Abstract
Objective
To analyze the natural history of small asymptomatic pancreatic neuroendocrine tumors (PanNET) and to present a matched comparison between groups who underwent either initial observation or resection. Management approach for small PanNET is uncertain.
Methods
Incidentally discovered, sporadic, small (<3 cm), stage I–II PanNET were analyzed retrospectively between 1993 and 2013. Diagnosis was determined either by pathology or imaging characteristics. Intention-to-treat analysis was applied.
Results
A total of 464 patients were reviewed. Observation was recommended for 104 patients (observation group), and these patients were matched to 77 patients in the resection group based on tumor size at initial imaging. The observation group was significantly older (median 63 vs. 59 years, p = 0.04) and tended towards shorter follow-up (44 vs. 57 months, p = 0.06). Within the observation group, 26 of the 104 patients (25 %) underwent subsequent tumor resection after a median observation interval of 30 months (range 7–135). At the time of last follow-up of the observation group, the median tumor size had not changed (1.2 cm, p = 0.7), and no patient had developed evidence of metastases. Within the resection group, low-grade (G1) pathology was recorded in 72 (95 %) tumors and 5 (6 %) developed a recurrence, which occurred after a median of 5.1 (range 2.9–8.1) years. No patient in either group died from disease. Death from other causes occurred in 11 of 181 (6 %) patients.
Conclusions
In this study, no patient who was initially observed developed metastases or died from disease after a median follow-up of 44 months. Observation for stable, small, incidentally discovered PanNET is reasonable in selected patients.
Overdiagnosis has become an evolving challenge for several cancer subtypes. It is defined as the identification of tumors that otherwise would not progress to cause symptoms or death.1 The leading cause for overdiagnosis is the increased use of high-resolution diagnostic imaging. Support for this statement can be found in the reported threefold increase in Medicare beneficiaries who have undergone abdominal CT during the past decade.2 Overdiagnosis, if not recognized, frequently results in overtreatment. Studies that have estimated the extent of overdiagnosis have found this phenomenon in approximately 60 % of prostate-specific antigen–detected prostate cancers, 25 % of mammographically detected breast cancers, and 50 % of chest x-ray and/or sputum-detected lung cancers.3–5 Similar observations have been reported in patients with thyroid cancer, melanoma, and kidney cancer.6–9 Our group has recently reported the phenomenon of overdiagnosis in patients who present with cystic lesions of the pancreas.10 In that study, the 5-year risk of death from pancreatic cancer in those initially selected for radiographic surveillance was 2.5 %, whereas the 5-year risk of death from other causes in those initially selected for radiographic surveillance was more than 20 %.
Pancreatic neuroendocrine tumors (PanNET) are the second most common neoplasm of the pancreas after adenocarcinoma.11 The prevalence of PanNET in the general population is approximately 1/100,000, but higher rates have been documented in postmortem studies, ranging between 1 and 10 % of autopsies (depending on the number of sections performed).12–14 This gap suggests that the majority of PanNET never become clinically relevant and thus may serve as a potential reservoir for overdiagnosis. It can be speculated that this reservoir, coupled with the improved diagnostic imaging modalities, may account for the sevenfold increase in the incidence of small PanNET in the United States during the past two decades.15
We hypothesized that a substantial portion of the incidentally discovered, small PanNET are overtreated as a result of overdiagnosis and that an initial observation approach may be reasonable for selected patients. Herein, we present what we believe to be the first matched comparison between groups who underwent either initial observation or tumor resection. In addition, we performed a comprehensive analysis of the natural history of sporadic, incidentally discovered PanNET smaller than 3 cm.
METHODS
Study Design
Approval was obtained from Memorial Sloan Kettering Cancer Center’s (MSKCC) Institutional Review Board. The institutional cancer database was queried for patients with an initial working diagnosis of PanNET between 1993 and 2013. Patients were identified either by pathological diagnosis of PanNET or by keyword search through the clinic registry or imaging reports. Patients were only included if they had either a pathological diagnosis or imaging characteristics of PanNET. All patients who were included had an unambiguous clinic note from the attending surgeon that stated the tumor was PanNET. The following exclusion criteria were applied: (1) Patients without tissue diagnosis whose imaging characteristics also could be consistent with either pseudoaneurysm, splenule, serous cystadenoma, solid pseudopapillary tumor, metastatic tumor, or intraductal papillary mucinous neoplasm; (2) Patients with familial syndromes; (3) Stage III–IV tumors; (4) Largest tumor size greater than 3 cm at initial imaging; (5) Age younger than 18 years; (6) Symptomatic or functional PanNET as defined by the National Comprehensive Cancer Network (NCCN)16; (7) Patients who were not treatment-naïve; (8) Patients with fewer than two serial cross-sectional imaging studies; (9) Other (non-PanNET) stage IV tumor at initial diagnosis; and (10) Patients who did not attend two clinic visits with at least 3 months of follow-up between them.
