Abstract
Nicotine stimulates the cholinergic anti-inflammatory pathway and prevents excessive inflammation by inhibiting the release of inflammatory cytokines from macrophages. We have previously reported that heme oxygenase-1 (HO-1) and tristetraprolin (TTP) are induced by nicotine and mediate the anti-inflammatory function of nicotine in macrophages. However, it was not clear whether two molecules are functionally linked. In this study, we sought to determine whether HO-1 associates with TTP to mediate the anti-inflammatory effects of nicotine. Inhibition of HO-1 activity or HO-1 expression attenuated the effects of nicotine on STAT3 activation, TTP induction, and TNF-α production in LPS-treated macrophages. Induction of HO-1 expression increased the level of TTP in the absence of nicotine. In a LPS-induced endotoxemia model, HO-1 deficiency blocked the effects of nicotine on the STAT3 phosphorylation, TTP induction and LPS-induced TNF-α production in the liver. Downregulation of STAT3 by siRNA attenuated the effect of nicotine on TTP expression and TNF-α production but did not affect the nicotine-mediated induction of HO-1. In TTP knockout mice, nicotine treatment enhanced HO-1 expression and STAT3 activation but failed to inhibit LPS-induced TNF-α production. Our results suggest that HO-1 and TTP are functionally linked in mediating anti-inflammatory effects of nicotine; HO-1 is necessary for the induction of TTP by nicotine. This novel nicotine-HO-1-TTP signaling pathway provides new possibilities for the treatment of inflammatory diseases.
Keywords: Nicotine, anti-inflammatory effects, HO-1, STAT3, TTP
Introduction
The cholinergic nervous system controls the inflammatory response to prevent excessive inflammation [1, 2]. Nicotine exerts anti-inflammatory effects in endotoxin-stimulated macrophages through an α7 nicotinic acetylcholine receptor (α7nAChR)-dependent mechanism that has been shown to improve survival rate in an experimental sepsis model [3–6]. The anti-inflammatory effects of α7nAChR activation are mediated by the activation of the STAT3 [7]. Nicotine fails to inhibit TNF-α production in macrophages overexpressing STAT3 with decreased DNA binding capacity [7], suggesting that the DNA binding ability of STAT3 is important for its regulatory activity.
Heme oxygenase (HO)-1 is a stress-induced enzyme that degrades heme to bilirubin, carbon monoxide (CO), and iron [8]. HO-1 and its byproducts have strong anti-inflammatory effects and HO-1 has been shown to have therapeutic potential in inflammatory arthritis [9], psoriasiform skin lesions [10], neuroinflammation [11], and inflammatory bowel disease [12]. HO-1-deficient mice display an increased inflammatory state and transfection with HO-1 mediates anti-inflammatory effects [13–15], which support the anti-inflammatory role for HO-1. HO-1 enhances the phosphorylation of STAT3 and loses its protective effects in the presence of STAT3 siRNA or in STAT3-deficient mice, suggesting that the protective role of HO-1 is dependent on STAT3 [16, 17]. On the contrary, STAT3 can function upstream of HO-1 as it has been reported that STAT3 is required for the induction of HO-1 expression by interleukin-10 (IL-10), IL-6, and hyperoxia [18–20] suggesting the presence of a positive feedback loop between STAT3 and HO-1.
The inflammatory response is modulated by post-transcriptional control [21, 22]. The post-transcriptional control of inflammatory transcripts is dependent on AU-rich element (ARE) –mediated mechanisms [23–25]. Tristetraprolin (TTP) is an ARE-binding protein that has anti-inflammatory activity by binding and destabilizing pro-inflammatory mRNAs including TNF-α, IL-17, and IL-23 [26–29]. TTP knockout (KO) mice develop severe inflammatory arthritis, autoimmune dysfunction, and myeloid hyperplasia, demonstrating the importance of TTP in limiting the inflammatory response [30].
Recently, we demonstrated that nicotine-activated STAT3 binds to the TTP promoter and enhances TTP expression and TTP mediates the anti-inflammatory function of nicotine by destabilizing TNF-α mRNA in LPS-treated macrophages [31]. We have also reported that HO-1 is induced by nicotine and mediates the anti-inflammatory effect of nicotine in LPS-induced sepsis [32]. Although our previous reports suggest that both HO-1 and TTP are induced by nicotine and mediate the anti-inflammatory effects of nicotine, it is not clear whether two proteins are functionally linked. We show here for the first time that HO-1 induction is a prerequisite for the induction of TTP by nicotine. HO-1 deficiency significantly attenuated the effect of nicotine on STAT3 activation, TTP induction, and the inhibition of LPS-induced TNF-α production in macrophages both in vitro and in vivo. Furthermore, inhibition of STAT3 by siRNA blocked the effect of nicotine on the induction of TTP and inhibition of TNF-α production in macrophages. However, TTP deficiency did not affect the nicotine-mediated induction of STAT3 activity and HO-1 expression. Our novel findings suggest that HO-1-STAT3 functions upstream of TTP induction in mediating the anti-inflammatory effects of nicotine and modulation of the HO-1-STAT3-TTP signaling pathway may have wide-ranging clinical implication in controlling inflammatory diseases.
Methods and Materials
Reagents and antibodies
Anti-HO-1 antibody, anti-phospho-STAT3, anti-STAT3, anti-β-actin, and horseradish peroxidase conjugated secondary (anti-mouse IgG and anti-goat IgG) antibodies and small interfering RNA (siRNAs) against mouse HO-1, mouse STAT3, and control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Lipopolysaccharides (LPS), cobalt protoporphyrin (CoPP), hemin, tin protoporphyrin (SnPP), and protease inhibitor cocktail sets were purchased from Sigma Aldrich (St. Louis, MO, USA). Zinc protoporphyrin IX (ZnPP IX) was obtained from Frontier Scientific Inc (Logan, UT, USA). Dulbecco’s Modified Egale Medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin and sodium pyruvate were purchased from Invitrogen (Grand Island, NY, USA). All other chemicals were purchased from Sigma-Aldrich.
