Abstract
We sought to address the significance of isolated follicles that exhibit atypical morphologic features that may be mistaken for lymphoma in a background of reactive lymphoid tissue. Seven cases that demonstrated centroblast-predominant isolated follicles and absent BCL2 staining in otherwise-normal lymph nodes were studied. Four of seven cases showed clonal B-cell proliferations amid a polyclonal B cell background; all cases lacked the IGH-BCL2 translocation and BCL2 protein expression. Although three patients had invasive breast carcinoma at other sites, none were associated with systemic lymphoma up to 44 months after diagnosis. The immunoarchitectural features of these highly unusual cases raise the question of whether a predominance of centroblasts and/or absence of BCL2 expression could represent a precursor lesion or atypical reactive phenomenon. Differentiating such cases from follicular lymphoma or another mimic is critical, lest patients with indolent proliferations be exposed to unnecessarily aggressive treatment.
Introduction
Unusual growth patterns of atypical large cells associated with lymph node follicles have been previously described and range from reactive hyperplasias to germinotropic lymphomas. In recent years, recognition of aberrant strong coexpression of the germinal center marker CD10 and the anti-apoptotic protein BCL2, has been described within isolated secondary follicles of otherwise-normal lymph nodes.[1] Since its recognition, this phenomenon, variously termed ‘in situ localization of follicular lymphoma’, ‘follicular lymphoma in situ’ (FLIS) or ‘follicular lymphoma-like lesion of uncertain clinical significance’, has been described in histologically unremarkable lymph nodes from healthy patients, normal lymph nodes in patients with a prior or concurrent diagnosis of follicular lymphoma (FL), lymph nodes involved by a variety of other hematolymphoid malignancies, and lymph nodes removed during the evaluation of non-hematolymphoid neoplasms.[1–9]
The clinical significance of FLIS is still of uncertain significance, particularly because numerous studies have reported the presence of non-neoplastic cells that harbor IGH-BCL2 gene rearrangements in the peripheral blood and tissues of patients with no evidence of FL.[10–20] Diagnostic criteria for identifying FLIS and guidelines for distinguishing this pattern of involvement from cases of partial lymph node involvement by FL have been proposed in an attempt to stratify patients into risk groups.[21–23] To date the reported cases in the literature contain aberrant strong coexpression of CD10 and BCL2 within follicles containing predominantly centrocytes amid otherwise-normal lymph nodes.[3–9, 21]
Herein, we report seven cases characterized by isolated abnormal follicles composed of atypical centroblasts within otherwise-normal lymph nodes that show significant differences from previously described cases. Detailed morphologic and immunophenotypic characterization was undertaken, and in cases with sufficient tissue, cytogenetics and molecular studies for clonal IGH@ gene rearrangements were performed. The diagnostic challenges and clinical implications of these unusual findings are described.
Materials and Methods
Case Selection
A search of the Department of Pathology database at Stanford University Medical Center to include the terms “atypical follicles”, "follicular lymphoma in situ," and "follicular lymphoma-like B-cells", yielded cases received between 2010 and 2014. These showed either an abnormal pattern of strong CD10 and BCL2 coexpression within otherwise-normal secondary follicles or isolated abnormal follicles composed predominantly of centroblasts within lymph nodes with preserved architecture. The latter group of cases was selected for inclusion. Institutional Review Board approval was obtained from Stanford University for these studies. Clinical information including follow-up in the form of subsequent lymph node samples, staging bone marrow biopsies, imaging studies and clinical evaluations was recorded. Patient records and information were anonymized and de-identified prior to analysis.
Histologic Assessment
The morphologic characteristics of each case were recorded in a manner similar to reports of follicular lymphoma in situ,[21] including the total number of follicles within the lymph node, the number of abnormal follicles involved, and the grade (ie: number of centrocytes and centroblasts) of the abnormal follicles, as defined by the 2008 World Health Organization classification.[24]
Immunohistochemistry
Immunohistochemical stains were performed on 4-micron thick, formalin-fixed, paraffin-embedded whole tissue sections using automated staining platforms (BenchMark XT, Roche/Ventana Medical Systems, Tucson, AZ or Leica BOND-MAX, Leica Microsystems Inc, Buffalo Grove, IL). Primary antibodies and conditions used for immunohistochemistry are detailed in Table 1 and were performed in all cases where involved follicles were present on sufficient numbers of sections. Interpretation of staining intensity and patterns for CD10, BCL2, IgM, HGAL and Ki67 in lesional follicles was performed by comparison to background reactive secondary follicles, particularly, mantle zone B-cells and T-cells, respectively.
