Abstract
Advances in biomaterials science and engineering are crucial to translating regenerative engineering, an emerging field that aims to recreate complex tissues, into clinical practice. In this regard, citrate-based biomaterials have become an important tool owing to their versatile material and biological characteristics including unique antioxidant, antimicrobial, adhesive, and fluorescent properties. This review discusses fundamental design considerations, strategies to incorporate unique functionality, and examples of how citrate-based biomaterials can be an enabling technology for regenerative engineering.
Keywords: citric acid, elastomer, degradation, tissue engineering, medical device
Introduction
For the past three decades, tissue engineering has been focused primarily on the fabrication and repair of individual tissues. However, the integration of stem cell science and developmental biology with biomaterial-based tissue engineering strategies has produced the new field of regenerative engineering, which aims to fabricate and regenerate complex tissues and biological systems (1). Established thermoplastic poly(α-hydroxyacids), such as poly(lactic acid) (PLA), poly(glycolic acid), and their copolymers, were the biomaterials of choice for initial exploratory applications to establish the feasibility of tissue engineering concepts. This was mostly due to the extensive experience that textile and polymer manufacturers had with these materials and their use in products approved by the US Food and Drug Administration. However, polymers such as PLA, poly(glycolic acid), and related copolymers fall short when it comes to meeting the increasingly sophisticated demands of regenerative engineering applications (2). To fully realize the concept of complex multitissue regeneration, there is an increasing need for advanced biomaterials that proactively interact with the host tissue to modulate cell behavior and instruct tissue regeneration.
To address these limitations of biodegradable thermoplastics such as PLA, considerable research effort has been invested in the development of synthetic biodegradable thermoset polymers with elastomeric properties. Biodegradable elastomers can be beneficial for regenerative engineering due to their chemical and mechanical properties, which enable the transfer of mechanical stimuli from the degrading matrix to the newly formed tissue. The chemical and mechanical properties of materials influence cell spreading, proliferation, migration, gene expression, and differentiation (3–5).
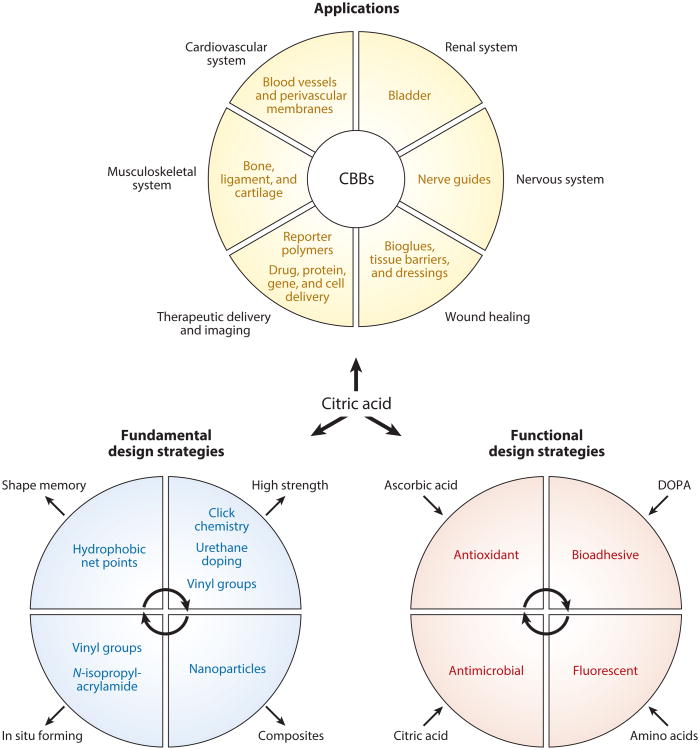
Poly(glycerol sebacate) (PGS) was the first noteworthy thermoset biodegradable elastomer to demonstrate the potential impact of this new class of biomaterials in tissue engineering a little over a decade ago and is now commercialized through Secant Medical, Inc., as its trademarked biomaterial for regenerative medicine applications, Regenerez™ (6). Since the publication of that landmark article in 2002 (6), many researchers have reported the synthesis, characterization, and application of various types of PGS-based materials, which have been reviewed elsewhere (7). However, despite the proof of concept of PGS in several tissue engineering applications and initial market entry as a biomaterial for commercial use, the harsh postpolymerization conditions (high temperature and vacuum) and relatively poor mechanical strength may inherently limit the versatility and functionality of this biomaterial for regenerative engineering applications. To address this challenge, Yang et al. (8, 9) investigated the use of citric acid as the main cross-linking monomer and described a new class of polyesters referred to as poly(diol citrates). Since then, citrate-based biomaterials (CBBs) have found widespread use owing to the freedom they provide in designing unique physical, chemical, and biological properties, which can be tuned in accordance with the requirements of specific tissues or medical applications (Figure 1). To meet the increasingly complex demands of regenerative engineering applications, innovative research has driven the evolution of CBBs to include unique antioxidant, antimicrobial, adhesive, and fluorescent properties. The aim of this review is to provide an insightful discussion of fundamental design considerations, strategies to incorporate unique functionality, and recent applications of CBBs in regenerative engineering in hopes of inspiring the next wave of biomaterials innovations to tackle unmet clinical challenges.
Figure 1.
Schematic illustrating the chemical, physical, and biological design versatility and applications of citrate-based biomaterials (CBBs). Together with the choice of diol and processing conditions, citric acid is the anchor monomer that can tailor the cross-link density, degradation rate, and chemical/physical functionalization of CBBs. These characteristics of CBBs enable the biomaterials engineer to target a variety of regenerative engineering and biomedical applications.
Fundamental Design Strategies
Rationale for Polymer Chain Cross-Linking
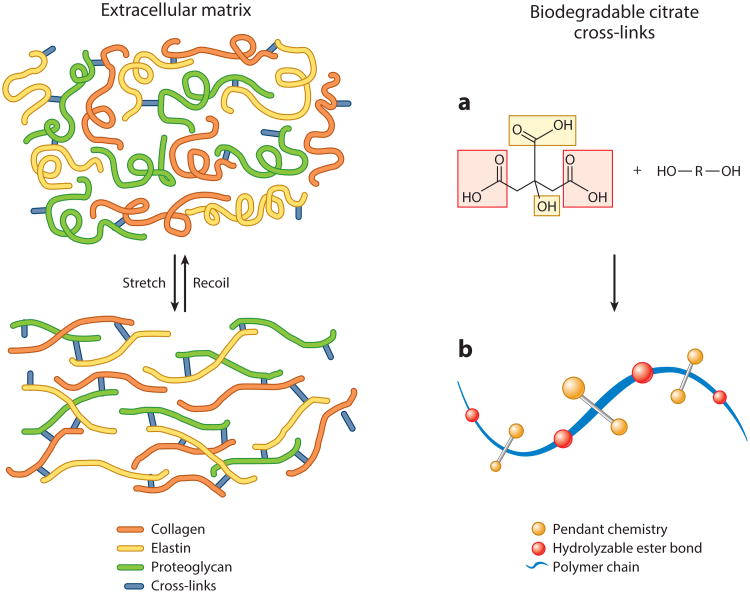
When tissue engineering was first introduced, its success in soft-tissue applications was hindered by the inability of scaffolds fabricated from the biomaterials available at that time to match the range of mechanical properties exhibited by the extracellular matrix (ECM) of the target tissue. Furthermore, mechanical irritation resulting from the compliance mismatch between the implanted bio-material and the host tissue caused inflammation leading to scar formation and limited integration of the biomaterial with the surrounding tissue (10, 11). These limitations and the surmounting clinical need to regenerate soft tissues motivated researchers to closely examine the structure-property relationships of the ECM to identify strategies for designing the next generation of mechanically compliant biomaterials (Figure 2). As a result, the rationale behind the design of a new biomaterial was inspired by the three-dimensional (3D) cross-linked network of collagen, glycosaminoglycans, and elastin, which provides the organizational framework, mechanical stability, tribology, and structural integrity for the formation of complex tissue (6, 12). Biomimicry of this structure-property relationship gives rise to a paradigm shift from the use of stiff, inelastic polylactone-based thermoplastics toward the development of novel cross-linkable biodegradable elastomers.
Figure 2.
Representative simplified schematic of the native extracellular matrix cross-linked network structure that inspired the design for thermoset biodegradable elastomers. (a) The multifunctionality of citric acid provides several reaction sites for prepolymer chain elongation (red) while preserving pendant chemistry for subsequent cross-linking or polymer modification (yellow). (b) Reacting citric acid with diol monomers produces degradable ester bonds (red) while preserving pendant carboxyl and hydroxyl chemistry (yellow) in the bulk of the material, which can be used for subsequent polymer chain cross-linking or material surface modification.
The concept of using polymer chain cross-linking to produce a mechanomimetic environment for cell function has revolutionized the field and provided new opportunities to engineer mechanically active tissues such as heart, blood vessels, lung, bladder, and nerves. In addition to modulating mechanical properties, the degree of cross-linking can also be used to tune the chemical functionality and degradation rate of a biomaterial. In contrast to previous tissue engineering strategies that relied on materials with limited properties repurposed for a variety of tissues, biodegradable elastomers could now be tuned to meet the requirements of a specific tissue. The ability to sustain and recover from cyclic deformations makes biodegradable elastomers uniquely suited for implantation in dynamic environments without causing significant irritation to the surrounding tissue. Another advantage of elastomeric materials is their ability to transfer mechanical stimuli to the newly formed tissue and create the mechanotransduction signals necessary to improve the overall quality of tissue regeneration. For example, mechanical cues influence cytoskeleton organization, cell adhesion, proliferation, differentiation, and cell-cell/cell-ECM communication (13–15).
Citric Acid: A Cornerstone Monomer
When a biodegradable elastomer is designed, at least one monomer should be multifunctional to create a homogeneous 3D cross-linked network structure. Citric acid, historically known as an intermediate in the Krebs cycle, is a multifunctional, nontoxic, readily available, and inexpensive monomer used in the design of all CBBs. As shown in Figure 2, the presence of three carboxyl groups and one hydroxyl group provides three key advantages for all CBBs:
Citric acid can participate in prepolymer formation with diol monomers using a simple, cost-effective, and catalyst-free thermal polycondensation reaction, which enables ester bond formation and facilitates degradation through hydrolysis.
During prepolymer synthesis, pendant carboxyl and hydroxyl chemistry can be partially preserved to provide inherent functionality in the bulk of the material for the conjugation of bioactive molecules. As discussed below, the free pendant chemistry of citric acid is vital in the design of novel CBBs with inherent antioxidant, adhesive, antimicrobial, and fluorescent properties.
The available pendant carboxyl and hydroxyl chemistry provides the necessary functionality for polymer chain cross-linking in an additional postpolymerization or polycondensation reaction to create a homogeneous cross-linked network of hydrolyzable ester bonds.
Thus, owing to the above attributes, citric acid is an interesting monomer that has given rise to a new paradigm for the design of functional biomaterials.
Diol and Diacid Selection
Another important design consideration for CBBs is the choice of the diol component to tune the material properties in order to meet the requirements of the target application. Previous studies have shown that the material properties of CBBs are dependent on the type of diol used during prepolymer synthesis. For example, in the initial development of poly(diol citrates), different aliphatic diols ranging from 4 to 12 carbon lengths were reacted with citric acid to produce materials that cover a wide range of mechanical properties, degradation profiles, and surface energies, which are all important parameters in controlling the biological response to an implanted material (8, 9). As shown in Table 1, peak stress values as high as 11.15 ± 2.62 MPa and elongation at break values of 502 ± 16% were obtained for the first generation of poly(diol citrates). As the aliphatic diol length increases, a reduction in material stiffness and an increase in the elasticity are observed. Conversely, shorter, more hydrophilic diols result in stiffer materials with faster degradation rates. Nitrogen-containing diols, such as N-methyldiethanolamine (MDEA), can be used to increase the mechanical strength, concomitantly speed the degradation rate, and modulate the charge state of the CBB network. As reported by Yang et al. (9), the introduction of 10% and 5% MDEA into the poly(diol citrate) network results in mass losses of 72% and 48%, respectively, after incubation in phosphate buffered saline at 37°C for 4 weeks. These degradation rates are significantly higher than those of MDEA-free poly(diol citrates).
Table 1. List of citrate-based biomaterials, mechanical properties (mean values),a and applications in regenerative engineering.
