Abstract
Sports-related head impact and injury has become a very highly contentious public health and medico-legal issue. Near-daily news accounts describe the travails of concussed athletes as they struggle with depression, sleep disorders, mood swings, and cognitive problems. Some of these individuals have developed chronic traumatic encephalopathy, a progressive and debilitating neurodegenerative disorder. Animal models have always been an integral part of the study of traumatic brain injury in humans but, historically, they have concentrated on acute, severe brain injuries. This review will describe a small number of new and emerging animal models of sports-related head injury that have the potential to increase our understanding of how multiple mild head impacts, starting in adolescence, can have serious psychiatric, cognitive and histopathological outcomes much later in life.
Keywords: animal models, cognitive dysfunction, neuropathological disorders, psychiatric outcomes, repetitive mild traumatic brain injury, Sports-related head injury
Traumatic brain injury (TBI) results from a blow to the head and the severity of injury can range along a continuum from mild (e.g., headache, dizziness) to severe (e.g., extended coma, amnesia) to fatal. TBI is one of the most common neurological diagnoses in the US (Rutland-Brown et al. 2006) and the Center for Disease Control and Prevention (CDC) has estimated that ~ 1.5 million people sustain TBI on an annual basis. Approximately, one-third of all injury deaths arise from TBI and roughly 90 000 experience long-term disability yearly as a result of brain injury (Masel and DeWitt 2010). The cost of lifetime care for survivors of TBI has been estimated at $0.6–$1 million per individual with a total annual cost of approximately $60 billion to the United States (Corso et al. 2006). Perhaps the form of TBI that has garnered the greatest amount of scrutiny recently is repetitive mild traumatic brain injury (rmTBI) resulting from sports-related head impacts. Frequent news accounts of concussed former athletes’ struggle with depression, sleep disorders, mood swings, and cognitive problems are directing much needed attention to this significant and emerging health issue. It has been estimated that 1.6–3.8 million sports-related TBIs occur each year (Langlois et al. 2006; Halstead and Walter 2010). Because many concussive-like injuries go unreported or are not recognized (McCrea et al. 2004; Langlois et al. 2006; Daneshvar et al. 2011), these figures are likely to represent a gross underestimation. From 2001 to 2005, there were ~ 500 000 emergency room visits for concussion among US children aged 8–19 years of age, half of which were sports-related. In organized high school football, concussions occur at rates of ~ 3.6–5.6% among the 1.2 million participants (Powell and Barber-Foss 1999; Guskiewicz et al. 2000), translating to ~ 43 000–67 000 adolescent head injuries. Epidemiological studies reveal that ~ 60% of retired National Football League (NFL) football players sustained at least one concussion during their careers (Guskiewicz et al. 2005) and ~ 25% experienced repeat injury (Pellman et al. 2004; Guskiewicz et al. 2005). The issue of how sports-related rmTBI may lead to chronic psychiatric and neuropathological disorders is highly contentious and has significant medico-legal ramifications. An overriding concern in rmTBI is the possibility that successive injuries, including subconcussive ones, may synergize with previous ones so that their effects become additive (Guskiewicz et al. 2003).
To place the terms ‘repetitive’ and ‘mild’ into the context of sports-related rmTBI, one must consider the rate and nature of head impacts as illustrated in American-style football. Beckwith et al. (2013a,b) studied collegiate and high school players and determined in a 6-year project that ~ 8% suffered concussive impacts, a rate of ~ 5 per season. A head impact was defined as one that was greater than or equal to 14.4 g. These same players also received ~ 280 non-concussive impacts each per season or ~ 11 per week. Players in these studies (Beckwith et al. 2013a,b) who were not concussed (92%) received ~ 210 impacts per season or ~ 8 per week. Altogether, the subjects in these studies received ~ 55 non-concussive impacts for each concussive impact. It has been estimated that elementary school-aged football players (ages 9–12 years) average 240 head impacts per season (Cobb et al. 2013). Individual high school football players can receive between 650 and 900 head impacts per season (Broglio et al. 2011a, 2012) or greater than 25 impacts per game plus practice sessions (Urban et al. 2013) and some collegiate football players have sustained upwards of 1440 head impacts in a single season (Crisco et al. 2010). Each of these studies derived the number of head impacts sustained by individual players by using helmets instrumented with the Head Impact Telemetry system. Forbes et al. (2012) summarized all studies that catalogd football head impacts, showing that individual athletes receive hundreds of head impacts on a regular basis. Injuries that may be caused by concussions are generally mild and often escape detection by brain imaging (Gardner et al. 2012; Slobounov et al. 2012; Terry et al. 2012), making detection of changes caused by non-concussive impacts even more challenging. As the aforementioned rates of head impact and injury focused on American-style football, participants in other sports including soccer, boxing, ice hockey, rugby, and lacrosse also have significant rates of head injury (Gessel et al. 2007; Hootman et al. 2007; Marar et al. 2012; Cusimano et al. 2013). The discussion in this review article is intended to apply generally to all sports events that involve frequent head contact.
Numerous attempts have been made to define ‘concussion’ in biomechanical terms. Although these very sophisticated studies have established the forces (e.g., translational and rotational acceleration) that can be delivered in athletic head impacts, it does not appear that these data can be used to predict whether a player will be concussed (Guskiewicz and Mihalik 2011; Forbes et al. 2012). A review by Forbes et al. (2012) calculated average head impact forces received by concussed players and found that a large number of nonconcussed players received impacts that exceeded forces encountered by concussed players. The Head Impact Telemetry system is known to have a large range of inaccuracy when measuring impact forces in the field (Jadischke et al. 2013), so attempts to relate specific impact forces to injury are limited. The issue of what defines a concussion clinically [or if a concussion represents injury (McCrory et al. 2013a)] is far too complex to be debated presently (e.g., see (Blennow et al. 2012; Slobounov et al. 2012), but it is clear that athletes are being exposed to very large numbers of head impacts over long periods of time, and less than 0.02% of these impacts account for concussions (Forbes et al. 2012). Emerging studies are establishing that football players can experience transient disruptions in the blood-brain barrier (evidenced by surges in serum levels of the astrocytic protein S100B) in the absence of concussion (Marchi et al. 2013). The authors of this study suggest further that the correlation of serum S100B levels and CNS changes seen using diffusion tensor imaging support a link between repeated blood-brain barrier disruption and future risk for cognitive changes (Marchi et al. 2013). Cognitive and neurophysiological impairments have been detected in high school football players without clinically diagnosed concussion (Talavage et al. 2014). The specific focus on concussions as the cause of persistent CNS deficits may be somewhat misplaced in light of the fact that most athletes are exposed to multiple subconcussive head impacts throughout their careers and these may also increase the risk of developing neurodegenerative insult (Stern et al. 2011).
It is fair to state that the full clinical picture of sports-related rmTBI has not yet been assembled. In general terms, rmTBI has symptoms that are similar to post-concussive syndrome that include headache, dizziness, confusion and fogginess, irritability, and drowsiness (Pellman et al. 2003b; Barlow et al. 2010; Halstead and Walter 2010; Jordan 2013). Most mildly concussed individuals return to normal shortly after injury (Pellman et al. 2005, 2006; Barlow et al. 2010; Halstead and Walter 2010). More significant health issues arise as athletes age and can include chronic traumatic encephalopathy (CTE; Omalu et al. 2010; Halstead and Walter 2010; Baugh et al. 2012; Mez et al. 2013). From a neuropathological perspective, CTE is associated with the appearance of foci of hyperphosphorylated tau tangles, disseminated microgliosis and astrocytosis and progressive neurodegeneration (McKee et al. 2009, 2013; Mez et al. 2013). CTE has been linked to increased mortality, shortened life-span and increased co-morbidity of neurodegenerative [e.g., Alzheimer's and Parkinson's disease (Masel and DeWitt 2010; Guskiewicz et al. 2007a; Langlois et al. 2006; Plassman et al. 2000; Sivanandam and Thakur 2012)] and psychiatric disorders (Guskiewicz et al. 2005, 2007a; De Beaumont et al. 2009, 2012; Rao et al. 2010; Hart et al. 2013; Mez et al. 2013)]. CTE may also reflect the culmination of long-term rmTBI (Omalu et al. 2005; McKee et al. 2009; Gavett et al. 2011). It is not yet possible to link repeated head impacts to the development of CTE directly and it is not clear that CTE causes behavioral or cognitive changes in retired athletes (Karantzoulis and Randolph 2013; Randolph et al. 2013).
