Abstract
The infections found in chronic obstructive pulmonary disease, cystic fibrosis, and bronchiectasis share a number of clinical similarities, the most striking of which is bacterial persistence despite the use of antibiotics. These infections have been clinically described using culture-based methods usually performed on sputum samples, and treatment has been directed towards the bacteria found in this manner. Unfortunately the clinical response to antibiotics is frequently not predictable based on these cultures, and the role of these cultured organisms in disease progression has been debated. The past 20 years have seen a revolution in the techniques used to describe bacterial populations and their growth patterns. These techniques have revealed these persistent lung infections are vastly more complicated than described by traditional, and still widely relied upon, sputum cultures. A better understanding of the initiation and evolution of these infections, and better clinical tools to describe them, will dramatically alter the way patients are cared for. While clinical tests to more accurately describe these infections are not yet available, the better appreciation of these infections afforded by current science should enlighten practitioners as to the care of their patients with these diseases.
Keywords: Chronic obstructive pulmonary disease, bronchiectasis, cystic fibrosis, microbiota, sputum culture
Persistent lung infections
Bacterial lung infections are usually categorized as acute or chronic depending upon the rate at which they evolve, but more likely related to the rate at which they resolve after antibiotic therapy. Acute infections such as community acquired pneumonia typically respond rapidly to antibiotics and usually leave little residual mark on the lung. Infections involving the conducting airways are more variable in their onset and resolution. While viruses and Bordatella pertussis, may initiate an acute “bronchitis” in healthy individuals, a vast number of patients with disruptions in lung immunity, or mucosal clearance, suffer from infections that typically don’t resolve even with antibiotic treatment. And unlike other forms of chronic infection, the immunologic response to bacterial infections of the airways does not appear to evolve to a classic, adaptive response, but rather maintains an unrelenting neutrophilic response, similar to those in acute infections.1 It is for this reason chronic lung infections may be better classified as persistent infections, as most bacteria involved in these infections have survived treatment that would normally be predicted to eradicate them, and may continue to persist lifelong in some patients. Most acute lung infections do uphold Koch’s postulates very well, and sputum cultures and staining techniques, when appropriately collected and culture positive, are usually helpful in determining which antibiotics to use. In time we have learned that patients with pneumonia will usually respond to empiric use of a handful of antibiotics and that sputum cultures are not necessary for their treatment, and may delay appropriate treatment if relied upon. This approach is applicable to COPD where routine culturing is not recommended;2 however, in other persistent lung infections like CF and non-CF bronchiectasis sputum cultures are considered a cornerstone of quality patient care.3
All of the persistent lung infections are characterized by periods of stability punctuated by periods of exacerbation. Exacerbations are loosely defined as a change in daily symptoms such as cough, dyspnea, sputum production, and fatigue; however defining these events for purposes of clinical study have been daunting.4, 5 Exacerbations of persistent lung infections are commonplace and have been shown to result in significant morbidity and loss of lung function if not treated. The microbiologic contributions to an exacerbation have traditionally been determined by culture results and antibiotic selection is based on these cultures. However there are a number of clinical situations that challenge the utility of this approach. Most clinicians who care for individuals with persistent airway infections will encounter many instances where a patient’s course is not predicted by sputum cultures. Many patients will not show a clinical response to antibiotics directed at the organism cultured in their sputum. On the contrary, many patients may respond to antibiotics not predicted to work; classically this is seen in patients whose sputum cultures reveal bacteria resistant to most, if not all, antibiotics tested in sensitivity panels. Some patients will culture the same organism repeatedly despite appropriate antibiotic therapy. In the cases of antibiotic ineffectiveness, it was presumed antibiotics could not reach every niche of the lung, leaving some bacteria untreated.6 This reasoning helped spawn the use of aerosolized antibiotics that could attack organisms at the air-liquid interface in the mucosal lining. Despite these approaches bacterial persistence, ineffectiveness of antibiotics, and unpredictable clinical responses have continued to frustrate practitioners.7
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is an inflammatory, non-reversible obstructive lung disorder caused primarily by exposure to tobacco smoke. Typical symptoms include dyspnea, wheezing, cough, and sputum production. COPD exacerbations are commonplace and patients who suffer frequent exacerbations experience poorer quality of life, accelerated loss of lung function, high health care utilization and costs, and higher morbidity and mortality.8–12 Studies relying on sputum culture predict 50% of COPD exacerbations are due to bacterial lung infection and 25–50% are due to viral infections.13 Acquisition of a new strain of Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae in the lung is associated with an increased risk of exacerbation.14 These pathogenic bacteria often colonize the lungs of COPD patients between exacerbations and typically reoccur on future sputum cultures, which is often referred to as colonization.13
Traditionally, clinicians understood that COPD exacerbations occurred as a result of lung infection with a new strain of bacteria (typically Haemophilus, Moraxella, or S. pneumoniae) or a virus. In this disease model, the new pathogen led to a lung infection and pulmonary inflammation; the latter of which resulted in cough, sputum production, dyspnea, and wheezing. Treatment with an antibiotic or oral corticosteroid was administered to eradicate the new organism and/or blunt the inflammatory response in order to decrease lung symptoms. When the patient responds to this therapy it is presumed to be due to the specific actions of the antibiotics, namely clearance of the new bacteria and the resultant prompt return to the baseline lung microbiota that could be cultured during periods of clinical stability. This model explains the disease course in those patients who respond to therapy; however this effect may be limited to only the sickest hospitalized patients. While antibiotics may improve symptoms in those admitted to the hospital, they do not improve overall mortality or length of stay for most of these patients.2 For these reasons it is not recommended to perform sputum cultures on patients with mild COPD exacerbation before beginning therapy with antibiotics. The widespread use of antibiotics, as they are currently selected, for all but the most severe COPD exacerbations remains debatable.
