Abstract
Background
Recent data suggest trait-like neurocognitive impairments in bipolar disorder (BPD), with deficits about 1 s.D. below average, less severe than deficits noted in schizophrenia. The frequency of significant impairment in BPD is approximately 60%, with 40% of patients characterized as cognitively spared. This contrasts with a more homogeneous presentation in schizophrenia. It is not understood why some BPD patients develop deficits while others do not.
Method
A total of 136 patients with BPD completed the MATRICS Consensus Cognitive Battery and data were entered into hierarchical cluster analyses to: (1) determine the optimal number of clusters (subgroups) that fit the sample; and (2) assign subjects to a specific cluster based on individual profiles. We then compared subgroups on several clinical factors and real-world community functioning.
Results
Three distinct neurocognitive subgroups were found: (1) an intact group with performance comparable with healthy controls on all domains but with superior social cognition; (2) a selective impairment group with moderate deficits on processing speed, attention, verbal learning and social cognition and normal functioning in other domains; and (3) a global impairment group with severe deficits across all cognitive domains comparable with deficits in schizophrenia.
Conclusions
These results suggest the presence of multiple cognitive subgroups in BPD with unique profiles and begin to address the relationships between these subgroups, several clinical factors and functional outcome. Next steps will include using these data to help guide future efforts to target these disabling symptoms with treatment.
Keywords: Bipolar disorder, cluster analysis, cognitive heterogeneity, functional outcome, neurocognition, schizophrenia
Introduction
Neurocognitive impairment has long been recognized as a core feature of schizophrenia (Green, 1996) and is the focus of a multitude of studies. In contrast, the importance of cognitive problems in bipolar disorder (BPD) has only very recently been recognized, with the initial publications emerging in the late 1990s.
While there are considerable effects of mood state on cognition, with acutely manic or depressed patients demonstrating profound, global deficits (Daban et al. 2006), individuals with BPD also demonstrate persistent, trait-like, deficits during remission. Impairment is most notable in attention, verbal learning and executive function (Bora et al. 2009), with performance falling 0.5–1 s.D. below average. Moreover, as has been repeatedly shown in schizophrenia (Green, 1996; Ventura et al. 2009; Shamsi et al. 2011), these cognitive deficits contribute significantly to functional disability in BPD (Martinez-Aran et al. 2007; Bowie et al. 2010; Burdick et al. 2010; Harvey et al. 2010).
Although BPD has been characterized as an episodic illness with resultant functional recovery (Murray & Lopez, 1997), data emerging over the past several years have documented that neither complete symptomatic nor functional recovery is the norm (Tohen et al. 2003). Early data suggest that, as in schizophrenia (Dion et al. 1988; Abood et al. 2002; Kupfer et al. 2002), BPD patients’ social, occupational and residential functioning is directly influenced by persistent symptomatology and cognitive dysfunction (Martinez-Aran et al. 2007; Bowie et al. 2010; Burdick et al. 2010; Depp et al. 2012). These data strongly support the importance of cognitive problems in relation to quality of life in BPD and highlight the need for treatment and prevention efforts targeting cognition (Burdick et al. 2007). However, relatively little is known about the structure and causes of these deficits.
It is accepted that schizophrenia is characterized by some degree of cognitive heterogeneity, as demonstrated by the co-existence of a minority of cognitively unimpaired subgroup of subjects alongside the severe impairment characterizing the majority of schizophrenia subjects (Kremen et al. 2000; Reichenberg et al. 2009). Previous studies have reported variable performance on both specific cognitive tasks (Heinrichs & Awad, 1993; Goldstein et al. 1996) and global measures (Seaton et al. 1999; Hill et al. 2002). Generally, four subgroups have been described with two extreme subgroups, one with almost normal and one with profoundly impaired cognitive performance, and two intermediate subgroups, each with a moderate level of dysfunction across the cognitive domains (Goldstein et al. 1998; Seaton et al. 1999; Hill et al. 2002). While these studies showed that cognitive heterogeneity is present in schizophrenia, relatively little is known about heterogeneity of cognitive functioning in BPD subjects.
When analysed at the group level, studies have confirmed that neurocognitive deficits are present in euthymic BPD patients but that they are significantly less severe than those reported in schizophrenia (Daban et al. 2006; Burdick et al. 2011). These data suggest the existence of a gradation of severity in cognitive functioning between schizophrenia and BPD along a continuum of severity, which implies that group differences in cognitive performance are quantitative rather than qualitative. The same continuum of severity may be present within each disorder; however, qualitatively distinct subgroups might also exist. Early data suggest that approximately 30–50% of BPD patients present as ‘neuropsychologically normal’ (performing similar to healthy controls) during periods of euthymia (Heinrichs & Awad, 1993; Goldstein et al. 1996, 1998; Seaton et al. 1999; Hill et al. 2002; Martino et al. 2008; Bora et al. 2009; Reichenberg et al. 2009; Burdick et al. 2011).
A study by Altshuler et al. (2004) highlights this issue where schizophrenia patients (n=20) were significantly impaired on nearly every cognitive task administered, while euthymic BPD patients (n=40) had significant deficits on only two tasks: the Wisconsin Card Sorting Task (WCST) and the California Verbal Learning Test. The BPD subjects, as a group, performed intermediate between the schizophrenia and healthy controls; however, BPD patients’ performance on the WCST showed a bimodal distribution, suggestive of two discrete subgroups – one with relatively normal and one with significantly impaired executive functioning (Altshuler et al. 2004). This is a critical issue in understanding the etiology of cognitive impairment in BPD, yet very little is currently known about why some patients with BPD develop significant cognitive deficits while others remain cognitively intact.
Several clinical factors (i.e. bipolar subtype, history of psychosis, number of prior episodes, age at onset and duration of illness) appear to increase the likelihood of significant cognitive impairment (Martinez-Aran et al. 2007; Bora et al. 2009). Some studies indicate that BPD I patients may be more impaired than BPD II patients (Simonsen et al. 2008), yet others argue that they are similarly impaired (Dittman et al. 2008; Rosa et al. 2010). This inconsistency may be due, in part, to psychosis in a subgroup of about 50% of BPD I patients v. much less frequent psychosis in BPD II (APA, 2000). Indeed, meta-analytic data suggest that BPD patients with psychosis perform worse than BPD patients who never experience psychosis, even during affective remission (Martinez-Aran et al. 2008; Bora et al. 2009). In addition the course of the illness varies considerably among BPD patients, particularly with regard to chronicity, and data suggest that more frequent severe episodes are associated with higher levels of cognitive dysfunction (Bora et al. 2009).
