Abstract
Sodium azide is toxic and widely used in agricultural, commercial products, and research laboratories. Thus it is of a significant environmental concern and there is a need for the development of a rapid detection method. A fluorogenic dibenzylcyclooctyne derivative (Fl-DIBO) is herein described as a fluorescent probe for the rapid detection of inorganic azide via Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC). Fl-DIBO was found to be highly selective toward NaN3 in comparison to other common anions with good sensitivity and detection limit of 10 μM.
Keywords: Sodium azide, Click chemistry, Fluorescent detection, Quantitative analysis
Graphical Abstract
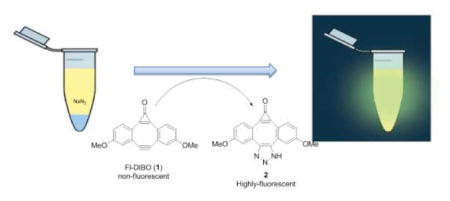
Sodium azide is a colorless, tasteless, odorless and salt-like solid that is widely employed in automobile airbags, airplane escape chutes, pest control, agriculture, and research laboratories.1 It is also often used as a preservative and biocide.2 Contamination by sodium azide is therefore a significant environmental concern. The US Environmental Protection Agency (EPA) and the Office of Occupational Health and Safety Administration (OSHA) have specified exposure limits at 0.3 mg/m3.3, 4 Exposures over 0.7 g (10 mg/kg) is considered lethal.5 Even at lower doses (0.004 to 2 mg/kg), sodium azide can cause substantial harm to human health.6 Azide is known to block cytochrome c oxidase leading to inhibition of mitochondrial respiration, and consequently impaired memory.7–9 Because of its acute toxicity, several azide-poisoning cases have been reported. In 1998, sodium azide was deliberately added to a teapot in Niigata, Japan, poisoning nine persons.10 Later, a Harvard University poisoning case in 200911 and a Dallas Richardson restaurant's tea poisoning case in 201012 were also reported as azide poisoning incidents. According to a report of the Center for Disease Control and Prevention (CDC), the FBI spent more than 5 months to figure out the chemical nature of the poison12 in the Harvard University case because of the limited ability for sodium azide detection. Symptoms of azide-induced mitochondrial poisoning are known to be similar to that of Alzheimer’s disease. Thus azide has been used in creating animal models of Alzheimer’s disease.13 For all these reasons, there is a need for a fast and accurate azide detection method.
Current laboratory methods for sodium azide determination mainly include chromatography14–17 and electrochemical detection.18–20 Various fluorescent probes for the detection of NaN3 have been recently reported.21–23 However, selectivity for NaN3 in the presence of other inorganic anions is an issue.21, 23 Metal complex-based methods have been reported.21 Herein we describe a metal-free fluorescent method for the detection of inorganic azide.
Although organic azido compounds have been widely known to react with terminal alkynes via copper (I)-catalyzed cycloaddition (CuAAC)24–26 and strained alkyne without the use of Cu(I),27 inorganic azide does not readily undergo the same reaction except in rare cases.15 4-Dibenzocyclooctynol (DIBO)28, 29 and its sulfated analogue S-DIBO30 have been used as probes for studying the subcellular location of glycoconjugates in living cells and labeling of azido-tagged proteins via SPAAC. Because of the fluorescent nature of the cycloaddition products, we were interested in examining whether they could be used to react with inorganic azide, and thus for their detection. Such work is along the line of our long interest in developing chemosensors for biologically important molecule such as hydrogen sulfide,31, 32 fluoride,33 and etc.
Since azide anion is known to react differently from their organic azido counterparts, we first examined the reactivity of the strained cyclooctyne FI-DIBO (1) with sodium azide (Scheme 1). Upon mixing Fl-DIBO with NaN3 at room temperature in a mixture of dioxane and HEPES buffer (1:1), a new fluorescent product was formed within minutes. The reaction was complete within 1 h (Scheme 1 and Figure 1). 1H- and 13C-NMR and mass spectrometric analyses of the isolated fluorescent product confirmed the formation of the desired triazole 2 (see SI for details).
Scheme 1.
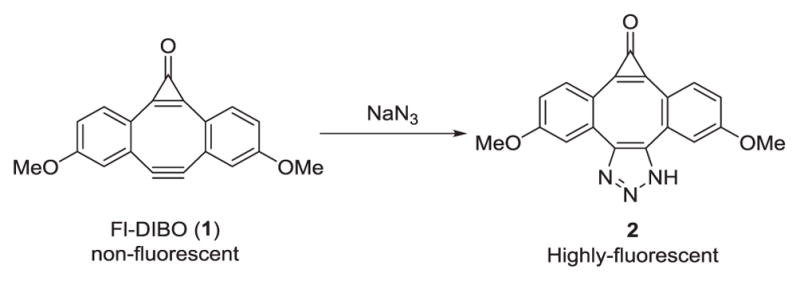
The reaction between inorganic azide and FI-DIBO
Figure 1.
