Abstract
Aquatic species inhabiting polluted estuaries are exposed to complex mixtures of xenobiotics which can alter normal reproduction. We previously reported that female Atlantic killifish (Fundulus heteroclitus) from the highly contaminated Newark Bay, NJ (USA) exhibited an inhibition of oocyte development due to reduced vitellogenin (egg-yolk precursor) levels. Our hypothesis was that the inhibition of oocyte development in Newark Bay killifish is due to (1) deficient levels of circulating 17β-estradiol, and (2) a decreased sensitivity of the vitellogenin pathway to physiological doses of 17β-estradiol. In the first study, adult naïve killifish from Tuckerton, NJ (reference) were caged at Tuckerton and Newark Bay. After 1 month, males caged at Newark Bay exhibited inductions of hepatic vitellogenin and estrogen receptor α, which were transient and returned to basal levels after 2 months (p ≤ 0.05). In the second study, fecundity and 17β-estradiol levels were measured in reproductively active adult females from Tuckerton and Newark Bay. Tuckerton females produced 140 eggs per female and Newark Bay females produced 11 eggs per female. Embryos from Newark Bay had 34% greater mortality and 28% less hatch, relative to Tuckerton. In addition, embryo mass and yolk-volume of Newark Bay embryos compared to Tuckerton embryos was 16% and 25% lower, respectively. Circulating 17β-estradiol levels in Newark Bay females (0.26 ng/mL) were measured to be 8-fold lower than Tuckerton females (2.25 ng/mL). In the third study, adult killifish from both sites were dosed with 17β-estradiol to assess the sensitivity of the vitellogenin pathway. At doses of 0.01, 0.1, 1 and 10 ng/g body weight, induction levels of circulating vitellogenin in Newark Bay males were significantly inhibited by 97, 99, 98 and 44%, respectively, compared to Tuckerton males. At doses of 0.01, 0.1, 1, 10 and 100 ng/g body weight, induction levels of circulating vitellogenin in Newark Bay females were inhibited by 89, 79, 61, 40 and 30%, respectively, compared to Tuckerton females. These differences in inducibility could not be explained by altered hepatic expression of estrogen receptors α, βa or βb. Based on the caged and dose-response studies, contaminants that down-regulate vitellogenin would interfere with its ability to be used as a biomarker for xeno-estrogen exposures. These studies demonstrate that contaminants within Newark Bay exert both estrogenic and anti-estrogenic responses which results in an overtly anti-estrogenic phenotype (reduced egg production due to inhibition of vitellogenesis).
Keywords: Fundulus heteroclitus, killifish, endocrine disruption, fish reproduction, fecundity, vitellogenin, adaptation, biomarkers, dose-response, attenuation
1.0. INTRODUCTION
Previous studies in our laboratory have demonstrated that female Atlantic killifish (Fundulus heteroclitus) inhabiting Newark Bay, NJ (USA) exhibited anti-estrogenic reproductive effects, when compared to a reference population from Tuckerton, NJ (Bugel et al., 2010). Decreased hepatic expression of vitellogenin (egg-yolk protein precursor) was proposed to be the etiology for the inhibition of normal oocyte development in Newark Bay female killifish. The purpose of the current study was to determine whether inhibition of oocyte development resulted in decreased fecundity, and to characterize contaminant effects on the regulation of circulating 17β-estradiol and vitellogenin. Atlantic killifish (Fundulus heteroclitus) were chosen for our studies because of its important role in the estuarine food web as a both predator and prey, and because it has a limited home range allowing for impacts to be related to locally found contaminants (Burnett et al., 2007).
Newark Bay and adjoining estuaries in the NY-NJ Harbor Complex contain mixtures of historical and emerging contaminants at elevated levels that pose a continued risk to the sustainability of aquatic populations. Newark Bay sediment concentrations of total polychlorinated dibenzo-p-dioxins and furans were reported to be 0.8 – 9.3 ng/g (parts per billion) and 0.2 – 3.7 ng/g, respectively (Dimou and Pecchioli, 2006). Total polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in sediments have been measured to be 44,000 and 756 ng/g, respectively (Huntley et al., 1995; Panero et al., 2005). Other contaminants of concern include pesticides, heavy metals, nonionic alkylphenol ethoxylate surfactants, flame retardants, bisphenol A, phthalates, and synthetic estrogens from effluent of approximately 30 wastewater treatment plants discharging into the NY-NJ Harbor Estuary (Litten, 2003; Muñoz et al., 2006; NY-NJ HEP, 2006; Wilson and Bonin, 2007; Iannuzzi et al., 2008). Little evidence to date has supported that the Newark Bay environment is estrogenic, although previous studies suggested that Newark Bay contaminants exert anti-estrogenic effects on killifish (McArdle et al., 2004; Bugel et al., 2010). For example, aryl hydrocarbon receptor (AhR) agonists modulate hormone signaling by cross-talk with the estrogen receptor and can affect vitellogenin gene regulation (Safe and Wormke, 2003; Wormke et al., 2003; Ohtake et al., 2008). Newark Bay is heavily contaminated with AhR agonists (dioxins, furans, PAHs, PCBs) that have resulted in elevated cytochrome P4501A (CYP1A) expression and a resistant AhR pathway in Newark Bay killifish (Prince and Cooper, 1995a/b; Elskus et al., 1999; Arzuaga and Elskus, 2002; Bugel et al., 2010; Nacci et al., 2010). Reproductive impacts by AhR agonists in Newark Bay killifish and other similarly impacted populations (New Bedford Harbor, MA; Elizabeth River, VA) are not clear.
Reproductive impacts in aquatic species exposed to complex mixtures are due to the dysregulation of multiple events in the hypothalamus-pituitary-gonad-liver axis (Arcand-Hoy and Benson, 1998; Rempel and Schlenk, 2008). Estrogen signaling and vitellogenin regulation are critical to oocyte development and are discussed below, although detailed reviews of teleosts oogenesis by Tyler and Sumpter (1996), Patiño and Sullivan (2002), and Thomas (2008), should be consulted. Gonadotropin hormone I secretion by the pituitary stimulates granulosa cells in the ovary to induce aromatase (cytochrome P450 19A1) and produce 17β-estradiol. Estrogen receptors (ER) in hepatocytes bind circulating 17β-estradiol, dimerize and translocate into the nucleus to induce transcription of vitellogenin genes (Menuet et al., 2005). Three killifish estrogen receptors have been identified: ER α, ER βa and ER βb (Greytak and Callard, 2007). All three ER isoforms are suspected to play a role in the induction of vitellogenin in teleosts, although the specific role of each isoforms in killifish vitellogenesis is not yet known (Nelson and Habibi, 2010). Vitellogenin proteins are large glycolipoproteins that are precursors to egg-yolk proteins. Two vitellogenin genes are known to exist in Fundulus heteroclitus: vitellogenins I and II (LaFleur et al., 1995; LaFleur et al., 1996). Vitellogenin I is the precursor for the majority of the known yolk-proteins in killifish. Circulating vitellogenin proteins are endocytosed into developing oocytes and cleaved by cathepsins into yolk-proteins required for oocyte growth and maturation (Kanungo et al., 1990; LaFleur et al., 2005). Yolk-proteins also act as a source of nutrients (lipids, inorganic ions, energy and free amino acids) during embryogenesis and eleuthero-embryo development. Estrogen and vitellogenin levels can be used for evaluating reproductive status in females. In male fish, vitellogenin genes are present but not expressed and therefore can serve as a biomarker of exposure to estrogenic contaminants (Sumpter and Jobling, 1995).
Three studies are presented within this paper. The first study was a caged study (2 month duration) designed to investigate impacts of Newark Bay contaminants on hepatic expression of ER α, ER βa, ER βb and vitellogenin using naïve killifish from Tuckerton, NJ. We hypothesized that transplantation of naïve killifish from the reference population (Tuckerton, NJ) to Newark Bay would result in estrogenic and anti-estrogenic responses in males and females, respectively. The caged study was used to evaluate sensitive responses in naïve killifish re-located to Newark Bay as an alternative approach to measuring impacts in the indigenous population that may have adapted responses. The second study assessed fecundity (egg production and embryo survival) as a measure of the reproductive capacity of killifish collected from Newark Bay and a reference site at Tuckerton. Circulating 17β-estradiol levels were also measured in killifish from both sites as an integrated measure of contaminant impacts on hormone signaling. In the third study, adult killifish from Newark Bay and Tuckerton were challenged with 17β-estradiol to assess the sensitivity and regulation of the vitellogenin pathway. Our working hypothesis was that impacts on egg development in Newark Bay female killifish were the result of decreased 17β-estradiol signaling and decreased responsiveness of the vitellogenin pathway to 17β-estradiol. These studies are the first to demonstrate that contaminants within Newark Bay exert both estrogenic and anti-estrogenic responses in killifish. We propose that contaminant exposure has led to the inhibition of egg development, which due to decreased steroid signaling and a decreased sensitivity of the vitellogenin pathway to 17β-estradiol.
2.0. MATERIALS AND METHODS
2.1 Site selection and necropsy and husbandry protocols
Killifish were collected from two estuaries in New Jersey, USA. Killifish were collected using baited minnow traps from a reference site in Tuckerton (Rutgers Marine Station, 39° 30' 32.52” N, 74° 19' 26.50” W) and the heavily contaminated Newark Bay (Richard Rutkowski Park, Bayonne, 40° 41' 17.0” N, 74° 06' 42.0” W). Fish were transported on the day of collection back to the laboratory in aerated water from each site. Studies coincided with the new or full moons, which are the peaks of the killifish's lunar dependent reproductive cycle (Taylor et al., 1979; Hsiao et al., 1994; Cerdá et al., 1996).
Killifish used for laboratory controlled study were housed in a recirculating system (500 gallon total, 20 ppt seawater, 25% water change daily, 20 °C), maintained on a 14:10 light:dark photoperiod and fed frozen ground squid muscle daily. System water was continually filtered through activated carbon, UV and bio-filters. Animals to be sacrificed and undergo necropsy were euthanized with an overdose of MS-222 (tricaine methanesulphonate), weighed and measured for body length. Blood was obtained by caudal severance and collected into heparinized tubes that were immediately centrifuged briefly at 14,000 × g. Plasma was transferred into a new tube and stored at −80 °C. The liver was removed, weighed, snap frozen in liquid nitrogen and maintained at −80 °C. All animal protocols were approved through the Rutgers Animal Care and Facilities Committee (Protocol #08-025).
