Abstract
Aims
Although macrophage phenotypes have been well studied in the myocardial infarction (MI) setting, this study investigated temporal neutrophil polarization and activation mechanisms.
Methods and results
Neutrophils isolated from the infarcted left ventricle (LV) of mice showed high expression of proinflammatory markers at Day 1 and anti-inflammatory markers at Days 5 and 7 post-MI, indicating distinct neutrophil phenotypes along the post-MI time continuum. Flow cytometry analysis revealed that although proinflammatory N1 neutrophils were always predominant (>80% of total neutrophils at each time point), the percentage of N2 neutrophils increased post-MI from 2.4 ± 0.6% at Day 1 to 18.1 ± 3.0% at Day 7. In vitro, peripheral blood neutrophils were polarized to proinflammatory N1 by lipopolysaccharide and interferon-γ or anti-inflammatory N2 by interleukin-4, indicating high plasticity potential. The in vivo post-MI relevant LV damage-associated molecular patterns (DAMPs) polarized neutrophils to a proinflammatory N1 phenotype by activating toll-like receptor-4. Transforming growth factor-β1 inhibited proinflammatory production in neutrophils. N1 neutrophils positively correlated with infarct wall thinning at Day 7 post-MI, possibly due to high production of matrix metalloproteinases-12 and -25.
Conclusion
This study is the first to identify the existence of N1 and N2 neutrophils in the infarct region and reveals that N1 polarization could be mediated by DAMPs.
Keywords: DAMPs, Inflammation, Myocardial infarction, Neutrophil polarization, Proteomics
1. Introduction
Following myocardial infarction (MI), the left ventricle (LV) undergoes a series of remodelling responses, which primarily consist of three sequential phases: acute inflammatory response, granulation tissue formation, and scar formation and maturation.1 How the infarcted heart heals over the first couple of weeks substantially affects the long-term outcome. Therefore, regulating this process may offer a potential therapeutic strategy for patients with MI.
Neutrophils play an important role in cardiac remodelling and repair post-MI, as key initiators of the inflammatory response that coordinate necrotic tissue removal.2 They are very early leucocytes recruited into the infarcted LV. Neutrophils produce a wide array of inflammatory mediators (e.g. cytokines and chemokines) and reactive oxygen species.2 Upon activation, neutrophils release granule components (e.g. proteinases) to coordinate the wound healing response, and this process is known as degranulation. Neutrophils can form neutrophil extracellular traps to contain inflammation.3 In addition, neutrophils coordinate activation and functions of innate and adaptive immune cells, including macrophages and lymphocytes.4 Pure neutrophil depletion studies in human patients have not been performed. Studies examining the effects of neutrophil depletion in animal models have produced contradictory results. In some cases, infarct size reduction has been observed, and in other studies, there was no effect on infarct size.5,6 Data on effects of neutrophil depletion in cardiac remodelling are lacking.
Macrophage phenotypes have been well studied in the last decade. Macrophages are polarized to a proinflammatory M1 phenotype at early time points post-MI and shift to an anti-inflammatory M2 subtype over time.7 M1 macrophages promote inflammation and tissue destruction, whereas M2 macrophages facilitate resolution of inflammation and tissue reconstruction.8 Unlike macrophages, neutrophil phenotypes are less studied, partly due to a short lifespan (∼10 h in the circulation) and non-adherent property when culturing.9 No information is available about neutrophil polarization in the infarcted myocardium.
Accordingly, this study focused on the various phenotypes of neutrophils and in particular on the distinction between N1 and N2 neutrophils. We hypothesized that proinflammatory N1 and anti-inflammatory N2 neutrophils are present in the infarcted LV. We reveal here the first report of temporal neutrophil polarization in the LV post-MI and investigate the in vitro and in vivo mechanisms of neutrophil polarization. These novel findings will initiate a new research direction in neutrophil biology, both in the post-MI setting specifically and during other inflammatory processes generally.
2. Methods
2.1. Mice
In this study, 3- to 6-month-old male C57BL/6J mice purchased from The Jackson Laboratory and housed in our Laboratory Animal Facility were used. All mice were housed in a facility with a 12/12 h light/dark cycle and given free access to water and standard rodent chow. All animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. Mice were euthanized with 5% isoflurane, followed by exsanguination and removal of the heart.
2.2. MI surgery
MI was induced by permanent ligation of the left anterior descending coronary artery, as described previously.10,11 Before surgery, buprenorphine (0.1 mg/kg) was administered intraperitoneally. Mice were anaesthetized with 1–2% isoflurane in a 100% oxygen mix, intubated, and ventilated with a standard rodent ventilator (Harvard Apparatus). The adequacy of anaesthesia was controlled by monitoring heart rate and respiratory rate. Using a minimally invasive surgery, the thoracic cavity was opened through an incision between the third and fourth intercostal space. The left anterior descending coronary artery was ligated with 8-0 prolene suture placed 1–2 mm distal to the left atrium. Infarction was confirmed by visualizing LV blanching and ST-segment elevation on the electrocardiogram. The thoracic cavity and skin were closed using 8-0 prolene suture.
2.3. Echocardiography examination
Cardiac function was measured using the Vevo 2100 system (VisualSonics).10,11 Mice were anaesthetized with 1–2% isoflurane in an oxygen mix. All images were captured at heart rates >400 b.p.m. to achieve physiologically relevant measurements. Measurements were acquired from the LV parasternal long-axis B-mode and short-axis M-mode. For each parameter, images from three cardiac cycles were analysed and averaged.
2.4. Immunohistochemistry for neutrophil staining
Paraffin-embedded LV sections were deparaffinized in CitriSolve (22-143-975, Fisher Scientific) and rehydrated in gradient ethanol and distilled water for 5 min each. After antigen retrieval was carried out using the Target Retrieval Solution (S1699, Dako), sections were incubated in 3% hydrogen peroxide (216763, Sigma) to quench endogenous peroxidase activity. The sections were blocked with rabbit serum and incubated with an anti-neutrophil antibody (1:100, CL8993AP, Cedarlane) or phosphate-buffered saline (negative control), followed by incubation with rabbit anti-rat IgG and ABC reagent (PK-6104, Vector Lab). HistoMark Black Peroxidase Substrate Kit (54-75-00, KPL) was used for colour development. The sections were counterstained with eosin, dehydrated, and mounted. Images were taken using an Olympus DP71 microscope. Staining quantification was calculated as the percentage of positively stained area to total area. Four to six random scans in the infarct region per section were analysed and averaged.12
2.5. LV neutrophil and macrophage isolation
Infarcted LV was visually separated from the remote region under the microscope. Freshly isolated whole LVs for Day 0 or infarcted LVs for Days 1, 3, 5, and 7 post-MI were minced and dissociated into single cell suspension with a cocktail of collagenase II (CLS-2, Worthington Biochemical) and DNase I (A3778, AppliCHem), as described previously.13 The cell suspension was applied over pre-separation filters (130-041-407, Miltenyi Biotec) to remove non-dissociated clumps and incubated with red blood cell lysis solution (130-094-183, Miltenyi Biotec) to remove red blood cells. The single cell suspension was incubated with anti-Ly-6G-Biotin and anti-Biotin Microbeads (130-092-332, Miltenyi Biotec). The cell suspension was applied over a magnetic MS column (130-042-201, Miltenyi Biotec). Ly-6G+ neutrophils were adhered to columns and harvested for future use. The flow-through (Ly-6G− cells) was incubated with CD11b Microbeads (130-049-601, Miltenyi Biotec). The cell suspension was applied over a magnetic MS column. The neutrophil-depleted CD11b+ cells (macrophages) were collected.13 Due to the small number of neutrophils in Day 0 no MI LVs, we pooled three to four LVs per sample.