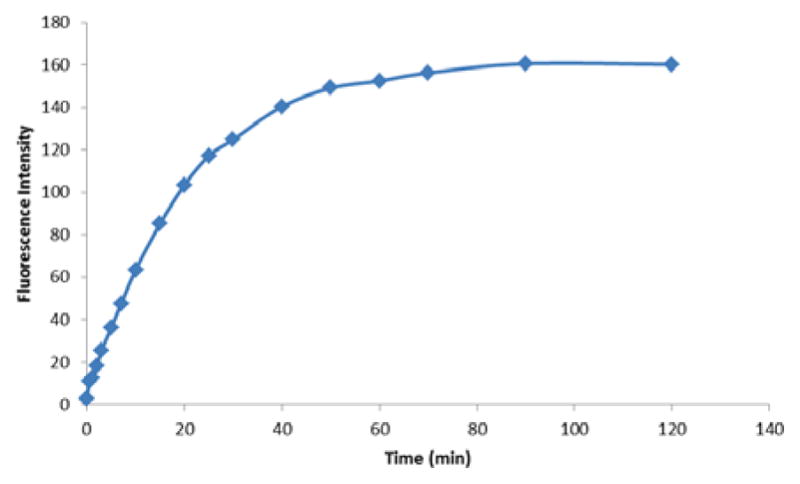
Time-dependent fluorescence intensity changes (λex= 363 nm, λem= 469 nm) of a mixture of Fl-DIBO (10 μM) and sodium azide (10 mM) at room temperature (25°C) in a mixture of 50 mM HEPES buffer and dioxane (1: 1) at pH 7.4
Triazole 2 exhibits a hypsochromic fluorescence emission as compared to 1-substituted triazole derivative with the emission maximum at 469 nm (λex 363 nm). Next, reaction kinetics was studied by following fluorescence intensity changes at 469 nm. In a typical pseudo first-order experiment, 10 μM of probe 1 was stirred with various amounts of NaN3 ranging from 1 mM to 10 mM at room temperature in a mixture of dioxane and HEPES buffer (1:1) and the fluorescence emission was recorded. The pseudo first-order rate constants were linearly dependent on the concentration of sodium azide and the second-order cycloaddition rate constant was estimated to be 0.0953 M−1s−1 (see SI for details).
Next, the influence of other anions on the fluorescence response of Fl-DIBO was evaluated. As shown in Figure 2, Fl-DIBO (100 μM) did not exhibit any fluorescence turn-on in the presence of 1 mM of various anions such as F−, Cl−, Br−, NO3−, AcO− and OH−.
Figure 2.
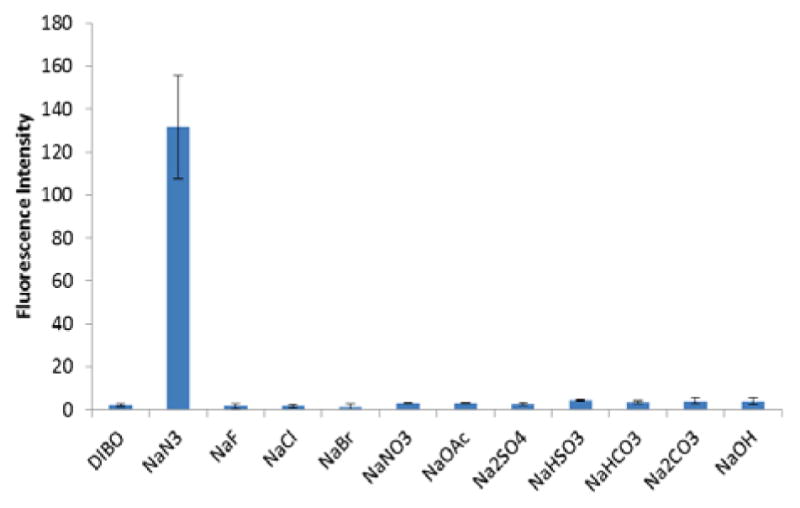
Fluorescence responses of Fl-DIBO to various salts. Fl-DIBO 10 μM, salts 1 mM in a mixture of 50 mM HEPES buffer and dioxane (1: 1) at pH 7.4. Fluorescence intensities were recorded after 10 h of incubation with the addition of salts at 25°C. Data represents the average of three independent experiments
The limit of detection is a critical factor when developing detection probes. Therefore, the quantitative analysis of NaN3 using Fl-DIBO was investigated. Thus, Fl-DIBO (100 μM) was reacted with NaN3 at various concentrations ranging from 5 μM to 100 μM (14 h, 60°C) in a mixture of HEPES/ dioxane (1: 1). The fluorescence emission intensity was found to be linearly dependent on the concentration of sodium azide (Figure 3). Using a 3:1 signal/background ratio as the cutoff, the limit of detection was found to be 10 μM (incubation at 60°C for 14 h, see details in SI). According to the CDC and OSHA, ingestion of 0.28–140 mg (0.004–2 mg/kg, adult weight: 70 kg) is sufficient to cause harm to human body. Take the Harvard coffee poisoning case as an example, only if the concentration of NaN3 in a cup of coffee reaches the range of 18.2 μM to 9.1 mM, the victims would have ingested enough to show poisoning symptoms. So this probe is sufficiently sensitive for detecting sodium azide in a pathologically relevant range.