Treatment-related variables were obtained from the database and cross-sectional imaging characteristics were reviewed by a radiologist (RKGD) with specific expertise in pancreatic tumors. Imaging characteristics were recorded from the first imaging report that documented the presence of PanNET, with the exception of size that was recorded both at initial diagnosis and at last follow-up or before resection.
Histopathologic assessment was performed and tumors were graded into low and intermediate groups according to the WHO 2010 definitions.17 Tumor stage was defined according to the American Joint Committee on Cancer (AJCC) definitions.16
Data Analysis
The study design was a matched case–control study. The observation and resection groups were determined according to an intention-to-treat principle. The observation group was defined as patients who were either followed by radiographic surveillance or patients who were resected after at least 6 months of radiographic surveillance. Patients from the observation group were matched with patients who had undergone tumor resection without an initial observation approach. Case matching was performed with initial tumor size at initial imaging. This variable was selected to reduce the confounding effect of clinically established factor that influence treatment recommendations and outcome.18 The match selection process was random and was performed by a statistician (MG) who was blinded to the clinical and outcome data.
Descriptive and comparative statistics were performed using Stata version 13.1 software. Continuous variables were compared using the Student t test or Mann–Whitney U test, as appropriate by the type of distribution. Categorical variables were compared using χ 2 or the Fisher exact test depending on the number of observations. A p value <0.05 was considered significant. Follow-up was determined from the initial imaging that identified the presence of PanNET and ended at the date of last imaging. Survival distributions were estimated using the Kaplan– Meier method. Time to event was calculated from the date of initial follow-up. An event for metastasis-free survival was defined as any locoregional or distant metastatic progression or death. Patients without the event of interest at last follow-up were censored.
RESULTS
During the study period, 464 patients with stage I–II PanNET were identified at MSKCC, from whom 377 underwent PanNET resection. Figure 1 illustrates the inverse correlation between the observed increase in the number of patients who have undergone tumor resection and the corresponding decrease in tumor size. The median tumor size was 3.9 cm (interquartile range (IQR) 2.5–5.5) during the first half of the study period (1993–2002) and decreased to 2.2 cm (IQR 1.4–4.0; p = 0.003) during the second half (2003–2013). During the study period (1993– 2013), a significant increase in the proportion of patients who were managed by initial observation approach was noted (1993–1999: 6 %; 2000–2006: 20 %; 2007–2013: 28 %; p = 0.001; Fig. 2); however, the majority of stage I–II PanNET patients continue to undergo resection.
FIG. 1.
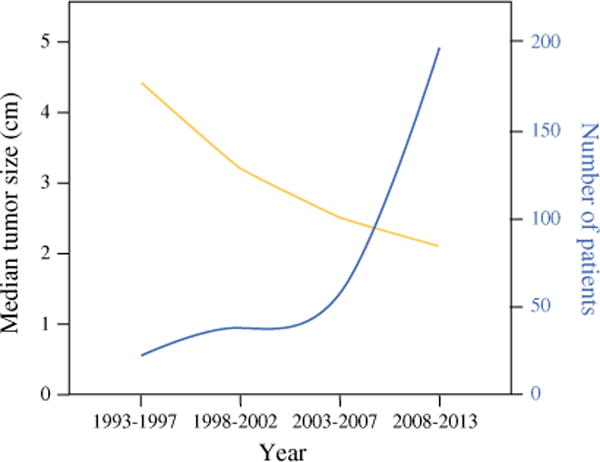
Distribution of tumor size over time in any patient whose PanNET was resected at MSKCC during the last two decades (n = 377). Yellow line represents the tumor size and blue line represents the number of patients
FIG. 2.