| Polymer | Peak stress (MPa) | Initial modulus (MPa) | Elongation at break (%) | Application | Reference(s) |
|---|---|---|---|---|---|
| Poly(diol citrates) synthesized with octanediol up to dodecanediol | 2.93–11.15 | 1.60–13.98 | 117–502 | Soft tissues | 9 |
| Cardiac | 143 | ||||
| Vascular graft coating | 68–70 | ||||
| Vascular graft | 74 | ||||
| Bone | 89, 91, 93, 94, 144, 145 | ||||
| Cartilage | 111–115 | ||||
| Tendon | 146 | ||||
| Craniofacial | 95 | ||||
| Bladder/protein delivery | 125–127 | ||||
| Drug delivery | 131, 134 | ||||
| Gene delivery | 135, 142 | ||||
| Poly(vinyl alcohol citrate) | 16.77 | 11.00 | 418 | Cardiovascular | 147 |
| Poly(sebacate glycerol citrate) | 0.63–1.46 | 0.61–3.26 | 51–170 | Soft tissues | 148, 149 |
| Poly(octamethylene citrate sebacate) | 0.20–0.63 | 0.19–1.10 | 168–231 | Soft tissues | 19–21 |
| Poly(poly(ethylene glycol)–co-citric acid) | 0.59–1.51 | 0.25–1.91 | 239–1,505 | Drug delivery | 16 |
| Poly(propanediol-cooctamethylene citrate) | 0.78–0.98 | N/A | 112–280 | Soft tissues | 17 |
| Poly(propanediolsebacate-citrate) | 0.87–2.12 | 0.60–1.23 | 225–431 | Soft tissues | 18 |
| Poly(octamethylene maleate citrate) | 0.29–0.88 | 0.07–1.06 | 55–322 | Soft tissues | 24 |
| Poly((ethylene glycol) maleate citrate) | 0.31–0.64 | 0.39–0.78 | 138–723 | Soft tissues | 26 |
| Orthopedic | 98, 99 | ||||
| Drug delivery | 133 | ||||
| Gastrointestinal | 128 | ||||
| Cross-linked urethane-doped polyesters | 9.56–41.07 | 2.53–38.35 | 206–450 | Cardiovascular | 29, 30, 75, 76 |
| Orthopedic | 88 | ||||
| Neural | 124 | ||||
| Citric acid-copolycaprolactone triol | 1.12–1.73 | 0.56–2.44 | 61–177 | Soft tissues | 150 |
| Poly(octamethylene citrate)–Click | 18.30–41.32 | 16.61–275.93 | 78–324 | Cardiovascular | 23 |
| Orthopedic | 97 | ||||
| Poly(octamethylene maleate (anhydride) citrate) | 0.25–0.99 | 0.04–1.52 | 51–441 | Soft tissues | 27 |
| Poly(octamethylene citrate acrylate/fumarate) | 1.8–15.7 | 5.3–75.9 | 86–260 | Soft tissues | 28 |
| Cross-linked urethane-doped poly(octamethylene maleate citrate) | 0.96–10.98 | 0.50–6.08 | 181–295 | Soft tissues | 62 |
| Injectable citrate-based mussel-inspired bioadhesives | 1.08–8.52 | 0.36–35.7 | 201–1,582 | Bioadhesive | 54 |
| Poly(octamethylene citrate)–ZnO | 6.52–45.75 | 0.68–6.34 | 94–272 | Drug delivery | 43 |
| Diazeniumdiolated poly(diol citrates) | 1.49–10.71 | 1.85–32.64 | 201–367 | Reduction of neointimal hyperplasia/antioxidant | 38 |
| Methacrylated poly(diol citrate) | 6.5–22 | N/A | 18–50 | N/A | 39 |
| Poly(1,8-octamethyleneco-citrate-co-ascorbate) | N/A | 2.12–2.24 | N/A | Reduction of neointimal hyperplasia/antioxidant | 36 |
| Poly(ethylene glycol citrate-co-N-isopropylacrylamide) | N/A | N/A | N/A | Soft tissue/drug delivery/antioxidant | 37 |
| Biodegradable photoluminescent polymers | 2.1–7.8 | 3.34–7.02 | 136–240 | Soft tissues Imaging | 57 |
| Urethane-doped biodegradable photoluminescent polymers | 0.8–49.41 | 0.1–52 | 198–456 | Soft tissue/imaging | 65 |
The mechanical properties listed may not be directly comparable owing to differences in methodology used to obtain them. N/A, values not reported.
Hydrophilic hydroxyl-containing polymers, such as poly(ethylene glycol) (PEG), 1,2-propanediol-sebacate, and poly(vinyl alcohol) can also be used to partially or fully replace hydrophobic aliphatic diols to create more hydrophilic or water-soluble CBB prepolymers (16–18). Designing water-soluble prepolymers is particularly advantageous in applications that involve protein, gene, and cell delivery where the use of toxic organic solvents is detrimental. In addition to the diol component, the partial replacement of citric acid with other diacids is another design strategy to modulate the material properties of CBBs. Aliphatic diacids such as sebacic acid have been introduced during prepolymer synthesis to partially replace citric acid and control the cross-linking density (19–21).
Mechanisms for Cross-Linking
Controlling the cross-linking degree or molecular weight between cross-links is a prevalent strategy to tune the mechanical properties, degradation rate, and chemical functionality of a polymer to meet the requirements of a specific application (22). For example, increasing the postpolymerization temperature and/or time increases the cross-linking density and mechanical strength, but it can reduce elasticity and slow the degradation rate. Polymer chain cross-linking via thermal poly-condensation sacrifices valuable pendant carboxyl and hydroxyl chemical groups. To address this issue, various approaches to incorporate novel cross-linking mechanisms have been introduced to preserve pendant chemistry and tune the resulting chemical functionality, mechanical properties, and degradation rates of CBBs.
Unsaturated compounds
By introducing azide- and alkyne-containing diols during prepolymer formation, click chemistry has been recently reported as an additional cross-linking mechanism in poly(octamethylene citrate)–click (POC-Click) elastomers (23). Researchers combined click chemistry with traditional thermal cross-linking strategies to create a novel thermal synchronous binary cross-linking mechanism: thermal click reaction between azide and alkyne groups and esterification between carboxyl and hydroxyl groups. As shown in Table 1, compared with the original poly(diol citrates), the mechanical properties of POC-Click are greatly increased, yielding tensile strengths as high as 41.32 MPa owing to the thermal synchronous binary and rigid property of the triazol rings. In addition to displaying enhanced strength for soft-tissue engineering, POC-Click elastomers may enable surface site-specific conjugation of heat-liable biomolecules via strain-promoted azide-alkyne cycloaddition (Supplemental Figure 1a). Monomers containing unsaturated chemical groups can be incorporated into CBB design to allow for free radical cross-linking mechanisms, which can rapidly cross-link the polymer chains together upon UV irradiation or redox initiation (24, 25). Via this strategy, carboxyl and hydroxyl groups that would have been used up in polycondensation reactions for thermal cross-linking can now be preserved for the bioconjugation of bioactive factors or used to introduce pH sensitivity into CBB hydrogels (26).
Vinyl-containing monomers have been incorporated into CBB design to provide a dual cross-linking mechanism: free radical polymerization between unsaturated groups and thermal esterification between carboxyl and hydroxyl groups (Supplemental Figure 1b) (27). For example, vinyl-containing CBBs including poly[octamethylene maleate (anhydride) citrate] (POMaC) and acrylated/fumarate-containing poly(diol citrates) may be cross-linked using free radical polymerization and/or thermal polycondensation. Such cross-linking strengthens the material and provides multiple options for fine-tuning the mechanical properties and degradation rates of the resulting elastomer (28).
Urethane doping
Urethane doping is a method to combine the advantages of elastic polyesters with the mechanical strength of polyurethanes. Introducing urethane chemistry into a poly(diol citrate) polyester network results in cross-linked urethane-doped polyesters (CUPEs), improving mechanical strength while maintaining softness and elasticity (Supplemental Figure 2). Poly(diol citrate) prepolymers have been reacted with hexamethylene diisocyanate, which serves as a chain extender and enhances hydrogen bonding within the network. Elastomers with tensile strength as high as 41.07 ± 6.85 MPa have been produced with corresponding elongation at break values of at least 200%, which may be tuned by varying the length of the diol used in the prepolymer synthesis, the diisocyanate-to-diol ratio, and the postpolymerization conditions (29, 30).
Nanostructures
To improve the mechanical strength of CBBs, the polymer network may be reinforced with nanostructures. The strength and stiffness of POC can be increased without sacrificing the material's elasticity by introducing a biodegradable polymeric nanophase into the network to function as additional cross-link points and reinforce the polymer's network chains. Nanoscale structures made from poly(l-lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA), hydroxyapatite (HA), multiwalled carbon nanotubes (MWCNTs), and modified nanofumed silica have all been incorporated into CBBs to increase the tensile strength and modulus of the material. Compared with POC controls, when PLLA or PLGA is incorporated as the nanophase into the POC network, an increase in the tensile strength and modulus from 1.51 ± 0.08 to 3.54 ± 0.35 MPa and 1.59 ± 0.13 to 17.73 ± 1.99 MPa, respectively, was observed (31). Nano-HA incorporated into poly[(1,2-propanediol-sebacate)-citrate] exhibits an 11.4- and 8.2-fold increase over controls in modulus and tensile strength, respectively (32). In addition to HA, MWCNT poly(glycerol-sebacate-citrate) (PGSC) composites have been developed to modulate material strength and stiffness. A 3-wt.% loading of MWCNTs into the PGSC results in a homogeneous dispersion, which increases the strength and modulus by 62.96 and 33.33%, respectively, when compared with PGSC without any nanophase reinforcement (33). Finally, modified nanofumed silica has been developed to improve the dispersion of the nanophase in CBBs. Although incorporation of modified nanofumed silica improves the tensile strength of PGSC from 0.9 MPa to 5.3 MPa, it also increases the material's cytotoxicity (34).
Hydrophobic domains
Alteration of the molar ratio of a hydrophobic diol to citric acid can create temperature-sensitive cross-links that consist of hydrophobic microdomains within the polymer network (35). These hydrophobic cross-links can lead to thermally induced shape-memory properties. Shape-memory polymers are a class of “smart materials” that can change shape in a programmable manner in response to an external stimulus. In the case of poly(diol citrates), thermosensitive mechanical and shape-memory properties can be induced between room and body temperature. The polyester network includes covalent net points that are responsible for the CBB's permanent shape, whereas the hydrophobic microdomains behave as reversible cross-links that function as switch structures to fix the temporary shape through intermolecular hydrophobic interactions. The hydrophobic microdomains within the shape-memory polymers can also be used as reservoirs to entrap and subsequently control the release of hydrophobic drugs.
Functional Design Strategies
In response to increasingly complex applications and requirements within regenerative engineering and related fields, CBBs have been engineered to incorporate advanced functionality to further increase their utility. As mentioned above, the multifunctionality of citric acid is key when designing CBBs with unique antioxidant, antimicrobial, adhesive, and fluorescent properties. The following section provides a brief discussion on recent developments regarding CBBs.
Antioxidant Properties
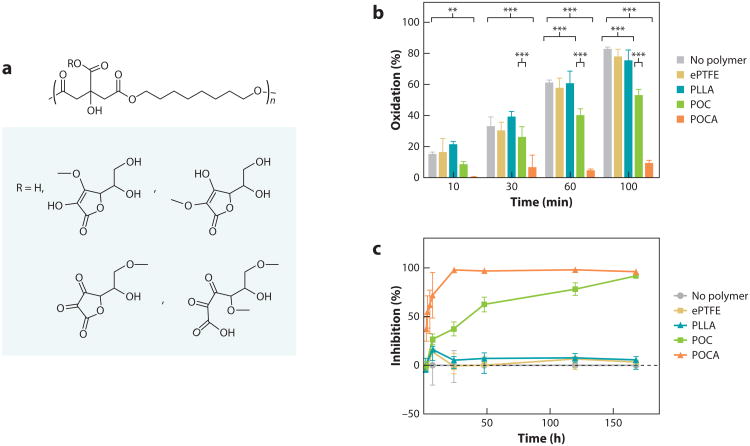
Our increased understanding of the biological response to synthetic biomaterial implants has provided new insights into the role of oxidative stress in the inflammatory response and the overall performance of medical devices. In biomaterial-induced inflammatory responses, cytokines and chemokines generate reactive oxygen species, which impair the normal function of cells through DNA, protein, and lipid damage. Oxidative stress has also been implicated in the failure of vascular interventions: By enhancing the proliferation of vascular smooth muscle cells, oxidative stress results in the progression of restenosis. Poly(diol citrates) have intrinsic antioxidant properties that can be significantly enhanced through various strategies. For example, ascorbic acid, a commonly used antioxidant vitamin compound, can be incorporated into the polymer chains of POC, thus generating poly(octamethylene citrate-co-ascorbate) (POCA) (Figure 3) (36). Citrate stabilizes ascorbic acid during polymer synthesis and also chelates iron ions, which catalyze the formation of hydroxyl radicals generated by the Fenton reaction during oxidative stress. Radical scavenging, iron chelation, and lipid peroxidation assays confirmed the potent antioxidant capacity of POCA. Furthermore, cells cultured on POCA exhibit reduced generation of cellular reactive oxygen species after oxidative challenge, and the material protects against oxidative stress–induced cell death over the course of material degradation. Therefore, unlike most strategies reported in the literature that simply add an antioxidant compound into the polymer to create a blend, POCA provides antioxidant protection during the entire polymer degradation process. The benefit of an antioxidant polymer was also evaluated in a vascular graft model: Expanded poly(tetrafluoroethylene) (ePTFE) grafts coated with the polymer and implanted in guinea pigs exhibited less neointimal hyperplasia than did grafts with only ePTFE (36).
Figure 3.
CBBs can be engineered to have enhanced antioxidant properties. (a) Representative synthesis schematic of POCA. (b) BothPOC and POCA inhibit lipid peroxidation, whereas ePTFE and PLLA do not have any effect on lipid peroxidation. N ≥ 3, mean ± SD.*p < 0.05, **p < 0.01, ***p < 0.001°.(c) DPPH assay shows free radical inhibition by both POC and POCA, whereas PLLA and ePTFE have no radical scavenging activity. Adapted with permission from figures 3 and 5 of Reference 36. Abbreviations: CBB, citrate-based biomaterial; ePTFE, expanded poly(tetrafluoroethylene); PLLA, poly(l-lactic acid); POC, poly(octamethylene citrate); POCA, POC-co-ascorbate.
Another example of antioxidant poly(diol citrates) was reported in a study by Yang et al. (37), which describes the development of thermoresponsive poly(polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN). PPCN, also referred to as Nanonets™, has a critical solution temperature that can be tailored to be within the range of 22°C to 33°C and exhibits intrinsic antioxidant properties given its ability to scavenge free radicals, chelate metal ions, and inhibit lipid peroxidation. Subcutaneous injections of PPCN in rats demonstrate that the material is resorbed by the body with a minimal inflammatory response. In addition, PPCN can entrap cells while maintaining high viability and function. It also entraps and slowly releases biologically active proteins and viruses. Taken together, these characteristics support the use of PPCN as a powerful intrinsically antioxidant therapeutic delivery platform that can also reduce oxidative stress in regenerative engineering applications where oxidative damage to cells or tissues may be a concern.
An interesting strategy to fight the consequences of oxidative stress in tissue, especially vascular tissue, is to release nitric oxide (NO). To this end, a secondary amine-containing diol such as N,N-bis(2-hydroxyethyl) ethylenediamine can be incorporated into POC and diazeniumdiolated to form a NO-releasing CBB, POC-DA (Supplemental Figure 3) (38). The tensile strength of POC-DA ranges from 1.49 to 10.71 MPa, the Young's modulus from 1.85 to 32.64 MPa, and elongation at break values from 201% to 367%. POC-DA elastomers can deliver NO for several days, and its duration and amount can be controlled by varying the amine content, the choice of diol, and the exposure time to pressurized NO gas without impacting the degradation rate. POC-DA is beneficial for vascular applications: It simultaneously enhances the proliferation of human umbilical vein endothelial cells while inhibiting the growth of human aortic smooth muscle cells. A longer duration of NO release and the ability to be rapidly cured in situ can be achieved by functionalizing POC with 2-aminoethyl methacrylate and incorporating a miscible NO donor, diazeniumdiolated N,N-diethyldiethylenetriamine, into the polymer matrix (39). The NO-containing polymer network can be cured within 3 min upon exposure to UV light and provides sustained release of NO for at least one week.