Table 1. Reagents and Conditions Used for Immunohistochemistry.
| Antibody | Clone/Source | Dilution | Platform and Pretreatment |
|---|---|---|---|
| CD20 | L26a | 1:1000 | BOND-MAX, ER2 retrieval |
| CD3 | Rabbit Polyclonalb | 1:100 | BenchMark XT, Standard retrieval |
| CD10 | 56C6c | 1:20 | BOND-MAX, ER2 Retrieval |
| BCL2 | 124a | 1:50 | BenchMark XT, Standard retrieval |
| BCL6 | GL191E/A8b | 1:100 | BenchMark XT; Standard retrieval |
| HGAL | MRQ-49b | 1:100 | BOND-MAX, ER2 retrieval |
| IgM | Polyclonala | 1:1250 | BenchMark XT; Standard retrieval |
| IRF4 | MUM1Pa | 1:40 | BOND-MAX, ER1 retrieval |
| Ki67 | MIB-1a | 1:200 | BOND-MAX, ER2 retrieval |
| CD21 | IF8a | 1:80 | BOND-MAX, ER2 retrieval |
| CD23 | 1B12c | 1:50 | BOND-MAX, ER2 retrieval |
| D2-40 | D2-40a | 1:80 | BOND-MAX, no retrieval |
| Kappa | Rabbit polyclonala | 1:1000 | BenchMark XT; Protease 2 retrieval |
| Lambda | Rabbit polyclonala | 1:4000 | BenchMark XT; Protease 2 retrieval |
aDako, Carpinteria, CA
bCell Marque, Rocklin, CA
cNovocastra, Newcastle upon Tyne, UK
Fluorescence In situ Hybridization (FISH)
Four micron formalin-fixed, paraffin-embedded tissue sections were analyzed for BCL2, IRF4 and BCL6 gene rearrangements by fluorescence in situ hybridization using a 5’/3’ breakapart probes for the BCL2 gene (ZytoVision, Bremerhaven, Germany), IRF4 gene (Empire Genomics, Buffalo, NY) and the BCL6 gene (ZytoVision). Briefly, using a Vysis VP2000™ slide pretreatment instrument and reagents (Abbott Molecular, Chicago, IL), slides were deparaffinized with CitroSolv™, digested with a 10% pepsin solution at 37°C, pre-treated with a sodium thiocyanate solution at 80°C, re-fixed in 10% buffered formalin, and dehydrated in an ethanol series. Dried, dehydrated slides were mounted with probe solution per manufacturer’s instructions, denatured with a Vysis® HYBrite instrument at 80°C for six minutes and hybridized for 48 hours at 37°C. Slides were washed with 2xSSC/0.3% NP-40 at 73°C for two minutes, counterstained with DAPI and analyzed with an Olympus BX51 microscope equipped with an 100x oil immersion objective, appropriate fluorescence filters and CytoVision® imaging software (LeicaBiosystems, Buffalo Grove, IL).
Genotyping
Polymerase chain reaction (PCR) for clonal IGH@ gene rearrangements was performed on DNA obtained from paraffin-embedded tissue samples in a subset of cases using the IGH Gene Rearrangement Assay (InvivoScribe, San Diego, CA) according to the manufacturer’s instructions. Three cases (cases #2, 3 and 5) in which unstained sections were available were further evaluated by microdissection. To accomplish this, unstained sections were overlaid on H&E stained sections and the area of interest marked and removed by scraping. Desired portions were extracted using proteinase K digestion followed by QIAgen DNA column purification (QIAgen Inc, Valencia, CA). Elution volume of 22uL with heat and shaking were used with a yield of 19uL. Clonality was assessed using Invivoscribe B-clonality kit, primer mix A (FR1-JH) and primer mix F (amplification control). Tube F was used to assess amplifiability and to estimate yield. No spectrophotometric assessment of yield was performed due to very low amount of sample available for these assays. Uninvolved nodes were subjected to clonality assessment in parallel. All except the uninvolved sample of case 5, among the three paired samples yielded amplifiable DNA. Analyses of amplicons were performed by fragment analysis using capillary electrophoresis on the ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Results
Clinical Features
The seven patients in this cohort included five women aged from 51 to 61 years old and two men aged six and 61. Anatomic sites of involvement included peripheral as well as internal sites such as periaortic lymph node and rectal mucosa-associated lymphoid tissue. Six cases were sampled during evaluation for unrelated medical conditions (three axillary lymph node dissections for invasive ductal carcinoma of the breast, one periaortic lymph node in a patient with cholecystitis, a tonsillectomy in a six-year old boy, and a colonoscopic biopsy for workup of intractable diarrhea) and seventh was a cervical lymph node removed for lymphadenopathy. Although three of the patients had invasive ductal carcinoma of the breast, none of the patients had associated follicular lymphoma (or other subtypes of lymphoma), detected either in the same lymph node or systemically at the time of biopsy. Bulky lymphadenopathy was not detected in any of the patients in our cohort. A summary of clinical features and follow-up is presented in Table 2.