Cells and mice
Mouse RAW 264.7 macrophage cells and peritoneal macrophages were collected from Balb/c wild type and HO-1 knockout (KO) mice and cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. Cells were seeded in 6-well plates at a density of 5×105/ml in DMEM medium supplemented with 10% FBS and incubated with 1 mM nicotine in the presence or absence of 1 μg/ml LPS for 24h. In the in vivo experiments, wild-type, TTP KO, and HO-1 KO mice (female, 7 to 8 weeks of age, 20–25 g) were intraperitoneally injected with PBS or 400 μg/kg nicotine for 2 h and endotoxemia was induced by intraperitoneal (i.p.) injection with LPS (12.5mg/kg) for 24 h. Peritoneal macrophages, blood serum and liver tissue were collected to determine the effects of nicotine on endotoxemia. The Animal Care Committee of the University of Ulsan, Ulsan, Korea approved all experiments with mice.
MTT assay
For 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) cell proliferation assay, cells were seeded in 96-well plate at 1×104 cells/well in DMEM and exposed to varying concentrations of nicotine for 24h. After removal of the supernatant from each well, DMEM containing 1 mg/ml of MTT was introduced. After incubation for 4 h, the supernatants were aspirated and the resultant formazan crystals were dissolved in 100 μl dimethyl sulfoxide for 30 min at 37°C and absorbance at 570 nm was determined for each well using a Victor 1420 Multilabel Counter (EG&G Wallac, Turku, Finland).
Transfection
Cells (5×105/ml) were cultured in 6-well plate for 3 h and transfected with HO-1 siRNA (100 nM) or STAT3 siRNA (100 nM) using Lipofectamine 2000 (Invitrogen). Cells were treated with 1 mM nicotine in the presence or absence of 1 μg/ml LPS for 24h. The expression levels of HO-1, STAT-3, TTP, or TNF-α mRNA and protein were analyzed by RT-PCR, Western blots, or ELISA, respectively.
Western blot assays
Cell lysates were prepared using RIPA buffer containing protease inhibitors and phosphatase inhibitors and total protein concentration of the lysates was measured using a BCA Protein Assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Proteins were resolved by SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membrane, and probed with appropriate dilutions of the following antibodies: anti-HO-1, anti-phospho-STAT3, anti-STAT3, and anti-β-actin. Immunoreactivity was detected using the ECL detection system (GE healthcare BioSciences Corp, NJ). Films were exposed at multiple time points to ensure that the images were not saturated.
Reverse transcription-polymerase chain reaction (RT-PCT)
Total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA) according to manufacturer’s instructions. DNase I-treated total RNA (2 μg) was reversed transcribed using M-MLV reverse transcriptase (Promega Corporation, WI, USA) and oligo-dT (Promega Corporation, WI, USA). Semi-quantitative RT-PCR was performed using Taq polymerase (Solgent, Daejeon, Korea) and PCR primer pairs as follows: GAPDH: 5′-aggccggtgctgagtatgtc-3′, 5′-tgcctgcttcaccttct-3′; HO-1: 5′-tcccagacaccgctcctccag-3′, 5′-ggatttggggctggtttc-3′; TTP: 5′-ctctgccatctacgagagcc-3′, 5′-gatggagtccgagtttatgttcc-3′; TNF-α, 5′-agcccacgtcgtagcaaaccaccaa-3′, 5′-acacccattcccttcacagagcaat-3′. The gene amplification reaction conditions were as follows: denaturation at 94°C for 0.5 min; annealing at 58–62°C (based on the melting temperature of each respective primer) for 0.5 min; extension at 72°C for 1 min: PCR cycle were determined according to a kinetic profile. GAPDH was used as an internal loading control.
Enzyme linked immunosorbent assay (ELISA)
TNF-α in the cell supernatants were analyzed using DuoSet ELISA Development Systems (R&D Systems).
Statistical analysis
Statistical differences between groups were evaluated by one-way ANOVA or student’s t-test. A p value of <0.05 was considered statistically significant.
