Abstract
Cord blood transplantation (CBT) is curative for many patients with hematologic malignancies but is associated with delayed immune recovery and an increased risk of viral infections compared to human leukocyte antigen (HLA) matched bone marrow or peripheral blood progenitor cell transplantation. In this study we evaluated the significance of lymphocyte recovery in 125 consecutive patients with hematologic malignancies who underwent double-unit CBT (DUCBT) with an anti-thymocyte globulin-containing regimen at our institution. A subset of 65 patients were prospectively evaluated for recovery of T, natural killer (NK) and B cells and in 46 patients we also examined viral-specific T cell recovery against Adenovirus, Epstein-Barr virus, cytomegalovirus, BK virus, respiratory syncytial virus and Influenza antigen.
Our results indicate that in recipients of DUCBT, the day 30 absolute lymphocyte count is highly predictive of non-relapse mortality (NRM) and overall survival (OS). Immune recovery post-DUCBT was characterized by prolonged CD8+ and CD4+ T lymphopenia associated with preferential expansion of B and NK cells. We also observed profound delays in quantitative and functional recovery of viral-specific CD4+ and CD8+ T-cell responses for the first year post-CBT. Taken together, our data support efforts aimed at optimizing viral-specific T cell recovery to improve outcomes post-CBT.
Introduction
Umbilical cord blood (CB) is being increasingly used as a source of hematopoietic stem and progenitor cells (HSPCs) for allogeneic stem cell transplant candidates lacking suitable matched donors. Although CB transplantation (CBT) is successful in many patients, its efficacy has been restricted by slow hematopoietic and immunologic reconstitution due to the quantitative and qualitative differences in the composition of CB grafts.1–5 While the frequency of HSPCs is greater in CB units, CB grafts contain an average of 1–2 logs fewer total cells compared to peripheral blood (PB) or bone marrow (BM) allografts. Moreover, the vast majority of T, B and dendritic cells in CB grafts are immature,6;7 which likely explains the low rates of graft-versus-host disease (GVHD) seen after CBT given the degree of HLA-mismatches typically used8;9. The use of dual CB grafts represents a potentially important approach to reducing non-relapse mortality (NRM) among patients undergoing double unit CBT (DUCBT), particularly in adult patients. In this setting, although two CB units are initially transplanted, only one provides prolonged engraftment and becomes the “dominant” engrafted unit. Yet, even following DUCBT, severe complications related to infections remain a major cause of morbidity and mortality.10–15 Although this may be a consequence of the lower cell dose in CB grafts, it also reflects the relative immaturity of cord blood immune subsets.
A number of studies have reported on immune reconstitution following single CBT,16–20 but few have studied immune recovery after DUCBT.21–23 Here we report the results of a prospective longitudinal study of immune recovery and viral-specific T-cell reconstitution in recipients of double CB grafts. Our results indicate that the day 30 absolute lymphocyte count (ALC30) is highly predictive of NRM and overall survival (OS) in recipients of DUCBT who receive serotherapy for GVHD prophylaxis, and that recovery of quantitative T cells as well as recovery of functional (cytokine-producing) viral-specific T cells is delayed.
Methods
Patient selection and management
A total of 125 consecutive adult patients undergoing DUCBT at our institution from January 2006 to November 2011 were studied (Table 1). Less than half (45%) of patients were in first or second complete remission or first or second chronic phase disease, while the rest had advanced disease at the time of transplant. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki for protocols approved by the MD Anderson Cancer Center Institutional Review Board (IRB). All patients received serotherapy with rabbit thymoglobulin 1.25 mg/kg on day −4 and 1.75 mg/kg on day −3. GVHD prophylaxis consisted of tacrolimus and mycophenolate mofetil (1 gram orally twice daily), with taper of mycophenolate mofetil at day 100 and tacrolimus at 6 months if no GVHD was present. In the event of confirmed or suspected GVHD, initial therapy consisted of methylprednisolone (2 mg/kg/day), with a taper based on clinical response. The surveillance for cytomegalovirus (CMV) was performed by antigenemia assay in patients with absolute neutrophil count (ANC) >1000/µL, or with quantitative polymerase chain reaction if ANC was lower. This was done twice weekly for the first 100 days after CBT, or longer if any complications were present. Other viruses including Adenovirus (AdV), Epstein Barr virus (EBV), BK virus (BKV), respiratory syncytial virus (RSV), human herpesvirus 6 (HHV6), influenza and parainfluenza were tested as clinically indicated. Donor engraftment was assessed using the polymerase chain reaction with primer sets flanking microsatellite repeats.
Table 1.
Patient characteristics.
| N=125 | (%) | |
|---|---|---|
| Age (years), median (range) | 49 (18–73) | |
| Gender | ||
| Female | 61 | 49% |
| Male | 64 | 51% |
| Diagnosis | ||
| AML | 64 | 51% |
| ALL | 20 | 16% |
| CML/CLL | 22 | 18% |
| NHL/HL/MM | 19 | 15% |
| Disease Status | ||
| CR1/CP1 | 26 | 21% |
| CR2/CP2 | 30 | 24% |
| Advanced disease | 69 | 55% |
| Recipient CMV serostatus | ||
| Seropositive | 109 | 87% |
| Seronegative | 16 | 13% |
| Conditioning | ||
| Bu/Clo/Thiotepa | 62 | 50% |
| Flu/Bu/Clo/TBI200 cGy | 46 | 37% |
| FM | 10 | 8% |
| Flu/Bu | 7 | 6% |
| GVHD Prophylaxis | ||
| Tacrolimus /MMF | 125 | 100% |
Abbreviations: AML; acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MM, multiple myeloma; CR, complete remission; CP, chronic phase; TBI, total body irradiation; FM, fludarabine and melphalan; Bu/Flu, fludarabine and busulfan; Clo: clofarabine GVHD, graft-versus-host disease; MMF, mycophenolate mofetil.