Animal models of sports-related rmTBI are essential to help minimize risk to human athletes
Animal models of TBI have always been an important element in the study of how the CNS responds to injury. Animal models have been developed to simulate virtually every form of human brain injury imaginable: penetrating, blast-related, hemorrhagic-related, and concussive/contusive. However, a substantial roadblock that is hampering efforts to achieve a better understanding of sports-related rmTBI is the shortage of effective animal models. If any form of human head injury could benefit from a new and useful model, it would certainly be sports-related head injury. At present, the vast majority of research on sports-related head injury is restricted to athletes. These studies, while recording impact forces experienced by participants, searching for CNS changes with brain imaging, determining health outcomes, and testing of new protective equipment, do not allow in-depth mechanistic approaches. Well-intentioned estimates of whether and when a head-injured athlete can return to play may be wrong and newly developed protective head gear may be no more or even less effective than what is presently available. The consequences of such estimates that turn out to be incorrect may not be known for 10–20 years, long after an athlete has ceased participation in a sports career that involves head impact, and would be dire. An animal model of sports-related rmTBI would have another important benefit. At present, it has been difficult to form a direct link between repeated head impacts in sports and the development of psychiatric and/or neuropathological disorders in athletes (McCrory et al. 2013b). Co-existing or complicating conditions such as genetic predispositions to disease, abuse of illicit drugs and alcohol, and intake of performance-enhancing steroids are often not cataloged in human athletes and each these factors, alone or in combination, could contribute to chronic and persistent health problems, including CNS disorders (Iverson 2014). The use of animal subjects would allow a determination of whether repeated mild head impacts can be linked to cognitive and neuro-pathological problems by controlling the conditions mentioned above.
Minimal characteristics of an animal model of sports-related head injury
Numerous animal models of TBI have been developed and a discussion of these models is well beyond the scope of this review [see (Lighthall et al. 1989; Park et al. 1999; Morales et al. 2005; LaPlaca et al. 2007; Weber 2007; O'Connor et al. 2011; Xiong et al. 2013; Dewitt et al. 2013) for reviews]. Just as these animal models have been very effective in characterizing the molecular and cellular bases of severe, acute TBI, an animal model of sports-related rmTBI would help fill many of the gaps in our understanding of this condition. Conservatively speaking, the key characteristics of head impact/injury in athletes that should guide the development and application of an animal model of this condition include:
-
(1)
The head (with scalp and skull intact) must be struck directly. It is not enough for a model to damage the subject's brain but to do so in a manner that closely approximates that seen in athletes when exposed to head impacts. Impacts directly to the head achieve higher accelerations and have shorter durations than those that involve non-head contacts (Kimpara and Iwamoto 2012).
-
(2)
Head impacts should occur with high velocity and involve a rapid change in head velocity and rapid acceleration of the head (both rotational and angular acceleration) (Pellman et al. 2003a,b; Viano and Pellman 2005; Viano et al. 2005, 2007, 2009; Guskiewicz et al. 2007b; Meaney and Smith 2011; Jordan 2013; Urban et al. 2013). The importance of head rotation (vs. striking a head fixed in place) in contributing to concussive-like brain injuries was recognized more than 70 years ago (Denny-Brown and Russell 1941) and more recent studies have shown that rhesus monkeys are rendered unconscious more readily when rapid head rotations were achieved via direct blows to the head versus contact involving whiplash without head contact (Ommaya et al. 1971; Ommaya and Gennarelli 1974). Emerging studies of head impacts sustained by football players have also suggested the importance of head movement, both rotational and angular acceleration, in determining clinical outcomes (Pellman et al. 2003a,b; Viano and Pellman 2005; Viano et al. 2005, 2007, 2009; Guskiewicz et al. 2007b; Meaney and Smith 2011; Jordan 2013; Urban et al. 2013).
-
(3)
Repeated impacts to the head are mild and single impacts do not cause moderate or severe injury such as edema, neuronal damage or loss, or hemorrhage. The majority of head impacts received by football players are non-concussive (Guskiewicz et al. 2007b; Broglio et al. 2011b; Guskiewicz and Mihalik 2011; Forbes et al. 2012; Beckwith et al. 2013a,b). Conventional brain imaging approaches generally lack the sensitivity to detect concussion-induced changes much less the milder changes that accompany non-concussive impacts (Gardner et al. 2012; Slobounov et al. 2012; Terry et al. 2012).
-
(4)
Head impacts should begin in adolescence and continue sporadically over a long period of time, generally into adulthood. Children play full contact football (e.g., Pop Warner from 5 to 16 years old) or participate in boxing (e.g., Golden Gloves from 10 to 15 years old). A cursory internet search reveals that ~ 1.2 million high school and ~ 65 000 college athletes are playing American-style football at any given time so the number of adolescent athletes exposed to sports-related head contacts is very large. The brain changes continuously through adolescence and into adulthood and the developing brain can respond differently to head impact than the mature brain (Babikian et al. 2010; Grady 2010; Choe et al. 2012; Prins and Giza 2012; Toledo et al. 2012). Indeed, all animal models of rmTBI that have addressed long-term neurodegenerative outcomes have involved injuries sustained in adulthood (see below).
-
(5)
The animal model of sports-related rmTBI should have psychiatric manifestations and result in mild cognitive difficulties as seen in former athletes. Findings emerging from the study of retired professional athletes are suggesting the possibility that repeated head impacts (concussive and non-concussive) can increase the risk for developing depression and other psychiatric disorders (Guskiewicz et al. 2007a; Kerr et al. 2012; Didehbani et al. 2013).
-
(6)
The animal model of sports-related rmTBI should lead to the gradual appearance of CTE-like neuropathology as has been seen in some retired athletes (Omalu et al. 2005, 2006, 2010; McKee et al. 2009; Gavett et al. 2011; Mez et al. 2013; Peskind et al. 2013). Documentation of increases in phosphorylated tau over time need not imply that these signs are causing the behavioral and psychiatric conditions being seen in retired athletes. Such results would suggest that the forces associated with sports-related head impacts in animal models can result in similar proteinopathies as seen in humans and may make phosphorylated tau, an useful biomarker of repeated mild head injuries.
Most existing animal models of TBI are not effective for the study of sports-related head impact
Most existing animal models of TBI achieve very few of the criteria for modeling sports-related rmTBI (see also (Viano et al. 2009) for related discussion). The first weight drop model developed for use in rats directly impacted the exposed dura and caused extensive hemorrhaging and cortical cavitation (Feeney et al. 1981). The Marmarou weight drop model applies impact to the exposed, intact skull. This method causes severe compressive deformation of the cranial vault, results in cortical injuries (e.g., contusions and bleeding) beneath the site of impact and its damage extends to the brainstem (resulting in respiratory arrest and apnea) and spinal cord (Foda and Marmarou 1994; Marmarou et al. 1994; Kallakuri et al. 2003). Variants of the weight drop method also result in cortical contusions, axonal damage, and in some cases high rates of mortality (Chen et al. 1996; Stahel et al. 2000; Flierl et al. 2009; Kilbourne et al. 2009; Namjoshi et al. 2013). TBI models other than closed-head, to include lateral fluid percussion, (Thompson et al. 2005) and controlled cortical impact methods (CCI, (Lighthall et al. 1989) involve head restraint (i.e., use of stereotaxic), skull trephaning, and direct impact of the brain. Recent modifications in each of these popular models have lead to the publication of methods that are referred to as repetitive and mild TBI. By striking the exposed skull directly (i.e., scalp reflected, no craniotomy), the CCI model has been used to deliver 2–3 impacts over 1–2 days (Laurer et al. 2001; Uryu et al. 2002; Conte et al. 2004; Longhi et al. 2005; Yoshiyama et al. 2005; Shitaka et al. 2011; Bennett et al. 2012; Mouzon et al. 2012). The use of dropped weights with lower masses to strike head 3–4 times over several days has also been tested (Allen et al. 2000; DeFord et al. 2002; Creeley et al. 2004). Each of these methods falls short of serving as effective models of sports-related TBI by using adult animals, by partially or totally restricting head movement at impact, by using small numbers of impacts over short periods of time, and by imparting injuries that are far more severe than seen in athletes. All of the above-referenced approaches reported cognitive deficits in their subjects whereas few if any have documented the development of psychiatric manifestations or CTE-like neuropathologies. Results from these studies have also been somewhat inconsistent in finding that by comparison to single injuries, multiple insults worsen (Kanayama et al. 1996; Laurer et al. 2001; Uryu et al. 2002; Longhi et al. 2005; Hamberger et al. 2009), make little difference (DeFord et al. 2002; Creeley et al. 2004) or actually improve outcome (DeRoss et al. 2002). Very young rats (11 days of age) exposed to three skull impacts show axonal injury and evidence of degeneration but no cognitive deficiency (Huh et al. 2007). Mice exposed to two impacts at 35 days of age show mild cognitive deficits and some axonal injury, gliosis and reduced metabolic rate (Prins et al. 2010, 2013). These studies using younger animals as subjects also restricted head movement at impact and delivered just two head impacts over short periods of time. A summary of the above-mentioned models that deliver more than a single head impact and satisfy few of the desired criteria of a model of human sports-related head injury is presented in Table 1.
Table 1.