Results
Nicotine induces both HO-1 and TTP expression in macrophages
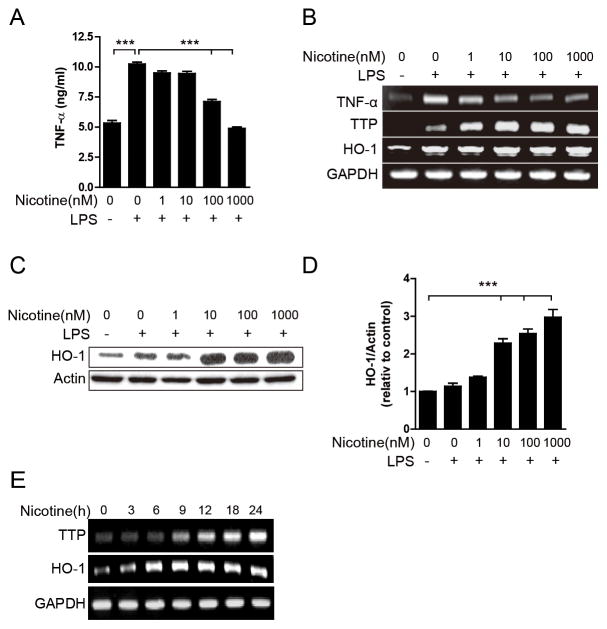
Both TTP and HO-1 were reported as induced by nicotine to mediate the anti-inflammatory effects of nicotine [31, 32], which suggests the potential relationship between HO-1 and TTP in the anti-inflammatory function of nicotine. To evaluate this hypothesis, we first determined whether nicotine induces both TTP and HO-1 in macrophages under the same conditions. Nicotine did not show any cytotoxic effect on RAW 264.7 cells at concentrations up to 1 mM (Supplementary Fig. 1). Therefore, 1 mM nicotine was used for subsequent experiments. RAW 264.7 cells were pretreated with nicotine for 20 min and incubated for an additional 24 h in the presence of LPS. Consistent with previous reports [7, 31], LPS-induced production of TNF-α protein and mRNA expression was significantly inhibited by nicotine in a dose-dependent manner (Fig. 1A and B). In addition, nicotine inhibited LPS-induced expression of IL-1β (Supplementary Fig. 2) and IFN-γ-induced expression of TNF-α (Supplementary Fig. 3), indicating that nicotine may exert a global effect on the stimulated macrophages. Consistent with our expectation, nicotine significantly increased the expression levels of both HO-1 and TTP mRNA (Fig. 1B) and protein (Fig. 1C and 1D) in a dose-dependent manner. Next, we determined the expression levels of HO-1 and TTP at different time point after treatment with1 mM nicotine. A time-dependent treatment with nicotine showed a dramatic increase in HO-1 and TTP level at 6h and 9 h, respectively (Fig. 1E). This result suggests that HO-1 is induced by nicotine before TTP in RAW 264.7 cells.
Figure 1. Nicotine induces the expression of both HO-1 and TTP in macrophages.
(A–D) RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml LPS for 24 h. (A) Cell supernatants were analyzed for TNF-α by ELISA. Values are mean ± SD (n = 3). ***p<0.001. (B) The levels of TNF-α, TTP, and HO-1 were measured by semi-qRT-PCR and the levels of HO-1 were measured by Western blot assays. The representative band is shown (C). Scion Image software was used for densitometry analysis of the HO-1 protein band (D). The relative density of the sample lane was divided by the control lane (β-actin). Values are mean ± SEM (n = 3). ***p<0.001. (E) RAW 264.7 cells were treated with 1 mM nicotine for indicated time. The levels of HO-1 and TTP were analyzed by semi-RT-PCR.
Inhibition of HO-1 activity blocks the effect of nicotine on the TTP expression
To determine whether HO-1 activity is required for the expression of TTP, we analyzed the effect of the HO-1 inhibitors ZnPP and SnPP on nicotine-mediated induction of TTP in macrophages. The HO-1 inhibitor ZnPP did not affect the nicotine-mediated induction of HO-1 but attenuated the effect of nicotine on the induction of TTP in a dose-dependent manner in RAW 264.7 cells (Fig. 2A). ZnPP blocked the inhibitory effect of nicotine on the LPS-induced production of TNF-α (Fig. 2B). The inhibitory effect of ZnPP on the nicotine-mediated induction of TTP was also detected in peritoneal macrophages (Fig. 2C). The SnPP, another HO-1 inhibitor, also blocked the induction of TTP in nicotine-treated peritoneal macrophages (Fig. 2C), confirming that HO-1 activity is required for nicotine-induced TTP expression.
Figure 2. Inhibition of HO-1 activity attenuates the effect of nicotine on the TTP induction in macrophages.
(A) RAW 264.7 cells were pretreated with the indicated concentration of ZnPP for 30 min and further incubated in the presence or absence of 1 mM nicotine for 24 h. The levels of TTP and HO-1 were analyzed by semi-qRT-PCR. (B) Peritoneal macrophages isolated from wild-type Balb/c mice were pretreated with ZnPP (20μM) or SnPP (50μM) for 30min and further incubated with 1 mM nicotine for 24h. The levels of TTP and HO-1 were analyzed by semi-qRT-PCR. (C) RAW 264.7 cells were pretreated with ZnPP (20μM) for 30min and further incubated with 1 mM nicotine and 1 μg/ml LPS for 24 h. The levels of TTP, HO-1, and TNF-α were analyzed by semi-qRT-PCR.
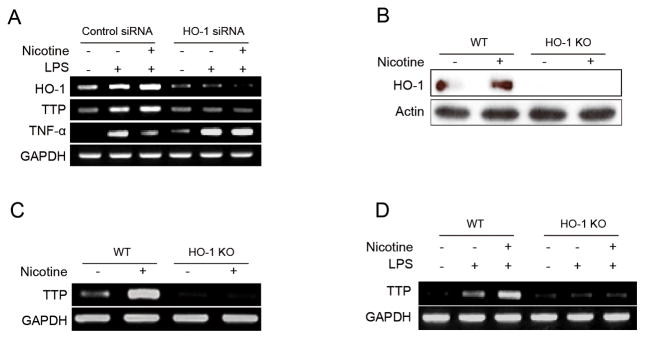
Gene silencing or knockdown of HO-1 suppressed nicotine-induced TTP expression
We next determined the effect of HO-1 knock-down on the nicotine-mediated induction of TTP. RAW 264.7 cells were transfected with siRNA against HO-1 and incubated with nicotine. Nicotine failed to induce TTP expression and inhibit LPS-induced TNF-α production in HO-1 siRNA-transfected cells (Fig. 3A). To confirm the effect of HO-1 on the nicotine-mediated induction of TTP, peritoneal macrophages were collected from HO-1 KO mice, treated with nicotine (1 mM, 18h) and subjected to analysis for TTP. Peritoneal macrophages from wild-type mice were used as a control. While nicotine enhanced the TTP expression in macrophages from wild-type mice, it did not increase these TTP levels in macrophages from HO-1 KO mice (Fig. 3B–3D). Collectively, our results suggest that HO-1 is required for the nicotine-mediated induction of TTP in macrophages.
