Abstract
Methamphetamine (Meth) is a neurotoxic drug of abuse that damages neurons and nerve endings throughout the central nervous system. Emerging studies of human Meth addicts using both postmortem analyses of brain tissue and noninvasive imaging studies of intact brains have confirmed that Meth causes persistent structural abnormalities. Animal and human studies have also defined a number of significant functional problems and comorbid psychiatric disorders associated with long-term Meth abuse. This review summarizes the salient features of Meth-induced neurotoxicity with a focus on the dopamine (DA) neuronal system. DA nerve endings in the caudate-putamen (CPu) are damaged by Meth in a highly delimited manner. Even within the CPu, damage is remarkably heterogeneous, with ventral and lateral aspects showing the greatest deficits. The nucleus accumbens (NAc) is largely spared the damage that accompanies binge Meth intoxication, but relatively subtle changes in the disposition of DA in its nerve endings can lead to dramatic increases in Meth-induced toxicity in the CPu and overcome the normal resistance of the NAc to damage. In contrast to the CPu, where DA neuronal deficiencies are persistent, alterations in the NAc show a partial recovery. Animal models have been indispensable in studies of the causes and consequences of Meth neurotoxicity and in the development of new therapies. This research has shown that increases in cytoplasmic DA dramatically broaden the neurotoxic profile of Meth to include brain structures not normally targeted for damage. The resistance of the NAc to Meth-induced neurotoxicity and its ability to recover reveal a fundamentally different neuroplasticity by comparison to the CPu. Recruitment of the NAc as a target of Meth neurotoxicity by alterations in DA homeostasis is significant in light of the numerous important roles played by this brain structure.
Keywords: dopamine, dopamine transporter, glutamate, methamphetamine, neurotoxicity, nucleus accumbens, tyrosine hydroxylase
Introduction
Methamphetamine (Meth1) is a member of the amphetamine family of psychostimulant drugs. Meth abuse and addiction have reached alarming proportions, leading numerous federal agencies including the National Institute on Drug Abuse, Office of National Drug Control Policy, and Drug Enforcement Administration to liken it to an epidemic. In fact, the UN Office on Drugs and Crime reported that amphetamine use has eclipsed that of cocaine and heroin on a global scale. In the United States the Rand Drug Policy Research Center has estimated that the economic cost of Meth is as high as $48 billion when factors such as increased morbidity and mortality, crime, lost productivity, health care and drug treatment, and illegal Meth production are considered (Rand 2009).
The medical, legal, and societal problems associated with any rampant drug of abuse are compounded in the case of amphetamines because many members of this pharmacological class cause persistent neuronal damage both in humans (Chang et al. 2007; McCann et al. 2008; Thompson et al. 2004; Tobias et al. 2010) and in animal models of neurotoxicity (Cadet et al. 2007; Fleckenstein et al. 2007; O’Callaghan and Miller 1994; Yamamoto and Bankson 2005). The consequences of this neurotoxicity are manifested as persistent depletions of dopamine (DA1), inhibition of tyrosine hydroxylase (TH1), inactivation of the DA transporter (DAT1), reduction in function of the vesicle monoamine transporter (VMAT1), degeneration of fine, unmyelinated axons, and apoptosis (Asanuma et al. 2000; Davidson et al. 2001; Jayanthi et al. 2002). As a neurotransmitter, DA plays an essential role in numerous physiological, neuronal, and behavioral processes. A persistent reduction in DA neuronal function resulting from chronic Meth abuse (Volkow et al. 2001a,b) could be expressed ultimately in the form of comorbid psychiatric or neurological diseases.
Methamphetamine Neurotoxicity: Overview of Mechanisms of Action
The mechanisms by which Meth damages the DA neuronal system are not yet fully understood. Space constraints prohibit an exhaustive coverage of Meth toxicity, and numerous excellent review articles are available (Davidson et al. 2001; Fleckenstein et al. 2000; Frost and Cadet 2000; Gibb et al. 1990; Yamamoto and Zhu 1998).
A great deal of attention has been focused on oxidative stress as a mediator of Meth toxicity. Meth increases the expression of nitric oxide synthase (NOS) (Deng and Cadet 1999), NOS inhibitors protect against Meth toxicity (Abekawa et al. 2001; Ali and Itzhak 1998; Itzhak and Ali 1996), and mice lacking the NOS gene are resistant to Meth toxicity (Itzhak et al. 1998, 1999), suggesting a potential role for nitric oxide. Furthermore, mice that overexpress superoxide dismutase are more resistant to Meth toxicity than wild-type mice (Cadet et al. 1994; Hirata et al. 1998; Maragos et al. 2000), suggesting a potential role for superoxide. Nitric oxide and superoxide can react at near diffusion-limited rates to form peroxynitrite, a well-known cytotoxicant, and reports have presented evidence of a role for peroxynitrite in Meth toxicity (Imam et al. 1999, 2000, 2001a,b; Imam and Ali 2001).
Glutamate excitotoxicity has also been implicated in Meth actions (Nash and Yamamoto 1992; Stephans et al. 1998) and mitochondrial dysfunction may be a final, common path by which nerve terminal function is diminished by the neurotoxic amphetamines (Burrows et al. 2000a,b; Nixdorf et al. 2001). However, the manner by which Meth causes oxidative stress and the cellular source and identity of the reactant species that mediate amphetamine-induced neuronal damage have largely eluded detection.
Another particularly dangerous outcome of acute Meth intoxication is hyperthermia. Meth can increase the body temperature of rodents from normal (37°C) to higher than 41°C when given on a schedule that simulates human binge intake (Bowyer et al. 1992, 1994). In humans high-dose Meth intake can lead to lethality and hyperthermia is likely the primary cause of death (Davidson et al. 2001).
Role of Nonneuronal Cells in Methamphetamine Neurotoxicity
Microglia
A clue to the possible cellular source of reactive oxygen species (ROS1) and reactive nitrogen species (RNS1) that mediate the neurotoxic actions of amphetamines emerged from studies using microarray analysis to examine striatal gene expression changes provoked by a neurotoxic regimen of Meth (Thomas et al. 2004a). This analysis revealed that numerous (20% of about 150 genes whose expression was changed significantly) “stress”- and “inflammation”-linked genes changed significantly 2 to 4 hours after Meth intoxication (Thomas et al. 2004a), well before the time when nerve ending damage is evident. One cell type, microglia, was an obvious candidate for so many apparently divergent genes.
Microglia are the primary antigen-presenting cells in the central nervous system (CNS1). These immunelike cells (Streit 2002) can be activated in response to injury, disease, or inflammation, leading to the secretion of a variety of factors such as proinflammatory cytokines, prostaglandins, and ROS/RNS, each of which can cause neuronal damage (Hanisch 2002). Activated microglia produce virtually all reactants that are essential for the toxicity associated with neurotoxic amphetamines, including nitric oxide and superoxide (Cadet et al. 2003; Davidson et al. 2001; Lyles and Cadet 2003). Many of the transcription factors (e.g., NFκB, c-Fos, AP-1) that are upregulated by Meth (Cadet et al. 2003; Davidson et al. 2001; Lyles and Cadet 2003) are also required for microglial activation (Nguyen et al. 2002; Pocock and Liddle 2001; Wyss-Coray and Mucke 2002).
Evidence of microglial involvement in amphetamine neurotoxicity has emerged at a rapid pace (Escubedo et al. 1998; Guilarte et al. 2003; LaVoie et al. 2004; Orio et al. 2004; Pubill et al. 2002, 2003), but the nature of that involvement is not clear. When Bowyer and colleagues (1994) first noted that Meth caused microglial activation, they surmised that microglia were not causing or mediating Meth damage but reacting to it. Therefore, an important question arises: Do microglia cause nerve ending damage related to Meth, or do they just react to it? The following points suggest that microglia contribute to drug-induced neurotoxicity:
Numerous genes related to microglia are upregulated in striatum within hours of initiation of a neurotoxic regimen of Meth, well before signs of nerve ending toxicity emerge (Thomas et al. 2004a).
The time course of microglial activation after Meth administration precedes the development of toxicity (Lavoie et al. 2004; Thomas et al. 2004b).