2.2 Study 1: Biomarker study of naïve killifish caged at Newark Bay, NJ
Adult killifish from Tuckerton, NJ (5–10 g, 7–10 cm) were caged at both Tuckerton and Newark Bay for up to 2 months. The beginning of the experiment coincided with a new moon on May 24th, 2009, and two collections were made at 1 and 2 months time, each corresponding with a new moon (June 22nd, 2009, and July, 21st, 2009). Three sets of thirty killifish (15 males, 15 females) were placed into stainless steel cages at both Tuckerton and Newark Bay. Cages were 2 cubic feet (30.5 × 30.5 × 61 cm) with 1/4” (0.64 cm) mesh and were anchored just below the low tide line so that cages would be 1 foot (30.5 cm) below the surface at low tide. At 1 and 2 months, 12–15 males and females were sacrificed from each site. Vitellogenin (hepatic mRNA and circulating protein) and hepatic estrogen receptor mRNA expression (ER α, ER βa, ER βb) was evaluated.
2.3 Study 2A: Fecundity study and embryo morphometric measurements
Adult male and female killifish (4-7 g, 6-7 cm) were collected and strip spawned from Tuckerton and Newark Bay to assess fecundity (number of mature eggs per female, viability, hatching success) and embryo morphometrics (mass and yolk-volume). Fish were collected less than 48 hours before a full moon spawning (May 27th, 2010), separated by gender and held for 24 hours to prevent early spawning. Twenty females from each site were strip spawned into individual Petri dishes containing egg water (20 ppt sea water, 0.22 μm filtered, 20 °C). Eggs were fertilized using minced testis from a pool of 5 males from the respective site. Egg water was replaced after 3 hours and was replaced daily until the end of the experiment. Dead embryos and newly hatched larvae were recorded and removed daily. Yolk-volume and embryo mass were determined at 24 hours post fertilization (hpf) so that measurements were made only on fertilized and viable embryos. Photomicrographs were taken of 21 embryos from each site (7 per female, 3 females per site) to measure the diameter of each yolk-sac for calculating volume. The mass of 24 hpf embryos were also measured using pools of 10 embryos from 6 females per site.
2.4 Study 2B: 17β-estradiol analysis
Blood was collected from 50 adult females (4-7 g, 6-7 cm) each from Tuckerton and Newark Bay to assess plasma levels of 17β-estradiol 3 days prior to a peak in the reproductive cycle on a new moon (July 8th, 2010). Blood plasma levels of 17β-estradiol have been shown to be highest 3 days prior to the peak of spawning (Cerdá et al., 1996). 17β-estradiol was quantified using Coat-A-Count Estradiol Radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Plasma from 5 individuals were combined in equal volumes to make 10 composite samples per site, which were diluted 20-fold and analyzed. The detection limit was 0.02 ng/mL of circulating 17β-estradiol in blood plasma. Samples below detection were recorded at the detection limit.
2.5 Study 3: Dose-response study - vitellogenin sensitivity to a 17β-estradiol challenge
Reproductively inactive adult killifish (3–5 g, 5–7 cm) were collected from Tuckerton and Newark Bay outside of the breeding season (October, 2009) and acclimated for 1–2 weeks to laboratory conditions. Fish were injected intra-peritoneally with graded doses of 17β-estradiol (≥ 98%, Sigma-Aldrich, St. Louis, MO) to assess site-specific differences in vitellogenin regulation. Six males and females each were injected with 17β-estradiol (0.01, 0.1, 1, 10 and 100 ng/g body weight) dissolved in corn oil (10 μL/g body weight). Killifish were sacrificed 4 days post-injection because circulating vitellogenin levels have been shown to peak 4–8 days post-injection when dosed with 17β-estradiol (Pait and Nelson, 2003). Controls for this experiment included a group that was sacrificed on day 0 (non-injected) and a group that was sacrificed on day 4 (injected with corn oil alone). Hepatic mRNA expression of ER α, ER βa and ER βb were evaluated for the day 0 control group and circulating vitellogenin protein was evaluated for all treatment groups.
2.6 Protein and mRNA expression analysis
Total RNA was isolated from liver using TRIzol® (Invitrogen, San Diego, CA), treated with DNA-free (DNA-free, Ambion, Austin, TX), and reverse transcribed using a High Capacity cDNA Reverse Transcript Kit (Applied Biosystems, Foster City, CA). Quantitative polymerase chain reaction was performed on 50 ng cDNA using Bio-Rad iQ SYBR Green Supermix with a Bio-Rad iCycler and iCycler iQ Detection System (Bio-Rad, Hercules, CA). Samples were analyzed in triplicate for each gene and normalized to the population's median β-actin value. After normalization, expression was quantified using a standard curve to calculate the nanogram amount of gene template per 50 ng RNA. Primers for β-actin are the same as those used previously and new primer sets for vitellogenin I and three estrogen receptors in killifish (ER α, ER βa and ER βb) were designed using criteria described previously (Bugel et al., 2010). Vitellogenin I (GenBank U07055.2) primers (234 bp product) were F: 5′-CAG CAC CAG GAA TAT CTC AG-3′ and R: 5′-GTG TAG AGT GTG TCT TCG AC-3′. Estrogen receptor α (ER α, GenBank AY571785.1) primers (195 bp product) were F: 5′-TTT CTT TCT GCA CCG GCA CAA TGG-3′ and R: 5′-GCT CCA TGC CTT TGT TGC TCA TGT-3′. Estrogen receptor βa (ER βa, GenBank AY570922.1) primers (112 bp product) were F: 5′-ATC TTT GAC ATG CTA ATC GCC GCC-3′ and R: 5′-TCA GGC ACA TGT TGG AGT TGA GGA -3′. Estrogen receptor βb (ER βb, GenBank AY570923.1) primers (162 bp product) were F: 5′-TTG ACG CTC TGG TTT GGG CTA TCT-3′ and R: 5′-ACA CAA GCA CCA CGT TCT TCC TCT-3′. The detection limit for vitellogenin was 1.0×10−8 ng per 50 ng total RNA. Data below these values were set as the detection limit.
Vitellogenin was detected in blood plasma using a modified immunoblotting technique described previously (Bugel et al., 2010). Blood plasma was diluted 1:50 or 1:100 and separated by electrophoresis using Novex 3–8% Tris-Acetate SDS polyacrylamide gels (Invitrogen, Carlsbad, CA). Protein was transferred to a 0.45 μm PVDF Transfer Membrane (Thermo Fisher Scientific, Waltham, MA) for 1 hour using 200 mA (constant) in 1X transfer buffer (25 mM Tris-base, 192 mM glycine, 10% methanol). Membranes were then incubated at 4 °C for 20 h in 1X TBST (200 mM NaCl, 50 mM Tris, 0.1% Tween-20) with vitellogenin (striped-bass) monoclonal antibody diluted 1:2500 (ND-1C8, Cayman Chemical, Ann Arbor, MI). Membranes were washed 3 times for 5 min each in 1X TBST and then incubated for 30 min at room temperature in 1X TBST with 5% non-fat milk and goat anti-mouse IgG1-HRP secondary antibody sc-2969 diluted 1:5000 (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were finally washed 5 times for 5 min each in 1X TBST and once with deionized ultrafiltered water for 5 min. The Amersham ECL Advance Western Blotting Detection Kit (RPN2135, GE Healthcare, Buckinghamshire, UK) was used to detect immunoreactivity with CL-X Posure Film (Thermo Fisher Scientific, Waltham, MA). Bands were quantified by densitometry with ImageJ (Abramoff et al., 2004) and converted to relative intensity to a standard rainbow trout vitellogenin sample (Vtg-51, Biosense Laboratories, Bergen, Norway).
2.7 Statistical analyses
Statistical tests were performed using SigmaPlot™ (v. 11.0). Data is reported as mean ± standard deviation. Fold-changes are calculated using median values. A p-value ≤ 0.05 was regarded as significantly different. Unpaired t-tests were used to compare different treatments. When normality failed the Student-Neuman-Keuhls test was used. When equal variance failed the Mann-Whitney Rank Sum test was used. Discrete data was compared using Fisher's Exact Test.
3.0. RESULTS
3.1. Study 1: Endocrine responses of Naïve Tuckerton killifish caged at Newark Bay
Adult naïve killifish from Tuckerton were caged at Tuckerton and Newark Bay for up to 2 months to investigate the effects of contaminant exposure on expression of vitellogenin, ER α, ER βa and ER βb. Mortality was low for fish caged at both sites for 1 month, 59 of 60 fish survived at Tuckerton (98% survival) and 57 of 60 fish survived at Newark Bay (95% survival). Survival was decreased at Newark Bay after 2 months, although not significant. After two months, 23 of 30 (77%) fish survived at Tuckerton and 30 of 54 fish (56%) survived at Newark Bay. Animals were feeding at both sites and during both collection times (determined by presence of food in the gastrointestinal tract). No significant differences or changes in body morphometrics (liver and gonad organ weights, body lengths, and organ to body weight ratios) were observed between killifish caged at Tuckerton and Newark Bay for either gender after 1 and 2 months caging time, or from month to month (data not shown).
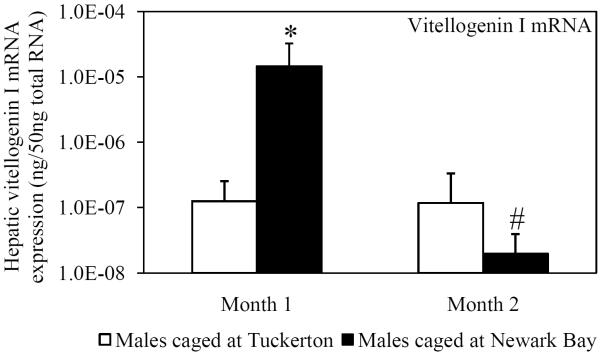
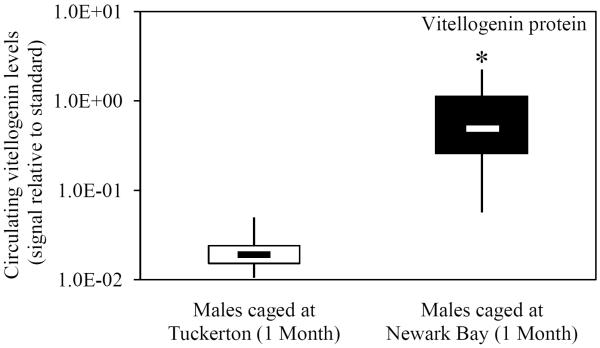
Vitellogenin expression (hepatic mRNA and circulating protein) was assessed in naïve Tuckerton male killifish caged at Tuckerton and Newark Bay, as a biomarker of exposure to estrogen mimics. Males caged at Newark Bay for 1 month exhibited significantly elevated levels of hepatic vitellogenin mRNA expression that were 53-fold higher than levels measured in killifish caged at Tuckerton (Fig. 1A). After 2 months, hepatic vitellogenin mRNA expression in males caged at Newark Bay returned to reference levels (Fig. 1A). Circulating vitellogenin protein was detected in 5 of 7 males caged at Newark Bay after 1 month while no males in the Tuckerton group (N = 7) expressed detectable levels (Fig. 1B). After 2 months, circulating vitellogenin protein was not detected in any males caged at either site (N = 7 per group).
