Abstract
Hydrogen sulfide (H2S), as a gaseous signalling molecule, has been found to play important roles in postconditioning (PostC)-induced cardioprotection. Similar to nitric oxide (NO)-mediated protein S-nitrosylation (SNO), recent studies suggest that H2S could regulate protein function through another redox-based post-translational modification on protein cysteine residue(s), i.e. S-sulfhydration (SSH). In this study, we examined whether there are changes in protein SSH associated with cardioprotection induced by treatment with H2S on reperfusion. In addition, we also examined whether there is cross talk between H2S and NO. Compared with control, treatment on reperfusion with NaHS (H2S donor, 100 µmol/L) significantly reduced post-ischaemic contractile dysfunction and infarct size. A comparable cardioprotective effect could be also achieved by reperfusion treatment with SNAP (NO donor, 10 µmol/L). Interestingly, simultaneous reperfusion with both donors had an additive protective effect. In addition, C-PTIO (NO scavenger, 20 µmol/L) eliminated the protection induced by NaHS and also the additive protection by SNAP + NaHS together. Using a modified biotin switch method, we observed a small increase in SSH following NaHS treatment on reperfusion. We also found that NaHS treatment on reperfusion increases SNO to a level comparable to that with SNAP treatment. In addition, there was an additive increase in SNO but not SSH when SNAP and NaHS were added together at reperfusion. Thus, part of the benefit of NaHS is an increase in SNO, and the magnitude of the protective effect is related to the magnitude of the increase in SNO.
Keywords: Postconditioning, Hydrogen sulfide, Nitric oxide, S-sulfhydration, S-nitrosylation
1. Introduction
Hydrogen sulfide (H2S) has been demonstrated to reduce myocardial infarction and cardiac ischaemia–reperfusion (I/R) injury.1–3 H2S-mediated cardioprotection has been reported to involve activation of Nrf2,4,5 increased glutathione levels,5 and activation of endothelial nitric oxide synthase (eNOS).6 However, the precise details of the mechanism by which H2S activates these pathways are poorly understood. Furthermore the observation that H2S can elicit protective effects when administered on reperfusion suggests a role for a rapid signalling mechanism.7,8
Similar to nitric oxide (NO) and S-nitrosylation (SNO) signalling, H2S has also been recently suggested to signal via protein S-sulfhydration (SSH), another important redox-based post-translational modification on protein cysteine residues.9,10 In liver, SSH is reported to be more abundant than SNO.11 NO/SNO signalling has been reported to play an important role in cardioprotection.12,13 However, at present, there are no reports of changes in protein SSH with I/R. Thus, a major aim of this study was to examine changes in protein SSH associated with cardioprotection following treatment with a H2S donor on reperfusion. In addition, H2S signalling has also been reported to alter NO bioavailability and to alter eNOS activity.6,14 Indeed the protective effects of H2S against I/R are lost in eNOS phosphomutant mice.6 Furthermore, the H2S activation of eNOS is reported to be mediated by SSH of eNOS.15 Taken together, these studies suggest that H2S-induced protection is NO signalling dependent. Therefore, in this study, we also examined the cross talk, i.e. SSH and SNO, between H2S and NO donors administrated at reperfusion.
2. Methods and materials
2.1. Animals and compounds
Male C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME, USA) were used for all experiments. Mice were between 12 and 16 weeks of age at the time of experimentation. All animals received humane treatment in accordance with National Institutes of Health guidelines and the ‘Guiding Principles for Research Involving Animals and Human Beings’. This study was reviewed and approved by the Institutional Animal Care and Use Committee of the National Heart Lung and Blood Institute. All compounds were obtained from Sigma (St Louis, MO, USA).
2.2. Langendorff-perfused mouse hearts and I/R protocol
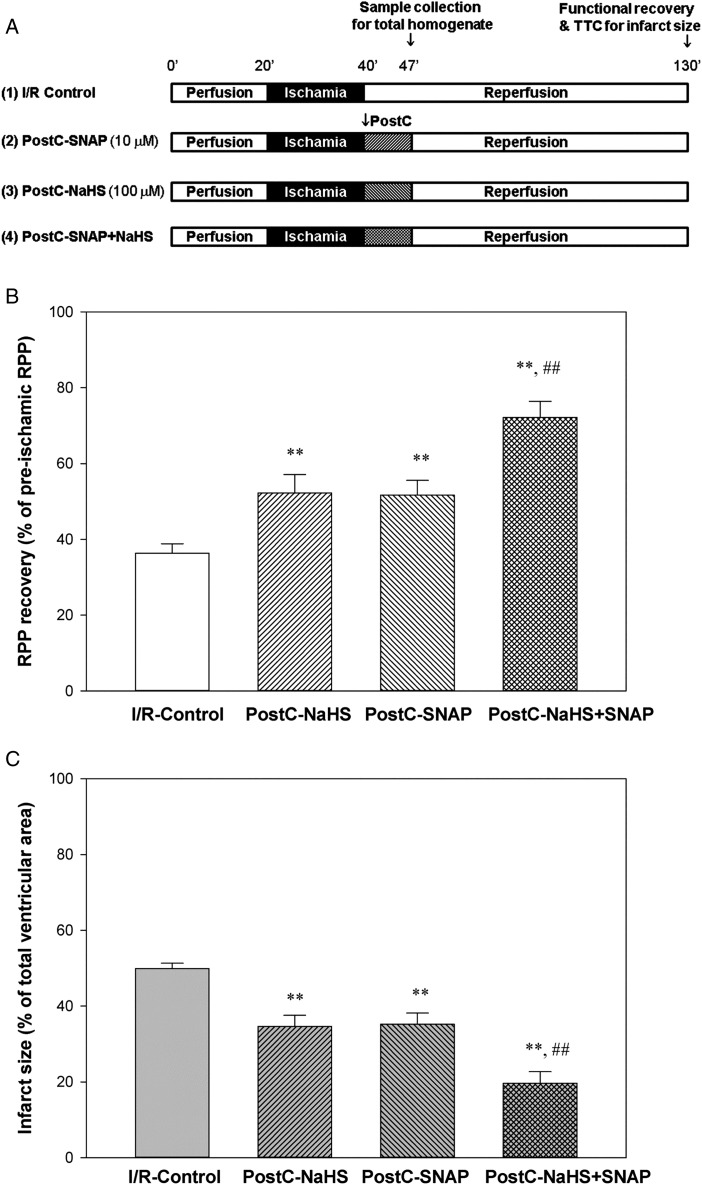
After anaesthesia with pentobarbital and anticoagulation with heparin, a thoracotomy was performed, and the heart was quickly excised and placed in ice-cold Krebs–Henseleit buffer (in mmol/L: 120 NaCl, 11 d-glucose, 25 NaHCO3, 1.75 CaCl2, 4.7 KCl, 1.2 MgSO4, and 1.2 KH2PO4). The aorta was cannulated, and the heart was perfused with Krebs–Henseleit buffer (oxygenated with 95% O2/5% CO2 and maintained at pH 7.4) in retrograde fashion at a constant pressure of 100 cm of water at 37°C. The perfusion was performed in the dark to prevent light-induced SNO decomposition. After equilibrium perfusion for 20 min, mouse hearts were subjected to 20 min of no-flow global ischaemia followed by 90 min of reperfusion. Pharmacological postconditioning (PostC) was performed at the beginning of reperfusion for 7 min with either 100 µmol/L sodium hydrosulfide (NaHS, H2S donor), 10 µmol/L S-nitroso-N-acetylpenicillamine (SNAP, NO/SNO donor), or both given as a simple mixture from an additional reservoir. In some experiments, C-PTIO (2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, an NO scavenger, 20 µmol/L) was also infused together with NaHS or NaHS + SNAP. Hearts were then reperfused with regular Krebs–Henseleit buffer for a total of 90 min of reperfusion. The I/R and PostC protocol is illustrated in Figure 1A.