In the current study, we hypothesized that there would be evidence of discrete cognitive subgroups in BPD based on neurocognitive profiles. We were specifically interested in addressing this empirically to determine: (1) whether specific subgroups exist; (2) the optimal number of subgroups that explain the heterogeneity; (3) the qualitative neurocognitive profiles associated with these subgroups; and (4) the clinical and functional correlates associated with each subgroup.
Method
Participants
Patients
A total of 136 BPD out-patients were recruited at two sites: 113 from the Zucker Hillside Hospital (ZHH) – North Shore Long Island Jewish Health System; and 23 patients from the Icahn School of Medicine at Mount Sinai. Inclusion criteria were: (1) diagnosis of BPD I or BPD II; (2) age 18–65 years; (3) current affective stability [<12 on the Hamilton Rating Scale for Depression (HAMD; Hamilton, 1960); and <8 on the Clinician-Administered Rating Scale for Mania (CARS-M; Altman et al. 1994)]. Exclusion criteria were: (1) history of central nervous system (CNS) trauma, neurological disorder, or attention deficit hyperactivity disorder; (2) diagnosis of recent substance abuse/dependence (past 3 months); and (3) electroconvulsive therapy (ECT) in the past 12 months.
A total of 105 patients were diagnosed with BPD I [n=60 (57.1%) with psychotic features] and 31 with BPD II [n=9 (29%) with psychotic features] based upon the Structured Clinical Interview for DSM-IV (SCID) and confirmed by a consensus panel. The mean age at the time of the assessment was 40.8 (S.D.=10.6) years, 50.0% (n=68) were female, and 49.3% (n=67) were Caucasian. The mean pre-morbid intelligence quotient (IQ) (Wide Range Achievement Test; WRAT-3; Wilkinson, 1993) was=97.6 (S.D.=10.8). The mean HAMD score (current depression) was 11.1 (S.D.=8.5) and the mean CARS-M (current mania) was 5.5 (S.D.=7.0), indicative of affective stability.
A total of 148 healthy controls (HCs) with no evidence of Axis I disorders (SCID) were recruited through advertisements at ZHH. Mean age was 41.58 (S.D.=15.1) years, 43.9% (n=65) were female, 47.3% (n=70) were Caucasian, and the mean pre-morbid IQ was 102.16 (S.D.=11.7). There were no significant differences between patients and controls (Table 1) in terms of age, sex or race (all p>0.05), while a difference was detected for pre-morbid IQ (F1,267 = 10.71, p=0.001).
Table 1.
Demographic, clinical and cognitive characteristics of the BPD patients and HC subjects
| Statistics
|
|||||
|---|---|---|---|---|---|
| BPD (n=136) | HC (n=148) | df | F or χ2 | p | |
| Diagnosis, n | |||||
| BPD I | 105 | – | |||
| BPD II | 31 | – | |||
| Sex, n (%) | |||||
| Males | 68 (50.0) | 83 (56.1) | |||
| Females | 68 (50.0) | 65 (43.9) | |||
| Race, n (%) | |||||
| Caucasian | 67 (49.3) | 70 (47.3) | 1 | 0.11 | 0.740 |
| Non-Caucasian | 69 (50.7) | 78 (52.7) | |||
| Age, years | 40.8 (10.6) | 41.6 (15.1) | 1, 283 | 0.26 | 0.607 |
| Age of onset, years | 20.6 (12.5)a | – | |||
| Duration of education, years | 14.1 (2.0)b | 14.9 (1.9)c | 1, 151 | 6.68 | 0.011 |
| Depressive symptoms: HAMD | 11.1 (8.5)d | 0.5 (1.3)c | 1, 276 | 223.56 | <0.001 |
| Manic symptoms: CARS-M | 5.5 (7.0)e | 0.3 (0.8) | 1, 251 | 81.56 | <0.001 |
| Pre-morbid IQ: WRAT-3 | 97.6 (10.8)f | 102.2 (11.7)g | 1, 267 | 10.71 | 0.001 |
| Cognitive domains, mean (Z score) | |||||
| Processing speed | −1.04 (1.18) | 0.0 (1.0) | 1, 283 | 65.17 | <0.001 |
| Attention | −0.98 (1.30) | 0.0 (1.0) | 1, 283 | 62.14 | <0.001 |
| Working memory | −0.76 (1.10) | 0.0 (1.0) | 1, 283 | 55.80 | <0.001 |
| Verbal learning | −0.51 (1.00) | 0.0 (1.0) | 1, 283 | 47.69 | <0.001 |
| Visual learning | −0.71 (1.24) | 0.0 (1.0) | 1, 283 | 81.32 | <0.001 |
| Reasoning and problem solving | −0.37 (0.99) | 0.0 (1.0) | 1, 283 | 30.08 | 0.002 |
| Social cognition | −0.43 (1.24) | 0.0 (1.0) | 1, 283 | 34.65 | 0.001 |
| Composite score | −1.11 (1.18) | 0.0 (1.0) | 1, 283 | 132.67 | <0.001 |
Data are given as mean (standard deviation).
BPD, Bipolar disorder; HC, healthy control; df, degrees of freedom; HAMD, Hamilton Rating Scale for Depression; CARS-M, Clinician-Administered Rating Scale for Mania; IQ, intelligence quotient; WRAT-3, Wide Range Achievement Test.
n=109;
n=84;
n=68;
n=129;
n=104;
n=121;
n=147.