Fig. 1.
(A) Hepatic vitellogenin mRNA expression for naïve males caged at Tuckerton and Newark Bay for 1 and 2 months. (B) Circulating levels of vitellogenin protein in naïve Tuckerton males after 1 month. No vitellogenin protein was detected in males after 2 months. Killifish were caged starting May 24th, 2009, for 1 or 2 months duration with collections on June 22nd and July, 21st, 2009. Bar graphs are presented as mean ± standard deviation. The box-and-whisker plot represents the minimum and maximum values and the median, lower and upper quartiles. N = 6-7 individuals per group. *Significantly different from Tuckerton (p ≤ 0.05). #Significantly different between months within the respective site and gender (p ≤ 0.05).
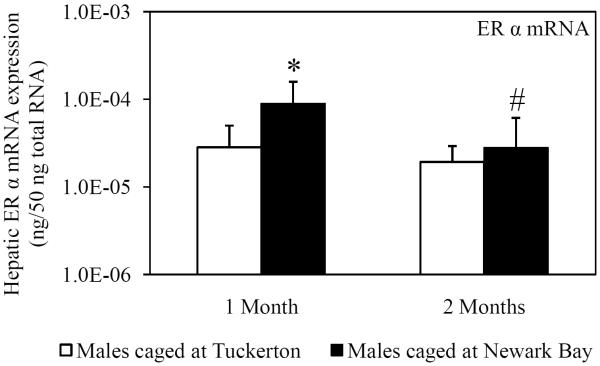
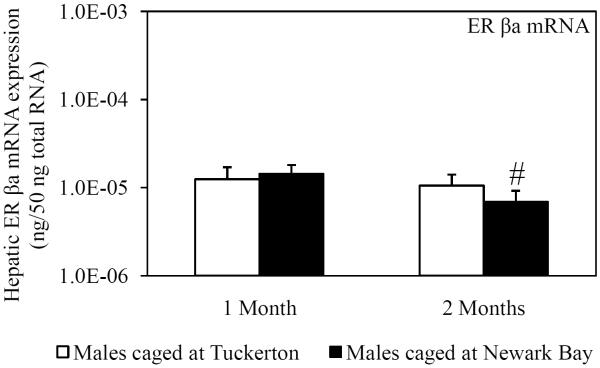
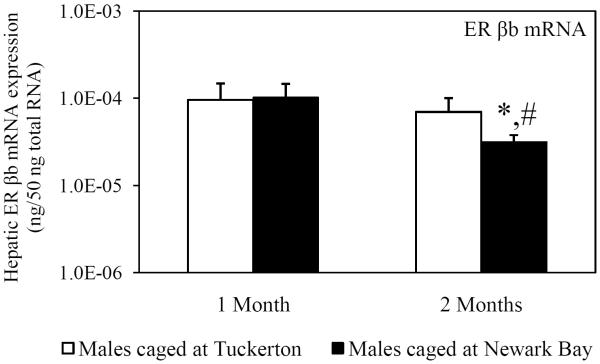
Hepatic mRNA expression of ER α, ER βa and ER βb was measured in killifish caged at both sites to investigate the potential for Newark Bay contaminants to alter expression of the estrogen receptors. ER α expression served as a second biomarker of xeno-estrogen exposure since ER α has been shown to be inducible by 17β-estradiol (Urushitani et al., 2003). Expression of ER α in males was significantly elevated 2.4-fold in males caged at Newark Bay for 1 month, relative to males caged at Tuckerton (Fig. 2A). The expression of ER α returned to basal levels after 2 months and was not different between males caged at Tuckerton and Newark Bay (Fig 2A). Expression of ER βa and ER βb was not affected in killifish caged at Newark Bay for 1 month (Fig. 2B and 2C). Hepatic expression of ER α, ER βa and ER βb decreased significantly in killifish caged at Newark Bay by 3.2, 2.0, and 3.0-fold, respectively, from month 1 to month 2 (Fig. 2A, 2B and 2C). However, only expression of ER βb was significantly lower than levels measured in killifish caged at Tuckerton for 2 months (Fig. 2C).
Fig. 2.
Hepatic mRNA expression of (A) ER α, (B) ER βa and (C) ER βb in naïve males caged at Tuckerton and Newark Bay for 1 and 2 months. Killifish were caged starting May 24th, 2009, for 1 or 2 months duration with collections on June 22nd and July, 21st, 2009. Bar graphs are presented as mean ± standard deviation. N = 6-8 individuals per group. *Significantly different from Tuckerton (p ≤ 0.05). #Significantly different between months within the respective site and gender (p ≤ 0.05).
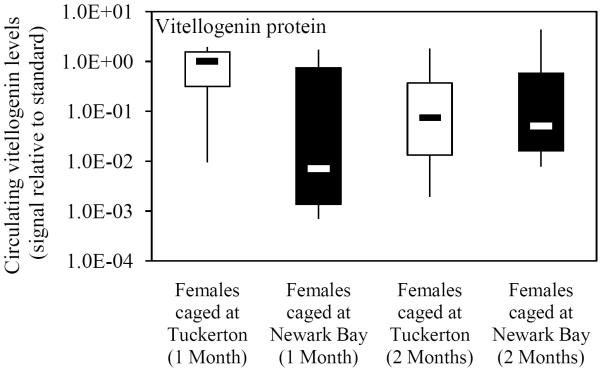
Hepatic vitellogenin mRNA expression and circulating protein levels were evaluated in females caged at Tuckerton and Newark Bay as a measure of contaminant impacts on vitellogenesis. Females caged at Newark Bay exhibited no significant change in hepatic vitellogenin mRNA expression or circulating protein levels after 1 and 2 months exposure time, relative to females caged at Tuckerton (Figs. 3A and 3B). Hepatic vitellogenin mRNA expression at each site decreased from month-to-month (Fig. 3A).
Fig. 3.
(A) Hepatic vitellogenin mRNA expression for naïve females caged at Tuckerton and Newark Bay for 1 and 2 months. (B) Circulating levels of vitellogenin protein in naïve females after 1 and 2 months. Killifish were caged starting May 24th, 2009, for 1 or 2 months duration with collections on June 22nd and July, 21st, 2009. Bar graphs are presented as mean ± standard deviation. The box-and-whisker plot represents the minimum and maximum values and the median, lower and upper quartiles. N = 6-7 individuals per group. #Significantly different between months within the respective site and gender (p ≤ 0.05).
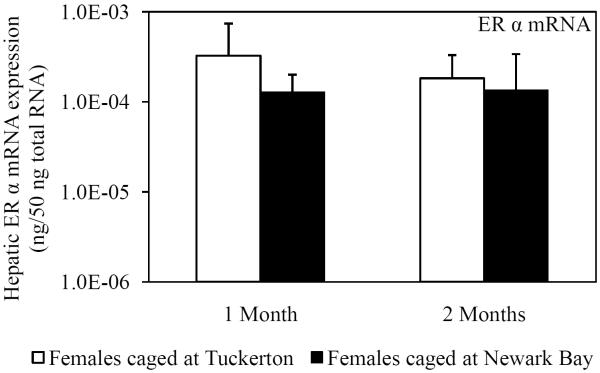
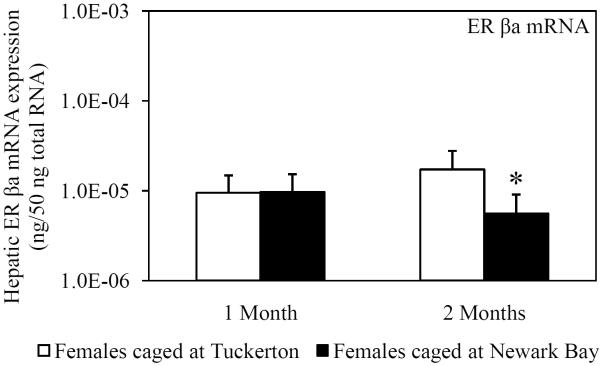
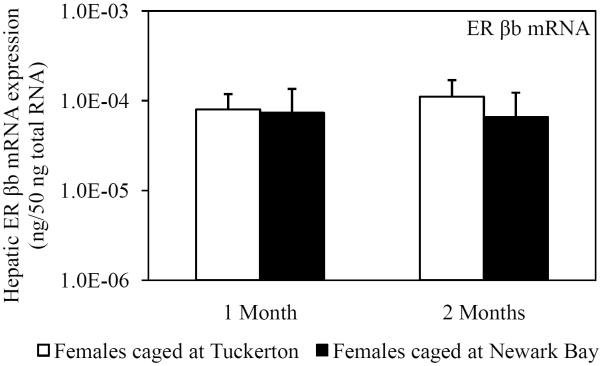
Hepatic mRNA expression of ER α, ER βa and ER βb was not affected in female killifish caged at Newark Bay after 1 month and did not change from month-to-month in killifish caged at either site (Fig. 4A, 4B and 4C). Expression of ER βa in females caged at Newark Bay for 2 months was significantly lower by 2.8-fold relative to levels measured in females caged at Tuckerton (Fig. 4B). There were no significant differences in expression of ER α and βb between females caged at Tuckerton and Newark Bay for 2 months.
Fig. 4.