Figure 1.
Additive cardioprotection induced by PostC with NaHS and SNAP. (A) I/R and PostC protocol. Mouse hearts were Langendorff perfused with Krebs–Henseleit buffer at a constant pressure of 100 cm of water at 37°C in the dark. After equilibrium perfusion for 20 min, mouse hearts were subjected to 20 min of no-flow ischaemia. PostC with either 10 µmol/L SNAP or 100 µmol/L NaHS, or both was applied immediately upon reperfusion for 7 min, followed by regular Krebs–Henseleit buffer till the total 90 min of reperfusion. (B) Post-ischaemic left ventricular RPP functional recovery. (C) Infarct size, measured at the end of reperfusion by 1% TTC staining. Results are expressed as mean ± SE (n = 8 in each group). Statistical significance was determined by two-way ANOVA followed by a post hoc Bonferroni test, **P < 0.01 vs. I/R-Control; ##P < 0.01 vs. PostC-NaHS or PostC-SNAP alone.
2.3. Haemodynamic and infarct size measurements
A latex balloon connected to a pressure transducer was inserted into the left ventricle of Langendorff-perfused mouse hearts to monitor left ventricular developed pressure (LVDP). LVDP was recorded and digitized using a PowerLab system (ADInstruments, Colorado Springs, CO, USA). The rate pressure product (RPP = LVDP × heart rate) was used as an index of cardiac contractile function. The post-ischaemic functional recovery was expressed as percentage of the pre-ischaemic RPP during the equilibrium period. For measurement of myocardial infarct size, at the end of the 90 min of reperfusion, hearts were perfused with 1% (w/v) of 2,3,5-triphenyltetrazolium chloride (TTC) and incubated in TTC at 37°C for 15 min, followed by fixation in 10% (w/v) formaldehyde. Infarct size was expressed as the percentage of the total cross-sectional area of the ventricles.
2.4. Mouse heart total homogenate preparation
Mouse heart total homogenate was prepared for in the dark to prevent SNO decomposition. Each snap-frozen mouse heart was powdered on liquid nitrogen followed by homogenization with a tight-fitting glass Dounce homogenizer on ice in 1.5 mL homogenate buffer containing (in mmol/L): 300 sucrose, 250 HEPES–NaOH (pH 7.8), 1 EDTA, 0.1 neocuproine (a copper chelating agent), and 0.1 deferoxamine (an iron chelating agent). An EDTA-free protease inhibitor tablet (Roche Diagnostics Corporation, Indianapolis, IN, USA) was added to the homogenate buffer just before use. Protein concentration of total heart homogenate was determined using the Bradford protein assay. Total heart homogenates were aliquoted in amber tubes, snap-frozen in liquid nitrogen, and stored at −80°C.
2.5. Identification of SSH/SNO proteins by maleimide-DyLight fluors
A modified biotin switch method11,16 using sulfhydryl-reactive DyLight-maleimide fluors (Thermo Scientific Pierce Biotechnology, Rockford, IL, USA) was applied to identify SSH/SNO proteins. Mouse heart total homogenate (200 µg) was blocked in HEND buffer (pH 8.0 in mmol/L: 250 HEPES–NaOH, 1 EDTA, 0.1 Neocuproine, and 0.1 Deferoxamine) containing 20 mmol/L methyl methanethiosulfonate (MMTS) or N-ethylmaleimide (NEM) and 2.5% (w/v) SDS at 50°C for 20 min with frequent vortex. The MMTS or NEM was then removed by cold acetone precipitation at −20°C for 20 min. After acetone removal, the protein pellets were resuspended in HEND buffer containing 1% (w/v) SDS and divided into two aliquots: (i) one was labelled with 5 mmol/L of DyLight-680-maleimide without Na-ascorbate; (ii) the other was labelled with 5 mmol/L of DyLight-800-maleimide with 1 mmol/L of Na-ascorbate. After incubation on a rotating device for 3 h at room temperature, the DyLight-labelled proteins were subjected to non-reducing 4–12% Bis–Tris SDS–PAGE in the dark. The gel was scanned using a Li-Cor Odyssey scanner (Li-Cor Biosciences, Lincoln, NB, USA) at 700 nm for SSH content (DyLight-680) and 800 nm for SSH/SNO total signalling (DyLight-800), respectively.
2.6. Measurement of SNO occupancy by iodoTMT sixplex label reagent set
We used a modified biotin switch method with iodoacetyl tandem mass tag (iodoTMT) sixplex label reagent set17–20 and an immobilized anti-TMT antibody resin enrichment kit (Thermo Scientific) to measure SNO occupancy in mouse heart total homogenates. Each sample (300 µg) was diluted in HEND buffer with 2.5% (w/v) SDS and protease inhibitor. Samples were incubated with 4.4 mmol/L of iodoTMTX for 2 h at 37°C on a rotation platform in the dark to block free SH and SSH groups. Following removal of iodoTMTX by cold (−20°C) acetone precipitation and centrifugation, iodoTMTX-labelled samples were incubated with 1 mmol/L Na-ascorbate and 4.4 mmol/L iodoTMTY for 2 h at 37°C to label SNO-derived SH groups. Excess iodoTMTY label was removed via cold acetone precipitation. Samples were then resuspended in buffer containing (in mmol/L) 50 NH4HCO3, 1 EDTA, and 0.05% (w/v) SDS, and subjected to trypsin digestion (Promega, Madison, WI, USA) overnight at 37°C with agitation. Samples were concentrated by SpeedVac (Thermo Scientific), diluted in Tris-buffered saline (TBS), and incubated with anti-iodoTMT resin (Thermo Scientific) overnight at 4°C with rotation. The supernatant was removed, and the resin was washed with 3 × 0.5 mL TBS, 3 × 0.5 mL 0.05% (w/w) CHAPS in TBS, 3 × 0.5 mL 2 mol/L of urea in TBS, and 3 × 0.5 mL HPLC-grade water. The iodoTMT-labelled peptides were eluted using 3 × 0.4 mL elution buffer (50% acetonitrile and 0.1% trifluoroacetic acid). All fractions were combined and concentrated through the SpeedVac at 45°C and then resuspended in 2.5% acetonitrile/0.1% formic acid. Each sample was cleaned with a C18 column ZipTip (Millipore, Billerica, MA, USA). Liquid chromatography–tandem mass spectrometry was performed using an LTQ Orbitrap Elite mass spectrometer (Thermo Scientific).