Table 2. Summary of Clinical and Histologic Findings.
| Case | Age/Sex | Anatomic site | Degree of Involvement by atypical follicles | Clinical Information and Follow-Up |
|---|---|---|---|---|
| 1 | 61 F | Axillary lymph node | <5 follicles; <50% | Remote history of radiation for cutaneous T-cell lymphoma; breast carcinoma; no cutaneous or systemic lymphoma at diagnosis or at 44 months. |
| 2 | 53 F | Axillary lymph node | <5 follicles; <50% | Breast carcinoma; no systemic lymphoma at diagnosis or at 14 months. |
| 3 | 58 F | Axillary lymph node | <5 follicles; <50% | Breast carcinoma; no systemic lymphoma detected at diagnosis |
| 4 | 53 F | Periaortic lymph node | <5 follicles; <50% | No systemic lymphoma detected at diagnosis |
| 5 | 6 M | Tonsil | <5 follicles; <50% | No systemic lymphoma detected at diagnosis or at 27 months. |
| 6 | 51 F | Rectum | <5 follicles; >50% | No systemic lymphoma detected at diagnosis |
| 7 | 61 M | Cervical lymph node | <5 follicles; <50% | No systemic lymphoma detected at diagnosis or at 2 months. |
Histologic Features
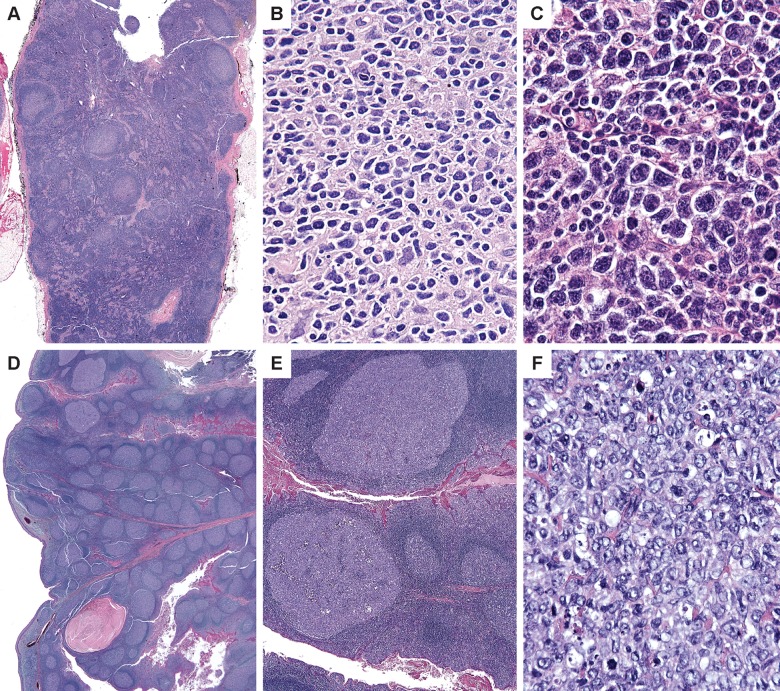
The seven cases in our series showed scattered follicles composed almost exclusively of centroblasts localized to isolated follicles in lymph nodes with preserved normal architecture (Fig 1). Morphologically, the cases demonstrate abnormal follicles composed of a cluster of cytologically atypical centroblasts. In each case, these follicles were few and comprised a minority of the node (<5%) with surrounding germinal centers demonstrating normal cytologic and architectural features. Although a diagnosis of partial involvement by follicular lymphoma was considered, these follicles were scattered singly throughout the nodes, a pattern that is not in keeping with that consideration. These follicles, however, lacked normal components of germinal centers with no tingible body macrophages and absent polarization (Fig 1).
Fig 1. Morphology of isolated atypical follicles.
An axillary lymph node dissection in a 61 year-old woman with breast carcinoma (case 1) shows one lymph node with scattered follicles containing sheets of centroblasts (A). The involved follicles exhibit sheets of large atypical cells with highly pleomorphic nuclear outlines and atypical mitoses (B). An axillary lymph node from a 53-year old woman (case 2) shows highly atypical large cells occupying an involved follicle (C). Sections of tonsil in a 6-year old boy (case 5) demonstrate a background of reactive follicular hyperplasia within which isolated follicles (upper left) show sheets of centroblasts (E and F).
Cytologically, the centroblasts were enlarged with vesicular chromatin and prominent nucleoli. They demonstrated nuclear membrane irregularity and deviated from the morphology seen in the surrounding normal lymph node. Furthermore, atypical mitoses were appreciated with admixed tingible body macrophages but lacking centrocytes. Overall, these features, in the context of follicular lymphoma, would warrant a grade 3B designation; however, they were not assigned a grade in the current clinical context.