Baucum and colleagues (2004) showed that Meth causes the formation of high molecular weight complexes of the DAT, an effect that is directly related to drug toxicity and that peaks 24 to 48 hours after treatment, the same time course over which Meth causes microglial activation.
The neurotoxic human immunodeficiency virus (HIV) Tat protein interacts synergistically with Meth and causes greater toxicity in mice than either agent alone (Cass et al. 2003; Maragos et al. 2002; Theodore et al. 2006a). HIV-positive individuals who abuse Meth suffer greater neuropathology than nonabusers (Langford et al. 2003). It is well known that the only cells in the brain that are productively infected with the AIDS virus are microglia (Morner et al. 2003) and it is also widely accepted that infection and activation of microglial cells play major roles in HIV-induced neuropathology (Gras et al. 2003).
Histopathological examination of brains from Meth-treated animals has actually provided the most compelling evidence that microglial activation (which peaks at 2 days post-Meth) precedes DA terminal degeneration. Ricaurte and colleagues (1982) showed that fine granular degeneration, which was not present 2 days after high-dose Meth treatment, peaked 4 days after treatment. Fluoro-Jade labeling of damaged neurons in the striatum also is maximal 3 to 5 days after Meth treatment (Schmued and Bowyer 1997; Yu et al. 2004). Evidence supporting microglial activation in the neurochemical deficits observed in human users is also accumulating (Sekine et al. 2008; Theodore et al. 2006b).
Despite findings that indicate an association between methamphetamine-induced toxicity in the striatum and microglial activation, attempts to interrupt the activation and prevent neurotoxicity have suggested that the relationship is complex. For instance, O’Callaghan and colleagues have shown that minocycline, a well-known inhibitor of microglial activation (Tikka et al. 2001), suppresses proinflammatory cytokine expression after Meth but does not prevent indices of DA nerve terminal damage (Sriram et al. 2006). Minocycline has also been shown to reduce microglial activation in the substantia nigra after Meth treatment but it did not attenuate Meth-induced reductions in tyrosine hydroxylase (Boger et al. 2009). These studies showed partially suppressive effects of minocycline on inflammatory processes but even this partial reduction did not correspond to any protection against Meth neurotoxicity. Together, these interesting studies suggest that Meth-induced neurotoxicity is not mediated by microglial activation.
Another recent study has shown that Meth-induced inflammation and hippocampal neuronal dysfunction are preventable by treatment of animals with the anti-inflammatory agent indomethacin (Gonçalves et al. 2010). Likewise, MK801 and dextromethorphan prevent microglial activation and DA nerve terminal damage caused by Meth (Thomas and Kuhn 2005). Results from these studies suggest that neuroprotection against Meth-induced damage may be dependent on the anti-inflammatory agent used and call for tests of a broader variety of protective drugs.
Astrocytes
Changes in astrocyte expression of glial fibrillary acidic protein (GFAP) have been used very effectively as an index of Meth-induced neurotoxicity (O’Callaghan and Miller 1993, 1994; O’Callaghan 1998; Pu and Vorhees 1995). However, a role for astrocytes as direct participants in Meth neurotoxicity has received only limited attention. This is disappointing in light of the many important roles of astrocytes in normal and pathological conditions in the brain (Buffo et al. 2010) as well as their ability to modulate neuronal activity and behavior through the release of the gliotransmitters ATP, d-serine, and glutamate (Halassa and Haydon 2010). One study has shown recently that the antiepileptic drug zonisamide is neuroprotective in a cellular model of Parkinson’s disease by increasing astrocyte levels of glutathione (Asanuma et al. 2010). Glutamate-mediated excitotoxicity is one of the major mechanisms by which Meth and the other neurotoxic amphetamines damage the CNS (Mark et al. 2004; Yamamoto and Bankson 2005; Yamamoto et al. 1998), and the cells that mediate glutamate release and uptake in brain areas showing damage after Meth intoxication could well be astrocytes.
Neuronal, Microglial, and Astrocyte Cross Talk and Meth Toxicity
Clearly, there is considerable cross talk between distressed neurons and microglia to fuel the very complex process of neuronal damage (Bruce-Keller 1999; Kerschensteiner et al. 2003; Polazzi and Contestabile 2002). It is also becoming increasingly evident that astrocytes form an essential part of the synapse along with the pre- and postsynaptic terminals and can exert profound influence on synaptic function (Halassa and Haydon 2010). When considering the close interplay among nerve endings, astrocytes, and microglia in brain areas targeted for damage by Meth (e.g., the caudate-putamen [CPu1], cortex, hippocampus) or in areas that are resistant to Meth (e.g., the nucleus accumbens [NAc1], substantia nigra, pars compacta [SNc1], see below), it may prove difficult to answer the foregoing rhetorical question of whether nonneuronal cells cause Meth neurotoxicity or react to it. The most likely scenario probably involves extensive cross talk among all of these cell types after Meth insult.
It could be that Meth, through its ability to cause such extensive disruption of DA homeostasis in nerve endings, leads to the formation of reactants that serve as false-positive distress signals. These signals could provoke changes in microglial cells and astrocytes that result in the secretion of excitatory and inflammatory species that are damaging to the nerve ending. What begins as a false distress signal from nerve endings in response to Meth intoxication leads to an “inappropriate” local response by nonneuronal cells to cause actual neuronal damage. The extent of nerve ending damage that results from Meth intake is therefore determined by the interplay among the nerve terminal and resident microglia and astrocytes. The pattern and extent of this cross talk could be brain-region specific and could indicate that Meth causes neurotoxicity via different mechanisms depending on the balance of this drug-induced communication among nerve terminals, microglia, and astrocytes, on the one hand, and, on the other, the resulting production of ROS, RNS, inflammatory and excitatory species, and neurotrophic factors.
Brain-Region Specificity of Meth Neurotoxicity: Nerve Endings versus Cell Body Damage
Caudate-Putamen
The neurotoxicity of Meth is remarkable in that ventral and lateral aspects of the CPu show greater damage (Eisch et al. 1992; Fukui et al. 1986; Harvey et al. 2000a; Hirata et al. 1996) and more microglial activation (Thomas et al. 2004b) than medial aspects. This gradient of susceptibility to amphetamine-induced toxicity is probably related to the heterogeneous distribution of DA uptake and release sites in the CPu and could also be influenced by local production of neurotrophic factors such as pleiotrophin. The importance of these elements of the DA neuronal system to Meth neurotoxicity is discussed below.
Nucleus Accumbens
DA nerve endings in the NAc are much more resistant to the damaging effects of Meth in comparison to the CPu. Meth-induced reductions in NAc DA levels are 20–40% of those in the CPu (Davidson et al. 2007; Morgan and Gibb 1980; O’Dell et al. 1991; Sabol et al. 2001; Wallace et al. 2001), and DAT binding sites (Eisch et al. 1992, 1996) and TH activity (Haughey et al. 1999) are not changed in the NAc. Furthermore, the Meth-induced formation of high molecular weight DAT complexes in the CPu (Baucum et al. 2004) does not occur in the NAc (Hadlock et al. 2009), and a non-toxic Meth dosing regimen that induces behavioral sensitization lowers DAT density in the CPu but not in the NAc (Bjorklund et al. 2008). The evoked release of DA is also reduced in the CPu (but not in the NAc) by prior exposure to a neurotoxic regimen of Meth (Cass 1997).
In contrast to these results showing greater sensitivity to Meth toxicity in the CPu versus the NAc, Broening and colleagues (1997) found that Meth reduces TH immunoreactivity in the core of the NAc, and Pereira and colleagues (2006) reported that it does not reduce CPu TH levels. The mechanisms determining the remarkably heterogeneous pattern of Meth-induced neurotoxicity are not understood, but brain-regional differences in expression of the DAT and VMAT, and the manner in which they interact with Meth to cause DA release, may play a role (Volz et al. 2007).
The differential effect of Meth on tyrosine hydroxylase loss in the NAc core while sparing the shell is notable (Broening et al. 1997) and suggests that the sensitivity of the NAc to damage is dependent on the species and doses used. We have not noted differential core versus shell effects in response to a Meth neurotoxic regimen in our studies of NAc in mice (Thomas et al. 2009), and the use of lower doses (i.e., 4 injections of 5 mg/kg) is probably responsible. The studies of Vorhees and colleagues (Broening et al. 1997) used higher doses of Meth (4 injections of 10 mg/kg) in rats and it is generally observed that mice show smaller responses in this regard by comparison to rats.
