Abstract
Nitrogen (N) fertilization can greatly improve plant productivity but needs to be carefully managed to avoid harmful environmental impacts. Nutrient management guidelines aimed at reducing harmful forms of N loss such as nitrous oxide (N2O) emissions and nitrate (NO3-) leaching have been tailored for many cropping systems. The developing bioenergy industry is likely to make use of novel cropping systems, such as polycultures of perennial species, for which we have limited nutrient management experience. We studied how a switchgrass (Panicum virgatum) monoculture, a 5-species native grass mixture and an 18-species restored prairie responded to annual fertilizer applications of 56 kg N ha-1 in a field-scale agronomic trial in south-central Wisconsin over a 2-year period. We observed greater fertilizer-induced N2O emissions and sub-rooting zone NO3- concentrations in the switchgrass monoculture than in either polyculture. Fertilization increased aboveground net primary productivity in the polycultures, but not in the switchgrass monoculture. Switchgrass was generally more productive, while the two polycultures did not differ from each other in productivity or N loss. Our results highlight differences between polycultures and a switchgrass monoculture in responding to N fertilization.
Introduction
Nitrogen (N) pollution from agricultural systems has local, regional, and global environmental impacts. Nitrate (NO3-) leaching can negatively impact drinking water quality [1] and contributes to eutrophication events such as the Gulf of Mexico hypoxic zone [2,3]. Ammonia volatilization contributes to acid precipitation, while nitrous oxide (N2O) is the single greatest ongoing source of ozone depletion [4] and an extremely potent greenhouse gas [5]. These forms of N pollution are commonly linked to excessive or misapplied N fertilizer [6,7]. To combat this, nutrient application guidelines and best management practices have been developed for many major cropping systems (e.g. [8]). These guidelines rely on knowledge about a cropping system and its performance under diverse conditions; however, such knowledge may be lacking for novel cropping systems such as perennial polycultures. Generating data on N dynamics and idiosyncrasies is thus a key element in the development of a novel cropping system.
The ongoing development of cellulosic (i.e. “second generation”) biofuels has spurred interest in the use of diverse mixtures of perennial species as biomass feedstock cropping systems [9]. Second generation biofuels use cellulose and other non-edible plant materials, in contrast to first generation fuels, which are produced from edible sugars, starches, or oils [10]. This creates the possibility of commercializing land covers, such as diverse assemblages of tallgrass prairie species, which have previously been valued purely for their aesthetic and ecological properties [11]. Despite their benefits, these systems lack the wealth of agronomic knowledge available for more conventional crops [12]. A growing body of research and experience informs management of certain biomass feedstock species, most notably switchgrass (Panicum virgatum) [13,14], but knowledge of the agronomic behaviors of polycultures in bioenergy feedstock cropping systems is much less developed [15]. As fertilization will likely be necessary for sustaining high levels of productivity in these systems [16], it will be critical to understand the agronomic and ecological dynamics of polyculture cropping systems.
A sizeable body of ecological research and theory explores the role of plant community diversity and composition in ecosystem functioning. Many studies find increasing the number of species present correlates to greater plant productivity [17,18], although multispecies assemblages rarely out-produce monocultures of highly productive species [19]. In some cases, increasing the number of species and functional groups in a plant community decreases soil N2O emissions [20,21] and reduces soil NO3- pools [22,23]. The effect of species diversity on ecosystem dynamics is frequently attributed to complementarity, where differences in species-specific resource utilization result in more complete exploitation of ecosystem resources [24,25]. Nutrient management strategies designed for particular species in monoculture may thus yield different results in diverse species assemblages.
In this study, we explore how nitrogen fertilization impacts N loss pathways and plant productivity in three biomass production systems based on grasses and forbs native to North America. The systems we studied were a switchgrass monoculture, a mixture of five grasses, and a mixture of 18 prairie species. All systems were managed at agronomic scale and split into plots receiving either replacement-level N fertilizer or no fertilizer. For each system, we measured aboveground productivity, N2O emissions, and potential NO3- leaching, with the aim of determining how these properties were impacted by N fertilization in the three systems.
Materials and Methods
Field sites
The study was conducted at the Great Lakes Bioenergy Research Center’s Biofuel Cropping Systems Experiment (BCSE) at the Arlington Agricultural Research Station (Arlington, WI, 43°17’45” N, 89°22’48” W, 315 masl) during the 2011 and 2012 field seasons. Predominant soils are classified as Plano silt loam (Fine-silty, mixed, superactive, mesic Typic Argiudoll). Mean annual air temperature and precipitation are 6.8°C and 869 mm respectively [26]. Cumulative precipitation and average daily temperature for 2011 and 2012 are given in the supporting information (S1 Fig).
The BCSE was established in 2008 and contains eight cellulosic biofuel feedstock production systems managed at agronomic scale in a randomized complete block design with five replicates (five blocks). We selected three blocks where other studies were conducting more intensive biophysical parameter modeling from which to sample. From the eight cropping systems, we selected three systems that formed a spectrum of species diversity: monoculture switchgrass (var. ‘Cave-in-Rock’), a mixture of five grasses native to the region (“native grasses”), and a mixture of 18 grass and forb species native to the region (“restored prairie”, see Table 1 for composition and diversity details). Switchgrass and mixed grass plots received early-season weed management through 2011; switchgrass received weed management again in 2012. The restored prairie received weed control in its establishment year, but none in subsequent years.