2.6. In vivo proliferation assay
Day 0 no MI mice were injected with bromodeoxyuridine (BrdU, 00-0103, Invitrogen, 10 mL/kg body weight) intraperitoneally 2 h before sacrifice. The LV was collected to isolate neutrophils and macrophages as described earlier. Neutrophils and macrophages were attached onto slides and fixed with 70% ethanol, permeabilized with 0.25% Triton X-100, and stained with anti-BrdU FITC antibody (1:20, 11-5071-42, eBioscience). The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, H-1200, Vector Laboratories). The images were captured using an Olympus IX81 microscope.
2.7. Quantitative RT-PCR
Total RNA was isolated from neutrophils using TRIzol reagent (15596026, Life Technologies) plus total RNA purification kit (12183018A, Life Technologies). cDNA was synthesized using a High-Capacity RNA-to-cDNA Kit (4837406, Life Technologies). The expression levels of Ccl3 (Mm00441259_g1), Ccl5 (Mm01302427_m1), Il1β (Mm01336189_m1), Il6 (Mm00843434_s1), Il12a (Mm00434165_m1), Tnfα (Mm00443258_m1), Arg1 (Mm00475988_m1), Cd206 (Mm00485148_m1), Il10 (Mm00439614_m1), Tgfβ1 (Mm01178820_m1), Ym1 (Mm00657889_mH), Mmp8 (Mm00439509_m1), Mmp9 (Mm00442991_m1), Mmp12 (Mm00500554_m1), Mmp25 (Mm01309189_m1), and Proteinase 3 (Mm00478323_m1) were measured with the Taqman gene expression master mix (4369016, Life Technologies). Hprt1 (Mm01545399_m1) or Gapdh (Mm99999915_g1) was used as the reference gene.14 MIQE guidelines were followed for all the PCR experiments and analysis.15
2.8. Flow cytometry
Single-cell suspensions isolated from LV or blood were blocked with 5% heat-inactivated mouse serum (M5905, Sigma) and incubated with an APC anti-Ly-6G antibody (1:500, 127614, Biolegend) and an Alexa Fluor 488 anti-CD206 antibody (1:25, MCA2235A488T, AbD Serotec). A second set of cells were labelled with a Vioblue anti-CD45 antibody (1:10, 130-102-775, Miltenyi Biotech), an FITC anti-CD11b antibody (1:11, 130-098-085, Miltenyi Biotech), and an APC anti-Ly-6G antibody or an APC anti-Ly-6G antibody only. The unstained cells and/or cells stained with isotype-matched IgG were used as negative controls. The flow cytometry experiments were performed using a MACSQuant Analyzer 10 and the data analysed using the MACSQuantify software. N1 neutrophils were defined as Ly-6G+CD206− and N2 neutrophils were defined as Ly-6G+CD206+.16
2.9. Immunofluorescence staining
Paraffin-embedded LV sections were deparaffinized and rehydrated, and antigen retrieval was performed as described earlier. The sections were blocked with 5% bovine serum albumin and incubated with an anti-Ly-6G antibody (1:100, ab25377, Abcam) or an anti-Mac-3 antibody (1:100, CL8943AP, Cedarlane) and an anti-CD206 antibody (1:200, ab64693, Abcam), followed by incubation with Alexa Fluor 488 donkey anti-rat (1:200, A-21208, Life Technologies) and Alexa Fluor 546 donkey anti-rabbit secondary antibodies (1:200, A10040, Life Technologies). Ly-6G+ cells isolated from Day 1 post-MI hearts were stained with an anti-Ly-6G antibody or an anti-Mac-3 antibody, followed by incubation with Alexa Fluor 488 donkey anti-rat and Alexa Fluor 546 donkey anti-rabbit secondary antibodies. The nuclei were stained with DAPI. The images were captured using an Olympus IX81 microscope.
2.10. Peripheral blood neutrophil isolation and stimulation
Peripheral blood neutrophils were isolated as described previously.17 Mice were anaesthetized with 1–2% isoflurane in a 100% oxygen mix. Heparinized mouse blood was collected and layered on top of an equal volume of Lymphocyte®-poly (CL5071, Cedarlane) in a 15 mL Falcon tube, followed by centrifugation at ×500 g for 35 min at room temperature. The cellular layers were collected and applied over pre-separation filters (130-041-407, Miltenyi Biotec) to remove blood clots. Erythrocytes were lysed with a red cell lysis buffer (130-094-183, Miltenyi Biotec). The single-cell suspension was used to evaluate neutrophil polarization by flow cytometry or incubated with anti-Ly-6G-Biotin and anti-Biotin Microbeads (130-092-332, Miltenyi Biotec). The cell suspension was applied over a magnetic MS column (130-042-201, Miltenyi Biotec). Ly-6G+ neutrophils were adhered to columns and collected for future use.
Neutrophils isolated from mouse peripheral blood were stimulated with lipopolysaccharide (LPS) (1 µg/mL, L 2880, Sigma) and interferon (IFN)-γ (20 ng/mL, 485-MI, R&D), interleukin (IL)-4 (20 ng/mL, 404-ML, R&D), damage-associated molecular pattern (DAMP) lysate (20 μg/mL), IL-10 (50 ng/mL, 417-ML, R&D), or transforming growth factor (TGF)-β1 (10 ng/mL, T7039, Sigma) for 4 h and collected to measure the expression of proinflammatory and anti-inflammatory markers. To acquire a sufficient number of neutrophils, we pooled three to six mice per sample for the stimulation experiments.
2.11. DAMP enrichment
Freshly isolated LVs from Day 0 no MI mice were used to enrich for DAMPs using an established protocol with minor modifications.18,19 Briefly, the LVs were minced into small pieces using sterile scalpels and added to phosphate-buffered saline (5.8 µL/mg tissue) with 1× protease inhibitor cocktail (04693159001, Roche). The samples were put into liquid nitrogen for 5 min and placed on ice for 30 min. After thawing, the samples were briefly vortexed. The freeze–thaw cycle was repeated three times. Control samples were kept on ice for the whole procedure without undergoing repeated freeze–thaw cycles. The cell suspension was centrifuged at ×10 000 g at 4°C for 10 min, and the supernatant was collected.
2.12. Immunoblotting
The same volume (2 μL) for all samples was loaded and separated on 4–12% Criterion™ XT Bis–Tris gels (#345-0124, Bio-Rad), transferred to nitrocellulose membrane, and stained with an MemCode™ Reversible Protein Stain Kit (PI-24580, Thermo Scientific) to verify total protein amount. After blocking with 5% non-fat milk (#170-6404XTU, Bio-Rad), the membrane was incubated overnight at 4°C with primary antibody against high mobility group box (HMGB) 1 (1:1000, ab79823, Abcam) or heat shock protein (HSP) 60 (1:1000, #4870, CST), followed by incubation with the secondary antibody and signal detection using the Amersham ECL prime western blotting detection reagent (RPN2236, GE Healthcare). Protein levels were quantified by densitometry using the IQ-TL image analysis software.