Figure 3. HO-1 gene silencing blocks the effects of nicotine on TTP induction in macrophages.
(A) RAW 264.7 cells were transfected with siRNA against HO-1 (HO-1-siRNA) or scrambled siRNA (scRNA). After treatment with 1 mM nicotine and 1 μg/ml LPS for 24 h, the levels of TTP, HO-1, and TNF-α were analyzed by semi-qRT-PCR. (B–D) Peritoneal macrophages were harvested from HO-1 KO and wild-type mice. (B & C) Cells were treated with 1 mM nicotine for 24 h. The level of TTP and HO-1 were determined by semi-qRT-PCR (B) and Western blot assays (C). (D) Cells were treated with 1 mM nicotine and 1 μg/ml LPS for 24 h and TTP levels were analyzed by semi-qRT-PCR.
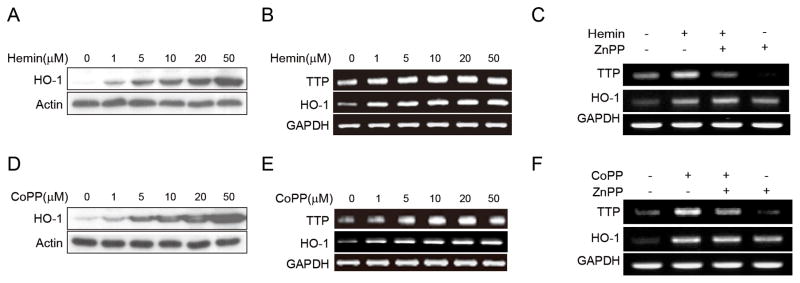
Hemin- and CoPP-induced HO-1-mediated inhibition of the nicotine inflammatory effect through TTP expression
Next, we tested the effect of HO-1 inducer on TTP expression in macrophages. Hemin, a vital constituent of hemoglobin, is known to have an anti-inflammatory function or by inducing HO-1 levels [33, 34]. RAW 264.7 cells were incubated in the presence of various hemin concentrations and were analyzed for HO-1 and TTP expression. Coinciding with previous report [33, 34], the hemin induced HO-1 expression in a dose-dependent manner (Fig. 4A and 4B). TTP expression was also increased by hemin treatment in a dose-dependent manner (Fig. 4A and 4B). Treatment with a HO-1 inhibitor (ZnPP, 20μM) did not affect the HO-1 level but abolished the effect of hemin on the induction of TTP (Fig. 4C), suggesting that HO-1 mediates the hemin-induced TTP expression. We also tested the effect of cobalt protoporphyrin (CoPP), a well-known inducer of HO-1 [35, 36], on the expression of TTP. Similar to hemin, CoPP induced the expression of both HO-1 and TTP (Fig. 4D and 4E) and ZnPP blocked the effect of CoPP on the induction of TTP (Fig. 4F). These results further support the role of HO-1 in the induction of TTP in macrophages.
Figure 4. Induction of HO-1 increases TTP levels in macrophages.
(A & B) RAW 264.7 cells were treated with the indicated concentration of hemin for 18h. HO-1 levels were analyzed by Western blot (A) and semi-qRT-PCR (B) and TTP levels were analyzed by semi-qRT-PCR (B). (C) RAW 264.7 cells were pretreated with 20μM ZnPP for 30min and further incubated with 10 μM hemin for 18h. The levels of TTP and HO-1 were analyzed by semi-qRT-PCR. (D & E) RAW 264.7 cells were treated with the indicated concentration of CoPP for 18h. HO-1 levels were analyzed by Western blot (D) and semi-qRT-PCR (E) and TTP levels were analyzed by semi-qRT-PCR (E). (F) RAW 264.7 cells were pretreated with 20μM ZnPP for 30min and further incubated with 10μM CoPP for 18h. The levels of TTP and HO-1 were analyzed by semi-qRT-PCR.
STAT-3 is required for HO-1-mediated induction of TTP
It has been reported that HO-1 enhances the STAT3 activity [16, 17] which is required for the induction of TTP by nicotine [31]. Our next goal was to determine whether HO-1 induces TTP expression by enhancing STAT3 activity. Consistent with previous reports [7, 31], nicotine increased the phosphorylation of STAT3 in a dose-dependent manner (Fig. 5A). Inhibition of HO-1 activity by ZnPP (Fig. 5B) or HO-1 expression by siRNA (Fig. 5C) blocked the effect of nicotine on the phosphorylation of STAT3 (Fig. 5B and 5C). In addition, nicotine-induced STAT3 phosphorylation was inhibited in peritoneal macrophages derived from HO-1 KO mice (Fig. 5D). Our results indicate that HO-1 is required for nicotine-induced STAT3 phosphorylation. To determine whether STAT3 is required for the induction of TTP by HO-1, RAW 264.7 cells were transfected with STAT3 siRNA and further treated with the HO-1 inducer, CoPP. Inhibition of STAT3 (Fig. 5E) did not affect the HO-1 induction by CoPP. However, it blocked the effect of CoPP on TTP induction (Fig. 5F). We also determined the effect of STAT3 inhibition on the induction of TTP in nicotine-treated macrophages. RAW 264.7 cells were transfected with STAT3 siRNA and further treated with nicotine in the presence or absence of LPS. While inhibition of STAT3 did not affect the HO-1 induction by nicotine (Fig. 5G), it blocked the effect of nicotine on the expression of TTP and LPS-induced TNF-α (Fig. 5G and 5H). These results suggest that STAT3 is required for the induction of TTP by the nicotine-HO-1 pathway.