Antimicrobial Properties
Despite the significant advances made in aseptic surgical practices, infection due to microbial contamination remains a challenge for in vivo application of synthetic biomaterials. Significant efforts have been made in recent years to avoid or control infection by introducing antimicrobial drugs, nanoparticles, quaternary ammonium salts (QAS), and nanomiddleography to reduce bacterial adhesion and biofilm formation on polymeric surfaces (40, 41). These strategies can also be applied to CBBs. For example, QAS have been tethered onto the pendant carboxyl group of citric acid in the development of POC-QAS. Surface analysis studies confirmed the presence of the biocide, which provided high antimicrobial activity (5–7 log kill of Staphylococcus aureus versus controls) for all materials containing tethered QAS (42). Several researchers have also described the development of antibiotic-releasing antibacterial poly(diol citrates) (43–45). Nevertheless, poly(diol citrates) may also have intrinsic antimicrobial activity. Citric acid is a highly germicidal compound that researchers may be able to exploit accordingly (46).
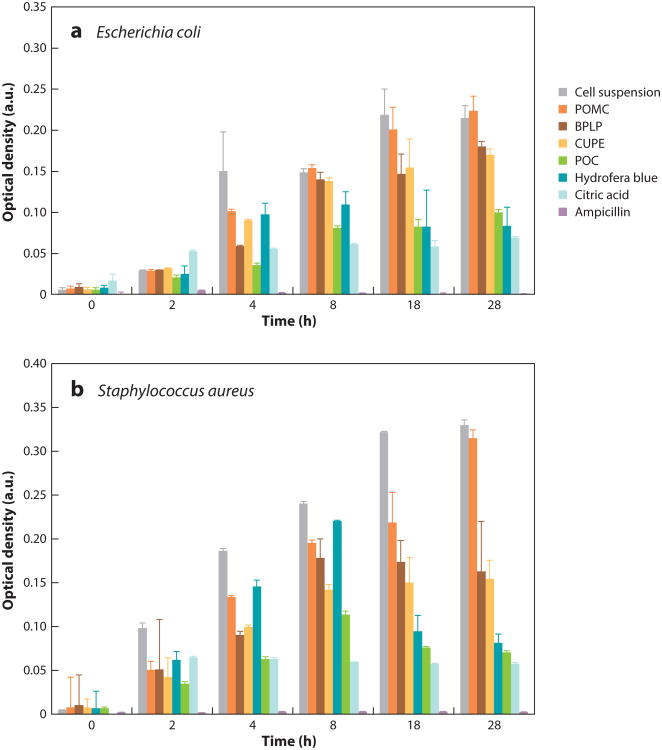
Studies assessing the antimicrobial activity of poly(diol citrates) demonstrated that POC is the most potent antibacterial poly(diol citrate). This effect is comparable with those observed when citric acid solutions were tested on both Escherichia coli and S. aureus (Figure 4). One possible reason is that during degradation, citric acid is released and transverses cell membranes to lower intracellular pH and suppress nicotinamide adenine dinucleotide oxidation, resulting in bacterial death. Another possible reason is that citric acid alters the local pH environment and/or chelates the metal ions in the cell wall. As a result, the microorganisms may not be able to absorb essential nutrients because the permeability of the cell wall has been altered, thus causing damage and hence cell death, especially in gram-negative bacteria (46). Therefore, the incorporation of citric acid into biodegradable polymer chains is a rational approach to potentially circumvent the use of antibiotics, silver nanoparticles, and other traditional bactericidal agents and provide intrinsic antimicrobial properties when the material is in the body.
Figure 4.
Bacterial growth assessment via turbidity (optical density) through culturing (a) Escherichia coli and (b) Staphylococcus aureus with various citrate-based polymers (50 mg in 1-ml solution) for 28 h. Cell suspensions without any treatment are the negative controls. Free ampicillin (2.5 μg/ml), citric acid (29 mg/ml), and the commercially available bacteriostatic agent Hydrofera Blue (50 mg in 1-ml solution) are positive controls. p < 0.01. Adapted from Reference 46. Abbreviations: BPLP, biodegradable photoluminescent polymer; CUPE, cross-linked urethane-doped polyester; POC, poly(octamethylene citrate); POMC, poly(octamethylene maleate citrate).
Adhesive Properties
Tissue adhesives are gaining popularity in diverse areas of clinical applications, including wound closure and healing, drug delivery, implantation of medical devices, tissue engineering, and orthopedic applications (47). Although the commercially available tissue bioadhesives based on fibrin glues, cyanoacrylate, gelatin, and polyurethanes have been used in many clinical applications, widespread adoption of these bioadhesives is hindered by their toxicity or poor mechanical and adhesive strength in wet conditions (48–52). To improve adhesion in wet and mechanically dynamic conditions, researchers have looked to nature for inspiration in designing adhesive biodegradable elastomers. Mussels, such as Mytilus edulis, secrete adhesive materials that enable them to adhere firmly to various underwater surfaces under harsh conditions. Studies have shown that this strong wet adhesion is due primarily to the presence of the posttranslationally modified catechol-containing amino acid 3,4-dihydroxy-phenylalanine (DOPA) (53). Under oxidizing or alkaline conditions, DOPA may promote cross-linking reactions through the oxidation of catechol hydroxyl side chains, subsequently triggering intermolecular cross-linking as well as cross-linking capacity for bulk cohesive strength (47). Oxidized DOPA also contributes to the strong adhesion ability of biological surfaces, as these surfaces form covalent bonds with available nucleophilic groups such as -NH2, -SH, -OH, and -COOH (47). Motivated by this unique structure-property relationship, researchers have designed CBBs functionalized with catechol groups to enhance adhesive properties in wet and mechanically dynamic environments (Figure 5).
Figure 5.
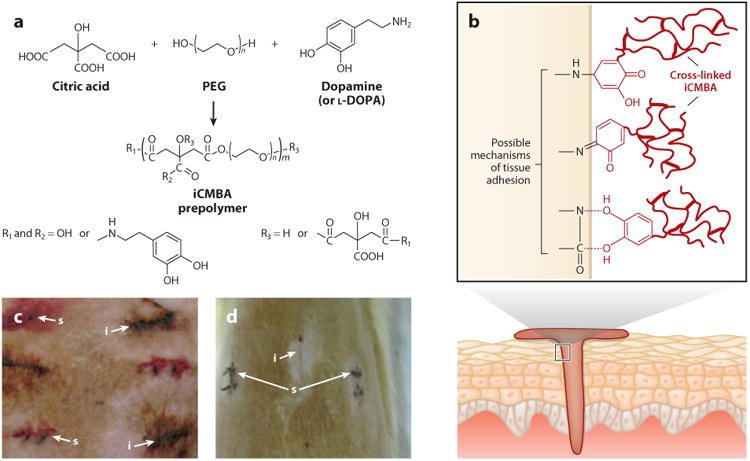
(a,b) Schematic representation of (a) injectable citrate-based mussel-inspired bioadhesive (iCMBA) synthesis and (b) adhesion mechanism to wet tissue. Panels a and b adapted with permission from figure 7 of Reference 47. (c,d) Images of rat dorsum skin wounds closed using iCMBA (i) and sutures (s) (c) 2 min and (d) 7 days after surgery. Panels c and d adapted with permission from figure 6 of Reference 54.
Injectable citrate-based mussel-inspired bioadhesives (iCMBAs) are a novel family of biodegradable and strong wet-tissue adhesives, which were recently developed by introducing DOPA into water-soluble citrate-based elastomer prepolymers. iCMBAs are synthesized using a facile and cost-effective polycondensation reaction using citric acid and PEG concurrent with the pendant modification of dopamine in a one-pot synthesis process (54). The presence of hydrolytically degradable ester bonds formed via polycondensation in the backbone of iCMBA prepolymers renders this family of adhesives completely biodegradable, which makes iCMBA more attractive than other mussel-inspired bioadhesives (54). iCMBAs display a wet-tissue adhesion strength that is 2.5–8.0-fold stronger than that of clinically used fibrin glue, and they have tunable degradability and tissue-like elastomeric mechanical properties. In addition, the material properties of iCMBAs can be tuned by adjusting the molecular weight of PEG and the feeding ratio of dopamine.
Fluorescent Properties
Biodegradable fluorescent polymers have attracted significant attention in theranostic health care and regenerative engineering (55, 56). Most of the reported fluorescent materials are made through the conjugation or encapsulation of organic dyes or quantum dots. However, the low photobleaching resistance of organic dyes and unacceptable toxicity of inorganic quantum dots largely limits the applications of these fluorescent biodegradable polymers and has motivated the design of fully degradable and biocompatible fluorescent polymers. As shown in Figure 6, an intrinsic fluorescent property can be incorporated into CBBs by reacting essential α-amino acids with citric acid and aliphatic diols to create aliphatic biodegradable photoluminescent polymers (BPLPs) (57, 58). Unlike previously developed aromatic fluorescent polymers or organic dyes that are not degradable, BPLPs are synthesized from biocompatible monomers through a convenient and cost-effective thermal polycondensation reaction to create a biodegradable fluorescent material with soft and elastic mechanical properties. BPLPs display quantum yields as high as 62.3% with fluorescence that can be tuned from blue to red by using different amino acids in BPLP synthesis (59, 60).
Figure 6.
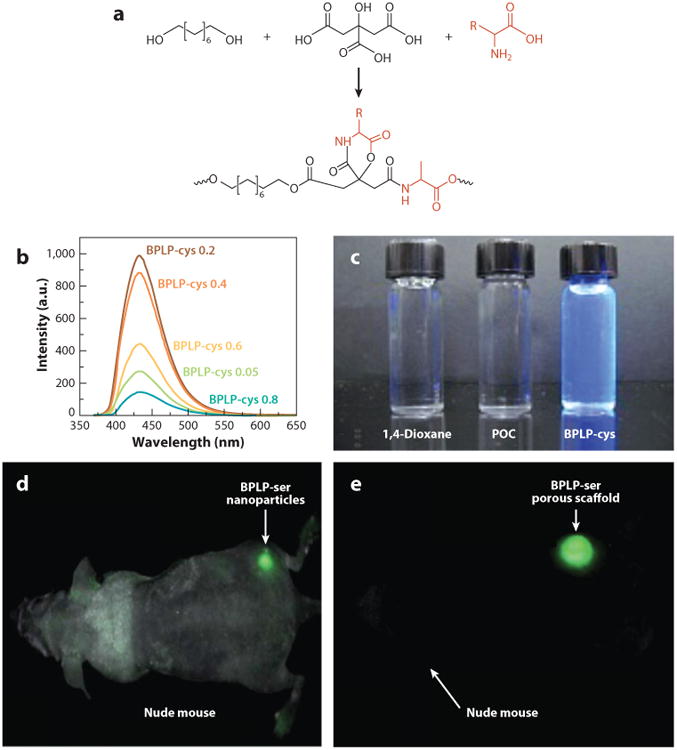
(a) Representative synthesis schematic of the biodegradable photoluminescent polymer (BPLP). (b) BPLPcysteine emission spectra in 1,4-dioxane solution with various molar ratios of l-cysteine excited at 350 nm. (c) 1,4-Dioxane solution, poly(octamethylene citrate) (POC), and BPLP solution to show inherent fluorescent property. (d,e) Fluorescence image of (d) BPLP-serine nanoparticles and (e) scaffold implanted subcutaneously in a nude mouse. Adapted with permission from figure 1 of Reference 58 and figures 2 and 4 of Reference 57.
The development of BPLP not only enables new applications for CBBs, such as in bioimaging and targeted drug delivery, but also has inspired the development of biodegradable photoluminescent aliphatic polylactone biomaterials. BPLPs can initiate ring-opening polymerization in the development of biodegradable photoluminescent polylactones (BPLPLs), exemplified with photoluminescent polylactide (BPLP-PLA) (61). Ring-opening polymerizations were performed with BPLPs as initiators and lactone monomers to create triblock copolymers that have thermal properties similar to those of commercialized PLA. BPLP-PLAs exhibit strong tunable fluorescent emission from 360 to 700 nm with a quantum yield up to 51%. BPLPLs also enable noninvasive fluorescence imaging for tracking the degradation of the material in vivo and the fabrication of nanoparticles for theranostic applications. The incorporation of BPLP units into biodegradable polymer design could be an effective way to integrate intrinsically photoluminescent properties into other biodegradable polymers. The use of polylactone materials has already generated significant impact. In vivo detection, photobleaching resistance, and photoluminescence of these materials represent further innovations that should advance the understanding, design, and use of biodegradable polymers in a broad range of biomedical and biological applications where fluorescence imaging and sensing have gained increasing importance.
Combinatorial Design Strategies
The fundamental and functional design strategies used to generate CBBs are modular and can be utilized independently or in combination to drive the development of novel biomaterials with unique properties (Figures 1 and 7). By starting with a versatile and robust monomer, citric acid, researchers can design an array of CBBs to fit the needs of particular regenerative engineering applications. For example, previous studies have shown that tough and elastic photo-cross-linkable CBBs can be designed using vinyl-containing groups in combination with urethane doping (62). Hydrophilic fluorescent materials have also been designed by partially or fully replacing hydrophobic diols with PEG to produce hydrophilic BPLPs, which were then used to create amphiphilic copolymers for self-assembling micelles for potential use in theranostic applications (63, 64). Unsaturated functional groups have also been incorporated into BPLP design to cross-link the polymer network using free radical polymerization for injectable, minimally invasive applications, and urethane bonds have been introduced in the polymer network to dramatically increase the strength of the resulting elastomer (63, 65).
Figure 7.