Table 1. Seeded species and measures of realized diversity and composition in three biofuel feedstock production systems.
| Switchgrass | Native grasses | Restored prairie | |
|---|---|---|---|
| Species richness | 3.6 ± 1.9 | 6.0 ± 2.0 | 10.9 ± 1.7 |
| Shannon’s diversity index | 0.7 ± 0.4 | 1.4 ± 0.3 | 1.9 ± 0.2 |
| C3 grass cover (%) | 5.0 ± 11.0 | 72.7 ± 29.8 | 90.2 ± 22.7 |
| C4 grass cover (%) | 127.2 ± 49.2 | 103.2 ± 47.5 | 21.1 ± 22.5 |
| Non-legume forb cover (%) | 11.9 ± 18.8 | 6.8 ± 11.3 | 86.5 ± 24.9 |
| Legume cover (%) | none observed | none observed | 10.4 ± 14.2 |
| Seeded species | Panicum virgatum L. | Panicum virgatum L. | Panicum virgatum L. |
| Andropogon gerardii Vitman | Andropogon gerardii Vitman | ||
| Elymus canadensis L. | Elymus canadensis L. | ||
| Schizachyrium scoparium (Michx.) Nash | Schizachyrium scoparium (Michx.) Nash | ||
| Sorghastrum nutans L. | Sorghastrum nutans L. | ||
| Koeleria macrantha (Ledeb.) Schult. | |||
| Anemone canadensis L. | |||
| Asclepias tuberosa L. | |||
| Baptisia alba (L.) Vent. var. macrophylla (Larisey) Isley | |||
| Desmodium canadense (L.) DC. | |||
| Lespedeza capitata Michx. | |||
| Monarda fistulosa L. | |||
| Oligoneuron rigidum (L.) Small var. rigidum | |||
| Ratibida pinnata (Vent.) Barnhart | |||
| Rudbeckia hirta L. | |||
| Silphium perfoliatum L. | |||
| Solidago erecta Pursh | |||
| Symphyotrichum novae-angliae (L.) G.L.Nesom. |
Cover measurements allowed multiple species hits per point; functional group cover is summed over all species and can thus exceed 100%. Values are means ± standard deviation.
Plots were 27 m wide × 43 m long (0.12 ha), with a 17 m wide main plot and two 5 m wide subplots (S2 Fig). The switchgrass and mixed grass main plots as well as the restored prairie subplot received annual applications of 56 kg ha-1 N as ammonium nitrate. Fertilizer was applied with a vacuum spreader when C4 grasses were 30–45 cm tall (2011-05-27 and 2012-05-11) in keeping with University of Wisconsin recommendations for management of established switchgrass stands [8]. The restored prairie main plot and the subplots of switchgrass and native grasses received no fertilization after establishment. The BCSE was established in spring 2008 and all systems have been harvested to a residual stubble height of 10 cm annually since 2009. We sampled at three randomly positioned 1.5 × 1.5 m quadrats (subsamples) within both main and subplots. Quadrats remained in place during the growing season, but were repositioned in the subsequent year (S2 Fig).
Nitrous oxide emissions
Soil N2O fluxes were measured twice monthly from 9 May to 14 September 2011 and 7 May to 6 September 2012. N2O measurement and flux estimation largely followed the approach of Oates et al. [27]. Briefly, 27.15 cm diameter stainless steel non-through flow chambers were inserted 5.5 cm into the soil to an effective height of 17.3 cm for a headspace volume of 10 l. Chambers were placed in the center of marked quadrats. Plants growing within the chambers were clipped flush with the chamber top at least 24 h before measurement to allow lid closure [28]. Gas was measured by sealing the chamber and sampling headspace gas at 10 minute intervals for 30 minutes. Gas concentrations were measured by gas chromatography with a 15 mCi 63Ni electron capture detector for N2O (Agilent 7890A GC system) and infra-red gas analyzer for CO2 (LI-COR LI-820 CO2 analyzer).
N2O fluxes were estimated through the HMR method [29], implemented in the R package 'HMR' (v0.3.1, [30]). We previously found the HMR method to be more appropriate than linear regression at long chamber deployment times [31]. Package recommendations were followed, with secondary visual inspection for samples where linear and nonlinear fluxes differed by ≥ 0.01 mg N2O-N m-2 h-1. Linear interpolation between measurement dates was used to estimate cumulative N2O emission over the growing season. Emissions factor (EF), the percentage of fertilizer N released as N2O, was calculated as:
where N2OF and N2OU are cumulative N2O emissions (kg N ha-1) for adjacent fertilized and unfertilized subplots respectively and FN is the nitrogen content (kg N ha-1) of fertilizer applied [32,33].
Soil inorganic nitrogen
We sampled extractable soil NO3- at the 50–80 cm depth. This was interpreted as potentially leachable NO3-, as root biomass and plant nutrient uptake are concentrated in the top 50 cm of soil [34,35]. We sampled periodically throughout the growing season in both years (S3 Fig), but focused our analysis on samples taken near peak plant biomass. The N content of C4 grasses reaches a maximum concurrent with peak biomass [36], so our samples should represent N that escaped below the rooting zone during the period of greatest plant N utilization.
