SUMMARY
BACKGROUND
Excess alcohol use among tuberculosis (TB) patients complicates TB control strategies.
OBJECTIVES
To characterize the role of excess alcohol use in TB control, we describe the epidemiology of excess alcohol use and TB in the United States among those aged ≥15 years.
DESIGN
Using data reported to the National Tuberculosis Surveillance System, 1997–2012, we examined associations between excess alcohol use and TB treatment outcomes and markers for increased transmission (involvement in a local genotype cluster of cases) using multivariate logistic regression. We used Cox proportional hazards regression analysis to examine the relationship between excess alcohol use and the rate of conversion from positive to negative in sputum culture results.
RESULTS
Excess alcohol use was documented for 31 207 (15.1%) of 207 307 patients. Prevalence of excess alcohol use was greater among male patients (20.6%) and US-born patients (24.6%). Excess alcohol use was associated with a positive sputum smear result (aOR 1.23, 95%CI 1.18–1.28) and death during treatment (vs. completion of treatment) (aOR 1.16, 95%CI 1.10–1.22). The rate of culture conversion was higher among patients without excess alcohol use (adjusted hazard ratio 1.20, 95%CI 1.18–1.23).
CONCLUSIONS
Excess alcohol use was common among patients with TB, and was associated with TB transmission, lower rates of sputum culture conversion, and greater mortality.
Keywords: substance use, Mycobacterium tuberculosis, directly observed therapy, sputum culture, culture conversion
Tuberculosis (TB) incidence continues to decrease in the United States, with fewer than 10 000 cases reported nationwide in 2012;1 however, excess alcohol use remains common. Excess alcohol use accounted for an estimated 88 000 deaths in the United States each year during 2006–2010,2 and an estimated US$224 billion in economic costs in 2006.3 Excessive alcohol use includes binge drinking, exceeding weekly limits, and any use by pregnant women or persons aged <21 years. In 2011, binge drinking was reported by 18.3% of US adults, who reported doing so an average of four times a month and consuming an average of 8 drinks per occasion.4 Substance use (including excess alcohol use) among patients with TB remains highly prevalent in the United States5 and abroad.6
Alcohol use might be responsible for a large proportion of TB program burden,7 which is represented by a strong association between excessive alcohol use and TB.8 This association may be explained by the progression of latent tuberculous infection to TB disease due to decreased immune system function among excess alcohol users,9 and exacerbated by other associated factors such as homelessness10 and smoking.11 Studies have shown excess alcohol use to be associated with a positive acid-fast bacilli (AFB) sputum smear microscopy result,12 delay in TB diagnosis,13 anti-tuberculosis treatment,13 and poor TB treatment outcomes.14 Excess alcohol use is also associated with an increased risk of belonging to a localized TB genotype cluster,15,16 suggesting an association between alcohol use and recent TB transmission.
Despite the high prevalence of excess alcohol use described among TB patients, and the associated comorbidity and mortality, no recent nationwide analysis examining the prevalence of excess alcohol use and potential associations has been published. We investigated the prevalence of excess alcohol use among TB patients reported in the United States from 1997 to 2012. We assessed the association of excess alcohol use with markers for TB transmission and with treatment outcomes, while adjusting for the effects of other demographic and clinical characteristic variables routinely reported in US TB surveillance. We also assessed whether time to sputum culture conversion was associated with excess alcohol use among patients with pulmonary TB.
STUDY POPULATION AND METHODS
Data collection
We used data from the National Tuberculosis Surveillance System (NTSS), which contains demographic and clinical information for each reported TB case in the United States and the District of Columbia. Incident cases of TB disease in persons aged ≥15 years reported to the NTSS for 1997–2012 were selected for this analysis. Molecular genotype data of Mycobacterium tuberculosis complex isolates were linked to NTSS case-based records, as described elsewhere.17 For analyses involving genotyping data, incident culture-positive TB cases in the NTSS for 2009–2012 with matched genotype results were used. Similar to a previous analysis, a genotype cluster was defined as two or more cases of TB with the same genotype matched using 24-locus mycobacterial interspersed repetitive unit (MIRU) and spacer oligonucleotide typing, and reported in the same county and state.18
As data for the NTSS are collected as part of routine public health practice and not for the purposes of human subjects’ research, the study proposal was reviewed by the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers of Disease Control and Prevention, Atlanta, GA, and it was determined that institutional review board approval was not required.
Excess alcohol use is defined as having used alcohol in excess within the past 12 months.19 Information for this variable is either self-reported or medically documented. If excess alcohol use is not self-reported by the patient, the health provider or TB controller is tasked with determining whether excess alcohol use occurs. This determination can be made over the course of numerous appointments. Homelessness, injection drug use, and non-injection drug use are defined as any period of self-reported behavior in the 12 months before the diagnosis of TB disease. Poor treatment outcomes were defined as being lost to follow-up, not complying with or refusing treatment, among those for whom treatment was stopped vs. treatment completion. Patients who died during treatment or whose completion status was unknown or missing were not included in the analysis of poor treatment outcomes. To be included in the variable ‘pulmonary cavity diagnosed by X-ray’, individuals also had to have documentation of an abnormal X-ray. Only those with a positive sputum culture result and initial drug susceptibility testing results were considered for analyses of drug resistance. Patients with documented human immunodeficiency virus (HIV) infection were coded as ‘known positive’, whereas patients with negative or unknown status were coded as ‘other’.