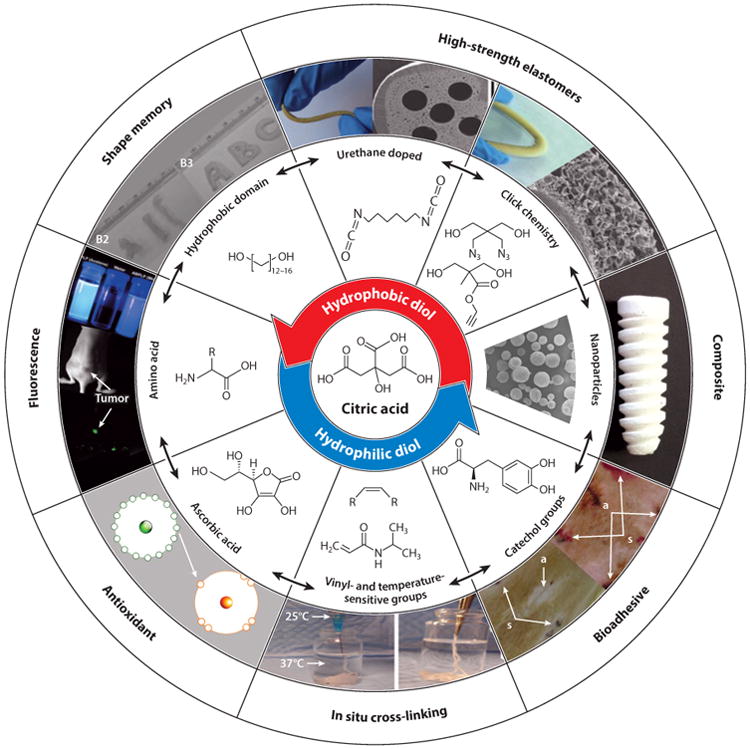
Schematic representation of the modular design strategy enabled by citrate-based biomaterials (CBBs). All CBBs are synthesized from a common multifunctional monomer, citric acid (center). Next, citric acid is reacted with diol monomers to tune the hydrophilicity of the resulting prepolymer with inherent functionality in the bulk of the material. The free pendant chemistry of citrate-based prepolymers can be used to introduce unique properties that are interchangeable, thus showing the modularity of CBB design.
The versatility of the CBB can also be extended to incorporate multiple functional design strategies. For example, the modular design strategies allow for the development of CBBs with multiple functional properties, including adhesives with tunable antioxidant properties, fluorescent shape-memory elastomers, and antimicrobial and osteoinductive composites. In addition, any of the newly developed multifunctional materials may be further enhanced: Incorporation of PEG-based diols could make them water soluble, vinyl-containing groups could make them in situ cross-linkable, and/or urethane doping or click chemistry could make them ultrastrong. Given the modularity of these design strategies, CBBs are a highly versatile class of biomaterials that will likely continue to play a large role in regenerative engineering.
Applications in Regenerative Engineering
Cardiovascular System: Blood Vessels
The long-term patency of prosthetic small-diameter vascular grafts remains a challenge owing to the possibility of thrombosis and neointimal hyperplasia, thus prompting extensive research into strategies that involve regenerative engineering (66). Bioengineered vascular grafts may one day provide a solution to many of the existing limitations; however, identifying a suitable combination of cell source and compliant biomaterial scaffold to re-create the architecture and mechanical properties of native blood vessels remains a challenge. Due to their elasticity, strength, hemo-compatibility, capacity to elute NO, and support of endothelial cell functions, CBBs have been investigated for a variety of vascular applications including surface modification of prosthetic vascular grafts, tissue-engineered vascular grafts, and perivascular delivery of therapeutics to reduce neointimal hyperplasia.
The hemocompatibility of POC was confirmed in previous studies that described a significant reduction of platelet adhesion and activation on POC films versus PLGA and ePTFE (67). The results prompted the application of POC as a coating on the lumen of ePTFE vascular grafts using a spin-shearing method to improve the hemocompatibility of these grafts. Coated grafts were implanted end to side in a porcine carotid artery bypass graft model and evaluated at 4 weeks. The addition of POC to the ePTFE did not negatively affect graft compliance, and thrombogenicity and inflammation were significantly reduced while endothelial cell compatibility was enhanced (68, 69). To further increase the thromboresistance of ePTFE vascular grafts, heparan was covalently bound to pendant carboxyl functional groups of POC coated on ePTFE vascular grafts. Results showed that heparan immobilization to POC significantly reduced whole blood clot mass and platelet adhesion while supporting the adhesion and proliferation of blood outgrowth endothelial cells and, to a much lesser extent, human aortic smooth muscle cells over the course of a 28-day period (70).
CBB-based strategies to release therapeutics to prevent neointimal hyperplasia have also been developed. For example, novel NO-releasing perivascular membranes based on POC elastomers modified with diazeniumdiolate groups in the polymer backbone significantly reduce neointimal hyperplasia (71). Compared with controls, NO-containing POC films also significantly reduce the proliferation of human aortic smooth muscle cells without affecting the proliferation of human umbilical vein endothelial cells in vitro. After implantation into rat carotid arteries, NO release from the POC membranes has reduced intimal area and the intima-to-media (I/M) area ratio by 45% and 38%, respectively. In addition to NO, all-trans retinoic acid has been loaded into POC perivascular membranes to prevent neointimal hyperplasia following arterial injury. Histomorphometry analysis showed that all-trans retinoic acid–POC membranes inhibit neointimal formation after balloon injury, with a 56%, 57%, and 50% decrease in the intimal area, I/M area ratio, and percent stenosis, respectively (72).
To avoid the disadvantages associated with finding a suitable cell source and long in vitro culture times, researchers have used CBBs to fabricate blood vessel scaffolds that harness the body's inherent regenerative capabilities to populate the graft and initiate vessel formation. For example, POC has been used as a substitute for elastic fibers in the development of biomimetic fiber-based scaffolds. Electrospun fibers containing various combinations of POC, collagen, decorin, and aggrecan have been chosen to mimic the native artery ECM composition. On average, compared with samples without POC, electrospun fibers containing POC display a 3.2-fold increase in elasticity without affecting human embryonic palatal mesenchymal fibroblast attachment or proliferation (73). These results demonstrate that the incorporation of POC and proteoglycans along with collagen can produce a viscoelastic nanofibrous material that may be suitable for the fabrication of small-diameter blood vessels. Along these lines, biodegradable and mechanically compliant POC small-diameter blood vessels with a biphasic connected nonporous and porous phase have been developed to mimic blood vessel intimal and medial layers, respectively (74). Mechanical testing results show increased peak stress and burst-pressure values, which have been attributed to the strength provided by the nonporous intimal layer. In addition to enhancing the mechanical strength of the highly porous graft, the biphasic design also supports cell compartmentalization, coculture, and cell differentiation of human aortic endothelial cells and smooth muscle cells in the intimal and medial layers, respectively. To further enhance the mechanical strength of small-diameter blood vessels to meet the strict requirements of in vivo tissue regeneration, CUPE biphasic scaffolds have also been fabricated (75, 76). A small doping of urethane bonds into the POC network results in tensile strengths of 5.02 ± 0.70 MPa, which are greater than those of native vessels (1.43 ± 0.60 MPa). CUPE scaffolds exhibit tunable burst pressure ranging from 1,500-mm Hg to 2,600-mm Hg and adequate suture retention values (2.45 ± 0.23 N) while maintaining hemocompatibility. Biphasic POC-Click vascular grafts have also been developed and display tensile strengths comparable with those of CUPE grafts but burst-pressure values that are significantly higher—approximately 5,000-mm Hg (23).
CBBs are also amenable to host-cell recruitment strategies to guide re-endothelialization, vascular remodeling, and integration of the scaffold with the native blood vessel. The feasibility of using endothelial progenitor cells found in circulating blood to generate a functional endothelium on POC films has been assessed (77). Endothelial-like cells differentiated from endothelial progenitor cells cultured on POC films express NO synthase, prostacyclin, and tissue plasminogen activator levels comparable to those of aortic endothelial cells. In addition, the seeded endothelial-like endothelial progenitor cells can withstand physical shear stress of 10 dynes/cm2 when cultured on POC-modified ePTFE vascular grafts. In a different study, the endothelialization of POC-coated ePTFE vascular grafts was assessed in porcine carotid artery circulation as end-to-side bypass grafts. After 4 weeks, the POC-coated ePTFE grafts were found to be biocompatible and to support endothelial cell proliferation, again confirming the potential of using POC to enhance the hemocompatibility of blood-contacting surfaces (78).
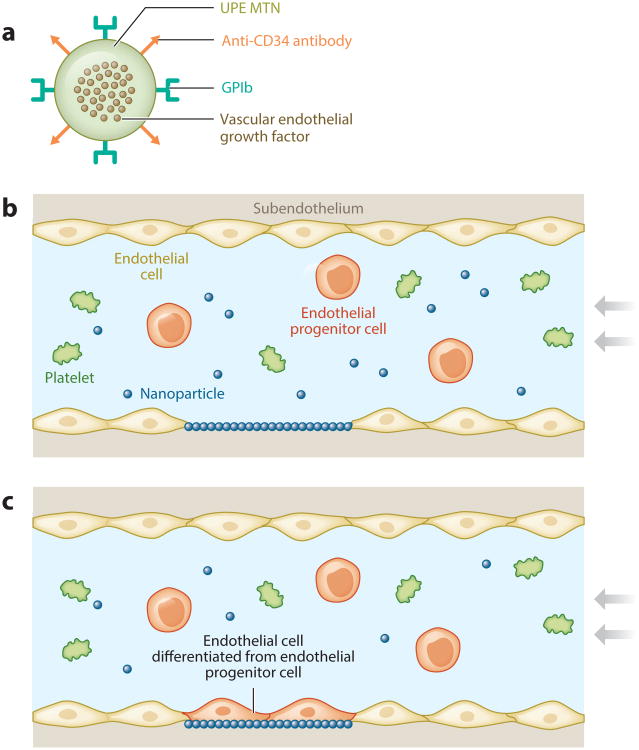
Targeted surface modification of biomaterials reduces platelet adhesion and promotes endothelialization, improving the antithrombogenic properties of small-diameter vascular grafts, and CBBs that incorporate click chemistry (POC-Click) enable surface site-specific conjugation of heat-liable biomolecules via strain-promoted azide-alkyne cycloaddition. A collagen mimetic peptide, p15, has been conjugated into the lumen of POC-Click small-diameter vascular grafts to promote the attachment and proliferation of human umbilical vein endothelial cells (23). A novel minimally invasive in situ technique has also recently been developed to re-endothelialize damaged areas of the artery (Figure 8). By using regenerative engineering and ligand receptor targeting strategies, researchers have generated a biodegradable urethane-doped polyester (UPE) multifunctional targeting nanoparticle (MTN) scaffold system with dual ligands: (a) Glycoprotein Ib (GPIb) targets the injured arterial endothelium and subendothelium, and (b) anti-CD34 antibodies capture endothelial progenitor cells for endothelium regeneration (79). Studies monitoring platelet adhesion, activation, and blood clotting show that fabricated spherical UPE MTNs of 400 nm are both cytocompatible and hemocompatible (79). UPE MTNs have strong binding specificity and effectively compete with platelets binding to glass slides coated with von Willebrand factor, an in vitro model used to mimic arterial damage. In a rat balloon angioplasty injury model (79), single delivery of MTNs upon vascular injury reduced neointimal hyperplasia by 57% and increased endothelium regeneration by ∼60% in 21 days.
Figure 8.
(a) Schematic representation of citrate-based multifunctional targeting nanoparticles (MTNs) loaded with growth factors and functionalized with glycoprotein Ib (GPIb) and anti-CD34 antibodies. (b) The MTNs can be targeted to the injured endothelium and serve as a substrate to capture endothelial progenitor cells through (c) platelet mimicking. Adapted with permission from figure 1 of Reference 79.
Musculoskeletal System: Bone
Despite the capacity of the human skeletal system to regenerate, more than 2.2 million bone-grafting procedures are performed worldwide annually to treat orthopedic pathological conditions such as fractures, tumor resection, and osteoporosis (80, 81). Bone has become the second-most transplanted tissue with more than $28 billion spent annually in orthopedic-related medical costs, which are projected to increase with the demands of an aging population (82, 83). To fulfill the increasing clinical demand for an effective off-the-shelf bone graft, the introduction of inorganic bioceramics such as HA, tricalcium phosphate, and biphasic calcium phosphates into synthetic biodegradable elastomers has been a prevailing strategy to match the composition and strength of native bone. Although the concept of polymer-ceramic composites is not new, carefully selecting the candidate polymer and ceramic to blend into a composite provides opportunities for innovation in biomaterials science for bone-regenerative engineering.
Since the 1940s, it has been well known that citrate, an ionic form of citric acid, is naturally abundant in bone tissue. Although its function in bone formation remains largely unknown, recent research has discovered that citrate plays a large role in bone's unique stability, strength, and resistance to fracture by regulating apatite nanocrystal growth (84–86). These recent findings have created a renewed interest in the role of citrate in bone physiology and development and provided a new hypothesis that osteoblasts are specialized citrate-producing cells that provide the necessary levels of citrate for proper bone formation (85, 87). A recent study has shown that citrate, whether presented on a biomaterial surface or supplemented into cell culture media, has unique effects on gene expression: It upregulates osterix, alkaline phosphatase, and osteopontin and downregulates osteocalcin in C2C12 myoblasts and human mesenchymal stem cells (88). Therefore, citrate may be closely associated with early-stage (osterix and alkaline phosphatase) as well as mid- or late-stage (osteopontin and osteocalcin) bone markers. In terms of orthopedic biomaterials, CBB composites contain inherent carboxyl chemistry in the bulk of the polymer, facilitating polymer-HA interactions and allowing for a high mass percentage of HA (>50 wt.%) to be incorporated into the composite. These composites, which can be engineered to be elastomeric for sponge-like orthobiologic grafts or to be very stiff for plate or screw devices, are in contrast to composites that use biodegradable lactide-based polymers, which are typically limited to <30 wt.% HA.
Citrate-based composites can be fabricated with any ceramic or glass micro- or nanoparticle, but most studies to date have investigated composites based on HA owing to its natural presence in bone (89, 90). POC-HA-composite cylindrical plugs implanted into rabbit medial femoral condyle critical-sized defects displayed no chronic inflammation and were well integrated with the surrounding tissue after 6 weeks of implantation, suggesting healthy and normal bone remodeling (91). The ability of a polymer nanophase to increase the mechanical properties of POC networks has inspired the use of nanoscale HA to produce citrate-based composites with mechanical properties within the range of those reported for trabecular bone (92). An increase in mechanical properties has been observed as the particle size decreases, which could be attributed to the physical interlock that is created between the spindle-shaped HA nanocrystal nanophase and the polymer macrophase. To evaluate the long-term in vivo response, POC nanocomposites were implanted in a rabbit osteochondral defect for up to 26 weeks: Compared with PLLA control implants, these composites were shown to be biocompatible with increased areas of tissue ingrowth (93, 94). Tissue samples removed 1 year post implantation show significant bone formation in POC-HA implants when compared with the PLLA implants.