Immunophenotyping
Immunophenotyping was performed by the flow laboratory at MDACC on peripheral blood (PB) samples collected at days +30, +100, +180 and 1 year post-CBT. PB mononuclear cells (PBMC) were surface stained with monoclonal antibodies against CD3, CD4, CD8, CD19 and CD56 (all BD Bioscences, San Jose, CA). Cells were acquired on a Cyan flow cytometer (Dako, Fort Collins, CO), and data analyzed with the FlowJo software (Tree Star, Ashland, OR).
Enzyme-Linked Immunospot (ELIspot) assay
In a subset of 46 patients, IFNγ ELIspot analysis was used to quantitate the frequency of T-cells that secreted IFNγ in response to Hexon and penton (Adv), IE1 and pp65 (CMV), EBNA1, EBNA3a-c, LMP1, LMP2, and BZLF1 (EBV), VP1 and large T (BKV), N and F (RSV), and MP1 and NP1 (Influenza) pepmixes (JPT Peptide Technologies GmbH, Berlin, Germany), all diluted to 1µg/peptide/ml. Staphylococcal Enterotoxin B (SEB) (1 µg/ml) (Sigma-Aldrich Corporation, Spring, TX) was used as positive control. PBMCs collected before and after transplant were resuspended at 2×106/ml in T cell media [Advanced RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 45% Click’s medium (Irvine Scientific, Santa Ana, CA), 2 mM GlutaMAX (Life Technologies, Grand Island, NY), and 10% Fetal Bovine Serum (Hyclone, Logan, Utah)]. Each condition was run in duplicate. After 20 hours of incubation, plates were developed, dried overnight at room temperature in the dark, and then sent to Zellnet Consulting for quantification. Spot-forming cells (SFC) and input cell numbers were plotted, and the frequency of T-cells specific to each antigen was expressed as specific SFC per input cell numbers.
Statistical analyses
Actuarial OS was estimated using the Kaplan-Meier method. The cumulative incidence of NRM was estimated considering disease progression or death attributable to malignancy as competing risks. Predictors of NRM were evaluated in landmark analysis using Cox proportional-hazards regression analysis. Engrafted patients who were alive and progression-free on the date ALC30 was measured were eligible for the risk factor analysis. ALC30 was evaluated in quartiles. All analyses were performed using STATA 11 [StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP] and statistical significance was defined at the 0.05 level.
Results
Clinical characteristics and clinical outcome
A total of 125 adult patients with high risk hematologic malignancy who underwent DUCBT during the study period were assessed, with a median follow-up of 979 (range 56–1907) days in surviving patients. Details of the conditioning regimens are included in Table 1. Thirty percent of patients received two unmanipulated CB units while 70% received one unmanipulated and one CB unit which was expanded prior to infusion as previously described.24 Successful neutrophil engraftment, as defined by the first date of three consecutive days of absolute neutrophil count ≥ 0.5×109/L, was achieved in 110 patients, while 9 patients experienced primary graft failure. Early death occurred in 6 patients before donor engraftment could be evaluated. The median times to neutrophil and platelet engraftment were 15 (range 4–45) and 36 (range 7–126) days, respectively. Donor engraftment data at day 30 were evaluable for 92 patients (Table 2) and revealed predominance of a single CB unit in 41 patients, while 50 patients had evidence of persistent host hematopoiesis. In 65 patients, full donor engraftment was achieved with a combination of both CB units. One patient could not be assessed due to delayed engraftment. At day 100 post-DUCBT, the median percent of donor-derived cells detected from the dominant unit was 100% (n=70), ranging from 14–100% (inter-quartiles range 82–100%).
Table 2. Donor engraftment at days +30 and +100 post-DUCBT.
Donor engraftment for total, T-cell (T) and myeloid cells (M) fractions are presented as median% (interquartile range).