Animal models using more than one head impact but satisfying few of the desired criteria of a model of human sports-related rmTBI
| Animal modela and reference | Subject | Direct hit on intact scalp and skull/craniotomy | Head free to move upon impact | No. impactsb, time between impacts | Accommodates young subjects | Psychiatric (Psy) and/or cognitive (Cog) manifestations | CTE-like tauopathy; other injuries |
|---|---|---|---|---|---|---|---|
| Marmarou and related weight drop variants | |||||||
| Allen et al. 2002 | Adult rat | Yes/yes | No-in stereotax | 3 @ 3 daysc | NDd | ND-Cog | ND; cortical damage, necrosis, gliosis |
| ND-Psy | |||||||
| DeFord et al. 2002 | Adult mouse | Yes/no | No-cushion used | 1-4 @ 24 h | ND | Yes - Cog | ND; no overt pathology |
| ND-Psy | |||||||
| Creeley et al. 2004 | Adult mouse | Yes/no | No-firm mold | 1-3 @ 24 h | ND | Yes - Cog | ND; neurodegeneration |
| ND-Psy | |||||||
| Namjoshi et al. 2013 | Adult mouse | Yes/no | No | 2 @ 24 h | ND | Yes - Cog | ND; axonal damage, cortical contusions |
| ND-Psy | |||||||
| Controlled cortical impact and variants | |||||||
| Laurer et al. 2001 | Adult mouse | Yes/no | No-in stereotax | 1-2 @ 24 h | ND | Neither | No; axonal injury |
| Uryu et al. 2002 | Adult Tg mouse | Yes/no | No-in stereotax | 1-2 @ 24 h | ND | Yes - Cog | ND; Aβ ↑ |
| ND-Psy | |||||||
| Conte et al. 2004 | Adult Tg mouse | Yes/no | No-in stereotax | 1-2 @ 24 h | ND | Yes - Cog | ND; Aβ ↑ |
| ND-Psy | |||||||
| Longhi et al. 2005 | Adult mouse | Yes/no | No-in stereotax | 1-2 @ 3,5, or 7 days | ND | Yes - Cog | ND; cytoskeletal, neuronal and axonal damage |
| ND-Psy | |||||||
| Yoshiyama et al. 2005 | Adult Tg mouse | Yes/no | No-in stereotax | 4 or 16 @ 20 min | ND | No-Cog | yes |
| ND-Psy | |||||||
| Huh et al. 2007 | PND11 rat | Yes/no | No-foam pad | 1, 2, or 3 @ 5 min | Yes | Yes - Cog | ND; axonal swelling, degeneration |
| ND-Psy | |||||||
| Prins et al. 2010 | PND35 rat | Yes/no | No-head against wooden block | 1-2 @ 24 h | Yes | Yes - Cog | ND; axonal injury; gliosis |
| ND-Psy | |||||||
| Shitaka et al. 2011 | Adult mouse | Yes/no | No-in stereotax | 1-2 @24 h | ND | Yes - Cog | ND; cytoskeletal and axonal damage, gliosis |
| ND-Psy | |||||||
| Mouzon et al. 2012 | Adult mouse | Yes/no | No-in stereotax | 1 or 5 @ 48 h | ND | Yes - Cog | No; axonal injury |
| ND-Psy | |||||||
| Bennett et al. 2012 | Adult mouse | Yes/no | No-in stereotax | 1-2 @24 h | ND | ND-Cog | ND; DTI deficits; axonal injury |
| ND-Psy | |||||||
| Prins et al. 2013 | PND35 rat | Yes/no | No-head against wooden block | 1-2 @ 24 or 120 h | Yes | Yes-novel object deficits | ND; metabolic depression |
| Lateral fluid percussion and variants | |||||||
| Kanayama et al. 1996 | Adult rats | Yes/yes | No-in stereotax | 1 or 7 @ 24 h | ND | Neither | yes |
| DeRoss et al. 2002 | Adult rat | Yes/yes | No-in stereotax | 1, 2 or 3 @ ? | ND | Yes - Cog | ND |
| ND-Psy | |||||||
See Xiong et al. (2013) for representative review of animal models of TBI and their nomenclature.
Many recent variants of the models under discussion have now administered 2 or 3 injuries and refer to these as mild and repeated. However, these injuries are still much more severe than those seen in athletes who have received hundreds of subconcussive head impacts. For the sake of the present discussion, studies that used 3 or more head impacts were included as satisfying this criterion because they represent a minority. The ‘correct’ number of head impacts for use in animal models is not known (see text for discussion).
Shading in a cell indicates fulfillment of a criterion of an effective animal model of sports-related head injury.
ND, not determined; Tg, transgenic; Aβ, amyloid beta; PND, postnatal day; DTI, diffusion tensor imaging.
Animal models with the potential to serve as models of sports-related TBI in humans
A small number of newer animal models using three distinct methodological approaches have the potential to serve as models of sports-related head injury, based on their ability to satisfy the minimal criteria described above. These include a momentum exchange method, two weight drop methods, and a modified CCI method. The essential characteristics of these approaches will be described along with brief discussions of their advantages and limitations.
Momentum exchange
Viano et al. (2009) developed the momentum exchange method to simulate the high velocity impacts and rapid change in head velocities seen in reconstructed head impacts sustained by professional football players. The human head impacts being modeled resulted in concussions. The apparatus consists of a free-moving ballistic impactor directed at the lateral aspect of a rat's skull. A padded aluminum plate is affixed to the side of the head of the subject just prior to impact to serve as a protective ‘helmet’. Pneumatic pressure is used to drive the impactor at the desired velocity (7.4, 9.3, or 11.2 m/s, corresponding to conditions in professional players deemed as threshold, average or elite in human head impacts, respectively) and the mass of the impactor was varied (50 or 100 g) to achieve different impact severities. A key facet of this model is that the subject's head and body are free to move after impact. The lateral head impact also produced primarily translational motion as confirmed by high speed video. The authors scaled the model to deliver impacts that closely matched those known to cause concussion in professional football players in terms of impact velocity, head ΔV, translational acceleration, rotational velocity, rotational acceleration, and impact duration. A series of studies using this model was undertaken to characterize the biomechanical (Viano et al. 2009), histopathological (Hamberger et al. 2009; Viano et al. 2012), and behavioral outcomes (Bolouri et al. 2012) caused by head impacts. Having established excellent concurrence of the model with human head impacts, these investigators delivered single or a series of three impacts (6 h interval between impacts) and assessed brain injury at various times after the last one. In the initial study (Viano et al. 2009), brain injury was defined primarily in terms of vascular-related damage (e.g., subdural bleeds, focal contusions, and petechial hemorrhages on the cortical surface). These ballistic impacts did not cause skull fracture or musculoskeletal injury and animals appeared normal upon waking. Viano et al. (2009) observed a clear relationship between increases in impact velocity and force, and the frequency of injury. Repeated head impacts caused greater injuries than single impacts and these effects were magnified when the impactor mass was increased from 50 to 100 g. A subsequent study showed that single impacts with the 50 g impactor at impact velocities of 7.4 and 9.3 m/s caused minimal neuronal injury (as judged by accumulation of NF-200 protein accumulation) or astrocytosis (Hamberger et al. 2009; Viano et al. 2012). When rats sustained three impacts or when the 100 g impactor was used to deliver a single impact, injuries were more severe and resulted in widespread neuronal injury and astrocytosis. Evidence of edema was observed in the cortex, brainstem, and cerebellum (Hamberger et al. 2009). Bolouri et al. (2012) next determined that the momentum exchange method transiently but significantly increased intracranial pressure after three head impacts (3 min interval between impacts) using the 50 g impactor at a velocity of 11.2 m/s. Similar impact conditions resulted in impaired cognitive performance on the Morris water maze and also decreased spontaneous exploratory activity in the open field test, a potential sign of anxiety (Bolouri et al. 2012). Using the threshold impact conditions associated with a 50 g impactor propelled at a velocity of 9.3 m/s to achieve the target head velocity of 7.2 m/s, these investigators did not observe cognitive impairments or changes in intracranial pressure, indicating that these mildest conditions which can result in concussion in human football players do not cause injury in rats (Bolouri et al. 2012).
The advantages of this model that make it relevant for the study of human sports-related head impact are numerous and are summarized in Table 2. This model was scaled to actual human head impacts. The impact conditions of the momentum exchange method are very tightly controlled and are highly reproducible. Use of a protective padded helmet helps avoid skull fractures and musculoskeletal injuries. Upon impact to the lateral side of the skull, the head and body of the subject rat are free to move and do so in a translational manner. The investigators have used this method to deliver one or three head impacts and the interval between head impact when repeated has varied from 6 h to 3 min between impacts. This method has also been very extensively characterized in biomechanical terms. The limitations of this model are few in number. Scaling of head impacts from humans to rodents is always difficult and is a limitation of all animal models of human TBI, whether or not they are directed at the study of sports-related head impacts. The apparatus for applying head impacts to rats is complex and its sophisticated design is not easily replicated (nor is it commercially available), limiting the extent to which other investigators could construct and use the momentum exchange method. The number of head impacts delivered to any subject has been limited to three, so the extent to which greater numbers of impacts can be delivered is not yet known. Higher forces of impact that are still within the ranges sustained by concussed football players generally cause more severe injuries in animals (neuronal damage, astrocytosis) than are seen in athletes. It is also not known if the momentum exchange method causes CTE-like signs of increases in phosphorylated tau. Finally, this model was scaled to head impacts that cause concussion (Viano et al. 2009). It is becoming clear that at least football players sustain far more subconcussive head impacts than concussive ones, so it would be interesting to examine higher numbers of lower impact forces in the momentum exchange method to determine if repetitive head impacts will cause behavioral and pathological effects in rats like those seen in retired professional athletes. The compliance of the momentum exchange method with the desired criteria for an animal model of sports-related rmTBI is summarized in Table 2.