2.7. LC–MS/MS analysis and database search
MS analysis was performed using an Eksigent nanoLC-Ultra 1D plus system (Dublin, CA, USA) coupled to an LTQ Orbitrap Elite mass spectrometer (Thermo Scientific) using HCD fragmentation. Peptides were first loaded onto a Zorbax 300SB-C18 trap column (Agilent, Palo Alto, CA, USA) at a flow rate of 6 mL/min for 6 min and then separated on a reversed-phase PicoFrit analytical column (New Objective, Woburn, MA, USA) using a 40-min linear gradient of 5–40% acetonitrile. Full MS spectra were collected at a resolution of 60 K (for ions at m/z 400) over the range of m/z 300–2000. MS/MS was performed in the data-dependent mode. The six most abundant parent ions were selected for MS/MS using high-energy collisional dissociation with a normalized collision energy setting of 35%. The MS/MS spectra were collected at a resolution of 15 K (m/z 400). Precursor ion activation was performed with an isolation width of 2 Da and a minimal intensity of 5000 counts.
The raw files generated from the LTQ Orbitrap Elite were analysed with Proteome Discoverer v1.4 software (Thermo Scientific), and all MS/MS spectra were searched with Sequest HT algorithm against the Mus musculus (mouse) sequence database (UniProt, released on 15 March 2015). The search parameters used were 15 ppm tolerance for precursor ion masses, 0.1 Da for fragment ion masses, a maximum of two missed tryptic cleavages, variable modifications: oxidation (M), acetyl (peptide N-term), deamidation (N,Q), and iodoTMT on cysteine (+329.227 Da). Each peptide spectrum match was validated with a false discovery rate of 1%.
2.8. Data analysis
Results are expressed as mean ± SE. Statistical significance was determined by two-way ANOVA followed by a post hoc Bonferroni test.
3. Results
3.1. Cardioprotection at reperfusion with a H2S donor
H2S donors are protective in several experimental models, including PostC against I/R injury.7,21 Although some of the signalling pathways that are mediated by H2S have been elucidated, the mechanistic molecular basis for this protective effect remains unclear. SSH appears to be an important element in these molecular mechanisms, but this has not been tested.
NaHS, a fast H2S-releasing donor with ∼20% forming undissociated H2S in aqueous solution, was employed in this study. Previous studies have shown that NaHS at 100 µmol/L exhibited the maximal cardioprotective effect in an isolated rat heart PostC model.21 We first confirmed that addition on reperfusion of 100 µmol/L NaHS was cardioprotective. As shown in Table 1 and Figure 1, treatment at reperfusion with NaHS significantly increased the post-ischaemic functional recovery, as the cardiac RPP recovery was 52.3 ± 4.8% (n = 8) in PostC-NaHS hearts compared with 36.4 ± 2.5% (n = 8) in I/R control hearts. Infarct size was also significantly reduced by NaHS treatment on reperfusion, i.e. 34.6 ± 2.9% (n = 8) in PostC-NaHS hearts vs. 49.9 ± 1.4% (n = 8) in I/R control hearts.
Table 1.
Haemodynamic parameters
| Heart samples | n | BW (g) | Pre-ischaemic equilibration |
End of reperfusion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR (mL/min) | HR (bpm) | LVDP (cmH2O) | +dp/dt | −dp/dt | FR (mL/min) | HR (bpm) | LVDP (cmH2O) | +dp/dt | −dp/dt | |||
| (cmH2O/ms) | (cmH2O/ms) | |||||||||||
| I/R-Control | 8 | 27.7 ± 0.6 | 2.7 ± 0.1 | 398 ± 11 | 123 ± 5 | 7.8 ± 0.2 | −5.6 ± 0.2 | 2.1 ± 0.1 | 360 ± 14 | 50 ± 3 | 4.3 ± 0.2 | −3.2 ± 0.1 |
| PostC-NaHS | 8 | 27.5 ± 0.6 | 2.5 ± 0.1 | 395 ± 13 | 121 ± 4 | 7.6 ± 0.5 | −5.4 ± 0.4 | 1.9 ± 0.1 | 356 ± 16 | 71 ± 5* | 5.7 ± 0.4* | −4.3 ± 0.3* |
| PostC-SNAP | 8 | 26.7 ± 0.5 | 2.5 ± 0.1 | 410 ± 10 | 121 ± 5 | 7.8 ± 0.5 | −5.2 ± 0.2 | 1.9 ± 0.1 | 350 ± 10 | 73 ± 6* | 5.7 ± 0.2* | −4.1 ± 0.2* |
| PostC-NaHS + SNAP | 8 | 27.2 ± 0.5 | 2.6 ± 0.1 | 381 ± 13 | 129 ± 4 | 8.1 ± 0.3 | −5.7 ± 0.2 | 2.0 ± 0.1 | 360 ± 10 | 96 ± 6** | 7.2 ± 0.3** | −5.4 ± 0.3** |
Values are mean ± SE.
n, number of hearts; BW, body weight; FR, flow rate; HR, heart rate (bpm); LVDP, left ventricular developed pressure; ±dp/dt, rates of pressure rise and fall, respectively.
*P < 0.01, vs. I/R-Control.
**P < 0.01 vs. PostC-NaHS or PostC-SNAP.
3.2. Additive cardioprotection induced by PostC with H2S and NO donors
Our previous study showed that PostC with an NO donor, SNAP (10 µmol/L) elicited a comparable cardioprotection to that induced by ischaemic PostC.22 Because recent studies have suggested crosstalk between H2S and NO signalling,6,23,24 we next investigated whether PostC with NaHS and SNAP together would elicit an additive cardioprotection. In addition to treating hearts on reperfusion with NaHS, we also treated hearts with SNAP (10 µmol/L) and with 100 µmol/L NaHS and 10 µmol/L SNAP together. As shown in Table 1 and Figure 1, 10 µmol/L of SNAP was as protective as 100 µmol/L of NaHS, improving recovery of function on reperfusion. The RPP recovery for PostC-SNAP was 51.7 ± 3.9% (n = 8) and the infarct size was 35.2 ± 2.9% (n = 8). Interestingly, treatment on reperfusion with both NaHS and SNAP simultaneously led to additional protection compared with that induced by either donor treatment alone, i.e. RPP recovery was increased to 72.29 ± 4.2% (n = 8) and infarct size was decreased to 19.7 ± 3.0% (n = 8). Therefore, similar to the findings reported by others,7,21,22,25 PostC with either H2S or NO donor was protective against I/R injury. Interestingly, PostC with both H2S and NO donors simultaneously elicited an additive cardioprotection against I/R injury.