Hepatic mRNA expression of (A) ER α, (B) ER βa and (C) ER βb in naïve females caged at Tuckerton and Newark Bay for 1 and 2 months. Killifish were caged starting May 24th, 2009, for 1 or 2 months duration with collections on June 22nd and July, 21st, 2009. Bar graphs are presented as mean ± standard deviation. N = 6-8 individuals per group. *Significantly different from Tuckerton (p ≤ 0.05).
3.2 Study 2A: Population productivity: egg production, quality and embryonic survival
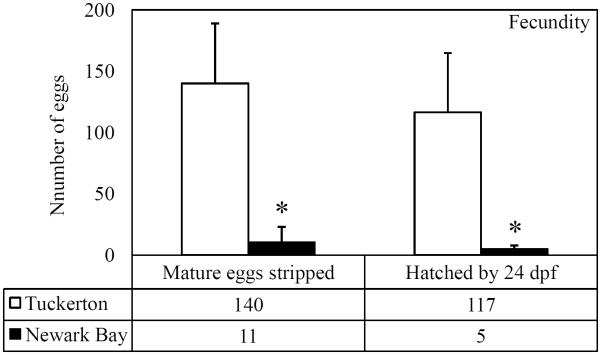
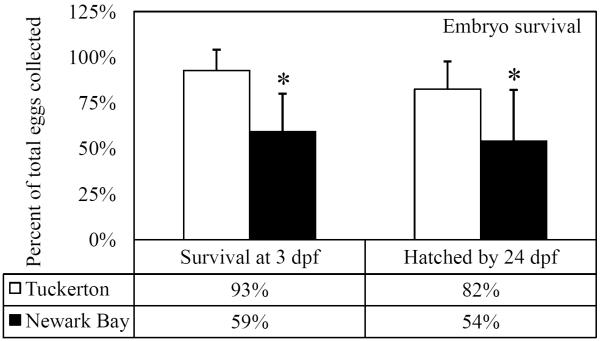
A fecundity study was performed using adult killifish collected from Tuckerton and Newark Bay to directly measure the reproductive capacity of each population and to assess the quality of eggs and embryos. All eggs that were evaluated were fertilized and proceeded through early embryonic stages. Newark Bay females produced significantly fewer mature eggs compared to Tuckerton females (Fig. 5A). In addition, significantly fewer embryos from Newark Bay successfully hatched by 24 days post fertilization (dpf), relative to Tuckerton embryos (Fig. 5A). Tuckerton killifish produced 140 ± 49 mature eggs per female and Newark Bay females produced 11 ± 13 mature eggs per female (92% fewer). Tuckerton females averaged 117 ± 48 embryos hatch per female by 24 dpf while Newark Bay females averaged 5 ± 3 embryos hatch per female by 24 dpf (95% fewer). Survival was not significantly affected in Tuckerton embryos, however Newark Bay embryos had a significant decrease in survival before 3dpf (Fig. 5B). Mortality in Newark Bay embryos was primarily observed throughout the first 3 days of development (59% of total mature eggs were viable compared to Tuckerton's 93%) and had little change in survival by 24 dpf. Of the total eggs strip spawned, 82% at Tuckerton hatched by 24 dpf and 54% hatched from Newark Bay (Fig. 5B).
Fig 5.
(A) Total number of eggs stripped from each site on the peak of the lunar cycle and number of embryos to hatch by 24dpf. (B) Percentage of total stripped-spawned eggs to survive 3 dpf and to hatch by 24 dpf. Data are presented as mean ± standard deviation. N = 20 females stripped per site. Eggs were fertilized with minced testis from 5 males from the respective site. *Significantly different from Tuckerton (p ≤ 0.05).
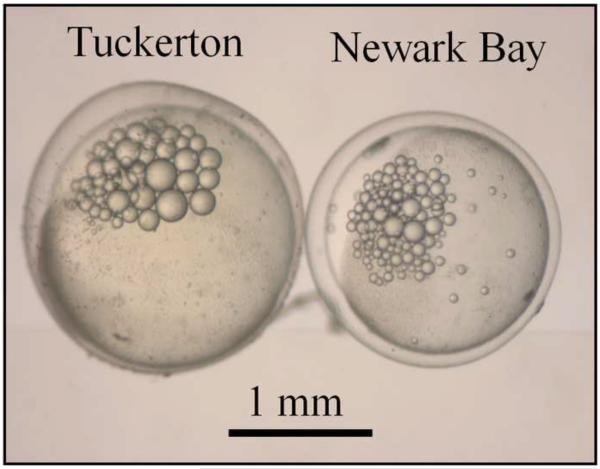
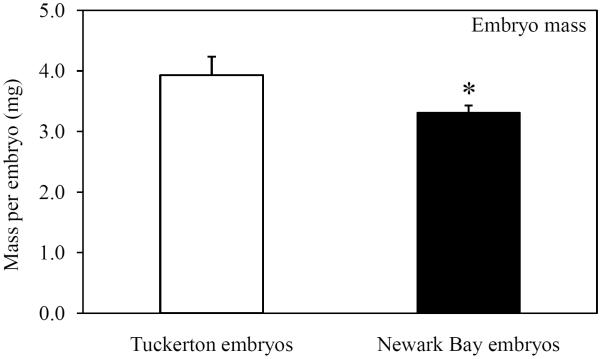
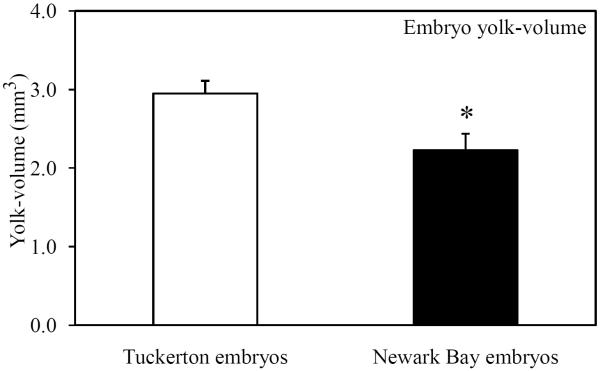
Embryo morphometric parameters (yolk volume and embryo mass) were measured at 24 hpf to investigate impacts on yolk development. Newark Bay embryos were visually smaller than Tuckerton embryos and appeared to have decreased yolk-volume (Fig. 6A). Quantitative measurements were made at 24 hpf so that only viable embryos were used. Embryo mass and yolk-volume were significantly decreased in Newark Bay embryos by 16 and 25%, respectively (Fig. 6B and 6C). The mean embryo mass at Newark Bay was 3.3 ± 0.1 mg per embryo, while at Tuckerton it was 3.9 ± 0.3 mg per embryo (Fig. 6B). The mean yolk-volume at Newark Bay was 2.2 ± 0.2 mm3 per embryo compared to Tuckerton embryos which were 3.0 ± 0.2 mm3 per embryo (Fig. 6C).
Fig. 6.
(A) Representative photomicrograph of embryos taken side-by-side from each site at 24 hpf. (B) Embryo-mass and (C) yolk-volume of viable embryos at 24 hpf. Data are presented as mean ± standard deviation. N = 6 pools of 10 embryos for mass measurements (6 different females from each site). N = 21 for yolk-volume measurements (7 embryos from 3 different females from each site). *Significantly different from Tuckerton (p ≤ 0.05).
3.3 Study 2B: Circulating 17β-estradiol prior to spawning
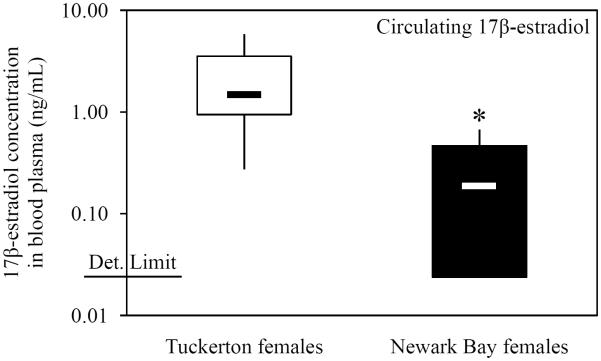
Circulating 17β-estradiol levels were measured in females from Tuckerton and Newark Bay three days prior to the peak of spawning to investigate the role of 17β-estradiol levels in the inhibition of oocyte development and decreased vitellogenin levels in Newark Bay females. Circulating levels of 17β-estradiol are highest at this point in the killifish reproductive cycle (Cerdá et al., 1996). Concentrations of 17β-estradiol in blood plasma of Newark Bay females were 8-fold significantly lower (than Tuckerton females (Fig. 7). Mean concentrations of circulating 17β-estradiol were 2.25 ± 1.78 ng/mL in Tuckerton females and 0.26 ± 0.26 ng/mL in Newark Bay females. Five of 10 composite samples analyzed from Newark Bay fell below the detection limit (0.02 ng/mL) and were recorded as the detection level.
Fig. 7.
Circulating 17β-estradiol levels in the blood plasma of female killifish 3 days prior to the peak of spawning. The box-and-whisker plot represents the minimum and maximum values and the median, lower and upper quartiles. The detection limit is shown on the axis (0.02 ng/mL). N = 10 pools of 5 individuals from each site. *Significantly different from Tuckerton (p ≤ 0.05).
3.4 Study 3: Regulation of vitellogenin protein by a 17β-estradiol challenge
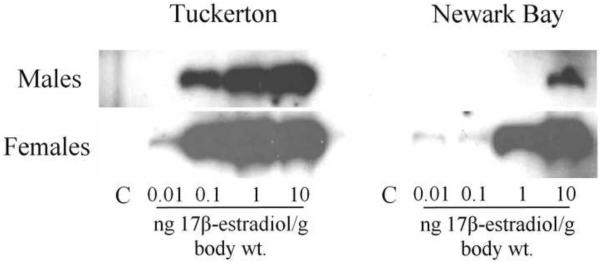
Adult male and female killifish from the Tuckerton and Newark Bay populations were given intra-peritoneal injection with graded doses of 17β-estradiol to assess the sensitivity and ability of the vitellogenin pathway to produce protein in response to an estrogen challenge. Vitellogenin was not detected in either control groups on day 0 (no injection) and day 4 (oil injection) for both males and females from both sites. Males and females from both sites were inducible with all doses tested (0.01, 0.1, 1, 10 and 100 ng/g body weight). However, killifish from Newark Bay were less responsive and had a dose-response shifted to the right, compared to Tuckerton killifish (Fig 8A).