2.13. Statistical analysis
Data are expressed as mean ± SEM. Multiple group comparisons were performed using the one-way analysis of variance (ANOVA), followed by the Student–Newman–Keuls post-test (when the Bartlett variation test passed), or using the non-parametric Kruskal–Wallis test followed by Dunn's post-test (when the Bartlett variation test did not pass). Comparisons between two groups were carried out using Student's t-test. Pearson's correlation was performed to determine correlation coefficients. A value of P < 0.05 was considered statistically significant. GraphPad Prism 6 was used for the statistical analyses.
3. Results
3.1. Neutrophils expressed high proinflammatory markers at Day 1 and high anti-inflammatory markers at Days 5 and 7 post-MI
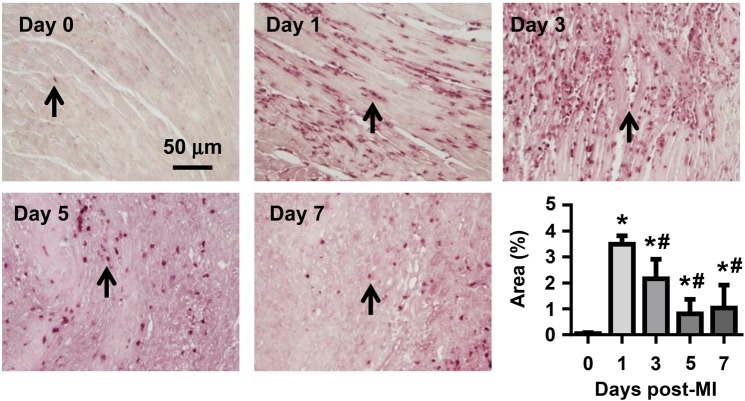
To examine temporal neutrophil infiltration, we performed immunohistochemical staining for LV neutrophils at Days 0, 1, 3, 5, and 7 post-MI. As shown in Figure 1, there were few resident neutrophils in the Day 0 no MI myocardium. Post-MI, neutrophil infiltration peaked at Days 1–3, substantially dropped at Day 5, and was low (but still significantly elevated) at Day 7 (all P < 0.05 vs. Day 0). Accordingly, this study evaluated the first 7 days post-MI.
Figure 1.
Temporal neutrophil infiltration. Time course of neutrophil infiltration post-MI. At Day 0 (no MI), very few neutrophils were observed in the LV. After MI, neutrophil infiltration peaked at Days 1–3, declined at Day 5, and was low by Day 7. Arrows indicate neutrophils. n = 8–16 per group. One-way ANOVA was used.
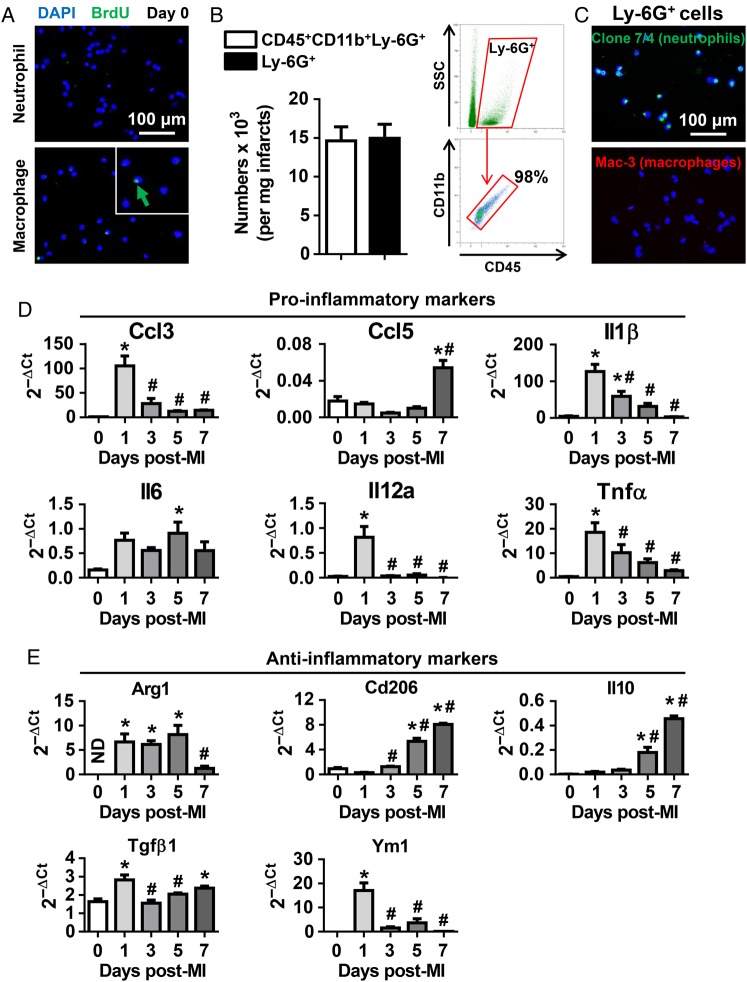
The Nahrendorf laboratory has demonstrated that cardiac resident macrophages proliferate and renew locally.20 To determine whether neutrophils renew locally, we evaluated neutrophil proliferation using in vivo BrdU staining (Figure 2A). Compared with the positive control macrophages, Day 0 cardiac neutrophils were BrdU-negative, indicating that these cells may continually derive from the circulation rather than proliferating locally.
Figure 2.
The expression of proinflammatory and anti-inflammatory markers in neutrophils. (A) Representative images demonstrated that cardiac resident neutrophils (Day 0) did not proliferate, as evidenced by BrdU-negative staining. Macrophages served as positive controls. (B) The numbers of CD45+CD11b+Ly-6G+ cells and Ly-6G+ cells at Day 1 post-MI evaluated by flow cytometry. n = 6 per group. (C) Ly6G+ cells were positive for the neutrophil clone 7/4, but negative for the macrophage marker Mac-3. Representative images of four biological samples. (D and E) Neutrophils expressed high levels of proinflammatory markers at Day 1 and anti-inflammatory markers at Days 5 and 7 post-MI, as assessed by quantitative RT–PCR. ND, not determined (value below detection). We assigned ND as zero when performing statistical analysis. Due to the small number of neutrophils in Day 0 LVs, we pooled three to four LVs per sample. n = 4–6 samples per group. *P < 0.05 vs. Day 0 and #P < 0.05 vs. Day 1 post-MI. One-way ANOVA was used.
Neutrophils are commonly defined as CD45+CD11b+Ly-6G+ cells.7 To assess whether Ly-6G is sufficient to label neutrophils, we incubated Day 1 post-MI neutrophils with CD45, CD11b, and Ly-6G antibodies or Ly-6G antibody only and compared the numbers of positively stained cells using flow cytometry. As shown in Figure 2B, both methods demonstrated similar numbers of neutrophils (14 627 ± 1811 CD45+CD11b+Ly6G+ cells vs. 14 965 ± 1804 Ly6G+ cells per milligram infarct tissue). Consistently, 98% of the Ly-6G+ cells were CD45+CD11b+ (Figure 2B). Combined, these findings indicate that 98% of the Ly6G+ cells are neutrophils. In addition, our immunofluorescence staining revealed that Ly-6G+ cells were highly pure and had no macrophage contamination (Figure 2C). Therefore, we used Ly-6G microbeads or antibody in this study to isolate or label neutrophils.