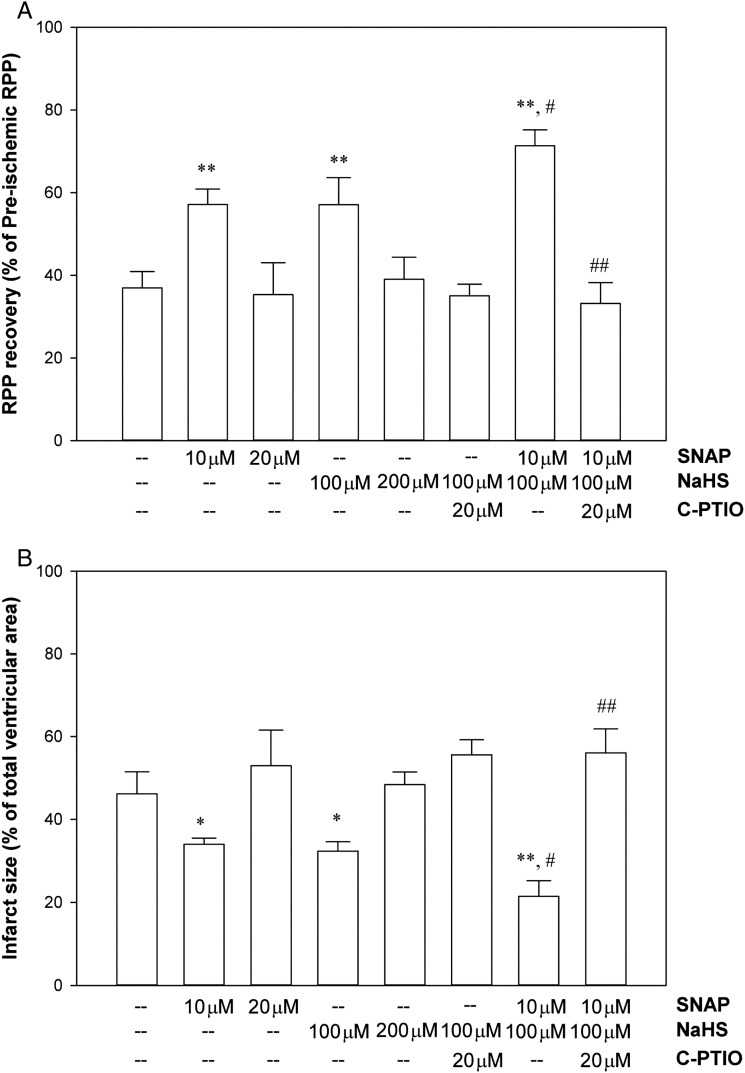
The cardioprotection with H2S has been reported to be lost in eNOS phosphomutant mice, and H2S is reported to alter eNOS activity,6 suggesting H2S might enhance cardioprotection via an NO-dependent signalling pathway. To further address whether the additive protection induced by PostC with NaHS + SNAP is via the same signalling pathway, we carried out another set of experiments to test (i) whether simply increasing the dose of each donor would enhance protection to the level of the additive protection by PostC with both donors together; (ii) whether an NO scavenger, C-PTIO (20 µmol/L), would block the protection. As shown in Figure 2, a higher dose of either SNAP (20 µmol/L) or NaHS (200 µmol/L) alone actually led to a loss rather than enhanced protection compared with the lower doses (10 µmol/L of SNAP or 100 µmol/L of NaHS). These results are consistent with data in the literature showing that these drugs can have detrimental effects at higher doses.7,20 In addition, co-infusion of C-PTIO with NaHS or SNAP + NaHS blocked the protection induced by NaHS and also the additive protection by SNAP + NaHS together, further suggesting that the cardioprotection induced by PostC-NaHS or PostC-SNAP + NaHS is depended on NO signalling.
Figure 2.
Dose- and NO-dependent cardioprotection induced by PostC with H2S/NO donors. (A) Post-ischaemic left ventricular RPP functional recovery. (B) Infarct size, measured at the end of reperfusion by 1% TTC staining. Results are expressed as mean ± SE (n = 4 in each group). Statistical significance was determined by two-way ANOVA followed by a post hoc Bonferroni test, *P < 0.05 and **P < 0.01 vs. Control; #P < 0.05 vs. PostC-NaHS (100 µmol/L) or PostC-SNAP (10 µmol/L) alone; ##P < 0.01 vs. PostC-NaHS + SNAP.
3.3. Identification of SSH/SNO by Dylight-maleimide fluors switch method
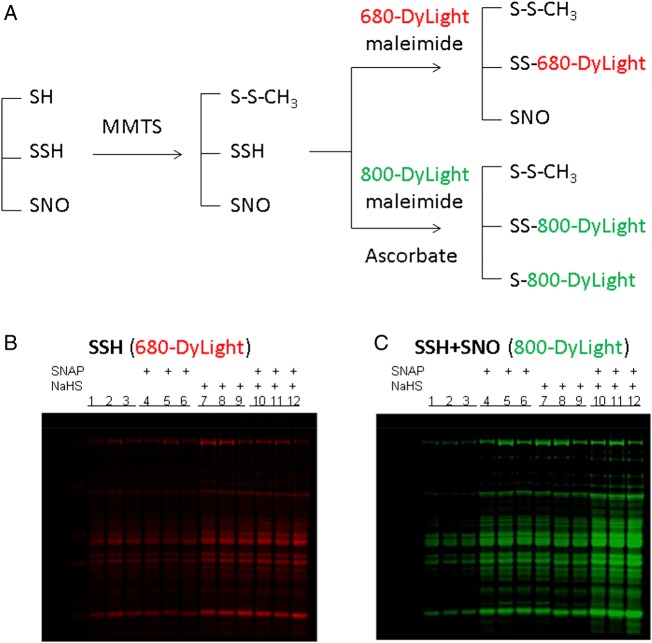
It has been suggested that both H2S and NO can regulate protein function through redox-based post-translational modification on protein cysteine residue(s), i.e. SSH and SNO.9,10,12 We next investigated whether the cardioprotection that is observed with addition of NaHS on reperfusion leads to an increase in SSH. We adapted the modified biotin switch method described by Synder's group in which MMTS is used as thiol-blocking agent.11 As illustrated in the protocol (Figure 3A, top panel), MMTS was added to the extract to block free SH groups and followed by addition of DyLight-680-maleimide to label SSH. As shown in Figure 3B, I/R-untreated hearts had a low level of DyLight-680 intensity, which might represent the endogenous SSH content in these hearts. Treatment with NaHS on reperfusion significantly increased DyLight-680/SSH intensity.
Figure 3.
SSH/SNO detection by modified biotin switch method with DyLight-maleimide fluors. A modified DyLight-maleimide fluors switch protocol (A) using MMTS as thiol-blocking agent was used to label different cysteine residues derived from protein SSH (top panel) and SSH/SNO (bottom panel). After non-reducing SDS–PAGE, the gel was scanned using a Li-Cor Odyssey scanner (Li-Cor Biosciences, Lincoln, NB, USA) at 700 nm for SSH/DyLight-680-maleimide (B) and 800 nm for SSH + SNO/DyLight-800-maleimide signalling (C), respectively.