Although POC-HA materials display excellent biocompatibility, osteoconductivity, and osteointegration in vivo, none of the investigated formulations has provided sufficient mechanical strength to match that of human cortical bone. To improve the mechanical strength of the previously reported POC-HA composites, a new generation of citrate-based polymer blends (CBPB-HA) based on POC, CUPE, and HA has been developed (88). Utilizing CUPE as the major fraction of the polymer blend exploits urethane-doping strategies to enhance the mechanical strength of the resulting composite while maintaining valuable free pendant carboxyl chemistry for HA chelation. CBPB-HA networks composed of 90% CUPE and 10% POC produce materials with a compressive strength of 116.23 ± 5.37 MPa, which falls within the range of human cortical bone (100–230 MPa) and is a significant improvement over POC-HA composites. CBPB-HA promotes mineralization and increases gene expression of C2C12 osterix and alkaline phosphatase in vitro. After 6 weeks of implantation in a rabbit lateral femoral condyle defect model, CBPB-HA composites elicited minimal fibrous tissue encapsulation and were well integrated with the surrounding bone tissues.
The promising osteogenic and in vivo bone responses from material characterization studies have recently motivated the development of prefabricated CBB-HA-composite constructs for specific orthopedic regenerative engineering applications such as craniofacial repair, long-bone regeneration, and bone-tunnel healing during ligament reconstruction. For example, the elasticity of POC-HA nanocomposites has prompted researchers to use them to apply contractive forces for manipulating craniofacial bones (95). Levi-Polyachenko et al. (95) demonstrated that POC composites synthesized with only 3-wt.% HA nanocrystals exhibit mechanical properties optimal (1.21-MPa elastic modulus and 13.17-N maximum load) for inducing contraction forces capable of facilitating osteogenesis and craniofacial repair. Anterior cruciate ligament tears are a common sports injury that can lead to chronic pain if not treated properly. To provide an alternative off-the-shelf ligament graft to surgeons, POC-HA has also been used in the development of synthetic biodegradable tricomponent grafts to promote bone-tunnel healing (96). Porous scaffolds composed of POC and 40-wt.% HA nanocrystals have been fabricated onto the ends of braided PLA fibers and used to reconstruct the anterior cruciate ligament of rabbits (Figure 9). After 6 weeks, all animals displayed good functionality, and histological analysis confirmed tissue infiltration and growth throughout the entire POC-HA scaffold to promote bone-to-bone healing and interlocking of the braided PLA fibers. Given the strong and osteogenic characteristics of POC-Click-HA composites, this new generation of CBB composites has been used to repair large segmental defects. To mimic the bimodal distribution of cancellous and cortical bone, biomimetic biphasic POC-Click-HA scaffolds have been developed with 70% internal phase porosity and various external phase porosities (between 5% and 50%) (97). Compared with scaffolds of uniform porosity as well as autologous bone grafts, the biphasic scaffolds display excellent osteointegration, enhanced new-bone formation, higher bone densities, and improved biomechanical support in the initial stages after implantation to repair 10-mm-long segmental radial defects in rabbits.
Figure 9.
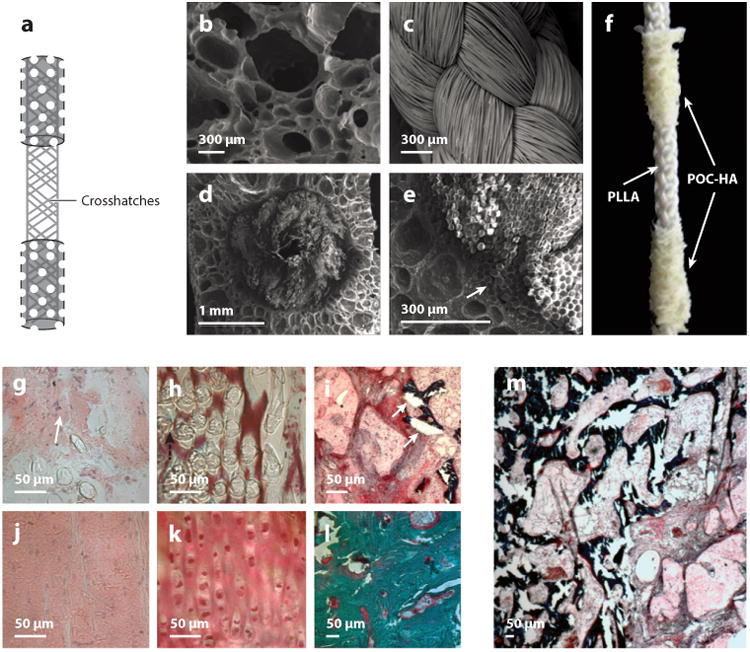
Structure of the poly(octamethylene citrate)–hydroxyapatite (POC-HA) tricomponent graft and 6-week histological images of a POC-HA tricomponent graft used to reconstruct a rabbit's anterior cruciate ligament. (a) Schematic depicting the graft with POC-HA bone-like ends (dotted area) and the intra-articular (crosshatches) poly(l-lactic acid) (PLLA) braided region. (b–d) Scanning electron microscope images of (b) porous POC-HA bone-like ends, (c) PLLA braid, and (d) cross section of PLLA braids embedded in the POC-HA. (e) High magnification of bone-like ends shows integration at the interface (arrow). (f) Sample POC-HA graft. (g,h) HE (hematoxylin and eosin) staining of the PLLA braid within (g) the intra-articular region and (h) the articular cartilage. The arrow in panel g indicates new fibrous tissue. (i) Masson-Goldner trichrome staining of the POC-HA bone-like ends within the bone tunnels shows tissue ingrowth and integration. The arrows point to mineralized bone and osteoid. (j) Mid-section of the intra-articular component of the native anterior cruciate ligament. (k) Cartilaginous transition zone near the bone. (l) Subchondral bone, where the ligament attaches in the contralateral knee. (m) Low magnification of the POC-HA-tissue interface showing ligament fibroblasts and bone ingrowth into the POC-HA bone-like ends. Adapted with permission from figures 1 and 4 of Reference 96.
Prefabricated polymer composites are not an option for certain orthopedic surgery procedures in hard-to-reach areas of the body, as in the case of femoral head osteonecrosis, where complete evacuation of the necrotic tissue is performed by core decompression. To address this need, bioactive citrate-based injectable composites based on biodegradable poly(ethylene glycol maleate citrate) (PEGMC)-HA have been developed. Such composites provide support while delivering cells and therapeutics to irregular bone defects (98, 99). The water-soluble PEGMC prepolymer can be mixed with HA, delivered using minimally invasive techniques, and cross-linked in situ using free radical polymerization to deliver bioactive payloads such as cells and drugs to the surgical site. For example, human fetal osteoblasts encapsulated in PEGMC-HA gels proliferated during the 3 weeks of culture with a significant increase in alkaline phosphatase production after 2 weeks. Ex vivo studies (98) also confirmed the feasibility of injecting PEGMC-HA using minimally invasive techniques into collapsed femoral heads for reinforcement.
Musculoskeletal System: Cartilage
Osteoarthritis is a debilitating joint disease characterized by articular cartilage degeneration that affects more than 27 million people in the United States (100). For professional athletes and elderly patients over 65, osteoarthritis due to the complete loss of cartilage tissue at the joint surface is one of the most frequent causes of physical disability (101). Complicating matters, the limited capacity of cartilage to regenerate and the highly variable outcome of techniques to repair damaged cartilage such as mosaicplasty and autologous chondrocyte implantation have been major roadblocks in the adequate treatment of osteoarthritis and cartilage injuries (102–104). Previous strategies to regenerate cartilage have been unable to replicate the normal physiological environment and functions of chondrocytes. To increase the quality of engineered cartilage constructs, mechanical stimuli in the form of cyclic compression and shear forces, which are found during natural joint movement, have been applied to improve cartilage glycosaminoglycan and collagen synthesis (105– 107). However, the biomaterials used in these mechanical conditioning regimens offer limited strength and elasticity and are prone to plastic deformation after cyclic compressive strains (108– 110). The cross-linked network structure of CBBs provides a completely elastic substrate, which can be used in mechanical conditioning regimens to enhance cartilage regeneration, and has prompted researchers to use them as scaffold materials in cartilage engineering.
Kang et al. (111) investigated the use of POC to engineer elastomeric scaffolds for cartilage regeneration. In their study, porous POC scaffolds were fabricated via a salt-leaching technique and characterized for their ability to support chondrocyte attachment, proliferation, cell differentiation, and matrix synthesis. The glycosaminoglycan and collagen content of chondrocytes cultured on POC scaffolds at 28 days was 36% and 26%, respectively, of that found in bovine knee cartilage explants. Histology and immunohistochemistry evaluations confirmed that chondrocytes were able to attach to the pore walls within the scaffold, maintain their cell phenotype, and form a cartilaginous tissue during the 28 days of culture. The elastomeric qualities of POC support the application of long-term cyclic and shear strains to increase the glycosaminoglycan and collagen content of engineered cartilage.
Researchers have also investigated the effect of material choice on cartilage regeneration (112). 3D scaffolds of the same microarchitectural design were fabricated using PCL, PGS, or POC (113). These studies show that PGS is the least favorable material for cartilage regeneration, as determined by high dedifferentiation (Col1), hypertrophic mRNA expression (Col10), and high matrix degradation (MMP13, MMP3). Although a majority of the cells seeded on PCL remained on the scaffold periphery, the PCL scaffolds showed moderate cellular activity and caused de-differentiation (Col1) of chondrocytes within the scaffold. Interestingly, after 4 weeks of culture, POC provided the best support for cartilage regeneration: It yielded the highest tissue ingrowth (cell penetration), matrix production, relative mRNA expressions for chondrocyte differentiation (Col2/Col1), and DNA and glycosaminoglycan content. The results (113) demonstrate that POC can outperform other biodegradable elastomers used for cartilage tissue engineering and warrants further in vivo studies. Motivated by their previous studies, these researchers published successive reports to evaluate the coupled effects of 3D POC scaffold pore shape and permeability on chondrogenesis using primary chondrocytes in vivo (114, 115). The results indicated that low-porosity POC scaffolds composed of spherical pores showed a significantly greater increase in cartilage matrix formation over 6 weeks in vivo when compared with highly porous POC scaffolds composed of cuboidal pores. As with bone, the significantly increased cartilage matrix production on lowpermeability spherical-pore POC scaffolds suggests that scaffold architecture is also important to the success of cartilage regeneration strategies.
Nervous System: Peripheral Nerves
Although peripheral nerves have an innate propensity to regenerate following injury, surgical intervention in the form of nerve autografting is necessary when the nerve is severed beyond the point of repair. Unfortunately, this form of repair is associated with many disadvantages, including the need for multiple surgical procedures, limited availability of suitable grafts, loss of function at the donor site, and the potential for neuroma formation (116–119). These limitations have motivated the development of biomaterial-based regenerative engineering strategies focused on the fabrication of artificial guides with intricate internal structures to promote axon elongation through mechanisms of contact guidance and basement membrane microtube theory for nerve regeneration (120, 121). For example, longitudinally oriented channels have been used to fill the interior of a conduit to provide internal support to prevent conduit collapse and support the body's natural pattern of growth by providing better nerve target reinnervation and a greater surface area to direct the growth of regenerating nerve fibers (bands of Büngner) (122, 123).
Although promising, many of the existing engineered peripheral nerve guides are stiff and in-elastic. Owing to their elasticity, strength, and biocompatibility, CBBs have been investigated as a potential material for the regeneration of peripheral nerves. Porous and elastic nerve-guide scaffolds based on CUPEs have been fabricated with multiple internal longitudinally oriented channels as well as an external nonporous sheath to mimic the native endoneurial microtubular structure and epineurium (124). The fabrication technique provides versatility for designing the scaffold channel geometry, porosity, and mechanical properties. The authors (124) hypothesized that peripheral nerve guides fabricated using CUPEs would have the strength and elasticity required to withstand physiological tensions and strains as well as surgical handling. CUPE multichanneled scaffolds displayed an ultimate peak stress of 2.83 ± 0.24 MPa with corresponding elongations at break values of 259.60 ± 21.49%, which are in the range of native nerve mechanical properties. CUPE multichanneled scaffolds to repair 1-cm rat sciatic nerve defects have also been evaluated in vivo. After 8 weeks of implantation, CUPE multichanneled scaffolds compared favorably with nerve autograft in terms of fiber number, density, and diameter.
Renal System: Bladder
In standard surgical treatment to augment or replace damaged or diseased bladder, vascularized gastrointestinal tissue is used as a substitute. However, this practice is associated with several complications including stone formation, increased mucus secretion, and increased probability of developing cancer. Attempts to use other materials such as collagen scaffolds and small-intestinal submucosa have had limited success owing to mismatch of mechanical properties and a deleterious inflammatory response. In a novel approach, highly elastic thin POC films were seeded with (a) human mesenchymal stem cells and urothelial cells, (b) normal bladder smooth muscle cells and urothelial cells, or (c) no cells as control (125). After 10 weeks of implantation to augment partially cystectomized nude rats, POC-augmented bladders seeded with mesenchymal stem and urothelial cells displayed normal tissue architecture and 1.75 times greater muscle/collagen ratios than did films seeded with smooth muscle and urothelial cells, thus demonstrating their potential to support partial bladder regeneration in vivo. To promote angiogenesis and improve bladder regeneration, the carboxylic acid groups of POC were modified with heparan sulfate to create heparan-binding domains for the subsequent conjugation of proangiogenic growth factors (126). A threefold increase in release of vascular endothelial growth factor, fibroblast growth factor 2, and insulin-like growth factor 1 was observed for heparan-modified POC versus controls during a 30-day time course in vitro. Immunostaining for CD31 and von Willebrand factor demonstrates that subcutaneous implantation of the heparan-modified elastomers within the dorsa of nude rats yields increased vascularization. POC films have also been used as the substrate to determine the efficacy of combining populations of bone marrow–derived mesenchymal and hemamiddleoietic stem cells. POC scaffolds seeded with multipotent mesenchymal stem cells and CD34+ hemamiddleoietic stem/progenitor cells lead to superior angiogenesis, peripheral nerve formation, and urothelium regeneration in vivo, thus highlighting the benefits of using these stem cell types as a cell source for bladder regeneration (127).
Gastrointestinal Tissue
CBBs have also been used to aid in the removal of gastrointestinal (GI) cancers. GI cancers frequently occur in industrialized countries, with new cases of esophageal, gastric, and colorectal cancers affecting 3.60%, 11.4%, and 30.1%, respectively, of the developed world's population. Surgical procedures aimed at the removal of GI cancers include endoscopic mucosal resection (EMR), which is a minimally invasive endoscopic procedure developed to remove dysplastic and malignant lesions limited to the mucosa and middle part of the submucosal layer of the GI tract. Originally, EMR was accomplished by mechanically separating the mucosal layer from the underlying muscle; however, perforation, bleeding, and damage to the muscle layer are common occurrences.