Three soil samples were removed from each quadrat and composited. Samples were homogenized and sieved to 2 mm, with rocks and biomass removed. Within 48 h of field sampling, 10 g soil samples were extracted with 2 M KCl [37]. Extracts were frozen prior to colorimetric NO3- determination (according to USEPA Method 353.2, O-I-Analytical Method #2648) using a Flow Solution 3100 segmented flow injection analyzer (OI Analytical, College Station, TX). Soil subsamples (15 g) were weighed, oven-dried for 48 h at 60°C, and then reweighed to obtain soil gravimetric water content.
Ancillary environmental measurements
Soil temperature and moisture were measured concurrently with trace gas sampling to identify potential abiotic drivers of flux differences among systems [38,39]. Soil temperature measurements were taken with a 10 cm probe (Checktemp 1C, Hanna Instruments, Smithfield, RI); soil volumetric water content was measured using a 6 cm ML3 ThetaProbe sensor (Dynamax, Houston, TX). In both cases, we took 3 measurements within 25 cm of the sampling chamber and averaged them. Volumetric water content was converted to water-filled pore space (WFPS) using bulk densities measured at the plot level in 2008 and a standard soil particle density of 2.65 g cm-3. WFPS is commonly linked to microbial nitrification and denitrification [40].
Aboveground productivity and biomass properties
After all field measurements were completed (23 to 30 September 2011 and 21 to 27 August 2012), all aboveground biomass from each quadrat was hand-harvested to ground level and then dried and weighed to estimate aboveground net primary productivity (ANPP). ANPP peaked earlier in 2012 due to above-average March temperatures and an early summer drought (S1 Fig). Plant species cover was measured immediately prior to harvest using the point-intercept method [41]. Each quadrat was divided into 49 evenly-spaced points at which each unique plant species intercepted by a dropped rod was recorded. The percent cover for each species was estimated as the percentage of points at which the species was observed. Multiple species could be counted at each point, so percent cover summed over all species could exceed 100% [41]. In addition to quadrat-level data, entire plots (main and subplot) were harvested to 10 cm stubble height following a killing frost (10 October 2011 and 7 November 2012). Total N content of a representative subsample of the harvested material was determined by combustion using a Flash EA 1112 Automated Elemental Analyzer (Thermo Finnigan, Milan, Italy).
Statistical analysis
Data analysis was conducted in the R statistical environment (v3.1.1, [42]). Values for N2O emissions and NO3- concentrations were log-transformed prior to analysis to meet assumptions of normality. Negative NO3- concentrations were replaced with half the smallest positive concentration observed (0.04 μg N g-1 dry weight soil). All analyses used linear mixed effect models from the R package 'nlme' (v3.1.1, [43]). We employed a nested random effects structure of subsample within subplot within plot within block (S2 Fig) to properly account for lack of independence in our data. We allowed variances to differ among treatments, identifying the most parsimonious grouping structure by likelihood ratio comparison of nested models. Significance of treatment differences was done separately by year using the R package 'lsmeans' (v1.06, [44]) and a Tukey-corrected P < 0.05 cutoff.
Results
Fertilization increased nitrous oxide emissions more in switchgrass than in polycultures
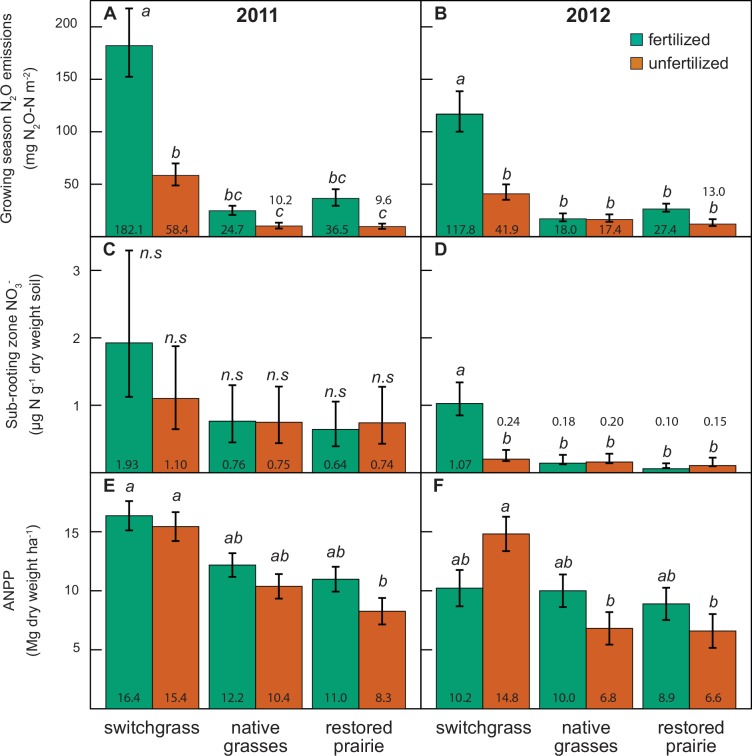
Crop type and fertilizer effects on total N2O emissions were significant in both years (Fig 1A and 1B). Fertilization increased emissions in all cases except for native grasses in 2012, where it had no effect. With equal fertilization, N2O emissions were comparable between the polycultures and lower than switchgrass. In 2011, N2O emissions from all crop types were approximately 3 times greater from the fertilized plots when compared to the unfertilized plots (Fig 1A). Emission factors in 2011 were 2.4% (s.e. ± 0.65%), 0.26% (s.e. ± 0.13%), and 0.56% (s.e. ± 0.26%) for switchgrass, native grasses, and restored prairie, respectively. In 2012, nitrogen fertilization similarly tripled N2O emissions from switchgrass, but did not impact the polycultures (Fig 1B). Emission factors for 2012 were 1.5% (s.e. ± 0.48%), 0.018% (s.e. ± 0.041%), and 0.23% (s.e. ± 0.025%) for switchgrass, native grasses, and restored prairie, respectively.