| D30 | D100 | |||||
|---|---|---|---|---|---|---|
| Total | T | M | Total | T | M | |
| Overall | 100% (97–100) | 100% (100) | 100% (99–100) | 100% (100) | 100% (100) | 100% (100) |
| CB1 | 82% (39–100) | 99% (75–100) | 91% (42–100) | 99% (60–100) | 100% (69–100) | 100% (55–100) |
| CB2 | 0% (0–31) | 0% (0–16) | 0% (0–28) | 0% (0–16) | 0% (0–4) | 0% (0–15) |
Predictors of Non-Relapse Mortality and Overall Survival
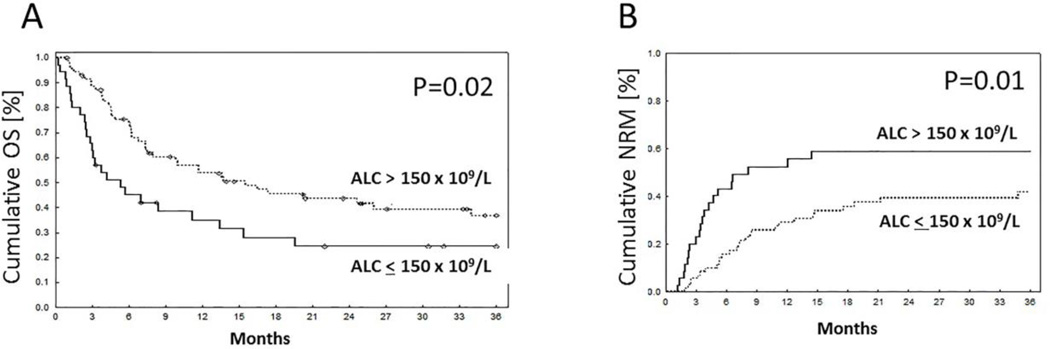
A total of 108 engrafted patients were eligible for assessment of predictors of 3-year NRM in a landmark analysis starting on day 30. In univariate analysis, two factors that significantly affected NRM were day 30 absolute lymphocyte count (ALC30), treated as dichotomous variables above or below 150 × 106/L (upper limit of first quartile), and a diagnosis of acute lymphoblastic leukemia (ALL). The median (range) of ALC30 was 240 (10–2420) × 106/L. There was no significant impact of age, sex, disease stage at the time of DUCBT, CMV serostatus, the occurrence of grade II-IV acute GVHD before day 30, total nucleated cell dose (TNC) dose, total CD34 dose, preparative regimen or CB manipulation on NRM (Table 3). In multivariate analysis, ALC30 (HR = 2.3, P = 0.01) and diagnosis of ALL (HR=2.6, P = 0.007) emerged as independent factors strongly associated with NRM. Figure 1 shows the impact of ALC30 on NRM and OS in all patients. For patients with ALC30 > 150× 106/L, OS at 3 years was 37% (95% CI 25–49) compared with 25% (95% CI 11–40) for those with counts ≤ 150×106/L (P = 0.02). Similarly, ALC30 > 150×106/L was related to a lower risk of NRM, 42% (95% CI 31–56%) vs 59% (95% CI 44–78%) (P = 0.01) in patients with ALC30 ≤ 150×106/L. The leading causes of NRM in both the groups (ALC30≤150 vs. ALC30 >150) included infections (50% vs. 52%, P=0.9) and GVHD (40% vs 30%, P=0.5). There were no differences in the type of infections noted between the groups. Moreover, there was no significant impact of ALC30 on the rate of grade II–IV aGVHD (P = 0.4), disease progression (P = 0.7) or progression-free survival (P = 0.07) at 2 years. (Table 4)
Table 3.
Predictors of non-relapse mortality (NRM) by univariate and multivariate analysis.
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Total *N=108 |
3 years HR |
95% CI | p | 3 years HR | 95% CI |
p | |
| Day 30 ALC (×106/L), quartiles | |||||||
| ≤150 | 35 | Ref. | |||||
| >150–250 | 22 | 0.4 | 0.2–1.02 | 0.05 | |||
| >250–400 | 22 | 0.5 | 0.2–1.2 | 0.1 | |||
| >400 | 29 | 0.4 | 0.2–0.9 | 0.04 | |||
| ≤150 vs >150 | 2.1 | 1.2–3.8 | 0.01 | 2.3 | 1.3–4.1 | 0.01 | |
| Diagnosis | |||||||
| AML | 57 | Ref. | |||||
| ALL | 17 | 2.2 | 1.1–4.7 | 0.03 | |||
| CML/CLL | 3/13 | 0.8 | 0.3–2.1 | 0.7 | |||
| NHL/HL/MM | 18 | 1.1 | 0.5–2.6 | 0.8 | |||
| ALL vs. all other | 2.3 | 1.2–4.5 | 0.02 | 2.6 | 1.3–5.1 | 0.01 | |
| aGVHD grade II-IV before Day 30 | |||||||
| No | 89 | Ref. | |||||
| Yes | 17 | 1.7 | 0.8–3.4 | 0.15 | N/A | ||
| Unknown | 2 | excluded | |||||
| Gender | |||||||
| Male | 53 | Ref. | |||||
| Female | 55 | 1.6 | 0.9–2.8 | 0.1 | N/A | ||
| Age, years | |||||||
| ≤45 | 51 | Ref. | |||||
| >45 | 57 | 1.2 | 0.7–2.1 | 0.5 | N/A | ||
| ≤50 | 61 | Ref. | |||||
| >50 | 47 | 0.9 | 0.5–1.5 | 0.6 | N/A | ||
| ≤55 | 76 | Ref. | |||||
| >55 | 32 | 0.9 | 0.4–1.7 | 0.7 | N/A | ||
| Disease Status | |||||||
| CR1/CP1 | 19 | Ref. | N/A | ||||
| CR2/CP2 | 29 | 2.1 | 0.8–5.3 | 0.1 | |||
| Advanced disease | 60 | 1.5 | 0.6–3.6 | 0.4 | |||
| CR1/CP1 vs all other | 0.6 | 0.2–1.4 | 0.2 | ||||
| Total TNC dose infused | |||||||
| QRT1 | 30 | Ref. | N/A | ||||
| QRT2 | 23 | 1.3 | 0.6–3.1 | 0.5 | |||
| QRT3 | 28 | 1.2 | 0.5–2.6 | 0.7 | |||
| QRT4 | 27 | 1.6 | 0.5–2.6 | 0.7 | |||
| QRT1 vs >QRT1 | 0.8 | 0.4–1.6 | 0.5 | ||||
| Total CD34 dose infused | |||||||
| QRT1 | 26 | Ref. | N/A | ||||
| QRT2 | 26 | 1.2 | 0.5–2.9 | 0.7 | |||
| QRT3 | 28 | 1.4 | 0.6–3.2 | 0.4 | |||
| QRT4 | 28 | 1.1 | 0.5–2.7 | 0.8 | |||
| QRT1 vs >1 | 0.8 | 0.4–1.6 | 0.5 | ||||
| Recipient CMV serostatus | |||||||
| Reactive | 95 | 1.1 | 0.4–2.8 | 0.8 | N/A | ||
| Non-reactive | 13 | Ref. | |||||
excluded from this analysis are patients who had primary graft failure or who died or progressed before day 30 ALC was measured.