Substantia Nigra
Contrasting results have been published concerning the impacts of Meth on DA-containing neurons of the SNc and the ventral tegmental area (VTA). Some studies have reported that Meth causes loss of TH-containing neurons of the SNc (Brown et al. 2006; Sonsalla et al. 1996), whereas others have shown no losses of either TH (Boger et al. 2007; Theodore et al. 2006a; Thomas et al. 2009) or DAT (Brunswick et al. 1992) and few, if any, signs of neuronal degeneration or gliosis (O’Callaghan and Miller 1994; Sriram et al. 2006). In vervet monkeys, nigrostriatal DA deficits caused by Meth recover without SNc cell loss (Harvey et al. 2000b).
Hippocampus and Cortex
Meth-induced neurotoxicity is by no means limited to brain areas rich in DA nerve terminals. In fact, significant damage and apoptosis (Deng et al. 2001) are widespread in the CNS and also seen in brain areas that are important in learning and memory, including the hippocampus and frontal cortex, both of which show Meth-induced neuroinflammation and increased gliosis (Gonçalves et al. 2010) and neuronal degeneration (Eisch et al. 1998; Kuczenski et al. 2007). As a result of Meth-induced neurotoxicity in these brain regions, substantial deficits in recognition memory have been uncovered by Marshall and colleagues (Belcher et al. 2008; Izquierdo et al. 2010; O’Dell et al. 2010; Schroder et al. 2003), and Vorhees and colleagues have detailed impairments in path integration and novel object recognition (Herring et al. 2008, 2010; Vorhees et al. 2010). Results from these animal models have been extended to abstinent human Meth users, who exhibit persistent cognitive deficits (McCann et al. 2008).
Role of Dopamine, Serotonin, and Norepinephrine in Meth Neurotoxicity
Monoamine neurotransmitters endogenous to the striatum (i.e., DA, serotonin, and norepinephrine) can profoundly influence the neurotoxicity associated with Meth. Not only does Meth cause a dramatic change in the homeostatic mechanisms that normally regulate these neurotransmitters in nerve endings (i.e., it alters their synthesis, storage, and release), it can also create additional neurotoxic species via reaction of the transmitters with ROS and RNS generated locally by this drug. For this reason, a brief description of how the monoamine neurotransmitters of the striatum interact with Meth to influence its neurotoxic actions is appropriate.
Dopamine
DA has long been implicated as a key factor in Meth neurotoxicity. Wagner and colleagues (1983) first showed that inhibition of tyrosine hydroxylase (TH) with α-methyl-p-tyrosine prevented Meth-induced neurotoxicity. In contrast, reserpine, which disrupts vesicle storage of DA while leaving the Meth-releasable pool intact, enhances its neurotoxicity (Albers and Sonsalla 1995; Thomas et al. 2008; Wagner et al. 1983). The combined effects of Meth—causing DA release and blocking its reuptake—expose extracellular DA to ROS-induced nonenzymatic degradation (Cadet et al. 2007; Yamamoto and Bankson 2005). Oxidant attack on DA leads to the formation of DA quinone (Graham 1978; Nappi and Vass 2001), a redox-active species that is implicated in Meth-induced toxicity (LaVoie and Hastings 1999) and that alters several of the same critical proteins inhibited by Meth, such as TH (Kuhn et al. 1999) and the DAT (Park et al. 2002; Whitehead et al. 2001). DA quinone also causes microglial activation (Kuhn et al. 2006; Le et al. 2001; Thomas et al. 2006), an effect that is emerging as an important element of Meth toxicity. The ability of reserpine, which reduces steady-state DA to near zero, to increase Meth toxicity indicates that Meth need mobilize only a very small pool of cytoplasmic DA to exert damaging effects.
A role for DA in mediating Meth-induced toxicity has been challenged by studies showing that changes in core body temperature (Yuan et al. 2001, 2010), more than alterations in intracellular DA status, can explain modifications in Meth neurotoxicity.
Serotonin
Meth disrupts striatal serotonin (5HT1) homeostasis in much the same manner as is seen for DA. Although an essential role for endogenous DA in Meth-induced toxicity to the DA system has been established (see above), a similar role for 5HT has scarcely been studied. The powerful release of 5HT from vesicles into the cytoplasm, and the synapse under conditions of heightened oxidative stress caused by Meth, would expose the neurotransmitter to a variety of reactants (e.g., superoxide, hydroxyl radical, nitric oxide, peroxynitrite) that could lead to the local production of numerous 5HT-derived neurotoxins (Wrona and Dryhurst 1988, 1998, 2001; Wrona et al. 1995, 1997). 5HT itself can even bind to nitric oxide synthase and increase its production of oxygen-based radicals (Breard and Grillon 2009; Breard et al. 2007). However, a series of experiments that tested 5HT for a role in Meth toxicity were overwhelmingly negative. Neither increases in neuronal 5HT levels with injections of its precursor 5-hydroxy-tryptophan nor partial reductions in 5HT content with parachlorophenylalanine had any effect on Meth toxicity (Thomas et al. 2010). In fact, mice with a null mutation in the gene for tryptophan hydroxylase 2 totally lack brain 5HT and responded to a neurotoxic dosing regimen of Meth in a manner that was identical to controls (Thomas et al. 2010).
Norepinephrine
A role for endogenous norepinephrine (NE) in the toxic actions of Meth on striatal DA nerve endings has also been indicated and derives from the general neuroprotective and antioxidant properties attributed to this monoamine (Heneka et al. 2002, 2003; Kalinin et al. 2006). Fornai and colleagues have shown that conditions associated with reduced striatal NE lead to substantial increases in drug-induced toxicity to DA nerve endings. These conditions include treatment of mice and rats with DSP-4, a selective NE neurotoxin (Fornai et al. 1995, 1996, 1999); ablation of the gene for dopamine β-hydroxylase (DBH); and inhibition of NE synthesis with the DBH inhibitor fusaric acid (Weinshenker et al. 2008). The input of NE-containing nerve endings to the striatum is very small by comparison to DA and 5HT, at levels that are 2–5% of the other monoamines, and DBH levels in striatum are among the lowest in the CNS (Ross and Reis 1974). DSP-4 lesions of the locus coeruleus reduce striatal NE levels by about 30% (Fornai et al. 1999), indicating an extremely powerful influence of NE on the sensitivity of DA nerve endings to Meth-induced toxicity.
Changes in the Nucleus Accumbens in Response to Meth Neurotoxicity
The NAc plays critical roles in reward mechanisms and decision making. It is at the center of a neuroanatomical locus that receives glutamate input from the medial prefrontal cortex, hippocampus, and basolateral amygdala as well as substantial DA input from the VTA. In turn, there are axonal projections from the NAc to important motor areas where reward information is translated into motivated actions (Day et al. 2007). The control of DA and glutamate release in the NAc is an essential determinant in the addictive actions of numerous drugs of abuse (Di Chiara and Imperato 1988; Kalivas 2009). Therefore, the NAc is important in Meth addiction and the reinstatement of Meth seeking after withdrawal (Rocha and Kalivas 2010), both of which may be reflective of the large role of the NAc in regulating impulsivity and response inhibition (Basar et al. 2010). Paradoxically, Meth-induced damage to DA nerve endings of the NAc might also have an effect on the rewarding or addictive properties of Meth or other abused drugs such as cocaine.