Measuring neurocognitive functioning
The MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al. 2008; Matrics Assessment Inc., USA) was used to measure neurocognitive functioning. The MCCB was originally developed to be used in clinical trials targeting cognition in schizophrenia; however, recent studies demonstrated its suitability to effectively capture neurocognitive deficits in BPD patients (Yatham et al. 2010; Burdick et al. 2011). The MCCB is composed of several individual tests that give rise to seven cognitive domains:
Processing speed (Brief Assessment of Cognition in Schizophrenia and Trail Making Test-A);
Attention/vigilance (Continuous Performance Test–Identical Pairs);
Working memory (Wechsler Memory Scale: Spatial Span Letter-Number Span);
Verbal learning (Hopkins Verbal Learning Test–Revised);
Visual learning (Brief Visuospatial Memory Test–Revised);
Reasoning and problem solving (Neuropsychological Assessment Battery Mazes);
Social cognition (Mayer–Salovey–Caruso Emotional Intelligence Test).
The battery was completed in a single session of about 70 min.
Measuring clinical features and functional disability
For a subsample of patients (sample sizes in tables) data on current symptoms, history of psychosis, number of mood episodes and lifetime history of co-morbid substance use disorders were collected using the SCID interview and standardized mood ratings.
A comprehensive functional outcome evaluation was done using the Multidimensional Scale of Independent Functioning (MSIF; Jaeger et al. 2003) and the Social Adjustment Scale-II (SAS-II; Schooler et al. 1979). The MSIF is able to separate out the contribution of role position, level of support, and adequacy of performance, providing anchors for rating functioning in work and residential domains. The SAS-II assesses social functioning through use of a semi-structured interview, resulting in a social/leisure rating. Higher scores on each of these scales correspond to worse community functioning.
Statistical analysis
Initial analyses were carried out to compare demographic and clinical characteristics, and cognitive performance of the BPD patients and HCs using analysis of variance (ANOVA) and χ2 as appropriate (Table 1). All cognitive data were standardized to a Z-scale score with a mean of 0 and S.D. of 1, based upon the HCs’ performance.
In order to identify homogeneous subgroups of BPD patients based on their cognitive performance, we conducted a hierarchical cluster analysis. Similarity between cases was computed with the squared Euclidian distance and the Ward linkage was selected as the agglomeration procedure. Since the variables (scores for each MCCB domain) had the same metrics (t-scores with a mean of 50 and S.D. of 10) no pre-standardization was necessary. The inspection of the dendogram was used as criterion to establish the appropriate number of clusters to retain. Therefore in a second step of the analysis the model was forced to include a meaningful number of clusters (deduced from the dendogram) and cluster membership was saved as a grouping variable. Stability of the clusters was ascertained by repeating the hierarchical cluster analysis in a split half of the sample. Then in order to test the validity of the clusters and to have a better understanding of the relationship between cognition and allocation of BPD patients into specific clusters, we conducted a discriminant function analysis (DFA). The DFA explored the predictive power of each of the seven MCCB domains in differentiating subjects into the discrete neurocognitive groups obtained from the hierarchical cluster analysis. The neurocognitive profiles of the BPD clusters and HCs were then compared using an ANOVA. Planned contrasts using the HC group as a comparison category was done to detect differences between groups and results were corrected for multiple comparisons with least significant difference (LSD) correction. We acknowledge that the clustering procedures were applied with the intent to separate the BPD sample into subgroups based on cognitive performance; therefore, it would follow that secondary testing for subgroup differences would be significant. The purpose of these analyses was not to prove that differences exist between the BPD subgroups. Rather, we carried out these analyses specifically in an effort to determine the degree of impairment versus HCs, to illustrate the qualitative differences among the BPD clusters, and to allow for a visualization of the profiles by subgroup.
Finally, to begin to understand the clinical and functional correlates of cluster membership, descriptive analyses (ANOVA and χ2 applied as appropriate) were carried out to investigate differences on demographic characteristics, clinical features, and functional disability between the identified BPD clusters.
Results
Results from the ANOVA revealed that BPD patients (full sample) had significantly worse performance than HCs on all seven of the MCCB domains and on the MCCB composite score (all p<0.002; Table 1). These data are consistent with prior work from our group (Burdick et al. 2007) and others (Bora et al. 2009) and suggest that our BPD sample is representative of other samples in previous studies of neurocognition.
Clustering BPD patients
Results from the hierarchical cluster analysis showed that the 136 BPD patients are optimally clustered (according to MCCB performance) into three discrete subgroups. The first cluster included 54 subjects (39.7%), the second cluster included 43 individuals (31.6%) and the third cluster included 39 subjects (28.7%).
To test the validity of these clusters, approximately 50% of the 136 BPD patients (n=65) from the original sample were randomly selected and hierarchical cluster analysis was repeated on the split-half sample. Results were consistent with the initial analysis with the emergence of three clusters in the split sample. The ANOVA revealed no statistically significant differences between the two split samples in term of age, sex, diagnosis and neurocognitive performance.
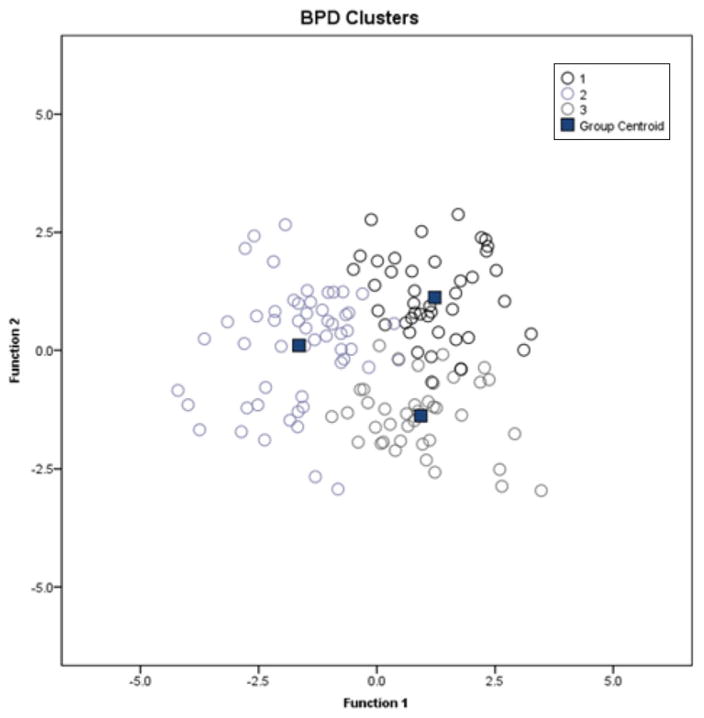
Results from the DFA using the three BPD clusters revealed the presence of two discriminant functions explaining 65.6% and 34.4% of the variance, respectively (Wilks’ λ = 0.18, , p<0.001; after removal of the first function the Wilk’s λ = 0.51, , p<0.001). Visual learning and working memory domains showed the strongest correlation coefficients (0.78 and 0.51, respectively), suggesting that they contributed more than the other cognitive domains to classify subjects into neurocognitive clusters. Subjects grouping into the three neurocognitive clusters are shown in Fig. 1.