Abbreviations: ALC, absolute lymphocyte count; HR, hazard ratio; QRT, quartile; CI, confidence interval.
Figure 1. Survival outcome based on ALC30.
Impact of ALC30 ≤ or > 150× 106/L on (A) OS; (B) NRM.
Table 4.
Impact of day 30 ALC on disease progression and aGVHD outcomes at 2 years
| Progression | PFS | Grade II–IV Acute GVHD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 30 ALC | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| QRT1 | Ref. | Ref. | Ref | ||||||
| QRT2 | 1.3 | 0.5–3.7 | 0.6 | 0.7 | 0.4–1.2 | 0.2 | 0.8 | 0.3–1.9 | 0.6 |
| QRT3 | 1.1 | 0.4–3.3 | 0.8 | 0.7 | 0.4–1.3 | 0.3 | 0.9 | 0.4–2.0 | 0.8 |
| QRT4 | 1.1 | 0.4–2.9 | 0.9 | 0.6 | 0.3–1.1 | 0.09 | 0.6 | 0.2–1.4 | 0.2 |
| QRT 1 vs all other | 0.85 | 0.4–1.9 | 0.7 | 1.5 | 0.96–2.4 | 0.07 | 1.3 | 0.7–2.6 | 0.4 |
Abbreviations: PFS, progression free survival; ALC, absolute lymphocyte count; HR, hazard ratio; QRT, quartile; CI, confidence interval.
Lymphocyte subset analysis
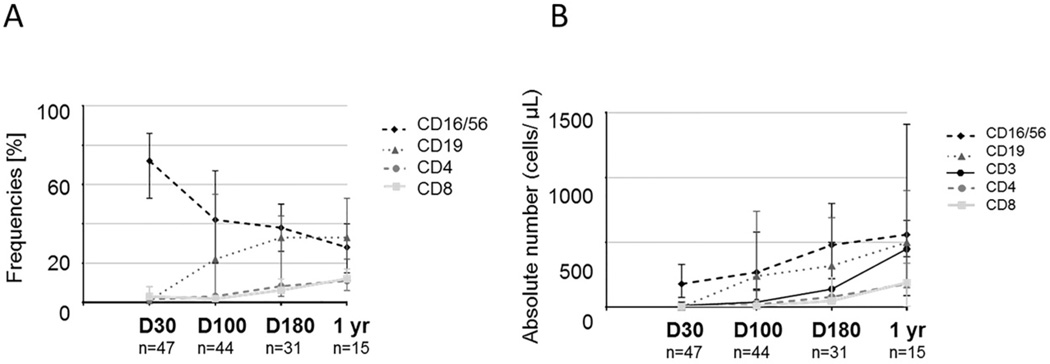
In 65 patients for whom PB samples had been collected at days +30, +100, +180 or 1 year post-DUCBT, we further characterized immune subset recovery by measuring the frequencies and absolute numbers of CD4+ T cells, CD8+ T cells, CD56+CD3− NK cells, and CD19+ B cells (Figure 2). The absolute number of each cell subset was calculated by multiplying their frequencies as determined by flow cytometry by the absolute lymphocyte number (cells/µl) obtained from a diagnostic complete blood count performed on the same day. Lymphocyte reconstitution following DUCBT began with a rapid increase in both the absolute number and frequencies of NK cells over baseline norms, and remained increased at the different study intervals, though the percentage of NK cells declined as T-cell counts recovered. The absolute B cell recovery followed a similar pattern to NK cells, with an initially rapid recovery followed by a return to baseline by 1 year post-DUCBT. T cell reconstitution on the other hand was delayed. CD4+ and CD8+ T cells declined after conditioning and were significantly reduced by day 30 post-DUCBT. The median absolute number of CD8+ cytotoxic T cells was 6 × 106/L (range 0–170) at 30 days and 11 × 106/L (range 0–1900) at 100 days post-transplant. The corresponding numbers of CD4+ helper T cells were 4 × 106/L (range 0–100) and 22 × 106/L (range 0–390), respectively. These results confirm that quantitative T cell recovery is delayed after DUCBT, with an inverted CD4/CD8 T cell ratio, and that this delay in T cell immunity is associated with a preferential rapid reconstitution of non-T lymphoid cells (eg, NK cells and B cells). Patients who developed acute GVHD had a slower T and B cell recovery (data not shown), in keeping with previous reports following allogeneic stem cell transplantation.25
Figure 2. Prolonged T lymphopenia and relative expansion of NK cells and B cells following DUCBT.