Figure 5. STAT3 is required for HO-1-induced TTP expression in macrophages.
(A–C) Nicotine-HO-1 enhances STAT3 phosphorylation. (A) RAW 264.7 cells were treated with 1 mM nicotine for the indicated times and the levels of total STAT3 and phospho-STAT3 were analyzed by Western blot assays. (B) RAW 264.7 cells were pretreated with 20 μM ZnPP for 30 min and further incubated with the indicated concentration of nicotine for 24 h. The levels of total STAT3 and phospho-STAT3 were analyzed by Western blot assays. (C) RAW 264.7 cells were transfected with HO-1-siRNA. After treatment with 1 mM nicotine for 24 h, the levels of STAT3, phospho-STAT3, and HO-1 were analyzed by Western blot assays. (D) Peritoneal macrophages were harvested from HO-1 KO and wild-type mice. Cells were treated with 1 mM nicotine for 24 h. The level of HO-1, STAT3, and phospho-STAT3 were determined by Western blot assays. (E–H) STAT3 is required for nicotine-induced TTP expression. RAW 264.7 cells were transfected with STAT3-siRNA. (E) The level of STAT3 was analyzed by Western blot assays. (F) Cells were incubated with 10μM CoPP for 18h and the levels of TTP, HO-1, and STAT3 were analyzed by semi-qRT-PCR. (G) After treatment with 1 mM nicotine for 24 h, the levels of TTP, STAT3, and HO-1 were analyzed by semi-qRT-PCR. (H) After treatment with 1 mM nicotine and 1 μg/ml LPS for 24 h, the levels of TTP, STAT3, HO-1, and TNF-α were analyzed by semi-qRT-PCR.
TTP knockout does not alter the effect of nicotine on HO-1 induction and STAT3 phosphorylation but blocks the inhibitory effects of nicotine on LPS-induced TNF-α
The experiments above indicate that TTP is induced by the nicotine-HO-1-STAT3 signaling pathway and decreases LPS-induced TNF-α levels. To confirm this hypothesis, wild-type and TTP KO mice were administrated LPS and/or nicotine and liver tissues were analyzed for HO-1, STAT3 and TNF-α. In wild-type mice, nicotine treatment increased the level of HO-1, phosphorylated STAT3, and TTP but decreased the level of LPS-induced TNF-α (Fig. 6A, 6C, 6D, Supplementary Fig. 4A). Interestingly, in TTP KO mice, while nicotine increased HO-1 expression and STAT3 phosphorylation to the similar level as in wild-type mice, it failed to decrease LPS-induced TNF-α production (Fig. 6B-6D, Supplementary Fig. 4B). This result suggests that TTP is down-stream of nicotine-HO-1-STAT3 signaling pathway and mediates the inhibitory effect of nicotine on LPS-induced TNF-α expression.
Figure 6. TTP deficiency does not affect nicotine-induced HO-1 expression in the LPS-induced endotoxemia mouse model.
(A–C) Wild type (WT) and TTP knockout (TTP KO) mice were pretreated with nicotine (400 μg/kg, i.p.) for 2h and administrated with LPS (12.5 mg/kg, i.p.) for 24h. (A & B) Liver tissues were analyzed for the levels of HO-1 and TNF-α by semi-qRT-PCR. One representative band is shown (A & B). (C) Liver tissues were analyzed for the levels of HO-1, STAT-3, and phosphor-STAT3 by Western blot assays. One representative band is shown. (D) Blood serum was analyzed for the TNF-α levels by ELISA. Values are mean ± SEM (n = 3). **p<0.01.
HO-1-mediated TTP is responsible for the anti-inflammatory effects in the LPS-induced endotoxemia mouse model
Sepsis is a systemic inflammatory response resulted from excessive production of pro-inflammatory cytokines such as TNF-α stimulation by pathogen components such as LPS [37]. Nicotine has been reported to suppress LPS-induced TNF-α production by enhancing the expression HO-1 and TTP [31, 32]. Because our results indicate that HO-1 and TTP function in the same pathway in nicotine-mediated anti-inflammatory function, it is possible that depletion of either HO-1 or TTP could block the anti-inflammatory function of nicotine. To test this hypothesis, we investigated the effect of nicotine on the expression of LPS-induced TNF-α as an indicator of sepsis in the liver tissues of wild-type, HO-1 KO, and TTP KO mice. Nicotine treatment significantly increased the level of HO-1 and TTP and suppressed the production of TNF-α in the liver tissues of LPS-treated wild-type mice (Fig. 6A, Fig. 7A, and Supplementary Fig. 4A and B). However, nicotine failed to decrease LPS-induced TNF-α production in the liver tissue of both HO-1 KO and TTP KO mice (Fig. 6B, 7B, and Supplementary Fig. 5A and B). We also analyzed sera from wild-type, HO-1 KO, and TTP KO mice for TNF-α levels 24 h after treatment with nicotine and LPS. Coinciding with the results of liver tissues, nicotine significantly suppressed TNF-α levels in wild-type serum but it did not reduce the serum TNF-α levels in HO-1 or TTP KO mice (Fig. 6D and Fig. 7C). These results suggest that HO-1 and TTP are not complementary in mediating the anti-inflammatory function of nicotine.
Figure 7. HO-1 deficiency blocks the effect of nicotine on TTP expression and TNF-α production in the LPS-induced endotoxemia mouse model.