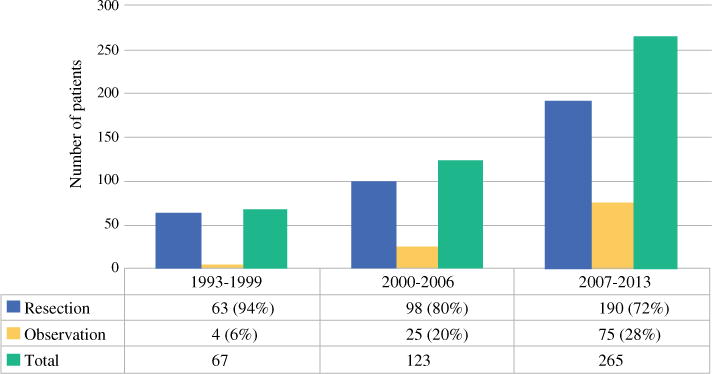
Stage I–II PanNET management strategies during the study period (1993–2013) by intention-to-treat analysis* (n = 455). *Excluding nine patients with familial syndromes who were not operated
Patient Selection
Of the 464 patients with stage I–II PanNET, 113 patients were initially observed radiographically (Fig. 3). Within this group of 113 patients, there were 9 who were diagnosed with familial syndromes and were excluded from further analysis. Thus, a total of 104 patients were included in the observation group. During the same time period, a total of 351 stage I–II patients were treated with initial resection. Within this group of 351 patients, there were 37 with familial syndromes who were excluded from further analysis. Based on initial tumor size, a matched control group (resection group) was selected, which included 77 patients who had undergone tumor resection.
FIG. 3.
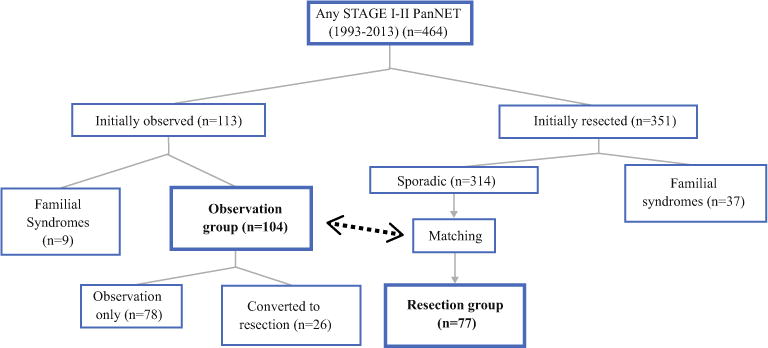
Study design flow chart. Overall 464 stage I–II PanNET patients were identified. After applying the exclusion criteria, the observation group (n = 104) was identified, and then a resection group (n = 77) was matched
Clinical Characteristics
The observation group was older (median 63 years, IQR 55–72) than the resection group (median 59 years, IQR 51– 68; p = 0.04). Table 1 details the clinical characteristics at initial diagnosis. Pathologic tissue diagnosis was obtained in 68 (65 %) patients in the observation group. Median initial tumor size was similar between the observation and resection groups [1.2 cm (IQR 0.8–1.7) and 1.4 cm (IQR 1–2), respectively, p = 0.07].
TABLE 1.
Clinical characteristics stratified by management strategy (intention-to-treat analysis)
| Characteristics | Observation group (n = 104) | Resection group (n = 77) | p value |
|---|---|---|---|
| Age at initial diagnosis (years) | 63 (55–72) | 59 (51–68) | 0.04 |
| Female | 54 (52 %) | 32 (42 %) | 0.2 |
| Imaging characteristics | |||
| Initial tumor size, cm | 1.2 (0.8–1.7) | 1.4 (1–2) | 0.07 |
| Single lesion | 99 (95 %) | 74 (96 %) | 0.8 |
| Tumor location | 0.9 | ||
| Proximal | 40 (38 %) | 30 (39 %) | |
| Distal | 64 (62 %) | 47 (61 %) | |
| Tumor consistency | 0.7 | ||
| Solid | 90 (87 %) | 64 (84 %) | |
| Cystic | 14 (13 %) | 12 (16 %) | |
| Calcifications | 10 (10 %) | 9 (12 %) | 0.6 |
| Pancreatic duct cutoff | 3 (3 %) | 7 (9 %) | 0.1 |
| Common bile duct dilation | 1 (1 %) | 0 | 1 |
| Evidence of pancreatitis | 3 (3 %) | 2 (3 %) | 1 |
| Staging | |||
| Initial tumor stage | 0.1 | ||
| Ia | 90 (87 %) | 60 (78 %) | |
| Ib | 14 (13 %) | 17 (22 %) | |
| Initial primary tumor (T) | 0.1 | ||
| T1 | 90 (87 %) | 60 (78 %) | |
| T2 | 14 (13 %) | 17 (22 %) | |
| Initial regional lymph nodes (N) | NA | ||
| N0 | 104 (100 %) | 77 (100 %) |
Continuous variables are expressed as median (Interquartile range); categorical variables are expressed as n (%)
NA not analyzed
Outcomes
Table 2 summarizes the outcome characteristics and Table 3 details the surgical and pathological characteristics. The median follow-up of the observation group and resection group was 44 months (range 4–223) and 57 months (range 10–176), respectively (p = 0.06). During observation, the median tumor size did not change (initial: 1.2 cm; final: 1.2 cm; p = 0.7) in the observation group, and at last radiographic follow-up, no patient had developed radiographic evidence of metastases (locoregional or distant).