Immunohistologic Features
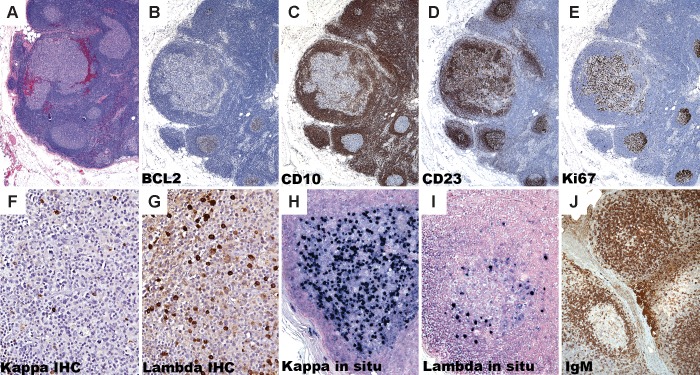
The presence of a subset of markedly atypical follicles intermixed with normal reactive follicular hyperplasia led us to seek further immunohistochemical and molecular characterization of these follicles. The immunohistologic findings are summarized in Table 3 and representative images are illustrated in Figs 2 and 3. The germinal center origin of the large cell proliferations was demonstrated by positivity for one or more of the following germinal center B-cell markers, CD10 (6 of 7), BCL6 4 of 4) and HGAL (2 of 2). All seven cases lacked coexpression of BCL2 with a germinal center marker by immunohistochemistry, which is typical of the cases of follicular lymphoma in situ described thus far (Fig 2, panels A-B). CD10 showed normal expression in five cases; however, CD10 was absent or weak in the neoplastic follicles of cases 3 and 4, in comparison to the surrounding normal follicles in each case (Fig 2, panel C). The atypical centroblasts were present within well-organized follicular dendritic networks as demonstrated by CD21, CD23 or D2-40 immunostaining in five of the seven cases, supporting the follicular nature and excluding a small focus of diffuse large B cell lymphoma (Fig 2, panel D). The morphologic absence of polarization was probed with a Ki-67 stain, which demonstrated a strong but patchy staining pattern and yielded no subtle support for even poorly delineated light and dark zones (Fig 2, panel E).
Table 3. Summary of Immunohistologic Findings.
| Case | CD20 | CD3 | CD10 | BCL2 | BCL6 | HGAL | IGM | IRF4 | Ki67 | Dendritic markers CD21, CD23, D240 | IG Light Chain Restriction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | POS | NEG | Normal | NEG | ND | ND | POS | ND | ND | Dendritic meshwork present (CD21) | Lambda (IHC) |
| 2 | POS | NEG | Normal | NEG | POS | POS, strong | POS | NEG | 50%, lack of polarization | Dendritic meshwork present (all three) | ND |
| 3 | POS | NEG | NEG | NEG | POS | ND | NEG | ND | 50%, lack of polarization | Dendritic meshwork present (CD23) | Kappa (in situ) |
| 4 | POS | NEG | Weak | NEG | POS | ND | POS | ND | ND | ND | Non-contributory (IHC and in situ) |
| 5 | POS | NEG | Normal | NEG | POS | POS, strong | NEG | NEG | ND | Dendritic meshwork present (all three) | Lambda (IHC) |
| 6 | POS | NEG | Normal | NEG | ND | ND | NEG | ND | 60%, lack of polarization | Dendritic meshwork present (CD21) | Non-contributory (IHC) |
| 7 | POS | Weak | Normal | NEG | ND | ND | ND | ND | ND | ND | ND |
Table legend 3. Atypical large cells in involved follicles were scored POS (positive) or NEG (negative); ND: Not done due to insufficient tissue
Fig 2. Immunohistochemistry of isolated atypical follicles.
An axillary lymph node dissection in a 58 year-old woman with breast carcinoma (case 3) shows a lymph node with a cluster of follicles (A). BCL2 expression is absent in both the involved and uninvolved follicles (B) and the involved follicle shows diminished CD10 expression relative to the surrounding normal germinal centers (C). CD23 demonstrates an intact follicular dendritic network around the involved follicle (D). Ki-67 is polarized in surrounding reactive follicles, but is not polarized in the involved follicles (E). The involved follicle in case 1 shows lambda light chain-restricted B-cells (F and G). Case 2 shows highly atypical large cells that by situ hybridization (ISH) for immunoglobulin kappa and lambda light chains show kappa-specific RNA in the majority of the atypical cells, confirming light chain restriction in the involved follicle (H and I). A periaortic lymph node from a 53-year old woman (case 4) shows abnormal strong IgM protein expression in the centroblasts of an involved follicle whereas the uninvolved follicle shows a weak dendritic pattern of IgM reactivity, which is typically seen in normal follicles (J).
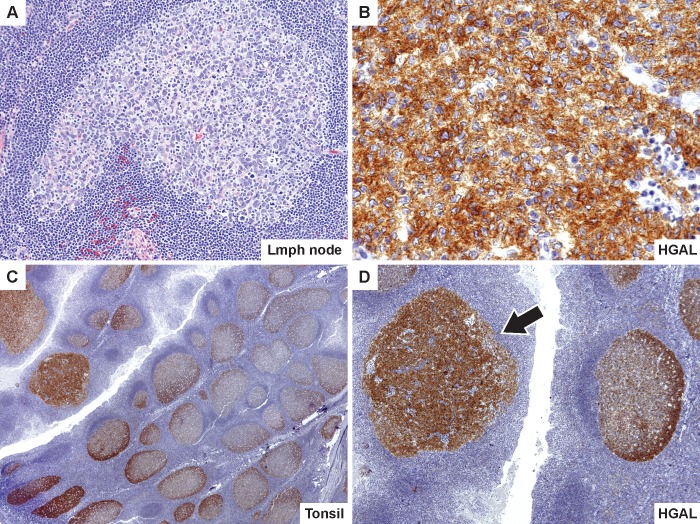
Fig 3. Atypical expression of HGAL in isolated atypical follicles.