Wild type and HO-1 KO mice were pretreated with nicotine (400 μg/kg, i.p.) for 2h and administrated with LPS (12.5 mg/kg, i.p.) for 24h. (A & B) Liver tissues were analyzed for TTP and TNF-α mRNA levels by semi-qRT-PCR. One representative band is shown. (C) Blood serum was analyzed for the level of TNF-α by ELISA. Values are mean ± SEM (n = 3). ***p<0.001. ns, not significant.
Discussion
HO-1, nicotine, and TTP all have anti-inflammatory function and are likely to provide intrinsic protection against inflammatory diseases. Nicotine inhibits TNF-α production from LPS-stimulated macrophages [4] through α7nAChR-mediated STAT3 activation [7]. HO-1, an important antioxidant molecule, is induced by nicotine and mediates the anti-inflammatory function of nicotine [32]. TTP suppresses inflammation by destabilizing the mRNAs of pro-inflammatory cytokines [38]. TTP is induced by nicotine and mediates the anti-inflammatory function of nicotine [31]. Despite evidence for the roles of HO-1 and TTP in nicotine-mediated anti-inflammatory function, nothing is known about the functional links between HO-1 and TTP. In this report, we demonstrate that HO-1 and TTP function in the same nicotine-mediated anti-inflammatory signaling pathway. Our data provide evidence for the requirement of HO-1 in nicotine-induced upregulation of TTP in macrophages and found that HO-1 inhibitors prevent the effects of nicotine on TTP production in macrophages. In addition, depletion of HO-1 blocks the effect of nicotine on the induction of TTP and inhibits LPS-induced TNF-α production both in vitro and in vivo. On the contrary, HO-1 inducers were shown to enhance TTP levels in the absence of nicotine. While depletion of TTP blocks the effect of nicotine on the inhibition of LPS-induced TNF-α, it does not affect the nicotine-induced expression of HO-1 in vivo. Collectively, our data suggest that HO-1 and TTP mediate the anti-inflammatory function of nicotine in the nicotine-HO-1-TTP signaling pathway.
The mechanism by which HO-1 affects TTP production remains to be elucidated. Previous reports suggest the presence of a positive feedback loop between STAT3 and HO-1 where STAT3 enhances the expression of HO-1 [18–20], which in turn activates STAT3 [16, 17, 39]. Activated STAT3 has been reported to bind to the TTP promoter and induce TTP transcription [31]. Thus, it is possible that HO-1 enhances TTP expression through STAT3 activation. We found that inhibition of STAT3 by siRNA blocked the induction of TTP by nicotine and HO-1 inducers. In addition, we provided evidence supporting the role of the HO-1-STAT3-TTP signaling pathway in the anti-inflammatory function of nicotine Inhibition of HO-1 activity or expression was shown to block the effect of nicotine on STAT3 phosphorylation, TTP expression, and LPS-induced TNF-α production and depletion of TTP did not change the effect of nicotine on HO-1 expression and STAT3 phosphorylation but yet blocked the inhibitory effect of nicotine on LPS-induced TNF-α production. Our results suggest that the HO-1-STAT3 signaling pathway induces TTP expression, which mediates the anti-inflammatory function of nicotine.
It has been reported that nicotine upregulates IL-10 in T lymphocytes [40] and IL-10 induces TTP expression through STAT3 activation in macrophages [41]. This suggests that nicotine may induce TTP expression using the IL-10-STAT-3 signaling pathway in addition to the HO-1-STAT3 signaling pathway in macrophages. However, we found that nicotine did not significantly increase IL-10 levels in macrophages (Supplementary Fig. 6). In addition, depletion of HO-1 almost completely abrogated the effect of nicotine on the induction of TTP both in vitro and in vivo, indicating that the HO-1-STAT3 signaling pathway is the main pathway for nicotine-mediated TTP induction. It is still unclear as to why nicotine does not increase IL-10 levels in macrophages. Unlike T lymphocytes [40], macrophages may not enhance the transcription of IL-10 in the presence of nicotine. Otherwise, IL-10 production may be prevented by TTP as a negative feedback loop [42]. Further study on the mechanism of nicotine-mediated control of IL-10 production is necessary.
Heme oxygenase-1 (HO-1) is known to have immune protective effects [9–12]. Despite the recognized cytoprotective effects of HO-1, the molecular pathways mediating these protective effects are still not well defined. In this study, we showed that HO-1 exerts its anti-inflammatory function by inducing the expression of TTP which destabilizes pro-inflammatory cytokines mRNA. However, it is not likely that TTP alone mediate the full anti-inflammatory effect of HO-1. The immunoprotective effects of HO-1 are attributed to the removal of pro-inflammatory agent heme and the anti-inflammatory function of three metabolites resulting from heme degradation by HO-1 (i.e., CO, ferrous iron, and biliverdin) [43]. Among the HO-1 metabolites, CO has been found to be responsible for the induction of TTP [44]. It is possible that HO-1 modulates multiple downstream anti-inflammatory pathways, of which TTP-mediated destabilization of pro-inflammatory cytokines mRNA may be one pathway. However, in the LPS-induced endotoxemia mice model, either HO-1 or TTP deficiency blocked the inhibitory effect of nicotine on LPS-induced TNF-α production in the liver. These results indicate that, even though HO-1 exerts its anti-inflammatory function by both TTP-dependent and independent mechanisms, the former may be the main mechanism for HO-1-mediated anti-inflammatory function.