Statistical analysis
Prevalence of excess alcohol use among TB patients in the United States
Trends in the prevalence of excess alcohol use were assessed using a Mantel-Haenszel extension of the χ2 test for trend.20 The prevalence of excess alcohol use was also stratified by state and categorized by quartile.
Bivariate associations between excess alcohol use, select characteristics and TB outcomes
Bivariate associations between excess alcohol use and demographic and clinical variables were assessed using crude odds ratios (ORs) and 95% confidence intervals (CIs).
Multivariate associations between excess alcohol use and TB outcomes
Multivariate logistic regression analysis was conducted to assess the association of excess alcohol use and select variables. Adjusted odds ratios (aORs) significant at the 95% confidence level are displayed. We did not include the multidrug-resistant and extensively drug-resistant variables in multivariate analysis due to large amounts of missing data in these variables. Analyses of genotype data were restricted to 2009–2012, as 24-locus MIRU data were only available for 2009 onward.
Analyses of time to sputum culture conversion
We conducted a Kaplan-Meier analysis to assess whether time to and rate of sputum culture conversion differed between patients with and those without documented excess alcohol use. Time to conversion was defined as the number of days between the start of treatment and the date of the first consistently negative result from sputum culture. Analysis was limited to patients with pulmonary TB who were culture-positive; had confirmed susceptibility to isoniazid, rifampin, and ethambutol; had no known resistance to pyrazinamide; were started on a standard four-drug regimen;21 had documentation of a negative sputum culture result; had a temporally valid documentation of date of sputum culture conversion; and, for those who culture converted, before stopping treatment and within 4 months (standard time after which treatment is considered failed);22 and were reported as either ‘yes’ or ‘no’ to the excess alcohol use question.
We plotted time to sputum culture conversion within 4 months of the start of anti-tuberculosis treatment by excess alcohol use using Kaplan-Meier curves. Differences were assessed using log-rank statistics, while hazard ratios (HRs) and 95%CIs for rate of culture conversion were calculated using Cox proportional hazard regression analyses to obtain adjusted hazard ratios (aHRs). The adjusted analysis controlled for age group, sex, race/ethnicity, and homelessness.
RESULTS
Prevalence of excess alcohol use among confirmed TB patients in the United States
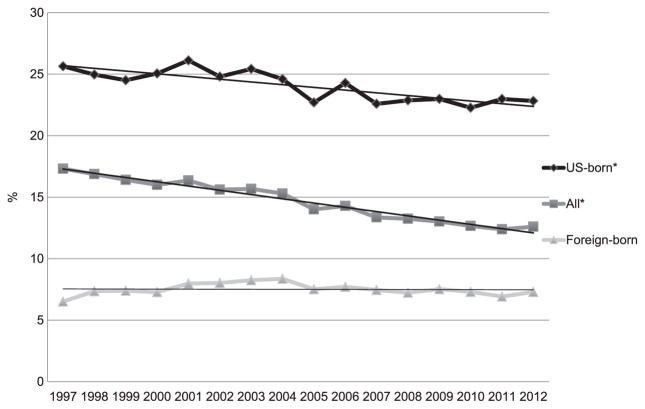
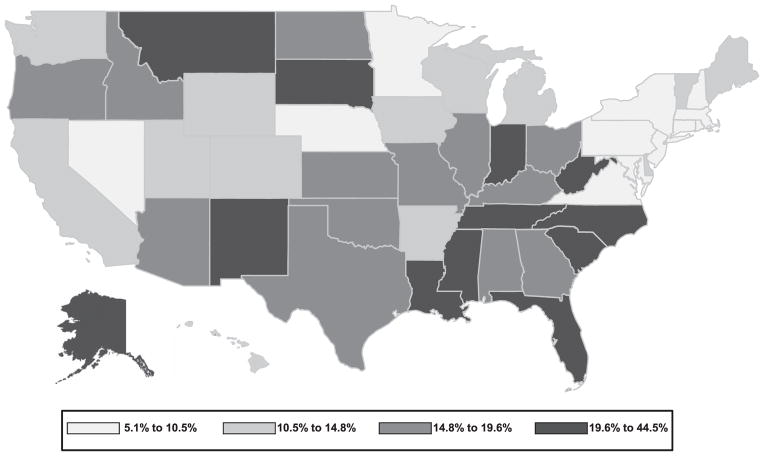
During the study period, 207 307 TB cases were reported among persons aged ≥15 years (Figure 1). Of these, excess alcohol use was documented for 31 207 (15.1%; yearly range 12.4–17.3) (data not shown); 22 733 (72.8%) were born in the United States (US-born) and 8474 (28.2%) outside the United States (foreign-born). For 1997–2012, the prevalence of excess alcohol use differed significantly between US-born and foreign-born patients (24.3%, range 22.8–25.6 vs. 7.5%, range 6.5–8.4; P < 0.001; Figure 1). The prevalence of excess alcohol use for 1997–2012 decreased significantly, from 25.3% to 23.1%, among US-born persons (P < 0.001), but did not change significantly among foreign-born persons (P = 0.842). Beginning in 2005 for US-born (P = 0.429) and in 2008 for all patients with TB (P = 0.051), there was no significant change in excess alcohol use prevalence. Excess alcohol use prevalence among TB patients varied by state by a factor of nearly nine, ranging from 5.1% in Rhode Island to 44.5% in Montana (Figure 2).