Table 2.
Animal models satisfying most desired criteria of a model of human sports-related rmTBI
| Animal Modela and reference | Subject | Direct hit on intact scalp and skull/craniotomy | Head free to move upon impact | No. impacts, time between impacts | Accommodates young subjects | Psychiatric (Psy) and/or cognitive (Cog) manifestations | CTE-like tauopathy; other injuries |
|---|---|---|---|---|---|---|---|
| Momentum exchange | |||||||
| Viano et al. 2009 | Adult rat | Yes/no | Yes | 1 or 3 @ 6 h | NDb | ND | ND; some SDH |
| Hamberger et al. 2009 | Adult rat | Yes/no | Yes | 1 or 3 @ 6 h | ND | ND | ND; mild DAI and gliosis |
| Bolouri et al. 2012 | Adult rat | Yes/no | Yes | 3 @ 3 min | ND | Yes-Cog yes-Psy |
|
| Weight drop methods | |||||||
| Kane et al. 2012 | Adult mice | Yes/no | Yes | 1, 5 or 10 @ 24 h | Yes | Yes-Cog yes-Psy |
Yes; mild gliosis |
| Meehan et al. 2012 | Adult mice | Yes/no | Yes | 1, 3, 5 or 10 @ 24 h | ND | Yes-Cog | ND |
| no-Psy | |||||||
| Mannix et al. 2013 | Adult mice | Yes/no | Yes | 5, 7, 10 varying time intervals | ND | Yes-Cog | No; mild gliosis |
| no-Psy | |||||||
| ‘hit and run’ method | |||||||
| Ren et al. 2013 | Adult mice | Yes/no | Yes | 1 | ND | Yes-Cog | No; some infarcts, edema, loss of cortical tissue |
| no-Psy |
Shading in a cell indicates fulfillment of a criterion of an effective animal model of sports-related head injury.
ND, not determined; SDH, subdural hematoma; DAI, diffusion tensor imaging.
Weight drop methods
Wayne state method
Our lab developed a method whereby a 95 g weight was dropped down a guide tube from a height of 1 m onto the head of a lightly anesthetized mouse suspended on a ‘stage’ of aluminum foil (Kane et al. 2012). The foil stage was slit to allow unrestricted movement of the mouse's head and body at impact. By stopping the descent of the weight at impact with a string, this method avoids re-hits of the head by the falling weight. Mice recover consciousness very quickly, show no respiratory distress and do not experience seizures or paralysis after single impacts. Animals exposed to five head impacts (1 per day for 5 days) showed delays in recovery of the righting reflex (recovery of consciousness) by comparison to controls and a transient decrement in balance and coordination was also seen. Repeated mild impacts also caused outcomes 30 days after the last impact that are similar to injuries seen in athletes. These include gliosis and a CTE-like increase in phosphorylated tau levels (Kane et al. 2012). We did not see evidence of microglial activation or disruption of the blood brain barrier, but a mild astrocytosis was documented (Kane et al. 2012).
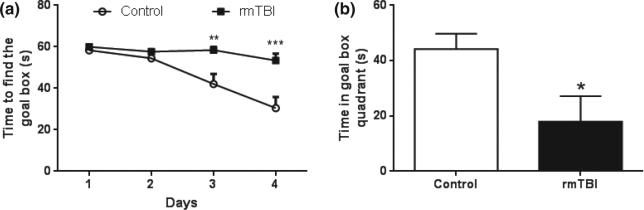
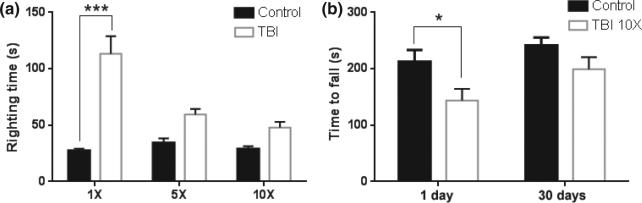
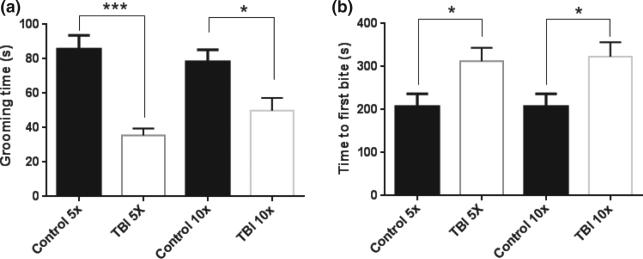
Since publication of our method, we have collected additional data supporting our contention that it can serve as an animal model of sports-related head impact. Adult C57BL/six male mice (7–8 weeks of age) were exposed to five head impacts using a 95 g weight dropped from 1 m and then tested 30 days after the last impact for cognitive performance using the Barnes maze (Barnes 1979). Results presented in Fig. 1 shows that mice exposed to rmTBI have a significant deficit in cognitive performance by comparison to the control group. Adolescent male mice (4 weeks of age) were exposed to 1, 5, or 10 head impacts (1 per day for 1, 5 or 10 days) using a weight of 30 g dropped from 1 m. All groups contained 12 mice and the survival rates for each group were 100% for the 1X group and 92% for the 5X and 10X groups (1 mouse per group died after the last impact). In agreement with our previous results using adult mice, adolescent mice showed significant increases in the time needed to regain the righting reflex, but this response decreased with successive impacts such that the impacted groups were not different from controls after the fifth head impact. These results are presented in Fig. 2a. We can only speculate that this response represents a form of tolerance to the isoflurane anesthetic and its interaction with repeated head impacts. Figure 2b shows that 10 head impacts caused a significant reduction in performance on the rotarod test, indicating problems with balance and coordination, but this effect recovered by the 30 day test point, as was seen in adult mice (Kane et al. 2012). Adolescent mice were also tested for psychiatric-like dysfunction 30 days after the last head impact and the results are presented in Fig. 3. The splash test was used to determine if mice develop depression-like behaviors after repeated head impacts. This test involves spraying a mist of 10% sucrose on the coats of mice to provoke self-grooming and the time spent grooming in a 5 min test session is recorded (Surget et al. 2008). A treatment-induced reduction in time spent grooming is indicative of depression-like behavior (i.e., self-neglect). It can be seen in Fig. 3a that adolescent mice subjected to 5 or 10 head impacts showed significant decreases in time spent grooming in the splash test by comparison with controls. Next, we tested mice for anxiety-like behavior using the novelty suppressed feeding test. In this test, mice are deprived of food for 18 h and then placed into a novel open field containing a centrally located pellet of food. The time required for the hungry test subject to take the first bite from the pellet is recorded in a 10 min test session. Treatment-induced increases in the time to eat the food pellet are indicative of anxiety-like behavior (Elsayed et al. 2012). Results in Fig. 3b indicate that adolescent mice exposed to either 5 or 10 head impacts show significant increases in the time required to eat in a novel environment, consistent with anxiety-like behavior. These results confirm a recent report by Mychasiuk et al. (2014) showing that a single impact to the head of juvenile rats using the Wayne State method resulted in the development of affective-like disorders and cognitive dysfunction. It will be interesting to see if multiple impacts increase the magnitude of these effects in young rats.
Fig. 1.
Repetitive mild traumatic brain injury reduces cognitive performance in adult mice. Mice were exposed to five head impacts over 3 days (1 on the first day and 2 on each of the subsequent days) using a 95 g weight dropped from a height of 1 m. Controls were treated in the same manner as experimental mice but were not exposed to head impacts. (a) Acquisition training. Fifteen days after the last head impact, mice were tested for acquisition over four training days (three trials per day) on the Barnes maze. Controls show a steady reduction in the amount of time required to find the goal box using external visual cues whereas mice exposed to five head impacts required significantly longer times. Data are presented as time required to find the goal box on each training and represent mean ± SEM (N = 6 for each group). Acquisition data were analyzed with a repeated measures one-way anova (F7,119 = 12.12; p < 0.0001) followed by Tukey's post-hoc test (b) Memory probe test. On day 5, the goal box was removed and the time spent by mice in the quadrant of the maze where the goal box was located during acquisition training was measured as a test of memory. Mice exposed to five head impacts were significantly impaired by comparison to controls. Data represent mean ± SEM (N = 6 for each group) and were analyzed with a student's t test [t(10) = 2.432; p = 0.0353]. *p < 0.05, **p < 0.001, ***p< 0.0001.
Fig. 2.