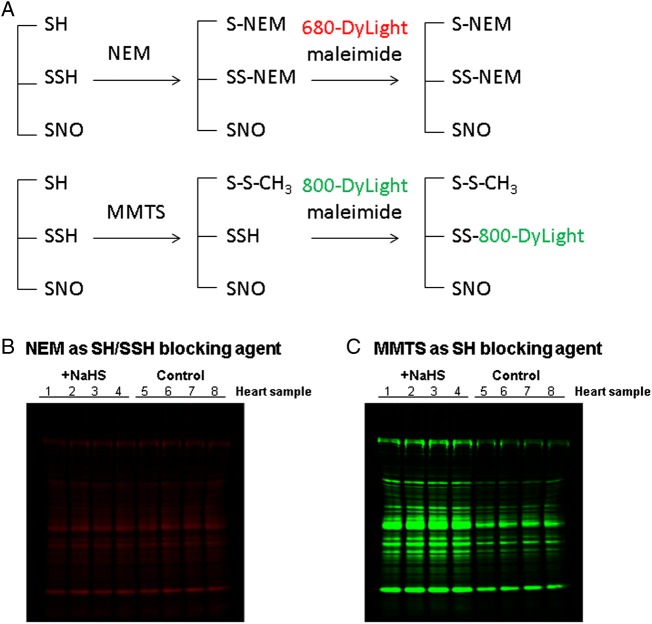
A question that has been raised for SSH detection is whether MMTS selectively blocks SH but not SSH.17,26 The data in Figure 3 indicate that this is possible, since we observed an increase of labelling with exogenous NaHS treatment in the perfused heart, and this would not be seen if MMTS blocked both SH and SSH groups. Furthermore, this method has been successfully used to detect SSH in several recent publications.15,27 To further test whether MMTS blocks SSH, we compared blocking with NEM to MMTS. Compared with MMTS, NEM is generally agreed to irreversibly block both SH and SSH groups. If we see an increase in fluorescent labelling on addition of NaHS only with MMTS, this would lend support for the concept that either MMTS does not block SSH groups, or that it forms a labile intermediate modification that is replaced by the DyLight-maleimide, or that H2S leads to a modification other than SSH which is not blocked by MMTS. We pretreated heart total homogenate with or without NaHS and then compared SH/SSH blocking with NEM vs. MMTS. When we blocked with NEM, no difference in DyLight-680-maleimide fluorescence was detected between samples with or without NaHS pretreatment, suggesting that SH and SSH were both blocked by NEM (Figure 4B). However consistent with our data in Figure 3 and other published studies,11,28 when we blocked with MMTS, we observed a significant increase of DyLight-800-maleimide fluorescence intensity in NaHS-treated protein samples compared with their individual control (Figure 4C), consistent with the concept that MMTS blocking can differentiate SH and SSH.
Figure 4.
SSH detection by DyLight-maleimide fluors switch method using MMTS or NEM as SH/SSH-blocking agent. (A) NEM (top panel) vs. MMTS (bottom panel) was used as SH/SSH-blocking agent in the modified DyLight-maleimide fluors switch method. NaHS-pretreated heart total homogenate samples were split into two aliquots, which are subjected to SH/SSH blocking by NEM and DyLight-600-maleimide labelling (B) or SH blocking by MMTS and DyLight-800-maleimide labelling (C), respectively.
3.4 Crosstalk between SSH and SNO signalling
We next wanted to compare the changes in SSH/SNO among the hearts reperfused with NaHS or SNAP alone vs. NaHS + SNAP. As previously discussed addition of NaHS on reperfusion increased SSH. As further shown in Figure 3B, addition of SNAP on reperfusion did not alter SSH levels; however, addition of NaHS + SNAP resulted in an increase in SSH which was similar to that observed with NaHS treatment alone. We next examined the effect of these reperfusion treatments on SNO using the traditional dye switch method. As shown in Figure 3C, we found that reperfusion treatment with NaHS or SNAP resulted in similar increases in DyLight-800-maleimide intensity, which were both higher than that in untreated hearts. Interestingly, reperfusion treatment with the two donors together dramatically increased DyLight-800 intensity. One caveat with using the traditional dye switch method (which uses MMTS to block) is that the method will measure both SSH and SNO. By blocking with MMTS, we therefore cannot determine whether the increase in fluorescence that we observe with NaHS is due to an H2S-dependent increase in SSH or whether the increase in fluorescence is the sum of SNO + SSH. Therefore to be sure that we were measuring only changes due to SNO, we used iodoTMT to block, which would be similar to NEM and block both SH and SSH groups. This method would also have the advantage of allowing us to measure SNO occupancy.18–20
We used the newly available iodoTMT sixplex label reagent set iodoTMT126 to 131 (Thermo Scientific) to evaluate changes in SNO occupancy using a method we previously developed.18–20 We measured SNO occupancy in I/R control, PostC-NaHS, and PostC-SNAP + NaHS hearts. As data shown in Table 2, we found that treatment on reperfusion with NaHS led to an increase of SNO occupancy level. Furthermore, reperfusion treatment with H2S and NO donors together led to an additional increase in SNO occupancy, almost a two-fold increase compared with the level found in PostC-NaHS hearts. In addition, PostC simultaneously with NaHS and SNAP together elicited an additive increase only in SNO (Table 2) but not in SSH (Figure 3).
Table 2.
SNO proteins identified by iodoTMT proteomic analysis in I/R control, PostC-NaHS, and PostC-NaHS + SNAP hearts
| Protein name | ID | Peptide sequence | SNO-Cys | SNO occupancy [iodoTMTy/(iodoTMTx + iodoTMTy)] |
||
|---|---|---|---|---|---|---|
| I/R control | PostC-NaHS | PostC-NaHS + SNAP | ||||
| Aconitate hydratase | Q99KI0 | VGLIGScTNSSYEDMGR | 385 | 0.013 ± 0.001 | 0.032 ± 0.005* | 0.048 ± 0.019* |
| ADP/ATP translocase 1 | P48962 | YFAGNLASGGAAGATSLcFVYPLDFAR | 129 | 0.016 ± 0.004 | 0.031 ± 0.006** | |
| Aspartate aminotransferase | P05202 | NLDKEYLPIGGLAEFcK | 106 | 0.028 ± 0.014 | 0.062 ± 0.003* | 0.074 ± 0.004* |