Figure 1.
Proportion of excess alcohol use among confirmed TB cases aged ≥15 years reported to the National TB Surveillance System, United States, 1997–2012 (n = 207 307).* Trend P < 0.0001.
Figure 2.
Excess alcohol use in previous year among persons aged ≥15 years with reported tuberculosis diagnosis, United States, 1997–2012.
Bivariate associations between excess alcohol use and select characteristics and TB outcomes
Excess alcohol use was more common among males than females, among patients aged 45–64 years than among other age groups, among American Indians or Alaska Natives than other races, and among US-born than foreign-born patients (Table 1). TB patients with excess alcohol use were more likely to be homeless and to have used injection or non-injection drugs during the past year. Those with excess alcohol use were also more likely to be HIV-positive.
Table 1.
Factors associated with excess alcohol use among persons aged ≥15 years reported with a TB diagnosis, United States, 1997–2012 (n = 207 307)
| Variable | n (%)* | Subjects with documented excess alcohol use n (%)* | Crude OR (95%CI) | Adjusted OR (95%CI)† |
|---|---|---|---|---|
| Demographic characteristics and behaviors | ||||
| Male sex | 128 713 (62.10) | 27 051 (20.62) | 4.31 (4.17–4.45) | 3.34 (3.21–3.47 |
| Age group, years | ||||
| 15–24 | 22 559 (10.88) | 1 317 (5.84) | 1.0 (reference) | 1.0 (reference) |
| 25–44 | 74 706 (36.04) | 12 117 (16.22) | 3.12 (2.95–3.31) | 2.82 (2.63–3.02) |
| 45–64 | 63 789 (30.77) | 14 920 (23.39) | 4.92 (4.65–5.214) | 4.094 (3.82–4.389) |
| ≥65 | 46 253 (22.31) | 2 604 (5.63) | 0.96 (0.90–1.03) | 1.21 (1.12–1.32) |
| Racial/ethnic group | ||||
| White | 41 959 (20.30) | 7 947 (18.94) | 1.0 (reference) | 1.0 (reference) |
| American Indian | 2 544 (1.23) | 1 040 (40.88) | 2.96 (2.72–3.21) | 2.73 (2.45–3.03) |
| Asian | 49 922 (24.15) | 1 662 (3.33) | 0.15 (0.14–0.16) | 0.49 (0.45–0.52) |
| Black | 58 149 (28.13) | 12 507 (21.51) | 1.17 (1.14–1.21) | 0.97 (0.94–1.01) |
| Hispanic | 52 622 (25.46) | 7 919 (15.05) | 0.76 (0.73–0.78) | 1.35 (1.29–1.43) |
| Multiple | 676 (0.33) | 49 (7.32) | 0.34 (0.25–0.45) | 0.80 (0.56–1.14) |
| Hawaiian/Pacific Islander | 829 (0.40) | 72 (8.68) | 0.41 (0.36–0.46) | 0.97 (0.72–1.32) |
| US born vs. foreign born | 92 489 (44.69) | 22 733 (24.58) | 3.95 (3.85–4.06) | 2.51 (2.40–2.63) |
| Homeless during last year | 12 762 (6.21) | 7 186 (56.31) | 9.27 (8.93–9.62) | 2.83 (2.70–2.97) |
| Injection drug use, past year | 4 621 (2.25) | 2 699 (58.41) | 9.03 (8.52–9.57) | 1.80 (1.67–1.95) |
| Non-injection drug use, past year | 15 956 (7.78) | 9 438 (59.15) | 12.00 (11.59–12.41) | 5.29 (5.05–5.53) |
| Clinical and epidemiological characteristics | ||||
| Known HIV-positive | 17 600 (8.49) | 4 247 (24.13) | 1.77 (1.70–1.84) | 1.40 (1.34–1.48) |
| Sputum smear result | ||||
| Negative | 85 789 (44.13) | 10 664 (12.43) | 1.0 (reference) | 1.0 (reference) |
| Positive | 76 156 (39.17) | 16 297 (21.40) | 1.92 (1.87–1.97) | 1.23 (1.18–1.28) |
| Not tested | 32 477 (16.70) | 2 137 (6.58) | 0.50 (0.47–0.52) | 1.06 (0.91–1.23) |
| Sputum culture result | ||||
| Negative | 44 080 (22.80) | 4 544 (10.31) | 1.0 (reference) | 1.0 (reference) |
| Positive | 115 929 (59.95) | 22 358 (19.31) | 2.08 (2.01–2.15) | 1.36 (1.29–1.43) |