To evaluate neutrophil phenotypes, we measured the mRNA expression of proinflammatory (Ccl3, Ccl5, Il1β, Il6, Il12a, and Tnfα) and anti-inflammatory (Arg1, Cd206, Il10, Tgfβ1, and Ym1) markers in neutrophils. Day 0 LVs served as no MI controls. Due to the small number of neutrophils in Day 0 LVs, we pooled three to four LVs per sample for RNA extraction and quantitative RT–PCR analysis. All MI time points used one mouse per sample. At Day 0, intra-LV neutrophils displayed low levels of both proinflammatory and anti-inflammatory markers (Figure 2D and E), indicating an unactivated naive status. Neutrophils at Day 1 post-MI showed significantly higher levels of proinflammatory markers (Ccl3, Il1β, Il12a, and Tnfα), compared with Day 0 no MI or Days 3, 5, or 7 post-MI (Figure 2D, all P < 0.05), indicating a proinflammatory N1 phenotype that peaked at Day 1 comprised specific proinflammatory genes. In contrast, neutrophils showed increased Il6 at Day 5 and increased Ccl5 at Day 7 post-MI, indicating that Ccl5 and Il6 are not good markers for N1 neutrophils. Consistent with this finding, IL-6 has been demonstrated to be a marker for both M1 and M2 macrophages.21,22
Anti-inflammatory markers demonstrated distinct expression patterns following MI. Neutrophil Arg1 was at levels below detection (not determined) under Day 0 no MI conditions; however, its expression increased from Days 1 to 5 and decreased at Day 7 post-MI (Figure 2E). Interestingly, Tgfβ1 expression in neutrophils increased at Day 1, decreased at Days 3 and 5, and increased again at Day 7 post-MI, consistent with the fact that TGF-β1 exerts biphasic proinflammatory and anti-inflammatory roles.23 Ym1 expression in neutrophils peaked at Day 1 and dramatically decreased thereafter in accordance to a role in the N1 neutrophil. Although being a marker of anti-inflammatory M2 macrophages, Ym1 is also highly present in neutrophils.24 Our results reveal that it may serve as an N1 marker, indicating disparate polarization phenotypes between macrophages and neutrophils. Both CD206 and IL-10 have been utilized as neutrophil N2 markers,16,25 and our findings demonstrated that the mRNA expression of Cd206 and ll10 gradually increased from Days 0 to 7 post-MI (Figure 2E, P < 0.05), indicating a shift from N1 to N2 neutrophil phenotype over the course of MI. Taken together, our data demonstrated that neutrophils display a proinflammatory N1 subtype at Day 1 and gradually shift to the anti-inflammatory N2 phenotype from Days 1 to 7 post-MI.
3.2. Although N1 was the predominant neutrophil phenotype in the infarcted LV, the percentage of N2 neutrophils increased over time after MI
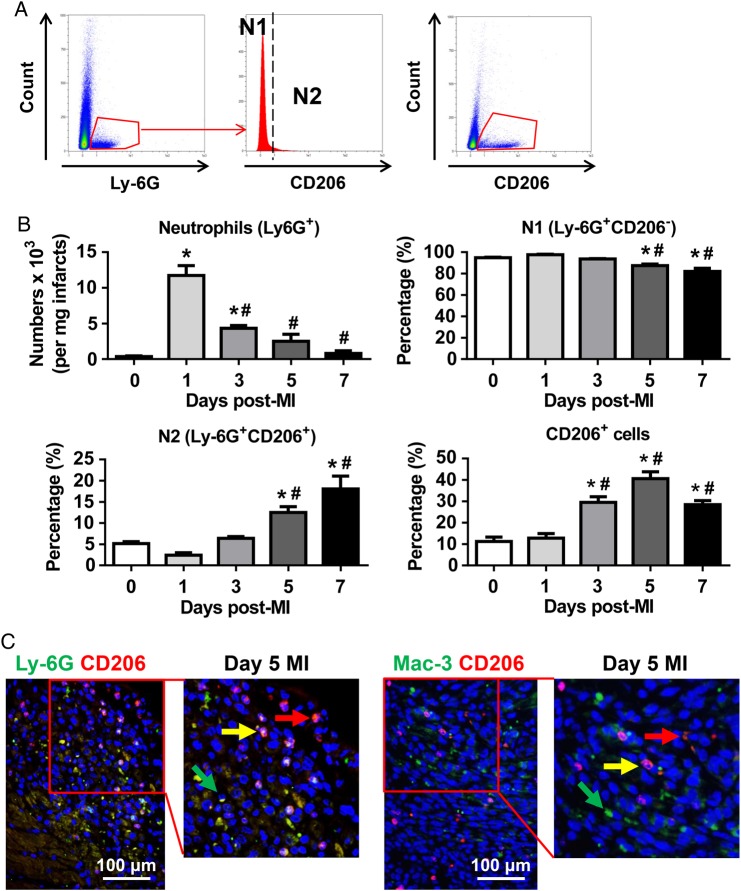
Global neutrophil analysis indicated the existence of different neutrophil polarization phenotypes in the infarcted LVs when all neutrophils were isolated as a combined cell pool. We further evaluated individual neutrophil phenotypes using flow cytometry to examine cell heterogeneity. N1 neutrophils were defined as Ly6G+CD206−, and N2 neutrophils were defined as Ly6G+CD206+ (Figure 3A). Consistent with the neutrophil immunohistochemistry results, total LV neutrophil numbers peaked at Day 1 and decreased at Days 3–7 post-MI (Figure 3B). Interestingly, the percentage of N1 neutrophils decreased from 97.6 ± 0.6% at Day 1 to 87.5 ± 1.4% at Day 5 and 81.9 ± 3.0% at Day 7 post-MI (P < 0.05 vs. Days 0 and 1); yet still were the predominant neutrophil phenotypes, accounting for >80% of the total neutrophils during the first 7 days post-MI (Figure 3B). In contrast, the percentage of N2 neutrophils significantly increased post-MI from 2.4 ± 0.6% at Day 1 to 18.1 ± 3.0% at Day 7 (Figure 3B, P < 0.05). CD206 is directly involved in cell phagocytic function.26 Here, we showed that the percentage of CD206+ cells increased at Days 3, 5, and 7, peaking at Day 5 post-MI (Figure 3B, P < 0.05 vs. Days 0 and 1). The higher CD206+ cell numbers, which include M2 macrophages and N2 neutrophils, may facilitate phagocytosis of apoptotic cells, resolution of inflammation, and cardiac repair.8,27 Dual immunofluorescence staining confirmed the existence of Ly-6G+CD206− N1 (green arrow, left panel), Ly-6G+CD206+ N2 (yellow arrow, left panel), Mac-3+CD206- M1 (green arrow, right panel), Mac-3+CD206+ M2 (yellow arrow, right panel), and CD206+ cells (red arrow) in Day 5 infarcted LVs (Figure 3C).
Figure 3.