In summary, these results suggest that HO-1 and TTP are functionally linked in mediating the anti-inflammatory function of nicotine. Nicotine enhances the expression of HO-1 and HO-1-STAT3 signaling increases TTP levels which in turn inhibits LPS-induced TNF-α production in vivo and in vitro (Fig. 8). This study identified a novel nicotine-HO-1-STAT3-TTP signaling pathway responsible for the inhibition of LPS-driven inflammation and potentially provides the rationale for novel therapeutic approaches for the management of inflammatory diseases.
Figure 8. Schematic diagram of the nicotine-mediated inhibition of LPS-induced TNF-α production.
Binding of nicotine to α7nAChR enhances the HO-1 expression and HO-1-STAT3 signaling increases TTP level in macrophages. TTP then inhibits the expression of LPS-induced TNF-α and IL-1β by enhancing their mRNA degradation.
Supplementary Material
Cells were incubated with nicotine (0, 1, 10, 100 and 1000nM) for 24h. After 24h, MTT assay was performed to measure the cell viability. Data indicating mean ± SEM of three independent experiments with triplicate wells.
RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml LPS for 24 h. The levels of IL-1β were measured by semi-qRT-PCR. The representative band is shown.
RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml IFN-γ for 24 h. (A) Cell supernatants were analyzed for TNF-α by ELISA. Values are mean ± SD (n = 3). ***p<0.001.
Wild type and TTP KO mice were pretreated with nicotine (400 μg/kg, i.p.) for 2h and administrated with LPS (12.5 mg/kg, i.p.) for 24h. Liver tissues were analyzed for the levels of TNF-α (A) and HO-1 (B) by semi-qRT-PCR. Scion Image software was used for densitometry analysis of TNF-α and HO-1 mRNA bands. The relative density of the sample lane was divided by the control lane (18S, internal control). The expression levels obtained from mice without nicotine and LPS treatment were set to 1. Values are mean ± SEM (n = 3). *p<0.05; **p<0.01. ns, not significant.
Wild type and HO-1 KO mice were pretreated with nicotine (400 μg/kg, i.p.) for 2h and administrated with LPS (12.5 mg/kg, i.p.) for 24h. Liver tissues were analyzed for TNF-α (A) TTP (B) mRNA levels by semi-qRT-PCR. Scion Image software was used for densitometry analysis of TNF-α and TTP mRNA bands. The relative density of the sample lane was divided by the control lane (18S, internal control). The expression levels obtained from mice without nicotine and LPS treatment were set to 1. Values are mean ± SEM (n = 3). *p<0.05; **p<0.01; ***p<0.001. ns, not significant.
RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml LPS for 24 h. The levels of IL-10 were measured by semi-qRT-PCR. The representative band is shown.
Acknowledgments
This study was supported by a Korea Research Foundation grant funded by the Korean government (MOEHRD, BRL- 2009-0087350) and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2012M3A9C3048687).
List of abbreviations
- HO-1
heme oxygenase-1
- TTP
tristetraprolin
- LPS
lipopolysaccharide
- STAT3
signal transducer and activator of transcription 3
- TNF
tumor necrosis factor
- ECL
enhanced chemiluminescence
- CoPP
cobalt protoporphyrin
- ZnPP
zinc protoporphyrin
- SnPP
Tin protoporphyrin
- HO-1 KO
HO-1 knockout
Footnotes
Conflict of interest
We declare that no conflicts of interest exist.
References
- 1.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 6.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 8.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 9.Benallaoua M, Francois M, Batteux F, Thelier N, Shyy JY, Fitting C, Tsagris L, Boczkowski J, Savouret JF, Corvol MT, Poiraudeau S, Rannou F. harmacologic induction of heme oxygenase 1 reduces acute inflammatory arthritis in mice. Arthritis Rheum. 2007;56:2585–2594. doi: 10.1002/art.22749. [DOI] [PubMed] [Google Scholar]
- 10.Ma LJ, You Y, Bai BX, Li YZ. Therapeutic effects of heme oxygenase-1 on psoriasiform skin lesions in guinea pigs. Arch Dermatol Res. 2009;301:459–466. doi: 10.1007/s00403-009-0956-4. [DOI] [PubMed] [Google Scholar]
- 11.Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naito Y, Takagi T, Yoshikawa T. Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 1):177–184. doi: 10.1111/j.1365-2036.2004.01992.x. [DOI] [PubMed] [Google Scholar]
- 13.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 14.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aziz MT, el-Asmar MF, el-Miligy D, Atta H, Shaker O, Ghattas MH, Hosni H, Kamal N. Retrovirus-mediated human heme oxygenase-1 (HO-1) gene transfer into rat endothelial cells: the effect of HO-1 inducers on the expression of cytokines. Int J Biochem Cell Biol. 2003;35:324–332. doi: 10.1016/s1357-2725(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 2006;20:2156–2158. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- 17.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, Busuttil RW, Kupiec-Weglinski JW. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 56:359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deramaudt TB, da Silva JL, Remy P, Kappas A, Abraham NG. Negative regulation of human heme oxygenase in microvessel endothelial cells by dexamethasone. Proc Soc Exp Biol Med. 1999;222:185–193. doi: 10.1046/j.1525-1373.1999.d01-130.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee PJ, Camhi SL, Chin BY, Alam J, Choi AM. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L175–182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- 20.Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76:719–726. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- 21.Stoecklin G, Anderson P. Posttranscriptional mechanisms regulating the inflammatory response. Adv Immunol. 2006;89:1–37. doi: 10.1016/S0065-2776(05)89001-7. [DOI] [PubMed] [Google Scholar]