| Branched-chain amino acid aminotransferase | O35855 | AWIGGVGDcK | 229 | 0.038 ± 0.017 | 0.082 ± 0.004* | 0.120 ± 0.028*,** |
| Carnitine O-palmitoyltransferase 1, muscle isoform | Q924X2 | GVYPGSPTSWLVVVMATVGSNYcK | 75 | 0.015 ± 0.010 | 0.040 ± 0.004* | 0.079 ± 0.023*,** |
| Creatine kinase M-type | P07310 | AGHPFMWNEHLGYVLTcPSNLGTGLR | 317 | 0.017 ± 0.003 | 0.063 ± 0.004* | 0.110 ± 0.021*,** |
| Creatine kinase S-type | Q6P8J7 | SEVELVQIVIDGVNYLVDcEK | 397 | 0.025 ± 0.001 | 0.056 ± 0.011** | |
| Cysteine and glycine-rich protein 3 | P50462 | SLESTNVTDKDGELYcK | 168 | 0.026 ± 0.012 | 0.069 ± 0.006* | 0.093 ± 0.004*,** |
| Cytochrome b-c1 complex subunit 1 | Q9CZ13 | YFYDQcPAVAGYGPIEQLPDYNR | 453 | 0.017 ± 0.002 | 0.044 ± 0.009* | 0.066 ± 0.015*,** |
| Cytochrome c oxidase subunit 5B | P19536 | IVGcIcEEDNCTVIWFWLHKGESQR | 90, 92 | 0.034 ± 0.002 | 0.062 ± 0.008** | |
| Dihydrolipoyl dehydrogenase | O08749 | AEVITcDVLLVcIGR | 306, 312 | 0.039 ± 0.012 | 0.093 ± 0.015* | 0.127 ± 0.017*,** |
| Electron transfer flavoprotein subunit-β | Q9DCW4 | HSMNPFcEIAVEEAVR | 42 | 0.032 ± 0.005 | 0.073 ± 0.007** | |
| Electron transfer flavoprotein oxidoreductase | Q921G7 | FcPAGVYEFVPLEQGDGFR | 560 | 0.039 ± 0.004 | 0.080 ± 0.009** | |
| ES1 protein homologue | Q9D172 | VALVLSGcGVYDGTEIHEASAILVHLSR | 50 | 0.035 ± 0.014 | 0.090 ± 0.010* | 0.124 ± 0.008*,** |
| F1-ATP synthase subunit-α | Q03265 | YTIVVSATASDAAPLQYLAPYSGcSMGEYFR | 294 | 0.035 ± 0.007 | 0.064 ± 0.001* | 0.091 ± 0.017*,** |
| Fructose-bisphosphate aldolase A | P05064 | cPLLKPWALTFSYGR | 290 | 0.011 ± 0.003 | 0.059 ± 0.004* | 0.079 ± 0.010*,** |
| Glyceraldehyde-3-phosphate dehydrogenase | P16858 | VPTPNVSVVDLTcR | 245 | 0.020 ± 0.002 | 0.034 ± 0.003* | 0.050 ± 0.006*,** |
| Heat shock protein HSP 90-β | P11499 | LVSSPccIVTSTYGWTANMER | 589, 590 | 0.010 ± 0.001 | 0.030 ± 0.006* | 0.052 ± 0.002*,** |
| Heparin-binding growth factor 1 | P61148 | LLYcSNGGHFLR | 31 | 0.175 ± 0.007 | 0.263 ± 0.061* | 0.336 ± 0.025*,** |
| High mobility group protein B3 | O54879 | MSAYAFFVQTcR | 23 | 0.040 ± 0.003 | 0.114 ± 0.016* | 0.142 ± 0.011*,** |
| Histidine triad nucleotide-binding protein 1 | P70349 | cLAFHDISPQAPTHFLVIPK | 38 | 0.033 ± 0.007 | 0.105 ± 0.016* | 0.145 ± 0.012*,** |
| Hydroxyacyl-coenzyme A dehydrogenase | Q61425 | TFESLVDFcK | 201 | 0.179 ± 0.008 | 0.219 ± 0.006* | 0.256 ± 0.029*,** |
| 3-hydroxyisobutyrate dehydrogenase | Q99L13 | IcNNMLLAISMIGTAEAMNLGIR | 210 | 0.046 ± 0.008 | 0.082 ± 0.003** | |
| Isocitrate dehydrogenase [NADP] | P54071 | VcVQTVESGAMTK | 402 | 0.069 ± 0.012 | 0.103 ± 0.005* | 0.110 ± 0.011* |
| Long-chain specific acyl-CoA dehydrogenase | P51174 | LLIAELAISAcEFMFEETR | 303 | 0.017 ± 0.007 | 0.064 ± 0.011* | 0.084 ± 0.005*,** |
| MACRO domain-containing protein 1 | Q922B1 | ScYLSSLDLLLEHR | 244 | 0.032 ± 0.006 | 0.141 ± 0.015* | 0.202 ± 0.018*,** |
| Malate dehydrogenase | P08249 | GcDVVVIPAGVPR | 93 | 0.018 ± 0.002 | 0.044 ± 0.011* | 0.049 ± 0.003* |
| Mitochondrial carnitine/acylcarnitine carrier protein | Q9Z2Z6 | LQTQPPSLSGQPPMYSGTLDcFR | 58 | 0.029 ± 0.002 | 0.054 ± 0.009** | |
| Myoglobin | P04247 | HGcTVLTALGTILK | 67 | 0.031 ± 0.004 | 0.089 ± 0.013* | 0.118 ± 0.011*,** |
| Myosin-6 | Q02566 | MESDLTQLQTEVEEAVQEcR | 1750 | 0.043 ± 0.004 | 0.076 ± 0.005** | |
| NADH dehydrogenase [ubiquinone] flavoprotein 1 | Q91YT0 | LKPPFPADVGVFGcPTTVANVETVAVSPTIcR | 238, 255 | 0.015 ± 0.004 | 0.033 ± 0.007* | 0.068 ± 0.018*,** |
| Peptidyl-prolyl cis-trans isomerase F | Q99KR7 | HVGPGVLSMANAGPNTNGSQFFIcTIK | 156 | 0.014 ± 0.002 | 0.054 ± 0.009* | 0.079 ± 0.011*,** |
| Peroxiredoxin-2 | Q61171 | KLGcEVLGVSVDSQFTHLAWINTPR | 70 | 0.024 ± 0.011 | 0.073 ± 0.010* | 0.083 ± 0.008* |
| Phosphoglucomutase-1 | Q9D0F9 | LSLcGEESFGTGSDHIR | 374 | 0.042 ± 0.031 | 0.105 ± 0.003* | 0.152 ± 0.010*,** |
| Profilin-1 | P62962 | cYEMASHLR | 128 | 0.102 ± 0.028 | 0.151 ± 0.019* | 0.198 ± 0.021*,** |
| Protein DJ-1 | Q99LX0 | KGLIAAIcAGPTALLAHEVGFGcK | 106, 121 | 0.024 ± 0.010 | 0.089 ± 0.013* | 0.113 ± 0.009*,** |
| Pyruvate dehydrogenase E1 component subunit β | Q9D051 | TNHLVTVEGGWPQFGVGAEIcAR | 306 | 0.014 ± 0.012 | 0.094 ± 0.008* | 0.099 ± 0.012* |
| 40S ribosomal protein S11 | P62281 | cPFTGNVSIR | 60 | 0.073 ± 0.040 | 0.135 ± 0.011* | 0.199 ± 0.012*,** |
| Short-chain specific acyl-CoA dehydrogenase | Q07417 | AcASTGVIMSVNNSLYLGPILK | 109 | 0.013 ± 0.003 | 0.045 ± 0.003* | 0.060 ± 0.005*,** |
| Succinate dehydrogenase flavoprotein subunit | Q8K2B3 | TYFScTSAHTSTGDGTAMVTR | 266 | 0.019 ± 0.007 | 0.082 ± 0.012* | 0.124 ± 0.019*,** |
| Trifunctional enzyme subunit α | Q8BMS1 | ALMGLYNGQVLcK | 349 | 0.033 ± 0.001 | 0.053 ± 0.016** | |
| Triosephosphate isomerase | P17751 | IIYGGSVTGATcK | 268 | 0.036 ± 0.010 | 0.078 ± 0.014** | |
SNO cysteine residues (SNO-Cys) are labelled by iodoTMTy and shown in lower case (c). Protein identifications were accepted based on two or more unique peptides with a false discovery rate (FDR) of 99% or higher. SNO-modified proteins and peptides listed in the Table were identified from each of three individual samples.