Effects on Dopamine Levels
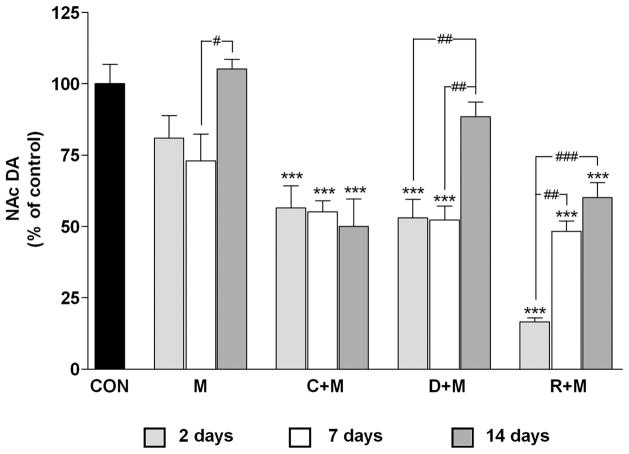
In light of the general finding that the NAc is highly resistant to the damaging effects of Meth (see above), we attempted to determine whether changes in DA homeostasis would alter the response of the NAc to a neurotoxic regimen of Meth, much as is seen in the dorsal striatum (Thomas et al. 2008). The effects of Meth on NAc DA levels are presented in Figure 1. Meth alone caused only a minor and nonsignificant reduction (~15–20%) 2 days after treatment. This result contrasts sharply with the effects of the same Meth treatment regimen in the CPu, where DA reductions reach 65–75% over the same time course (Thomas et al. 2008). The effects of Meth on NAc DA showed a time-dependent response, and these results are also presented in Figure 1. It can be seen that Meth itself caused a slight but significant reduction in DA levels (to 73% of control) at day 7 and this response recovered to control levels by day 14.
Figure 1.
Effects of a neurotoxic methamphetamine (Meth) regimen on dopamine (DA) depletion in the nucleus accumbens (NAc) when administered alone and in conjunction with clorgyline, L-DOPA, or reserpine. Mice (n = 5–8 per group) were treated with Meth alone (M: 4 × 5 mg/kg; 2 hr between injections) or in conjunction with clorgyline (C + M: 10 mg/kg; t = −1 h), L-DOPA (D + M: 50 mg/kg, t = −1 and 3 hr), or reserpine (R + M: 2.5 mg/kg; t = −24 h). NAc DA levels were measured 2, 7, and 14 days post-Meth. Results are presented as means ± SEM relative to controls (CON). Significant differences were determined via one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, and are indicated as follows: **, p < 0.01, and ***, p < 0.001 relative to control; #, p < 0.05, ##, p < 0.01, and ###, p < 0.001 indicate significant differences between indicated treatment conditions. Reprinted with permission from Thomas et al. (2009).
Drugs that increase the cytoplasmic (or Meth-releasable) pool of DA significantly enhance Meth-induced neurotoxicity and microglial activation in the CPu (Thomas et al. 2008). Therefore, the effects of L-DOPA1 (L-3,4-dihydroxypheny-lalanine, the immediate precursor to DA), clorgyline (an irreversible monoamine oxidase inhibitor that prevents DA catabolism), and reserpine on DA levels in the NAc were tested to determine whether Meth neurotoxicity is extended anatomically under these conditions as well. Each treatment significantly potentiated the effects of Meth 2 days after treatment (Figure 1). Clorgyline or L-DOPA in combination with Meth depleted NAc DA by almost 50%, and reserpine + Meth depleted it by more than 80%.
The enhancement of Meth toxicity caused by L-DOPA, clorgyline, and reserpine showed differential recoveries as well. By day 7, NAc DA levels in mice treated with clor-gyline + Meth or L-DOPA + Meth remained about the same as at day 2 (Figure 1) and each was significantly different from control. By day 14, the L-DOPA + Meth group showed near-total recovery (88%) to control DA levels, whereas the clorgyline + Meth group did not show any recovery over the 2 to 14 days. DA levels in mice treated with reserpine + Meth recovered to almost 50% at day 7 and to about 60% by day 14.
Effects on Tyrosine Hydroxylase Levels
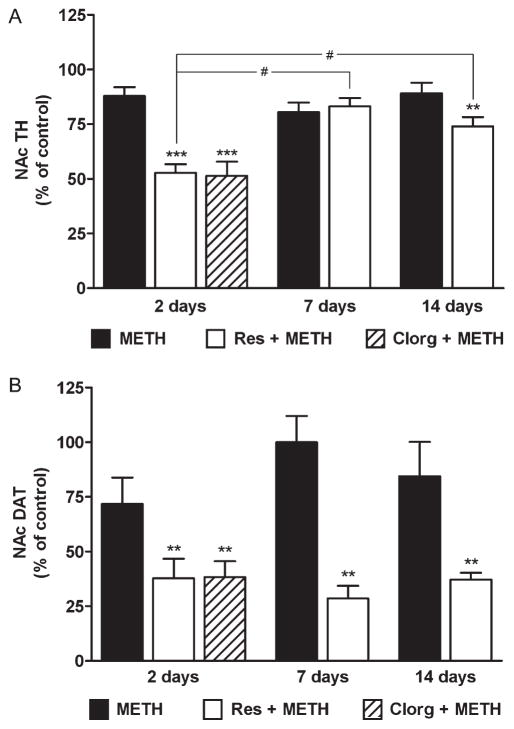
In view of the response of the NAc DA system to Meth by comparison to that of the CPu, it was important to confirm the effects of alterations in cytoplasmic DA on drug toxicity with the use of other markers for DA nerve ending status. Therefore, we measured TH and DAT protein levels in NAc but limited the analysis to groups treated with Meth, reserpine + Meth, or clorgyline + Meth because the drug combinations caused the most persistent enhancement of Meth-induced depletion of DA2 (Figure 1; mice treated with clorgyline + Meth were studied only at the 2d time point because of the lack of recovery after this treatment).
The results in Figure 2A show that Meth alone caused a slight reduction (~15%) in NAc TH content at day 2. The level fell slightly by day 7 and returned to about 90% of control level by day 14. Although these effects trended toward reductions, they did not reach statistical significance. In contrast, the combined treatment of mice with reserpine + Meth resulted in a much greater reduction in NAc TH—approximately 50% at day 2 (Figure 2A), although TH expression recovered between days 7 and 14 to about 70% of control. Thus the time-dependent recovery of TH in the reserpine + Meth group was significant. The effect of clorgyline + Meth on NAc TH 2 days after treatment was the same as that of reserpine + Meth, reducing TH by about 50% (Figure 2A).
Figure 2.
Effects of reserpine or clorgyline on levels of (A) tyrosine hydroxylase (TH) and (B) dopamine transporter (DAT) in the nucleus accumbens (NAc) of mice treated with a neurotoxic methamphetamine (Meth) regimen. Mice (n = 5–8 per group) were treated with Meth alone, reserpine (Res) + Meth, or clorgyline (Clorg) + Meth as described in the text. NAc TH and DAT protein levels were determined by western blot analysis at the indicated times post-Meth and are presented as means ± SEM relative to control. Mice treated with clorgyline + Meth were tested only at the 2d time point. Significant differences were determined via one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test: **, p < 0.01, and ***, p < 0.001 relative to control; #, p < 0.05 indicates significant differences between indicated treatment conditions. Reprinted with permission from Thomas et al. (2009).
The effects of reserpine or clorgyline in combination with Meth on DAT levels in the NAc are shown in Figure 2B. As with TH, Meth alone did not significantly reduce DAT, notwithstanding a decline at the 2-day time point. The combined treatment of reserpine + Meth caused a significant reduction at days 2 (37% of control), 7 (29%), and 14 (35%). Clorgyline + Meth reduced NAc DAT levels at day 2 to the same extent as reserpine (35% of control). NAc DAT levels did not recover as was seen for TH after the same treatments. By comparison to the NAc, treatments that increase the Meth-reactive pool of DA in the nerve terminal did not cause losses in DA cell bodies of the SNc (Thomas et al. 2009).