| Not tested | 33 360 (17.25) | 2 135 (6.40) | 0.60 (0.57–0.63) | 0.85 (0.73–0.99) |
| Disease site | ||||
| Extra-pulmonary | 41 367 (19.96) | 2 622 (6.34) | 1.0 (reference) | 1.0 (reference) |
| Pulmonary | 147 540 (71.20) | 25 568 (17.33) | 3.10 (2.97–3.23) | 1.31 (1.23–1.39) |
| Both | 18 316 (8.84) | 2 744 (14.98) | 2.60 (2.46–2.75) | 1.20 (1.11–1.29) |
| Pulmonary cavity diagnosed by X-ray | 47 160 (27.69) | 10 238 (21.71) | 1.55 (1.51–1.59) | 1.21 (1.16–1.25) |
| Cluster involvement‡ | 7 209 (23.60) | 1 010 (14.01)† | 2.52 (2.35–2.70) | 1.36 (1.23–1.50) |
| First-line drug resistance | 16 893 (8.15) | 2 083 (12.33) | 0.79 (0.75–0.82) | 0.90 (0.85–0.95) |
| Multidrug-resistant TB§ | 1 558 (1.35%) | 164 (10.55) | 0.563 (0.48–0.66) | — |
| Extensively drug-resistant TB§ | 31 (0.36) | 4 (12.90) | 1.40 (0.57–3.42) | — |
| Directly observed therapy | ||||
| No (self-administered) | 32 694 (16.79) | 2 056 (6.29) | 1.0 (reference) | 1.0 (reference) |
| Yes | 106 140 (54.49) | 18 829 (17.74) | 3.21 (3.06–3.37) | 1.92 (1.81–2.03) |
| Both | 55 941 (28.72) | 8 251 (14.75) | 2.58 (2.45–2.71) | 1.82 (1.71–1.93) |
| Poor treatment outcomes | 5 929 (3.41) | 1 309 (22.09) | 1.64 (1.54–1.75) | 1.55 (1.04–2.31) |
| Reason for stopping treatment | ||||
| Completed | 167 985 (86.92) | 24 576 (14.63) | 1.0 (reference) | 1.0 (reference) |
| Died | 15 057 (7.79) | 2 569 (17.06) | 1.20 (1.15–1.25) | 1.16 (1.10–1.22) |
| Lost | 4 678 (2.42) | 1 100 (23.51) | 1.79 (1.68–1.92) | 1.25 (1.15–1.37) |
| Moved | 4 189 (2.17) | 547 (13.05) | 0.88 (0.80–0.96) | 1.11 (0.99–1.24) |
| Refused treatment | 1 251 (0.65) | 209 (16.73) | 1.17 (1.01–1.36) | 1.35 (1.11–1.62) |
Numbers may not reflect 100% of data due to missing data.
Variables with adjusted ORs were adjusted for all other variables in the table except for multidrug resistance, extensive drug resistance, and cluster involvement.
Variables that had >10% missing data were not included in the adjusted analysis.
Data from 2009–2012 only.
TB = tuberculosis; OR = odds ratio; CI = confidence interval; HIV = human immunodeficiency virus.
Multivariate associations between excess alcohol use and TB outcomes
Excess alcohol use was independently associated with positive AFB sputum smear microscopy results (aOR 1.23, 95%CI 1.18–1.28), pulmonary vs. extra-pulmonary site of disease (aOR 1.31, 95%CI 1.23–1.39), having a pulmonary cavity diagnosed on X-ray (aOR 1.21, 95%CI 1.16–1.25), and genotype cluster involvement (aOR 1.36, 95%CI 1.23–1.50). With regard to measures of anti-tuberculosis treatment, excess alcohol use was associated with receiving directly observed therapy (DOT) vs. self-administered therapy (SAT) (aOR 1.92, 95%CI 1.81–2.03). Compared with completion of treatment, rates of death (aOR 1.16, 95%CI 1.10–1.22) or loss to follow-up (aOR 1.25, 95%CI 1.15–1.37) were significantly higher in those with excess alcohol use (Table 1). A total of 67 patients (0.03%) had treatment outcome information missing and were excluded from further analyses. The proportion of patients with missing treatment outcome information and reported excess alcohol use was not significantly different from that observed among those with no reported excess alcohol use (P > 0.99).