A potential solution to address these problems has recently been reported: the development of an injectable drug eluting elastomeric polymer (iDEEP) system based on PEGMC (128). Whereas previous applications of PEGMC were formulated to optimize mechanical strength and porosity for regenerative engineering applications, the novel injectable cross-linking mechanism uniquely suits EMR applications. iDEEP utilizes both viscosity and gel formation through redox-initiated cross-linking to overcome the limitations of previous designs. Water-soluble iDEEP-A, which is composed of PEGMC and is more viscous than saline, remains a viscous liquid until combined with water-soluble iDEEP-B to produce a soft biodegradable hydrogel. Dividing the system into two separate components offers significant advantages over previous designs: The surgeon can precisely control the gel-setting location and time and avoid premature gelling inside the delivery tools. In addition, using a redox-initiated cross-linking mechanism does not require additional equipment such as UV light for the gel formation to occur.
PEGMC formulations used in the study (128) demonstrated a tunable transition from a viscous flowable liquid to a cross-linked soft biodegradable hydrogel within 5 min. Researchers used minimally invasive techniques to inject the water-soluble prepolymer and used redox-initiated mechanisms to form it into a hydrogel in situ. The elastomeric hydrogel simultaneously separates the diseased submucosal tissue from the underlying muscle layers and delivers a therapeutic dose of rebamipide, which is a mucosal protective and ulcer-healing drug that stimulates prostaglandin generation and improves the speed of ulcer healing to aid in the management of EMR-induced damage. The in vitro drug release profile studies of iDEEP hydrogels using rebamipide revealed an initial burst release followed by a sustained release for up to 2 weeks; such releases may be controlled through PEGMC monomer ratios. Future studies will be dedicated to comparative long-term evaluations in living animals, with pathological analysis to confirm the efficacy, depth of resection, and degree of submucosal regeneration using the iDEEP system.
Dermal Tissue
Wound healing in skin is a dynamic and complex biological process that involves the orchestrated differentiation, migration, proliferation, and apoptosis of various cell types to recreate the multilaminated skin architecture (129). Clinicians often use materials to aid this process and help the wound heal. However, the inability of currently used biomaterials to adhere and effectively integrate with irregular-shaped defects in a wet and dynamic environment remains a problem for which regenerative engineering strategies may offer a solution (130). To address this issue, CBBs with inherent adhesive properties, iCMBAs, have been developed for sutureless wound closure and healing. The mussel-inspired adhesion of iCMBAs is uniquely suited for wound-healing applications because the iCMBA prepolymer is water soluble and can be applied to conform easily to the irregular-shaped defects (54). Once the iCMBA is applied, the DOPA-based adhesion chemistry allows the CBB to adhere strongly to the wound in the presence of blood and effectively stabilize the tissue and integrate with it. Within the formulations investigated, iCMBAs showed wet-tissue adhesion strength 2.5–8.0-fold stronger than that of clinically used fibrin glue. iCMBAs have greater controlled degradability, compliant elastomeric mechanical properties, and excellent cytocompatibility both in vitro and in vivo. iCMBAs also smiddle bleeding instantly and have closed wounds (2 cm long × 0.5 cm deep) created on the backs of Sprague-Dawley rats without the use of sutures (Figure 5). This task is difficult to achieve using currently available products such as fibrin glue owing to weak wet-tissue adhesion strength. The new bioadhesives facilitate wound healing and are completely absorbed without eliciting a significant inflammatory response. Per such results, iCMBA technology is highly translational, as it could potentially have a significant impact on wound healing and wound care management.
Controlled Release of Therapeutic Compounds
An elastomer-based drug delivery system that combines mechanomimetics with drug-based biological signals could play an important role in regenerative engineering. Nanoporous POC films have been developed: In the presence of a solvent porogen such as PEG dimethyl ether, POC cross-links may be separated during the polymerization process. The resulting nanoporous construct contains pores on the order of hundreds of nanometers, which can be collapsed to encapsulate drugs through lyophilization (131). Via a system of deep eutectic solvents, POC elastomers effectively transport lidocaine, which is a local anesthetic considered to be an attractive anti-inflammatory compound (132). Nuclear magnetic resonance studies confirmed that lidocaine is not degraded during synthesis using this system: Although a burst release was evident after 3 h (132), the sustained release of lidocaine lasted 4 days.
CBB hydrogels such as PEGMC have also been applied in therapeutic drug delivery to repair exposed dental pulp (133). The in situ cross-linkable nature of PEGMC is especially suitable for this application because drugs and cells can be safely encapsulated within the hydrogel and delivered to facilitate the formation of reparative dentin. In vitro release studies showed a cumulative release of calcium from PEGMC for up to 96 h, and L929 cells encapsulated within PEGMC showed viability when exposed to UV light for 60 s. CBBs have also been used to develop a biocompatible intravaginal ring to deliver nonhormonal contraceptives (134). Nanoporous POC was loaded with ferrous gluconate to cause spermiostasis, with ascorbic acid to increase cervical mucus viscosity, and with a mixture of ampholines to maintain an acidic environment to cause spermiostasis within 30 s for the prevention of undesired pregnancy. Researchers found that levels of the nonhormonal contraceptive released in a 30-day period by a POC intravaginal ring stored for 1 year were comparable to those of a freshly made POC intravaginal ring.
In addition to providing a physical substrate for cellular growth, regenerative engineering constructs should also facilitate the delivery of cell signaling factors to repair and/or integrate with the diseased tissues in the body. The delivery vehicle should allow for both short- and long-term delivery and enable researchers to control dosing without compromising the biological activity of the factor. Traditionally, the physical adsorption and release of a protein from a biomaterial has been the primary means to deliver proteins to the surrounding scaffold environment. However, this method may not translate well in vivo as a result of the protein's short half-life and limited loading capacity, two challenges to maintaining a prolonged therapeutic effect.
An alternative to directly releasing proteins from a depot is to deliver genes encoding the protein of interest. Such genes are internalized by cells and then trigger the production of the desired protein for extended periods. In this regard, POC has been studied as a substrate for plasmid immobilization and cellular transduction of colonizing cells (135). The rationale behind this approach is that plasmid DNA in its native form or in complex with a cationic polymer such as polyethylenimine could be physically adsorbed onto POC. Polyethylenimine can disrupt the endosome via the proton sponge effect and enhance the efficiency of DNA delivery (135–137). Compared with naked, plasmid DNA–containing scaffolds, polyplex-containing CBB scaffolds display a higher loading efficiency and slower initial release rates. HEK293 cells and porcine aortic smooth muscle cells seeded onto polyplex-loaded POC scaffolds also demonstrated cell proliferation and transfection for up to 12 days in vitro. However, in vivo studies of a mouse intraperitoneal fat model showed that only the use of naked, plasmid DNA–containing scaffolds yielded successful long-term transgene delivery, which was determined by higher expression levels of both luciferase and green fluorescent protein. Several other groups (138–141) have also reported this contradiction between in vitro and in vivo results, which may be due to the interactions among carriers, host tissue, and immunity that cannot be adequately replicated in an in vitro setting. In addition to the delivery of nonviral vectors, POC has been investigated for use as a substrate to deliver genes via a lentivirus. To obtain sustained and localized transgene expression, a lentivirus was immobilized on porous POC scaffolds through the negative charges naturally present on the surface of POC (142). Cells seeded on lentivirus-loaded POC showed titer-dependent transgene expression with lentivirus activity up to 5 days in vitro and up to 5 weeks in vivo.
Conclusions
In this review, we summarize important design considerations, strategies to introduce and enhance functionality, and examples of the use of CBBs in applications that are relevant to regenerative engineering. Due to their versatile and functional material properties, easy synthesis, and scalability, CBBs will continue to play an important role in regenerative engineering. Whether researchers attempt to engineer soft or hard tissues, CBBs possess tunable chemical functionalities, mechanical properties, and degradation rates that can meet the increasingly sophisticated demands of regenerative engineering applications. When making the next generation of biodegradable elastomers, investigators should explore designing CBBs that guide and provide feedback on the tissue regeneration process. Such CBBs should recruit host cells to the implanted material; respond to changes in the cellular environment to accommodate tissue maturation; and alert the clinician, patient, or scientist to these effects.
Supplementary Material
Supplemental Figure 1. Citrate-based biomaterial combinational crosslinking mechanisms. A) Poly(octamethylene citrate) – Click (POC-Click) materials can be crosslinked using a thermal synchronous binary (TSB) crosslinking mechanism: thermal click reaction between azide and alkyne groups and esterification between –COOH and –OH groups. B) Citrate-based biomaterials synthesized with vinyl containing monomers offer a dual crosslinking mechanism (DCM): free radical polymerization between unsaturated functional groups and/or thermal esterification between –COOH and –OH groups.
Supplemental Figure 2. Representative synthesis schematic of crosslinked urethane-doped polyesters (CUPE). A) First, citric acid is reacted with 1,8-octanediol to create a poly(octamethylene citrate) (POC) pre-polymer. B) Next, hexamethylene diisocyanate (HDI) is doped into the pre-polymer network and acts as a chain extender for pre-POC. The resulting material is a soft and elastic polyester network containing degradable ester bonds with urethane linkages. Adapted from Figure 1 of reference (29) with permission.
Supplemental Figure 3. Schematic of the synthesis of diazeniumdiolated CBBs for the prolonged release of nitric oxide (NO). NO release profile can be controlled with the choice of diol, with hydrophobic diols resulting in slower NO release.
Acknowledgments
The authors thank Dianna Y. Nguyen for her assistance in creating the figures of this review. This work was supported in part by National Institutes of Health awards (NIBIB EB012575, NIBIB EB017129-02, NCI CA182670, NHLBI HL118498) and a National Science Foundation award (DMR1313553).
Footnotes
Disclosure Statement: All authors are listed as coinventors on patents that disclose CBB. G. Ameer is a cofounder of VesselTek BioMedical, LLC, a company that is developing CBB for commercial use. R. Tran, formerly at Pennsylvania State at the time the article was written, is currently employed at Acuitive Technologies, Inc. (ATI). CBB technologies for musculoskeletal applications have been licensed to ATI.
Contributor Information
Jian Yang, Email: jxy30@psu.edu.
Guillermo A. Ameer, Email: g-ameer@northwestern.edu.