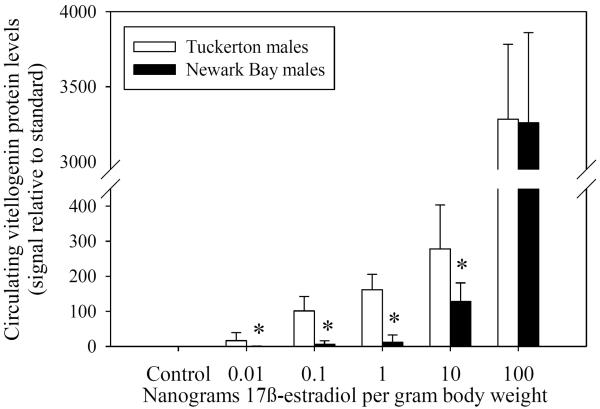
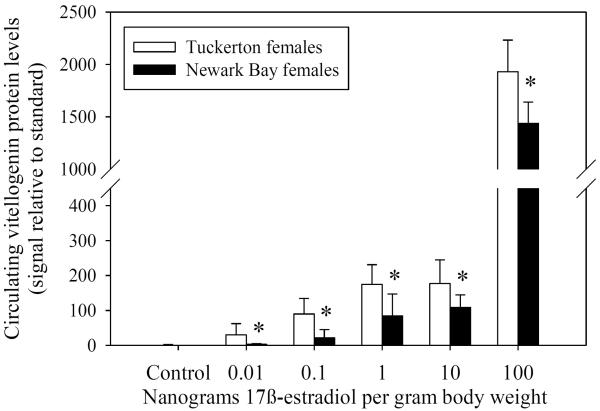
Fig. 8.
(A) Western blots for doses 0.01 ng – 10 ng 17β-estradiol/g body weight in males and females. Each sample is a composite of 3 individuals for each treatment group. Dose-response of vitellogenin protein induction by 17β–estradiol in reproductively inactive (B) males and (C) females from both Tuckerton and Newark Bay. N = 6 per treatment group. Data are reported as mean ± standard deviation. *Significantly different from the Tuckerton at p ≤ 0.05 among each treatment group. All treatment groups were significantly higher than control groups (no vitellogenin detected).
Newark Bay males produced significantly less circulating vitellogenin protein in response to a 17β-estradiol challenge than males from Tuckerton at concentrations of 0.01 to 10 ng 17β-estradiol per gram body weight (Fig. 8B). At doses of 0.01, 0.1, 1 and 10 ng/g body weight, Newark Bay males exhibited induction levels of circulating vitellogenin protein that were lower by 32.6, 139.0, 53.9 and 1.8-fold, respectively, than induction levels in Tuckerton males. Induction of vitellogenin in Newark Bay males at doses of 0.01, 0.1, 1 and 10 ng/g body weight were therefore inhibited by 96.9, 99.3, 98.1 and 44.2%, respectively. At the highest dose tested (100 ng/g body weight), there were no differences in vitellogenin levels between Tuckerton and Newark Bay males.
Female killifish from Newark Bay also produced significantly less circulating vitellogenin protein in response to 17β-estradiol at all doses tested, compared to Tuckerton females (Fig. 8C). At doses of 0.01, 0.1, 1, 10 and 100 ng/g body weight, females from Newark Bay exhibited induction levels of circulating vitellogenin protein that were lower by 9.0, 4.8, 2.5, 1.7 and 1.4-fold, respectively, than induction levels in Tuckerton females. Induction of vitellogenin in Newark Bay females at doses of 0.01, 0.1, 1, 10 and 100 ng/g body weight were therefore inhibited by 88.9, 79.3, 60.5, 39.5 and 30.2%, respectively.
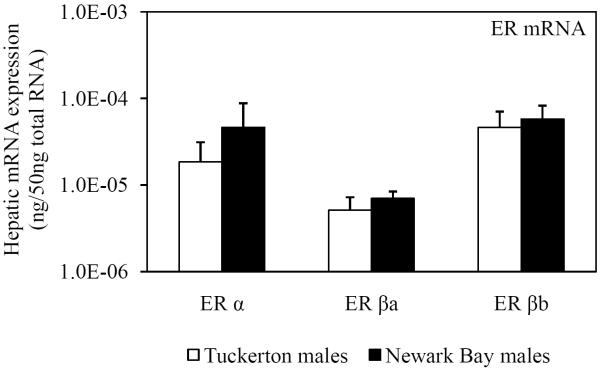
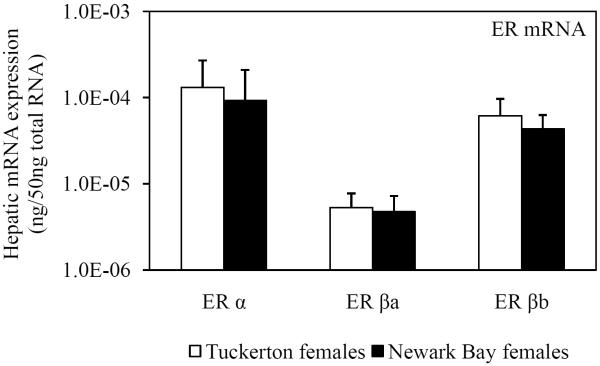
As a result of the shifted-dose response for induction of circulating vitellogenin protein by 17β-estradiol, hepatic mRNA expression of ER α, ER βa and ER βb was assessed in Tuckerton and Newark Bay killifish. Expression prior to treatment (day 0 controls) acted as a surrogate measure of basal estrogen receptor levels. However, no significant differences were found between Tuckerton and Newark Bay killifish for either gender on day 0 for ER α, ER βa and ER βb (Fig. 9).
Fig. 9.
Day 0 (pre-injection) hepatic mRNA expression of three estrogen receptors (ER α, ER βa, ER βb) in reproductively inactive (A) male and (B) female killifish from both Tuckerton and Newark Bay. N = 6 per treatment group. Data are reported as mean ± standard deviation.
4.0. DISCUSSION
Endocrine disruption at Newark Bay is a complex interaction of estrogenic and anti-estrogenic stressors that result in an overtly anti-estrogenic phenotype in Newark Bay killifish. Estrogenic responses (vitellogenin and ER α expression) in naïve males transplanted to Newark Bay were transiently induced before returning to basal levels over time. This demonstrated that Newark Bay is an acutely estrogenic environment with anti-estrogenic impacts resulting from prolonged exposure. In addition, we demonstrated that female killifish inhabiting Newark Bay exhibited decreased fecundity (number of mature eggs per female, viability, hatching success), as a result of (1) decreased 17β-estradiol signaling and (2) a decreased sensitivity of the vitellogenin pathway to a 17β-estradiol challenge. Impacts on oogenesis (yolk-development) in female killifish are proposed to result from contaminants that inhibit steroidogenesis and vitellogenesis, which cumulatively result in decreased vitellogenin expression.
4.1. Endocrine responses in naïve Tuckerton killifish caged at Newark Bay
Elevated levels of vitellogenin have not been detected in male killifish inhabiting Newark Bay or from naïve killifish injected intra-peritoneally with Newark Bay sediment extracts (McArdle et al., 2004; Bugel et al., 2010). These two separate studies speculated that the lack of an estrogenic response was due to either (1) insufficient levels of xeno-estrogens in Newark Bay to induce a vitellogenin response or (2) the presence of contaminants in Newark Bay that act antagonistically with xeno-estrogens (i.e. AhR agonists). These studies concluded that Newark Bay was not an overtly estrogenic environment, despite the presence of known xeno-estrogens (i.e. dioxin like compounds, PCBs, PAHs, synthetic estrogens, etc.). The caged study presented in this paper addressed the limitations of measuring vitellogenin expression in indigenous males that may have developed altered responses from chronic exposure to contaminants. We demonstrated that Newark Bay is an estrogenic environment that can transiently induce hormonal responses, which were down-regulated over time. Elevated expression levels of vitellogenin and ER α in naïve male Tuckerton killifish caged at Newark Bay for 1 month were evidence of feminization (Figs. 1 and 2). However, these inductions were transient and expression of both vitellogenin and ER α returned to basal levels after 2 months (Figs. 1 and 2). It is unclear whether the down-regulation of ER α plays a role in the down-regulation of vitellogenin because ER α expression did not decrease below basal levels after 2 months (Fig. 2). In addition, the impacts on expression of the ER β isoforms and relevance to vitellogenin regulation are not clear but may indicate contaminant effects on these receptors. These results help to explain the lack of feminization of the indigenous male killifish population in Newark Bay and suggest that the vitellogenin pathway has adapted to be non-responsive in this estrogenic environment. This is the first evidence to our knowledge to suggest that vitellogenin may not be an appropriate biomarker for xeno-estrogen exposure in a wild fish population chronically exposed to complex mixtures of contaminants. Therefore, we recommend that studies, such as transplant studies, should follow negative vitellogenin results (not up-regulated) to reduce the occurrence of false-negatives in environments known to contain xeno-estrogens.
Our caged study was unable to determine the basis for the down-regulation of the elevated vitellogenin and ER α expression in males caged at Newark Bay. However, studies by Nash et al., (2004) reported that male zebrafish develop attenuated vitellogenic responses to 17α-ethynylestradiol after life-long exposure to environmentally relevant concentrations (5 ng/L). Desensitization of estrogenic responses may therefore be a normal outcome of chronic exposure to xeno-estrogens. Alternatively, reductions in hepatic expression of vitellogenin and ER α have been reported in female fathead minnows (Pimephales promelas) exposed to sediments impacted by pesticides, resulting in a similar down-regulation of vitellogenin and ER α (Sellin et al., 2010). Further work is necessary to determine the chemical and biological reasons for down-regulation of estrogenic responses in Newark Bay killifish.