An axillary lymph node in a 53-year old woman (case 2) shows a single atypical follicle with pleomorphic large cells overexpressing HGAL (A and B). Sections of the tonsil in a 6-year old boy stained with HGAL, show preservation of overall architecture with numerous normal reactive follicles and a gradient of HGAL staining with higher intensity in the dark zone. In the atypical follicle (indicated by arrow in panel D), HGAL staining is abnormal and shows overexpression throughout the affected follicle (C and D).
A more direct assessment of clonality was pursued with kappa and lambda light chain staining. Although not informative in every case, three cases showed distinct staining patterns between the involved and uninvolved follicles. Cases 1 and 5 show lambda light chain restriction in the involved follicles and case 3 shows kappa light chain restriction (Fig 2, panels F–I). In each case, this provides further support for the clonal nature of the B-cells in the involved follicles.
An IgM immunostain was performed in six cases of which three showed an abnormal membranous pattern of staining (Fig 2, panel J). Whereas the surrounding unremarkable germinal centers show the expected weak dendritic pattern of IgM reactivity, the involved follicles show strong cytoplasmic staining, a feature associated with malignant follicles. One case (case 7) showed aberrant coexpression of CD3 in the atypical cells, a finding absent in the surrounding germinal centers.
Two cases (2 and 5) demonstrated altered expression of germinal center B-cell marker, HGAL, which show overexpression of the protein throughout the affected follicle. In comparison, the surrounding reactive follicles show a gradient of HGAL staining with higher intensity in dark zones, which matches the polarization of normal germinal centers (Fig 3).
Fluorescence in situ hybridization and genotypic features
Six of seven cases with available material were tested by FISH for the presence of BCL2 gene rearrangement. None were positive, which is in keeping with over 50 percent of grade 3 follicular lymphomas lacking the IGH-BCL2 rearrangement. Two cases were further tested by FISH for BCL6 and IRF4 gene rearrangements (case 2, lymph node; case 5, tonsil) and were found to be negative (Table 4).
Table 4. Summary of FISH and Molecular Findings.
| Case | BCL2 FISH | BCL6 FISH | IRF4 FISH | Molecular Findings |
|---|---|---|---|---|
| 1 | NEG | ND | ND | Polyclonal; not microdissected due to lack of tissue |
| 2 | NEG | NEG | NEG | Clonal population detected in microdissected sample |
| 3 | NEG | ND | ND | Polyclonal, although apparent paucity of B-cells in microdissected sample |
| 4 | NEG | ND | ND | ND |
| 5 | NEG | NEG | NEG | Polyclonal in microdissected sample |
| 6 | NEG | ND | ND | Polyclonal; not microdissected due to lack of tissue |
| 7 | ND | ND | ND | ND |
Table 4 legend. Atypical large cells in involved follicles were scored POS (positive) or NEG (negative); ND: Not done due to insufficient tissue
Molecular PCR studies for IGH gene rearrangements were pursued in five cases, all of which initially failed to show a B-cell receptor gene rearrangement. In three cases with available material molecular studies were pursued on microdissected samples. Among the three cases analyzed by microdissection, two cases had only one follicle each that was involved. The third case had two closely apposed follicles, which were pooled. Two of these cases were successfully amplified, and a clonal amplification was further detected on a microdissected sample of case 2 among the B-cells comprising the involved follicles but not in the uninvolved lymph node follicles from the same case (Table 4).
Discussion
Isolated follicles enriched for centroblasts in otherwise reactive lymph nodes pose a distinct diagnostic challenge. These cases are difficult to classify and in the current diagnostic framework, the differential diagnoses would include a focus of diffuse large B cell lymphoma, partial involvement by grade 3 follicular lymphoma, follicular lymphoma in situ, or an unusual reactive phenomenon. The seven cases we describe are distinctly different from previously reported cases of follicular lymphoma in situ in that they demonstrate a predominance of centroblasts and lack expression of BCL2 protein.
The cases presented herein, despite the absence of the IGH-BCL2 translocation and the corresponding BCL2 protein overexpression, demonstrate aberrant morphologic and immunophenotypic features that are typical of lymphoma. These cases are characterized by pleomorphic cytology with irregular nuclear contours, large nuclear size and atypical mitotic figures. These cells are arranged in clusters amid intact follicular dendritic networks and lack key features of the normal germinal center, including tingible body macrophages and polarization. Furthermore, these cases demonstrate altered expression of germinal center markers CD10 and HGAL, co-expression of CD3, abnormal proliferation indices and disturbed IgM staining patterns. Each of these features can be seen in the setting of lymphoma, but is absent in the physiologic germinal center. Clonality studies, including light chain expression analysis and molecular assessment of the IGH@ gene rearrangement provide further support for the clonal nature of these follicles in the majority of the cases. Three cases had kappa or lambda light chain restricted B-cells and a fourth case was clonal by gene rearrangement studies following microdissection. The caveat that clonality studies in limited samples may demonstrate spurious clones when only a few B-cells are present should be kept in mind. Moreover, even non-neoplastic conditions can demonstrate clonal populations in the context of normal reactive germinal centers and hyperplastic marginal zones. [25–26] Therefore, our diagnoses are based predominantly on the altered morphology and immunoarchitecture, coupled with the markedly atypical cytologic findings, in the involved follicles. Immunohistochemistry shows decreased to absent CD10 expression in two cases, increased HGAL reactivity compared to background follicles in two cases, and light chain-restricted cells confined to the aberrant follicles in three cases of which one was also confirmed by microdissection of abnormal follicles with detection of clonal amplification and suggests that these findings represent a distinct pathologic process.