Repetitive mild head impacts alter recovery of consciousness and impair motor coordination and balance in adolescent mice. Mice (4 weeks of age) were exposed to 1, 5, or 10 head impacts (1 per day for 1, 5 or 10 days) using a 30 g weight dropped from a height of 1 m. Controls were treated in the same manner as experimental mice but were not exposed to head impacts. (a) Time required for mice to recover the righting reflex was recorded on each day that the respective groups received a head impact. Data are presented as mean ± SEM (N = 12–14 mice per group). The main effects of 1 (1X), 5 (5X), and 10 (10X) impacts (one-way anova, F5,103 = 9.902; p < 0.0001) were only significant for 1X versus its corresponding control (Tukey's test p < 0.0001). The 5X and 10X groups were not significantly different from their controls. (b) Adolescent mice exposed to 10 head impacts were tested for motor coordination and balance on the rotarod 1 day or 30 days after the last impact. Mice exposed to repeated head impacts had a significant reduction in rotarod performance at the 1 day time point [student's t test t(21) = 2.270; p = 0.0339] and recovered by 30 days. Results are mean ± SEM (N = 12–14 mice per group). *p < 0.05, ***p < 0.0001.
Fig. 3.
Effects of repeated mild head impacts on affective behavior in adolescent mice. Mice (4 weeks of age) were exposed to 5 or 10 head impacts (1 per day for 5 or 10 days) using a 30 g weight dropped from a height of 1 m. Controls were treated in the same manner as experimental mice but were not exposed to head impacts. Tests for the expression of affective-like behaviors were carried out 30 days after the last head impact. (a) Grooming time in the splash test of depression-like behavior. A 10% solution of sucrose was sprayed onto the backs of mice and the time spent self-grooming was measured. Mice exposed to 5 (Student's t test t(13) = 6.331; p < 0.0001) or 10 (Student's t test t(20) = 2.738; p = 0.0127) head impacts showed significantly less time grooming by comparison to controls, indicative of depression-like behavior. Data is presented as mean ± SEM (N = 6–12 mice for each group). ***p < 0.001, *p < 0.05. (b) Novelty suppressed feeding test of anxiety-like behavior. Mice were deprived of food for 18 h and then placed into a novel open-field environment containing a normal mouse food pellet. The time taken for mice to take the first bite from the pellet was recorded. Mice exposed to 5 [Student's t test t(16) = 2.442; p = 0.0266] or 10 (Student's t test t(18)= 2.408; p = 0.027) head impacts showed a significant increase in the amount of time to take the first bite by comparison with controls, indicative of anxiety-like behavior. Data represents mean ± SEM (N = 6–12 mice per group). *p < 0.01.
The key characteristics of the Wayne State model that set it apart from other closed-head, weight drop models, giving it numerous advantages for studies of sports-related rmTBI include the following: (i) it can be used to deliver repeated, mild injuries to the same subject; (ii) the body and head of the subject are not constrained (e.g., not resting on a cushion) and the mouse falls freely at impact; (iii) the dropped weight does not ‘drive’ the head into a cushion and thereby avoids the additional loading factors and midbrain injuries imparted by it; (iv) the falling weight is restricted in the length of its downward traverse (glancing blow, not crushing) and cannot strike the head of the subject multiple times (e.g., after mouse and weight recoil post-impact); (v) the impact causes a very rapid and translational acceleration of the head; (vi) only light anesthesia is required and incisions of the scalp or other surgical preparations (e.g., emplacement of protective skull helmet) are not needed; (vii) post-injury support and/or resuscitation are not required as the animals recover the righting reflex spontaneously; (viii) the procedure is simple, inexpensive, and rapid (can be carried out in ~ 60 s per mouse); (ix) it is suitable for high-throughput screening of therapeutic compounds, and (x) it appears to recapitulate the human condition of sports-related rmTBI. This method can be used with adolescent mice as subjects by using impacting weights with lower masses (e.g., 30 g vs. 95 g for adults). Adolescent mice readily survive multiple head impacts, showing slight increases in the time required to recover the righting reflex. Likewise, adolescent mice exposed to 5–10 head impacts display depression-and anxiety-like behaviors 30 days after treatment. These results are consistent with findings of psychiatric conditions, to include affective disorders, in amateur (Kontos et al. 2012) and former professional athletes (Guskiewicz et al. 2007a; Stern et al. 2013) and make our method highly relevant as a model of sports-related rmTBI.
Harvard method
A second weight drop approach very similar to our published method involved dropping a metal bolt (54 g) down a guide tube either 38 or 42 inches in length onto the head of a lightly anesthetized mouse. In this method, mice were placed on a laboratory kimwipe which also allows relatively unrestricted movement of the mouse head and body after impact (Meehan et al. 2012). These investigators subjected mice to 1, 3, 5, or 10 head impacts, each a day apart and other mice were exposed to 5 repeated head impacts spaced apart daily, weekly, or monthly. Mice were assessed for behavioral and neuropathological outcomes 1 day, 1 month, or 1 year after the last impact. Results from this approach are very similar to those obtained with our method (Kane et al. 2012) and indicated that mice did not experience skull fractures, intracranial hemorrhage, or edema. A large percentage of mice (22–26%) showed tonic-clonic seizures after head impact. Mice also did not exhibit cell loss, degeneration or death, nor did the investigators observe evidence of axonal injury or disruption of the blood brain barrier (Meehan et al. 2012). Interestingly, as the number of head impacts increased, greater decrements of cognition were seen using the Morris water maze. As the interval between head impacts was increased, the persistence of cognitive problems was reduced. Mice receiving five head impacts at the rate of 1 per day for 5 days exhibited poorer cognitive performance for up to 1 year after the last impact (Meehan et al. 2012). A subsequent study by Mannix et al. (2013) using this same approach (54 g weight dropped from a height of 28 inches) subjected mice to five daily impacts, seven impacts over 9 days, one impact per week for 5 weeks, one impact biweekly for 10 weeks, one impact per month for 5 months, or a single head impact. The use of a shorter drop-distance in this latest study (28 inches vs. 38 or 42 inches) avoided seizure development. As before, it was observed that mice impacted daily or weekly showed long-term cognitive deficits for up to 1 year after the last impact. Longer rest intervals between head impacts guarded against the development of cognitive deficits (Mannix et al. 2013). This form of repetitive TBI did not result in changes in white matter volume as assessed by magnetic resonance imaging, nor did it cause increases in brain levels of phosphorylated tau, amyloid β, neuronal death, axonal pathology, or microglial activation. Increases in the number of glial fibrillary acidic protein-positive astrocytes were seen in cortex, corpus callosum, and hippocampus (Mannix et al. 2013). This weight drop method has all of the advantages discussed above for our model and, additionally, the investigators established that increasing times between head impacts lessens the deficits seen in cognitive performance (Meehan et al. 2012; Mannix et al. 2013). The results from this model did not cause an increase in phosphorylated tau, a neuropathological outcome that has been associated with sports-related rmTBI in some retired athletes (McKee et al. 2009, 2013; Mez et al. 2013). This would be a limitation if phosphorylated tau is confirmed as a biomarker of repeated head impacts. It is also not known if adolescent mice can endure repetitive head impacts with the Harvard model without sustaining severe injuries.
The compliance of these two weight drop methods with the desired criteria for a model of sports-related rmTBI is summarized in Table 2. Unfortunately, neither weight drop method has been characterized from a biomechanical perspective as has been completed for the momentum exchange method. Studies in our lab to carry this out are well underway.
‘Hit and Run’ model of closed-skull TBI
Ren et al. (2013) modified the commercially available CCI apparatus by rotating the impacting rod to a horizontal position. The subject mice were suspended head-up by their incisors using a metal ring. The impactor, with a polished stainless steel tip, was aimed between the eye and the external ear canal. Impact velocities of 4.8 (termed mild injury) and 5.2 m/s (termed moderate injury) were used whereras impact depth (10 mm) and contact times (0.1 s) were held constant. Mice were exposed to a single impact and were assessed for outcomes 3 h, 24 h, 3, 7, 14, or 28 days after head impact. This method is much like the weight drop methods described above in that it can be completed very quickly in lightly anesthetized mice and skull fractures were not observed. These investigators carried out an extensive battery of tests and found, by and large, that mild injury impact conditions had few effects that were different from controls [e.g., recovery of consciousness, number of infarcts, edema, blood brain barrier status, intracranial pressure, open filed activity, novel object test, Barnes maze test of cognition, and brain volume changes (Ren et al. 2013)]. The mild injury did significantly increase the neuroscore and impaired rotarod performance, and it significantly elevated glial fibrillary acidic protein-positive astrocytes and axonal degeneration. Overall, the moderate injury was significantly elevated above the mild injury for all outcomes mentioned above with the exception of the rotarod, axonal degeneration, and aquaporin 4 dysregulation, where the moderate level of injury was the same as the mild level. This new adaptation of the standard CCI approach has potential as a model of sports-related head impact. However, the investigators have not established that the head impacts can be repeated, using only single impacts in their initial report. It remains to be seen if adolescent mice can endure multiple head impacts using this model without sustaining severe injuries. The difference in injury magnitude between the mild (4.8 m/s impact velocity) and the moderate injury conditions (5.2 m/s impact velocity) is quite small with the mild form causing little observable damage. On the other hand, the moderate level of injury is much more severe in outcome and raises some doubt about its usefulness as a model of sports-related rmTBI. In many cases, the mild and moderate injury outcomes were the same. It is not yet known if the ‘hit and run’ method causes CTE-like neuropathological changes after single impacts. The apparatus required for carrying out the ‘hit and run’ method is commercially available but is much more expensive to employ than the weight drop methods. The compliance of the ‘hit and run’ method with the desired criteria for a model of sports-related rmTBI is summarized in Table 2.