Our caged study is the first to demonstrate that killifish in Newark Bay are exposed to xeno-estrogens that can illicit estrogenic responses. We previously reported decreased spermatocytes and interstitial fibrosis in testis of male killifish from Newark Bay (Bugel et al., 2010). Exposure to estrogen mimics may help explain these reproductive impacts in the indigenous male population. Several studies have demonstrated that exposure to estrogenic compounds impairs gonadal development and testis function in fish (Jobling et al., 1996; Nash et al., 2004; Kidd et al., 2007). Other studies investigating endocrine disruption within the NY-NJ Harbor Estuary have also reported feminization of nearby fish populations. Studies by Baldigo et al. (2006) reported elevated vitellogenin levels and altered 17β-estradiol/11-ketotestosterone ratios in common carp (Cyprinus carpio), bass (Micropterus salmoides and Micropterus dolomieui) and bullhead (Ameiurus nebulosus) in the nearby Hudson River (NY). Studies by McArdle et al. (2000) and Todorov et al. (2002) demonstrated that larval and juvenile sunshine bass (Morone saxatilis x M. Chrysops) exposed to effluent from several sewage treatment plants serving New York City exhibited significant elevations in plasma vitellogenin levels. Endocrine disruption resulting from xeno-estrogen exposure is likely to be widespread in fish populations throughout the NY-NJ Harbor Estuary.
The down-regulated vitellogenin response observed in naïve male killifish caged at Newark Bay could be related to the anti-estrogenic reproductive impacts (decreased vitellogenin mRNA and protein expression) that were previously reported in female killifish inhabiting Newark Bay (Bugel et al., 2010). However, the caged study was unable to replicate these effects in naïve female killifish caged at Newark Bay. Vitellogenin levels (mRNA and protein) in naïve female killifish caged at Newark Bay were not significantly different than those caged at Tuckerton after 1 and 2 months exposure (Figs. 3A and 3B). Hepatic expression of ER α, and ER βb in female killifish was unaffected by transplantation into Newark Bay while expression of ER βa was significantly lower after 2 months, but the biological significance of this change (2.8-fold) is not clear (Fig. 4A, 4B and 4C). We propose that vitellogenin and estrogen receptor regulation in females was relatively unaffected by caging at Newark Bay for two reasons. First, two months exposure to contaminants within Newark Bay may not be enough to alter the reproductive status of female naïve killifish. Second, killifish were caged at Newark Bay after the reproductive cycle had initiated, and in 2 months contaminants did not interfere with hormonal signaling that were already in place. Events during ovarian recrudescence may be more sensitive to disruption by contaminants. We therefore recommended that future studies consider beginning caged-studies prior to the onset of oogenesis when investigating impacts on female fish.
4.2. Impacts on fecundity and yolk development in Newark Bay killifish
The fecundity study demonstrated that Newark Bay killifish have a significantly reduced capacity for reproduction which was due primarily to the reduction in egg production, rather than loss of embryos during development. The inhibition of follicular development in Newark Bay killifish reported by Bugel et al., (2010) correlated with a 92% reduction in egg production, relative to Tuckerton females (Fig. 5). In addition, many Newark Bay embryos did not survive to hatching due to high mortality in the first 3 days of development (Fig. 5). The high mortality in Newark Bay embryos may have been due to both elevated contaminant levels and poor embryo quality (reduced size and yolk supply). Elevated body burdens of contaminants found in the sediments and wildlife of Newark Bay (i.e. 2,3,7,8-tetrachlorodibenzo-p-dioxin, furans, PCBs) correlate with early life stage mortality in other fish populations (Casillas et al., 1991; Fitzsimmons, 1995; Zabel et al., 1995). Despite dramatic impacts on egg production, the Newark Bay killifish population continues to sustain itself, as is evidenced by young-of-the-year. A small percentage of the population appears to perform the majority of the reproductive duties and may be why this population persists. The three females with the highest fecundity at Newark Bay produced 47, 25 and 22 eggs with a population mean of 11 ± 13 eggs per female. The three females with the lowest fecundity in the reference population at Tuckerton produced 91, 94 and 95 eggs each with a population average of 140 ± 49 eggs per female. The few females at Newark Bay reproducing at a relatively higher capacity may represent non-responders that are relatively resistant to contaminant effects.
An inverse relationship between vitellogenin expression and fecundity in the fathead minnow has been established, suggesting that impacts on vitellogenesis directly translates to a reduction in egg production and population growth (Miller et al., 2007; Thorpe et al., 2007). Reduced vitellogenin expression in Newark Bay killifish has been previously associated with underdeveloped ovaries. The inhibition of development was demonstrated by many follicles present at early pre-vitellogenic stages and few that progressed through vitellogenin-dependent stages (Bugel et al., 2010). The relatively few eggs that are produced by Newark Bay females are less robust and lower quality, than those from Tuckerton (Fig. 6). Newark Bay embryo mass and yolk-volume was demonstrated to be reduced by 25% and 16%, respectively, relatively to Tuckerton embryos. Vitellogenin accumulation by developing oocytes largely determines size and quality of eggs and is estimated to account for 50% of the final egg size in killifish (Selman and Wallace, 1983). Therefore, the reduced vitellogenin levels in Newark Bay females are proposed to be the basis for the impacts on egg and yolk-development.
4.3. Role of altered 17β-estradiol signaling in reproductive impacts of Newark Bay killifish
Decreased vitellogenin expression in Newark Bay females is proposed to be the cumulative result of (1) deficiency of circulating 17β-estradiol and (2) desensitization of the vitellogenin pathway to induction by 17β-estradiol. Newark Bay females had an 8-fold decrease in circulating 17β-estradiol levels relative to Tuckerton females, indicating impacts on hormone regulation (Fig. 7). Studies with the fathead minnow by Ankley et al. (2008) established a relationship between circulating 17β-estradiol levels and both vitellogenin and fecundity. These studies demonstrate that impacts on circulating 17β-estradiol levels correlate with reductions in vitellogenin and fecundity. In addition, our challenge studies showed that vitellogenin was less inducible in Newark Bay killifish relative to Tuckerton killifish, which indicated a decrease in sensitivity of the vitellogenin pathway to 17β-estradiol (Fig. 8). This shifted dose-response confounds the use of vitellogenin as a biomarker for xeno-estrogen exposure in males and helps to explain why vitellogenin has not been previously detected in indigenous Newark Bay male killifish. In females, a decreased capacity to produce vitellogenin offers a unique explanation for the decreased vitellogenin expression levels and impaired egg development. Newark Bay females exhibited inhibited induction at levels of 17β-estradiol (0.1 – 100 ng/g body weight) that are relevant to those measured during reproduction in Tuckerton killifish (2.25 ng/mL blood plasma). A linear regression model formulated to predict fecundity from measured vitellogenin levels in fathead minnow estimated that a 50% decrease in vitellogenin concentration in females translates to an approximate 50% decrease in fecundity (Miller et al., 2007). The direct relationship between vitellogenin and fecundity is important considering vitellogenin levels in Newark Bay females were 88.9, 79.3 and 60.5% the induction levels of Tuckerton females, at doses of 0.01, 0.1, 1 ng 17β-estradiol/g (Fig. 8). The decrease in vitellogenin inducibility of Newark Bay killifish is biologically important and will act in conjunction with the 17β-estradiol deficiency to result in decreased vitellogenin expression and reduced egg production.
The decreased 17β-estradiol levels in Newark Bay females were contrary to what was expected considering ovarian aromatase (cytochrome P450 19A) mRNA expression that was previously measured to be 210-fold higher than Tuckerton females (Bugel et al., 2010). Gonadal aromatase is stimulated by gonadotropin hormone I and was expressed at elevated levels, but failed to produce normal circulating levels. Circulating levels of 17β-estradiol are balanced by aromatase synthesis in granulosa cells of the ovary, and clearance by the liver and kidneys. Levels of 17β-estradiol in blood are an integrated measure of the ability of Newark Bay killifish to achieve normal circulating sex steroid levels. Our studies are unable to determine whether the decreased 17β-estradiol levels were due to a decrease in aromatase activity (synthesis), or an increase in 17β-estradiol metabolism (clearance). Future studies will need to examine the basis for this deficiency.
The expression levels of ER α, ER βa and ER βb in killifish prior to treatment with 17β-estradiol in the challenge study are unlikely to explain differences in vitellogenin inducibility. No differences in expression levels for any of the isoforms measured were found between killifish from Newark Bay and Tuckerton, for males or females (Fig. 9). However, in the caged study, exposure to Newark Bay contaminants led to the induction and down-regulation of ER α expression to basal levels after 1 and 2 months, respectively (Fig. 1). Therefore, contaminants at Newark Bay have the ability to modulate ER α responses but with unclear consequences on the regulation of vitellogenin regulation.
Overall, we conclude that impacts on egg production and oocyte development in Newark Bay females are due to insufficient vitellogenin expression levels in the liver. The reduced vitellogenin expression is inadequate for yolk-development, and results from a combination of both altered regulation of vitellogenin and 17β-estradiol deficiency. Contaminants at Newark Bay alter various reproductive pathways that cumulatively result in an anti-estrogenic phenotype in females. The contaminants responsible for these effects are unknown. However, Newark Bay is heavily contaminated by AhR agonists, and reproductive processes in the liver and the ovary are known to be direct targets for a variety of AhR agonists (Hutz, 1999). Killifish within Newark Bay exhibit elevated basal levels of hepatic CYP1A expression (mRNA, protein, activity) indicative of exposure to AhR agonists (Prince and Cooper, 1995a/b; Bugel et al., 2010). Chronic exposure to AhR agonists at Newark Bay has also led to a resistance of CYP1A induction, although the mechanisms for this resistance are currently unknown (Prince and Cooper, 1995a/b; Elskus et al., 1999; Arzuaga and Elskus, 2002; Nacci et al., 2010). We have previously reported an inverse relationship between hepatic expression of CYP1A protein and vitellogenin mRNA demonstrating a potential relationship between the AhR and ER pathways in Newark Bay female killifish (Bugel et al., 2010). Exposure to AhR agonists may therefore play a role in the reproductive impacts of the Newark Bay population. For example, rodents exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin exhibited decreased serum levels of 17β-estradiol and early transition to reproductive senescence (Petroff et al., 2000; Franczak et al., 2006; Shi et al., 2007). Zebrafish chronically exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin exhibited decreased sensitivity to gonadotropins, decreased 17β-estradiol and vitellogenin synthesis, and ultimately an inhibition of follicle development (King Heiden et al., 2006; King Heiden et al., 2008; King Heiden, 2009). There is mounting evidence from in vitro studies that AhR agonists can directly affect vitellogenin levels by inhibiting induction by 17β-estradiol in teleost hepatocytes (Anderson et al., 1996; Navas and Segner, 2000; Bemanian et al., 2004; Mortensen and Arukwe, 2007; Gräns et al., 2010). However, in vivo studies reporting direct effects of AhR agonists on liver regulation of vitellogenin is limited. For example, Vaccaro et al. (2005) demonstrated that acute exposure to PCB 126 can inhibit E2-induced vitellogenin synthesis in sea bass (Dicentrarchus labrax). The attenuation of vitellogenin in males caged at Newark Bay for 2 months may be explained by increased exposure to AhR agonists. The shifted dose-response in Newark Bay killifish may also be explained by inhibition from the highly active AhR pathway in this population. Reproductive impacts in the Newark Bay killifish population are phenotypic of dioxin toxicity and further studies are necessary to establish direct evidence for the role of AhR agonists in the endocrine disruption of this population.