Fig 1. Average N losses and aboveground net primary productivity (ANPP) for each crop and fertilizer treatment in 2011 and 2012.
Within each panel, samples sharing a letter are not significantly different (P > 0.05), n.s. indicates no significant differences among samples. (a) Average total N2O emissions for the measurement period (May-September) are given as back-transformed geometric means ± standard error. (b) Average sub-rooting zone NO3- concentrations are given as back-transformed geometric means ± standard error. (c) Average aboveground productivity values are given as arithmetic means ± standard error.
Potential nitrate leaching increased with fertilization in switchgrass
In both years, fertilized switchgrass had higher NO3- concentrations below the rooting zone than the polycultures, while all other cropping system × fertilization combinations were comparable (Fig 1C and 1D). Across all systems, NO3- concentrations were lower in 2012 than 2011. In 2011, there was no significant effect of fertilizer on potential leaching (Fig 1C). In 2012 there was a crop × fertilizer effect with higher potential leaching in fertilized versus unfertilized switchgrass, but no fertilizer effect in the polycultures (Fig 1D). Regardless of fertilizer use, early season soil NO3- was higher in switchgrass than either polyculture, while later-season values converged among crops (S3 Fig). The exception was the unfertilized crops in 2012 where NO3- values differed among all three systems in the early season but converged by peak biomass.
Aboveground net primary productivity of polycultures increased by fertilization
Fertilization increased ANPP in polycultures, although the increase was only significant for restored prairie in 2011 and native grasses in 2012 (Fig 1E and 1F). In contrast, fertilization did not significantly increase switchgrass ANPP in 2011 and reduced it in 2012. Switchgrass was generally more productive than the polycultures, which were not significantly different from each other within the fertilization treatment.
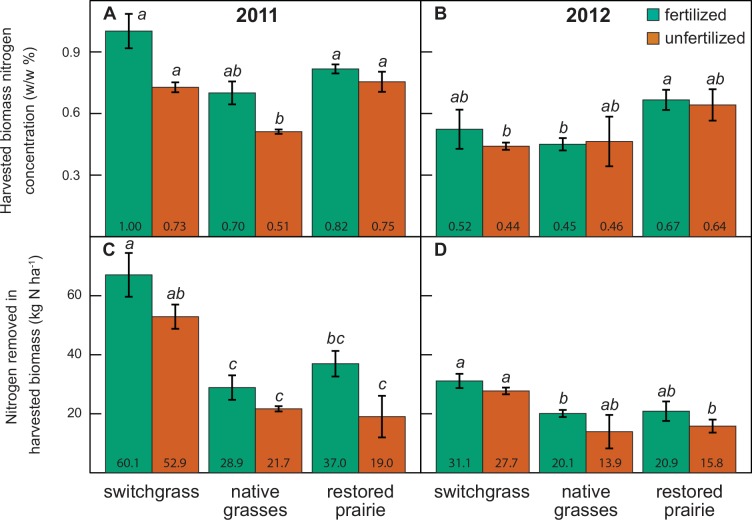
Fertilization did not increase the ratio of N2O emissions or sub-rooting zone NO3- concentrations to ANPP (Table 2). In most cases, fertilized polycultures had significantly lower ratios than fertilized switchgrass. All systems had similar aboveground biomass N concentrations in 2011, while in 2012 restored prairie had the highest concentrations (Fig 2A and 2B). Fertilization increased aboveground N concentrations only in switchgrass and the native grasses, and then only in 2011. Fertilization increased N removed in harvested biomass for switchgrass in 2011 and restored prairie in both years (Fig 2C and 2D). Switchgrass had the greatest N removal in 2011, while in 2012 switchgrass and fertilized restored prairie had equivalent removal rates.
Table 2. Relationship between nitrogen loss and aboveground net primary productivity (ANPP) production in three bioenergy cropping systems.
| g N loss Mg-1 biomass | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | Crop | Fertilizer treatment | N2O | NO3- | ||||
| 2011 | Switchgrass | Fertilized | 114 | (94–139) | a | 513 | (295–892) | a |
| Unfertilized | 39 | (31–48) | ab | 312 | (179–541) | a | ||
| Native grasses | Fertilized | 20 | (17–25) | b | 277 | (162–472) | a | |
| Unfertilized | 10 | (7–13) | b | 321 | (185–559) | a | ||
| Restored prairie | Fertilized | 34 | (26–43) | ab | 253 | (153–421) | a | |
| Unfertilized | 12 | (9–15) | b | 391 | (225–680) | a | ||
| 2012 | Switchgrass | Fertilized | 125 | (95–164) | a | 483 | (344–677) | a |
| Unfertilized | 29 | (23–36) | b | 71 | (47–106) | b | ||
| Native grasses | Fertilized | 18 | (15–22) | b | 81 | (51–129) | ab | |
| Unfertilized | 26 | (20–32) | b | 130 | (84–200) | ab | ||
| Restored prairie | Fertilized | 31 | (26–38) | b | 49 | (33–73) | b | |
| Unfertilized | 31 | (16–27) | b | 99 | (61–161) | ab | ||
Values are geometric means, with ± 1 s.e. in parentheses. Within a year and column, values sharing a letter are not significantly different (P > 0.05).