Cystic Fibrosis
Of those lung conditions with persistently positive bacterial cultures, the infections of the CF lung have arguably been the best described in the medical literature, despite it being far less common than COPD or non-CF bronchiectasis. CF is a genetic disease that results in bronchiectasis from the inability to clear dehydrated mucous from the airways.15 This mucous becomes too thick for the mucociliary elevator to move, trapping most inhaled particles and infecting organisms in the airways. CF is a multi-system disease, and children with CF who survive the gastrointestinal manifestations were noted to succumb to bacterial infections of their lungs. The sputum culture technique revealed the presence of H. influenzae and Staphylococcus aureus, and these were presumed to be the source of the lung morbidity in children with CF.16 Treating these two organisms with antibiotics prolonged their lives, but did not eradicate these infections, which frequently demonstrated persistent culture positivity through their lives. With more aggressive antibiotic approaches, patients were noted to have an unusual but easily cultured organism in their sputum—Pseudomonas aeruginosa. Early studies demonstrated children with CF who acquired P. aeruginosa had lower survival rates than those children whose cultures remained free of it.17 Screening the sputum, throat, or bronchoalveolar lavage fluid for these organisms became the standard of care on the presumption that early identification could result in early intervention to prevent establishment of pathogens known to be associated with worse outcomes. These presumptions were tested in the Early Pseudomonal Infection Control trial in which children with CF were treated on first acquisition of P. aeruginosa.18 This trial demonstrated that cultures could be made clear of P. aeruginosa but follow up studies did not demonstrate fewer hospitalizations than historical controls,19 or improved lung functions20 in those with successful eradication from culture. Furthermore some in the treated groups clearly acquired more resistant strains of P. aeruginosa and started culturing more resistant organisms like Stenotrophomonas maltophila, than those not treated.18 The Cystic Fibrosis Foundation has created consensus guidelines that still recommend all of the centers in its care network culture all patients with CF every 3 months if possible and begin inhaled tobramycin upon acquisition of P. aeruginosa in sputum culture.3
Non CF bronchiectasis
Non CF bronchiectasis is far more common than CF, and has a multiple etiologies. The most common etiology is idiopathic, with the rest ranging from infectious, immune deficiency, micro-aspiration, autoimmune, and genetic.21 While the underlying disease and the areas of the lung differ, the underlying pathology remains the same, inability to adequately clear secretions. Much the same as other highlighted diseases with persistent lung infections, the hallmark of the non CF bronchiectasis is characterized by progressively worsening infections and exposure to the resulting inflammatory response leading to further respiratory function decline. Traditional culture methods reveal H. influenza and P. aeruginosa as the most common pathogens found in sputum of non CF bronchiectasis patients.
The treatment of non CF bronchiectasis is based on the underlying cause, however, the treatment of the infectious complications is very similar. In the pediatric populations, treatment of the underlying cause is important, as it was previously felt that the most common etiology of bronchiectasis was post-infectious, but is now demonstrated to be immune deficiency and aspiration.21 Beyond this, the mainstay of treatment has been airway clearance, bronchodilators, and antibiotics. The antibiotic regimens used are targeted at cultured organisms, and are usually based on previous sputum cultures from the patient and the most common organisms cultured in the community. As the treatment of non CF bronchiectasis lacks any conventional guidelines given the heterogeneity of its clinical presentation, most physicians rely on experiences with CF and COPD to dictate management.
Sputum culture: history and current use
Sputum production is a hallmark of lung infection and is the least invasive way to sample the secretions of the lower airways for medical studies. Generations of medical scientists have studied the physical, biochemical, and cellular components of sputum hoping to better understand lung pathology. Translations of Hippocrates reveal his deduction that expectorated sputum that sank in sea water was associated with a poor prognosis.22 In likely the first microscopic study of sputum, Van Leeuwenhoeck described “odd corpuscles and globules” in his own fasting “spittle.” Two hundred years later Koch used microscopy to describe bacilli from lung tubercles, but was flummoxed by his inability to grow these organisms using his solid media preparations.23 With persistence he developed a coagulated blood agar that could grow what is now known as Mycobacterium tuberculosis after two weeks of incubation.
Expectorated sputum readily grows micro-organisms on non-selective medias, however care has to be taken to distinguish organisms that have been incidentally introduced during expectoration, such as oral bacteria, and those actually inhabiting the lower airways. This is done through a series of tests to distinguish samples that are truly sputum vs saliva, and through the use of subjective selection of organisms “likely to cause” lower respiratory tract infections.24 Decades of medical research on lung infections using classic microbiological techniques, guided by Koch’s postulates, have determined particular species of bacteria are attributable to particular lung infections. Their pathogenic mechanisms have been elucidated to reveal how disease is created, how this relates to symptoms, and testing with antibiotics have shown how to treat these diseases. The ability to culture a pathogen, select an antibiotic that can kill that pathogen, and cure a patient with that antibiotic is a very satisfying outcome for both the patient and physician and has validated culture techniques as a gold standard in the medical literature.
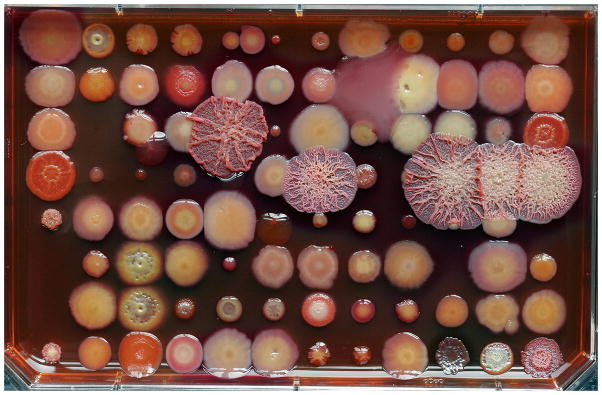
Today’s sputum cultures are performed using protocols derived during the 1970’s and 1980’s with some slight adaptations to account for newly recognized pathogens.25 After a quality check to validate the sample as sputum and not saliva, the sample is diluted in a sterile saline solution to make it transferrable by pipet and plated to a number of selected medias to grow known pathogens. There are separate protocols recommended by the CF foundation for isolation of particular pathogens associated with CF, and these may be useful in non-CF bronchiectasis as well.3, 26 If there are different morphotypes of colonies after speciation, they are considered for separate MIC tests as presumably any of these could be the cause of the infection. In reality this becomes very difficult as some species, like P. aeruginosa, can take on many different morphotypes (see Figure 1), and frequently more than one isolate is found in each sputum sample. Each isolate is then tested to establish its minimal inhibitory concentrations (MIC) of several antibiotics. The tests for MIC occur in vitro in rich media and are shown to be most reliable in infections where rapid growth conditions are thought to occur, such as the blood stream. MICs are less useful in infections with prolonged courses, or those with multiple, culturable organisms, such as bronchiectasis in the lung.27 The reasons for this lack of clinical utility are likely founded in both the highly subjective nature of the sputum culture and MIC determination, and newer evidence concerning the biology of these infections which is described below.28
Figure 1. The inherent challenge in morphotype selection for further testing and clinical report.
All of the colonies on this image are various morphotypes of P. aeruginosa, and each patient with persistent lung disease may harbor dozens of these morphotypes if carefully screened. Typically one or two are subjectively chosen for MIC testing and clinical report. Bacterial medium is a Congo red agar, image and background discussion kindly provided by H. Kulasekara and L. Hoffman.