Temporal neutrophil polarization in the infarcted LVs. (A) Representative flow cytometry plots illustrate gating strategy to identify neutrophil N1 and N2 phenotypes and CD206+ cells. (B) Although proinflammatory N1 was the predominant neutrophil in the infarcted LVs at all times examined, the percentage of anti-inflammatory N2 neutrophils increased over the course of MI. n = 6–12 per group. *P < 0.05 vs. Day 0 and #P < 0.05 vs. Day 1 post-MI. One-way ANOVA was used. (C) Dual immunofluorescence images revealed the presence of Ly-6G+CD206− N1 (green arrow, left panel), Ly-6G+CD206+ N2 (yellow arrow, left panel), Mac-3+CD206− M1 (green arrow, right panel), Mac-3+CD206+ M2 (yellow arrow, right panel), and CD206+ cells (red arrow) in day 5 infarcted LVs. Representative images of three to six biological samples.
3.3. Neutrophils demonstrated high plasticity in vitro
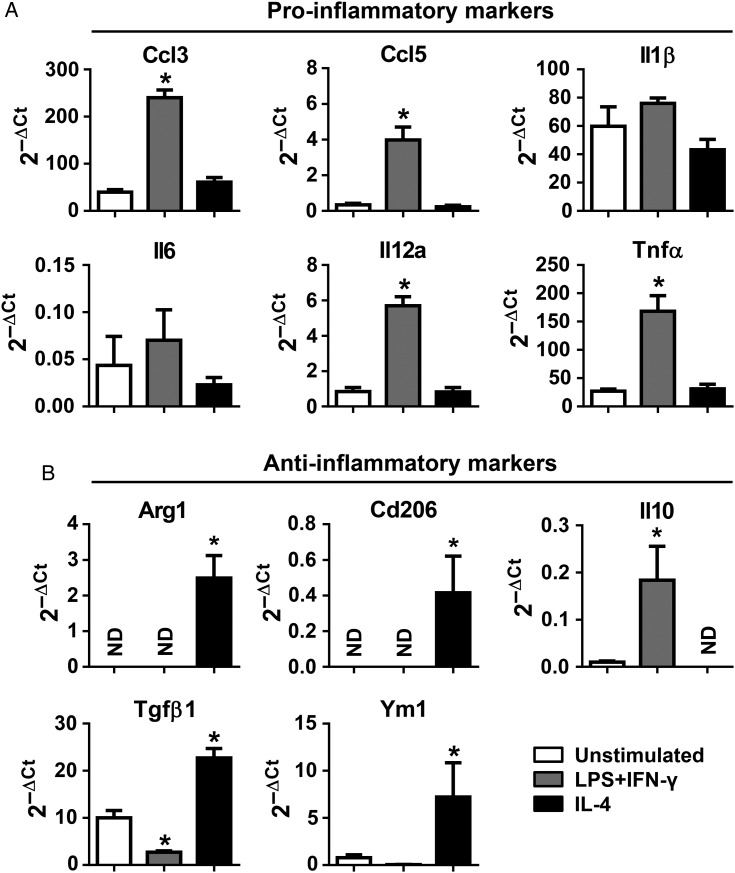
Combined LPS and IFN-γ stimulation is commonly used to prime macrophages to proinflammatory M1 and IL-4 stimulation to anti-inflammatory M2 phenotypes.10,28 To assess whether neutrophils show a similar response, we stimulated peripheral blood neutrophils with LPS (1 µg/mL) and IFN-γ (20 ng/mL) or IL-4 (20 ng/mL) for 4 h and measured the expression of the same proinflammatory and anti-inflammatory markers shown previously. As expected, LPS and IFN-γ significantly stimulated the production of proinflammatory markers (Ccl3, Ccl5, Il12a, and Tnfα, all P < 0.05) and attenuated the expression of the anti-inflammatory marker Tgfβ1 (Figure 4A and B, P < 0.05). In contrast, IL-4 treatment induced the expression of anti-inflammatory markers (Arg1, Cd206, Tgfβ1, and Ym1, all P < 0.05). Of note, Il10 expression was induced by LPS and IFN-γ but not IL-4 (Figure 4B, P < 0.05). Combined, these data revealed that neutrophils exhibit high plasticity in vitro. Because LPS and IFN-γ or IL-4 are not post-MI neutrophil stimuli, we next evaluated ex vivo stimuli from the LV as the source of neutrophil polarization factors.
Figure 4.
LPS and IFN-γ polarized neutrophils to a proinflammatory N1 phenotype, whereas IL4 polarized neutrophils to an anti-inflammatory N2 phenotype. (A and B) LPS (1 μg/mL) and IFN-γ (20 ng/mL) up-regulated the expression of proinflammatory markers; on the contrary, IL-4 (20 ng/mL) treatment up-regulated the expression of anti-inflammatory markers in neutrophils. ND, not determined (value below detection). We assigned ND as zero when performing statistical analysis. n = 5 mice per sample. n = 5–6 samples per group. *P < 0.05 vs. unstimulated. One-way ANOVA was used.
3.4. DAMPs activated N1 neutrophil polarization in vitro
Following MI, cardiac myocytes undergo irreversible necrosis and apoptosis. Dead myocytes release intracellular components to the extracellular space, among which is a group of proteins known as DAMPs. DAMPs are produced from host tissue or immune cells in response to stress or injury. DAMPs can initiate and perpetuate the inflammatory response through binding to their receptors on leucocytes.29 As such, DAMPs are very early potentiators of MI injury.30 We hypothesized that in the post-MI setting, DAMPs polarize neutrophils to a proinflammatory N1 phenotype.
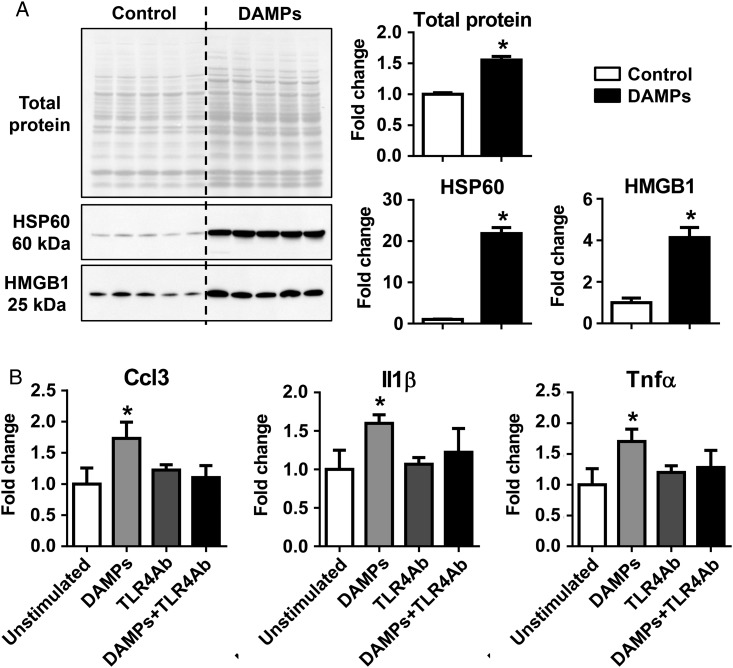
We generated DAMPs ex vivo by repeated freeze–thaw injury of Day 0 no MI LVs and used them to stimulate neutrophils.19 To evaluate whether the protein lysate had the expected higher total protein and DAMPs released into the lysate, we determined the protein amount and carried out immunoblotting for two well-known DAMPs (HSP60 and HMGB1). As shown in Figure 5A, the protein lysate in the DAMP group contained significantly higher amounts of total protein (1.6-fold), HSP60 (22-fold), and HMGB1 (4.1-fold), compared with controls (all P < 0.05). This indicates the successful enrichment of DAMPs in the lysate.