Literature Cited
- 1.Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med. 2012;4:160e. doi: 10.1126/scitranslmed.3004467. d9. [DOI] [PubMed] [Google Scholar]
- 2.Bettinger CJ. Biodegradable elastomers for tissue engineering and cell-biomaterial interactions. Macromol Biosci. 2011;11:467–82. doi: 10.1002/mabi.201000397. [DOI] [PubMed] [Google Scholar]
- 3.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 5.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–49. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 7.Rai R, Tallawi M, Grigore A, Boccaccini AR. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): a review. Prog Polym Sci. 2012;37:1051–78. [Google Scholar]
- 8.Yang J, Webb AR, Ameer GA. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv Mater. 2004;16:511–16. [Google Scholar]
- 9.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27:1889–98. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 10.He H, Matsuda T. Arterial replacement with compliant hierarchic hybrid vascular graft: biomechanical adaptation and failure. Tissue Eng. 2002;8:213–24. doi: 10.1089/107632702753724987. [DOI] [PubMed] [Google Scholar]
- 11.Xue L, Greisler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37:472–80. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]
- 12.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–32. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment: implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–39. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano MC, Chung EJ, Ameer GA. Advances and applications of biodegradable elastomers in regenerative medicine. Adv Funct Mater. 2010;20:192–208. [Google Scholar]
- 16.Ding T, Liu QY, Shi R, Tian M, Yang H, Zhang LQ. Synthesis, characterization and in vitro degradation study of a novel and rapidly degradable elastomer. Polym Degrad Stab. 2006;91:733–39. [Google Scholar]
- 17.Li J, Zheng W, Pan P, Sun X, Zhang Y. Synthesis and characterization of poly(1,2-propanediol-co-1,8-octanediol-co-citrate) biodegradable elastomers for tissue engineering. Biomed Mater Eng. 2014;24:619–24. doi: 10.3233/BME-130849. [DOI] [PubMed] [Google Scholar]
- 18.Lei LJ, Ding T, Shi R, Liu QY, Zhang LQ, et al. Synthesis, characterization and in vitro degradation of a novel degradable poly((1,2-propanediol-sebacate)-citrate) bioelastomer. Polym Degrad Stab. 2007;92:389–96. [Google Scholar]
- 19.Djordjevic I, Choudhury NR, Dutta NK, Kumar S. Synthesis and characterization of novel citric acid-based polyester elastomers. Polymer. 2009;50:1682–91. [Google Scholar]
- 20.Djordjevic I, Choudhury NR, Dutta NK, Kumar S. Poly[octanediol-co-(citric acid)-co-(sebacic acid)] elastomers: novel bio-elastomers for tissue engineering. Polym Int. 2011;60:333–43. [Google Scholar]
- 21.Djordjevic I, Choudhury NR, Dutta NK, Kumar S, Szili EJ, Steele DA. Polyoctanediol citrate/sebacate bioelastomer films: surface morphology, chemistry and functionality. J Biomater Sci Polym Ed. 2010;21:237–51. doi: 10.1163/156856209X415558. [DOI] [PubMed] [Google Scholar]
- 22.Webb AR, Yang J, Ameer GA. A new strategy to characterize the extent of reaction of thermoset elastomers. J Polym Sci. 2008;46:1318–28. [Google Scholar]
- 23.Guo J, Xie Z, Tran RT, Xie D, Jin D, et al. Click chemistry plays a dual role in biodegradable polymer design. Adv Mater. 2014;26:1906–11. doi: 10.1002/adma.201305162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J. Citric-acid-derived photo-cross-linked biodegradable elastomers. J Biomater Sci Polym Ed. 2010;21:1761–82. doi: 10.1163/092050609X12567178204169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin DS, Guhanathan S. Synthesis and characterization of citric acid-based pH-sensitive biopolymeric hydrogels. Polym Bull. 2014;71:93–110. [Google Scholar]
- 26.Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, et al. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials. 2010;31:9092–105. doi: 10.1016/j.biomaterials.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran RT, Thevenot P, Gyawali D, Chiao JC, Tang L, Yang J. Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism. Soft Matter. 2010;6:2449–61. doi: 10.1039/C001605E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao HC, Ameer GA. Modulating the mechanical properties of poly(diol citrates) via the incorporation of a second type of crosslink network. J Appl Polym Sci. 2009;114:1464–70. [Google Scholar]
- 29.Dey J, Xu H, Shen J, Thevenot P, Gondi SR, et al. Development of biodegradable crosslinked urethane-doped polyester elastomers. Biomaterials. 2008;29:4637–49. doi: 10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey J, Tran RT, Shen J, Tang L, Yang J. Development and long-term in vivo evaluation of a biodegradable urethane-doped polyester elastomer. Macromol Mater Eng. 2011;296:1149–57. doi: 10.1002/mame.201100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb AR, Kumar VA, Ameer GA. Biodegradable poly(diol citrate) nanocomposite elastomers for soft tissue engineering. J Mater Chem. 2007;17:900–06. [Google Scholar]
- 32.Lei LJ, Li L, Zhang LQ, Chen DF, Tian W. Structure and performance of nano-hydroxyapatite filled biodegradable poly((1,2-propanediol-sebacate)-citrate) elastomers. Polym Degrad Stab. 2009;94:1494–502. [Google Scholar]
- 33.Liu QY, Wu JY, Tan TW, Zhang LQ, Chen DF, Tian W. Preparation, properties and cytotoxicity evaluation of a biodegradable polyester elastomer composite. Polym Degrad Stab. 2009;94:1427–35. [Google Scholar]
- 34.Wu Y, Shi R, Chen DF, Zhang LQ, Tian W. Nanosilica filled poly(glycerol-sebacate-citrate) elastomers with improved mechanical properties, adjustable degradability, and better biocompatibility. J Appl Polym Sci. 2012;123:1612–20. [Google Scholar]
- 35.Serrano MC, Carbajal L, Ameer GA. Novel biodegradable shape-memory elastomers with drug-releasing capabilities. Adv Mater. 2011;23:2211–15. doi: 10.1002/adma.201004566. [DOI] [PubMed] [Google Scholar]
- 36.van Lith R, Gregory EK, Yang J, Kibbe MR, Ameer GA. Engineering biodegradable polyester elastomers with antioxidant properties to attenuate oxidative stress in tissues. Biomaterials. 2014;35:8113–22. doi: 10.1016/j.biomaterials.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, van Lith R, Baler K, Hoshi RA, Ameer GA. A thermoresponsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules. 2014;15:3942–52. doi: 10.1021/bm5010004. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Serrano MC, Popowich DA, Kibbe MR, Ameer GA. Biodegradable nitric oxide-releasing poly(diol citrate) elastomers. J Biomed Mater Res A. 2010;93:356–63. doi: 10.1002/jbm.a.32536. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Kibbe MR, Ameer GA. Photo-crosslinked biodegradable elastomers for controlled nitric oxide delivery. Biomater Sci. 2013;1:625–32. doi: 10.1039/C3BM00169E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karahaliloglu Z, Ercan B, Chung S, Taylor E, Denkbas EB, Webster TJ. Nanostructured antibacterial poly-lactic-co-glycolic acid films for skin tissue engineering applications. J Biomed Mater Res A. 2014;102:4598–608. doi: 10.1002/jbm.a.35141. [DOI] [PubMed] [Google Scholar]
- 41.Machado MC, Tarquinio KM, Webster TJ. Decreased Staphylococcus aureus biofilm formation on nanomodified endotracheal tubes: a dynamic airway model. Int J Nanomed. 2012;7:3741–50. doi: 10.2147/IJN.S28191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coneski PN, Fulmer PA, Wynne JH. Thermal polycondensation of poly(diol citrate)s with tethered quaternary ammonium biocides. RSC Adv. 2012;2:12824–34. [Google Scholar]
- 43.Kompany K, Mirza EH, Hosseini S, Pingguan-Murphy B, Djordjevic I. Polyoctanediol citrate-ZnO composite films: preparation, characterization and release kinetics of nanoparticles from polymer matrix. Mater Lett. 2014;126:165–68. [Google Scholar]
- 44.Halpern JM, Urbanski R, Weinstock AK, Iwig DF, Mathers RT, von Recum HA. A biodegradable thermoset polymer made by esterification of citric acid and glycerol. J Biomed Mater Res A. 2014;102:1467–77. doi: 10.1002/jbm.a.34821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Arguelles S, Serrano MC, Gutierrez MC, Ferrer ML, Yuste L, et al. Deep eutectic solvent-assisted synthesis of biodegradable polyesters with antibacterial properties. Langmuir. 2013;29:9525–34. doi: 10.1021/la401353r. [DOI] [PubMed] [Google Scholar]
- 46.Su LC, Xie Z, Zhang Y, Nguyen KT, Yang J. Study on the antimicrobial properties of citrate-based biodegradable polymers. Front Bioeng Biotechnol. 2014;2:23. doi: 10.3389/fbioe.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehdizadeh M, Yang J. Design strategies and applications of tissue bioadhesives. Macromol Biosci. 2013;13:271–88. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuocolo T, Haddad R, Edwards GA, Lyons RE, Liyou NE, et al. A highly elastic and adhesive gelatin tissue sealant for gastrointestinal surgery and colon anastomosis. J Gastrointest Surg. 2012;16:744–52. doi: 10.1007/s11605-011-1771-8. [DOI] [PubMed] [Google Scholar]
- 49.Oda S, Morita S, Tanoue Y, Eto M, Matsuda T, Tominaga R. Experimental use of an elastomeric surgical sealant for arterial hemostasis and its long-term tissue response. Interact Cardiovasc Thorac Surg. 2010;10:258–61. doi: 10.1510/icvts.2009.217620. [DOI] [PubMed] [Google Scholar]
- 50.Lurtz C, Voss K, Hahn V, Schauer F, Wegmann J, et al. In vitro degradation and drug release of a biodegradable tissue adhesive based on functionalized 1,2-ethylene glycol bis(dilactic acid) and chitosan. J Mater Sci Mater Med. 2013;24:667–78. doi: 10.1007/s10856-012-4826-9. [DOI] [PubMed] [Google Scholar]
- 51.Rohm HW, Lurtz C, Wegmann J, Odermatt EK, Behrend D, et al. Development of a biodegradable tissue adhesive based on functionalized 1,2-ethylene glycol bis(dilactic acid). II. J Biomed Mater Res B. 2011;97:66–73. doi: 10.1002/jbm.b.31787. [DOI] [PubMed] [Google Scholar]
- 52.Sternberg K, Rohm HW, Lurtz C, Wegmann J, Odermatt EK, et al. Development of a biodegradable tissue adhesive based on functionalized 1,2-ethylene glycol bis(dilactic acid). I. J Biomed Mater Res B. 2010;94:318–26. doi: 10.1002/jbm.b.31654. [DOI] [PubMed] [Google Scholar]
- 53.Waite JH. Nature's underwater adhesive specialist. Int J Adhes Adhes. 1987;7:9–14. [Google Scholar]
- 54.Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials. 2012;33:7972–83. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–63. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine. 2008;3:137–40. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Zhang Y, Gautam S, Liu L, Dey J, et al. Development of aliphatic biodegradable photo-luminescent polymers. PNAS. 2009;106:10086–91. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Yang J. Design strategies for fluorescent biodegradable polymeric biomaterials. J Mater Chem B. 2013;1:132–48. doi: 10.1039/C2TB00071G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serrano CA, Zhang Y, Yang J, Schug KA. Matrix-assisted laser desorption/ionization mass spec-trometric analysis of aliphatic biodegradable photoluminescent polymers using new ionic liquid matrices. Rapid Commun Mass Spectrom. 2011;25:1152–58. doi: 10.1002/rcm.4974. [DOI] [PubMed] [Google Scholar]
- 60.Kasprzyk W, Bednarz S, Bogdal D. Luminescence phenomena of biodegradable photoluminescent poly(diol citrates) Chem Commun. 2013;49:6445–47. doi: 10.1039/c3cc42661k. [DOI] [PubMed] [Google Scholar]
- 61.Xie Z, Zhang Y, Liu L, Weng H, Mason RP, et al. Development of intrinsically photoluminescent and photostable polylactones. Adv Mater. 2014;26:4491–96. doi: 10.1002/adma.201306070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Tran RT, Gyawali D, Yang J. Development of photocrosslinkable urethane-doped polyester elastomers for soft tissue engineering. Int J Biomater Res Eng. 2011;1:18–31. doi: 10.4018/ijbre.2011010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gyawali D, Zhou S, Tran RT, Zhang Y, Liu C, et al. Fluorescence imaging enabled biodegradable photostable polymeric micelles. Adv Healthc Mater. 2014;3:182–86. doi: 10.1002/adhm.201300145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wadajkar AS, Menon JU, Kadapure T, Tran RT, Yang J, Nguyen KT. Design and application of magnetic-based theranostic nanoparticle systems. Recent Pat Biomed Eng. 2013;6:47–57. doi: 10.2174/1874764711306010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Tran RT, Qattan IS, Tsai YT, Tang L, et al. Fluorescence imaging enabled urethane-doped citrate-based biodegradable elastomers. Biomaterials. 2013;34:4048–56. doi: 10.1016/j.biomaterials.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niklason LE, Langer RS. Advances in tissue engineering of blood vessels and other tissues. Transpl Immunol. 1997;5:303–6. doi: 10.1016/s0966-3274(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 67.Motlagh D, Allen J, Hoshi R, Yang J, Lui K, Ameer G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J Biomed Mater Res A. 2007;82:907–16. doi: 10.1002/jbm.a.31211. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, et al. Modulating expanded polytetrafluoroethylene vascular graft host response via citric acid-based biodegradable elastomers. Adv Mater. 2006;18:1493–98. [Google Scholar]
- 69.Kibbe MR, Martinez J, Popowich DA, Kapadia MR, Ahanchi SS, et al. Citric acid-based elastomers provide a biocompatible interface for vascular grafts. J Biomed Mater Res A. 2010;93:314–24. doi: 10.1002/jbm.a.32537. [DOI] [PubMed] [Google Scholar]
- 70.Hoshi RA, Van Lith R, Jen MC, Allen JB, Lapidos KA, Ameer G. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials. 2013;34:30–41. doi: 10.1016/j.biomaterials.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serrano MC, Vavra AK, Jen M, Hogg ME, Murar J, et al. Poly(diol-co-citrate)s as novel elastomeric perivascular wraps for the reduction of neointimal hyperplasia. Macromol Biosci. 2011;11:700–9. doi: 10.1002/mabi.201000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregory EK, Webb AR, Martinez-Vercammen J, Flynn ME, Ameer GA, Kibbe MR. Periadventital atRA via citrate-based polyester membranes reduces neointimal hyperplasia and restenosis after carotid injury in rats. Am J Physiol Heart Circ Physiol. 2014;307:H1419–29. doi: 10.1152/ajpheart.00914.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Argenta L, Morykwas M, Wagner WD. Properties of single electrospun poly(diol citrate)-collagen-proteoglycan nanofibers for arterial repair and in applications requiring viscoelasticity. J Bio-mater Appl. 2014;28:729–38. doi: 10.1177/0885328213477893. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Motlagh D, Webb AR, Ameer GA. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue Eng. 2005;11:1876–86. doi: 10.1089/ten.2005.11.1876. [DOI] [PubMed] [Google Scholar]
- 75.Dey J, Xu H, Nguyen KT, Yang J. Crosslinked urethane-doped polyester biphasic scaffolds: potential for in vivo vascular tissue engineering. J Biomed Mater Res A. 2010;95:361–70. doi: 10.1002/jbm.a.32846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tran RT, Naseri E, Kolasnikov A, Bai X, Yang J. A new generation of sodium chloride porogen for tissue engineering. Biotechnol Appl Biochem. 2011;58:335–44. doi: 10.1002/bab.44. [DOI] [PubMed] [Google Scholar]
- 77.Allen J, Khan S, Serrano MC, Ameer G. Characterization of porcine circulating progenitor cells: toward a functional endothelium. Tissue Eng Part A. 2008;14:183–94. doi: 10.1089/ten.a.2007.0265. [DOI] [PubMed] [Google Scholar]
- 78.Allen JB, Khan S, Lapidos KA, Ameer GA. Toward engineering a human neoendothelium with circulating progenitor cells. Stem Cells. 2010;28:318–28. doi: 10.1002/stem.275. [DOI] [PubMed] [Google Scholar]
