Abstract
Cytomegalovirus (CMV) IgM indicates recent active CMV infection. CMV IgM seroprevalence is a useful marker for prevalence of transmission. Using data from the National Health and Nutrition Examination Survey (NHANES) III 1988–1994, we present estimates of CMV IgM prevalence by race/ethnicity, provide a comparison of IgM seroprevalence among all women and among CMV IgG positive women, and explore factors possibly associated with IgM seroprevalence, including socioeconomic status and exposure to young children. There was no difference in IgM seroprevalence by race/ethnicity among all women (3.1%, 2.2%, and 1.6% for non-Hispanic white, non-Hispanic black and Mexican American, respectively; P = 0.11). CMV IgM seroprevalence decreased significantly with increasing age in non-Hispanic black women (P<0.001 for trend) and marginally among Mexican American women (P = 0.07), while no apparent trend with age was seen in non-Hispanic white women (P = 0.99). Among 4001 IgG+ women, 118 were IgM+, resulting in 4.9% IgM seroprevalence. In IgG+ women, IgM seroprevalence varied significantly by age (5.3%, 7.3%, and 3.7% for women of 12–19, 20–29, and 30–49 years; P = 0.04) and race/ethnicity (6.1%, 2.7%, and 2.0% for non-Hispanic white, non-Hispanic black, and Mexican American; P<0.001). The factors reported associated with IgG seroprevalence were not associated with IgM seroprevalence. The patterns of CMV IgM seroprevalence by age, race/ethnicity, and IgG serostatus may help understanding the epidemiology of congenital CMV infection as a consequence of vertical transmission and are useful for identifying target populations for intervention to reduce CMV transmission.
Introduction
Cytomegalovirus (CMV) is a common human viral pathogen that typically causes minimal, if any, symptoms in immunocompetent individuals.[1] However, CMV infection can cause severe outcomes and even death in immunocompromised individuals and infants infected in utero.[2–4] Active CMV infection during pregnancy is the leading viral cause of birth defects and developmental disabilities in developed countries.[2]
An individual develops lifetime CMV IgG seropositivity (IgG+) after primary infection (the first infection in life), after which CMV establishes latency with intermittent reactivation. CMV IgG+ individuals can be reinfected with another strain of CMV. CMV IgM can be produced after primary infection and after non-primary infection (reactivation or reinfection).[5] It typically is detectable for only a few months,[6] and indicates recent active CMV infection. The transiency of IgM makes CMV IgM seroprevalence rates a useful marker for prevalence of transmission in a population at the time of testing.
In the U.S., CMV IgM seroprevalence in the general population have been briefly described among women aged 12–49 years from the National Health and Nutrition Examination Survey (NHANES) III 1988–1994.[7] The lack of temporal changes in CMV IgG seroprevalence from 1988–1994 to 1999–2004 [8] suggests that the factors associated with CMV transmission have remained fairly consistent over time and that findings on IgM seroprevalence from NHANES III are still informative for understanding the epidemiology of acute CMV infection and risk of transmission. We expand on the previous analysis of IgM seroprevalence among US women [7] by presenting estimates by race/ethnicity and by re-categorized age groups. We also present a comparison of IgM seropositivity by race/ethnicity and age among all women and among CMV IgG positive women to investigate whether future assessments of IgM seropositivity could be conducted using a less expensive, more streamlined approach. In addition, we explore factors possibly associated with IgM seroprevalence, including those previously identified as being associated with CMV IgG seroprevalence such as socioeconomic status and exposure to young children.[5]
Materials and Methods
Publically accessible data on CMV IgG and IgM of NHANES III 1998–1994 were analyzed and IgM was only tested on women aged 12–49 years of age while IgG data available for all NHANES III participants.[9] NHANES III was conducted by the Centers for Disease Control and Prevention from 1988 to 1994 and was a complex, stratified, multistage probability cluster sample of the noninstitutionalized civilian of the United States. The detailed methodology and response rates of NHANES III are publically accessible.[10] In contrast to a previously published analysis of all female NHANES participants,[7] only women of three racial/ethnic groups (non-Hispanic white, non-Hispanic black and Mexican American) were included in the current analysis in order to provide estimates by racial/ethnic groups that were consistent with previous analyses of CMV IgG seroprevalence.[5, 8] NHANES methods for serum sample selection and laboratory testing for CMV IgG and IgM have been reported previously.[5, 7]
Age was categorized as 12–19, 20–29, and 30–49 years with varied intervals to ensure that there were at least 5 individuals in each subgroup as fewer subjects were IgM+ in those aged ≥30 years. Nationally representative estimates of IgM seroprevalence were calculated using the same modified weights as in the prior report [7] after accounting for the proportion of available serum samples by age groups and race/ethnicity with SAS Survey Procedures (SAS Inc., Cary, NC). Risk factors associated with CMV IgG seroprevalence such as socioeconomic status and exposure to young children were categorized as reported previously [5] and examined for their association with CMV IgM seroprevalence in all and CMV IgG+ women. Home exposure to young children was defined as reporting having at least one child aged ≤6 years at home.