Fig 2. Harvested biomass properties for each crop and fertilizer treatment in 2011 and 2012.
Within each panel, samples sharing a letter are not significantly different (P > 0.05). All values are arithmetic means ± standard error.
Soil moisture and temperature
Average soil temperatures to 10 cm exhibited a crop × fertilizer interaction with significantly higher temperatures in 2012 than 2011 (S1 and S2 Tables). In native grasses and restored prairie systems, unfertilized subplots were warmer. Soil WFPS exhibited a crop × year effect and was consistently higher in unfertilized subplots (S1 and S2 Tables). Within each crop, WFPS in 2012 was lower than 2011. In 2011, WFPS in the native grasses was significantly lower than other crops while in 2012 WFPS in the native grasses was lower than restored prairie but not significantly different from switchgrass.
Discussion
We observed a cropping systems-level tradeoff between biomass production and loss of environmentally harmful forms of nitrogen (N), with the native grasses and prairie species mixtures having lower N2O emissions, potential NO3- leaching, and aboveground productivity. N losses resulting from fertilization were greater and more consistent in switchgrass than in the two polycultures, while productivity gains in response to fertilization were observed only in the polycultures. Both mechanisms of N loss we considered in this study are heavily influenced by precipitation and soil moisture; our measurements in the drought year of 2012 allowed us to compare the interplay of soil moisture and labile N availability across the three cropping systems. Given that all three systems were managed identically following best practices for switchgrass production, our findings suggest that perennial grass-based polycultures handle fertilizer-applied N more robustly and efficiently than a switchgrass monoculture.
We hypothesize that many of our findings result from differences in how quickly and completely the cropping systems we studied are able to immobilize applied N. This immobilization primarily entails incorporation of labile N into plant and microbial biomass [45]. Ideally, N fertilizer application should coincide with periods of heavy plant uptake, ensuring as much N as possible is immobilized into plant tissues [8]. Predicting phenology in perennial species is extremely challenging; in perennial bunch-forming grasses, like switchgrass, windows for conducting field operations are further constrained by the need to avoid damage to plant crowns. In this context, polycultures benefit greatly from seasonal complementarity, which has been demonstrated to reduce labile N levels in soil [25]. Polycultures incorporate species with a range of phenologies, so timing of plant N demand overlap to provide a broader time window for efficient N uptake. In addition, both polycultures in our study contained significant components of cool-season grasses and/or forbs. These taxa have higher N demands and greater early-season activity than a warm-season grass like switchgrass, and thus should have been well-positioned to assimilate applied N.
Our N application rates were low, approximately half the rate recommended by University of Wisconsin Extension for established switchgrass [8] and below the agronomically optimal fertilization rate for a mixture of prairie species grown for biomass [15]. Despite this, the amount of N we applied exceeded the amount removed as harvested biomass in all cases except for the fertilized switchgrass in 2011. One guideline for N management in bioenergy crops is to match application rates to the amount of N anticipated to be removed by biomass [46]. This calculation can be complicated, as many perennial species, including switchgrass, are capable of progressively relocating N from their aboveground biomass to their belowground tissues over the period of senescence [47]. The N content of biomass can thus potentially vary interannually, depending on when in the senescence process the plants were harvested, as we observed with broadly lower N contents in 2012. That said, we did not observe consistent, significant differences in biomass N concentrations across cropping systems. Rates of N removal were largely due to differences in harvested biomass. It is curious that the polycultures had a greater excess of N fertilization but lower levels of N loss. Most likely, much of the excess N was immobilized into microbial biomass. Prairies in south-central Wisconsin support greater microbial biomass than switchgrass fields [48], and in these soils biomass and necromass constitute a significant component of the organic N pool [49]. It would be important to investigate the practical limits of organic N accumulation in soils, as these would determine how these polycultures would behave with long-term excessive N application.
Concentrations of NO3- at 50–80 cm responded strongly to cropping systems, N application, and yearly precipitation dynamics. These concentrations were dramatically higher in the switchgrass system early in the season, decreasing sharply over time. Notably, while fertilization increased concentrations, the dynamic was still present in unfertilized switchgrass, indicating considerable free NO3- in the system early in the season. Given the limited root activity occurring at these depths [34,35], these decreases likely represent loss of NO3- from the system, rather than growing season uptake by plants. Concentrations were much lower in 2012, where the lack of precipitation limited the percolation of water needed to transport NO3-. The contrast to the polycultures is stark and informative. In both systems, NO3- concentrations did not increase in the early season, did not increase with fertilization, and were largely the same in the normal and drought years. These observations strongly suggest that, even following fertilization in a year with normal precipitation, soil NO3- levels in the polycultural systems were low enough to avoid significant leaching. This demonstrated the capacity of these polycultural systems to rapidly and efficiently immobilize fertilizer N.