The new paradigm in persistent airway infections
Diverse, polymicrobial infections
With the introduction of molecular microbiology (the study of microbes through genetic techniques) it became possible to detect known pathogens with greater speed and sensitivity and with lower costs. In a study by van Belkum et al., sputum from patients with CF was analyzed for the presence of “small subunit” ribosomal DNA, now referred to as 16S rDNA.29 All bacteria contain this gene and its sequence can be used to identify bacteria. PCR primers to amplify this gene were used, followed by a hybridization with probes specific for “known” airway pathogens like P. aeruginosa and S. aureus. The authors discovered that many samples contained 16S rDNA sequences that did not hybridize to the pathogen-specific probes, indicating that many more species may be present in sputum. In 2003 Rogers et al. performed sequencing on the 16S rDNA extracted from sputum and bronchoalveolar lavage samples and demonstrated a large number of species representing vastly different genera including Bacillus, Bacteroides, Stenotrophomonas, and Abiotrophia.30 This technique could not provide quantitative data but it did demonstrate that sputum and BAL fluid contained far more species than are typically shown on selective culture. The “microbiota” of the airways began to take shape with succeeding papers that demonstrated similar results with differing molecular techniques.31, 32 Furthermore, looking at the clones present within a single species, such as P. aeruginosa, demonstrates that patients may carry dozens of clonal types with varying degrees of relatedness.33 Penterman et al. has demonstrated that many of the P. aeruginosa found in this manner aren’t capable of growing under standard culture techniques.34
Some of the observations from these initial studies received criticism. Many of the species found were shown to exist in the “normal” oral flora, and were likely only in the sputum samples during the process of expectoration through the mouth. Another observation was the presence of a large number of anaerobic bacteria in what was considered a strictly aerobic environment of the lung. It was also suggested that while there is DNA from these species, they may not be alive or have active metabolisms capable of causing a disease phenotype. In addition, genetic techniques are unable to determine if the identified bacteria had formed a biofilm in the airways as biofilm formation affords a persistence phenotype that includes tolerance to antibiotics and avoidance of neutrophil killing (discussed below).
The notion of sputum contamination by oral flora has been addressed in a number of studies that use “clean” BAL methods to extract samples from deep within the lung without chance of contamination by oral flora.35 These studies demonstrate some species are not found in BAL, that are otherwise found in normal flora, but many of the “non-traditional” organisms and anaerobic organisms were persistently found. Recent studies were performed on explanted lungs from lung transplants which not only demonstrate diversity amongst species, but regional diversity as well. Brown et al. demonstrated that samples from each lobe contain distinct populations with some dramatically different than others.36 Erb-Downward et al. also demonstrated microanatomic differences in community composition and diversity even within the same explanted lobe.37
The question of anaerobic niches within the lung can be readily demonstrated by measuring the oxygen tension in fresh sputum at varying depths.38 Bacterial consumption of oxygen can exceed oxygen diffusion and this can create microaerophilic environments that support the growth of organisms that would otherwise perish in an oxygenated environment. P. aeruginosa was long assumed to be a strict aerobe, although it is now clear it can grow anaerobically.39 It is noteworthy in this instance because antibiotics can be dramatically less effective against the same organism when grown anaerobically.40 These discoveries also suggest the complicated interplay between bacterial communities, as seen in the gut and in the environment, are at play in the lung as well.
The current technique for sputum culture is very selective as described above. Broadening that technique without knowledge of what could possibly grow is not straightforward as many species require highly specialized nutrients, or isolation from other bacteria that could prevent their growth. To show that the diversity of the airway microbiome represented living organisms, Sibley et al. used the molecular data to design a culturing technique to grow most of the known organisms from sputum.41 This technique relied heavily on anaerobic technique, and over 50 different types of media to accurately describe the microbiome using culture technique. Studies using RNA profiles and metabolomics have further demonstrated that anaerobes and other difficult to culture organisms do participate in metabolic processes and contribute much to the chemical milieu in the lung.42
Studies of the lung microbiota have led to the realization that healthy lungs are not sterile either; rather, they too harbor a polymicrobial microbiota. Erb-Downward et al. showed that the lung microbiota of healthy non-smokers consisted principally of Pseudomonas, Streptococcus, Prevotella, and Fusobacterium.37 Charlson et al. demonstrated that the lung microbiota of healthy smokers and non-smokers consisted principally of Veillonella, Prevotella, and Streptococcus.43 Hilty et al. similarly showed that healthy subjects’ lung microbiota consisted principally of Prevotella, Streptococcus, and Veillonella.44 Many of the lung bacteria are anaerobic (Prevotella, Veillonella, Fusobacterium) and are known to inhabit the oral cavity, suggesting that aspiration may influence the lung microbiota. The roles of these normal lung bacteria in disease pathogenesis or prevention is an area with many research opportunities.
Origins of infection
A universally accepted method to prevent infections in humans is to avoid exposure to known niches containing pathogens. The sources of the classically cultured organisms from persistent lung infections have typically been linked to either the environment or other patients. There has been a clear demonstration of harm with the transmission of some pathogens such as Burkholder cepacia between CF patients, but many patients develop these unusual infections without contact with other individuals with lung disease. Many of the organisms encountered in the persistently infected lung are considered “environmental isolates,” however very few of the clinical isolates genetically resemble the environmental isolates typically found outside of the patient’s home. Furthermore, patients with genetic causes of persistent lung infections frequently develop evidence of inflammation or lung injury before classic pathogens are cultured.
With the development of molecular methods described above, a number of groundbreaking discoveries have been made to better understand the origin of these infections, which may help us to better make treatment decisions for our patients. As described above, these molecular methods are better at detecting multiple species, including known pathogens. It is thus not surprising that pathogens can be detected by molecular means well before they are detected by cultures in many cases. It is becoming clear that the microbial communities of the upper and likely lower respiratory tracts are not initiated by these classic pathogens but by microbes considered a component of the “normal flora” that are not typically reported on sputum cultures.45 And while the human lower respiratory tract is now not considered sterile, it is clearly not normally in a state of inflammation. The role of the “normal flora” in disease development of abnormal lungs is starting to be revealed. In a study of human neonates with CF, the gut and upper respiratory microbiomes were tracked and found to both increase in numbers and diversity over time.46 And while it is understood that colonization of the gut microflora is a normal process, this same process occurs in the upper, and presumably lower respiratory tract. In fact, many of the same species initially occupy both niches, and are influenced by diet and other environmentally modifiable features. To better study the lower respiratory tract, the use of the CF pig model has been instrumental in obtaining samples that cannot be obtained from human infants safely, as sputum production may not occur for years in patients with genetic diseases. Stoltz and colleagues analyzed bronchoalveolar lavage fluid from piglets with defective CFTR and determined that this animal model did indeed develop early lung disease with inflammation and evidence of early bronchiectasis formation, but that bacterial infection likely precedes this as there was no evidence of this inflammation immediately after birth.47 The bacterial species present in both CF and non-CF pigs were diverse and dynamic over time, however the CF pigs carried larger amounts of bacteria and never cleared them unlike their non-CF counterparts. These data suggest that the very early colonization with “normal” flora from the upper respiratory tract and GI tract is normal in infants but it may be that a failure to establish a normal homeostasis leads to increased inflammation and injury, potentially priming the lungs for more infections from species typically associated with lung disease.