Results
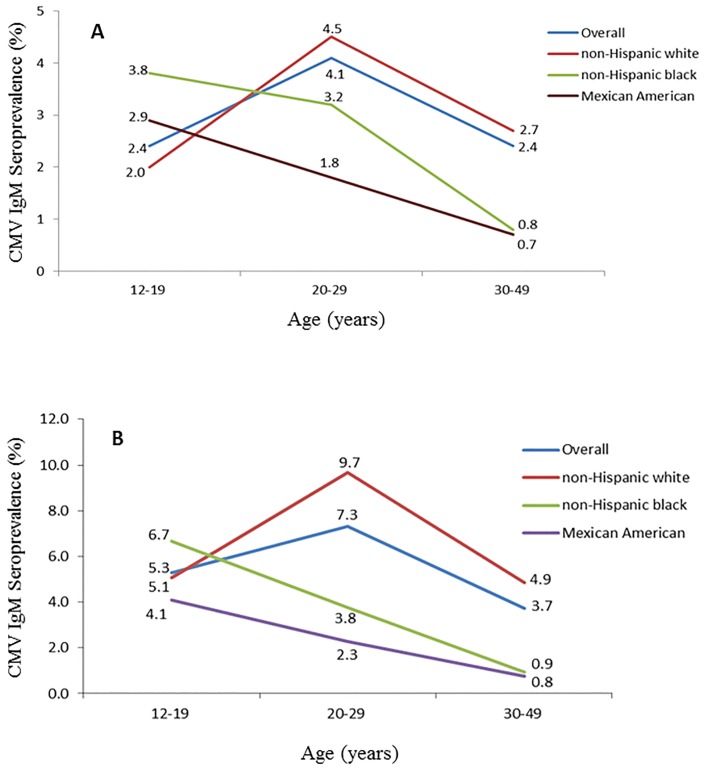
Among the 5714 women included in the analysis, approximately half were aged 30–49 years (2695, 47.2%) and similar proportions were aged 12–19 years and 20–29 years (1445 and 1574, 25.3% and 27.6%, respectively). Non-Hispanic white, non-Hispanic black and Mexican American participants each represented about one third of the study population (1825, 2013, and 1876; 31.9%, 35.2% and 32.8%, respectively). The majority of participants were IgG+ (4001, 70%). A total of 121 women were CMV IgM+ for an overall prevalence estimate of 2.8% [95% confidence interval (CI): 2.1–3.6%] among these three racial/ethnic groups. Overall, CMV IgM seroprevalence did not differ significantly by race/ethnicity (3.1%, 2.2%, and 1.6% for non-Hispanic white, non-Hispanic black and Mexican American, respectively; P = 0.11). When stratified by age, there was no difference by race/ethnicity among women aged 12–19 years (P = 0.38), but non-Hispanic white women had higher IgM seroprevalence compared to Mexican American women at 20–29 years of age (4.5% vs. 1.8%, P = 0.02), and higher IgM seroprevalence compared to non-Hispanic black and Mexican American women at age of 30–49 years (2.7% vs. 0.8% and 0.7% for non-Hispanic white, non-Hispanic black, and Mexican American, respectively; P = 0.03 and 0.01). IgM seroprevalence marginally varied by age with the highest IgM seroprevalence among those aged 20–29 years and lower seroprevalence among those aged 12–19 or 30–49 years (4.1%, 2.4% and 2.4%, respectively; P = 0.05). The patterns of CMV IgM seroprevalence with age differed by race/ethnicity. CMV IgM seroprevalence decreased significantly with increasing age in non-Hispanic black women (P<0.001 for trend) and marginally among Mexican American women (P = 0.07), while no apparent trend with age was seen in non-Hispanic white women (P = 0.99) (Fig 1, Panel A).