Analyses of time to culture conversion
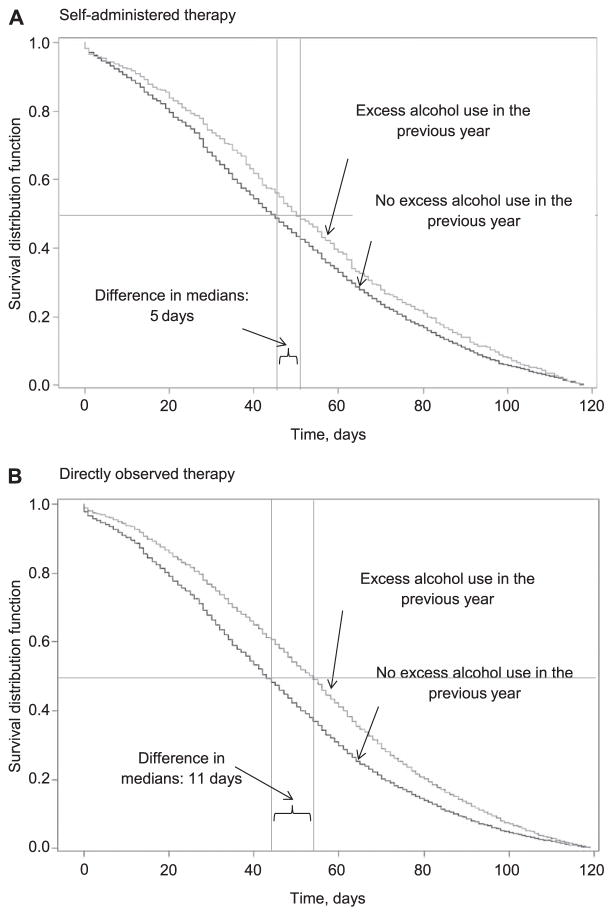
The proportion of patients with culture conversion was significantly lower for those who reported excess alcohol use (88.6%) than those who did not (92.3%, P < 0.001; Table 2). Analysis of time to culture conversion indicated a significant difference between median days to culture conversion for those for whom excess alcohol use was reported vs. those for whom it was not (54 days, interquartile range [IQR] 31–76 vs. 43 days, IQR 25–66; P = 0.001). After adjustment for race/ethnicity, age, sex, and homelessness, the rate of sputum culture conversion remained significantly higher for those without excess alcohol use (aHR 1.20, 95%CI 1.18–1.23). When the adjusted analysis was stratified by therapy, the rate of conversion among those without excess alcohol use was significantly higher for those treated with DOT (aHR 1.22, 95%CI 1.19–1.25), but not for those who underwent SAT (aHR 1.11, 95%CI 0.99–1.25) (Figure 3).
Table 2.
HRs for sputum culture conversion from start of treatment for incident TB cases* and excess alcohol use by DOT vs. SAT
| Total† (n = 60 034)‡ | Total converted by 4 months n (%) | Time to culture conversion, days, median [IQR] | HR (95%CI) | Adjusted HR§ | |
|---|---|---|---|---|---|
| All | |||||
| No excess alcohol use | 46 925 | 43 311 (92.3) | 43 [25–66] | 1.23 (1.22–1.26) | 1.20 (1.18–1.23) |
| Excess alcohol use | 12 512 | 11 082 (88.6) | 54 [31–76] | Reference | Reference |
| SAT | |||||
| No excess alcohol use | 3 392 | 2 907 (85.7) | 45 [26–69] | 1.14 (1.01–1.27) | 1.11 (0.99–1.25) |
| Excess alcohol use | 308 | 255 (82.8) | 50 [31–76] | Reference | Reference |
| DOT | |||||
| No excess alcohol use | 30 041 | 27 751 (92.4) | 43 [24–65] | 1.27 (1.23–1.30) | 1.22 (1.19–1.25) |
| Excess alcohol use | 8 887 | 7 878 (88.6) | 54 [31–75] | Reference | Reference |
Limited to US cases aged ≥15 years, 1997–2012, with culture positivity, confirmed susceptibility to isoniazid, rifampin, and ethambutol, no known resistance to pyrazinamide, alive at the time of diagnosis, started on four-drug regimen, had documentation of negative sputum, had valid documentation date of conversion, culture converted before stopping treatment, culture converted within 4 months, responded either ‘yes’ or ‘no’ to excess alcohol use.
Columns may not sum to 100% due to missing data.
Total with documented conversion.
Adjusted for race/ethnicity, sex, age and homelessness. Missing cases excluded from analysis.
HR = hazard ratio; TB = tuberculosis; IQR = interquartile range; CI = confidence interval; DOT = directly observed therapy; SAT =self-administered therapy.
Figure 3.
Time (in days) from start of treatment to conversion to negative sputum culture by excess alcohol use vs. no excess alcohol use in past year in individuals treated with A) self-administered therapy and B) directly observed therapy, among persons reported with TB, United States, 1997–2012. (Horizontal line indicates the median time point and vertical lines indicate the median time point to culture conversion.)
DISCUSSION
Nearly one in six TB patients consumes alcohol in excess, and this behavior complicates TB control at every level. These findings echo research from abroad and from other US states and cities.5,6,23,24 The burden of alcohol and TB is distributed disproportionately in the United States, with 12 states reporting an overall prevalence of excess alcohol use among TB patients ranging between 19% and 44%. Among US-born patients with TB, the prevalence of excess alcohol use has been >20% each year since 1997, suggesting that excess alcohol use needs to be considered when addressing TB control in the United States. The prevalence of excess alcohol use among US-born patients with TB has remained steady since 2005 and among foreign-born patients with TB since 1997. While the prevalence of excess alcohol use among TB patients appears to be lower than that of binge drinking in the general population of the United States, it should be noted that these measures of alcohol use are not equivalent, and that the proportion of foreign-born TB patients in the United States is approaching 50%, among whom only 7.5% had excess alcohol use reported. This drives down the overall prevalence of excess alcohol use compared to that of the general population in the United States. As a common factor among patients with TB, excess alcohol use may represent a large portion of the morbidity and mortality associated with TB in the United States, and may be a marker for other factors that were not included in this analysis, including smoking and poverty.