Patterns of N2O emissions similarly reflected differences in N immobilization efficiency across cropping systems, fertilization managements, and years. Soils can produce N2O through both the processes of nitrification and denitrification, but in soils with adequate moisture, such as at our study site, flux dynamics are dominated by denitrification [50]. Denitrification uses free NO3- as an alternative electron acceptor under low oxygen conditions. Typically, large N2O fluxes are observed when precipitation events, which reduce oxygen availability by restricting gas movement in the soil, coincide with high concentrations of NO3-, which is usually the limiting substrate for the reaction [27]. The lower N2O emissions observed in the fertilized polycultures in the normal precipitation year of 2011 illustrate how effectively these systems were able to immobilize N between the time it was applied and the next major precipitation event. The lack of spring precipitation in 2012, by contrast, provided greater time for N immobilization. This was sufficient to completely erase the fertilizer effect in the polycultures, but the effect was still clearly visible in the switchgrass, suggesting that the systems differed both in the speed and the thoroughness of their N immobilization. It should be noted that in 2011 switchgrass had slightly higher soil moisture, potentially accounting for some of the increase in emissions, but soil moistures were comparable across systems in 2012 while emission differences remained.
In light of the N immobilization dynamics we observed, the productivity responses to N fertilization suggest the polycultures were mildly N-limited, while the switchgrass was not. Switchgrass responds unevenly to N fertilization [51], which can even decrease switchgrass productivity under high soil N conditions [52]. Fertilization may indirectly inhibit switchgrass growth by stimulating forb and cool-season grass weeds, which can be detrimental to switchgrass productivity [53] and stand establishment [13,14]. The polycultures, by contrast, deliberately included highly productive forbs and cool-season grasses, whose stimulation by N addition contributed to the system's productivity. It needs to be noted, however, that fertilized and unfertilized switchgrass were at least as productive as the polycultures, as has been previously reported [54]. The polycultures juxtaposed low N losses with productivity gains in response to fertilization, suggesting these systems were N limited. It is possible that the microbial biomass in these systems competed with the plants for nitrogen [55], an interaction that may complicate management of these systems.
Our findings hold significant implications for the selection and management of perennial bioenergy cropping systems. Selecting a bioenergy cropping system may entail balancing biomass production needs with N pollution risks, although productivity gains through optimization of polyculture species assemblages may be possible. Our results show, with respect to nitrogen processing, polycultural biomass production systems can be resilient in the face of annual weather fluctuations which indicates the environmental and management benefits of these systems should not be overlooked.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank T.D. Burhop, M.J. Cruse, A. David, T. Davis, S.M. Greenler, J.D. Lechelt, N. Lee, C. Liu, and A.C. Perillo for their assistance in field sampling, and G.R. Sanford for agronomic management. Comments from K. Stahlheber, S.M. Marion, L.C. Smith, A. Cates, and multiple anonymous reviewers were helpful improving this manuscript.
Data Availability
All data have been submitted to Dryad, with the following DOI: http://dx.doi.org/10.5061/dryad.m717n
Funding Statement
This work was funded in part by the DOE Great Lakes Bioenergy Research Center (https://www.glbrc.org/)(DOE BER Office of Science DE-FC02-07ER64494; http://science.energy.gov/) and DOE OBP Office of Energy Efficiency and Renewable Energy (DE-AC05-76RL01830; http://energy.gov/eere/office-energy-efficiency-renewable-energy), the Water Sustainability and Climate Program of the National Science Foundation (DEB-1038759), a Multi-State Hatch Grant (#WISO1420), the UW Department of Agronomy, and the D.C. Smith Wisconsin Distinguished Graduate Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: A review. Ann Appl Biol 162: 145–173. 10.1111/aab.12014 [DOI] [Google Scholar]
- 2.Mississippi River/Gulf of Mexico Watershed Nutrient Task Force (2009) Moving forward on Gulf hypoxia annual report 2009 Washington, D.C.: U.S. Environmental Protection Agency. [Google Scholar]
- 3.Rabalais NN, Turner RE, Wiseman WJ (2001) Hypoxia in the Gulf of Mexico. J Environ Qual 30: 320–329. [DOI] [PubMed] [Google Scholar]