Fig. 1.
Graphical agglomeration of bipolar disorder (BPD) subjects using discriminant function analysis. The figure represents the agglomeration of subjects using the three clusters emerged from the hierarchical cluster analysis. The centroids (■) are the mean score for each cluster. Cluster 1 is the global impairment group, cluster 2 is the selective impairment group and cluster 3 is the intact group (see text).
Comparison between BPD clusters and HCs on neurocognitive functioning
Multivariate ANOVA revealed a significant main effect of group when comparing the three BPD clusters and HCs (F21,828 =15.30, p <0.001) (Table 2). Based on the level of cognitive impairment, the BPD clusters were labeled as follows:
Table 2.
Comparison between the three neurocognitive bipolar disorder clusters and HC subjects across cognitive domains (Z scores)
| Cluster
|
|||||
|---|---|---|---|---|---|
| Global (n=54) | Selective (n=39) | Intact (n=43) | HC (n=148) | Significancea | |
| Processing speed | −1.70 (0.91) | −1.10 (0.88) | −0.15 (1.16) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p<0.001 HC v. intact p=0.355 Global v. selective p=0.005 Global v. intact p<0.001 Selective v. intact p<0.001 |
| Attention | −1.61 (1.23) | −1.30 (1.01) | 0.11 (0.88) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p<0.001 HC v. intact p=0.569 Global v. selective p=0.141 Global v. intact p<0.001 Selective v. intact p<0.001 |
| Working memory | −1.52 (0.99) | −0.38 (0.96) | −0.16 (0.73) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p=0.028 HC v. intact p=0.329 Global v. selective p<0.001 Global v. intact p<0.001 Selective v. intact p=0.306 |
| Verbal learning | −0.91 (0.82) | −0.64 (0.79) | 0.08 (1.07) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p=0.020 HC v. intact p=0.637 Global v. selective p=0.172 Global v. intact p<0.001 Selective v. intact p=0.001 |
| Visual learning | −1.81 (0.87) | 0.32 (0.78) | −0.27 (0.83) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p=0.057 HC v. intact p=0.079 Global v. selective p<0.001 Global v. intact p<0.001 Selective v. intact p=0.004 |
| Reasoning and problem solving | −0.84 (0.77) | −0.24 (1.01) | 0.09 (0.97) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p=0.166 HC v. intact p=0.565 Global v. selective p=0.003 Global v. intact p=0.001 Selective v. intact p=0.115 |
| Social cognition | −0.70 (1.30) | −1.11 (0.96) | 0.52 (0.77) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p<0.001 HC v. intact p=0.004 Global v. selective p=0.056 Global v. intact p<0.001 Selective v. intact p=0.001 |
| Composite score | −2.08 (0.12) | −1.07 (0.14) | 0.05 (0.14) | 0.0 (1.0) | HC v. global p<0.001 HC v. selective p<0.001 HC v. intact p=0.722 Global v. selective p<0.001 Global v. intact p<0.001 Selective v. intact p<0.001 |
Data are given as mean (standard deviation).
HC, Healthy control.
All p values are adjusted for multiple comparisons with least significant difference (LSD) correction.
Cluster 1, the global impairment group (global), presented with diffuse and severe cognitive dysfunction, with performance falling between 1 and 2 s.D.s below the HCs’ mean;
Cluster 2, the selective impairment group (selective), presented with more modest deficits on specific domains, with performance ranging between normal and –1 s.D. below average;
Cluster 3, the intact group (intact), performed comparably to HCs on all domains (all p values>0.07), with significantly superior performance v. HCs on social cognition (p=0.004) (Table 2).
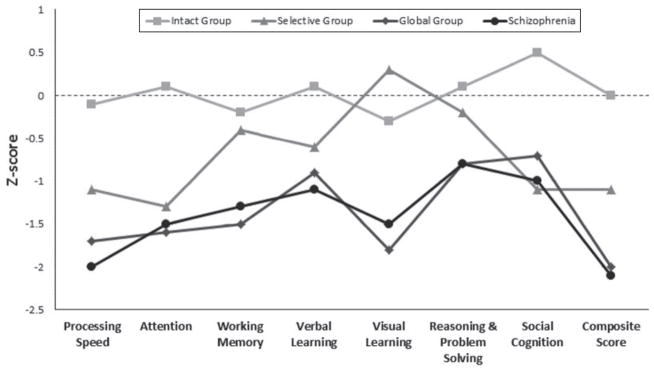
Fig. 2 illustrates the cognitive profiles of the three BPD clusters and the schizophrenia sample. Z scores were computed based upon our HC sample instead of using the MCCB normative sample, as our HCs were demographically matched to our BPD sample on demographic characteristics (see Table 1) than the community sample used for standardization of the MCCB battery (Kern et al. 2008).
Fig. 2.
Neurocognitive profiles of bipolar disorder clusters and the schizophrenia sample. The X-axis indicates the MATRICS Consensus Cognitive Battery (MCCB) domains. The Y-axis depicts a Z-scale score with a mean of 0 and a standard deviation of 1. Z scores were computed based upon the healthy control sample. Patients are divided into lines based on scoring for each cognitive domain. Statistical comparisons among groups are specified in Table 2.
After LSD correction for multiple testing, the global group performed significantly worse than HCs across all cognitive domains. The selective group performed significantly worse than HCs on all domains except for visual learning and reasoning and problem solving. The intact group performed within the normal range for all domains v. HCs, apart from social cognition (p=0.004) where intact BPD subjects scored significantly higher than HCs (Table 2).