Figure 3.
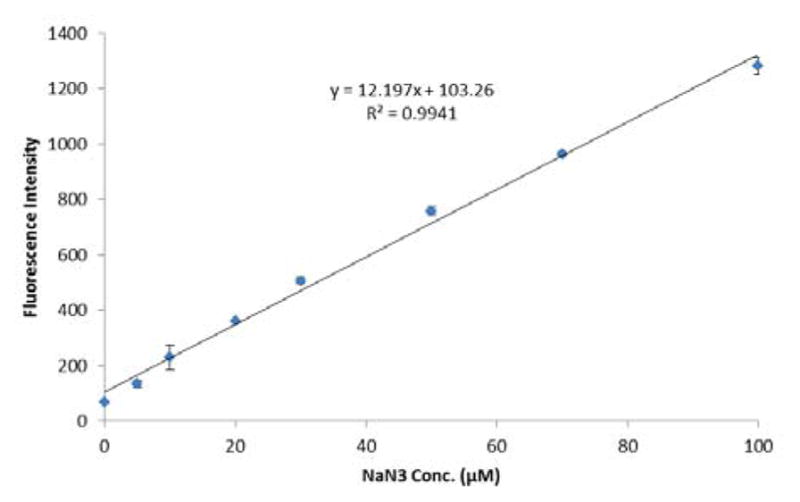
Calibration curve for NaN3. Fl-DIBO 100 μM, NaN3 0–100 μM in a mixture of 50 mM HEPES buffer and dioxane (1: 1) at pH 7.4. Fluorescence intensities at 469 nm were recorded after 14 h of incubation at 60°C with the addition of NaN3. Data represents the average of three independent experiments
To test the feasibility of using the Fl-DIBO probe for detection of azide in tea, different concentrations of NaN3 dissolved in tea samples were prepared. 100 μM of Fl-DIBO was incubated with various amounts of NaN3 ranging from 0.14 mg (9.1 μM in a cup of tea) to 1.12 mg (72.7 μM in a cup of tea) at 60°C in a mixture of dioxane and tea solution (1:1, 237 mL) and the fluorescence emission was recorded. As shown in SI, this probe showed concentration-dependent fluorescence intensity changes upon addition of sodium azide with the signal to background ratio being about 2:1 at the low end of the threshold concentration (about 20 μM), which is pathologically relevant.
In conclusion, Fl-DIBO was successfully applied to the detection of inorganic azide in aqueous medium via strain-promoted alkyne-azide cycloaddition. The fluorescence response of the triazole product was linearly dependent on the azide concentration with an excellent detection limit of 10 μM. In addition, Fl-DIBO proved to be selective to azide with no fluorescence detected in the presence of other anions. The rapid rate, high sensitivity and selectivity, and simplicity of the procedure make Fl-DIBO an excellent turn-on probe for the routine quantification of NaN3 in analytical labs.