Effects on Microglial Activation
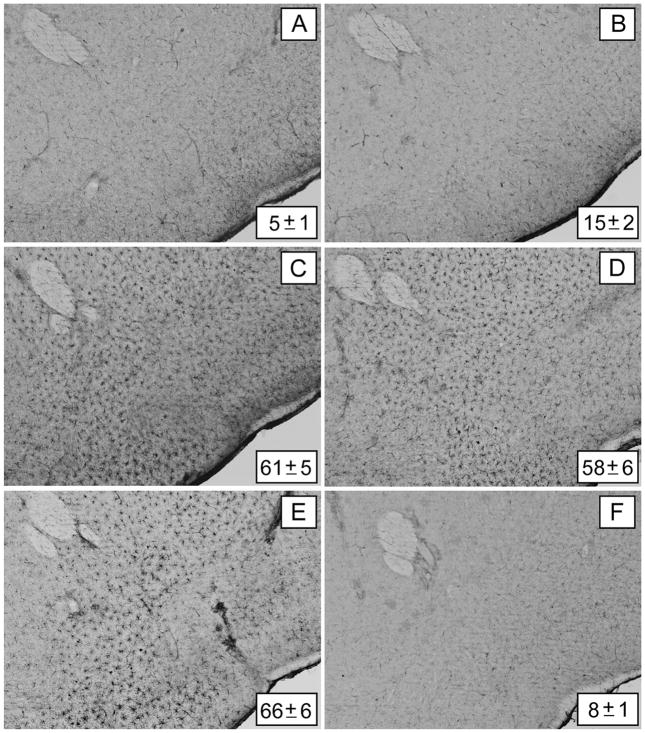
Microglial activation in the NAc after Meth treatment showed a very different pattern in comparison to the CPu, where robust response occurs within 1 or 2 days (Thomas et al. 2004b). Meth treatment alone (Figure 3B) did not change NAc microglial status from control levels (Figure 3A). However, when clorgyline (Figure 3C), L-DOPA (Figure 3D), or reserpine (Figure 3E) was combined with Meth, extensive microglial activation was evident in the NAc at day 2. The increases in microglial activation were significantly different from controls and from Meth alone, but not from each other. In agreement with previous results (Thomas et al. 2004b), the activation subsided to control levels by days 7 and 14 (data not shown). As with the effects on DA and TH levels, treatment with L-DOPA, clorgyline, or reserpine without Meth did not cause changes in NAc microglial activation; a representative image from mice treated with reserpine alone is included in Figure 3F.
Figure 3.
Effects of clorgyline, L-DOPA, and reserpine on microglial activation in the nucleus accumbens (NAc) caused by a neurotoxic methamphetamine (Meth) regimen. Mice (n = 3–5 per group) were treated as described in the text and analyzed for microglial activation in the NAc 48 h after the last Meth injection. Microglia counts are presented as means ± SEM. Treatment conditions and microglia counts for each panel are (A) control (5 ± 1), (B) Meth (15 ± 2), (C) clorgyline + Meth (61 ± 5), (D) L-DOPA + Meth (58 ± 6), (E) reserpine + Meth (66 ± 6), and (F) reserpine only (8 ± 1). Significant differences were determined via one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test: p < 0.001 for clorgyline + Meth, L-DOPA + Meth, and reserpine + Meth relative to control. No significant differences were determined for Meth or reserpine only relative to control (p > 0.05). Reprinted with permission from Thomas et al. (2009). The color image is available in the online posting of this article at www.ilarjournal.com.
Figure 3 also shows that the enhancement of Meth-induced microglial activation caused by L-DOPA, clorgyline, and reserpine extended in a ventral direction beyond the NAc and was evident in areas where DA fibers of passage in the median forebrain bundle and the olfactory tubercle traverse at the level of the NAc. These effects did not occur in control mice or in mice treated with Meth alone (Thomas et al. 2009). In a series of very elegant experiments, Bonci and colleagues established that DA terminals in the NAc, but not in the dorsal striatum, corelease glutamate (Stuber et al. 2010). Although the Meth-induced release of DA from nerve terminals is not thought to be exocytotic (i.e., it does not involve depolarization and calcium-mediated release from synaptic vesicles), it is still possible that it could result in glutamate release along with that of DA.
Effects of Kainic Acid versus Meth
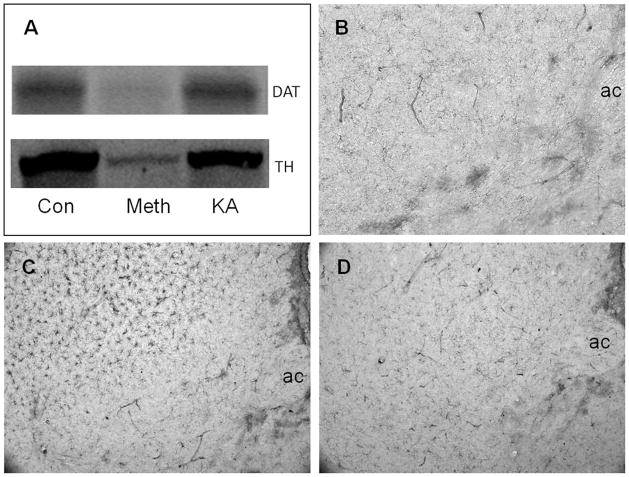
In an attempt to test the possibility that glutamate release could cause Meth-like effects in the NAc, mice were treated with kainic acid (25 mg/kg) to simulate hyperglutamate conditions in the brain. The area of the NAc and surrounding striatum was tested for signs of toxicity to DA terminals as seen after Meth administration (reductions in DA and DAT) and for microglial activation. Kainic acid did not alter the levels of DAT or TH (Figure 4A) as measured by immunoblotting nor did it result in microglial activation in the NAc (Figure 4D) using histochemical staining. By contrast, Meth caused significant reductions in DAT and TH (Figure 4A) and resulted in extensive microglial activation in the CPu, but not in the NAc (Figure 4C), in agreement with our previous study (Thomas et al. 2009).
Figure 4.
Effects of kainic acid (KA) on dopamine (DA) nerve terminals and microglial activation. Mice (n = 3–5 per group) were injected intraperitoneally with kainic acid (25 mg/kg) or with a neurotoxic regimen of methamphetamine (Meth) (4 × 5 mg/kg, 2 hr between injections) and analyzed 48 hr after treatment for expression levels of the dopamine transporter (DAT) and tyrosine hydroxylase (TH) and for microglial activation. Levels of DAT and TH were determined in combined caudate-putamen/nucleus accumbens (CPu/NAc) samples by western blot analysis (A). Microglial activation was determined by histochemical staining using Isolectin B4. The anterior commissure (ac) is labeled for orientation. Very little microglial activation is seen in the NAc or CPu of control mice (B) whereas Meth treatment causes extensive microglial activation in the CPu but not in the NAc (C). Kainic acid does not lead to microglial activation in either the NAc or CPu (D). Con, control. The color image is available in the online posting of this article at www.ilarjournal.com.
These findings with kainic acid are highly reminiscent of results published by O’Dell and colleagues (1994) showing that excitotoxic lesions with quinolinic acid do not cause damage to the DA nerve terminals of the striatum. These investigators also made the very interesting observation that prior excitotoxic lesions protected against subsequent Meth-induced DA depletion, an effect attributed in part to alterations in local glial function (O’Dell et al. 1994). The doses of kainic acid used in our study were convulsant but these effects did not last as long (1–2 hr) as the normal psychostimulant and hyperthermic effects of Meth (8–10 hr) and could offer an explanation for the failure to see damage to DA terminals of the NAc after treatment of mice with the excitotoxin.
Comparison of Effects in the NAc and the Striatum
The insensitivity of the NAc to Meth-induced damage to intrinsic DA terminals in this brain region is hard to explain when considering the status of the DA neuronal system in this area versus the striatum. After all, the static levels of DA in these two regions are not appreciably different, yet their responses to Meth are. Clues to a better understanding of this disparity may lie in subtle differences in DA neurochemistry between the NAc and striatum. For instance,
the density and activity of the DAT are lower in the NAc than in the striatum (McElvain and Schenk 1992; Meiergerd and Schenk 1994; Povlock and Schenk 1997);
Meth-induced inhibition of the VMAT in the striatum (Brown et al. 2000) is greater than in the NAc (Volz et al. 2007); and
amphetamine-induced release of DA is less in the NAc than in the striatum (Cass 1997; Hernandez et al. 1987).
Thus it appears that Meth-induced disruptions in presynaptic homeostasis are far less drastic in the NAc than in the striatum, resulting in lower net release of DA after a neurotoxic Meth regimen. By extension, increases in the Meth-releasable pool of DA in the NAc, as discussed above, would result in the emergence of toxicity in this critical brain area.
Conclusions
Animal models of Meth-induced neurotoxicity have added substantially to understanding of how this powerful and addictive drug of abuse can alter brain function. In this overview we have highlighted research that has focused on brain regionally specific effects of Meth as a neurotoxin and activator of gliosis, with emphasis on how endogenous neurotransmitters can influence the course of its damaging effects.