For purposes of reference to the severity of cognitive impairment noted in schizophrenia, we have included in Fig. 2 data from a sample of 185 schizophrenia patients ascertained at ZHH using the same recruitment procedures as were used for the BPD sample selection. The mean age at the time of the assessment was 42.9 years (S.D.=1 0.1), mean age at onset was 19.5 years (S.D.=8.7), and, on average, schizophrenia subjects had 12.7 years (S.D.=2.8) of education. Of the subjects, 57 (31.5%) were female and 77 (42.5%) were Caucasian. The mean pre-morbid IQ (WRAT-3) was=90.8 (S.D.=12.7) and the mean depressive symptoms score (as measured by the HAMD) was 10.4 (S.D.=7.0). Additional details regarding the schizophrenia sample have been previously described and published (Shamsi et al. 2011).
When comparing the global BPD group with the schizophrenia patients, the two groups did not differ on six of seven of the cognitive domains, nor did they differ on the MCCB composite score (all p<0.09). The only exception was for social cognition where the BPD global group outperformed the schizophrenia group [mean −0.70 (S.D.=1.30) v. −1.06 (S.D.=1.16)].
Non-cognitive characteristics of the three BPD clusters (Table 3)
Table 3.
Demographic and clinical characteristics of BPD clusters
| Cluster
|
Statistics
|
|||||
|---|---|---|---|---|---|---|
| Global (n=54) | Selective (n=39) | Intact (n=43) | df | F or χ2 | p | |
| Age, years (n=136) | 39.8 (9.8) | 43.8 (10.9) | 39.3 (11.1) | 2, 135 | 2.24 | 0.110 |
| Sex, n (%) | ||||||
| Males (n=68) | 30 (55.6) | 22 (56.4) | 16 (37.2) | 2 | 4.12 | 0.127 |
| Females (n=68) | 24 (44.4) | 17 (43.6) | 27 (62.8) | |||
| Race, n (%) | ||||||
| Caucasian (n=67) | 24 (44.4) | 18 (46.2) | 25 (58.1) | 2 | 2.01 | 0.366 |
| Non Caucasian (n=69) | 30 (55.6) | 21 (53.8) | 18 (41.9) | |||
| Pre-morbid IQ: WRAT-3 (n=121) | 93.1 (10.1)b | 98.9 (9.1)c | 102.0 (11.3)d | 2, 120 | 8.46 |
p<0.001a Global v. selective p=0.012a Global v. intact p<0.001a Selective v. intact p=0.182 |
| Depressive symptoms: HAMD (n=129) | 11.9 (8.2)e | 10.1 (7.8)f | 11.0 (9.5)g | 2, 128 | 0.44 | 0.645 |
| Manic symptoms: CARS-M (n=104) | 6.6 (8.1)h | 4.2 (5.8)i | 5.5 (6.5)j | 2, 103 | 0.99 | 0.376 |
| Diagnosis, n (%) | ||||||
| BPD I (n=105) | 45 (83.3) | 29 (74.4) | 31 (72.1) | 2 | 1.97 | 0.373 |
| BPD II (n=31) | 9 (16.7) | 10 (25.6) | 12 (27.9) | |||
| History of psychosis, n (%) | ||||||
| Yes (n=69) | 24 (44.4) | 18 (46.2) | 27 (62.8) | 2 | 3.68 | 0.159 |
| No (n=67) | 30 (55.6) | 21 (53.8) | 16 (37.2) | |||
| Substance disorder, n (%) | ||||||
| Yes (n=49) | 19 (54.9) | 14 (51.9) | 16 (50.0) | 2 | 0.24 | 0.887 |
| No (n=44) | 15 (44.1) | 13 (48.1) | 16 (50.0) | |||
| Number of depressive episodes (n=76) | 10.5 (14.3)k | 14.1 (20.2)l | 4.5 (3.5)m | 2, 75 | 2.70 |
p=0.074 Global v. selective p=0.371 Global v. intact p=0.135 Selective v. intact p=0.025a |
| Number of manic episodes (n=95) | 6.2 (9.1)n | 6.7 (15.4)k | 2.1 (2.6)k | 2, 94 | 1.74 |
p=0.181 Global v. selective p=0.827 Global v. intact p=0.120 Selective v. intact p=0.095 |
| Total number of mood episodes (n=80) | 16.6 (19.3)o | 22.5 (35.1)l | 6.4 (4.0)p | 2, 79 | 3.30 |
p=0.042a Global v. selective p=0.344 Global v. intact p=0.089 Selective v. intact p=0.014a |
| Medications | ||||||
| Total number of medications (n=116) | 2.2 (1.2)q | 1.8 (1.1)r | 1.9 (1.1)s | 2, 115 | 1.04 | 0.355 |
| Type of medications | ||||||
| Anticonvulsant, n (%) | ||||||
| Yes (n=53) | 24 (50.0) | 13 (39.4) | 16 (45.7) | 2 | 0.89 | 0.642 |
| No (n=63) | 24 (50.0) | 20 (60.6) | 19 (54.3) | |||
| Antipsychotic, n (%) | ||||||
| Yes (n=86) | 37 (77.1) | 25 (75.8) | 24 (68.6) | 2 | 0.83 | 0.661 |
| No (n=30) | 11 (22.9) | 8 (24.2) | 11 (31.4) | |||
| Antidepressant, n (%) | ||||||
| Yes (n=39) | 17 (35.4) | 12 (36.4) | 10 (28.6) | 2 | 0.58 | 0.748 |
| No (n=77) | 31 (64.6) | 21 (63.6) | 25 (71.4) | |||
| Lithium, n (%) | ||||||
| Yes (n=33) | 12 (25.0) | 11 (33.3) | 10 (28.6) | 2 | 0.67 | 0.716 |
| No (n=83) | 36 (75.0) | 22 (66.7) | 25 (71.4) | |||
| MSIF-Work (n=53) | 6.8 (1.7)t | 6.4 (1.4)u | 4.5 (2.6)v | 2, 52 | 7.01 |
p=0.002a Global v. selective p=0.574 Global v. intact p=0.001a Selective v. intact p=0.008a |
| MSIF-Residential (n=53) | 3.5 (1.4)t | 3.7 (1.5)u | 3.9 (1.8)v | 2, 52 | 0.28 | 0.760 |
| SAS-II – social functioning (n=50) | 3.7 (1.1)t | 3.6 (1.2)w | 3.1 (1.4)x | 2, 49 | 1.14 | 0.329 |
Data are given as mean (standard deviation).