Figure 5.
DAMPs activated N1 neutrophil polarization in vitro via TLR4. (A) LV tissues that underwent freeze–thaw cycles (the DAMPs group) contained significantly higher total protein, HSP60, and HMGB1, compared with controls, as evaluated by immunoblotting. n = 5 LVs per group. The same volume of all samples was loaded (2 µL). (B) DAMPs stimulated the production of proinflammatory markers Ccl3, Il1β, and Tnfα, which was abolished by the anti-TLR4 neutralizing antibody. The data were expressed as fold change (normalized to control or unstimulated group). n = 3–6 mice per sample and n = 4–9 samples per group; *P < 0.05 vs. controls. One-way ANOVA was used.
DAMPs, including HMGB1 and HSP60, can engage toll-like receptor (TLR) 4 on neutrophils and promote proinflammatory cytokine production.29 To investigate whether DAMPs can activate N1 neutrophils, we stimulated peripheral blood neutrophils with 20 μg/mL of DAMP lysate for 4 h with or without anti-TLR4 neutralizing antibody and measured proinflammatory and anti-inflammatory marker expression. DAMP treatment significantly up-regulated the expression of proinflammatory markers (Figure 5B, Ccl3, Il1β, and Tnfα, all P < 0.05). The effect of DAMPs was blunted by the addition of the anti-TLR4 antibody, which had no effect by itself (Figure 5B). These data indicate that DAMPs polarize neutrophils to a proinflammatory N1 subtype by activating TLR4. The expression of Ccl5, Il12a, Il10, Tgfβ1, and Ym1 did not show statistical difference among any groups. The expression of Il6, Arg1, and Cd206 was below detection.
3.5. CD206+ N2 neutrophils were activated in the post-MI LV, but not in circulation
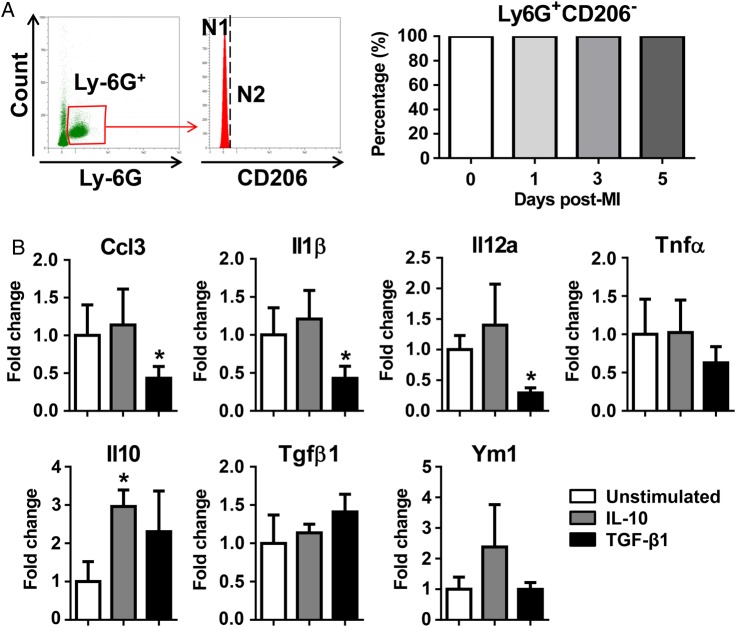
Our findings revealed that the percentage of CD206+ N2 neutrophils increased over time after MI. To investigate whether the infarct CD206+ N2 neutrophils derive from circulating N2 or are activated locally, we evaluated the phenotype of circulating neutrophils. Circulating neutrophils at Days 0, 1, 3, or 5 post-MI were all CD206− (Figure 6A). This indicates that CD206+ N2 neutrophils were locally activated in the LV infarct.
Figure 6.
CD206+ N2 neutrophils were activated in the post-MI LV, but not in circulation. (A) Circulating neutrophils at different time points post-MI were CD206−. n = 6–8 per group. (B) IL-10 (50 ng/mL) up-regulated the expression of the anti-inflammatory marker Il10; TGF-β1 (10 ng/mL) inhibited the expression of proinflammatory markers (Ccl3, Il1β, and Il12a) in neutrophils. The data were expressed as fold change (normalized to the unstimulated group). n = 4 mice per sample and n = 4 samples per group. *P < 0.05 vs. unstimulated. One-way ANOVA was used.
IL-10 and TGF-β1 are important anti-inflammatory factors in post-MI LV remodelling.31–33 To investigate whether IL-10 and TGF-β1 mediate post-MI CD206+ N2 neutrophil activation, we stimulated peripheral blood neutrophils. Out of the 11 markers measured, IL-10 treatment up-regulated Il10 expression only, forming a positive feedback for Il10 induction (Figure 6B, P < 0.05). TGF-β1 treatment significantly inhibited the expression of proinflammatory markers (Figure 6B, Ccl3, Il1β, and Il12a, all P < 0.05). Neither IL-10 nor TGF-β1 affected Cd206 expression (all were below detection). This indicates that an additional factor, but not IL-10 or TGF-β1, facilitates post-MI CD206+ N2 activation.
3.6. N1 neutrophils were a significant contributor to LV infarct wall thinning
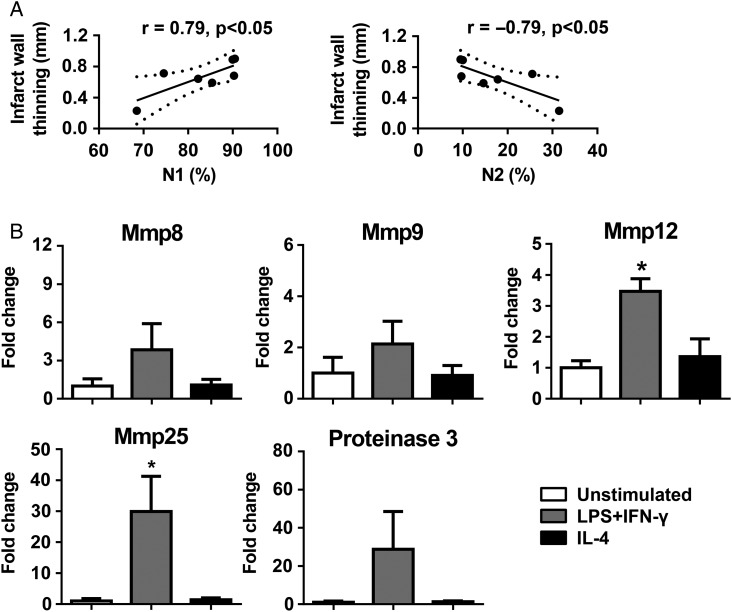
We performed Pearson's correlation analysis to compare N1 and N2 neutrophils to various LV function variables (Table 1). Out of the nine variables measured, the thinning of the LV posterior wall in systole most significantly correlated with neutrophil polarization (Figure 7A, both P < 0.05). Infarcted wall thinning is a hallmark of cardiac remodelling post-MI. Enhanced wall thinning reduces LV tensile strength and increases LV dilation.10,34 This indicates that N1 neutrophils are a significant source of wall thinning, and N2 prevents LV wall thinning and adverse infarct remodelling.