Nonneuronal cells are emerging as important modulators of Meth neurotoxicity probably because they become involved in a complex cross talk with DA nerve terminals soon after Meth intoxication. The consequences of Meth neurotoxicity also reflect the widespread expression of its damaging properties. Cortical and hippocampal degeneration have been linked to deficits in learning and memory. The NAc is privileged in that it is highly resistant to Meth-induced neurotoxicity by comparison to the CPu, and this resistance is fortunate considering the NAc’s very important roles in reward and executive function as well as in the addictive properties of many abused drugs. However, it is becoming clear that even small changes in the disposition of DA in nerve terminals of the NAc can increase the susceptibility of this structure to Meth-induced damage.
It may also be the case that neurotrophic factors such as pleiotrophin play a role in NAc resistance to Meth neurotoxicity. Preclinical studies reveal that expression of this interesting cytokine increases after dosing with amphetamine (Le Greves 2005), and mice lacking pleiotrophin show enhanced amphetamine neurotoxicity in the striatum and damage in the SNc (Gramage et al. 2010), effects that are somewhat reminiscent of the manner in which alterations in DA homeostasis can alter NAc sensitivity to Meth.
The value of animal models in the study of substituted amphetamine neurotoxicity can be appreciated when considering the rapid growth in the study of human abusers of these dangerous drugs. More than two decades of study using animal models of Meth-induced neurotoxicity have contributed to a much better understanding of both the nature of brain damage caused by this drug and the mechanisms underlying its neurotoxicity. Furthermore, research on the neurotoxic amphetamines using animal models now has numerous parallel studies on Meth-induced neuronal abnormalities (Berman et al. 2008; Chang et al. 2007; McCann et al. 2008; Schwartz et al. 2010; Thompson et al. 2004; Tobias et al. 2010) and glial activation (Sekine et al. 2008; Theodore et al. 2006b) in human abusers. These emerging studies have also resulted in growing interest in the development of new pharmacotherapies for treating Meth addiction and restoring neuronal function in individuals with persistent behavioral and neuronal deficits (De La Garza et al. 2010; Zorick et al. 2009).
Animal models not only have contributed significantly to a better understanding of the causes and consequences of Meth neurotoxicity but also continue to inform clinical researchers about prevention strategies and more effective treatments for neurotoxic drugs of abuse. Future studies can usefully focus on how Meth may suppress endogenous protective factors (e.g., pleiotrophin) to exert its neurotoxic properties.
Acknowledgments
The research described in this article was supported by National Institutes of Health grants (DA020680 and DA010756, Thomas; and DA017327, Kuhn) and by the Department of Veterans Affairs (Kuhn and Thomas). We are indebted to Dr. Roxanne A. Vaughan for providing DAT antibodies. Acknowledgment is also extended to the Journal of Neurochemistry, the International Society for Neurochemistry, and Blackwell Publishing for granting permission to reuse figures from our published article.
Footnotes
Abbreviations that appear ≥3x throughout this article: CNS, central nervous system; CPu, caudate-putamen; DA, dopamine; DAT, dopamine transporter; L-DOPA, L-3,4-dihydroxyphenylalanine; Meth, methamphetamine; NAc, nucleus accumbens; RNS, reactive nitrogen species; ROS, reactive oxygen species; 5HT, serotonin; SNc, substantia nigra, pars compacta; TH, tyrosine hydroxylase; VMAT, vesicle monoamine transporter
Neither reserpine nor clorgyline alone changed the expression of either TH or DAT in the NAc at any time point (data not shown).
Contributor Information
Donald M. Kuhn, Professor in the Department of Psychiatry and Behavioral Neurosciences at Wayne State University School of Medicine, and a Research Career Scientist in the Research and Development Service at the John D. Dingell VA Medical Center in Detroit, Michigan.
Mariana Angoa-Pérez, Postdoctoral fellow in the Department of Psychiatry and Behavioral Neurosciences at Wayne State University School of Medicine.
David M. Thomas, Assistant professor in the Department of Pharmaceutical Sciences, Eugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, and a research scientist at the John D. Dingell VA Medical Center.
References
- Abekawa T, Ohmori T, Honda M, Ito K, Koyama T. Effect of low doses of L-NAME on methamphetamine-induced dopaminergic depletion in the rat striatum. J Neural Transm. 2001;108:1219–1230. doi: 10.1007/s007020100000. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: Pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Ali SF, Itzhak Y. Effects of 7-nitroindazole, an NOS inhibitor on methamphetamine-induced dopaminergic and serotonergic neurotoxicity in mice. Ann N Y Acad Sci. 1998;844:122–130. [PubMed] [Google Scholar]
- Asanuma M, Hayashi T, Ordonez SV, Ogawa N, Cadet JL. Direct interactions of methamphetamine with the nucleus. Brain Res Mol Brain Res. 2000;80:237–243. doi: 10.1016/s0169-328x(00)00128-5. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, Miyoshi K, Murata M. Neuroprotective effects of zonisamide target astrocyte. Ann Neurol. 2010;67:239–249. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci. 2004;24:3436–3443. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: Comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund NL, Sorg BA, Schenk JO. Neuronal dopamine transporter activity, density and methamphetamine inhibition are differentially altered in the nucleus accumbens and striatum with no changes in glycosylation in rats behaviorally sensitized to methamphetamine. Synapse. 2008;62:736–745. doi: 10.1002/syn.20528. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Granholm AC, McGinty JF. Minocycline restores striatal tyrosine hydroxylase in GDNF heterozygous mice but not in methamphetamine-treated mice. Neurobiol Dis. 2009;33:459–466. doi: 10.1016/j.nbd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Breard M, Grillon C. Serotonin binds to purified neuronal nitric oxide synthase: A possible explanation for ROS production induced by 5HT in the presence of nNOS. Free Radic Res. 2009;43:206–213. doi: 10.1080/10715760802676662. [DOI] [PubMed] [Google Scholar]
- Breard M, Sari MA, Frapart Y, Boucher JL, Ducrocq C, Grillon C. The endogenous neurotransmitter, serotonin, modifies neuronal nitric oxide synthase activities. Free Radic Res. 2007;41:413–423. doi: 10.1080/10715760601105681. [DOI] [PubMed] [Google Scholar]
- Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem. 2000;74:2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- Brown JM, Gouty S, Iyer V, Rosenberger J, Cox BM. Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J Neurochem. 2006;98:495–505. doi: 10.1111/j.1471-4159.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ. Microglial-neuronal interactions in synaptic damage and recovery. J Neurosci Res. 1999;58:191–201. doi: 10.1002/(sici)1097-4547(19991001)58:1<191::aid-jnr17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Brunswick DJ, Benmansour S, Tejani-Butt SM, Hauptmann M. Effects of high-dose methamphetamine on monoamine uptake sites in rat brain measured by quantitative autoradiography. Synapse. 1992;11:287–293. doi: 10.1002/syn.890110404. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: Molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010;79:77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur J Pharmacol. 2000a;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Nixdorf WL, Yamamoto BK. Central administration of methamphetamine synergizes with metabolic inhibition to deplete striatal monoamines. J Pharmacol Exp Ther. 2000b;292:853–860. [PubMed] [Google Scholar]
- Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: Cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: Molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007; 102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Davidson C, Chen Q, Zhang X, Xiong X, Lazarus C, Lee TH, Ellinwood EH. Deprenyl treatment attenuates long-term pre- and post-synaptic changes evoked by chronic methamphetamine. Eur J Pharmacol. 2007;573:100–110. doi: 10.1016/j.ejphar.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2010;106:173–180. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Cadet JL. Methamphetamine administration causes overexpression of nNOS in the mouse striatum. Brain Res. 1999;851:254–257. doi: 10.1016/s0006-8993(99)02087-9. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: Evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O’Dell SJ, Marshall JF. Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res. 1992;598:321–326. doi: 10.1016/0006-8993(92)90201-j. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, O’Dell SJ, Marshall JF. Striatal and cortical NMDA receptors are altered by a neurotoxic regimen of methamphetamine. Synapse. 1996;22:217–225. doi: 10.1002/(SICI)1098-2396(199603)22:3<217::AID-SYN3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with Fluoro-Jade labeling after a neurotoxic regimen of methamphetamine. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jimenez A, Pubill D, Pallas M, Camins A, Camarasa J. Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain Res. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: Pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fornai F, Bassi L, Torracca MT, Scalori V, Corsini GU. Norepinephrine loss exacerbates methamphetamine-induced striatal dopamine depletion in mice. Eur J Pharmacol. 1995;283:99–102. doi: 10.1016/0014-2999(95)00313-a. [DOI] [PubMed] [Google Scholar]