Supplementary Material
Acknowledgments
Financial support from Georgia State University Molecular Basis of Disease Program (MBD) through a fellowship to KW and the National Institutes of Health (R01CA88986 to G-JB and GM084933 to BW) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Frederick KA, Babish JG. Regul Toxicol Pharmacol. 1982;2:308. doi: 10.1016/0273-2300(82)90004-6. [DOI] [PubMed] [Google Scholar]
- 2.Lichstein HC, Soule MH. J Bacteriol. 1944;47:221. doi: 10.1128/jb.47.3.221-230.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agengy, U. S. E. P. Agengy, U. S. E. P, editor. Intergrate Rish Information System. 1987. [Google Scholar]
- 4.Administration, O. S. H. 2004 [Google Scholar]
- 5.Chang S, Lamm SH. Int J Toxicol. 2003;22:175. doi: 10.1080/10915810305109. [DOI] [PubMed] [Google Scholar]
- 6.Kleinhofs A, Owais WM, Nilan RA. Mutat Res-Rev Genet. 1978;55:165. doi: 10.1016/0165-1110(78)90003-9. [DOI] [PubMed] [Google Scholar]
- 7.Keilin DE, Hartree EF. Proc R Soc London Ser B. 1939;167 [Google Scholar]
- 8.Robertson HE, Boyer PD. J Biol Chem. 1954;229:295. [PubMed] [Google Scholar]
- 9.Bingham E, Cohrssen B, Powell CH, editors. In Patty's industrial hygiene and toxicology. Interscience; New York: 1990. [Google Scholar]
- 10.Okumura T, Ninomiya N, Ohta M. Prehosp Disaster Med. 2003;18:189. doi: 10.1017/s1049023x00001047. [DOI] [PubMed] [Google Scholar]
- 11.Gussow L. Emergency Med News. 2010;32:8. [Google Scholar]
- 12.CDC. Morbidity and Mortality Weekly Report. 2012;61:457. [PubMed] [Google Scholar]
- 13.Szabados T, Dul C, Majtenyi K, Hargitai J, Penzes Z, Urbanics R. Behav Brain Res. 2004;154:31. doi: 10.1016/j.bbr.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Oshima H, Ueno E, Saito I, Matsumoto H. J AOAC Int. 2000;83:1410. [PubMed] [Google Scholar]
- 15.Wang L, Dai C, Chen W, Wang SL, Wang B. Chem Commun. 2011;47:10377. doi: 10.1039/c1cc11199j. [DOI] [PubMed] [Google Scholar]
- 16.Annable PL, Sly LA. J Chromatogr. 1991;546:325. doi: 10.1016/s0021-9673(01)93030-4. [DOI] [PubMed] [Google Scholar]
- 17.Kage S, Kudo K, Ikeda N. J Anal Toxicol. 2000;24:429. doi: 10.1093/jat/24.6.429. [DOI] [PubMed] [Google Scholar]
- 18.Sezgintürk MK, Göktuğ T, Dinçkaya E. Biosen Bioelectron. 2005;21:684. doi: 10.1016/j.bios.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Ivandini TA, Kamiya A, Nomura S, Yamanuki M, Matsumoto K, Fujishima A, Einaga Y. Sensor Actuat B-Chem. 2007;120:500. [Google Scholar]
- 20.Leech D, Daigle F. Analyst. 1998;123:1971. doi: 10.1039/a803313g. [DOI] [PubMed] [Google Scholar]
- 21.Dhara K, Saha UC, Dan A, Sarkar S, Manassero M, Chattopadhyay P. Chem Commun. 2010;46:1754. doi: 10.1039/b919937c. [DOI] [PubMed] [Google Scholar]
- 22.Sahana A, Banerjee A, Guha S, Lohar S, Chattopadhyay A, Mukhopadhyay SK, Das D. Analyst. 2012;137:1544. doi: 10.1039/c2an16180j. [DOI] [PubMed] [Google Scholar]
- 23.Kim HW, Choi MG, Park H, Lee JW, Chang SK. RSC Adv. 2015;5:4623. [Google Scholar]
- 24.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 27.Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 28.Ning X, Guo J, Wolfert MA, Boons GJ. Angew Chem Int Ed. 2008;47:2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friscourt F, Fahrni CJ, Boons GJ. J Am Chem Soc. 2012;134:18809. doi: 10.1021/ja309000s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friscourt F, Ledin PA, Mbua NE, Flanagan-Steet HR, Wolfert MA, Steet R, Boons GJ. J Am Chem Soc. 2012;134:5381. doi: 10.1021/ja3002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang B. Angew Chem Int Ed. 2011;50:9672. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang K, Peng H, Ni N, Dai C, Wang B. J Fluoresc. 2014;24:1. doi: 10.1007/s10895-013-1296-5. [DOI] [PubMed] [Google Scholar]
- 33.Ke B, Chen W, Ni N, Cheng Y, Dai C, Dinh H, Wang B. Chem Commun. 2013;49:2494. doi: 10.1039/c2cc37270c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


