RW, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med. 1995;122(4):286–295. doi: 10.7326/0003-4819-122-4-199502150-00009. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Ahn C. Postprandial hypotension in 499 elderly persons in a long-term health care facility. J Am Geriatr Soc. 1994;42(9):930–932. doi: 10.1111/j.1532-5415.1994.tb06582.x. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Fisher AA, Davis MW, McLean AJ. Postprandial systolic blood pressure responses of older people in residential care: association with risk of falling. Gerontology. 2003;49(4):260–264. doi: 10.1159/000070408. [DOI] [PubMed] [Google Scholar]
- Puisieux F, Bulckaen H, Fauchais AL, Drumez S, Salomez-Granier F, Dewailly P. Ambulatory blood pressure monitoring and postprandial hypotension in elderly persons with falls or syncopes. J Gerontol A Biol Sci Med Sci. 2000;55(9):M535–M540. doi: 10.1093/gerona/55.9.m535. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Ahn C. Association of postprandial hypotension with incidence of falls, syncope, coronary events, stroke, and total mortality at 29-month follow-up in 499 older nursing home residents. J Am Geriatr Soc. 1997;45(9):1051–1053. doi: 10.1111/j.1532-5415.1997.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Kohara K, Jiang Y, Igase M, Takata Y, Fukuoka T, Okura T. et al. Postprandial hypotension is associated with asymptomatic cerebrovascular damage in essential hypertensive patients. Hypertension. 1999;33(1 Pt 2):565–568. doi: 10.1161/01.hyp.33.1.565. [DOI] [PubMed] [Google Scholar]
- Tabara Y, Okada Y, Uetani E, Nagai T, Igase M, Kido T. et al. Postprandial hypotension as a risk marker for asymptomatic lacunar infarction. J Hypertens. 2014;32(5):1084–1090. doi: 10.1097/HJH.0000000000000150. discussion 90. [DOI] [PubMed] [Google Scholar]
- Luciano GL, Brennan MJ, Rothberg MB. Postprandial hypotension. Am J Med. 2010;123(3):281. doi: 10.1016/j.amjmed.2009.06.026. e1-6. [DOI] [PubMed] [Google Scholar]
- Jansen RW, Peeters TL, Van Lier HJ, Hoefnagels WH. The effect of oral glucose, protein, fat and water loading on blood pressure and the gastrointestinal peptides VIP and somatostatin in hypertensive elderly subjects. Eur J Clin Invest. 1990;20(2):192–198. doi: 10.1111/j.1365-2362.1990.tb02268.x. [DOI] [PubMed] [Google Scholar]
- Senard JM, Chamontin B, Rascol A, Montastruc JL. Ambulatory blood pressure in patients with Parkinson’s disease without and with orthostatic hypotension. Clin Auton Res. 1992;2(2):99–104. doi: 10.1007/BF01819664. [DOI] [PubMed] [Google Scholar]
- Idiaquez J, Benarroch EE, Rosales H, Milla P, Rios L. Autonomic and cognitive dysfunction in Parkinson’s disease. Clin Auton Res. 2007;17(2):93–98. doi: 10.1007/s10286-007-0410-7. [DOI] [PubMed] [Google Scholar]
- Idiaquez J, Rios L, Sandoval E. Postprandial hypotension in Alzheimer’s disease. Clin Auton Res. 1997;7(3):119–120. doi: 10.1007/BF02308837. [DOI] [PubMed] [Google Scholar]
- Parry SW, Kenny RA. The role of tilt table testing in neurocardiocascular instability in older adults. Eur Heart J. 2001;22(5):370–372. doi: 10.1053/euhj.1999.2046. [DOI] [PubMed] [Google Scholar]
- Marrison VK, Fletcher A, Parry SW. The older patient with syncope: practicalities and controversies. Int J Cardiol. 2012;155(1):9–13. doi: 10.1016/j.ijcard.2010.10.055. [DOI] [PubMed] [Google Scholar]
- Frewen J, King-kallimanis B, Boyle G, Kenny RA. Recent syncope and unexplained falls are associated with poor cognitive performance. Age Ageing. 2015;44(2):282–286. doi: 10.1093/ageing/afu191. [DOI] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ. et al. The rebirth of neuroscience in psychosomatic medicine, Part I: historical context, methods, and relevant basic science. Psychosom Med. 2009;71(2):117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]