- Fornai F, Torracca MT, Bassi L, D’Errigo DA, Scalori V, Corsini GU. Norepinephrine loss selectively enhances chronic nigrostriatal dopamine depletion in mice and rats. Brain Res. 1996;735:349–353. doi: 10.1016/0006-8993(96)00891-8. [DOI] [PubMed] [Google Scholar]
- Fornai F, Giorgi FS, Alessandri MG, Giusiani M, Corsini GU. Effects of pretreatment with N-(2-chloroethyl)-N-ethyl-2- bromobenzylamine (DSP-4) on methamphetamine pharmacokinetics and striatal dopamine losses. J Neurochem. 1999;72:777–784. doi: 10.1046/j.1471-4159.1999.0720777.x. [DOI] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: A hypothesis. Brain Res Brain Res Rev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Fukui K, Kariyama H, Kashiba A, Kato N, Kimura H. Further confirmation of heterogeneity of the rat striatum: Different mosaic patterns of dopamine fibers after administration of methamphetamine or reserpine. Brain Res. 1986;382:81–86. doi: 10.1016/0006-8993(86)90113-7. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Hanson GR. Neurochemical basis of neurotoxicity. Neurotoxicology. 1990;11:317–321. [PubMed] [Google Scholar]
- Gonçalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: Preventive effect of indomethacin. Eur J Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Gramage E, Rossi L, Granado N, Moratalla R, Herradon G. Genetic inactivation of Pleiotrophin triggers amphetamine-induced cell loss in the substantia nigra and enhances amphetamine neurotoxicity in the striatum. Neuroscience. 2010;170:308–316. doi: 10.1016/j.neuroscience.2010.06.078. [DOI] [PubMed] [Google Scholar]
- Gras G, Chretien F, Vallat-Decouvelaere AV, Le Pavec G, Procheray F, Bossuet C, Leone C, Mialocq, Dereuddre-Bosquet N, Clayette P, Le Grand R, Creminon C, Dormont D, Rimaniol AC, Gray F. Regulated expression of sodium-dependent glutamate transporters and synthetase: A neuroprotective role for activated microglia and macrophages in HIV infection? Brain Pathol. 2003;13:211–222. doi: 10.1111/j.1750-3639.2003.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: Distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Baucum AJ, King JL, Horner KA, Cook G, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. Mechanisms underlying methamphetamine-induced dopamine transporter complex formation. J Pharmacol Exp Ther. 2009;329:169–174. doi: 10.1124/jpet.108.145631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Melegan WP. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res. 2000a;133:349–358. doi: 10.1007/s002210000386. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000b;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Galea E, Gavrilyuk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: Implications for Alzheimer’s disease. J Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Gavrilyuk V, Landreth GE, O’Banion MK, Weinberg G, Feinstein DL. Noradrenergic depletion increases inflammatory responses in brain: Effects on IkappaB and HSP70 expression. J Neurochem. 2003;85:387–398. doi: 10.1046/j.1471-4159.2003.01694.x. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Lee F, Hoebel BG. Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: Increase in extracellular dopamine and serotonin. Brain Res Bull. 1987;19:623–628. doi: 10.1016/0361-9230(87)90047-5. [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Gudelsky GA, Vorhees CV, Williams MT. (+)-Methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia. Synapse. 2010;64:773–785. doi: 10.1002/syn.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: Attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996;714:95–103. doi: 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- Hirata H, Asanuma M, Cadet JL. Superoxide radicals are mediators of the effects of methamphetamine on Zif268 (Egr-1, NGFI-A) in the brain: Evidence from using CuZn superoxide dismutase transgenic mice. Brain Res Mol Brain Res. 1998;58:209–216. doi: 10.1016/s0169-328x(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Aging increases the susceptiblity to methamphetamine-induced dopaminergic neurotoxicity in rats: Correlation with peroxynitrite production and hyperthermia. J Neurochem. 2001;78:952–959. doi: 10.1046/j.1471-4159.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Crow JP, Newport GD, Islam F, Slikker W, Jr, Ali SF. Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: Protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837:15–21. doi: 10.1016/s0006-8993(99)01663-7. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Islam F, Itzhak Y, Slikker W, Ali SF. Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: Implications for the prevention of methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci. 2000;914:157–171. doi: 10.1111/j.1749-6632.2000.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W, Jr, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity: Role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann N Y Acad Sci. 2001a;939:366–380. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W, Ali SF. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: Evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem. 2001b;76:745–749. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Gandia C, Huang PL, Ali SF. Resistance of neuronal nitric oxide synthase-deficient mice to methamphetamine-induced dopaminergic neurotoxicity. J Pharmacol Exp Ther. 1998;284:1040–1047. [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. Methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity in inducible nitric oxide synthase-deficient mice. Synapse. 1999;34:305–312. doi: 10.1002/(SICI)1098-2396(19991215)34:4<305::AID-SYN6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: Possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Ladenheim B, Cadet JL. Methamphetamine causes coordinate regulation of Src, Cas, Crk, and the Jun N-terminal kinase-Jun pathway. Mol Pharmacol. 2002;61:1124–1131. doi: 10.1124/mol.61.5.1124. [DOI] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Madrigal JL, Gavrilyuk V, Sharp A, Chauhan N, Marien M, Colpaert F, Feinstein DL. Beta-amyloid-dependent expression of NOS2 in neurons: Prevention by an alpha2-adrenergic antagonist. Antioxid Redox Signal. 2006;8:873–883. doi: 10.1089/ars.2006.8.873. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Stadelmann C, Dechant G, Wekerle H, Hohlfeld R. Neurotrophic cross-talk between the nervous and immune systems: Implications for neurological diseases. Ann Neurol. 2003;53:292–304. doi: 10.1002/ana.10446. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE, Jr, Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catecholquinones and converted to a redox-cycling quinoprotein: Possible relevance to Parkinson’s disease. J Neurochem. 1999;73:1309–1317. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: Relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. JAIDS. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: Evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: An in vitro model relevant to Parkinson’s disease. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Greves P. Pleiotrophin gene transcription in the rat nucleus accumbens is stimulated by an acute dose of amphetamine. Brain Res Bull. 2005;65:529–532. doi: 10.1016/j.brainresbull.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: Cellular and molecular mechanisms. Brain Res Brain Res Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Jakel R, Chesnut D, Pocernich CB, Butterfield DA, St Clair D, Cass WA. Methamphetamine toxicity is attenuated in mice that overexpress human manganese superoxide dismutase. Brain Res. 2000;878:218–222. doi: 10.1016/s0006-8993(00)02707-4. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palero M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McElvain JS, Schenk JO. A multisubstrate mechanism of striatal dopamine uptake and its inhibition by cocaine. Biochem Pharmacol. 1992;43:2189–2199. doi: 10.1016/0006-2952(92)90178-l. [DOI] [PubMed] [Google Scholar]
- Meiergerd SM, Schenk JO. Striatal transporter for dopamine: Catechol structure-activity studies and susceptibility to chemical modification. J Neurochem. 1994;62:998–1008. doi: 10.1046/j.1471-4159.1994.62030998.x. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Gibb JW. Short-term and long-term effects of methamphetamine on biogenic amine metabolism in extra-striatal dopaminergic nuclei. Neuropharmacology. 1980;19:989–995. doi: 10.1016/0028-3908(80)90010-6. [DOI] [PubMed] [Google Scholar]