TABLE 2.
Outcome characteristics stratified by management strategy (intention-to-treat analysis)
| Characteristics | Observation group (n = 104) | Resection group (n = 77) | p value |
|---|---|---|---|
| Final tumor sizea (cm) | 1.2 (0.8–1.9) | 1.4 (1.1–1.9) | 0.3 |
| Final Primary tumor (T)b | 0.3 | ||
| T0 | 3 (3 %) | 0 | |
| T1 | 84 (81 %) | 67 (87 %) | |
| T2 | 17 (16 %) | 10 (13 %) | |
| Final regional lymph nodes (N)b | 0.4 | ||
| N0 | 104 (100 %) | 76 (99 %) | |
| N1 | 0 | 1 (1 %) | |
| Final tumor stageb | 0.3 | ||
| 0 | 3 (3 %) | 0 | |
| Ia | 84 (81 %) | 66 (86 %) | |
| Ib | 17 (16 %) | 10 (13 %) | |
| IIb | 0 | 1 (1 %) | |
| Follow-up (months) | 44 (14–77) | 57 (35–87) | 0.06 |
| Oncological status at last follow-up | NA | ||
| No evidence of disease | 27 (26 %) | 66 (86 %) | |
| Alive with disease | 73 (70 %) | 4 (5 %) | |
| Dead of other causes | 2 (2 %) | 4 (5 %) | |
| Dead of unknown causes | 2 (2 %) | 3 (4 %) | |
| 5Y overall survival, % (95 % CI) | 99 (97–100) | 91 (84–97) | 0.05 |
| 5Y metastasis-free survival, % (95 % CI) | 99 (97–100) | 88 (79–96) | 0.01 |
| Recurrencec | 0 | 5 (6 %) | NA |
Continuous variables are expressed as median (interquartile range); categorical variables are expressed as n (%)
NA not analyzed
Final tumor size in the observation group was measured from the last imaging taken at the end of the observation period. Final tumor size in the resection group was measured from the pathological report
Defined as the pathological staging for patients who underwent resection. For patients who were observed without resection, staging was defined clinically at last imaging follow-up
One patient that developed recurrence in the portal lymph node underwent re-resection and was NED (no evidence of disease) on last follow-up. The other four patients developed recurrences in the following sites: mesenteric lymph nodes (two patients), liver (one patient), and pelvis (one patient)
TABLE 3.
Resection group: surgical and pathological characteristics (intention-to-treat analysis)
| Characteristics | Resection group (n = 77) |
|---|---|
| Surgery | |
| ASAa | |
| 1 | 6 (7 %) |
| 2 | 47 (63 %) |
| 3 | 22 (30 %) |
| Length of hospital stay, days | 7 (6–10) |
| Procedure type | |
| Pancreaticoduodenectomy | 18 (24 %) |
| Distal pancreatectomy | 44 (57 %) |
| Central pancreatectomy | 7 (9 %) |
| Enucleation | 8 (10 %) |
| Any complicationsb | |
| Grade 1 | 2 (3 %) |
| Grade 2 | 6 (8 %) |
| Grade 3 | 16 (21 %) |
| Leak-related complicationsb | |
| Grade 1 | 0 |
| Grade 2 | 3 (4 %) |
| Grade 3 | 13 (17 %) |
| Pathology | |
| Size (cm) | 1.4 (1.1–1.9) |
| Grade | |
| Low grade (G1) | 72 (95 %) |
| Intermediate grade (G2) | 5 (5 %) |
| Lymphovascular invasionc | 11 (18 %) |
| Perineural invasiond | 7 (11 %) |
| Necrosise | 1 (1 %) |
| Pancreatitis | 12 (16 %) |
| Positive resection margin | 9 (12 %) |