Conclusions and perspectives
The majority of animal models used presently for the study of TBI are able to recapitulate only 1–2 head impacts and satisfy few of the desired criteria of an animal model of sports-related head injury (see Table 1). Perhaps the most significant limitation of the models in Table 1 for serving as models of sports-related head injury is the restriction of head movement upon impact. Denny-Brown and Russell (1941) were perhaps the first investigators to note the importance of head movement upon impact in their early studies of concussion when they wrote ‘it was considered essential, in causing concussion, that the animal's head should be subjected to a sudden change of velocity. For this reason, we discarded the method, used by many workers, of striking downwards on the head supported by a table’ (Denny-Brown and Russell 1941, p. 100). More than 70 years after this prescient observation, the majority of animal models of rmTBI still impact the head which is fixed in place, unable to move in reaction to impact. Recent studies of head impacts in football players have documented the importance of high velocity impacts and rapid changes in head velocity (Pellman et al. 2003a,b; Viano and Pellman 2005; Guskiewicz et al. 2007b; Viano et al. 2007; Meaney and Smith 2011; Jordan 2013; Urban et al. 2013). The Marmarou weight drop method, which allows some movement of the struck head, actually increases the load forces on the head through the use of a cushion and which can worsen injury (Viano et al. 2009, 2012). Studies that have used impact forces similar to those imparted by the Marmarou method but which allowed free movement of the animal's head and body after impact have observed consistently that injuries are absent or much lower in severity (Nilsson et al. 1977; Viano et al. 2012).
It remains to be seen if the models of rmTBI in Table 1 can use adolescent animals as subjects. Adult subjects in these models show rather severe injuries after 1–2 head impacts making it unlikely that younger animals could be used. Most investigators use adult animals and future studies should consider the fact that the vast majority of participants in sports that involve head impact begin participation around the time of early adolescence. Large population-based epidemiological studies have reported that the incidence of sports-related head injury is greatest in adolescents (Selassie et al. 2013). Young athletes are not just small adults in terms of their response to brain injury (Giza et al. 2007). This is a particularly vulnerable period in the development of the human brain and it may well be the case that the adolescent brain is much more sensitive to long-term injury than the adult brain (Babikian et al. 2010; Grady 2010; Prins and Giza 2012). The methods summarized in Table 1 that tested animals for learning and memory show without exception that rmTBI causes cognitive dysfunction. Many of these methods may well cause psychiatric manifestations, but few have tested subjects for these outcomes. Finally, as many of the models in Table 1 are referred to as ‘mild’ in terms of injuries caused, it is clear that single and especially double head impacts result in quite severe injuries to include neuronal, axonal, and cytoskeletal damage. Methods that use scalp incisions and craniotomies are also unrealistic as models of sports-related head injury for obvious reasons. With all things considered, the models summarized in Table 1 have far more limitations than advantages for serving as models of sports-related head injuries.
The three methods summarized in Table 2 suggest that these models hold great promise for advancing the study of sports-related head injury. All allow free movement of the head, most can deliver numerous head impacts over varying times with remarkably few discernible injuries and at least Wayne State weight drop method can use adolescent mice as subjects (Kane et al. 2012; Mychasiuk et al. 2014). The injuries that are seen, to include mild gliosis and increases in phosphorylated tau, are also seen in postmortem brains of some former professional football players. It is clear that pre-clinical studies of sports-related head injury are in their infancy and lag far behind those directed at acute, severe brain injury. A cursory search of the PubMed database reveals that ~ 4600 papers have been published on animal models of TBI. Within this total, ~ 1100 papers have been published using the lateral fluid percussion method, 780 using the CCI approach, and 300 on various weight drop methods. Only ~ 21 papers have been published on rmTBI using animals to include 13 papers that used 2 blows to the head and 8 papers that used greater than or equal to 3 head impacts. Removal of review articles from this database reveals that less than 0.5% of the total has been directed at the study of rmTBI. The number of individuals exposed to sports-related rmTBI at any given time is very large by comparison to the number injured moderately or severely as modeled by existing animal methods. Of all sports-related head injuries, more than 90% are classified as mild (Selassie et al. 2013). Considering the fact that increasing numbers of individuals are being exposed to this insidious form of head injury [e.g., the number of injuries has doubled over the past 20 years, (Selassie et al. 2013)], greater emphasis on this problem is warranted.
Hopefully, the methods summarized in Table 2 will be used to expand the study of some of the most pressing issues relating to sports-related head injuries and these include:
A determination of the relationship between the number and frequency of head impacts, the duration of time over which impacts are delivered, post-injury survival time and the chronic development of psychiatric and neuro-pathological outcomes. This point is particularly challenging. It has been possible to ‘scale’ human head impacts to animals, in terms of biomechanics, but it will be difficult to determine how many total head impacts in an animal model best mimics the very large number of head impacts being sustained by human athletes. As many investigators refer to head impacts in animals as ‘concussive’, this term could be misleading. The definition of a concussion in humans is extremely difficult and use of terms like ‘concussive impact’ or ‘concussion’ should be avoided because all animals are anesthetized when subjected to head impact. Thus, as the ‘correct’ number of head impacts in an animal model that accurately simulates what is being seen in human athletes cannot be probably determined, it is clear that 1–2 are far too few in number. The methods outlined in Table 2 can, at a minimum, determine the threshold number of head impacts that lead eventually to the development of psychiatric and neuropathological outcomes. Future pre-clinical studies should follow the lead of studies of animal (Viano et al. 2009) and human athlete head collisions (Viano and Pellman 2005; Broglio et al. 2009, 2012; Beckwith et al. 2013a,b) by measuring head impacts in more biomechanical detail in an attempt to link the summed total linear acceleration (measured as g forces) and rotational/angular acceleration (measured as rad/s2) for all impacts delivered to the emergence of behavioral and/or CTE-like neuropathological changes.
A determination of whether males and females differ in terms of injury and long-term outcomes.
A search for peripheral and central biomarkers of truly repetitive, mild head injury.
An exploration for new neuro-protective materials and conditions that could prevent or minimize long-term injury.
Identification of factors that put some individuals at heightened risk for the development of more severe injury.
High throughput tests of potential therapeutic agents that can prevent or minimize the long-term development of psychiatric and neuropathological outcomes. This approach has high face validity because, unlike accidental head injuries, sports-related head impacts are predictable and medications could be given prior to participation.
No animal model of a human disease is perfect. With regard to the study of acute or repeated moderate to severe TBI, a vast number of animal models exist and these have been used to develop therapies for brain injuries in animal subjects. However, to date, none of the treatments shown to be neuroprotective in animal models has translated to the successful treatment of human TBI (Maas et al. 2007; Marklund and Hillered 2011; Xiong et al. 2013). Why should a model of sports-related head injury be any different? First, most existing animal models of TBI have studied acute, severe injury to the brain and the chances of successful treatment of these injuries in humans remain low. Sports-related head injury is very mild and could be more amenable to treatment and prevention. Second, and as mentioned above, attempts to link repeated head impacts to the development of cognitive, psychological, psychiatric, and neuropathological outcomes in human athletes have been difficult and remain quite contentious. Co-existing factors such as drug and alcohol abuse or steroid intake could cause health problems of their own. However, if an animal model could establish that repeated mild head impacts in the absence of drug or steroid intake leads to the development of the same health issues being seen in retired athletes, these factors could be viewed in a different light. Perhaps some of these factors may lessen the long-term outcomes of repeated blows to the head and some will probably worsen them. Without effective animal models to explore the mechanistic underpinnings of sports-related head impacts, a better understanding of this significant health problem will progress at a much slower rate.
Acknowledgements
This work was supported by the Department of Veterans Affairs.
Abbreviations used
- CCI
controlled cortical impact
- CTE
chronic traumatic encephalopathy
- GFAP
glial fibrillary acidic protein
- LFP
lateral fluid percussion
- rmTBI
repetitive mild traumatic brain injury
- TBI
traumatic brain injury
Footnotes
conflict of interest disclosure
The authors have no conflict of interest to declare.