The pathogenesis of COPD, unlike CF and non-CF bronchiectasis, is currently not thought to be due to lung infection. COPD develops in smokers following significant tobacco exposure—however only approximately 20% of tobacco users develop COPD and the severity of disease does not correspond to the intensity of tobacco exposure. In addition, some patients have a rapid decline in lung function while others have a more indolent course. Failure of immune regulation in the lung, enhanced dendritic cell function, genetic predisposition, and autoimmunity have all been suggested as potential mechanisms of COPD pathogenesis.48 Another potential mechanism is inflammation triggered by lung microbiota dysbiosis (defined as disruption of the microbiota, potentially due to repeated lung infections, antibiotic courses, corticosteroids, and aspiration). Although there is currently no conclusive evidence that dysbiosis is responsible for the development of COPD, it is well known that COPD exacerbations often result from lung infections.14, 49, 49, 50 We are only just beginning to understand complex relationship between the lung microbiota and lung inflammation during exacerbations as compared to the “steady-state” that exists between COPD exacerbations.
The lower airways as an ecosystem
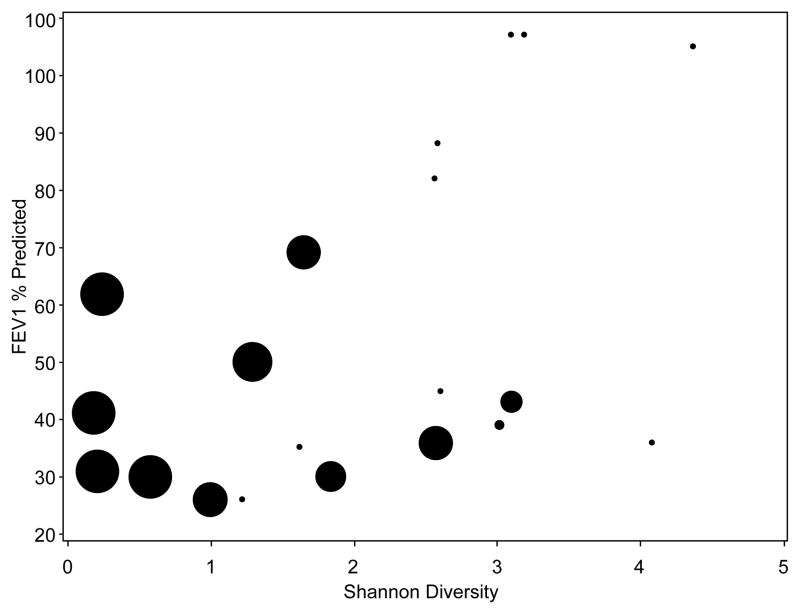
The complex nature of the lower airways with multiple species interacting has been described as an ecosystem.51, 52 Ecologic systems thrive on diversity.53 Diversity stabilizes ecosystems in times of stress as the forms of stressors can take on a multitude of forms. The loss of diversity can wreak havoc on this stability and lead to loss of structure and entire populations. In the human gastrointestinal system we have learned that loss of bacterial diversity and density with the use of antibiotics can lead to overgrowth with Clostridium difficile which can be a lethal infection.54 As patients with persistent lung infections are frequently monitored for disease progression, they typically generate numerous sputum and blood samples over years or decades which can be analyzed for changes with correlations made to their disease state. As molecular microbial analyses can be performed on frozen samples with minimal preservation techniques (unlike culture methods) scientists have been able to retrospectively track how patients’ microbiomes change over the years. Zhao et al. analyzed 126 sputum samples generated by 6 CF patients over 8–9 years of clinic visits.55 They were able to determine the abundance of individual species as well as the diversity of species in each sample and learned that patients with more “diverse” microbiomes that could maintain that diversity had little change in their baseline lung function over time. The patients with loss of diversity over those years had significant reductions in lung function. Antibiotic use was heavily correlated with loss of diversity, but it is not clear if this was the cause of the loss, or the response to changing symptoms. Zemanick et. al also determined that patients with the worse lung function had the less diverse microbiota dominated by P. aeruginosa (See Figure 2).56 These findings have been corroborated in a number of follow up studies performed in varying techniques with different CF patient populations.31, 55, 57
Figure 2. Diversity of the microbiota is correlated with lung function.
In this study by Zemanick, bacterial diversity was determined in patients undergoing an exacerbation.56 The Shannon Diversity score is a measure of the richness and eveness of populations. Patients with lower FEV1% predicted scores typically contain less diversity. The size of the circle represents the relative abundance of P. aeruginosa with larger circles indicating patients dominated by P. aeruginosa. Reproduced with permission from the authors, obtained via open access from PLOS one.
Unlike CF, the relationship between COPD severity and lung microbiota diversity has not been firmly established. However, it appears that COPD patients harbor a more diverse microbiota than do healthy controls. Lung microbiota diversity appears to be maintained in mild, moderate, and severe COPD, but this association may be primarily driven by age or medication use.37, 44, 58 Diversity appears decreased in very severe COPD.59 Diversity appears to be low in COPD-affected lungs at the time of explantation for lung transplantation, although it is not clear to what degree this reflects COPD severity vs. antibiotic and corticosteroid use in end-stage lung disease.37, 60
Exacerbations and the effect of treatment with antibiotics
If the lower airways can be thought of as an ecosystem, one may conclude a disruption in this ecosystem occurs during periods of exacerbation. A number of research groups have studied the microbiota changes that occur around exacerbations.61, 62 The use of antibiotics is associated with mortality benefits when treating severe exacerbations in both CF and COPD, however their use would seemingly disrupt a healthy diversity in the lower lung fields. This question has been addressed by Price and others who tracked CF patients during treatment of an exacerbation and monitored the relative amounts of individual species and the change in diversity over the course of a single exacerbation.63 They determined that antibiotics do not substantially change the amount of bacteria or diversity in sputum and that diversity does not decrease at the height of an exacerbation. Furthermore, this population contained mostly Pseudomonas dominated microbiomes, and this population did not show expansion at the beginning of an exacerbation, or a reduction after treatment. This study, and similar studies,64 suggest that the clinical efficacy of antibiotics may not be due to the targeted clearance of what has been considered the classical pathogens of the lower respiratory tract. However previous studies have clearly demonstrated antibiotics reduce the number of “culturable” pathogens from sputum, which suggests that molecular techniques are identifying a much larger percentage of non-culturable bacteria, or unable to distinguish alive from dead bacteria. Together these data suggest species like Pseudomonas are not always the cause of exacerbations, or that the effect of antibiotics is not in altering the absolute amounts of these species.