- 79.Su LC, Xu H, Tran RT, Tsai YT, Tang L, et al. In situ re-endothelialization via multifunctional nanoscaffolds. ACS Nano. 2014;8:10826–36. doi: 10.1021/nn504636n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Praemer A, Furner S, Rice DP. Am Acad Orthop Surg. Park Ridge, NJ: 1999. Musculoskeletal Conditions in the United States. [Google Scholar]
- 81.Nardecchia S, Serrano MC, Gutierrez MC, Portoles MT, Ferrer ML, del Monte F. Osteoconductive performance of carbon nanotube scaffolds homogeneously mineralized by flow-through electro-deposition. Adv Funct Mater. 2012;22:4411–20. [Google Scholar]
- 82.Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res. 1998;355(Suppl.):22–30. doi: 10.1097/00003086-199810001-00004. [DOI] [PubMed] [Google Scholar]
- 83.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 84.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. PNAS. 2010;107:22425–29. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costello LC, Franklin RB, Reynolds MA, Chellaiah M. The important role of osteoblasts and citrate production in bone formation: “osteoblast citration” as a new concept for an old relationship. Open Bone J. 2012;4:27–34. doi: 10.2174/1876525401204010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davies E, Muller KH, Wong WC, Pickard CJ, Reid DG, et al. Citrate bridges between mineral platelets in bone. PNAS. 2014;111:E1354–63. doi: 10.1073/pnas.1315080111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costello LC, Franklin RB. A review of the important central role of altered citrate metabolism during the process of stem cell differentiation. J Regen Med Tissue Eng. 2013;2:1. doi: 10.7243/2050-1218-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran RT, Wang L, Zhang C, Huang M, Tang W, et al. Synthesis and characterization of biomimetic citrate-based biodegradable composites. J Biomed Mater Res A. 2014;102:2521–32. doi: 10.1002/jbm.a.34928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shirazi FS, Moghaddam E, Mehrali M, Oshkour AA, Metselaar HS, et al. In vitro characterization and mechanical properties of beta-calcium silicate/POC composite as a bone fixation device. J Biomed Mater Res A. 2014;102:3973–85. doi: 10.1002/jbm.a.35074. [DOI] [PubMed] [Google Scholar]
- 90.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 91.Qiu H, Yang J, Kodali P, Koh J, Ameer GA. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials. 2006;27:5845–54. doi: 10.1016/j.biomaterials.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 92.Chung EJ, Sugimoto MJ, Ameer GA. The role of hydroxyapatite in citric acid-based nanocomposites: surface characteristics, degradation, and osteogenicity in vitro. Acta Biomater. 2011;7:4057–63. doi: 10.1016/j.actbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Chung EJ, Qiu H, Kodali P, Yang S, Sprague SM, et al. Early tissue response to citric acid-based micro- and nanocomposites. J Biomed Mater Res A. 2011;96:29–37. doi: 10.1002/jbm.a.32953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung EJ, Kodali P, Laskin W, Koh JL, Ameer GA. Long-term in vivo response to citric acid-based nanocomposites for orthopaedic tissue engineering. J Mater Sci Mater Med. 2011;22:2131–38. doi: 10.1007/s10856-011-4393-5. [DOI] [PubMed] [Google Scholar]
- 95.Levi-Polyachenko N, Rosenbalm T, Kuthirummal N, Shelton J, Hardin W, et al. Development and characterization of elastic nanocomposites for craniofacial contraction osteogenesis. J Biomed Mater Res B. 2015;103:407–16. doi: 10.1002/jbm.b.33220. [DOI] [PubMed] [Google Scholar]
- 96.Chung E, Sugimoto M, Koh J, Ameer G. A biodegradable tri-component graft for anterior cruciate ligament reconstruction. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1966. [DOI] [PubMed] [Google Scholar]
- 97.Guo Y, Tran RT, Xie D, Wang Y, Nguyen DY, et al. Citrate-based biphasic scaffolds for the repair of large segmental bone defects. J Biomed Mater Res A. 2014;103:772–81. doi: 10.1002/jbm.a.35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gyawali D, Nair P, Kim HK, Yang J. Citrate-based biodegradable injectable hydrogel composites for orthopedic applications. Biomater Sci. 2013;1:52–64. doi: 10.1039/C2BM00026A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiao Y, Gyawali D, Stark JM, Akcora P, Nair P, et al. A rheological study of biodegradable injectable PEGMC/HA composite scaffolds. Soft Matter. 2012;8:1499–507. doi: 10.1039/C1SM05786C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112:S13–19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 101.Corti MC, Rigon C. Epidemiology of osteoarthritis: prevalence, risk factors and functional impact. Aging Clin Exp Res. 2003;15:359–63. doi: 10.1007/BF03327356. [DOI] [PubMed] [Google Scholar]
- 102.Hunziker EB. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr Cartil. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 103.LaPrade RF, Swiontkowski MF. New horizons in the treatment of osteoarthritis of the knee. JAMA. 1999;281:876–78. doi: 10.1001/jama.281.10.876. [DOI] [PubMed] [Google Scholar]
- 104.Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26:309–24. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 105.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 106.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223–37. [PubMed] [Google Scholar]
- 107.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr Cartil. 2003;11:879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 109.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 110.Rahman MS, Tsuchiya T. Enhancement of chondrogenic differentiation of human articular chondrocytes by biodegradable polymers. Tissue Eng. 2001;7:781–90. doi: 10.1089/107632701753337726. [DOI] [PubMed] [Google Scholar]
- 111.Kang Y, Yang J, Khan S, Anissian L, Ameer GA. A new biodegradable polyester elastomer for cartilage tissue engineering. J Biomed Mater Res A. 2006;77:331–9. doi: 10.1002/jbm.a.30607. [DOI] [PubMed] [Google Scholar]
- 112.Jeong CG, Hollister SJ. Mechanical, permeability, and degradation properties of 3D designed poly(1,8 octanediol-co-citrate) scaffolds for soft tissue engineering. J Biomed Mater Res B. 2010;93:141–49. doi: 10.1002/jbm.b.31568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jeong CG, Hollister SJ. A comparison of the influence of material on in vitro cartilage tissue engineering with PCL, PGS, and POC 3D scaffold architecture seeded with chondrocytes. Biomaterials. 2010;31:4304–12. doi: 10.1016/j.biomaterials.2010.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jeong CG, Hollister SJ. Mechanical and biochemical assessments of three-dimensional poly(1,8-octanediol-co-citrate) scaffold pore shape and permeability effects on in vitro chondrogenesis using primary chondrocytes. Tissue Eng Part A. 2010;16:3759–68. doi: 10.1089/ten.tea.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeong CG, Zhang H, Hollister SJ. Three-dimensional poly(1,8-octanediol-co-citrate) scaffold pore shape and permeability effects on sub-cutaneous in vivo chondrogenesis using primary chondrocytes. Acta Biomater. 2011;7:505–14. doi: 10.1016/j.actbio.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 116.Bender MD, Bennett JM, Waddell RL, Doctor JS, Marra KG. Multi-channeled biodegradable polymer/CultiSpher composite nerve guides. Biomaterials. 2004;25:1269–78. doi: 10.1016/j.biomaterials.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 117.Ao Q, Wang A, Cao W, Zhang L, Kong L, et al. Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro. J Biomed Mater Res A. 2006;77:11–18. doi: 10.1002/jbm.a.30593. [DOI] [PubMed] [Google Scholar]
- 118.Bozkurt A, Brook GA, Moellers S, Lassner F, Sellhaus B, et al. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007;13:2971–79. doi: 10.1089/ten.2007.0116. [DOI] [PubMed] [Google Scholar]
- 119.Flynn L, Dalton PD, Shoichet MS. Fiber templating of poly(2-hydroxyethyl methacrylate) for neural tissue engineering. Biomaterials. 2003;24:4265–72. doi: 10.1016/s0142-9612(03)00334-x. [DOI] [PubMed] [Google Scholar]
- 120.Brayfield CA, Marra KG, Leonard JP, Cui XT, Gerlach JC. Excimer laser channel creation in polyethersulfone hollow fibers for compartmentalized in vitro neuronal cell culture scaffolds. Acta Biomater. 2008;4:244–55. doi: 10.1016/j.actbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 121.Zhang M, Yannas IV. Peripheral nerve regeneration. Adv Biochem Eng Biotechnol. 2005;94:67–89. doi: 10.1007/b100000. [DOI] [PubMed] [Google Scholar]
- 122.Hu X, Huang J, Ye Z, Xia L, Li M, et al. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:3297–308. doi: 10.1089/ten.TEA.2009.0017. [DOI] [PubMed] [Google Scholar]
- 123.Li J, Rickett TA, Shi R. Biomimetic nerve scaffolds with aligned intraluminal microchannels: a “sweet” approach to tissue engineering. Langmuir. 2009;25:1813–17. doi: 10.1021/la803522f. [DOI] [PubMed] [Google Scholar]
- 124.Tran RT, Choy WM, Cao H, Qattan I, Chiao JC, et al. Fabrication and characterization of biomimetic multichanneled crosslinked-urethane-doped polyester tissue engineered nerve guides. J Biomed Mater Res A. 2014;102:2793–804. doi: 10.1002/jbm.a.34952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31:6207–17. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 126.Sharma AK, Bury MI, Fuller NJ, Rozkiewicz DI, Hota PV, et al. Growth factor release from a chemically modified elastomeric poly(1,8-octanediol-co-citrate) thin film promotes angiogenesis in vivo. J Biomed Mater Res A. 2012;100:561–70. doi: 10.1002/jbm.a.33306. [DOI] [PubMed] [Google Scholar]
- 127.Sharma AK, Bury MI, Fuller NJ, Marks AJ, Kollhoff DM, et al. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. PNAS. 2013;110:4003–8. doi: 10.1073/pnas.1220764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tran RT, Palmer M, Tang SJ, Abell TL, Yang J. Injectable drug-eluting elastomeric polymer: a novel submucosal injection material. Gastrointest Endosc. 2012;75:1092–97. doi: 10.1016/j.gie.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70:2059–81. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoshi RA, Behl S, Ameer GA. Nanoporous biodegradable elastomers. Adv Mater. 2009;21:188–92. [Google Scholar]
- 132.Serrano MC, Gutierrez MC, Jimenez R, Ferrer ML, del Monte F. Synthesis of novel lidocaine-releasing poly(diol-co-citrate) elastomers by using deep eutectic solvents. Chem Commun. 2012;48:579–81. doi: 10.1039/c1cc15284j. [DOI] [PubMed] [Google Scholar]
- 133.Komabayashi T, Wadajkar A, Santimano S, Ahn C, Zhu Q, et al. Preliminary study of light-cured hydrogel for endodontic drug delivery vehicle. J Investig Clin Dent. 2014 doi: 10.1111/jicd.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saxena BB, Koldras KE, Singh M, Nguyen N, Rathnam P, et al. Development of a nanoporous elastomere intra-vaginal ring (IVR) for the sustained release of non-hormonal contraceptives. J Pharm Drug Deliv Res. 2012;1:1–4. [Google Scholar]
- 135.Zhang XQ, Tang H, Hoshi R, De Laporte L, Qiu H, et al. Sustained transgene expression via citric acid-based polyester elastomers. Biomaterials. 2009;30:2632–41. doi: 10.1016/j.biomaterials.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–63. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 137.Zhang XQ, Wang XL, Huang SW, Zhuo RX, Liu ZL, et al. In vitro gene delivery using polyamidoamine dendrimers with a trimesyl core. Biomacromolecules. 2005;6:341–50. doi: 10.1021/bm040060n. [DOI] [PubMed] [Google Scholar]
- 138.Steinstraesser L, Hirsch T, Beller J, Mittler D, Sorkin M, et al. Transient non-viral cutaneous gene delivery in burn wounds. J Gene Med. 2007;9:949–55. doi: 10.1002/jgm.1099. [DOI] [PubMed] [Google Scholar]
- 139.Huang SW, Wang J, Zhang PC, Mao HQ, Zhuo RX, Leong KW. Water-soluble and nonionic polyphosphoester: synthesis, degradation, biocompatibility and enhancement of gene expression in mouse muscle. Biomacromolecules. 2004;5:306–11. doi: 10.1021/bm034241l. [DOI] [PubMed] [Google Scholar]
- 140.Gharwan H, Wightman L, Kircheis R, Wagner E, Zatloukal K. Nonviral gene transfer into fetal mouse livers (a comparison between the cationic polymer PEI and naked DNA) Gene Ther. 2003;10:810–17. doi: 10.1038/sj.gt.3301954. [DOI] [PubMed] [Google Scholar]
- 141.Rolland AP. From genes to gene medicines: recent advances in nonviral gene delivery. Crit Rev Ther Drug Carrier Syst. 1998;15:143–98. doi: 10.1615/critrevtherdrugcarriersyst.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- 142.Jen MC, Baler K, Hood AR, Shin S, Shea LD, Ameer GA. Sustained, localized transgene expression mediated from lentivirus-loaded biodegradable polyester elastomers. J Biomed Mater Res A. 2013;101:1328–35. doi: 10.1002/jbm.a.34449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hidalgo-Bastida LA, Barry JJ, Everitt NM, Rose FR, Buttery LD, et al. Cell adhesion and mechanical properties of a flexible scaffold for cardiac tissue engineering. Acta Biomater. 2007;3:457–62. doi: 10.1016/j.actbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 144.Moradi A, Dalilottojari A, Pingguan-Murphy B, Djordjevic I. Fabrication and characterization of elastomeric scaffolds comprised of a citric acid-based polyester/hydroxyapatite microcomposite. Mater Des. 2013;50:446–50. [Google Scholar]
- 145.Chung EJ, Sugimoto M, Koh JL, Ameer GA. Low-pressure foaming: a novel method for the fabrication of porous scaffolds for tissue engineering. Tissue Eng Part C. 2012;18:113–21. doi: 10.1089/ten.TEC.2011.0289. [DOI] [PubMed] [Google Scholar]
- 146.Thaker H, Sharma AK. Engaging stem cells for customized tendon regeneration. Stem Cells Int. 2012;2012:309187. doi: 10.1155/2012/309187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thomas LV, Arun U, Remya S, Nair PD. A biodegradable and biocompatible PVA-citric acid polyester with potential applications as matrix for vascular tissue engineering. J Mater Sci Mater Med. 2009;20(Suppl. 1):259–69. doi: 10.1007/s10856-008-3599-7. [DOI] [PubMed] [Google Scholar]
- 148.Liu QY, Wu SZ, Tan TW, Weng JY, Zhang LQ, et al. Preparation and properties of a novel biodegradable polyester elastomer with functional groups. J Biomater Sci Polym Ed. 2009;20:1567–78. doi: 10.1163/092050609X12464345064325. [DOI] [PubMed] [Google Scholar]
- 149.Liu Q, Tan T, Weng J, Zhang L. Study on the control of the compositions and properties of a biodegradable polyester elastomer. Biomed Mater. 2009;4:025015. doi: 10.1088/1748-6041/4/2/025015. [DOI] [PubMed] [Google Scholar]
- 150.Thomas LV, Nair PD. (Citric acid-co-polycaprolactone triol) polyester: a biodegradable elastomer for soft tissue engineering. Biomatter. 2011;1:81–90. doi: 10.4161/biom.1.1.17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Citrate-based biomaterial combinational crosslinking mechanisms. A) Poly(octamethylene citrate) – Click (POC-Click) materials can be crosslinked using a thermal synchronous binary (TSB) crosslinking mechanism: thermal click reaction between azide and alkyne groups and esterification between –COOH and –OH groups. B) Citrate-based biomaterials synthesized with vinyl containing monomers offer a dual crosslinking mechanism (DCM): free radical polymerization between unsaturated functional groups and/or thermal esterification between –COOH and –OH groups.
Supplemental Figure 2. Representative synthesis schematic of crosslinked urethane-doped polyesters (CUPE). A) First, citric acid is reacted with 1,8-octanediol to create a poly(octamethylene citrate) (POC) pre-polymer. B) Next, hexamethylene diisocyanate (HDI) is doped into the pre-polymer network and acts as a chain extender for pre-POC. The resulting material is a soft and elastic polyester network containing degradable ester bonds with urethane linkages. Adapted from Figure 1 of reference (29) with permission.
Supplemental Figure 3. Schematic of the synthesis of diazeniumdiolated CBBs for the prolonged release of nitric oxide (NO). NO release profile can be controlled with the choice of diol, with hydrophobic diols resulting in slower NO release.