Fig 1.
Cytomegalovirus IgM seroprevalence among All Women (Panel A) and IgG+ Women only (Panel B) by Age and Racial/ethnic Group, NHANES III, 1988–1994.
Among the 121 CMV IgM+ women, 118 (97.5%) were CMV IgG+ and three (2.5%) were IgG-. Among the 4001 CMV IgG+ women, overall IgM seroprevalence was 4.9% (95% CI: 3.4–6.4%), and CMV IgM seroprevalence varied significantly by race/ethnicity (6.1% vs. 2.7% and 2.0% for non-Hispanic white vs. non-Hispanic black and Mexican American, respectively; P = 0.002 and 0.005). When stratified by age, there was no difference by race/ethnicity among women aged 12–19 years (P = 0.58), but non-Hispanic white women had higher IgM seroprevalence than women of other two racial/ethnic groups at age of 20–29 years (9.7% vs. 3.8% and 2.3% for non-Hispanic white, non-Hispanic black, and Mexican American, respectively; P = 0.01 and <0.001) and age of 30–49 years (4.9% vs. 0.9% and 0.8% for non-Hispanic white, non-Hispanic black, and Mexican American, respectively; P = 0.003 and 0.001). IgM seroprevalence also varied significantly by age (5.3%, 7.3%, and 3.7% for women of 12–19, 20–29, and 30–49 years, respectively; P = 0.04) among IgG+ women. Similar to findings from the analysis of CMV IgM among all women, there were significant patterns of decreasing IgM seroprevalence with age in non-Hispanic black and Mexican American women (P<0.001 and = 0.03, respectively), while lack of trend among women of non-Hispanic white (P = 0.37) (Fig 1, Panel B).
The factors previously reported as associated with CMV IgG seroprevalence [5] such as education level, poverty level, insurance, family size, area of residence, census region, or having a child ≤6 years of age at home were not associated with IgM seroprevalence (Table 1). Marital status was associated with IgM seroprevalence among all women aged ≥20 years (4.5% vs. 2.2% for single vs. married women, P = 0.04); however this association was not statistically significant when the analysis was restricted to IgG+ women (6.8% vs. 3.8%, P = 0.11). Association between CMV IgM seroprevalence and sexual behaviors such as number of lifetime sex partners and age at initiation of sexual activity, which were reported as being associated with IgG seroprevalence,[11] could not be examined due to sparsity of the data (approximately 50–60% missing values for these factors).
Table 1. Differences in CMV IgM Seroprevalence among Women Aged 12–49 Years by Selected Demographic Factors, NHANES III, 1988–1994.
| All women | IgG+ women | |||||||
|---|---|---|---|---|---|---|---|---|
| IgM+ | Sample size | Prevalence (95% CI) | P value | IgM+ | Sample size | Prevalence (95% CI) | P value | |
| Overall | 121 | 5714 | 2.8 (2.1–3.6) | NA | 118 | 4001 | 4.9 (3.4–6.4) | NA |