*P < 0.05 vs. I/R control.
**P < 0.05 vs. PostC-NaHS; n = 3 in each group.
4. Discussion
Both H2S and NO signalling molecules have been suggested to play important roles in PostC-induced protection.8,29 It has been long known that addition of NO donors on reperfusion is cardioprotective.20,30,31 A recent study suggested that ischaemic PostC prolongs early acidosis, and this would favour the formation of protein SNO.32 We have recently demonstrated that ischaemic PostC leads to an increase in protein SNO.22 In addition, Penna et al.33 showed that pharmacological PostC by diazoxide induced mitochondrial protein SNO. Cardioprotection similar to that obtained with ischaemic PostC could be also achieved with pharmacological PostC with NO/SNO donors20,22,25,30,31 and mito-SNO34 (a mitochondria-targeted S-nitrosothiol agent). In murine hearts, it has been shown that the protection afforded by ischaemic PostC or mito-SNO was independent of the NO/cGMP/PKG signalling pathway, suggesting an important role for SNO signalling in PostC.22,34
H2S has also been shown to be cardioprotective when added on reperfusion.7,8,35 Recent studies suggest crosstalk between H2S and NO.23,24,36,37 It has been shown that eNOS inhibition led to the abrogation of H2S-induced cardioprotection in mice,38 and that H2S increased NO production via activation of eNOS in endothelial cells.39 Minamishima et al.40 found that H2S improves survival after cardiac arrest and cardiopulmonary resuscitation in mice, and that this is also through activation of eNOS. King et al. found that mice lacking the H2S-producing enzyme cystathionine γ-lyase (CSE) exhibit dysfunctional eNOS, diminished NO levels, and exacerbated I/R injury. Acute H2S therapy in CSE-deficient mice restored eNOS function, NO bioavailability, and attenuated I/R injury while H2S fails to protect in eNOS (S1179A) phosphomutant inactive mice.6 These results suggest that H2S-mediated cardioprotection against I/R injury is dependent on eNOS activation and NO generation. However, the dependence of H2S on eNOS maybe species dependent as a recent study using rabbit found that H2S protection was not blocked by inhibition of eNOS.41 Interestingly, this same study reported that inhibition of eNOS blocked cardioprotection of NaHS in mouse.41
To further examine the crosstalk between NO and H2S, we measured protein SNO and SSH following addition of NaHS and SNAP added on reperfusion. We found that PostC-NaHS results in a modest formation of SSH, but primarily led to an increase of SNO to a level comparable to that found in pharmacological PostC-SNAP hearts. Furthermore, pharmacological PostC with SNAP + NaHS had an additive protection compared with pharmacological PostC-SNAP or PostC-NaHS alone, correlating with an additive increase of SNO found in PostC-SNAP + NaHS hearts. These results provide evidence to support the hypothesis that H2S-mediated cardioprotection is dependent on NO/SNO signalling. In further support of this concept, we find that C-PTIO blocked the protection afforded by NaHS and by NaHS + SNAP.
The application H2S and NO donors simultaneously in vitro could lead to formation of reactive species such as thionitrous acid (HSNO), a powerful SNO donor,42,43 which might be an explanation for the additive effects by PostC-NaHS + SNAP-induced cardioprotection against I/R injury (Table 1 and Figure 1) and SNO formation (Table 2). In addition, a possible involvement of nitroxyl (HNO) has also been suggested as a mechanism of crosstalk between H2S and NO,24,44 which might also play an important role in the regulation of cardiac function.23,24,45 NO and H2S can generate a number of additional reactive compounds,37,46 and it is possible that one of these compounds might react with free thiols to generate a thiol modification that is blocked by MMTS but not by NEM, and this might account for the increase in fluorescence observed with H2S treatment. Using the same strategy as our previous study,19 we were able to measure SNO occupancy and detect SNO-cysteine sites by using an iodoTMT sixplex label reagent set (Table 2). This study has identified a number of proteins (Table 2) that undergo SNO with pharmacological PostC with H2S/NO donors, and most of them are mitochondrial proteins that have been also found in ischaemic PostC hearts, such as aconitate hydratase, creatine kinase, cytochrome b-c1 complex subunit 1, electron transfer flavoprotein oxidoreductase, F1-ATP synthase subunit α, heat shock protein HSP90-β, isocitrate dehydrogenase, malate dehydrogenase, peptidyl-prolyl cis-trans isomerase F (cyclophilin D), succinyl-CoA dehydrogenase flavoprotein subunit.22 SNO of these mitochondrial proteins has been shown to elicit cardioprotective effects against I/R injury.12,47 For example, cardiac resynchronization therapy led to SNO of F1-ATP synthase subunit α, which has been found to prevent Cys294 disulfide formation and protect against oxidative stress in dyssynchronous heart failure.48 Nguyen et al.49 found that SNO of cyclophilin D prevents oxidative stress-induced activation of mitochondrial permeability transition pore opening thus cell death.
The Langendorff-perfused heart model allows us to test the effect of the NaHS and SNAP directly on heart. Addition of these donors in vivo could affect the heart via indirect mechanisms, which could complicate a mechanistic interpretation. Therefore, for the initial study, we examined the direct effect of these donors on the heart. The perfused heart model also allows a model of global ischaemia, which has advantages for measuring proteomic changes in SNO and SSH, because all the tissue is uniform. With an in vivo model, one needs to be sure that one is only sampling ischaemic tissue, which can be difficult in a small mouse heart. Although the perfused heart model has advantages for elucidating mechanisms, one should be cautious about translating findings from a perfused heart to an in vivo heart.
In summary, this study provides several novel findings. We report for the first time that NaHS addition results in an increase in SNO. We further show that co-addition on reperfusion of NaHS with SNAP leads to a synergistic increase in cardioprotection with improved functional recovery and reduced infarct size. This additive protection correlates with an additive increase in SNO; there was no additional increase in SSH. Taken together, these data suggest the protection afforded by NaHS and the additive protection with NaHS and SNAP are largely due to an increase in SNO.
Conflict of interest: none declared.
Funding
This work was supported by the NIH/NHLBI Intramural Program (J.S., A.A., S.M., M.G., and E.M.) and NIH 5R01HL039752 (C.S.).