- 4.Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science (80-) 326: 123–125. 10.1126/science.1176985 [DOI] [PubMed] [Google Scholar]
- 5.Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, et al. (2007) Changes in atmospheric constituents and in radiative forcing In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, et al. , editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press; pp. 129–234. [Google Scholar]
- 6.Robertson GP, Paul EA, Harwood RR (2000) Greenhouse gases in intensive agriculture: Contributions of individual gases to the radiative forcing of the atmosphere. Science (80-) 289: 1922–1925. 10.1126/science.289.5486.1922 [DOI] [PubMed] [Google Scholar]
- 7.Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, et al. (2008) Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science (80-) 320: 889–892. 10.1126/science.1136674 [DOI] [PubMed] [Google Scholar]
- 8.Laboski CAM, Peters JB, Bundy LG (2012) Nutrient application guidelines for field, vegetable, and fruit crops in Wisconsin Madison, WI: UW-Madison Cooperation Extension Publications. [Google Scholar]
- 9.Sanderson MA, Adler PR (2008) Perennial forages as second generation bioenergy crops. Int J Mol Sci 9: 768–788. 10.3390/ijms9050768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries BJM, van Vuuren DP, Hoogwijk MM (2007) Renewable energy sources: Their global potential for the first-half of the 21st century at a global level: An integrated approach. Energy Policy 35: 2590–2610. 10.1016/j.enpol.2006.09.002 [DOI] [Google Scholar]
- 11.Robertson GP, Dale VH, Doering OC, Hamburg SP, Melillo JM, et al. (2008) Sustainable biofuels redux. Science (80-) 322: 49–50. 10.1126/science.1161525 [DOI] [PubMed] [Google Scholar]
- 12.Zegada-Lizarazu W, Elbersen HW, Cosentino SL, Zatta A, Alexopoulou E, et al. (2010) Agronomic aspects of future energy crops in Europe. Biofuels, Bioprod Biorefining 4: 674–691. 10.1002/bbb.242 [DOI] [Google Scholar]
- 13.Mitchell R, Vogel KP, Sarath G (2008) Managing and enhancing switchgrass as a bioenergy feedstock. Biofuels, Bioprod Biorefining 2: 530–539. 10.1002/bbb.106 [DOI] [Google Scholar]
- 14.Vogel KP, Brejda JJ, Walters DT, Buxton DR (2002) Switchgrass biomass production in the Midwest USA: Harvest and nitrogen management. Agron J 94: 413–420. [Google Scholar]
- 15.Jungers JM, Sheaffer CC, Lamb JA (2015) The effect of nitrogen, phosphorus, and potassium fertilizers on prairie biomass yield, ethanol yield, and nutrient harvest. BioEnergy Res 8: 279–291. 10.1007/s12155-014-9525-6 [DOI] [Google Scholar]
- 16.U.S. Department of Energy (2011) Billion-ton update: Biomass supply for a bioenergy and bioproducts industry Perlack, Stokes BS, editors Oak Ridge, TN: Oak Ridge National Laboratory. 227 p. [Google Scholar]
- 17.Tilman D, Knops JMH, Wedin D, Reich PB, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science (80-) 277: 1300–1302. 10.1126/science.277.5330.1300 [DOI] [Google Scholar]
- 18.Weigelt A, Weisser WW, Buchmann N, Scherer-Lorenzen M (2009) Biodiversity for multifunctional grasslands: Equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 6: 1695–1706. 10.5194/bg-6-1695-2009 [DOI] [Google Scholar]
- 19.Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, et al. (2007) Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci U S A 104: 18123–18128. 10.1073/pnas.0709069104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein HE, Burke IC, Mosier AR, Hutchinson GL (1998) Plant functional type effects on trace gas fluxes in the shortgrass steppe. Biogeochemistry 42: 145–168. 10.1023/A:1005959001235 [DOI] [Google Scholar]
- 21.Niklaus PA, Wardle DA, Tate KR (2006) Effects of plant species diversity and composition on nitrogen cycling and the trace gas balance of soils. Plant Soil 282: 83–98. 10.1007/s11104-005-5230-8 [DOI] [Google Scholar]
- 22.Oelmann Y, Wilcke W, Temperton VM, Buchmann N, Roscher C, et al. (2007) Soil and plant nitrogen pools as related to plant diversity in an experimental grassland. Soil Sci Soc Am J 71: 720–729. 10.2136/sssaj2006.0205 [DOI] [Google Scholar]
- 23.Palmborg C, Scherer-Lorenzen M, Jumpponen A, Carlsson G, Huss-Danell K, et al. (2005) Inorganic soil nitrogen under grassland plant communities of different species composition and diversity. Oikos 110: 271–282. 10.1111/j.0030-1299.2005.13673.x [DOI] [Google Scholar]
- 24.Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, et al. (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science (80-) 294: 804–808. 10.1126/science.1064088 [DOI] [PubMed] [Google Scholar]
- 25.Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, et al. (2005) Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr 75: 3–35. 10.1890/04-0922 [DOI] [Google Scholar]
- 26.NOAA (2012) National Weather Service Milwaukee/Sullivan 30 year climate averages: 1981–2010.