Although there is some support for neoplasia, the appropriate classification is unclear in the currently used system. As the proliferations are found only within follicular dendritic networks, diffuse large B cell lymphoma was excluded. The germinal center origin of the cells was supported by expression of CD10, BCL6, and/or HGAL, helping exclude lymphomas of non-germinal center origin. The atypical follicles were single or scattered amongst the germinal centers of a reactive pattern lymph node, which would be atypical for a definitive diagnosis of follicular lymphoma demonstrating partial involvement. To further exclude partial involvement by follicular lymphoma, and as recommended by Carbone and Santoro,[23] each patient received a complete staging workup to exclude additional sites of lymphadenopathy and associated involvement by indolent or aggressive lymphoma. In a subset of patients this included a bone marrow biopsy. None of the patients had evidence of systemic lymphoma at time of diagnosis or at available follow-up (up to 44 months later). Despite the concerning morphologic features that raise consideration of an aggressive lymphoma, such as diffuse large B cell lymphoma, not otherwise specified, the presence of these scattered atypical follicles did not require aggressive clinical management. Our previous studies on the germinal center B-cell associated protein, HGAL, showed that increased expression of HGAL is correlated with low stage disease and decreased capacity for dissemination.[27–28] None of the cases with increased HGAL expression were associated with systemic lymphoma, supporting this finding.
The t(14;18)(q32;q21) translocation involving the IgH and BCL2 genes that results in the overexpression of the anti-apoptotic protein BCL2, is the initiating event and genetic hallmark of FL.[29] Up to 10% of FL lack the t(14;18) translocation and the vast majority of these cases also lack expression of the BCL2 protein. Lack of BCL2 expression is particularly enriched in grade 3 FL, where only 50–70% of cases express the BCL2 protein in neoplastic B-cells within the follicle.(24) Recent investigations have shown that t(14;18)-negative FL is characterized by distinct microRNA profiles, an increased proliferative capacity and a late-germinal center immunophenotype, while t(14;18)-positive FL that lack BCL2 retain the full germinal center phenotype similar to t(14;18)-positive, BCL2-positive FL.[30]
Jegalian and colleagues also reported t(14;18)/IGH-BCL2 translocation-positive cases that lacked BCL2 reactivity in cases of FL associated with FLIS.[1] In addition, a single case report documented an additional point mutation within the BCL2 breakpoint region in the FL component of a case with simultaneous FL and FLIS.[31]These findings suggest a process of clonal evolution in cases with FLIS associated with FL. Recent gene array comparative genomic hybridization studies have demonstrated a continuum of cytogenetic findings ranging from none in reactive follicular hyperplasia, few in FLIS and more numerous chromosomal aberrations in fully developed follicular lymphoma.[32–33] These findings support a model of increasing cytogenetic complexity with progression of follicular lesions. Such studies would be interesting in cases similar to the ones we describe to ascertain if the miRNA and cytogenetic profiles more closely resemble those of FLIS, partial involvement by FL or fully developed follicular lymphoma.
FLIS is recognized as a follicular proliferation of monoclonal BCL2+ CD10+ lymphoid cells within scattered germinal centers of a lymph node with otherwise intact architecture. Cases described to date have been comprised of predominantly centrocytes and contained the IGH-BCL2 translocation. The seven cases we describe are distinctly different from the previously reported cases in that they demonstrate a predominance of centroblasts and lack expression of BCL2.
While the clinical significance of FLIS will depend largely on the results of long-term follow-up, initial studies suggest progression to follicular lymphoma is an infrequent event.[34] It may be many years before the true relationship to systemic lymphoma is uncovered given that even low-grade FL tends to be an indolent disease that many oncologists manage with minimal therapy such as rituximab or a ‘watch-and-wait’ approach.[35–37] Conceivably, decades of follow-up may be necessary in prospective trials to determine if there is an increased risk for the development of lymphoma in these patients, and, even then, early diagnosis in this setting may not alter clinical management. The relationship between the current cases and follicular lymphoma in situ is unclear and whether these could represent a variant of follicular lymphoma in situ with grade 3A or 3B morphology should be evaluated in future investigations.
Early detection of an aggressive B-cell lymphoma or carcinoma may lead to immediate intervention. Our cases showed involvement of rare scattered follicles in an architecturally intact lymph node, but contained increased centroblasts histologically consistent with grade 3B FL. Just as in typical FLIS, these cases were incidental findings in lymph nodes removed for various reasons and the patients did not show clinical or radiological evidence of lymphoma at the time of presentation. It is critical to recognize such cases, both for further study, and to potentially avoid overly aggressive treatment of an indolent lesion.
Acknowledgments
Preliminary results contained in this paper were presented in a poster format at the United States and Canadian Academy of Pathology annual meeting, March 2012, Vancouver, British Columbia. We would like to thank Kristen Jensen and Norman Cyr for aiding in the preparation of figures.