A population of killifish from the heavily PCB-contaminated New Bedford Harbor (MA) has exhibited similar impacts on vitellogenesis and steroidogenesis as those from Newark Bay, although with several distinct differences. Male killifish from New Bedford Harbor are feminized, unlike those from Newark Bay, indicating contaminants at New Bedford Harbor result in a stable up-regulation of vitellogenin expression (Greytak et al., 2005). Our caged study demonstrated that Newark Bay is estrogenic, although estrogenic responses (vitellogenin and ER α expression) are transient and down-regulate over time. Female killifish from New Bedford Harbor, similar to those from Newark Bay, exhibit decreased vitellogenin expression (mRNA and protein), decreased gonadal somatic index, and decreased circulating 17β-estradiol levels (Greytak et al., 2005). Challenge studies with 17β-estradiol demonstrated that larval killifish from New Bedford Harbor have a shifted dose-response for ER α expression, but exhibit normal vitellogenin inducibility (Greytak et al., 2010). The relevance of these impacts to the formation of eggs and reproductive success of females at New Bedford Harbor are not clear because fecundity has been shown to be normal and not impacted (Black et al., 1998). Naïve killifish caged at Newark Bay were shown to down-regulate ER α expression to basal levels over time, and impacts on ER α regulation are not as clear as those in New Bedford Harbor killifish. Also strikingly different, is that killifish from New Bedford Harbor did not exhibit a shifted dose-response for induction of vitellogenin in larval fish or adult males. Decreased vitellogenin levels in adult female killifish from New Bedford Harbor are likely related to altered regulation of ER α and a deficiency in 17β-estradiol levels (Greytak and Callard, 2007). Impacts on oocyte development at Newark Bay are thought to be due to both altered regulation of vitellogenin and 17β-estradiol deficiency. Future studies in these two populations will generate new interest in site-specific comparisons of contaminant impacts on killifish reproduction.
CONCLUSIONS
We propose that the inhibition of ovarian follicle development in Newark Bay killifish has led to a reduction of egg production due to insufficient vitellogenin expression. The decrease in hepatic vitellogenin synthesis is due to a combination of (1) deficiency of circulating 17β-estradiol levels, and (2) a decreased sensitivity of the vitellogenin pathway to 17β-estradiol. Contaminants at Newark Bay result in a multi-faceted disruption of reproductive events in the ovary and liver, which ultimately impact fecundity. Further studies are necessary to determine the mechanism for 17β-estradiol deficiency and the inhibition of vitellogenin induction by 17β-estradiol. Contaminant impacts on killifish reproduction are relevant to ecologically and commercially important species that inhabit or migrated through Newark Bay or for spawning. In addition, contaminants at Newark Bay interfere with the ability of vitellogenin to be a reliable biomarker of exposure to estrogen mimics in males due to the down-regulation of the vitellogenin pathway with chronic exposure. Killifish are a relatively insensitive species, and because teleost oogenesis is well conserved between species, contaminant impacts in other fish populations may be similar and widespread in estuaries with similar contaminant profiles.
ACKNOWLEDGEMENT
This research was carried out at the New Jersey Agricultural Experiment Station (NJAES) with funding from NJAES (01201) through Cooperative State Research, Education, and Extension Services, The Environmental and Occupational Health Sciences Institute (ES05022), the New Jersey Water Resources Research Institute (2009NJ198B), New Jersey Department of Environmental Protection Agency, Division of Science, Research and Technology (SR09-019) and The National Oceanic and Atmospheric Administration (432742).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton. Int. 2004;11(7):36–42. [Google Scholar]
- Anderson MJ, Miller MR, Hinton DE. In vitro modulation of 17β-estradiol-induced vitellogenin synthesis: Effects of cytochrome P4501A1 inducing compounds on rainbow trout (Oncorhynchus mykiss) liver cells. Aquat. Toxicol. 1996;34:327–350. [Google Scholar]
- Ankley GT, Miller DH, Jensen KM, Villeneuve DL, Marinović D. Relationship of plasma sex steroid concentrations in female fathead minnows to reproductive success and population status. Aquat. Toxicol. 2008;88:69–74. doi: 10.1016/j.aquatox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Arcand-Hoy LD, Benson WH. Fish Reproduction: An ecologically relevant indicator of endocrine disruption. Environ. Toxicol. Chem. 1998;17(1):49–57. [Google Scholar]
- Arzuaga X, Elskus A. Evidence for resistance to benzo[a]pyrene and 3,4,3',4'-tetrachlorobiphenyl in a chronically polluted Fundulus heteroclitus population. Mar. Environ. Res. 2002;54(3–5):247–251. doi: 10.1016/s0141-1136(02)00184-8. [DOI] [PubMed] [Google Scholar]
- Baldigo BP, Sloan RJ, Smith SB, Denslow ND, Blazer VS, Gross TS. Polychlorinated biphenyls, mercury, and potential endocrine disruption in fish from the Hudson River, New York, USA. Aquat. Sci. 2006;68:206–228. [Google Scholar]
- Bemanian V, Male R, Goksøyr A. The aryl hydrocarbon receptor-mediated disruption of vitellogenin synthesis in the fish liver: Cross-talk between AhR- and ERα-signaling pathways. Comp. Hepatol. 2004;3(2) doi: 10.1186/1476-5926-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DE, Gutjahr-Gobell R, Pruell RJ, Bergen B, Mills L, McElroy AE. Reproduction and polychlorinated biphenyls in Fundulus heteroclitus (Linnaeus) from New Bedford Harbor, Massachusetts, USA. Env. Toxicol. Chem. 1998;17(7):1405–1414. [Google Scholar]
- Bugel SM, White LA, Cooper KR. Impaired reproductive health of killifish (Fundulus heteroclitus) inhabiting Newark Bay, NJ, a chronically contaminated estuary. Aquat. Toxicol. 2010;96(3):182–193. doi: 10.1016/j.aquatox.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gómez-Chiarri, Hahn ME, Hoover CA, Karchner SI, Katoh F, Maclatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL. Fundulus as the premier teleost model in environmental biology: opportunities for new insights using genomics. Comp. Biochem. Physiol. Part D: Genomics Proteomics. 2007;2(4):257–286. doi: 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas E, Misitano DA, Johnson LL, Rhodes LD, Collier TK, Steine JE, McCain BB, Varanasi U. Inducibility of spawning and reproductive success of female English sole (Parophys vetulus) from urban and nonurban areas of Puget Sound, Washington. Mar. Environ. Res. 1991;21:99–122. [Google Scholar]
- Cerdá J, Calman BG, LaFleur GJ, Jr., Limesand S. Pattern of vitellogenesis and follicle maturational competence during the ovarian follicular cycle of Fundulus heteroclitus. Gen. Comp. Endocrinol. 1996;103:24–35. doi: 10.1006/gcen.1996.0090. [DOI] [PubMed] [Google Scholar]
- Dimou KN, Pecchioli JA. The New Jersey toxics reduction workplan for NY-NJ Harbor: Distribution of PCDD/Fs in ambient waters. Water, Air, Soil Pollut.: Focus. 2006;6:5–16. [Google Scholar]
- Elskus AA, Monosson E, McElroy AE, Stegeman JJ, Woltering DS. Altered CYP1A expression in Fundulus heteroclitus adults and larvae: A sign of pollutant resistance? Aquat. Toxicol. 1999;45:99–113. [Google Scholar]
- Fitzsimons JD. A critical review of the effects of contaminants on early life stage mortality of lake trout in the Great Lakes. J. Great Lakes Res. 1995;21(Suppl. 1):267–276. [Google Scholar]
- Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biol. Reprod. 2006;74:125–130. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- Gräns J, Wassmur B, Celander MC. One-way inhibiting cross-talk between arylhydrocarbon receptor (AhR) and estrogen receptor (ER) signaling in primary cultures of rainbow trout hepatocytes. Aquat. Toxicol. 2010;100:263–270. doi: 10.1016/j.aquatox.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Champlin D, Callard GV. Isolation and characterization of two cytochrome P450 aromatase forms in killifish (Fundulus heteroclitus): Differential expression in fish from polluted and unpolluted environments. Aquat. Toxicol. 2005;71:371–389. doi: 10.1016/j.aquatox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Callard GV. Cloning of three estrogen receptors (ER) from killifish (Fundulus heteroclitus): Differences in populations from polluted and reference environments. Gen. Comp. Endocrinol. 2007;150:174–188. doi: 10.1016/j.ygcen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Tarrant AM, Nacci D, Hahn ME, Callard GV. Estrogen responses in killifish (Fundulus heteroclitus) from polluted and unpolluted environments are site- and gene-specific. Aquat. Toxicol. 2010;99:291–299. doi: 10.1016/j.aquatox.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao S, Greeley MS, Jr., Wallace RA. Reproductive cycling in female Fundulus heteroclitus. Biol. Bull. 1994;186:271–284. doi: 10.2307/1542273. [DOI] [PubMed] [Google Scholar]
- Huntley SL, Bonnevie NL, Wenning RJ. Polycyclic aromatic hydrocarbon and petroleum hydrocarbon contamination in sediment from the Newark Bay Estuary, New Jersey. Arch. Environ. Contam. Toxicol. 1995;28:93–107. [Google Scholar]
- Hutz RJ. Reproductive endocrine disruption by environmental xenobiotics that modulate the estrogen-signaling pathway, particularly tetrachlorodibenzo-p-dioxin (TCDD) J. Reprod. Dev. 1999;45(1):1–12. [Google Scholar]
- Iannuzzi TJ, Armstrong TN, Long ER, Iannuzzi J, Ludwig DF. Sediment quality triad assessment of an industrialized estuary of the northeastern USA. Environ. Monit. Assess. 2008;139:257–275. doi: 10.1007/s10661-007-9832-x. [DOI] [PubMed] [Google Scholar]
- Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ. Toxicol. Chem. 1996;15(2):194–202. [Google Scholar]