This study has limitations. First, the variable ‘excess alcohol use’ collected in the NTSS and used in this study lacks a specific definition. The specificity of this variable could be increased by systematically implementing a reliable, valid use measure, such as the single-question screen measure used by the National Institute on Alcohol Abuse and Alcoholism (National Institutes of Health, Bethesda, MD).25 However, the current, broad definition remains in place due to requests from TB programs that are striving to ensure that all patients with excess alcohol use are captured. Second, the reliability of self-reported or medically documented data on excess alcohol use is unknown. As a potentially stigmatized behavior, it is probable that the prevalence of excess alcohol use is underreported. Importantly, our analysis was unable to analyze the potential confounding effects of smoking on the excess alcohol use/TB outcome association. Unfortunately, as the NTSS does not collect information on tobacco smoking, we were not able to perform an analysis that controlled for smoking.
Alcohol use is associated with the development of TB disease,8 probably due to impaired immune function.26 In addition, excess alcohol use among TB patients has been linked to TB transmission27 and adverse treatment outcomes.28 Our results are consistent with these findings, as excess alcohol use was associated with pulmonary vs. extra-pulmonary disease, a positive sputum smear result, having a diagnosed pulmonary cavity and being included in a local genotype cluster. Each of these factors can influence transmission, including clustering, which has been linked to recent transmission.29 Furthermore, excess alcohol use was associated with non-injection drug use and homelessness, both of which negatively impact our ability to control TB through the difficulty in locating contacts and ensuring treatment adherence.30,31
The odds of documented excess alcohol use were greater among those with poor treatment outcomes and for those who died vs. those who completed treatment. These findings support those from past studies that reported that patients who used alcohol in excess were more likely to experience adverse treatment outcomes.32,33 A recent study in India found that persons who consumed alcohol during treatment missed on average 18 (95%CI 13–22) more intensive phase doses than those who did not.34 A variety of factors probably account for poor treatment outcomes: TB patients who drink excessively may be less likely to have adequate social support, housing, and transportation to appointments for care, and may be unable to attend appointments for treatment due to factors related to substance use.35 Furthermore, medication failure is possible due to immunosuppression36 and changes in pharmacokinetics8,37 caused by alcohol consumption. In the current study, among patients who culture converted within 4 months, those for whom excess alcohol use was not documented completed treatment more than a week sooner on average, demonstrating that patients who use alcohol in excess may be more likely to fail treatment and require additional resources from TB control programs.
Further complicating anti-tuberculosis treatment among TB patients who consume alcohol in excess is the additional hepatic damage caused by the metabolism of alcohol. The majority of anti-tuberculosis medications are processed in the liver,38 where additive hepatotoxic effects can occur.39 This outcome is more commonly found in those who consume alcohol in excess than in those who do not.40 For people who are known to be currently using alcohol in excess or have a history of alcohol abuse, the American Thoracic Society recommends treatment with fewer TB medications to reduce the hepatotoxic effects from the additive effects of excess alcohol use and anti-tuberculosis treatment.41 Cigarette smoking is more common among those who use excess alcohol than among those who do not, and is also associated with tuberculous infection and progression to TB disease.11 Smoking might complicate anti-tuberculosis treatment through poor TB outcomes (e.g., relapse after treatment).42 The association between smoking and TB may also be responsible for some of the effects observed between excess alcohol use and the outcomes in the current study.
In addition to being less likely to complete anti-tuberculosis treatment, a significantly higher proportion of those who used alcohol in excess were prescribed DOT than those who did not use alcohol in excess; those who consumed alcohol in excess were nearly twice as likely to receive DOT as SAT. As DOT requires active supervision by a health care worker, the cost in terms of human resources is higher for each TB patient treated with DOT.43 Despite this extra attention from the system, TB patients who used alcohol in excess were still less likely to complete treatment than those who did not. After adjusting for race/ethnicity, sex, age, and homelessness, the rate of culture conversion among those who did not use alcohol in excess was significantly greater. The conversion rate maintained statistical significance after stratification among those who did not use alcohol in excess who were receiving DOT, which indicates that, even with supervised treatment, those who used alcohol in excess take longer to cure. The increased treatment time under DOT ostensibly means that those who use alcohol in excess may require an even larger proportion of resources during anti-tuberculosis treatment than those who do not.
One strategy that may provide an opportunity for clinical intervention is alcohol screening and brief intervention. Since 2004, the US Preventive Services Task Force (Rockville, MD) has recommended alcohol screening and behavioral counseling (also known as alcohol screening and brief intervention [ASBI]) for all adults in primary care to address excessive alcohol use. This review of evidence indicated that brief (6–15 min) intervention sessions were effective in significantly reducing weekly alcohol consumption (by 3.6 fewer drinks/week for adults) and binge level episodes (reported by 12% fewer participants) and increasing adherence to recommended drinking limits (achieved by 11% more participants).44 Furthermore, the effects can last for years and show improvement in health care utilization outcomes, including fewer hospital days and lower costs.44
Another strategy consists of an integrated approach to concurrently treating both excessive alcohol use and TB. This is an approach that represents a dual-purpose opportunity. While very few programs appear to have utilized such an approach to co-treating excess alcohol use and TB,45 and few current programs with TB and alcohol treatment exist, this model is fairly well established in the literature on injection drug use in populations at high risk for transmission of HIV-TB.46 A similar model might utilize a holistic approach that addresses a variety of factors, such as the concomitant social (e.g., homelessness), psychological (e.g., unmet mental health needs), physiological (e.g., alcohol dependence), and physical health issues (e.g., TB) that are simultaneously affecting a disproportionately large number of TB persons. This strategy presents an opportunity for the health system to manage multiple related issues, which could result in an overall cost savings to the system and respond to calls in the literature for integrative approaches to co-treating infectious disease and substance use disorders.46–49
Footnotes
Conflicts of interest: none declared.