Data Availability
All relevant data are within the text, figures, and tables contained in the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99(9):3376–3382. [DOI] [PubMed] [Google Scholar]
- 2.Sotomayor EA, Shah IM, Sanger WG, Mark HF. In situ follicular lymphoma with a 14;18 translocation diagnosed by a multimodal approach. Exp Mol Pathol. 2007;83(2):254–258. [DOI] [PubMed] [Google Scholar]
- 3.Handa T, Maki K, Segawa A, Masawa N, Mitani K. In situ follicular lymphoma associated with progressive transformation of germinal centers. Int J Surg Pathol. 2011;19(4):521–523. 10.1177/1066896909350174 [DOI] [PubMed] [Google Scholar]
- 4.Cheung MC, Bailey D, Pennell N, Imrie KR, Berinstein NL, Amato D, et al. In situ localization of follicular lymphoma: evidence for subclinical systemic disease with detection of an identical BCL-2/IGH fusion gene in blood and lymph node. Leukemia. 2009;23(6):1176–1179. 10.1038/leu.2009.9 [DOI] [PubMed] [Google Scholar]
- 5.Montes-Moreno S, Castro Y, Rodriguez-Pinilla SM, Garcia JF, Mollejo M, Castillo ME, et al. Intrafollicular neoplasia/in situ follicular lymphoma: review of a series of 13 cases. Histopathology. 2010;56(5):658–662. 10.1111/j.1365-2559.2010.03529.x [DOI] [PubMed] [Google Scholar]
- 6.Roullet MR, Martinez D, Ma L, Fowler MH, McPhail ED, Judkins A, et al. Coexisting follicular and mantle cell lymphoma with each having an in situ component: A novel, curious, and complex consultation case of coincidental, composite, colonizing lymphoma. Am J Clin Pathol. 2010;133(4):584–591. 10.1309/AJCP5RT4MRSDGKSX [DOI] [PubMed] [Google Scholar]
- 7.Carbone A, Della Libera D, Zannier L, Selva A, Ceolin P, Gualeni A, et al. In situ follicular lymphoma associated with overt B- or T-cell lymphomas in the same lymph node. Am J Hematol. 2011;86(12):E66–70. 10.1002/ajh.22169 [DOI] [PubMed] [Google Scholar]
- 8.Carbone A, Gloghini A. Coexisting follicular and mantle cell lymphoma with each having an in situ component. Am J Clin Pathol. 2011;136(3):481–483. 10.1309/AJCPDSG2J5IBEYFB [DOI] [PubMed] [Google Scholar]
- 9.Carbone A, Tibiletti MG, Canzonieri V, Rossi D, Perin T, Barnesconi B, et al. In situ follicular lymphoma associated with nonlymphoid malignancies. Leuk Lymphoma. 2011;53(4):603–608. 10.3109/10428194.2011.624229 [DOI] [PubMed] [Google Scholar]
- 10.Bell DA, Liu Y, Cortopassi GA. Occurrence of bcl-2 oncogene translocation with increased frequency in the peripheral blood of heavy smokers. J Natl Cancer Inst. 1995;87(3):223–224. [DOI] [PubMed] [Google Scholar]
- 11.Dolken G, Dolken L, Hirt C, Fusch C, Rabkin CS, Schuler F. Age-dependent prevalence and frequency of circulating t(14;18)-positive cells in the peripheral blood of healthy individuals. J Natl Cancer Inst Monogr. 2008(39):44–47. 10.1093/jncimonographs/lgn005 [DOI] [PubMed] [Google Scholar]
- 12.Dolken G, Illerhaus G, Hirt C, Mertelsmann R. BCL-2/JH rearrangements in circulating B cells of healthy blood donors and patients with nonmalignant diseases. J Clin Oncol. 1996;14(4):1333–1344. [DOI] [PubMed] [Google Scholar]
- 13.Ji W, Qu GZ, Ye P, Zhang XY, Halabi S, Ehrlich M. Frequent detection of bcl-2/JH translocations in human blood and organ samples by a quantitative polymerase chain reaction assay. Cancer Res. 1995;55(13):2876–2882. [PubMed] [Google Scholar]
- 14.Ladetto M, Drandi D, Compagno M, Astolfi M, Voena C, Novarino A, et al. PCR-detectable nonneoplastic Bcl-2/IgH rearrangements are common in normal subjects and cancer patients at diagnosis but rare in subjects treated with chemotherapy. J Clin Oncol. 2003;21(7):1398–403. [DOI] [PubMed] [Google Scholar]
- 15.Limpens J, de Jong D, van Krieken JH, Price CG, Young BD, van Ommen GJ, et al. Bcl-2/JH rearrangements in benign lymphoid tissues with follicular hyperplasia. Oncogene. 1991;6(12):2271–2276. [PubMed] [Google Scholar]
- 16.Limpens J, Stad R, Vos C, de Vlaam C, de Jong D, van Ommen GJ, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85(9):2528–2536. [PubMed] [Google Scholar]
- 17.Liu Y, Hernandez AM, Shibata D, Cortopassi GA. BCL2 translocation frequency rises with age in humans. Proc Natl Acad Sci U S A. 1994;91(19):8910–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt C, Balogh B, Grundt A, Buchholtz C, Leo A, Benner A, et al. The bcl-2/IgH rearrangement in a population of 204 healthy individuals: occurrence, age and gender distribution, breakpoints, and detection method validity. Leuk Res. 2006;30(6):745–750. [DOI] [PubMed] [Google Scholar]