Table 1.
LV function evaluated by echocardiography
| Days post-MI |
||
|---|---|---|
| Day 0 (n = 6) | Day 7 (n = 7) | |
| Heart rate (b.p.m.) | 529 ± 12 | 521 ± 12 |
| EDV (µL) | 63 ± 2 | 165 ± 17* |
| ESV (µL) | 21 ± 1 | 145 ± 19* |
| EF (%) | 66 ± 1 | 14 ± 4* |
| LVPW:d (mm) | 0.65 ± 0.01 | 0.34 ± 0.04* |
| LVPW:s (mm) | 0.87 ± 0.03 | 0.42 ± 0.08* |
| EDD (mm) | 3.66 ± 0.07 | 6.62 ± 0.30* |
| ESD (mm) | 2.36 ± 0.08 | 6.39 ± 0.36* |
| FS (%) | 36 ± 2 | 4 ± 2* |
Student's t-test was used.
EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; LVPW:d, left ventricular posterior infarct wall in diastole; LVPW:s, left ventricular posterior infarct wall in systole; EDD, end-diastolic dimension; ESD, end-systolic dimension; FS, fractional shortening.
*P < 0.05 vs. day 0.
Figure 7.
N1 neutrophils were a source of LV wall thinning due to high levels of Mmp12 and Mmp25. (A) At day 7 post-MI, N1 positively and N2 negatively correlated with infarct wall thinning. Infarct wall thinning = (days 0–7) LV posterior wall thickness in systole. n = 7 per group. Pearson's correlation analysis was used. (B) N1 neutrophils stimulated by LPS + IFN-γ expressed higher levels of Mmp12 and Mmp25, compared with unstimulated cells. The data were expressed as fold change (normalized to the unstimulated group). n = 4 per group. *P < 0.05 vs. unstimulated. One-way ANOVA was used.
Neutrophils are one of the major sources of proteinases, which can degrade extracellular matrix and lead to wall thinning.2,17 Accordingly, we measured the mRNA levels of known neutrophil proteinases Mmp8, Mmp9, Mmp12, Mmp25, and Proteinase 3 in unstimulated, LPS + IFN-γ-, and IL-4-treated neutrophils. Compared with unstimulated cells, neutrophils stimulated by LPS + IFN-γ (N1) demonstrated significantly higher Mmp12 and Mmp25 (Figure 7B, both P < 0.05). Mmp8, Mmp9, and Proteinase 3 levels were comparable among groups. MMP-12 and MMP-25 have proteolytic activity and can degrade wall extracellular matrix.2,17 This may, at least partially, explain why N1 neutrophils induce LV wall thinning.
4. Discussion
This study investigated temporal neutrophil polarization and potential mechanisms in the MI setting. The major findings were as follows: (i) post-MI, cardiac neutrophils demonstrated proinflammatory N1 and anti-inflammatory N2 phenotypes; (ii) although N1 was the predominant phenotype in the infarcted LV, N2 numbers increased over the course of MI; (iii) neutrophils displayed high plasticity in vitro and were polarized to N1 by LPS + IFN-γ or to N2 by IL-4; (iv) LV DAMPs polarized neutrophils to N1 by activating TLR4, which provides an MI-relevant activation mechanism; and (v) N1 neutrophils correlated with infarct wall thinning.
Although macrophage and lymphocyte phenotypes have been well established, the neutrophil phenotype is a very novel topic. Neutrophil polarization has only very recently been identified in cancer, bacterial infection, and stroke animal models, and this is the first report of neutrophil polarization in MI. Fridlender et al.35 revealed that TGF-β within the tumour microenvironment induces a pro-tumour phenotype of neutrophils, whereas TGF-β blockade shifts neutrophils to an anti-tumour phenotype, indicating differential neutrophil polarization. In a burn injury model of bacterial infection, N1 neutrophils express high levels of proinflammatory IL-12, whereas N2 neutrophils demonstrate high levels of anti-inflammatory IL-10 production.25 Both N1 (Ly-6G+CD206− or Ly-6G+Ym1−) and N2 (Ly-6G+CD206+ or Ly-6G+Ym1+) neutrophils are present in the brain after stroke.16 Here, we also showed the presence of proinflammatory N1 and anti-inflammatory N2 in the MI heart. Importantly, N1 is the predominant neutrophil phenotype in the MI heart (>80% of the total neutrophils), whereas the percentage of N2 increased over time after MI, supporting its role in the resolution of inflammation and wound repair (similar to M2 macrophages). We also demonstrated that CD206+ N2 neutrophils are locally activated because circulating neutrophils post-MI are CD206−. Programming neutrophils to the N2 subtype by a PPARγ agonist rosiglitazone reduces brain infarct volume, indicative of beneficial roles for anti-inflammatory N2 neutrophils.16 Consistently, our study demonstrated that N2 neutrophils negatively correlate with infarct wall thinning, indicating that N2 neutrophils may help to prevent further LV wall thinning. N1 neutrophils contribute to wall thinning possibly by generating high levels of MMP-12 and MMP-25.2,17 The anti-CD-18 antibody has been shown to reduce LV neutrophil numbers in the mouse ischaemia–reperfusion model.36 It would be very interesting to know whether it can prevent post-MI LV wall thinning.
The findings that neutrophils were polarized to N1 by LPS and IFN-γ or N2 by IL-4 indicate high plasticity potential of neutrophils, which supports neutrophils being differentially polarized in the MI setting. LPS or IL4 does not increase after MI, and therefore LPS and INF-γ or IL4 are not post-MI relevant stimuli of neutrophil N1 polarization.
Following MI, necrotic cardiac myocytes release a multitude of DAMPs, which include HSPs, HMGB1, hyaluronic acid, fibronectin-EDA, and S100 protein family.37,38 DAMPs, as secondary danger signals, are involved in the initiation and maintenance of the inflammatory response. HSP70 and HMGB1 have been shown to stimulate the production of cytokines in neutrophils in vitro by binding to TLRs.29 In line with this concept, we showed that LV DAMPs promoted the expression of proinflammatory mediators in neutrophils. This effect is mediated by TLR4, as the anti-TLR4 antibody blunted DAMPs induced N1 polarization. Whether other TLRs also mediate this effect needs to be verified in future studies.
In this study, we evaluated the role of DAMPs in neutrophil N1 polarization. Likely, DAMPs do not fully recapitulate the entire MI setting, and other unknown factors, including complement, cytokines, and growth factors, contribute as a mixture of stimuli to post-MI neutrophil N1 activation. For example, tumour necrosis factor-α, IL-1β, and complement C5a are proinflammatory mediators up-regulated very early after MI and are likely involved in neutrophil N1 polarization post-MI.39 The DAMPs we used are a mix of multiple proteins, including hyaluronic acid, HSP70, S100A8, and S100A9.37 Future studies are warranted to isolate the individual components and understand combined effects on neutrophil polarization.
The insufficient removal of neutrophils in the ischaemic heart enhances matrix degradation, delays collagen deposition, and increases susceptibility of the LV to cardiac rupture.40 Likewise, oncostatin M (OSM) regulates both cardiomyocyte dedifferentiation and cardiomyocyte-dependent regulation of macrophage trafficking. Release of OSM from infiltrating neutrophils and macrophages initiates a positive feedback loop to recruit additional OSM-secreting macrophages and to remove apoptotic neutrophils. In addition, OSM is known to induce production of inflammatory cytokines, which can activate three major innate immune cells: neutrophils, macrophages, and dendritic cells. The impact of OSM on neutrophils, macrophages, and dendritic cells and the interplay among innate immune leucocytes in the post-MI setting are variables that warrant further evaluation.