| Race/ethnicity | 0.11 | <0.001 | ||||||
| Non-Hispanic white | 52 | 1825 | 3.1 (2.0–4.1) | 50 | 892 | 6.1 (3.8–8.3) | ||
| Non-Hispanic black | 44 | 2013 | 2.2 (1.5–2.8) | 43 | 1582 | 2.7 (1.9–3.5) | ||
| Mexican American | 25 | 1876 | 1.6 (0.6–2.6) | 25 | 1527 | 2.0 (0.8–3.2) | ||
| Age | 0.05 | 0.04 | ||||||
| 12–19 years | 37 | 1445 | 2.4 (1.1–3.6) | 37 | 810 | 5.3 (2.4–8.2) | ||
| 20–29 years | 48 | 1574 | 4.1 (2.7–5.5) | 46 | 1104 | 7.3 (4.7–9.9) | ||
| 30–49 years | 36 | 2695 | 2.4 (1.2–3.5) | 35 | 2087 | 3.7 (1.8–5.7) | ||
| Household income level | 0.29 | 0.48 | ||||||
| High | 17 | 990 | 2.0 (0.5–3.5) | 17 | 516 | 4.5 (1.1–8.0) | ||
| Middle | 54 | 2185 | 3.3 (2.4–4.2) | 51 | 1468 | 5.4 (3.7–7.2) | ||
| Low | 37 | 2070 | 2.7 (1.7–3.8) | 37 | 1647 | 4.0 (2.4–5.5) | ||
| Education | 0.27 | 0.78 | ||||||
| Completed 11th grade or less | 39 | 2057 | 3.9 (2.3–5.4) | 36 | 1693 | 4.6 (2.2–6.9) | ||
| Completed high school | 47 | 1850 | 3.1 (2.1–4.1) | 47 | 1258 | 5.5 (3.7–7.2) | ||
| College or higher | 34 | 1767 | 2.2 (0.9–3.5) | 34 | 1018 | 4.7 (2.0–7.4) | ||
| Family size | 0.19 | 0.09 | ||||||
| 1–2 | 38 | 1207 | 3.9 (1.8–6.1) | 37 | 811 | 6.7 (3.1–10.2) | ||
| 3–4 | 46 | 2452 | 2.5 (1.6–3.4) | 45 | 1622 | 4.9 (3.1–6.7) | ||
| 5+ | 37 | 2055 | 2.0 (1.1–3.0) | 36 | 1568 | 2.9 (1.5–4.3) | ||
| Insurance | 0.74 | 0.88 | ||||||
| Government | 18 | 993 | 2.8 (0.4–5.2) | 18 | 787 | 4.0 (0.6–7.4) | ||
| Private | 79 | 3567 | 2.7 (1.8–3.7) | 76 | 2263 | 5.1 (3.1–7.0) | ||
| Uninsured | 24 | 1154 | 3.6 (1.5–5.7) | 24 | 951 | 4.9 (2.0–7.9) | ||
| Area of residence | 0.56 | 0.73 | ||||||
| Urban | 50 | 2875 | 2.5 (1.1–4.0) | 50 | 2074 | 4.6 (1.9–7.3) | ||
| Non-urban | 71 | 2839 | 3.1 (2.2–3.9) | 68 | 1927 | 5.2 (3.6–6.7) | ||
| Census region | 0.31 | 0.28 | ||||||
| Northeast | 18 | 609 | 3.6 (2.3–4.9) | 17 | 373 | 7.5 (3.4–11.5) | ||
| Midwest | 28 | 1104 | 3.0 (1.7–4.2) | 27 | 691 | 5.3 (3.1–7.6) | ||
| South | 48 | 2527 | 2.9 (1.1–4.6) | 47 | 1850 | 4.5 (1.8–7.2) | ||
| West | 27 | 1474 | 1.9 (0.9–2.9) | 27 | 1087 | 3.4 (1.3–5.4) | ||
| Birthplace | NA | NA | ||||||
| United States | 116 | 4656 | 3.0 (2.1–3.8) | 113 | 3028 | 5.3 (3.7–6.9) | ||
| Mexico | 3 | 836 | 0.3 (0.0–0.6) | 3 | 784 | 0.3 (0.0–0.7) | ||
| Other country | 2 | 208 | 2.2 (0.0–5.7) | 2 | 177 | 2.9 (0.0–7.6) | ||
| Have child aged ≤6 years at home | 0.79 | 0.96 | ||||||
| Yes | 47 | 2186 | 3.0 (1.8–4.2) | 47 | 1694 | 4.9 (3.0–6.9) | ||
| No | 74 | 3528 | 2.8 (1.8–3.7) | 71 | 2307 | 4.9 (2.9–6.8) | ||
| Marital status* | 0.04 | 0.11 | ||||||
| Married | 46 | 2713 | 2.2 (1.6–2.9) | 44 | 1972 | 3.8 (2.4–5.2) | ||
| Single | 38 | 1550 | 4.5 (1.7–7.4) | 37 | 1214 | 6.8 (2.5–11.1) | ||
NA: not applicable.
* in women aged ≥20 years only.