References
- Allen GV, Gerami D, Esser MJ. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience. 2000;99:93–105. doi: 10.1016/s0306-4522(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Prins ML, Cai Y, Barkhoudarian G, Hartonian I, Hovda DA, Giza CC. Molecular and physiological responses to juvenile traumatic brain injury: focus on growth and metabolism. Dev. Neurosci. 2010;32:431–441. doi: 10.1159/000320667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126:e374–e381. doi: 10.1542/peds.2009-0925. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, Nowinski CJ, Cantu RC, McKee AC, Stern RA. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Beckwith JG, Greenwald RM, Chu JJ, et al. Head impact exposure sustained by football players on days of diagnosed concussion. Med. Sci. Sports Exerc. 2013a;45:737–746. doi: 10.1249/MSS.0b013e3182792ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith JG, Greenwald RM, Chu JJ, et al. Timing of concussion diagnosis is related to head impact exposure prior to injury. Med. Sci. Sports Exerc. 2013b;45:747–754. doi: 10.1249/MSS.0b013e3182793067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RE, Mac Donald CL, Brody DL. Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci. Lett. 2012;513:160–165. doi: 10.1016/j.neulet.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Bolouri H, Saljo A, Viano DC, Hamberger A. Animal model for sport-related concussion; ICP and cognitive function. Acta Neurol. Scand. 2012;124:241–247. doi: 10.1111/j.1600-0404.2011.01614.x. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Sosnoff JJ, Shin S, He X, Alcaraz C, Zimmerman J. Head impacts during high school football: a biomechanical assessment. J. Athl. Train. 2009;44:342–349. doi: 10.4085/1062-6050-44.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Martini D, Sosnoff JJ, Kutcher JS, Randolph C. Cumulative head impact burden in high school football. J. Neurotrauma. 2011a;28:2069–2078. doi: 10.1089/neu.2011.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Surma T, Kutcher JS. Post-concussion cognitive declines and symptomatology are not related to concussion biomechanics in high school football players. J. Neurotrauma. 2011b;28:2061–2068. doi: 10.1089/neu.2011.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Kutcher JS. Field-based measures of head impacts in high school football athletes. Curr. Opin. Pediatr. 2012;24:702–708. doi: 10.1097/MOP.0b013e3283595616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Choe MC, Babikian T, DiFiori J, Hovda DA, Giza CC. A pediatric perspective on concussion pathophysiology. Curr. Opin. Pediatr. 2012;24:689–695. doi: 10.1097/MOP.0b013e32835a1a44. [DOI] [PubMed] [Google Scholar]
- Cobb BR, Urban JE, Davenport EM, Rowson S, Duma SM, Maldjian JA, Whitlow CT, Powers AK, Stitzel JD. Head impact exposure in youth football: elementary school ages 9–12 years and the effect of practice structure. Ann. Biomed. Eng. 2013;41:2463–2473. doi: 10.1007/s10439-013-0867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Pratico D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J. Neurochem. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj. Prev. 2006;12:212–218. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE, Wozniak DF, Bayly PV, Olney JW, Lewis LM. Multiple episodes of mild traumatic brain injury result in impaired cognitive performance in mice. Acad. Emerg. Med. 2004;11:809–819. doi: 10.1111/j.1553-2712.2004.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Fiore R, Beckwith JG, Chu JJ, Brolinson PG, Duma S, McAllister TW, Duhaime AC, Greenwald RM. Frequency and location of head impact exposures in individual collegiate football players. J. Athl. Train. 2010;45:549–559. doi: 10.4085/1062-6050-45.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusimano MD, Cho N, Amin K, Shirazi M, McFaull SR, Do MT, Wong MC, Russell K. Mechanisms of team-sport-related brain injuries in children 5 to 19 years old: opportunities for prevention. PLoS ONE. 2013;8:e58868. doi: 10.1371/journal.pone.0058868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin. Sports Med. 2011;30:1–17. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Henry LC, Gosselin N. Long-term functional alterations in sports concussion. Neurosurg. Focus. 2012;33:E8. doi: 10.3171/2012.9.FOCUS12278. [DOI] [PubMed] [Google Scholar]
- DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A, Bullock R, Hamm RJ. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J. Neurotrauma. 2002;19:427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D, Russell WR. Experimental brain concussion. Brain. 1941;64:93–164. [Google Scholar]
- DeRoss AL, Adams JE, Vane DW, Russell SJ, Terella AM, Wald SL. Multiple head injuries in rats: effects on behavior. J. Trauma. 2002;52:708–714. doi: 10.1097/00005373-200204000-00017. [DOI] [PubMed] [Google Scholar]
- Dewitt DS, Perez-Polo R, Hulsebosch CE, Dash PK, Robertson CS. Challenges in the development of rodent models of mild traumatic brain injury. J. Neurotrauma. 2013;30:688–701. doi: 10.1089/neu.2012.2349. [DOI] [PubMed] [Google Scholar]
- Didehbani N, Munro Cullum C, Mansinghani S, Conover H, Hart J., Jr Depressive symptoms and concussions in aging retired NFL players. Arch. Clin. Neuropsychol. 2013;28:418–424. doi: 10.1093/arclin/act028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol. Psychiatry. 2012;72:258–265. doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: Morphological characterization. J. Neurosurg. 1994;80:301–313. doi: 10.3171/jns.1994.80.2.0301. [DOI] [PubMed] [Google Scholar]
- Forbes JA, Awad AJ, Zuckerman S, Carr K, Cheng JS. Association between biomechanical parameters and concussion in helmeted collisions in American football: a review of the literature. Neurosurg. Focus. 2012;33:E10. doi: 10.3171/2012.9.FOCUS12288. [DOI] [PubMed] [Google Scholar]
- Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, Schofield P, Levi C, Jones DK. A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma. 2012;29:2521–2538. doi: 10.1089/neu.2012.2628. [DOI] [PubMed] [Google Scholar]
- Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 2011;30:179–188. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD. Concussions among United States high school and collegiate athletes. J. Athl. Train. 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Mink RB, Madikians A. Pediatric traumatic brain injury: not just little adults. Curr. Opin. Crit. Care. 2007;13:143–152. doi: 10.1097/MCC.0b013e32808255dc. [DOI] [PubMed] [Google Scholar]
- Grady MF. Concussion in the adolescent athlete. Curr. Prob. Pediatr. Adoles. Health Care. 2010;40:154–169. doi: 10.1016/j.cppeds.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Mihalik JP. Biomechanics of sport concussion: quest for the elusive injury threshold. Exerc. Sport Sci. Rev. 2011;39:4–11. doi: 10.1097/JES.0b013e318201f53e. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Weaver NL, Padua DA, Garrett WE., Jr Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 2000;28:643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 2007a;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Mihalik JP, Shankar V, Marshall SW, Crowell DH, Oliaro SM, Ciocca MF, Hooker DN. Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery. 2007b;61:1244–1252. doi: 10.1227/01.neu.0000306103.68635.1a. [DOI] [PubMed] [Google Scholar]
- Halstead ME, Walter KD. Sport-related concussion in children and adolescents. Pediatrics. 2010;126:597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- Hamberger A, Viano DC, Saljo A, Bolouri H. Concussion in professional football: morphology of brain injuries in the NFL concussion model–part 16. Neurosurgery. 2009;64:1174–1182. doi: 10.1227/01.NEU.0000316855.40986.2A. [DOI] [PubMed] [Google Scholar]
- Hart J, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, Conover H, Mansinghani S, Lu H, Cullum CM. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70:326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J. Athl. Train. 2007;42:311–319. [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: a preliminary report. J. Neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Chronic traumatic encephalopathy and risk of suicide in former athletes. Br. J. Sports Med. 2014;48:162–165. doi: 10.1136/bjsports-2013-092935. [DOI] [PubMed] [Google Scholar]
- Jadischke R, Viano DC, Dau N, King AI, McCarthy J. On the accuracy of the Head Impact Telemetry (HIT) System used in football helmets. J. Biomech. 2013;46:2310–2315. doi: 10.1016/j.jbiomech.2013.05.030. [DOI] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 2013;9:222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Kallakuri S, Cavanaugh JM, Ozaktay AC, Takebayashi T. The effect of varying impact energy on diffuse axonal injury in the rat brain: a preliminary study. Exp. Brain Res. 2003;148:419–424. doi: 10.1007/s00221-002-1307-2. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Takeda M, Niigawa H, Ikura Y, Tamii H, Taniguchi N, Kudo T, Miyamae Y, Morihara T, Nishimura T. The effects of repetitive mild brain injury on cytoskeletal protein and behavior. Method Find. Exp. Clin. Pharmacol. 1996;18:105–115. [PubMed] [Google Scholar]
- Kane MJ, Angoa-Perez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM. A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Meth. 2012;203:41–49. doi: 10.1016/j.jneumeth.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantzoulis S, Randolph C. Modern chronic traumatic encephalopathy in retired athletes: What is the evidence? Neuropsychol. Rev. 2013;23:350–360. doi: 10.1007/s11065-013-9243-4. [DOI] [PubMed] [Google Scholar]
- Kerr ZY, Marshall SW, Guskiewicz KM. Reliability of concussion history in former professional football players. Med. Sci. Sports Exerc. 2012;44:377–382. doi: 10.1249/MSS.0b013e31823240f2. [DOI] [PubMed] [Google Scholar]
- Kilbourne M, Kuehn R, Tosun C, Caridi J, Keledjian K, Bochicchio G, Scalea T, Gerzanich V, Simard JM. Novel model of frontal impact closed head injury in the rat. J. Neurotrauma. 2009;26:2233–2243. doi: 10.1089/neu.2009.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpara H, Iwamoto M. Mild traumatic brain injury predictors based on angular accelerations during impacts. Ann. Biomed. Eng. 2012;40:114–126. doi: 10.1007/s10439-011-0414-2. [DOI] [PubMed] [Google Scholar]