T (CD3+CD4+ and CD3+CD8+), B (CD19+), and NK (CD56+) cells were prospectively measured by multiparameter flow cytometry on fresh samples; (A) frequencies; (B) absolute numbers (× 106/L) for each immune subset are presented. At baseline and after DUCBT, CD4+ and CD8+ T cells were relatively reduced, whereas early B-cell and NK-cell recovery was evident. Surviving CB transplant recipients demonstrated rebound of CD4+ and CD8+ T cells at later intervals after transplantation. Error bars represent interquartile range.
Viral infections and viral-specific T cell recovery post-CBT
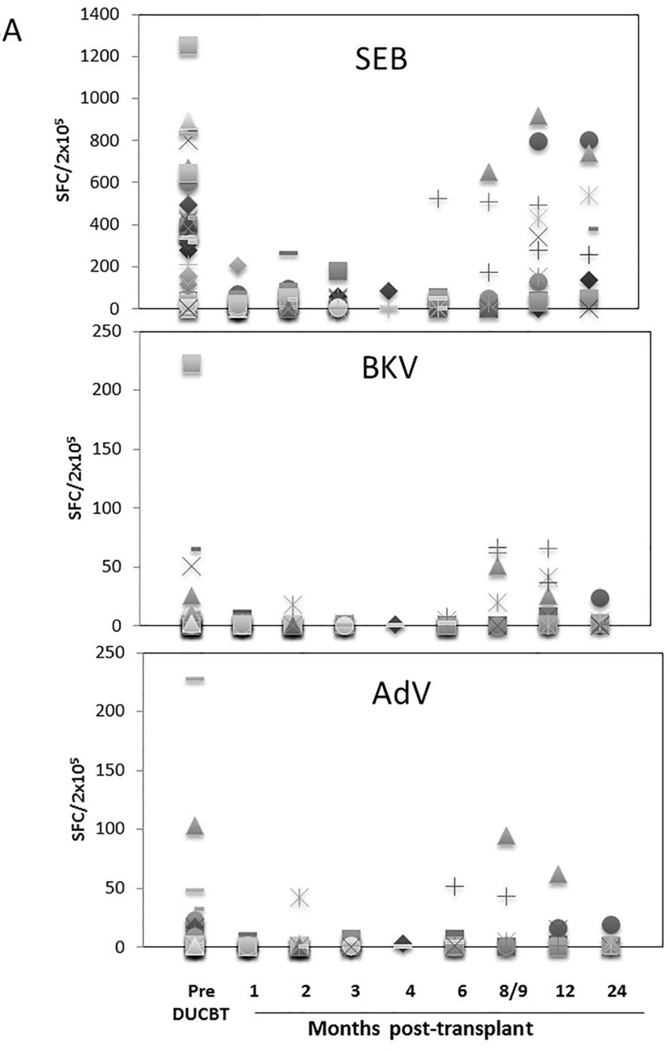
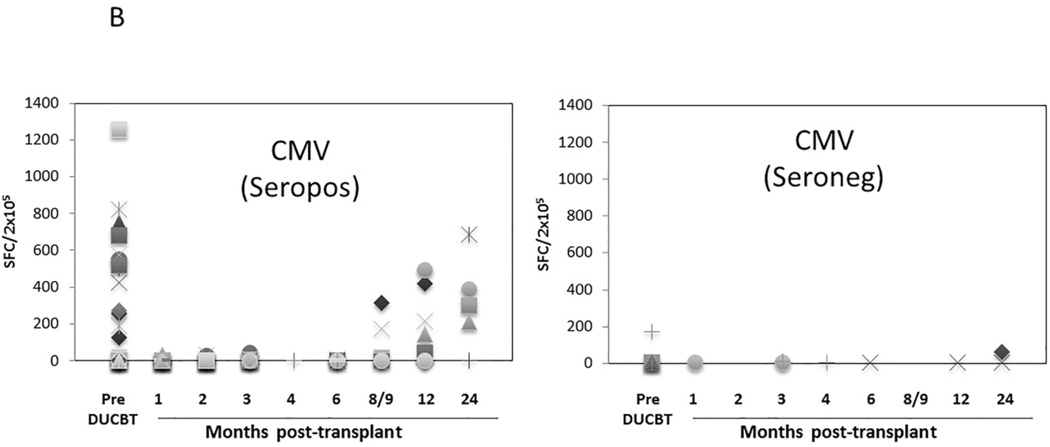
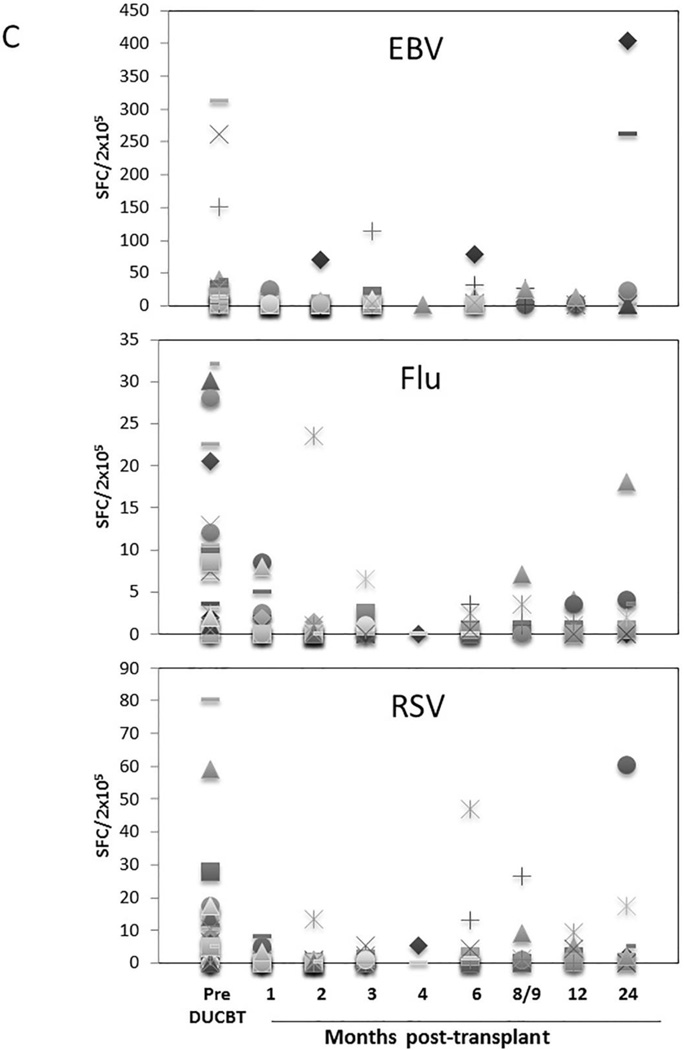
Functional immune reconstitutional studies were performed under a Baylor College of Medicine IRB-approved protocol. To assess the tempo of functional virus-specific T cell recovery after DUCBT, we stimulated PBMC from 46 transplant recipients with 15-mer overlapping peptides spanning T cell immunogenic antigens from a range of both latent (CMV, EBV, BKV) and community (AdV, Influenza and RSV) viruses as well as to SEB, which was used as a positive control. Prior to transplant the frequency of T cells reactive against SEB was highest (mean 424.5 SFC/2×105 PBMCs), followed by CMV (mean 258 SFC in 39 seropositive donors), EBV (mean 33 SFC), AdV (mean 17 SFC), BKV (mean 13 SFC), Influenza (mean 8 SFC), and RSV (mean 10 SFC) (Figure 3). As shown in Figures 3A and 3B, T cell activity against SEB, CMV, EBV, and AdV was delayed for at least 8–9 months following DUCBT (mean 200, 89, 28 and 24 