Our current understanding of the COPD lung microbiota and its role in COPD exacerbations is nicely summarized in two recent reviews.65, 66 Barker et al. used quantitative PCR (qPCR) to compare sputum H. influenzae, M. catarrhalis, and S. pneumoniae bacterial loads before and during COPD exacerbations. Of the three organisms studied, only M. catarrhalis demonstrated a correlation with lung inflammatory changes (as assessed by levels sputum IL1β, IL10 and TNFα) observed during exacerbations.67 Huang et al. studied the lung microbiota of 12 subjects before, during, and after a COPD exacerbation. They found that members of the Proteobacteria phylum (including non-typical COPD pathogens) were increased during exacerbations. Changes in the microbiota following exacerbation treatment differed significantly depending the regimen prescribed (antibiotics alone, oral corticosteroids alone, or both). Treatment with antibiotics alone decreased the abundance of Proteobacteria, with prolonged suppression observed in some cases. In contrast, treatment with corticosteroids alone led to enrichment for Proteobacteria. Those patients treated with both showed an increase in the abundance of Proteobacteria. This suggests that recovery of the lung microbiota is strongly affected by the therapy or therapies chosen to treat the exacerbation.49 Millares et al. analyzed the lung microbiota of 16 stage III or stage IV COPD patients before and during exacerbations.68 Five of the patients were chronically colonized with P.aeruginosa. During exacerbation, the differences between P.aeruginosa-colonized and non-colonized patients disappeared. Multiple bacteria, including Streptococcus, Pseudomonas, Moraxella, Haemophilus, Neisseria, Achromobacter, and Corynebacterium increased in abundance during exacerbations. Of particular interest, simultaneously-obtained sputum cultures did not identify the genera increased in abundance in one-third of exacerbations. These results suggest that exacerbations in P. aeruginosa-colonized patients are mainly related to other organisms and that traditional sputum cultures are an insensitive technique for monitoring bacterial changes during exacerbations.68 Molyneaux et al. recently evaluated alterations in the bacterial lung microbiota as a result of viral respiratory infection.50 The investigators experimentally infected 14 subjects with stage II COPD and 17 healthy controls with rhinovirus. All subjects exhibited symptomatic infection but none required treatment with antibiotics or steroids. By day 15 following infection, the COPD subjects’ lung microbiota contained more bacteria overall and significantly more Proteobacteria (specifically Haemophilus influenza) than did the control subjects’ microbiota. This change persisted at day 42 following infection.50
In non CF bronchiectasis it has also been shown repeatedly that the more diverse the lung microbiota, the better the overall lung function.69, 70 FEV1 is maintained in patient populations that have no bacteria on chronic sputum results, and those who maintained a more diverse “core” population.71 While several species are identified in the “core” sample, Pseudomonas is responsible for the majority of patients who have frequent exacerbations and lower than predicted lung function tests,69 Furthermore, Rogers was able to show that taxons dominated by Pseudomonas, and to a lesser extent Haemophilus, as identified by 16s rRNA were associated with decreased FEV1 and more frequent exacerbations.70 What is more important is that as the richness of these taxons were decreased, Pseudomonas and Haemophilus were more likely to be present. They then demonstrated that Pseudomonas was associated with decreased FEV1 and greater number of exacerbations per 12 month period than any other taxon. Interestingly, Haemophilus was associated with fewer exacerbations. Furthermore, the use of Azithromycin daily (BAT trial) in bronchiectasis demonstrated a decrease in infectious exacerbations and has led to the widespread use of this antibiotic for long-term treatment.72 Upon evaluation of the BLESS data, there was a significant change in the microbiota of taxons that were not dominated by Pseudomonas.73 This would suggest that chronic use of antibiotics may significantly change the microbiota such that the diversity is decreased, and a dominant taxon selected. At this time, there has been no investigation of overall microbiota changes in intermittent use of antibiotics in non-CF bronchiectasis.
Taken together, these studies suggest that exacerbations of persistent lung infections may also be polymicrobial, involving the outgrowth of several bacteria or viruses rather than the introduction of a novel pathogen. Sputum cultures are unable to detect important shifts in the lung microbiota that occur during exacerbations and following treatment. Additionally, the therapy chosen (antibiotics, steroids, or both) may have profoundly different effects on the lung microbiota. Furthermore, these treatment effects may persist long after these therapies are discontinued. These results suggest that lung microbiota dysbiosis—a microbial imbalance in the lung microbiome—may be responsible for COPD exacerbations. Furthermore, administration of antibiotics and corticosteroids may also disrupt the lung microbiota in an unintended and unanticipated manner.
Persistence afforded by growth as a biofilm
The field of microbiology is undergoing a paradigm shift in that the majority of the discoveries and characterizations performed prior to the 21st century were performed on bacteria grown “planktonically.”74 Planktonic growth is a mode of growth characterized as free living bacteria, typically in a nutrient rich environment that affords rapid growth of the colonies. All bacteria cultured in clinical microbiology labs are studied in planktonic forms of growth. With the advent of molecular techniques, vast populations of unculturable organisms have been identified. Many of these populations were considered easily studied by standard culture technique, but have taken on a new form of growth, clustered tightly to a surface or in tight-knit “microcolonies.”75 It is estimated that over 99% of the bacterial biomass on the planet actually exists in this “biofilm” form of growth rather than free living planktonic growth. There are a number of dramatic physiologic differences between planktonic and biofilm grown bacteria, and many of these differences help explain the clinical features of persistent lung infections.34, 76, 77
Bacteria growing as a biofilm are classically defined as growing in tightly packed cluster, attached to a surface and encased in an exopolysaccharide matrix produced by the bacteria. Bacteria living within a biofilm are more resilient than planktonic bacteria with respect to chemical, environmental, and antimicrobial insults. Without acquiring any new genetic traits, bacteria within a biofilm may “tolerate” antibiotic concentrations well above their MIC values when grown planktonically.76, 78 The mechanism of tolerance depends on the type of antimicrobial used, but the simplest explanation that applies to persistent lung infections, is that densely packed bacteria can overcome the effects of antibiotics through sacrifice of the outer layer of the colony. In persistent lung infections bacterial densities may reach 109 cfu/mL of sputum.79 This characteristic helps explain how bacteria with no classic “resistance” patterns to antibiotics on MIC, can survive the multi-week courses of antibiotics used to treat persistent lung infections.