References
- 1.Centers for Disease Control and Prevention. Trends in tuberculosis — United States, 2012. MMWR. 2013;62:201–295. [Google Scholar]
- 2.Gonzales K, Roeber J, Kanny D, et al. Alcohol-attributable deaths and years of potential life lost: 11 States, 2006–2010. MMWR. 2014;63:213–216. [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Kanny D, Liu Y, Brewer RD, Lu H Centers for Disease Control and Prevention (CDC) Binge drinking—United States, 2011. MMWR Surveill Summ. 2013;62(Suppl 3):77–80. [PubMed] [Google Scholar]
- 5.Oeltmann JE, Kammerer JS, Pevzner ES, Moonan PK. Tuberculosis and substance abuse in the United States, 1997–2006. Arch Intern Med. 2009;169:189–197. doi: 10.1001/archinternmed.2008.535. [DOI] [PubMed] [Google Scholar]
- 6.de la Haye B, Wild SH, Stevenson J, Johnston F, Blatchford O, Laurenson IF. Tuberculosis and alcohol misuse in Scotland: a population-based study using enhanced surveillance data. Int J Tuberc Lung Dis. 2012;16:886–890. doi: 10.5588/ijtld.11.0624. [DOI] [PubMed] [Google Scholar]
- 7.Creswell J, Raviglione M, Ottmani S, et al. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. Eur Respir J. 2011;37:1269–1282. doi: 10.1183/09031936.00084310. [DOI] [PubMed] [Google Scholar]
- 8.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Bagby G, Happel K, Summer W, Nelson S. Pulmonary host defenses and alcohol. Front Biosci. 2002;1:1314–1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- 10.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Davies PD, Yew WW, Ganguly D, et al. Smoking and tuberculosis: the epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg. 2006;100:291–298. doi: 10.1016/j.trstmh.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Yohanes A, Abera S, Ali S. Smear positive pulmonary tuberculosis among suspected patients attending Metehara Sugar Factory Hospital, eastern Ethiopia. Afr Health Sci. 2012;12:325–330. doi: 10.4314/ahs.v12i3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Non-adherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med. 1997:102. doi: 10.1016/s0002-9343(96)00402-0. [DOI] [PubMed] [Google Scholar]
- 15.Žolnir-Dovč M, Poljak M, Eržen D, Šorli J. Molecular epidemiology of tuberculosis in Slovenia: results of a one-year (2001) nation-wide study. Scand J Infect Dis. 2003;35:863–868. doi: 10.1080/00365540310017221. [DOI] [PubMed] [Google Scholar]
- 16.Kline SE, Hedemark LL, Davies SF. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N Engl J Med. 1995;333:222–227. doi: 10.1056/NEJM199507273330404. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Moonan P, Cowan L, Grant J, Kammerer S, Navin T. Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol. 2012;12:782–788. doi: 10.1016/j.meegid.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Bamrah S, Yelk Woodruff R, Powell K, Ghosh S, Kammerer J, Haddad M. Tuberculosis among the homeless, United States, 1994–2010. Int J Tuberc Lung Dis. 2013;17:1414–1419. doi: 10.5588/ijtld.13.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Report of verified case of tuberculosis self-study modules facilitator manual. Atlanta, GA, USA: CDC; 2009. [Accessed October 2014]. http://www.cdc.gov/tb/programs/rvct/InstructionManual.pdf. [Google Scholar]
- 20.Schlesselman S. Case-control studies. New York, NY, USA: Oxford University Press; 1982. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 22.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 23.Fiske CT, Hamilton CD, Stout JE. Alcohol use and clinical manifestations of tuberculosis. J Infect. 2008;57:385–391. doi: 10.1016/j.jinf.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brudney K, Dobkin J. Resurgent tuberculosis in New York City: human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis. 1991;144:745–749. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- 25.National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician’s guide. Bethesda, MD, USA: US Department of Health & Human Services, National Institutes of Health, NIAAA; 2005. [Accessed October 2014]. http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. [Google Scholar]
- 26.Happel K, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 27.Wendy A, Cronin, Golub JE, et al. Statewide molecular epidemiology of Mycobacterium tuberculosis transmission in a moderate- to low-incidence state: are contact investigations enough? Emerg Infect Dis. 2002;8:1271–1279. doi: 10.3201/eid0811.020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterling T, Zhao Z, Khan A, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis. 2006;10:542–549. [PubMed] [Google Scholar]