Discussion
Using data from NHANES III 1988–1994, we present estimates of CMV IgM seroprevalence by race/ethnicity in U.S. women of reproductive age. As a result of excluding “other or unknown” racial/ethnic groups, our analyses demonstrated a slightly lower rate in overall CMV IgM seroprevalence than previously reported (2.8% vs. 3.0%).[7] This small difference in point estimate of CMV IgM seroprevalence does not change our overall understanding of CMV IgM seroprevalence in the U.S. However, the estimates we present by age and racial/ethnic groups provide useful baseline reference values for future studies to monitor CMV rates of transmission over time and assess the relative contribution of different factors to IgM seroprevalence. In contrast to the prior study on IgM seroprevalence, [7] we found IgM seroprevalence did differ across age groups when collapsing 30–49 year olds into a single age group. Given that 98% of CMV IgM+ women were identified from among IgG+ individuals, future analyses of IgM seroprevalence using NHANES data could be more efficiently conducted by limiting the sample to CMV IgG+ subjects, as that would require testing of fewer participants. An additional benefit of this approach is that variations in IgM seroprevalence would be less likely to be diluted by the inclusion of IgG- participants, as evidenced by the significant differences by age and race/ethnicity observed in current analysis on IgG+ women only.
After primary infection, IgG seropositivity is lifelong and thus CMV IgG seroprevalence reflects the accumulation of primary infections. In contrast, CMV IgM is produced transiently as a result of primary infection and non-primary CMV infection (reinfection with a new strain of CMV or reactivation of a latent strain), and, therefore, can be a marker of recent transmission. CMV IgG seroprevalence is relatively low among non-Hispanic whites. Because a greater proportion are susceptible to primary infections, the higher rate of IgM positivity among white females compared to non-Hispanic black and Mexican American women is not surprising. Important sources for CMV transmission in adulthood are thought to include exposure to young children and sexual activity.[11, 12] In our analysis, these factors were not associated with IgM seropositivity, but this may reflect low statistical power due to the small number of IgM positive participants as well as a dilution of the importance of these factors in face of the combined contribution to overall IgM positivity from non-primary infections in addition to primary infections, in contrast to the sole contribution to IgG positivity from primary infection. Differences in the patterns of IgM and IgG seroprevalence with age suggest non-primary infection may account for a substantial proportion of IgM positivity, especially with increasing age. While factors associated with CMV IgM seroprevalence among those undergoing primary infection or reinfection may be similar to those associated with CMV IgG seroprevalence, factors associated with reactivation of latent infection may be very different. Understanding this will be difficult until there are better tools for differentiating reinfection from reactivation. Further study is needed to identify risk factors associated with reinfection and reactivation and to determine the effectiveness of strategies to prevent infection, particularly during pregnancy.
Our finding of 2.8% IgM seroprevalence in U.S. women of reproductive age is similar to estimates reported from other populations with moderate IgG seroprevalence such as France and Japan (4.1% and 2.5%, respectively).[13, 14] In contrast, populations with higher CMV IgG seroprevalence, such as Turkey and Korea (both above 98%), reported having much lower prevalence of IgM in women (0.2% and 1.3%, respectively), and none of the IgM+ subjects in those studies had low IgG avidity that would be suggestive of recent primary infection.[15, 16] Variations in IgM seroprevalence had been observed in populations with high IgG seroprevalence such as India, China, and Brazil, where similar IgG seroprevalence (98.6%, 95.5%, and 96.4%, respectively) had been reported but IgM seroprevalence varies dramatically (0.07%, 0.5%, and 2.3%, respectively).[17–19] Populations with higher maternal CMV IgG seroprevalence generally have higher rates of congenital CMV infection than populations with moderate IgG seroprevalence such as the U.S. [20, 21] The relative contributions of primary and non-primary maternal CMV infection to rates of congenital CMV infection across different populations are not well understood.
In summary, the patterns of CMV IgM seroprevalence differ by age and race/ethnicity in women of reproductive age in the US, and the differences may be useful for understanding the prevalence of transmission and congenital CMV epidemiology, therefore, potentially helpful in identifying target population for effective prevention efforts to reduce CMV transmission, in addition to providing baseline estimates for temporal monitoring on IgM seroprevalence over time.