- Morner A, Thomas JA, Bjorling E, Munson PJ, Lucas SB, McKnight A. Productive HIV-2 infection in the brain is restricted to macrophages/microglia. JAIDS. 2003;17:1451–1455. doi: 10.1097/00002030-200307040-00005. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E. The effects of nitric oxide on the oxidations of l-dopa and dopamine mediated by tyrosinase and peroxidase. J Biol Chem. 2001;276:11214–11222. doi: 10.1074/jbc.M009872200. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: Comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: The missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nixdorf WL, Burrows KB, Gudelsky GA, Yamamoto BK. Enhancement of 3,4-methylenedioxymethamphetamine neurotoxicity by the energy inhibitor malonate. J Neurochem. 2001;77:647–654. doi: 10.1046/j.1471-4159.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP. Astrocytes: Key players in mediation or modulation of neurotoxic responses? Commentary on forum position paper [comment] Neurotoxicology. 1998;19:35–36. discussion 37–38. [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Quantification of reactive gliosis as an approach to neurotoxicity assessment. NIDA Res Monogr. 1993;136:188–212. [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, McPherson RJ, Marshall JF. Excitotoxic striatal lesions protect against subsequent methamphetamine-induced dopamine depletions. J Pharmacol Exp Ther. 1994;269:1319–1325. [PubMed] [Google Scholar]
- O’Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2010;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Orio L, O’Shea E, Sanchez V, Pradillo JM, Escobedo I, Camarero J, Moro MA, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: Studies on the relationship with acute hyperthermia and 5-HT depletion. J Neurochem. 2004;89:1445–1453. doi: 10.1111/j.1471-4159.2004.02443.x. [DOI] [PubMed] [Google Scholar]
- Park SU, Ferrer JV, Javitch JA, Kuhn DM. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: Potential mechanism of neurotoxicity in dopamine neurons. J Neurosci. 2002;22:4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FC, Lourenco ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR, Ali SF. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60:185–193. doi: 10.1002/syn.20285. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Liddle AC. Microglial signalling cascades in neurodegenerative disease. Prog Brain Res. 2001;132:555–565. doi: 10.1016/S0079-6123(01)32103-9. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Contestabile A. Reciprocal interactions between microglia and neurons: From survival to neuropathology. Rev Neurosci. 2002;13:221–242. doi: 10.1515/revneuro.2002.13.3.221. [DOI] [PubMed] [Google Scholar]
- Povlock SL, Schenk JO. A multisubstrate kinetic mechanism of dopamine transport in the nucleus accumbens and its inhibition by cocaine. J Neurochem. 1997;69:1093–1105. doi: 10.1046/j.1471-4159.1997.69031093.x. [DOI] [PubMed] [Google Scholar]
- Pu C, Vorhees CV. Protective effects of MK-801 on methamphetamine-induced depletion of dopaminergic and serotonergic terminals and striatal astrocytic response: An immunohistochemical study. Synapse. 1995;19:97–104. doi: 10.1002/syn.890190205. [DOI] [PubMed] [Google Scholar]
- Pubill D, Verdaguer E, Sureda FX, Camins A, Pallas M, Camarasa J, Escubedo E. Carnosine prevents methamphetamine-induced gliosis but not dopamine terminal loss in rats. Eur J Pharmacol. 2002;448:165–168. doi: 10.1016/s0014-2999(02)01949-0. [DOI] [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Rand. Rand Drug Policy Research Center Research Brief. Santa Monica: Rand Corporation; 2009. [accessed on April 26, 2011]. The Costs of Methamphetamine Use: A National Estimate. Available online ( www.rand.org/pubs/research_briefs/2009/Rand_RB9438.pdf. [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Reis DJ. Effects of lesions of locus coeruleus on regional distribution of dopamine-beta-hydroxylase activity in rat brain. Brain Res. 1974;73:161–166. doi: 10.1016/0006-8993(74)91016-6. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Roach JT, Broom SL, Ferreira C, Preau MM. Long-term effects of a high-dose methamphetamine regimen on subsequent methamphetamine-induced dopamine release in vivo. Brain Res. 2001;892:122–129. doi: 10.1016/s0006-8993(00)03244-3. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Bowyer JF. Methamphetamine exposure can produce neuronal degeneration in mouse hippocampal remnants. Brain Res. 1997;759:135–140. doi: 10.1016/s0006-8993(97)00173-x. [DOI] [PubMed] [Google Scholar]
- Schroder N, O’Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED. Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res. 1996;738:172–175. doi: 10.1016/0006-8993(96)00995-x. [DOI] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: Role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Whittingham TS, Douglas AJ, Lust WD, Yamamoto BK. Substrates of energy metabolism attenuate methamphetamine-induced neurotoxicity in striatum. J Neurochem. 1998;71:613–621. doi: 10.1046/j.1471-4159.1998.71020613.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum core-lease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006a;137:925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Theodore S, Stolberg S, Cass WA, Maragos WF. Human immunodeficiency virus-1 protein Tat and methamphetamine interactions. Ann N Y Acad Sci. 2006b;1074:178–190. doi: 10.1196/annals.1369.018. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment: An oligonucleotide microarray approach. J Neurochem. 2004a;88:380–393. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage. FASEB J. 2006;20:515–517. doi: 10.1096/fj.05-4873fje. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem. 2009;109:1745–1755. doi: 10.1111/j.1471-4159.2009.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Perez MA, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology (Berl) 2010;209:13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiat. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: Implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, ±-methylenedioxymethamphetamine (MDMA), (+)-amphetamine and ±-fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2010;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Lucot JB, Schuster CR, Seiden LS. Alpha-methyltyrosine attenuates and reserpine increases methamphetamine-induced neuronal changes. Brain Res. 1983;270:285–288. doi: 10.1016/0006-8993(83)90602-9. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Neurotoxic regimen of methamphetamine produces evidence of behavioral sensitization in the rat. Synapse. 2001;39:1–7. doi: 10.1002/1098-2396(20010101)39:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Ferrucci M, Busceti CL, Biagioni F, Lazzeri G, Liles LC, Lenzi P, Pasquali L, Murri L, Paparelli A, Fornai F. Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. J Neurochem. 2008;105:471–483. doi: 10.1111/j.1471-4159.2007.05145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead RE, Ferrer JV, Javitch JA, Justice JB. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J Neurochem. 2001;76:1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Dryhurst G. Further insights into the oxidation chemistry of 5-hydroxytryptamine. J Pharm Sci. 1988;77:911–917. doi: 10.1002/jps.2600771102. [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Dryhurst G. Oxidation of serotonin by superoxide radical: Implications to neurodegenerative brain disorders. Chem Res Toxicol. 1998;11:639–650. doi: 10.1021/tx970185w. [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Dryhurst G. A putative metabolite of serotonin, tryptamine-4,5-dione, is an irreversible inhibitor of tryptophan hydroxylase: Possible relevance to the serotonergic neurotoxicity of methamphetamine. Chem Res Toxicol. 2001;14:1184–1192. doi: 10.1021/tx010037c. [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Yang Z, McAdams M, O’Connor-Coates S, Dryhurst G. Hydroxyl radical-mediated oxidation of serotonin: Potential insights into the neurotoxicity of methamphetamine. J Neurochem. 1995;64:1390–1400. doi: 10.1046/j.1471-4159.1995.64031390.x. [DOI] [PubMed] [Google Scholar]
- Wrona MZ, Yang Z, Zhang F, Dryhurst G. Potential new insights into the molecular mechanisms of methamphetamine-induced neurodegeneration. NIDA Res Monogr. 1997;173:146–174. [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease: A double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: Cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Gudelsky GA, Stephans SE. Amphetamine neurotoxicity: Roles for dopamine, glutatamate, and oxidative stress. In: Qureshi GA, editor. Progress in HPLC-HPCE. Vol. 7. Bombay: VSP; 1998. pp. 223–244. [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–131. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Callahan BT, McCann UD, Ricaurte GA. Evidence against an essential role of endogenous brain dopamine in methamphetamine-induced dopaminergic neurotoxicity. J Neurochem. 2001;77:1338–1347. doi: 10.1046/j.1471-4159.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Darvas M, Sotak B, Hatzidimitriou G, McCann UD, Palmiter RD, Ricaurte GA. Dopamine is not essential for the development of methamphetamine-induced neurotoxicity. J Neurochem. 2010;114:1135–1142. doi: 10.1111/j.1471-4159.2010.06839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Sevak RJ, Miotto K, Shoptaw S, Swanson AN, Clement C, De La Garza R, Newton TF, London ED. Pilot safety evaluation of varenicline for the treatment of methamphetamine dependence. J Exp Pharmacol. 2009;2010:13–18. [PMC free article] [PubMed] [Google Scholar]