- 22.Hollams EM, Giles KM, Thomson AM, Leedman PJ. MRNA stability and the control of gene expression: implications for human disease. Neurochem Res. 2002;27:957–980. doi: 10.1023/a:1020992418511. [DOI] [PubMed] [Google Scholar]
- 23.Clark A. Post-transcriptional regulation of pro-inflammatory gene expression. Arthritis Res. 2000;2:172–174. doi: 10.1186/ar83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kracht M, Saklatvala J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 2002;20:91–106. doi: 10.1006/cyto.2002.0895. [DOI] [PubMed] [Google Scholar]
- 25.Khabar KS. The AU-rich transcriptome: more than interferons and cytokines, and its role in disease. J Interferon Cytokine Res. 2005;25:1–10. doi: 10.1089/jir.2005.25.1. [DOI] [PubMed] [Google Scholar]
- 26.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 27.Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc Natl Acad Sci U S A. 2004;101:2011–2016. doi: 10.1073/pnas.0400148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park D, Lee EK, Jang EJ, Jeong HO, Kim BC, Ha YM, Hong SE, Yu BP, Chung HY. Identification of the dichotomous role of age-related LCK in calorie restriction revealed by integrative analysis of cDNA microarray and interactome. Age (Dordr) doi: 10.1007/s11357-012-9426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian X, Ning H, Zhang J, Hoft DF, Stumpo DJ, Blackshear PJ, Liu J. Posttranscriptional regulation of IL-23 expression by IFN-gamma through tristetraprolin. J Immunol. 186:6454–6464. doi: 10.4049/jimmunol.1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 31.Joe Y, Kim HJ, Kim S, Chung J, Ko MS, Lee WH, Chang KC, Park JW, Chung HT. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolysaccharide-stimulated macrophages. J Biol Chem. 286:24735–24742. doi: 10.1074/jbc.M110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, Kim HJ, Seo HG, Lee JH, Chung HT, Chang KC. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 14:2057–2070. doi: 10.1089/ars.2010.3555. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 34.Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 35.Ewing P, Hildebrandt GC, Planke S, Andreesen R, Holler E, Gerbitz A. Cobalt protoporphyrine IX-mediated heme oxygenase-I induction alters the inflammatory cytokine response, but not antigen presentation after experimental allogeneic bone marrow transplantation. Int J Mol Med. 2007;20:301–308. [PubMed] [Google Scholar]
- 36.Sardana MK, Kappas A. Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc Natl Acad Sci U S A. 1987;84:2464–2468. doi: 10.1073/pnas.84.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 38.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 40.Galitovskiy V, Qian J, Chernyavsky AI, Marchenko S, Gindi V, Edwards RA, Grando SA. Cytokine-induced alterations of alpha7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17- versus Th2-mediated colitis. J Immunol. 187:2677–2687. doi: 10.4049/jimmunol.1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaljo B, Kratochvill F, Gratz N, Sadzak I, Sauer I, Hammer M, Vogl C, Strobl B, Muller M, Blackshear PJ, Poli V, Lang R, Murray PJ, Kovarik P. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol. 2009;183:1197–1206. doi: 10.4049/jimmunol.0803883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaba A, Grivennikov SI, Do MV, Stumpo DJ, Blackshear PJ, Karin M. Cutting edge: IL-10-mediated tristetraprolin induction is part of a feedback loop that controls macrophage STAT3 activation and cytokine production. J Immunol. 189:2089–2093. doi: 10.4049/jimmunol.1201126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 44.Joe Y, Uddin J, Zeng M, Kim HJ, Chen Y, Yoon NA, Cho GJ, Park JW, Chung HT. Tristetraprolin mediates anti-inflammatory effect of carbon monoxide against DSS-induced colitis. Oxidative Medicine and Cellular Longivity. doi: 10.1371/journal.pone.0088776. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells were incubated with nicotine (0, 1, 10, 100 and 1000nM) for 24h. After 24h, MTT assay was performed to measure the cell viability. Data indicating mean ± SEM of three independent experiments with triplicate wells.
RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml LPS for 24 h. The levels of IL-1β were measured by semi-qRT-PCR. The representative band is shown.
RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml IFN-γ for 24 h. (A) Cell supernatants were analyzed for TNF-α by ELISA. Values are mean ± SD (n = 3). ***p<0.001.
Wild type and TTP KO mice were pretreated with nicotine (400 μg/kg, i.p.) for 2h and administrated with LPS (12.5 mg/kg, i.p.) for 24h. Liver tissues were analyzed for the levels of TNF-α (A) and HO-1 (B) by semi-qRT-PCR. Scion Image software was used for densitometry analysis of TNF-α and HO-1 mRNA bands. The relative density of the sample lane was divided by the control lane (18S, internal control). The expression levels obtained from mice without nicotine and LPS treatment were set to 1. Values are mean ± SEM (n = 3). *p<0.05; **p<0.01. ns, not significant.
Wild type and HO-1 KO mice were pretreated with nicotine (400 μg/kg, i.p.) for 2h and administrated with LPS (12.5 mg/kg, i.p.) for 24h. Liver tissues were analyzed for TNF-α (A) TTP (B) mRNA levels by semi-qRT-PCR. Scion Image software was used for densitometry analysis of TNF-α and TTP mRNA bands. The relative density of the sample lane was divided by the control lane (18S, internal control). The expression levels obtained from mice without nicotine and LPS treatment were set to 1. Values are mean ± SEM (n = 3). *p<0.05; **p<0.01; ***p<0.001. ns, not significant.
RAW 264.7 cells were pretreated with the indicated concentration of nicotine for 20 min and incubated with 1 μg/ml LPS for 24 h. The levels of IL-10 were measured by semi-qRT-PCR. The representative band is shown.