Continuous variables are expressed as median (interquartile range); categorical variables are expressed as n (%). Grade of complication is the highest grade for a specific patient
Two case not recorded
Operative morbidity was recorded and graded in the MSKCC surgical events database.33 This database uses a severity scale similar to others previously published and is consistent with the “Common Terminology Criteria for Adverse Events Version 4.0”, endorsed by the National Institutes of Health and the National Cancer Institute34
Seventeen cases not recorded
Sixteen cases not recorded
One case not recorded
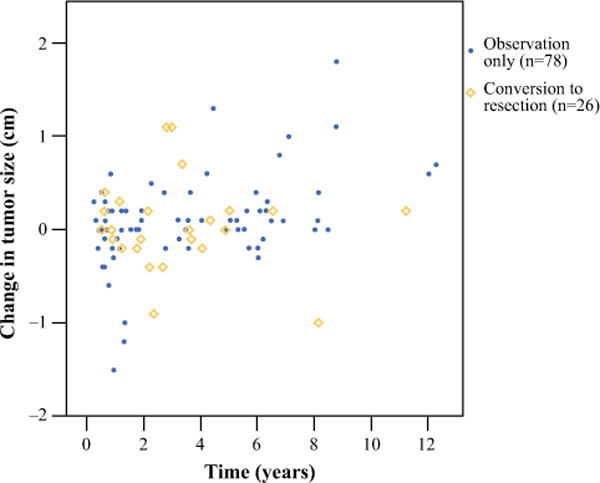
Twenty-six patients from the observation group underwent subsequent tumor resection after a median observation interval of 30 months (range 7–135). The following indications for subsequent resection were recorded: patient’s preference [n = 10 (38 %)], increasing tumor size [n = 8 (31 %)], physician’s preference [n = 7 (27 %)], and development of pancreatic duct dilatation [n = 1 (4 %)]. This group of 26 patients have been followed for a median of 6.6 years (range 1–18), and no patient has died of disease or developed radiographic evidence of metastases during follow-up. Figure 4 illustrates the change in tumor size for each patient in the observation group over time. The median change in tumor size during the observation period was similar between the patients who converted to resection (0 cm; IQR −0.2 to 0.2) and the patients who were observed with no conversion (0.1 cm; IQR −0.1 to 0.2; p = 0.6).
FIG. 4.
Change in tumor size over time in the observation group (n = 104, each dot/diamond represents a single patient). Fifty-three (51 %) patients experienced increase in tumor size, 19 (18 %) patients experienced no change in tumor size, and 32 (31 %) patients experienced decrease in tumor size
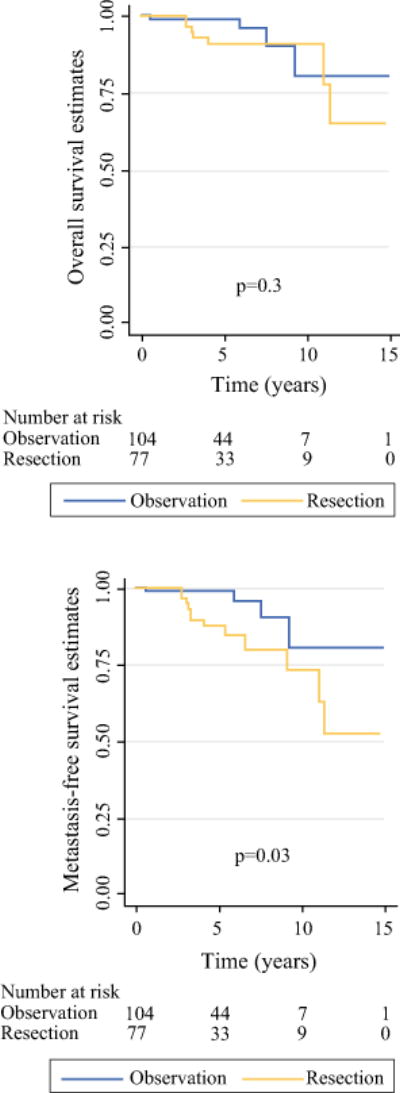
Within the resection group, low-grade (G1) pathology was recorded in 72 (95 %) tumors, 1 patient (1 %) had node positive disease, and 5 developed a recurrence (6 %), which occurred after a median of 5.1 years (range 2.9–8.1). No patient in either group died from disease. Death from other causes or unknown causes occurred in 11 of 181 (6 %) patients. Survival distributions are shown in Fig. 5. Tumor growth rate, in the observation group, was not associated with any of the clinical characteristics (Table S1).
FIG. 5.
Overall survival and metastasis-free survival Kaplan–Meier estimates stratified by management strategy (log-rank test)
Additionally, in Table 4 we performed a separate comparison between the resection group (n = 77) and the observation only group (n = 78; Fig. 3). No between-group differences were noted with regard to initial tumor size, final tumor size, and survival. It is noteworthy that compared to the resection group, the observation only group was older [median age (years) 65 vs. 59, p = 0.001] and the follow-up was shorter [median (months) 33 vs. 57, p < 0.001].
TABLE 4.