BPD, Bipolar disorder; df, degrees of freedom; IQ, intelligence quotient; WRAT-3, Wide Range Achievement Test; HAMD, Hamilton Rating Scale for Depression; CARS-M, Clinician-Administered Rating Scale for Mania; MSIF, Multidimensional Scale of Independent Functioning; SAS-II, Social Adjustment Scale-II.
p Values are adjusted for multiple comparisons with least significant difference (LSD) correction.
n=47.
n=36.
n=38.
n=49.
n=37
n=38.
n=38.
n=30.
n=36.
n=29.
n =23.
n=24.
n=37.
n=31.
n=26.
n=48.
n=33.
n=35.
n=20.
n=15.
n=18.
n=13.
n=17.
The three clusters did not differ on age, sex or race distributions; however, there were significant group differences for pre-morbid-IQ (F2,120 =8.46, p<0.001), with post-hoc tests indicating significant group differences between the global group and each of the other two clusters. There was no difference between the selective group and the intact group. As might be expected, subjects in the global group had the lowest pre-morbid IQ.
There were no significant differences for depressive or manic symptoms at the time of assessment. Diagnostic subtype distributions (BPD I v. BPD II) did not differ by cognitive group; nor did psychosis history (p=0.373 and p=0.159, respectively). There was no significant difference with regard to history of substance use disorder (inclusive of alcohol/drug abuse/dependence; p=0.887).
There was a main effect of group on total number of prior episodes (including both depressive and manic; p =0.042); post-hoc testing revealed significant differences between the intact and selective groups (p=0.014) and a trend-level difference between the global and intact groups (p =0.089). The selective group had the highest number of prior episodes but did not significantly differ from the global group (p=0.344). Likewise, while there was no main effect of group with regard to total number of previous depressive episodes (p=0.074), post-hoc results were significantly different between the intact and selective groups (p=0.025), with selective group subjects reporting the highest number of prior depressive episodes. There was no main effect of group on prior number of manic episodes (p=0.181) and no post-hoc group differences. There were no differences between the clusters on total number of medications or the types of medications prescribed.
When evaluating the influence of cluster membership on everyday functions, we found a significant main effect of group for MSIF occupational status (p=0.002), with significant post-hoc differences: global v. intact (p =0.001); selective v. intact (p=0.008). There were no significant effects on independent living status or social functioning.
Discussion
A convergence of anecdotal and clinically based evidence suggests that there is substantial heterogeneity in neurocognition and functional capacity in BPD patients. In this paper, we provide empirical support for statistically discrete neurocognitive subgroups in BPD, characterizing the structure of this heterogeneity.
Previous work has consistently shown that, as a group, remitted BPD patients are impaired about 0.5 to 1 s.D. below average, particularly on attention, verbal learning and executive functioning (Bora et al. 2009). While this level of impairment is considered modest, particularly in light of considerably more severe deficits in schizophrenia, these data fail to account for the substantial cognitive heterogeneity in BPD. Early data suggest that only a small proportion of patients with schizophrenia are considered ‘neuropsychologically normal’ (Wilk et al. 2005; Kremen et al. 2008), while nearly half of all BPD patients function within the average range (Martino et al. 2008; Bora et al. 2009; Reichenberg et al. 2009). Thus, prior group-based analyses comparing these two samples may have failed to capture the extent of the impairment that is present in a specific subgroup of BPD patients. Indeed, our data strongly support this notion.
Using hierarchical cluster analyses, we found evidence for three distinct, cognitively homogeneous subgroups in an unselected sample of 136 BPD patients. Specifically we found: (1) a cluster of BPD patients with normal cognitive functioning (intact group; 31.6% of the sample) who do not differ from demographically matched HCs; (2) a cluster of BPD patients whose cognitive profile is consistent with selective impairment (selective group 28.7% of the sample), with modest deficits on only a subset of the seven cognitive domains v. HCs; and (3) a cluster of BPD patients (global group; 39.7% of the sample) with severe impairment across all cognitive domains.
The emergence of an intact group is consistent with prior reports that used a −1 s.D. cut-off to define clinically significant impairment and found that approximately 40% of BPD patients were characterized as unimpaired (Reichenberg et al. 2009). Our data expand upon this to show that there is a specific subgroup that not only fail to meet this threshold of impairment but indeed do not differ from HCs on any of the MCCB cognitive domains. Moreover, in line with the anecdotal evidence of a high-performing, socially savvy BPD subgroup, we found that the intact group was actually superior to our HCs on the MCCB social cognition domain. In addition, the global group performed better than the schizophrenia group on social cognition although their cognitive profiles were comparable across all the other domains. This result is consistent with a very recent study (Lee et al. 2013) showing that social cognition in BPD is relatively intact. Lee et al. (2013) showed that BPD patients did not significantly differ from the comparison group on any social cognitive task and that BPD subjects performed significantly better on social compared with non-social domains, while the opposite pattern emerged in schizophrenia patients. Although our findings support the presence of less severe impairment in social cognition compared with other domains in BPD patients when considered at a group level, it might also suggest the potential for meaningful subgroups based upon social cognitive performance within BPD. Future studies including a more extensive social cognitive battery will be needed to expand upon these results in order to determine whether social cognition can be considered a key determinant of poor functional outcome for those subgroups of BPD patients showing very severe cognitive deficits.
Subjects who were clustered into the selective group were characterized by a profile similar to that which is commonly noted in BPD group-level data. Deficits were noted on some but not all cognitive domains (intact visual learning and reasoning and problem solving) and were generally in the mild to moderate range.