Human infections that take the form of biofilms include all infections of foreign devices such as catheters, endotracheal tubes, wires, and prosthetic implants.80 A recent European clinical guideline suggests evaluating for biofilm formation on all explanted foreign devices.81 If the infection results in symptoms or risk of spread, classically the only way to remove the infection is to remove the device, as it is well established that antimicrobials cannot sterilize the device, even if the device is in a location readily accessed by administered antibiotics. In persistent lung infections, biofilms have been observed growing within sputum and within the airways, but not attached to a surface.82 Tightly knit colonies are interspersed within the sputum or airway secretions, frequently surrounded by dead or dying neutrophils, unable to penetrate within the structure. Unlike other biofilm infections, removal of the attached device is not an option, but this understanding does help support the roles of mucolytics and pulmonary secretion clearance. The use of mucolytics is considered standard of care in CF where the use of DNAse has been demonstrated to improve clinical outcomes, reduce bacterial burden in sputum, and disrupt bacteria growing as biofilms in the laboratory.83
Evidence of biofilms are seen in CF, COPD and non-CF bronchiectasis.75, 82 The classic pathogens of these infections, Pseudomonas, Staphylococcus, Haemophilus, Moraxella, have all been demonstrated to form biofilms in the laboratory. There have been a number of studies to support the notion of polymicrobial biofilms, and a number of interactions between co-infecting species have been identified.84 It is not yet understood how the biofilms of some species interacts with others within the lung, or how they are spatially organized. Presumably species with specific nutrient requirements or ability to tolerate particular insults, would be enriched within certain niches within the milieu. Anaerobic species would likely be found deeper within the mucous layer, or in more hypoxic regions of the lung, whereas organisms resistant to oxidant attacks from neutrophils may risk a more superficial layer at the benefit of more nutrients. It has been demonstrated that some populations of bacteria support the growth of others through generation of unique metabolites, and conversely, that some bacteria specifically inhibit the presence of others through nutrient competition and direct bactericidal effects.84 All of these survival strategies have been described in other instances of polymicrobial communities and we suspect these models will help us better understand persistent lung infections.
Treatment implications
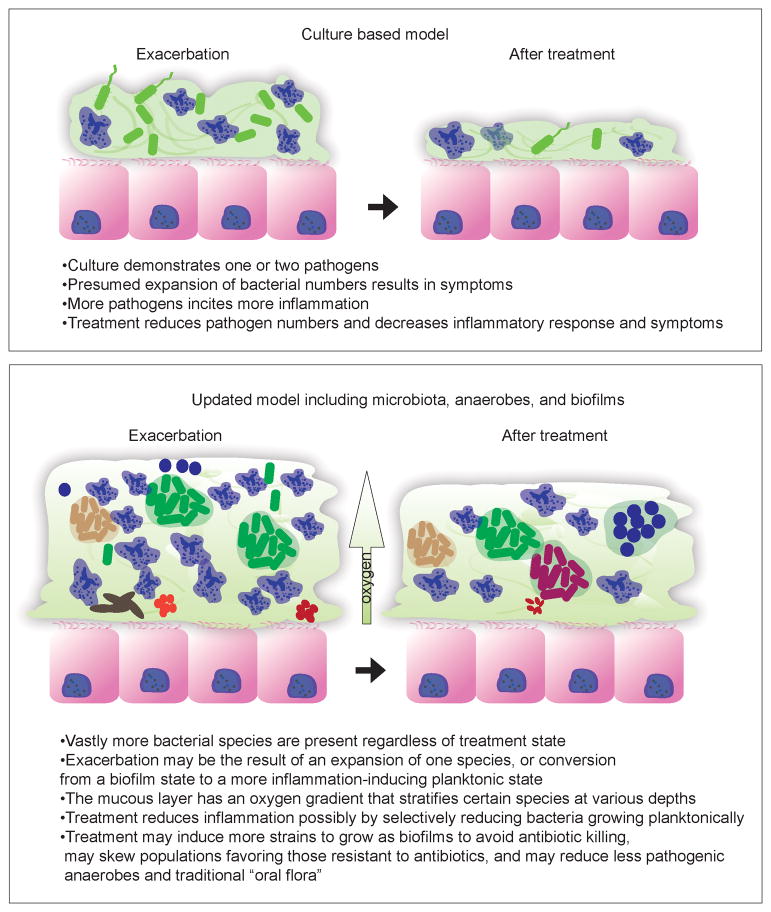
As one model to describe these infections is replaced with newer, more biologically correct models (See Figure 3), one may wonder how the treatment strategies we have developed, that typically improve patients, actually achieve this effect. Furthermore, do these new models offer insight into better treatment strategies? One presumed benefit of culturing an infection would be the theoretically benefit of “paring down” an antibiotic strategy to be more selective of the organism causing the infection in an effort to prevent resistance to broad spectrum antibiotics. This is not typically possible for highly resistant organisms like P. aeruginosa, but may readily apply to S. aureus as methicillin resistance requires very different treatment strategies. The conclusions drawn from the studies listed above demonstrate that culturing polymicrobial infections is painstaking work, highly subjective, and may not identify causative organisms, but rather those that simply “out-compete” their neighbors on the medias chosen by the microbiology lab. Furthermore the result of treatment may not be suppression of the organism seen in culture, but rather suppression of a large number of organisms that are typically not seen in culture. There is clearly a clinical benefit to the use of antibiotics in treating severe exacerbations of persistent lung disease, but the use of antibiotics in mild exacerbations, or in response to newly acquired pathogens without associated symptoms may have significant unrecognized effects on the stability of the lower airways ecosystem.
Figure 3.
An updated model of persistent lung infection informed by knowledge of microbiota and biofilms
Some practices that apply to acute infections should likely be disbanded in the treatment of persistent lung infections as described below:
Culture data should not be relied on to determine when a particular organism is acquired, but one might conclude that if found on culture, it might be in more abundance, and possibly more clinically relevant. Treatment of a newly cultured organism may be indicated when associated with symptoms or decline in lung function.
Culture data should not be relied on to determine if a species is “eradicated,” as it is safer to presume that species is “suppressed.” This is an important distinction, because the belief of eradication may skew the choices of empiric antibiotics in future exacerbations in which targeting these suppressed species may be useful in a patient not responding to culture based therapeutic strategies.
Minimal inhibitory concentrations should not be heavily relied upon in choosing antibiotics for persistent lung infections as they do not predict response. MIC data may have prognostic implications as to how likely the first antibiotic choice will work, and may also serve as a biomarker as to how often the patient has received antibiotics.
Changing antibiotic prescriptions based on new culture information in a patient responding to current therapies may have multiple unintended consequences including resistance from partially treated infections and loss of effectiveness if the cultured organism is not responsible for the current symptom set.
Tolerance to antibiotics is more likely in persistent lung infections and strategies to overcome these barriers such as use of higher doses and continuous infusion of beta-lactams may be clinically appropriate in patients not responding to conventional therapy.