Comparison of clinical and outcome characteristics between the observation only group (n = 78) and the resection group (n = 77)
| Characteristics | Observation only group (n = 78) | Resection group (n = 77) | P value |
|---|---|---|---|
| Age at initial diagnosis (years) | 65 (58–74) | 59 (51–68) | 0.001 |
| Female | 38 (49 %) | 32 (42 %) | 0.4 |
| Imaging characteristics | |||
| Initial tumor size (cm) | 1.2 (0.8–1.9) | 1.4 (1–2) | 0.07 |
| Single lesion | 74 (95 %) | 74 (96 %) | 1.0 |
| Tumor location | 0.9 | ||
| Proximal | 31 (40 %) | 30 (39 %) | |
| Distal | 47 (60 %) | 47 (61 %) | |
| Tumor consistency | 0.9 | ||
| Solid | 66 (85 %) | 64 (84 %) | |
| Cystic | 12 (15 %) | 12 (16 %) | |
| Calcifications | 8 (10 %) | 9 (12 %) | 0.5 |
| Pancreatic duct cutoff | 2 (3 %) | 7 (9 %) | 0.1 |
| Common bile duct dilation | 0 | 0 | NA |
| Evidence of pancreatitis | 2 (3 %) | 2 (3 %) | 0.7 |
| Staging | |||
| Initial tumor stage | 0.2 | ||
| Ia | 67 (86 %) | 60 (78 %) | |
| Ib | 11 (14 %) | 17 (22 %) | |
| Initial primary tumor (T) | 0.2 | ||
| T1 | 67 (86 %) | 60 (78 %) | |
| T2 | 11 (14 %) | 17 (22 %) | |
| Initial regional lymph nodes (N) | NA | ||
| N0 | 78 (100 %) | 77 (100 %) | |
| Outcome | |||
| Final tumor sizea (cm) | 1.2 (0.8–1.9) | 1.4 (1.1–1.9) | 0.4 |
| Final primary tumor (T)b | 0.1 | ||
| T0 | 3 (4 %) | 0 | |
| T1 | 61 (78 %) | 67 (87 %) | |
| T2 | 14 (18 %) | 10 (13 %) | |
| Final regional lymph nodes (N)b | 0.5 | ||
| N0 | 78 (100 %) | 76 (99 %) | |
| N1 | 0 | 1 (1 %) | |
| Final tumor stageb | 0.2 | ||
| 0 | 3 (4 %) | 0 | |
| Ia | 61 (78 %) | 66 (86 %) | |
| Ib | 14 (18 %) | 10 (13 %) | |
| IIb | 0 | 1 (1 %) | |
| Follow-up, mo | 33 (11–72) | 57 (35–87) | \0.001 |
| Oncological status at last follow-up | NA | ||
| No evidence of disease | 3 (4 %) | 66 (86 %) | |
| Alive with disease | 73 (94 %) | 4 (5 %) | |
| Dead of other causes | 1 (1 %) | 4 (5 %) | |
| Dead of unknown causes | 1 (1 %) | 3 (4 %) | |
| 5Y overall survival, % (95 % CI) | 99 (95–100) | 91 (84–97) | 0.3 |
| 5Y metastasis-free survival, % (95 % CI) | 99 (95–100) | 88 (79–96) | 0.08 |
| Recurrencec | 0 | 5 (6 %) | NA |
Continuous variables are expressed as median (Interquartile range); categorical variables are expressed as n (%)
NA not analyzed
Final tumor size in the observation group was measured from the last imaging taken at the end of the observation period. Final tumor size in the resection group was measured from the pathological report
Defined as the pathological staging for patients who underwent resection. For patients who were observed without resection, staging was defined clinically at last imaging follow-up
One patient that developed recurrence in the portal lymph node underwent re-resection and was NED (no evidence of disease) on last follow-up. The other four patients developed recurrences in the following sites: mesenteric lymph nodes (two patients), liver (one patient), and pelvis (one patient)
DISCUSSION
The incidence of small PanNET has increased sevenfold during the past two decades, and their relative proportion compared to all PanNET has doubled.15 We hypothesized that overdiagnosis explain, in part, this increase and that overtreatment is a potential concern. This report is a case–control study, which matches patients with small (<3 cm) PanNET based on initial tumor size in an attempt to evaluate the potential concern of overdiagnosis and to further describe the natural history of this uncommon and relatively indolent disease.