The globally impaired group (global group) showed a generalized pattern of impairment with significant deficits on all cognitive domains when compared with HCs. Deficits in the global group were severe, ranging from −1 to −2 s.D.s below the mean performance of a HC sample, directly comparable with the impairment in our schizophrenia cohort (Shamsi et al. 2011) (see Fig. 2) and in other samples of schizophrenia patients (Harvey & Serper, 1990; Heinrichs & Zakzanis, 1998). This overlapping profile is of critical importance when considering recent data supporting shared molecular genetic risk factors for BPD and schizophrenia (Lichtenstein et al. 2009; Williams et al. 2011). As illustrated in Fig. 2 the global measure of cognition (MCCB composite score) would suggest a continuum of severity across the full range of major psychiatric disease, such that all patients, regardless of diagnosis, could be characterized by mild, moderate or severe impairment. The overlap between the global BPD group and patients with schizophrenia argues that BPD patients do not simply lie intermediate between HCs and schizophrenia on this cognitive continuum. Moreover, when taking into account the performance on specific domains, the presence of qualitative differences across the three BPD clusters supports the existence of meaningfully unique subgroups within the illness.
The three BPD clusters did not differ with regard to age, sex or race distribution; however, pre-morbid IQ estimates were significantly different by group. The global group had the lowest pre-morbid IQ and although it was still considered to be within the normal range, it was significantly lower than each of the other two BPD clusters and the HCs. The other two BPD clusters did not differ from one another, nor were they different from the HCs. This suggests that lower IQ prior to the onset of BPD might place a patient at increased risk for developing more severe and generalized neurocognitive deficits in the context of the illness. This is consistent with mild pre-morbid deficits seen in patients with schizophrenia (Woodberry et al. 2008) and could be conceptualized in reference to the cognitive reserve theory, where higher cognitive capacity could serve as protective against decline (Stern, 2012). Longitudinal studies will be important to address this hypothesis.
Prior evidence suggests that diagnostic subtype within BPD may influence the extent of cognitive dysfunction; however, our data did not support this finding. The distribution of BPD I v. BPD II was comparable in all groups, as was the psychotic subtype of the illness. The lack of significant findings in our sample could be due to limited statistical power but it also speaks to the inconsistencies reported in previous studies (Dittmann et al. 2008; Simonsen et al. 2008) and the relative paucity of data on neurocognitive functioning across the entire BPD spectrum (Sole et al. 2012). As has been shown in several previous studies, the total number of affective episodes was related to neurocognitive impairment in our sample. We found that the selective group had the highest number of total episodes, including both depressive and manic polarities, with the global group falling intermediate to the selective and intact groups. These data further support the deleterious and potentially cumulative effects of full-blown affective episodes on neurocognitive functioning in BPD (Bora et al. 2009).
Although data were only available in a subset of the sample, thereby limiting our interpretation of these findings, our data also suggest that there are meaningful group differences with regard to everyday functioning in the community. Specifically we found that BPD patients in the global group were significantly more occupationally disabled than patients who were either in the selective or intact groups. These data are consistent with several prior reports that indicate that neurocognitive impairment is an important predictor of functional disability across many psychiatric disorders (Bowie et al. 2010) including BPD (Depp et al. 2012; Martinez-Aran et al. 2007; Burdick et al. 2010) and suggest that these clusters may have direct functional relevance.
As the MCCB was initially designed for assaying cognition in schizophrenia, the use of a more comprehensive, bipolar-specific, neurocognitive battery (e.g. Yatham et al. 2010) may have resulted in the emergence of different subgroups. Future studies including a larger number of tasks and measures of affective processing will be important in establishing and refining these profiles.
This is the first study describing the presence of empirically derived neurocognitive subgroups in BPD. The potential downstream implications of our findings are two-fold. By subgrouping BPD patients based on neurocognitive profiles we can reduce the heterogeneity of the phenotype to allow for a more targeted assessment of clinical and biological predictors of cognitive impairment in BPD. The identification of specific biomarkers and clinical factors that are associated with specific neurocognitive profiles will allow for the tailoring of treatments to correct the cognitive dysfunction in BPD. Preventative and early intervention strategies might also be suggested based upon future studies utilizing this approach.
Acknowledgments
Financial support for this work included grants from the National Institute of Mental Health (NIMH) to K.E.B. (K23MH077807; R01MH100125) and to A.K.M. (R01MH079800).
Footnotes
Declaration of Interest
K.E.B. has served on an advisory board for Dainippon Sumitomo Pharma. A.K.M. has served as consultant or speaker for Bristol-Myers Squibb, Merck, AstraZeneca, Vanda Pharmaceuticals and Clinical Data Inc., and has received research support from Pfizer, Janssen Pharmaceuticals, Bristol-Myers Squibb and Eli Lilly. S.F. serves on advisory boards for Enzymotec and Janssen-Cilag.