SFC/2×105, respectively), while activity against BKV, Influenza and RSV was even further delayed (Figure 3C). To assess whether delayed functional viral-specific T cell recovery had clinical consequences in these 46 patients, we correlated viral-specific immune recovery with viral infections post DUCBT. The majority (72%) of the patients assessed for functional recovery developed viral infections/ reactivations post-DUCBT (summarized in Table 5). The most common causes of viral infection included CMV (59%), BKV (20%) and AdV (11%). The majority of these infections occurred within the first 100 days post DUCBT and nearly 50% of patients had infections with multiple viruses. Within the constraints of relatively small number of patients assessed, we did not observe a difference in day 30 ALC among patients who did or did not develop viral infections/ reactivations post-DUCBT. Similarly we did not find any differences in patients who acquired infections and those who did not with respect to their viral immune reconstitution.
Figure 3. Virus-specific T cell activity following DUCBT.
(A) Frequencies of SEB, BKV (Large T and VP1) and AdV (Hexon and Penton)-reactive T cells, (B) frequency of CMV (IE1 and pp65)-specific T cells in seropositive and seronegative donors, and (C) frequencies of EBV (LMP1, LMP2, EBNA1, EBNA3a, EBNA3b, EBNA3c, BZLF1), Influenza (MP1 and NP1) and RSV (N and F) reactive T cells in PB samples collected from patients prior to and post-DUCBT using IFNγ ELIspot as readout. Each symbol represents an individual patient and results represent the SFC/2×105 input cells.
Table 5.