The adage to treat the patient and not the laboratory value is most relevant when the laboratory value carries little therapeutic implication. Mindful use of culture information in persistent lung infections should be practiced, but over-reliance on this data may have far reaching impacts in the ecosystem of the lower airways. The future of clinical microbiology will likely be shaped by molecular methods to better describe what is present in the infections we are treating.85 These tests may give relative abundances of certain bacterial family members, or may use a bacterial diversity score as a signifier of lung health.57 More accurately predicting which species, or cohorts, is expanding at the time of an exacerbation may help chose antibiotics for their treatment.51 Earlier discovery of known pathogens by molecular techniques might prompt new investigation into better ways to suppress these organisms with antibiotics or other modifications. These more sensitive techniques may also help us understand the impact of human-to-human transmission of species not only consider pathogenic, but also beneficial. Repopulation of oral and colonic flora is a known protective measure to prevent opportunistic infection in chronically ill individuals. A better understanding of the biology of persistent lung infections may suggest a role for selective “recolonization” of the upper airways after antibiotic use, or other instances where the ecosystem is considered destabilized.
Summary.
Recent breakthroughs in microbiology and molecular diagnostics have revealed the bacterial infections found in bronchitis and bronchiectasis to be far more complicated than sputum culture results suggest. A better appreciation of this complexity should bring question to reliance on culture results and motivate new approaches in making in treatment decisions.
Acknowledgments
AAP supported by NIH KL2 5KL2TR113, JB supported by NIH T32 HL07741, BJW supported by NIH K08 PA-10-059
Footnotes
The authors declare no conflicts of interests related to the contents of this article.
Reference List
- 1.Whitters D, Stockley R. Immunity and bacterial colonisation in bronchiectasis. Thorax. 2012;67(11):1006–1013. doi: 10.1136/thoraxjnl-2011-200206. [DOI] [PubMed] [Google Scholar]
- 2.Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257. doi: 10.1002/14651858.CD010257. [DOI] [PubMed] [Google Scholar]
- 3.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol. 2003;24(5 Suppl):S6–52. doi: 10.1086/503485. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld M, Emerson J, Williams-Warren J, et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(3):359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 5.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 6.Flume PA, VanDevanter DR. Clinical applications of pulmonary delivery of antibiotics. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorde R, Pahissa A, Rello J. Management of refractory Pseudomonas aeruginosa infection in cystic fibrosis. Infect Drug Resist. 2011;4:31–41. doi: 10.2147/IDR.S16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 9.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2 Suppl):5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 11.Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58(7):589–593. doi: 10.1136/thorax.58.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev. 2001;14(2):336–363. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 15.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 16.Smith A. Pathogenesis of bacterial bronchitis in cystic fibrosis. Pediatr Infect Dis J. 1997;16(1):91–95. doi: 10.1097/00006454-199701000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48(8):1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 18.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–856. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer-Hamblett N, Rosenfeld M, Treggiari MM, et al. Standard care versus protocol based therapy for new onset Pseudomonas aeruginosa in cystic fibrosis. Pediatr Pulmonol. 2013;48(10):943–953. doi: 10.1002/ppul.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemanick ET, Emerson J, Thompson V, et al. Clinical outcomes after initial Pseudomonas acquisition in cystic fibrosis. Pediatr Pulmonol. 2014 doi: 10.1002/ppul.23036. [DOI] [PubMed] [Google Scholar]
- 21.Li AM, Sonnappa S, Lex C, et al. Non-CF bronchiectasis: does knowing the aetiology lead to changes in management? Eur Respir J. 2005;26(1):8–14. doi: 10.1183/09031936.05.00127704. [DOI] [PubMed] [Google Scholar]
- 22.FINLAYSON R. The vicissitudes of sputum cytology. Med Hist. 1958;2(1):24–35. doi: 10.1017/s0025727300023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blevins SM, Bronze MS. Robert Koch and the 'golden age' of bacteriology. Int J Infect Dis. 2010;14(9):e744–e751. doi: 10.1016/j.ijid.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Campbell S, Forbes B. The Clinical Microbiology Laboratory in the Diagnosis of Lower Respiratory Tract Infections. J Med Microbiol. 2011;49(9):S30–S33. [Google Scholar]
- 25.Wong K, Roberts MC, Owens L, Fife M, Smith AL. Selective media for the quantitation of bacteria in cystic fibrosis sputum. J Med Microbiol. 1984;17(2):113–119. doi: 10.1099/00222615-17-2-113. [DOI] [PubMed] [Google Scholar]
- 26.Brown J. Use of the Cystic Fibrosis Foundation's extensive sputum-culturing protocol for patients without cystic fibrosis: implications for infection control and antimicrobial resistance. Am J Infect Control. 2014;42(5):546–547. doi: 10.1016/j.ajic.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Smith AL, Fiel SB, Mayer-Hamblett N, Ramsey B, Burns JL. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: lack of association in cystic fibrosis. Chest. 2003;123(5):1495–1502. doi: 10.1378/chest.123.5.1495. [DOI] [PubMed] [Google Scholar]
- 28.Foweraker JE, Laughton CR, Brown DF, Bilton D. Phenotypic variability of Pseudomonas aeruginosa in sputa from patients with acute infective exacerbation of cystic fibrosis and its impact on the validity of antimicrobial susceptibility testing. J Antimicrob Chemother. 2005;55(6):921–927. doi: 10.1093/jac/dki146. [DOI] [PubMed] [Google Scholar]
- 29.Van BA, Renders NH, Smith S, Overbeek SE, Verbrugh HA. Comparison of conventional and molecular methods for the detection of bacterial pathogens in sputum samples from cystic fibrosis patients. FEMS Immunol Med Microbiol. 2000;27(1):51–57. doi: 10.1111/j.1574-695X.2000.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 30.Rogers GB, Hart CA, Mason JR, Hughes M, Walshaw MJ, Bruce KD. Bacterial diversity in cases of lung infection in cystic fibrosis patients: 16S ribosomal DNA (rDNA) length heterogeneity PCR and 16S rDNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol. 2003;41(8):3548–3558. doi: 10.1128/JCM.41.8.3548-3558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zemanick ET, Wagner BD, Robertson CE, et al. Assessment of Airway Microbiota and Inflammation in Cystic Fibrosis Using Multiple Sampling Methods. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201407-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D, Hou C, Li Y, et al. Analysis of the bacterial community in chronic obstructive pulmonary disease sputum samples by denaturing gradient gel electrophoresis and real-time PCR. BMC Pulm Med. 2014;14(1):179. doi: 10.1186/1471-2466-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feliziani S, Marvig RL, Lujan AM, et al. Coexistence and within-host evolution of diversified lineages of hypermutable Pseudomonas aeruginosa in long-term cystic fibrosis infections. PLoS Genet. 2014;10(10):e1004651. doi: 10.1371/journal.pgen.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penterman J, Nguyen D, Anderson E, et al. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep. 2014;6(2):293–300. doi: 10.1016/j.celrep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goddard AF, Staudinger BJ, Dowd SE, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci U S A. 2012;109(34):13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown PS, Pope CE, Marsh RL, et al. Directly sampling the lung of a young child with cystic fibrosis reveals diverse microbiota. Ann Am Thorac Soc. 2014;11(7):1049–1055. doi: 10.1513/AnnalsATS.201311-383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the "healthy" smoker and in COPD. PLoS One. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tunney MM, Field TR, Moriarty TF, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177(9):995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 40.Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48(7):2659–2664. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sibley CD, Grinwis ME, Field TR, et al. Culture enriched molecular profiling of the cystic fibrosis airway microbiome. PLoS One. 2011;6(7):e22702. doi: 10.1371/journal.pone.0022702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twomey KB, Alston M, An SQ, et al. Microbiota and metabolite profiling reveal specific alterations in bacterial community structure and environment in the cystic fibrosis airway during exacerbation. PLoS One. 2013;8(12):e82432. doi: 10.1371/journal.pone.0082432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184(8):957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Gast CJ, Cuthbertson L, Rogers GB, et al. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann Am Thorac Soc. 2014;11(7):1039–1048. doi: 10.1513/AnnalsATS.201312-456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madan JC, Koestler DC, Stanton BA, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;3(4) doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2(29):29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 49.Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52(8):2813–2823. doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molyneaux PL, Mallia P, Cox MJ, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(10):1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conrad D, Haynes M, Salamon P, Rainey PB, Youle M, Rohwer F. Cystic fibrosis therapy: a community ecology perspective. Am J Respir Cell Mol Biol. 2013;48(2):150–156. doi: 10.1165/rcmb.2012-0059PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrison F. Microbial ecology of the cystic fibrosis lung. Microbiology. 2007;153(Pt 4):917–923. doi: 10.1099/mic.0.2006/004077-0. [DOI] [PubMed] [Google Scholar]
- 53.Ives AR, Carpenter SR. Stability and diversity of ecosystems. Science. 2007;317(5834):58–62. doi: 10.1126/science.1133258. [DOI] [PubMed] [Google Scholar]
- 54.Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124(10):4162–4165. doi: 10.1172/JCI78366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2012;109(15):5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zemanick ET, Harris JK, Wagner BD, et al. Inflammation and airway microbiota during cystic fibrosis pulmonary exacerbations. PLoS One. 2013;8(4):e62917. doi: 10.1371/journal.pone.0062917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zemanick ET, Sagel SD, Harris JK. The airway microbiome in cystic fibrosis and implications for treatment. Curr Opin Pediatr. 2011;23(3):319–324. doi: 10.1097/MOP.0b013e32834604f2. [DOI] [PubMed] [Google Scholar]
- 58.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7(10):e47305. doi: 10.1371/journal.pone.0047305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Nunez M, Millares L, Pomares X, et al. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52(12):4217–4223. doi: 10.1128/JCM.01967-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deschaght P, Schelstraete P, Van SL, et al. Is the improvement of CF patients, hospitalized for pulmonary exacerbation, correlated to a decrease in bacterial load? PLoS One. 2013;8(11):e79010. doi: 10.1371/journal.pone.0079010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daniels TW, Rogers GB, Stressmann FA, et al. Impact of antibiotic treatment for pulmonary exacerbations on bacterial diversity in cystic fibrosis. J Cyst Fibros. 2013;12(1):22–28. doi: 10.1016/j.jcf.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Price KE, Hampton TH, Gifford AH, et al. Unique microbial communities persist in individual cystic fibrosis patients throughout a clinical exacerbation. Microbiome. 2013;1(1):27. doi: 10.1186/2049-2618-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stressmann FA, Rogers GB, van der Gast CJ, et al. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax. 2012;67(10):867–873. doi: 10.1136/thoraxjnl-2011-200932. [DOI] [PubMed] [Google Scholar]
- 65.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384(9944):691–702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sze MA, Hogg JC, Sin DD. Bacterial microbiome of lungs in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:229–238. doi: 10.2147/COPD.S38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barker BL, Haldar K, Patel H, et al. Association Between Pathogens Detected Using Quantitative Polymerase Chain Reaction With Airway Inflammation in COPD at Stable State and Exacerbations. Chest. 2015;147(1):46–55. doi: 10.1378/chest.14-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millares L, Ferrari R, Gallego M, et al. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2014;33(7):1101–1111. doi: 10.1007/s10096-013-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purcell P, Jary H, Perry A, et al. Polymicrobial airway bacterial communities in adult bronchiectasis patients. BMC Microbiol. 2014;14:130. doi: 10.1186/1471-2180-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogers GB, van der Gast CJ, Cuthbertson L, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax. 2013;68(8):731–737. doi: 10.1136/thoraxjnl-2012-203105. [DOI] [PubMed] [Google Scholar]
- 71.Rogers GB, Zain NM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc. 2014;11(4):496–503. doi: 10.1513/AnnalsATS.201310-335OC. [DOI] [PubMed] [Google Scholar]
- 72.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–1259. doi: 10.1001/jama.2013.1937. [DOI] [PubMed] [Google Scholar]
- 73.Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med. 2014;2(12):988–996. doi: 10.1016/S2213-2600(14)70213-9. [DOI] [PubMed] [Google Scholar]
- 74.Harrison J, Turner R, Marques L, Ceri H. Biofilms. American Scientist. 2005;93(6):508–515. [Google Scholar]
- 75.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 76.Hoiby N, Krogh JH, Moser C, Song Z, Ciofu O, Kharazmi A. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 2001;3(1):23–35. doi: 10.1016/s1286-4579(00)01349-6. [DOI] [PubMed] [Google Scholar]
- 77.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002;184(4):1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies JC, Bilton D. Bugs, biofilms, and resistance in cystic fibrosis. Respir Care. 2009;54(5):628–640. doi: 10.4187/aarc0492. [DOI] [PubMed] [Google Scholar]
- 79.Palmer KL, Mashburn LM, Singh PK, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187(15):5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 81.Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015 doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 82.Bjarnsholt T, Jensen PO, Fiandaca MJ, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(6):547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 83.Tetz GV, Artemenko NK, Tetz VV. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother. 2009;53(3):1204–1209. doi: 10.1128/AAC.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect. 2013;19(2):107–112. doi: 10.1111/j.1469-0691.2012.04001.x. [DOI] [PubMed] [Google Scholar]
- 85.Yang DH, Zhang YY, DUPC, et al. Rapid Identification of Bacterial Species Associated with Bronchiectasis via Metagenomic Approach. Biomed Environ Sci. 2014;27(11):898–901. doi: 10.3967/bes2014.126. [DOI] [PubMed] [Google Scholar]