- 19.Schuler F, Dolken L, Hirt C, Keifer T, Berg T, Fusch G, et al. Prevalence and frequency of circulating t(14;18)-MBR translocation carrying cells in healthy individuals. Int J Cancer. 2009;124(4):958–963. 10.1002/ijc.23958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001;19(2):420–424. [DOI] [PubMed] [Google Scholar]
- 21.Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118(11):2976–2984. 10.1182/blood-2011-05-355255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbone A, Gloghini A, Santoro A. In situ follicular lymphoma: pathologic characteristics and diagnostic features. Hematol Oncol. 2012;30(1):1–7. 10.1002/hon.993 [DOI] [PubMed] [Google Scholar]
- 23.Carbone A, Santoro A. How I treat: diagnosing and managing "in situ" lymphoma. Blood. 2011;117(15):3954–3960. 10.1182/blood-2010-10-299628 [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow SH, International Agency for Research on Cancer., World Health Organization., Louis A. Duhring Fund. WHO classification of tumours of haematopoietic and lymphoid tissues 4th ed. Lyon, France: International Agency for Research on Cancer; 2008. 439 p. [Google Scholar]
- 25.Nam-Cha SH, San-Millan B, Mollejo M, Garcia-Cosio M, Garijo G, Gomez M, et al. Light-chain-restricted germinal centres in reactive lymphadenitis: report of eight cases. Histopathology. 2008;52(4):436–444. 10.1111/j.1365-2559.2008.02965.x [DOI] [PubMed] [Google Scholar]
- 26.Attygalle AD, Liu H, Shirali S, Diss TC, Loddenkemper C, Stein H, et al. Atypical marginal zone hyperplasia of mucosa-associated lymphoid tissue: a reactive condition of childhood showing immunoglobulin lambda light-chain restriction. Blood. 2004;104(10):3343–3348. [DOI] [PubMed] [Google Scholar]
- 27.Temmins C, Zhao S, Lossos IS, Natkunam Y. HGAL protein expression persists in disorders of germinal center dissolution: potential role of HGAL in the germinal center microenvironment. Appl Immunohistochem Mol Morphol. 2011;19(3):266–272. 10.1097/PAI.0b013e3181f89a4d [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Lu X, McNamara G, Liu X, Cubedo E, Sarosiek KA, et al. HGAL, a germinal center specific protein, decreases lymphoma cell motility by modulation of the RhoA signaling pathway. Blood. 2010;116(24):5217–5727. 10.1182/blood-2010-04-281568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature. 1991;349(6306):254–256. [DOI] [PubMed] [Google Scholar]
- 30.Leich E, Zamo A, Horn H, Haralambieva E, puppe B, Gascoyne RD, et al. MicroRNA profiles of t(14;18)-negative follicular lymphoma support a late germinal center B-cell phenotype. Blood. 2011;118(20):5550–5558. 10.1182/blood-2011-06-361972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonzheim I, Salaverria I, Haake A, Gastl G, Adam P, Siebert R, et al. A unique case of follicular lymphoma provides insights to the clonal evolution from follicular lymphoma in situ to manifest follicular lymphoma. Blood. 2011;118(12):3442–3444. 10.1182/blood-2011-07-368944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt J, Salaverria I, Haake A, et al. Increasing genomic and epigenomic complexity in the clonal evolution from in situ to manifest t(14;18)-positive follicular lymphoma. Leukemia. 2014;28(5):1103–1112. 10.1038/leu.2013.307 [DOI] [PubMed] [Google Scholar]
- 33.Mamessier E, Song JY, Eberle FC, Pack S, Drevet C, Chetaille B, et al. Early lesions of follicular lymphoma: a genetic perspective. Haematologica. 2014; 99(3):481–488. 10.3324/haematol.2013.094474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai RK, Surti U, Swerdlow SH. Follicular lymphoma-like B cells of uncertain significance (in situ follicular lymphoma) may infrequently progress, but precedes follicular lymphoma, is associated with other overt lymphomas and mimics follicular lymphoma in flow cytometric studies. Haematologica. 2013;98(10):1571–1580. 10.3324/haematol.2013.085506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin's lymphoma: long-term follow-up of no initial therapy. J Clin Oncol. 2004;22(8):1454–1459. [DOI] [PubMed] [Google Scholar]
- 36.Ardeshna KM, Smith P, Norton A, Hancock B, Hoskin PJ, MacLennan KA, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362(9383):516–522. [DOI] [PubMed] [Google Scholar]
- 37.Sapunar F, Catovsky D, Wotherspoon A, Matutes E. Follicular lymphoma. A series of 11 patients with minimal or no treatment and long survival. Leuk Lymphoma. 2000;37(1–2):163–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the text, figures, and tables contained in the paper.