Viral reactivation or infection in 46 patients post DUCBT in whom viral-specific T cell recovery was examined.
| Viral Infection type | Number of patients with incident infection |
Median days to diagnosis of infection |
Range | |
|---|---|---|---|---|
| Viremia/ DNAemia |
End organ involvement |
|||
| CMV | 19 | 8 | 31 | (13–77) |
| BKV | 9 | - | 48 | (12–97) |
| HHV6 | 2 | - | 66 | |
| Parvovirus | - | 1 | 69 | |
| Adenovirus | 2 | 3 | 70 | (44–85) |
| HSV | - | 1 | 139 | |
| EBV | - | 2 | 254 | |
| RSV | - | 1 | 425 | |
| Parainfluenza 1–3 | 1 | 3 | 455 | (98–1237) |
| Influenza B | - | 1 | 799 | |
| Total Number of incident infections | 33 | 20 | 44 | (12–1237) |
| Number of different types of viral infection / patient |
Number of patients | % |
|---|---|---|
| One | 18 | 55% |
| Two | 10 | 30% |
| Three | 5 | 15% |
| Total patients with at least 1 type of viral infection | 33 | 100% |
Abbreviations: CMV, cytomegalovirus; BKV, BK virus; EBV, Epstein Barr virus; HHV6, human herpesvirus 6; HSV, herpes simplex virus; RSV, respiratory syncytial virus
Discussion
Our study of 125 consecutive adult patients receiving DUCBT with a median follow-up of 32 months is the largest series to date reporting on the significance of lymphocyte recovery and the kinetics of immune reconstitution and viral-specific T cell immunity in this setting. Despite the heterogeneity of this population, this study confirms previous reports of early lymphocyte recovery as a prognostic factor for outcome after CBT.26–32
Lymphocyte subset analysis in 65 patients revealed that CD4+ and CD8+ T-cell reconstitution was significantly delayed, when compared to reported recovery in recipients of matched sibling or matched unrelated donor hematopoietic stem cell transplant recipients, in agreement with previous reports in smaller cohorts of patients in the setting of single or double CBT.16;21–23;27;27;33 Ruggeri and colleagues reported on clinical outcome and immune recovery in 35 patients with high-risk hematological diseases undergoing DUCBT.21 Immune reconstitution studies, including measures of thymopoiesis as assessed by T cell receptor excision circle (TREC) analysis, revealed delayed T cell recovery for the first 9 months post DUCBT.21 Studies in the non-myeloablative DUCBT setting by Somers and colleagues revealed early T and NK cell engraftment, followed by predominance of myeloid cells by day 18.23
As a direct consequence of delayed immune recovery, recipients of CBT are at significant risk of opportunistic infections, particularly viral infections including CMV, EBV, AdV, and BK virus.34–37 Indeed, when we prospectively and longitudinally assessed the frequency of functional virus-directed T cells directed against a range of immunogenic CMV (IE1, pp65), EBV (EBNA1, EBNA3a, 3b,3c, LMP1, LMP2, BZLF1), BKV (VP1, LT1), AdV (hexon and penton), Influenza (MP1, NP1) and RSV (N and F) antigens, we saw a significant delay (9–12 months) in the recovery of IFNγ-secreting precursors, demonstrating the inability of immature CB T cells to initiate early primary immune responses to pathogens. This explains the high rates of viral infections observed associated with post DUCBT. Interestingly, the observation that in the early posttransplantation period, most PB lymphocytes are NK cells, which can mediate cytotoxicity without prior sensitization, suggest that NK cells may be responsible for protection against viral reactivation early following CBT.
DUCBT is associated with a higher rate of aGVHD than single CBT.38 In our cohort the incidence of aGVHD grade II-IV was around 40%. Acute GVHD and its treatment with corticosteroids and other immunosuppressive agents may account for some of the delay in immune reconstitution seen following DUCBT. To improve engraftment and limit GVHD, we used in vivo T-cell depletion with ATG in all our conditioning regimens, which likely contributes further to delayed T cell recovery. The impact of ATG on infection outcomes after CBT is debated in the absence of a randomized trial. While some studies suggest an increased risk of opportunistic infections and viral reactivations with the use of high dose ATG after reduced intensity conditioning regimens, yet similar association was not observed after myeloablative conditioning regimens.35 Other studies using ATG did not reveal increased risk of infections after CBT.4 More recently, use of high dose rabbit ATG in pediatric patients undergoing CBT was shown to be associated with lower incidence of acute GVHD at the expense of higher rate of viral infections.39
Taken together, our results confirm that delayed immune reconstitution, and consequently infections, remain major complications after DUCBT when compared to HLA-matched marrow or peripheral blood progenitor cell transplantation, with slower quantitative recovery of T lineage immune cell populations and more rapid NK and B cell reconstitution5;22;38;40. This information is essential to improve our understanding of immune reconstitution, and to develop strategies to accelerate immune recovery after CB transplantation. Our group is exploring a number of novel approaches in preclinical and clinical studies, such as ex-vivo graft manipulation and the use of adoptively transferred viral-specific mature T cells,24;41 to improve immune reconstitution and clinical outcomes in patients undergoing CBT.
Highlights.
Immune reconstitution after double unit CBT begins with a rapid increase in NK cells.
Functional virus-specific T-cell recovery is delayed for at least 9–12 months post-transplant.
Day 30 absolute lymphocyte count predicts non-relapse mortality and overall survival.
Acknowledgments
The authors are grateful to the patients and their families. The authors would like to acknowledge our PharmDs, the nursing staff, research coordinators, and cell therapy laboratory staff.