Data Availability
All data files are available from the NHANES database: http://www.cdc.gov/nchs/nhanes/nh3data.htm.
Funding Statement
The authors have no support or funding to report.
References
- 1.Wreghitt TG, Teare EL, Sule O, Devi R, Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37(12):1603–6. [DOI] [PubMed] [Google Scholar]
- 2.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46 Suppl 4:S6–10. 10.1016/j.jcv.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Mwintshi K, Brennan DC. Prevention and management of cytomegalovirus infection in solid-organ transplantation. Expert Rev Anti Infect Ther. 2007;5(2):295–304. [DOI] [PubMed] [Google Scholar]
- 4.Bowen EF, Griffiths PD, Davey CC, Emery VC, Johnson MA. Lessons from the natural history of cytomegalovirus. AIDS. 1996;10 Suppl 1:S37–41. [PubMed] [Google Scholar]
- 5.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–51. [DOI] [PubMed] [Google Scholar]
- 6.Prince HE, Lape-Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clinical and vaccine immunology: CVI. 2014;21(10):1377–84. 10.1128/CVI.00487-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dollard SC, Staras SA, Amin MM, Schmid DS, Cannon MJ. National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity. Clinical and vaccine immunology: CVI. 2011;18(11):1895–9. 10.1128/CVI.05228-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47. 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. NHANES III Cytomegalovirus IgG and IgM Data. Available: http://www.cdc.gov/nchs/nhanes/nhanes3/data_files.htm#surplus2005. Accessed 10 December 2015.
- 10.Centers for Disease Control and Prevention. Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey, NHANES III (1988–1994). Available: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf. Accessed 10 December 2015.
- 11.Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE Jr, Cannon MJ. Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988–1994. Sex Transm Dis. 2008;35(5):472–9. 10.1097/OLQ.0b013e3181644b70 [DOI] [PubMed] [Google Scholar]
- 12.Pass RF, Little EA, Stagno S, Britt WJ, Alford CA. Young children as a probable source of maternal and congenital cytomegalovirus infection. N Engl J Med. 1987;316(22):1366–70. [DOI] [PubMed] [Google Scholar]
- 13.Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clin Infect Dis. 2013;56(10):1428–35. 10.1093/cid/cit059 [DOI] [PubMed] [Google Scholar]
- 14.Kaneko M, Sameshima H, Minematsu T, Kusumoto K, Yamauchi A, Ikenoue T. Maternal IgG avidity, IgM and ultrasound abnormalities: combined method to detect congenital cytomegalovirus infection with sequelae. J Perinatol. 2013;33(11):831–5. 10.1038/jp.2013.87 [DOI] [PubMed] [Google Scholar]
- 15.Uysal A, Taner CE, Cuce M, Atalay S, Gol B, Kose S, et al. Cytomegalovirus and rubella seroprevalence in pregnant women in Izmir/Turkey: follow-up and results of pregnancy outcome. Arch Gynecol Obstet. 2012;286(3):605–8. 10.1007/s00404-012-2353-z [DOI] [PubMed] [Google Scholar]
- 16.Seo S, Cho Y, Park J. Serologic screening of pregnant Korean women for primary human cytomegalovirus infection using IgG avidity test. The Korean journal of laboratory medicine. 2009;29(6):557–62. 10.3343/kjlm.2009.29.6.557 [DOI] [PubMed] [Google Scholar]
- 17.Das B, Kaur G, Basu S. Seroprevalence of cytomegalovirus antibodies among blood donors and Multitransfused recipients—a study from north India. Transfusion and apheresis science. 2014;50(3):438–42. 10.1016/j.transci.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 18.Souza MA, Passos AM, Treitinger A, Spada C. Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev Soc Bras Med Trop. 2010;43(4):359–61. [DOI] [PubMed] [Google Scholar]
- 19.Qi Y, Feng Z, Cai X, Ma J, Zhu S, Kong H, et al. Human cytomegalovirus infection status among reproductive aged women and hospitalized children in China. The 5th International Congenital CMV Conference; April 20–24, 2015; Brisbane, Australia, 2015.
- 20.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis. 2011;52(2):e11–3. 10.1093/cid/ciq085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data files are available from the NHANES database: http://www.cdc.gov/nchs/nhanes/nh3data.htm.