- Kanungo J, Petrino TR, Wallace RA. Oogenesis in Fundulus heteroclitus. VI. Establishment and verification of conditions for vitellogenin incorporation by oocytes in vitro. J. Exp. Zool. 1990;254:313–321. doi: 10.1002/jez.1402540310. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U.S.A. 2007;104(21):8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Heiden TC, Carvan MJ, III, Hutz RJ. Inhibition of follicular development, vitellogenesis, and serum 17ß-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chemosphere. 2006;90(2):490–499. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- King Heiden TC, Struble CA, Rise ML, Hessner MJ, Hutz RJ, Carvan MJ., III Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) within the zebrafish ovary: Insights into TCDD-induced endocrine disruption and reproductive toxicity. Reprod. Toxicol. 2008;25:47–57. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Heiden TC, Spitzhergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol. Sci. 2009;109(1):75–87. doi: 10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur GJ, Jr., Byrne MB, Kanungo J, Nelson LA, Greenberg RM, Wallace RA. Fundulus heteroclitus vitellogenin: The deduced primary structure of a piscine precursor to noncrystalline, liquid-phase yolk protein. J. Mol. Evol. 1995;41:505–521. doi: 10.1007/BF00160323. [DOI] [PubMed] [Google Scholar]
- LaFleur GJ, Jr., Byrne MB, Haux C, Greenberg R, Wallace RA. Liver-derived cDNAs: Vitellogenins and vitelline envelope protein precursors (choriogenins). In: Thomas P, Goetz F, editors. “Proceedings of the Fifth International Symposium on the Reproductive Physiology of Fish”; 1996. in press. [Google Scholar]
- LaFleur GJ, Jr., Raldúa D, Fabra M, Carnevali O, Denslow N, Wallace RA, Cerdá J. Derivation of major yolk proteins from parental vitellogenins and alternative processing during oocyte maturation in Fundulus heteroclitus. Biol. Reprod. 2005;73:815–824. doi: 10.1095/biolreprod.105.041335. [DOI] [PubMed] [Google Scholar]
- Litten S. New York/New Jersey Harbor Contaminant Assessment and Reduction Project (CARP) NewYork State Department of Environmental Conservation; Albany, New York: 2003. Available online: http://www.hudsonriver.org/ [Google Scholar]
- McArdle ME, McElroy AE, Elskus AA. Enzymatic and estrogenic responses in fish exposed to organic pollutants in the New York-New Jersey (USA) Harbor Complex. Environ. Toxicol. Chem. 2004;4:953–959. doi: 10.1897/03-82. [DOI] [PubMed] [Google Scholar]
- McArdle M, Elskus A, McElroy A, Larsen B, Benson W, Schlenk D. Estrogenic and CYP1A response of mummichogs and sunshine bass to sewage effluent. Mar. Environ. Res. 2000;50:175–179. doi: 10.1016/s0141-1136(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Menuet A, Adrio F, Kah O, Pakdel F. Regulation and function of estrogen receptors: Comparative aspects. In: Melamed P, Sherwood N, editors. Hormones and their receptors in fish reproduction. World Scientific Publishing Company; Singapore: 2005. 2005. [Google Scholar]
- Mortensen AS, Arukwe A. Interactions between estrogen- and Ah-Receptor signaling pathways in primary culture of salmon hepatocytes exposed to nonylphenol and 3,3',4,4'-tetrachlorobiphenyl (congener 77) Comp. Hepatol. 2007;6(2) doi: 10.1186/1476-5926-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Jensen KM, Villeneuve DL, Kahl MD, Makynen EA, Durhan EJ, Ankley GT. Linkage of biochemical responses to population-level effects: A case study with vitellogenin in the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 2007;26(3):521–527. doi: 10.1897/06-318r.1. [DOI] [PubMed] [Google Scholar]
- Muñoz GR, Panero MA, Powers CW. Pollution prevention and management strategies for dioxins in the New York/New Jersey Harbor. New York Academy of Sciences; New York, NY: 2006. Available online: http://www.nyas.org. [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S. Adaptation of the estuarine fish Fundulus heteroclitus (Atlantic killifish) to polychlorinated biphenyls (PCBs) Estuaries and Coasts. 2010;33:853–864. [Google Scholar]
- Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Stahlschmidt-Allner P, Tyler CR. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Perspect. 2004;112:1725–33. doi: 10.1289/ehp.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas JM, Segner H. Antiestrogenicity of ß-naphthoflavone and PAHs in cultured rainbow trout hepatocytes: Evidence for a role of the aryl hydrocarbon receptor. Aquat. Toxicol. 2000;51:79–92. doi: 10.1016/s0166-445x(00)00100-4. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Habibi HR. Functional significance of nuclear estrogen receptor subtypes in the liver of goldfish. Endocrinology. 2010;151(4):1668–1676. doi: 10.1210/en.2009-1447. [DOI] [PubMed] [Google Scholar]
- NY-NJ Harbor Estuary Program (NY-NJ HEP) Harbor-wide water quality monitoring report for the New York-New Jersey Harbor Estuary. 2006 Available online: http://www.harborestuary.org/
- Ohtake F, Baba A, Fujii-Kuriyama Y, Kato S. Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signaling. Biochem. Biophys. Res. Commun. 2008;370:541–546. doi: 10.1016/j.bbrc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Pait AS, Nelson JO. Vitellogenesis in male Fundulus heteroclitus (killifish) induced by selected estrogenic compounds. Aquat. Toxicol. 2003;64:331–342. doi: 10.1016/s0166-445x(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Panero M, Boehme S, Muñoz G. Pollution prevention and management strategies for polychlorinated biphenyls in the New York/New Jersey Harbor. New York Academy of Sciences; New York, NY: 2005. Available online: http://www.nyas.org. [Google Scholar]
- Patiño R, Sullivan CV. Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol. Biochem. 2002;26:57–70. [Google Scholar]
- Petroff BK, Gao X, Roxman KK, Terranova PF. Interaction of estradiol and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in an ovulation model: Evidence for systemic potentiation and local ovarian effects. Reprod. Toxicol. 2000;14:247–255. doi: 10.1016/s0890-6238(00)00075-7. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper KR. Comparison of the effects of 2,3,7,8- tetrachlorodibenzo-p-dioxin on chemically impacted and non-impacted subpopulations of Fundulus heteroclitus. I: TCDD toxicity. Environ. Toxicol. Chem. 1995a;14:579–587. [Google Scholar]
- Prince R, Cooper KR. Comparison of the effects of 2,3,7,8- tetrachlorodibenzo-p-dioxin on chemically impacted and non-impacted subpopulations of Fundulus heteroclitus. II: Metabolic considerations. Environ. Toxicol. Chem. 1995b;14:589–596. [Google Scholar]
- Rempel MA, Shlenk D. Effects of environmental estrogens and antiandrogens on endocrine function, gene regulation, and health in fish. Int. Rev. Cell Mol. Biol. 2008;267:207–252. doi: 10.1016/S1937-6448(08)00605-9. [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor α cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003;16(7):807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Sellin MK, Snow DD, Kolok AS. Reductions in hepatic vitellogenin and estrogen receptor alpha expression by sediments from an agriculturally impacted waterway. Aquat. Toxicol. 2010;96:103–108. doi: 10.1016/j.aquatox.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Selman K, Wallace RA. Oogenesis in Fundulus heteroclitus. III. Vitellogenesis. J. Exp. Zool. 1983;226:441–457. doi: 10.1002/jez.1402260315. [DOI] [PubMed] [Google Scholar]
- Shi Z, Valdez KE, Ting AY, Franczak A, Gum SL, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol. Reprod. 2007;76:198–202. doi: 10.1095/biolreprod.106.053991. [DOI] [PubMed] [Google Scholar]
- Sumpter JP, Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ. Health Perspect. 1995;103(Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MH, Leach GJ, DiMichele L, Levitan WM, Jacob WF. Lunar spawning cycle in the mummichog, Fundulus heteroclitus (Pisces Cyprinodontidae) Copeia. 1979;2:291–297. [Google Scholar]
- Thomas P. The Endocrine System. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. Taylor & Francis Group; Fl: 2008. pp. 457–488. [Google Scholar]
- Thorpe KL, Benstead R, Hutchinson TH, Tyler CR. Associations between altered vitellogenin concentrations and adverse health effects in fathead minnow (Pimephales promelas) Aquat. Toxicol. 2007;85:176–183. doi: 10.1016/j.aquatox.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Todorov JR, Elskus AA, Schlenk D, Ferguson PL, Brownawell BJ, McElroy AE. Estrogenic responses of larval sunshine bass (Morone saxatilis x M. Chrysops) exposed to New York city sewage effluent. Mar. Environ. Res. 2002;54:691–695. doi: 10.1016/s0141-1136(02)00197-6. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Sumpter JP. Oocyte growth and development in teleosts. Reviews in Fish Biology and Fisheries. 1996;6:287–318. [Google Scholar]
- Urushitani H, Nakai M, Inanaga H, Shimohigashi Y, Shimizu A, Katsu Y, Iguchi T. Cloning and characterization of estrogen receptor alpha in mummichog, Fundulus heteroclitus. Mol. Cell. Endocrinol. 2003;203:41–50. doi: 10.1016/s0303-7207(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Vaccaro E, Meucci V, Intorre L, Soldani G, Di Bello D, Longo V, Gervasi PG, Pretti C. Effects of 17β-estradiol, 4-nonylphenol and PCB 126 on the estrogenic activity and phase 1 and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax) Aquat. Toxicol. 2005;75(4):293–305. doi: 10.1016/j.aquatox.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Wilson TP, Bonin JL. Concentrations and loads of organic compounds and trace elements in tributaries to Newark and Raritan Bays, New Jersey. U.S. Geological Survey Scientific Investigations Report 2007-5059. 2007:177. Available online: http://www.usgs.gov.
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor α through activation of proteasomes. Mol. Cell. Biol. 2003;23(6):1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel EW, Cook PM, Peterson RE. Toxic equivalency factors of polychlorinated dibenzo-p-dioxin, dibenzofuran and biphenyl congeners based on early life stage mortality in rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 1995;21:315–328. [Google Scholar]