In general, routine resection has been recommended for patients who present with PanNET. The rationale for routine resection is based on the risk of malignant progression, as well as the ability to relieve symptoms. On the contrary, the concept of an initial observation approach in PanNET was first introduced in the setting of MEN1 syndrome, in which a “field-defect” is present throughout the gland and unless total pancreatectomy is performed, the pancreatic remnant is prone to develop new tumors.19–21
There are several theoretical reasons to consider an initial observation approach in sporadic small PanNET. The indolent course of small PanNET has been recently demonstrated by Lee et al. who evaluated 133 patients with sporadic PanNET < 4 cm.22 Within the group of 77 patients in their study who underwent observation there was no reported disease progression or disease-specific mortality after a mean follow-up of 45 months. The non-matched group that underwent tumor resection (n = 56) was followed for a mean of 52 months and experienced no recurrence or disease-specific mortality. A similar observation was demonstrated in a multi-institutional study that reported on 46 patients with small PanNET (<2 cm) in whom no nodal or distant metastases developed after a median follow-up of 34 months.23 In light of this indolent course, an initial observation approach has been suggested, as pancreatic resection continues to be associated with a risk of substantial morbidity and mortality. Additional reasons to consider an initial observation approach include exocrine and endocrine pancreatic insufficiency.24,25 Thus, as the incidence of PanNET increases dramatically, it is unclear what the clinical significance of small PanNET will be.15
Advocates of routine resection claim that an aggressive surgical approach should be practiced with any asymptomatic PanNET. Haynes et al. reviewed 139 patients who underwent resection of any asymptomatic PanNET and showed that 3 (8 %) of 39 patients with tumors 2 cm or smaller eventually developed disease recurrence and died of their disease.26 It is noteworthy that only 1 (2 %) of the three patients was initially diagnosed without gross local invasion and/or metastases. They concluded that resection should be offered regardless of tumor size and growth characteristics. However, a limitation of similar single-arm surgical case series is that patients who were initially approached by observation were not included. Therefore, it cannot be determined whether patients who progressed could have been identified during an initial observation approach, based on tumor size and growth over time, and only once the disease has declared a more aggressive behavior, may be managed by resection.
In the current study, the observation group was followed for a median of 44 months and demonstrated no tumor growth and no development of metastases (locoregional or distant). The matched group of patients who initially underwent tumor resection was followed for a median of 57 months and demonstrated low-grade pathology in 95 % tumors, node negative disease in 99 % of patients, and 6 % developed recurrence, which occurred after a median of 5 years. No patient in either group died from the disease and death from other causes occurred in 6 % of patients. These favorable results are clearly attributed to the rigorous selection process of “low-risk” tumors in both groups. In our opinion, it is evident that in selected patients the risks of pancreatic resection and non tumor-related death prevail over the risks of malignancy (tumor-related death and tumor progression). These considerations are accentuated, as the elderly population is steadily growing and age remains an established risk factor for postoperative complications and mortality.27,28
It is noteworthy that a quarter of the observation group crossed over to resection, mostly due to increasing tumor size, patient’s preference, and physician’s preference. None of these patients developed symptoms prior to resection. After a median follow-up of 7 years, none of these patients developed locoregional or distant metastases. These data suggest that delayed surgical intervention may not compromise long-term outcomes.
In the future, the management of these tumors may be improved by defining disease dynamics (behavior over time) and by molecular diagnostics that may predict whether a PanNET has a more aggressive or indolent phenotype. A recent collaborative study, which performed whole exome sequencing, found that 44 % of sporadic PanNET harbored somatic inactivating mutations in MEN-1 and that mutations in the MEN1 gene were associated with improved survival.29 In addition, a large multi-institutional study from Switzerland demonstrated that loss of DAXX/ATRX proteins was associated with poor survival in patients with PanNET.30 Validation of markers such as these may further guide the selection of patients for initial observation approach.
Limitations of the current study include its retrospective design and the relatively short follow-up of the observation group. Therefore, we stress that an initial observation approach must go along with a low threshold for surgical intervention if a tumor develops aggressive characteristics (e.g., tumor growth, regional lymph node involvement). On the contrary, this is the first case–control study, which matches patients based on initial tumor size in an attempt to elucidate the natural history of this rare disease. Ideally, the question of how to manage these PanNETs should be studied in a prospective randomized study. However, given the low prevalence of these tumors and their relatively indolent course, such a trial is unlikely to be accomplished. The intention-to-treat analysis enabled us to characterize disease dynamics in greater detail than previously reported.22,23 Notably, population-based data exists; however, their conclusions that tumor resection improves survival, regardless of tumor size and growth characteristics, are difficult to reach given the lack of data regarding familial predisposition, tumor growth characteristics, intent of surgical procedure (palliative or curative), resection margin status, comorbidities, performance status, the evolving grading terminology over time, and changing registry protocols.31,32
CONCLUSIONS
The results of this study suggest that not all sporadic PanNET require resection, and that it is reasonable and safe to initiate an initial observation approach with selected patients who are identified with small (<3 cm), stable, nonfunctional, incidentally discovered PanNET. A selective initial observation approach will lessen overtreatment by avoiding unnecessary operative morbidity and mortality in this subset of patients who may not benefit from resection. Further investigation into disease dynamics and molecular markers is warranted in order to better characterize management strategies.