References
- 1.Nicholson CK, Calvert JW. Hydrogen sulfide and ischemia–reperfusion injury. Pharmacol Res 2010;62:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 2014;114:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabó G, Veres G, Radovits T, Gerő D, Módis K, Miesel-Gröschel C, Horkay F, Karck M, Szabó C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide 2011;25:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvert J, Jha S, Gundewar S, Elrod J, Ramachandran A, Pattillo C, Kevil C, Lefer D. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 2009;105:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of keap1 and activation of Nrf2. Antioxid Redox Signal 2013;18:1906–1919. [DOI] [PubMed] [Google Scholar]
- 6.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao Y-X, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA 2014;111:3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Y, Pang Q-F, Xu G, Wang L, Wang J-K, Zeng Y-M. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol 2008;587:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y-E, Tang Z-H, Xie WEI, Shen X-T, Liu M-H, Peng X-P, Zhao Z-Z, Nie D-B, Liu L-S, Jiang Z-S. Endogenous hydrogen sulfide mediates the cardioprotection induced by ischemic postconditioning in the early reperfusion phase. Exp Ther Med 2012;4:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu C, Kavalier A, Lukyanov E, Gross SS. S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: a common paradigm for gasotransmitter signaling by H2S and NO. Methods 2013;62:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul BD, Snyder SH. H2S signaling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 2012;13:499–507. [DOI] [PubMed] [Google Scholar]
- 11.Mustafa A, Gadalla M, Sen N, Kim S, Mu W, Gazi S, Barrow R, Yang G, Wang R, Snyder S. H2S signals through protein S-sulfhydration. Sci Signal 2009;2:ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res 2010;106:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy E, Kohr M, Sun J, Nguyen T, Steenbergen C. S-nitrosylation: a radical way to protect the heart. J Mol Cell Cardiol 2012;52:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol 2012;302:H2410–H2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 2014;7:ra87. [DOI] [PubMed] [Google Scholar]
- 16.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001;86:PL1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park C-M, Carroll KS, Filipovic MR, Xian M. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl 2014;53:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray CI, Chung H, Uhrigshardt H, Van Eyk JE. Quantification of mitochondrial S-nitrosylation by cysTMT(6) switch assay. Methods Mol Biol 2013;1005:169–179. [DOI] [PubMed] [Google Scholar]
- 19.Kohr MJ, Aponte A, Sun J, Gucek M, Steenbergen C, Murphy E. Measurement of S-nitrosylation occupancy in the myocardium with cysteine-reactive tandem mass tags. Circ Res 2012;111:1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell RM, Maddock HL, Yellon DM. The cardioprotective and mitochondrial depolarising properties of exogenous nitric oxide in mouse heart. Cardiovasc Res 2003;57:405–415. [DOI] [PubMed] [Google Scholar]
- 21.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian J-S. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol 2008;295:H1330–H1340. [DOI] [PubMed] [Google Scholar]
- 22.Tong G, Aponte AM, Kohr MJ, Steenbergen C, Murphy E, Sun J. Postconditioning leads to an increase in protein S-nitrosylation. Am J Physiol 2014;306:H825–H832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yong Q-C, Cheong JL, Hua F, Deng L-W, Khoo YM, Lee H-S, Perry A, Wood M, Whiteman M, Bian J-S. Regulation of heart function by endogenous gaseous mediators–crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Signal 2011;14:2081–2091. [DOI] [PubMed] [Google Scholar]
- 24.Yong Q-C, Hu L-F, Wang S, Huang D, Bian J-S. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res 2010;88:482–491. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MV, Yang X-M, Liu Y, Solenkova NV, Downey JM. Cardioprotective PKG-independent NO signaling at reperfusion. Am J Physiol 2010;299:H2028–H2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan J, Carroll KS. Persulfide reactivity in the detection of protein S-sulfhydration. ACS Chem Biol 2013;8:1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mir S, Sen T, Sen N. Cytokine-induced gapdh sulfhydration affects PSD95 degradation and memory. Mol Cell 2014;56:786–795. [DOI] [PubMed] [Google Scholar]
- 28.Sen N, Paul Bindu D, Gadalla Moataz M, Mustafa Asif K, Sen T, Xu R, Kim S, Snyder Solomon H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 2012;45:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol 2015;172:1587–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabla R, Buda AJ, Flynn DM, Salzberg DB, Lefer DJ. Intracoronary nitric oxide improves postischemic coronary blood flow and myocardial contractile function. Am J Physiol 1995;269:H1113–H1121. [DOI] [PubMed] [Google Scholar]
- 31.Lefer DJ, Nakanishi K, Johnston WE, Vinten-Johansen J. Antineutrophil and myocardial protecting actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion of dogs. Circulation 1993;88:2337–2350. [DOI] [PubMed] [Google Scholar]
- 32.Penna C, Perrelli M-G, Tullio F, Moro F, Parisella M, Merlino A, Pagliaro P. Post-ischemic early acidosis in cardiac postconditioning modifies the activity of antioxidant enzymes, reduces nitration, and favors protein S-nitrosylation. Pflugers Arch 2011;462:219–233. [DOI] [PubMed] [Google Scholar]
- 33.Penna C, Perrelli MG, Tullio F, Angotti C, Camporeale A, Poli V, Pagliaro P. Diazoxide postconditioning induces mitochondrial protein S-nitrosylation and a redox-sensitive mitochondrial phosphorylation/translocation of risk elements: no role for SAFE. Basic Res Cardiol 2013;108:371. [DOI] [PubMed] [Google Scholar]
- 34.Methner C, Lukowski R, Grube K, Loga F, Smith RJ, Murphy M, Hofmann F, Krieg T. Protection through postconditioning or a mitochondria-targeted S-nitrosothiol is unaffected by cardiomyocyte-selective ablation of protein kinase G. Basic Res Cardiol 2013;108:337. [DOI] [PubMed] [Google Scholar]
- 35.Elrod J, Calvert J, Morrison J, Doeller J, Kraus D, Tao L, Jiao X, Scalia R, Kiss L, Szabo C. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 2007;104:15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King S. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Rad Biol Med 2013;55:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianco CL, Fukuto JM. Examining the reaction of NO and H2S and the possible cross-talk between the two signaling pathways. Proc Natl Acad Sci USA 2015;112:10573–10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sojitra B, Bulani Y, Putcha U, Kanwal A, Gupta P, Kuncha M, SK B. Nitric oxide synthase inhibition abrogates hydrogen sulfide-induced cardioprotection in mice. Mol Cell Biochem 2012;360:61–69. [DOI] [PubMed] [Google Scholar]
- 39.Kida M, Sugiyama T, Yoshimoto T, Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci 2013;48:211–215. [DOI] [PubMed] [Google Scholar]
- 40.Minamishima S, Bougaki M, Sips PY, De Yu J, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 2009;120:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bibli S-I, Andreadou I, Chatzianastasiou A, Tzimas C, Sanoudou D, Kranias E, Brouckaert P, Coletta C, Szabo C, Kremastinos DT, Iliodromitis EK, Papapetropoulos A. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc Res 2015;106:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovi-Burmazovi I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc 2012;134:12016–12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 2006;343:303–310. [DOI] [PubMed] [Google Scholar]
- 44.Filipovic MR, Eberhardt M, Prokopovic V, Mijuskovic A, Orescanin-Dusic Z, Reeh P, Ivanovic-Burmazovic I. Beyond H2S and NO interplay: hydrogen sulfide and nitroprusside react directly to give nitroxyl (HNO). A new pharmacological source of HNO. J Med Chem 2013;56:1499–1508. [DOI] [PubMed] [Google Scholar]
- 45.Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res 2012;111:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortese-Krott MM, Kuhnle GGC, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci USA 2015;112:E4651–E4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun J, Steenbergen C, Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal 2006;8:1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S-B, Foster DB, Rucker J, O'Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res 2011;109:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem 2011;286:40184–40192. [DOI] [PMC free article] [PubMed] [Google Scholar]