References
- Abood Z, Sharkey A, Webb M, Kelly A, Gill M. Are patients with bipolar affective disorder socially disadvantaged? A comparison with a control group. Bipolar Disorder. 2002;4:243–248. doi: 10.1034/j.1399-5618.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biological Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. DSM-IV-TR. [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. American Journal of Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Braga RJ, Goldberg JF, Malhotra AK. Cognitive dysfunction in bipolar disorder: future place for pharmacotherapy. CNS Drugs. 2007;21:971–981. doi: 10.2165/00023210-200721120-00002. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg JF, Harrow M. Neurocognitive dysfunction and psychosocial outcome in patients with bipolar I disorder at 15-year follow-up. Acta Psychiatrica Scandinavica. 2010;122:499–506. doi: 10.1111/j.1600-0447.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Cornblatt BA, Keefe RS, Gopin CB, Derosse P, Braga RJ, Malhotra AK. The MATRICS Consensus Cognitive Battery in patients with bipolar I disorder. Neuropsychopharmacology. 2011;36:1587–1592. doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabarés-Seisdedos R, Balanzá-Martínez V, Salazar-Fraile J, Selva-Vera G, Vieta E. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychotherapy and Psychosomatics. 2006;75:72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disorder. 2012;14:217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion GL, Tohen M, Anthony WA, Waternaux CS. Symptoms and functioning of patients with bipolar disorder six months after hospitalization. Hospital and Community Psychiatry. 1988;39:652–657. doi: 10.1176/ps.39.6.652. [DOI] [PubMed] [Google Scholar]
- Dittmann S, Hennig-Fast K, Gerber S, Seemüller F, Riedel M, Emanuel Severus W, Langosch J, Engel RR, Möller HJ, Grunze HC. Cognitive functioning in euthymic bipolar I and bipolar II patients. Bipolar Disorder. 2008;10:877–887. doi: 10.1111/j.1399-5618.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Allen DN, Seaton BE. A comparison of clustering solutions for cognitive heterogeneity in schizophrenia. Journal of the International Neuropsychological Society. 1998;4:353–362. [PubMed] [Google Scholar]
- Goldstein G, Beers SR, Shemansky WJ. Neuropsychological differences between schizophrenic patients with heterogeneous Wisconsin Card Sorting Test performance. Schizophrenia Research. 1996;21:13–18. doi: 10.1016/0920-9964(96)00019-9. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Serper MR. Linguistic and cognitive failures in schizophrenia. A multivariate analysis. Journal of Nervous and Mental Disease. 1990;178:487–493. [PubMed] [Google Scholar]
- Harvey PD, Wingo AP, Burdick KE, Baldessarini RJ. Cognition and disability in bipolar disorder: lessons from schizophrenia. Bipolar Disorder. 2010;12:364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Awad AG. Neurocognitive subtypes of chronic schizophrenia. Schizophrenia Research. 1993;9:49–58. doi: 10.1016/0920-9964(93)90009-8. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. Journal of Clinical and Experimental Neuropsychology. 2002;24:765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Berns SM, Czobor P. The multidimensional scale of independent functioning: a new instrument for measuring functional disability in psychiatric populations. Schizophrenia Bulletin. 2003;29:153–168. doi: 10.1093/oxfordjournals.schbul.a006987. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, Part 2: co-norming and standardization. American Journal of Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. Journal of Abnormal Psychology. 2000;109:743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. IQ decline in cross-sectional studies of schizophrenia: methodology and interpretation. Psychiatry Research. 2008;158:181–194. doi: 10.1016/j.psychres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Grochocinski VJ, Cluss PA, Houck PR, Stapf DA. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. Journal of Clinical Psychiatry. 2002;63:120–125. doi: 10.4088/jcp.v63n0206. [DOI] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. American Journal of Psychiatry. 2013;170:334–341. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, Sanchez-Moreno J, Manuel Goikolea J, Benabarre A, Colom F, Vieta E. Neurocognitive impairment in bipolar patients with and without history of psychosis. Journal of Clinical Psychiatry. 2008;69:233–239. doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Torrent C, Sanchez-Moreno J, Goikolea JM, Salamero M, Malhi GS, Gonzalez-Pinto A, Daban C, Alvarez-Grandi S, Fountoulakis K, Kaprinis G, Tabares-Seisdedos R, Ayuso-Mateos JL. Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar Disorder. 2007;9:103–113. doi: 10.1111/j.1399-5618.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Scápola M, Igoa A, Marengo E, Ais ED, Perinot L. Heterogeneity in cognitive functioning among patients with bipolar disorder. Journal of Affective Disorders. 2008;109:149–156. doi: 10.1016/j.jad.2007.12.232. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AR, Bonnín CM, Vázquez GH, Reinares M, Solé B, Tabarés-Seisdedos R, Balanzá-Martínez V, González-Pinto A, Sánchez-Moreno J, Vieta E. Functional impairment in bipolar II disorder: is it as disabling as bipolar I? Journal of Affective Disorders. 2010;127:71–76. doi: 10.1016/j.jad.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Schooler NR, Hogarty GE, Weissman MM. Social Adjustment Scale II (SAS II) In: Hargreaves WA, Attkisson CC, Sorenson JE, editors. Resource Materials for Community Mental Health Evaluators. Department of Mental Health, Education and Welfare; Washington, DC: 1979. pp. 290–330. [Google Scholar]
- Seaton BE, Allen DN, Goldstein G, Kelley ME, van Kammen DP. Relations between cognitive and symptom profile heterogeneity in schizophrenia. Journal of Nervous and Mental Disease. 1999;187:414–419. doi: 10.1097/00005053-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Shamsi S, Lau A, Lencz T, Burdick KE, DeRosse P, Brenner R, Lindenmayer JP, Malhotra AK. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophrenia Research. 2011;126:257–264. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Hansen CF, Jónsdóttir H, Ringen PA, Opjordsmoen S, Friis S, Andreassen OA. Neurocognitive profiles in bipolar I and bipolar II disorder: differences in pattern and magnitude of dysfunction. Bipolar Disorder. 2008;10:245–255. doi: 10.1111/j.1399-5618.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- Sole B, Bonnin CM, Torrent C, Martinez-Aran A, Popovic D, Tabarés-Seisdedos R, Vieta E. Neurocognitive impairment across the bipolar spectrum. CNS Neuroscience and Therapeutics. 2012;18:194–200. doi: 10.1111/j.1755-5949.2011.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, Mitchell PB, Centorrino F, Risser R, Baker RW, Evans AR, Beymer K, Dube S, Tollefson GD, Breier A. Efficacy of olanzapine and olanzapine–fluoxetine combination in the treatment of bipolar I depression. Archives of General Psychiatry. 2003;60:1079–1088. doi: 10.1001/archpsyc.60.11.1079. [DOI] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophrenia Research. 2009;113:189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk CM, Gold JM, McMahon RP, Humber K, Iannone VN, Buchanan RW. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19:778–786. doi: 10.1037/0894-4105.19.6.778. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test: Administration Manual. 3. Del Wide Range Inc; Wilmington, DE: 1993. [Google Scholar]
- Williams HJ, Craddock N, Russo G, Hamshere ML, Moskvina V, Dwyer S, Smith RL, Green E, Grozeva D, Holmans P, Owen MJ, O’Donovan MC. Most genome-wide significant susceptibility loci for schizophrenia and bipolar disorder reported to date cross-traditional diagnostic boundaries. Human Molecular Genetics. 2011;20:387–391. doi: 10.1093/hmg/ddq471. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry. 2008;65:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Torres IJ, Malhi GS, Frangou S, Glahn DC, Bearden CE, Burdick KE, Martínez-Arán A, Dittmann S, Goldberg JF, Ozerdem A, Aydemir O, Chengappa KN. The International Society for Bipolar Disorders-Battery for Assessment of Neurocognition (ISBD-BANC) Bipolar Disorder. 2010;12:351–363. doi: 10.1111/j.1399-5618.2010.00830.x. [DOI] [PubMed] [Google Scholar]