- 29.Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent Mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18:458–465. doi: 10.3201/eid1803.111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers of Disease Control and Prevention. Notes from the field: outbreak of tuberculosis associated with a newly identified Mycobacterium tuberculosis genotype: New York City, 2010–2013. MMWR Morb Mortal Wkly Rep. 2013;62:904. [PMC free article] [PubMed] [Google Scholar]
- 31.Gasner MR, Maw KL, Feldman GE, Fujiwara PI, Frieden TR. The use of legal action in New York City to ensure treatment of tuberculosis. N Engl J Med. 1999;340:359–366. doi: 10.1056/NEJM199902043400506. [DOI] [PubMed] [Google Scholar]
- 32.Siemion-Szcześniak I, Kuś J. Impact of social risk factors on treatment outcome in patients with culture positive pulmonary tuberculosis (CPPTB) Pneumonol Alergol Pol. 2012;80:412–421. [Polish] [PubMed] [Google Scholar]
- 33.Muture B, Keraka M, Kimuu P, Kabiru E, Ombeka V, Oguya F. Factors associated with default from treatment among tuberculosis patients in Nairobi Province, Kenya: a case control study. BMC Public Health. 2011;9:696. doi: 10.1186/1471-2458-11-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duraisamy K, Mrithyunjayan S, Ghosh S, et al. Does alcohol consumption during multidrug-resistant tuberculosis treatment affect outcome? A population-based study in Kerala, India. Ann Am Thorac Soc. 2014;11:712–718. doi: 10.1513/AnnalsATS.201312-447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Albuquerque Mde F, Ximenes R, Lucena-Silva N, et al. Factors associated with treatment failure, dropout, and death in a cohort of tuberculosis patients in Recife, Pernambuco State, Brazil. Cad Saúde Publica. 2007;23:1573–1582. doi: 10.1590/s0102-311x2007000700008. [DOI] [PubMed] [Google Scholar]
- 36.Wyatt T. Alcohol and the lung: an overview. Alcohol. 2007;41:291–292. [Google Scholar]
- 37.Koriakin V, Sokolova G, Grinchar N, Iurchenko L. Pharmacokinetics of isoniazid in patients with pulmonary tuberculosis and alcoholism. Probl Tuberk. 1986;12:43–46. [Russian] [PubMed] [Google Scholar]
- 38.Girling D. Adverse effects of antituberculosis drugs. Drugs. 1982;23:56–74. doi: 10.2165/00003495-198223010-00003. [DOI] [PubMed] [Google Scholar]
- 39.Moreno S, Podzamczer D, Blázquez R, et al. Treatment of tuberculosis in HIV-infected patients: safety and antiretroviral efficacy of the concomitant use of ritonavir and rifampin. AIDS. 2001;15:1185–1187. doi: 10.1097/00002030-200106150-00018. [DOI] [PubMed] [Google Scholar]
- 40.Hwang S, Wu J, Lee C, et al. A prospective clinical study of isoniazid-rifampicin-pyrazinamide-induced liver injury in an area endemic for hepatitis B. J Gastroenterol Hepatol. 1997;12:87–91. doi: 10.1111/j.1440-1746.1997.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 41.American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. Atlanta, GA, USA: CDC; 2003. [Google Scholar]
- 42.d’Arc Lyra Batista J, de Fatima Pessoa Militao de Albuquerque M, de Alencar Ximenes RA, Rodrigues LC. Smoking increases the risk of relapse after successful tuberculosis treatment. Int J Epidemiol. 2008;37:841–851. doi: 10.1093/ije/dyn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burman WJ, Dalton CB, Cohn DL, Butler JRG, Reves RR. A cost-effectiveness analysis of directly observed therapy vs self-administered therapy for treatment of tuberculosis. Chest. 1997;112:63–70. doi: 10.1378/chest.112.1.63. [DOI] [PubMed] [Google Scholar]
- 44.McKnight-Eily LR, Liu Y, Brewer RD, et al. Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR. 2014;63:16–22. [PMC free article] [PubMed] [Google Scholar]
- 45.Shin S, Livchits V, Connery HS, et al. Effectiveness of alcohol treatment interventions integrated into routine tuberculosis care in Tomsk, Russia. Addiction. 2013;108:1387–1396. doi: 10.1111/add.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18:306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krupitsky EM, Zvartau EE, Lioznov DA, et al. Co-morbidity of infectious and addictive diseases in St Petersburg and the Leningrad Region, Russia. Eur Addict Res. 2006;12:12–19. doi: 10.1159/000088578. [DOI] [PubMed] [Google Scholar]
- 48.Thomas B, Suhadev M, Mani J, et al. Feasibility of an Alcohol intervention programme for TB Patients with alcohol use disorder (AUD): a qualitative study from Chennai, South India. PLOS ONE. 2011;6:e27752. doi: 10.1371/journal.pone.0027752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Darraji H, Wong K, Yeow D, et al. Tuberculosis screening in a novel substance abuse treatment center in Malaysia: implications for a comprehensive approach for integrated care. J Subst Abuse Treat. 2014;46:144–149. doi: 10.1016/j.jsat.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]