Footnotes
Authors’ contribution: RS and KR contributed equally to this paper. Substantial contributions to conception and design (EJS, KR, AL, CB, JJ NS, CH, BO, AO, RM, RC), acquisition of data or analysis (RS, EJS, KR, AL, CB), interpretation of data (EJS, KR, AL, CB, JJ NS, CH, BO, AO, RM, RC), drafting the article or revising it critically for important intellectual content (EJS, KR, AL, CB, JJ NS, CH, BO, AO, RM) and final approval of the version to be published (EJS, KR, AL, CB, JJ NS, CH, BO, AO, RM, RC, IMS).
Conflicts of interest: The authors have no conflicts of interest to declare.
Reference List
- 1.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N. Engl. J. Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 2.Escalon MP, Komanduri KV. Cord blood transplantation: evolving strategies to improve engraftment and immune reconstitution. Curr. Opin. Oncol. 2010;22:122–129. doi: 10.1097/cco.0b013e328335a56e. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N. Engl. J. Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 4.Sanz J, Sanz MAFAU, Saavedra S, Saavedra SFAU, Lorenzo I, et al. Cord blood transplantation from unrelated donors in adults with high-risk acute myeloid leukemia. Biol. Blood Marrow Transplant. 2010;16:86–94. doi: 10.1016/j.bbmt.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2012;18:1664–1676. doi: 10.1016/j.bbmt.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YJ, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev. Oncol. Hematol. 2011;79:112–126. doi: 10.1016/j.critrevonc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danby R, Rocha V. Improving Engraftment and Immune Reconstitution in Umbilical Cord Blood Transplantation. Front Immunol. 2014;5:68. doi: 10.3389/fimmu.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha V, Wagner JE, Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N. Engl. J. Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N. Engl. J. Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 10.Brunstein CG. Umbilical cord blood transplantation for the treatment of hematologic malignancies. Cancer Control. 2011;18:222–236. doi: 10.1177/107327481101800403. [DOI] [PubMed] [Google Scholar]
- 11.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutman JA, Leisenring W, Appelbaum FR, Woolfrey AE, Delaney C. Low relapse without excessive transplant-related mortality following myeloablative cord blood transplantation for acute leukemia in complete remission: a matched cohort analysis. Biol. Blood Marrow Transplant. 2009;15:1122–1129. doi: 10.1016/j.bbmt.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118:5689–5696. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler C, Stevenson KF, Kim HTFAU, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Biol. Blood Marrow Transplant. 2011;46:659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komanduri KV, St John LS, de LM, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niehues T, Rocha V, Filipovich AH, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children -- a Eurocord analysis. Br. J. Haematol. 2001;114:42–48. doi: 10.1046/j.1365-2141.2001.02900.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- 20.Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol. Blood Marrow Transplant. 2001;7:454–466. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri A, Peffault de LR, Carmagnat M, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl. Infect. Dis. 2011;13:456–465. doi: 10.1111/j.1399-3062.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somers JA, Brand A, van HY, et al. Double umbilical cord blood transplantation: a study of early engraftment kinetics in leukocyte subsets using HLA-specific monoclonal antibodies. Biol. Blood Marrow Transplant. 2013;19:266–273. doi: 10.1016/j.bbmt.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 24.deLima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N. Engl. J. Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porrata LF, Gertz MAFAU, Inwards DJFAU, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson CA, Turki ATFAU, McDonough S, McDonough SMFAU, Stevenson K, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einsele H, Ehninger G, Steidle M, et al. Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood. 1993;82:1672–1678. [PubMed] [Google Scholar]
- 29.Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 30.Chakrabarti S, Collingham KE, Marshall T, et al. Respiratory virus infections in adult T cell-depleted transplant recipients: the role of cellular immunity. Transplantation. 2001;72:1460–1463. doi: 10.1097/00007890-200110270-00024. [DOI] [PubMed] [Google Scholar]
- 31.Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br. J. Haematol. 2004;125:217–224. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Chen MG, Gastineau DA, et al. Lymphocyte recovery after allogeneic bone marrow transplantation predicts risk of relapse in acute lymphoblastic leukemia. Leukemia. 2003;17:1865–1870. doi: 10.1038/sj.leu.2403055. [DOI] [PubMed] [Google Scholar]
- 33.Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp. Hematol. 2001;29:371–379. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- 34.Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol. Blood Marrow Transplant. 2010;16:215–222. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunstein CG, Weisdorf DJ, DeFor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheurer ME, Pritchett JC, Amirian ES, et al. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2013;48:574–580. doi: 10.1038/bmt.2012.180. [DOI] [PubMed] [Google Scholar]
- 37.Silva LP, Patah PA, Saliba RM, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storek J, Joseph AF, Espino GF, et al. Immunity of patients surviving 20 to 30 years after allogeneic or syngeneic bone marrow transplantation. Blood. 2001;98:3505–3512. doi: 10.1182/blood.v98.13.3505. [DOI] [PubMed] [Google Scholar]
- 39.Lindemans CA, Chiesa RF, Amrolia PJFAU, Rao K, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–132. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 40.Small TN, Papadopoulos EBFAU, Boulad FF, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. [PubMed] [Google Scholar]
- 41.Hanley PJ, Cruz CR, Shpall EJ, Bollard CM. Improving clinical outcomes using adoptively transferred immune cells from umbilical cord blood. Cytotherapy. 2010;12:713–720. doi: 10.3109/14653249.2010.517518. [DOI] [PMC free article] [PubMed] [Google Scholar]