Supplementary Material
Acknowledgments
Funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748, R21-CA158267.
Footnotes
Presented at the ASCO Gastrointestinal Cancers Symposium (January 15–17, 2015, San Francisco, CA) and at the SSO’s 68th Annual Cancer Symposium (March 25–28, 2015, Houston, TX).
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4986-1) contains supplementary material, which is available to authorized users.
DISCLOSURE All authors declare that they have no conflict of interest regarding this study.
References
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 2.Dartmouth Atlas of Health Care. The Dartmouth Institute for health policy and clinical practice. Raleigh: Lulu; 2008. [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Welch HG, Schwartz LM, Woloshin S. Ramifications of screening for breast cancer: 1 in 4 cancers detected by mammography are pseudocancers. BMJ (Clin Res ed) 2006;332(7543):727. doi: 10.1136/bmj.332.7543.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus PM, Bergstralh EJ, Zweig MH, Harris A, Offord KP, Fontana RS. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst. 2006;98(11):748–56. doi: 10.1093/jnci/djj207. [DOI] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 7.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–7. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 8.Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ (Clin Res ed) 2005;331(7515):481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Kang SK, Wang L, Touijer A, Hricak H. Distribution of renal tumor growth rates determined by using serial volumetric CT measurements. Radiology. 2009;250(1):137–44. doi: 10.1148/radiol.2501071712. [DOI] [PubMed] [Google Scholar]
- 10.Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg. 2011;212(4):590–600. doi: 10.1016/j.jamcollsurg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbeke C, Caroline SV, Fiona C. Pathology of the pancreas: a practical approach. New York: Springer Verlag; 2013. [Google Scholar]
- 12.Grimelius L, Hultquist GT, Stenkvist B. Cytological differentiation of asymptomatic pancreatic islet cell tumours in autopsy material. Virch Archiv A Pathol Anat Histol. 1975;365(4):275–88. doi: 10.1007/BF00471177. [DOI] [PubMed] [Google Scholar]
- 13.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36(7):933–42. doi: 10.1007/BF01297144. [DOI] [PubMed] [Google Scholar]
- 14.Klimstra DS, Perren A, Oberg K, et al. Pancreatic endocrine tumors: non-functioning tumors and microadenomas. In: DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and genetics of tumours of endocrine origin. Lyon, France: IARC Press; 2004. pp. 201–4. [Google Scholar]
- 15.Kuo EJ, Salem RR. Population-level analysis of pancreatic neu-roendocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;20(9):2815–21. doi: 10.1245/s10434-013-3005-7. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. :2014. doi: 10.6004/jnccn.2004.0021. version 2. http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed 20 July 2014. [DOI] [PubMed]
- 17.Bosman FT, Carneiro F, Hruban RH, Theise ND, World Health Organization . Classification of Tumours of the Digestive System. IARC; Lyon: 2010. [Google Scholar]
- 18.Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat. 2009 doi: 10.2202/1557-4679.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson NW, Lloyd RV, Nishiyama RH, et al. MEN I pancreas: a histological and immunohistochemical study. World J Surg. 1984;8(4):561–74. doi: 10.1007/BF01654938. [DOI] [PubMed] [Google Scholar]
- 20.Majewski JT, Wilson SD. The MEA-I syndrome: an all or none phenomenon? Surgery. 1979;86(3):475–84. [PubMed] [Google Scholar]
- 21.Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243(2):265–72. doi: 10.1097/01.sla.0000197715.96762.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee LC, Grant CS, Salomao DR, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152(6):965–74. doi: 10.1016/j.surg.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98(12):4784–9. doi: 10.1210/jc.2013-2604. [DOI] [PubMed] [Google Scholar]
- 24.Kendall DM, Sutherland DE, Najarian JS, Goetz FC, Robertson RP. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med. 1990;322(13):898–903. doi: 10.1056/NEJM199003293221305. [DOI] [PubMed] [Google Scholar]
- 25.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25(4):452–60. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 26.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146(5):534–8. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haigh PI, Bilimoria KY, DiFronzo LA. Early postoperative outcomes after pancreaticoduodenectomy in the elderly. Arch Surg. 2011;146(6):715–23. doi: 10.1001/archsurg.2011.115. [DOI] [PubMed] [Google Scholar]
- 28.Sukharamwala P, Thoens J, Szuchmacher M, Smith J, DeVito P. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB. 2012;14(10):649–57. doi: 10.1111/j.1477-2574.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146(2):453–60.e455. doi: 10.1053/j.gastro.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115(4):741–51. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 32.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14(3):541–8. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 33.Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204(3):356–64. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 34.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244(6):931–7. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