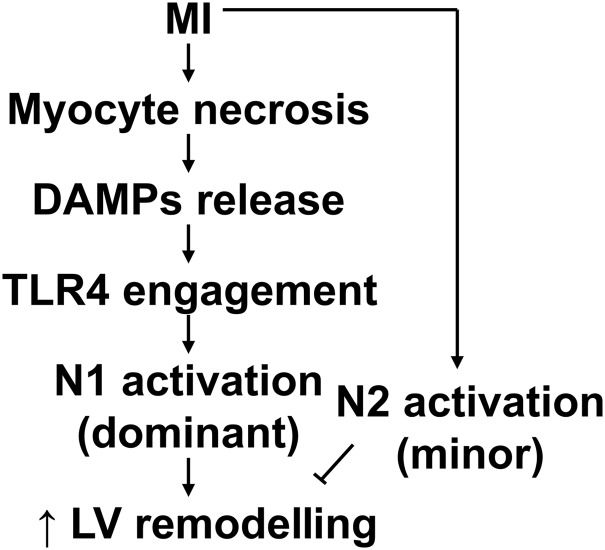
In summary, this study for the first time mapped the temporal progression of neutrophil polarization in the post-MI infarct LV and evaluated MI-relevant activation mechanisms of polarization. MI induces myocyte necrosis, which release DAMPs to stimulate neutrophil N1 polarization by binding to TLR4 (Figure 8). Excessive N1 neutrophils may exacerbate and N2 neutrophils may attenuate adverse LV remodelling. These innovative findings drive forward research on the mechanisms of LV remodelling post-MI by dissecting out the individual contribution of neutrophil polarization. Specifically inhibiting N1 or enhancing N2 neutrophils may represent novel therapeutic strategies for MI patients and has broad additional applicability across a number of inflammatory processes in which neutrophils are involved.
Figure 8.
Mechanisms of neutrophil N1 polarization post-MI. Following MI, necrotic myocytes release DAMPs to polarize neutrophils to a proinflammatory N1 phenotype by activating TLR4. N1 may exacerbate and N2 may attenuate adverse LV remodelling.
Funding
This work was supported by the American Heart Association [15SDG22930009 to Y.M. and 14POST18770012 to R.P.I.]; the National Institutes of Health [HHSN 268201000036C (N01-HV-00244), HL075360, and GM114833 to M.L.L. and P01HL051971 and P20GM104357]; and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award [5I01BX000505 to M.L.L.].
Acknowledgement
We thank Ryan Clark and Ashley DeCoux for technical assistance.
Conflict of interest: none declared.
References
- 1.Ghasemi O, Ma Y, Lindsey ML, Jin YF. Using systems biology approaches to understand cardiac inflammation and extracellular matrix remodeling in the setting of myocardial infarction. Wiley Interdiscip Rev Syst Biol Med 2014;6:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 2013;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui BB, Tan CY, Schorn C, Tang HH, Liu Y, Zhao Y. Neutrophil extracellular traps in sterile inflammation: the story after dying? Autoimmunity 2012;45:593–596. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011;11:519–531. [DOI] [PubMed] [Google Scholar]
- 5.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 1983;67:1016–1023. [DOI] [PubMed] [Google Scholar]
- 6.Chatelain P, Latour JG, Tran D, de Lorgeril M, Dupras G, Bourassa M. Neutrophil accumulation in experimental myocardial infarcts: relation with extent of injury and effect of reperfusion. Circulation 1987;75:1083–1090. [DOI] [PubMed] [Google Scholar]
- 7.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 2013;62:24–35. [DOI] [PubMed] [Google Scholar]
- 8.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 2014;102:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 2011;12:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 2013;112:675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Lindsey ML. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci 2015; 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halade GV, Ma Y, Ramirez TA, Zhang J, Dai Q, Hensler JG, Lopez EF, Ghasemi O, Jin YF, Lindsey ML. Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 2013;305:H1830–H1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, Jin YF. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res 2015;106:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez TA, Iyer RP, Ghasemi O, Lopez EF, Levin DB, Zhang J, Zamilpa R, Chou YM, Jin YF, Lindsey ML. Aliskiren and valsartan mediate left ventricular remodeling post-myocardial infarction in mice through MMP-9 effects. J Mol Cell Cardiol 2014;72:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55:611–622. [DOI] [PubMed] [Google Scholar]
- 16.Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbi AL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke 2013;44:3498–3508. [DOI] [PubMed] [Google Scholar]
- 17.Iyer RP, Patterson NL, Zouein FA, Ma Y, Dive V, de Castro Bras LE, Lindsey ML. Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution. Int J Cardiol 2015;185:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol 2001;167:4844–4852. [DOI] [PubMed] [Google Scholar]
- 19.Unlu S, Tang S, Wang E, Martinez I, Tang D, Bianchi ME, Zeh HJ III, Lotze MT. Damage associated molecular pattern molecule-induced microRNAs (DAMPmiRs) in human peripheral blood mononuclear cells. PLoS ONE 2012;7:e38899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014;115:284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007;110:4319–4330. [DOI] [PubMed] [Google Scholar]
- 22.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 2009;284:34342–34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 2009;9:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbord M, Novelli M, Canas B, Power D, Davis C, Godovac-Zimmermann J, Roes J, Segal AW. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J Biol Chem 2002;277:5468–5475. [DOI] [PubMed] [Google Scholar]
- 25.Neely CJ, Kartchner LB, Mendoza AE, Linz BM, Frelinger JA, Wolfgang MC, Maile R, Cairns BA. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12- neutrophil polarization. PLoS ONE 2014;9:e85623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie EJ, Taylor PR, Stillion RJ, Lucas AD, Harris J, Gordon S, Martinez-Pomares L. Mannose receptor expression and function define a new population of murine dendritic cells. J Immunol 2007;178:4975–4983. [DOI] [PubMed] [Google Scholar]
- 27.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012;110:159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 2009;28:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol 2011;11:397–403. [DOI] [PubMed] [Google Scholar]
- 30.de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm 2013;2013:206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 2009;104:e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, Kubota T, Takeshita A. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res 2004;64:526–535. [DOI] [PubMed] [Google Scholar]
- 33.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 2008;103:485–492. [DOI] [PubMed] [Google Scholar]
- 34.Spinale FG, Villarreal F. Targeting matrix metalloproteinases in heart disease: lessons from endogenous inhibitors. Biochem Pharmacol 2014;90:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’ TAN. Cancer Cell 2009;16:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka M, Brooks SE, Richard VJ, FitzHarris GP, Stoler RC, Jennings RB, Arfors KE, Reimer KA. Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation 1993;87:526–535. [DOI] [PubMed] [Google Scholar]
- 37.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 2011;8:292–300. [DOI] [PubMed] [Google Scholar]
- 38.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm 2013;2013:828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002;53:31–47. [DOI] [PubMed] [Google Scholar]
- 40.Lorchner H, Poling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nat Med 2015;21:353–362. [DOI] [PubMed] [Google Scholar]