- Kontos AP, Covassin T, Elbin RJ, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch. Phys. Med. Rehabil. 2012;93:1751–1756. doi: 10.1016/j.apmr.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Simon CM, Prado GR, Cullen DK. CNS injury biomechanics and experimental models. Prog. Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- Laurer HL, Bareyre FM, Lee VM, et al. Mild head injury increasing the brain's vulnerability to a second concussive impact. J. Neurosurg. 2001;95:859–870. doi: 10.3171/jns.2001.95.5.0859. [DOI] [PubMed] [Google Scholar]
- Lighthall JW, Dixon CE, Anderson TE. Experimental models of brain injury. J. Neurotrauma. 1989;6:83–97. doi: 10.1089/neu.1989.6.83. [DOI] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Fujimoto S, et al. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56:364–374. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J. Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Mannix R, Meehan WP, Mandeville J, et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 2013;74:65–75. doi: 10.1002/ana.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am. J. Sports Med. 2012;40:747–755. doi: 10.1177/0363546511435626. [DOI] [PubMed] [Google Scholar]
- Marchi N, Bazarian JJ, Puvenna V, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS ONE. 2013;8:e56805. doi: 10.1371/journal.pone.0056805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J. Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin. J. Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Echemendia RJ, Iverson GL, Dvorak J, Kutcher JS. What is the lowest threshold to make a diagnosis of concussion? Br. J. Sports Med. 2013a;47:268–271. doi: 10.1136/bjsports-2013-092247. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: behavioural, pathological and clinical outcomes? Br. J. Sports Med. 2013b;47:327–330. doi: 10.1136/bjsports-2013-092248. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney DF, Smith DH. Biomechanics of concussion. Clin. Sports Med. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan WP, 3rd, Zhang J, Mannix R, Whalen MJ. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery. 2012;71:885–891. doi: 10.1227/NEU.0b013e318265a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr. Neurol. Neurosci. Rep. 2013;13:407. doi: 10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Mouzon B, Chaytow H, Crynen G, Bachmeier C, Stewart J, Mullan M, Stewart W, Crawford F. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma. 2012;29:2761–2773. doi: 10.1089/neu.2012.2498. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Farran A, Esser MJ. Assessment of an experimental rordent model of pediatric mild traumatic brain injury. J. Neurotrauma. 2014 doi: 10.1089/neu.2013.3132. doi:10.1089/neu.2013.3132. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, Martin G, Donkin J, et al. The liver X receptor agonist GW3965 improves recovery from mild repetitive traumatic brain injury in mice partly through apolipoprotein E. PLoS ONE. 2013;8:e53529. doi: 10.1371/journal.pone.0053529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B, Ponten U, Voigt G. Experimental head injury in the rat. Part 1: mechanics, pathophysiology, and morphology in an impact acceleration trauma model. J. Neurosurg. 1977;47:241–251. doi: 10.3171/jns.1977.47.2.0241. [DOI] [PubMed] [Google Scholar]
- O'Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: a critical evaluation. Pharmacol. Ther. 2011;130:106–113. doi: 10.1016/j.pharmthera.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59:1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: case report and emerging medicolegal practice questions. J. Forens. Nurs. 2010;6:40–46. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain. 1974;97:633–654. doi: 10.1093/brain/97.1.633. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, Grubb RL, Jr, Naumann RA. Coup and contre-coup injury: observations on the mechanics of visible brain injuries in the rhesus monkey. J. Neurosurg. 1971;35:503–516. doi: 10.3171/jns.1971.35.5.0503. [DOI] [PubMed] [Google Scholar]
- Park HK, Fernandez II, Dujovny M, Diaz FG. Experimental animal models of traumatic brain injury: medical and biomechanical mechanism. Crit. Rev. Neurosurg. 1999;9:44–52. doi: 10.1007/s003290050108. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Tucker AM, Casson IR. Concussion in professional football: location and direction of helmet impacts-Part 2. Neurosurgery. 2003a;53:1328–1340. doi: 10.1227/01.neu.0000093499.20604.21. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003b;53:799–812. doi: 10.1093/neurosurgery/53.3.799. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Casson IR, Tucker AM, Waeckerle JF, Powell JW, Feuer H. Concussion in professional football: repeat injuries–part 4. Neurosurgery. 2004;55:860–873. doi: 10.1227/01.neu.0000137657.00146.7d. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football: players returning to the same game–part 7. Neurosurgery. 2005;56:79–90. [PubMed] [Google Scholar]
- Pellman EJ, Lovell MR, Viano DC, Casson IR. Concussion in professional football: recovery of NFL and high school athletes assessed by computerized neuropsychological testing–Part 12. Neurosurgery. 2006;58:263–274. doi: 10.1227/01.NEU.0000200272.56192.62. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military-and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J. Clin. Psychiat. 2013;74:180–188. doi: 10.4088/JCP.12011co1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958–963. doi: 10.1001/jama.282.10.958. [DOI] [PubMed] [Google Scholar]
- Prins ML, Giza CC. Repeat traumatic brain injury in the developing brain. Int. J. Dev. Neurosci. 2012;30:185–190. doi: 10.1016/j.ijdevneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 2010;32:510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Alexander D, Giza CC, Hovda DA. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma. 2013;30:30–38. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Karantzoulis S, Guskiewicz K. Prevalence and characterization of mild cognitive impairment in retired national football league players. J. Int. Neuropsychol. Soc. 2013;19:873–880. doi: 10.1017/S1355617713000805. [DOI] [PubMed] [Google Scholar]
- Rao V, Bertrand M, Rosenberg P, Makley M, Schretlen DJ, Brandt J, Mielke MM. Predictors of new-onset depression after mild traumatic brain injury. J. Neuropsychiat. Clin. Neurosci. 2010;22:100–104. doi: 10.1176/appi.neuropsych.22.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, Giese RN, Wang B, Shi X, Nedergaard M. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013;33:834–845. doi: 10.1038/jcbfm.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Selassie AW, Wilson DA, Pickelsimer EE, Voronca DC, Williams NR, Edwards JC. Incidence of sport-related traumatic brain injury and risk factors of severity: a population-based epidemiologic study. Ann. Epidemiol. 2013;23:750–756. doi: 10.1016/j.annepidem.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, Brody DL. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci. Biobehav. Rev. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Gay M, Johnson B, Zhang K. Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav. 2012;6:224–243. doi: 10.1007/s11682-012-9167-2. [DOI] [PubMed] [Google Scholar]
- Stahel PF, Shohami E, Younis FM, et al. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. Phys. Med. Rehab. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE, Feuer H, Leverenz LJ. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma. 2014;31:327–338. doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry DP, Faraco CC, Smith D, Diddams MJ, Puente AN, Miller LS. Lack of long-term fMRI differences after multiple sports-related concussions. Brain Inj. 2012;26:1684–1696. doi: 10.3109/02699052.2012.722259. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Toledo E, Lebel A, Becerra L, Minster A, Linnman C, Maleki N, Dodick DW, Borsook D. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci. Biobehav. Rev. 2012;36:1510–1531. doi: 10.1016/j.neubiorev.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JE, Davenport EM, Golman AJ, Maldjian JA, Whitlow CT, Powers AK, Stitzel JD. Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann. Biomed. Eng. 2013;41:2474–2487. doi: 10.1007/s10439-013-0861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viano DC, Pellman EJ. Concussion in professional football: biomechanics of the striking player–part 8. Neurosurgery. 2005;56:266–280. doi: 10.1227/01.neu.0000150035.54230.3c. [DOI] [PubMed] [Google Scholar]
- Viano DC, Casson IR, Pellman EJ, Bir CA, Zhang L, Sherman DC, Boitano MA. Concussion in professional football: comparison with boxing head impacts–part 10. Neurosurgery. 2005;57:1154–1172. doi: 10.1227/01.neu.0000187541.87937.d9. [DOI] [PubMed] [Google Scholar]
- Viano DC, Casson IR, Pellman EJ. Concussion in professional football: biomechanics of the struck player–part 14. Neurosurgery. 2007;61:313–327. doi: 10.1227/01.NEU.0000279969.02685.D0. [DOI] [PubMed] [Google Scholar]
- Viano DC, Hamberger A, Bolouri H, Saljo A. Concussion in professional football: animal model of brain injury–part 15. Neurosurgery. 2009;64:1162–1173. doi: 10.1227/01.NEU.0000345863.99099.C7. [DOI] [PubMed] [Google Scholar]
- Viano DC, Hamberger A, Bolouri H, Saljo A. Evaluation of three animal models for concussion and serious brain injury. Ann. Biomed. Eng. 2012;40:213–226. doi: 10.1007/s10439-011-0386-2. [DOI] [PubMed] [Google Scholar]
- Weber JT. Experimental models of repetitive brain injuries. Prog. Brain Res. 2007;161:253–261. doi: 10.1016/S0079-6123(06)61018-2. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature Rev. Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Uryu K, Higuchi M, Longhi L, Hoover R, Fujimoto S, McIntosh T, Lee VM, Trojanowski JQ. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J. Neurotrauma. 2005;22:1134–1141. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]