- 27.Oates LG, Duncan DS, Gelfand I, Millar N, Robertson GP, et al. (2015) Nitrous oxide emissions during establishment of eight alternative cellulosic bioenergy cropping systems in the North Central United States. GCB Bioenergy. [Google Scholar]
- 28.Grogan P, Chapin FS (1999) Arctic soil respiration: Effects of climate and vegetation depend on season. Ecosystems 2: 451–459. 10.1007/s100219900093 [DOI] [Google Scholar]
- 29.Pedersen AR, Petersen SO, Schelde K (2010) A comprehensive approach to soil-atmosphere trace-gas flux estimation with static chambers. Eur J Soil Sci 61: 888–902. 10.1111/j.1365-2389.2010.01291.x [DOI] [Google Scholar]
- 30.Pedersen AR (2012) HMR: Flux estimation with static chamber data. [Google Scholar]
- 31.Duran BEL, Kucharik CJ (2013) Comparison of two chamber methods for measuring soil trace-gas fluxes in bioenergy cropping systems. Soil Sci Soc Am J 77: 1601–1612. 10.2136/sssaj2013.01.0023 [DOI] [Google Scholar]
- 32.De Klein CAM, Novoa RSA, Ogle SM, Smith KA, Rochette P, et al. (2006) N2O emissions from managed soils, and CO2 emissions from lime and urea application In: Eggleston HS, Buendia L, Ngara T, Tanabe K, editors. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. National Greenhouse Gas Inventories Programme, IGES, Japan. [Google Scholar]
- 33.Stehfest E, Bouwman LF (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosystems 74: 207–228. 10.1007/s10705-006-9000-7 [DOI] [Google Scholar]
- 34.Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, et al. (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108: 389–411. 10.1007/BF00333714 [DOI] [PubMed] [Google Scholar]
- 35.Kucharik CJ, Fayram NJ, Cahill KN (2006) A paired study of prairie carbon stocks, fluxes, and phenology: Comparing the world’s oldest prairie restoration with an adjacent remnant. Glob Chang Biol 12: 122–139. 10.1111/j.1365-2486.2005.01053.x [DOI] [Google Scholar]
- 36.Beale C V, Long SP (1997) Seasonal dynamics of nutrient accumulation and partitioning in the perennial C4-grasses Miscanthus × giganteus and Spartina cynosuroides. Biomass and Bioenergy 12: 419–428. 10.1016/S0961-9534(97)00016-0 [DOI] [Google Scholar]
- 37.Robertson GP, Sollins P, Ellis BG, Lajtha K (1999) Exhangeable ions, pH, and cation exchange capacity In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P, editors. Standard Soil Methods for Long Term Ecological Research. Oxford: Oxford University Press; pp. 106–114. [Google Scholar]
- 38.Dobbie KE, Smith KA (2003) Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables. Glob Chang Biol 9: 204–218. 10.1046/j.1365-2486.2003.00563.x [DOI] [Google Scholar]
- 39.Rustad LE, Huntington TG, Boone RD (2000) Controls on soil respiration: Implications for climate change. Biogeochemistry 48: 1–6. 10.1023/A:1006255431298 [DOI] [Google Scholar]
- 40.Davidson EA (1991) Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems In: Rogers JE, Whitman WB, editors. Microbial production and consumption of greenhouse gases: methane, nitrogen oxides, and halomethanes. American Society for Microbiology; pp. 219–235. [Google Scholar]
- 41.Elzinga CL, Salzer DW, Willoughby JW (1998) Measuring and monitoring plant populations Denver, CO: U.S. Department of the Interior, Bureau of Land Management. [Google Scholar]
- 42.R Core Team (2014) R: A language and environment for statistical computing.
- 43.Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Development Core Team (2013) nlme: Linear and nonlinear mixed effects models.
- 44.Lenth R V (2013) lsmeans: Least-squares means. R Packag version 2.
- 45.Frank DA, Groffman PM (2009) Plant rhizospheric N processes: What we don’t know and why we should care. Ecology 90: 1512–1519. [DOI] [PubMed] [Google Scholar]
- 46.Robertson GP, Bruulsema TW, Gehl RJ, Kanter D, Mauzerall DL, et al. (2012) Nitrogen–climate interactions in US agriculture. Biogeochemistry 114: 41–70. 10.1007/s10533-012-9802-4 [DOI] [Google Scholar]
- 47.Jach-Smith LC, Jackson RD (2015) Nitrogen conservation decreases with fertilizer addition in two perennial grass cropping systems for bioenergy. Agric Ecosyst Environ 204: 62–71. 10.1016/j.agee.2015.02.006 [DOI] [Google Scholar]
- 48.Liang C, Jesus EC, Duncan DS, Jackson RD, Tiedje JM, et al. (2012) Soil microbial communities under model biofuel cropping systems in southern Wisconsin, USA: Impact of crop species and soil properties. Appl Soil Ecol 54: 24–31. 10.1016/j.apsoil.2011.11.015 [DOI] [Google Scholar]
- 49.Liang C, Duncan DS, Balser TC, Tiedje JM, Jackson RD (2013) Soil microbial residue storage linked to soil legacy under biofuel cropping systems in southern Wisconsin, USA. Soil Biol Biochem 57: 939–942. 10.1016/j.soilbio.2012.09.006 [DOI] [Google Scholar]
- 50.Vilain G, Garnier J, Decuq C, Lugnot M (2014) Nitrous oxide production from soil experiments: denitrification prevails over nitrification. Nutr Cycl Agroecosystems 98: 169–186. 10.1007/s10705-014-9604-2 [DOI] [Google Scholar]
- 51.Parrish DJ, Fike JH (2005) The biology and agronomy of switchgrass for biofuels. CRC Crit Rev Plant Sci 24: 423–459. 10.1080/07352680500316433 [DOI] [Google Scholar]
- 52.Hong CO, Owens VN, Bransby D, Farris R, Fike JH, et al. (2014) Switchgrass response to nitrogen fertilizer across diverse environments in the USA: A regional feedstock partnership report. BioEnergy Res 7: 777–788. 10.1007/s12155-014-9484-y [DOI] [Google Scholar]
- 53.Miesel JR, Renz MJ, Doll JE, Jackson RD (2012) Effectiveness of weed management methods in establishment of switchgrass and a native species mixture for biofuels in Wisconsin. Biomass and Bioenergy 36: 121–131. 10.1016/j.biombioe.2011.10.018 [DOI] [Google Scholar]
- 54.Wang D, Lebauer DS, Dietze MC (2010) A quantitative review comparing the yield of switchgrass in monocultures and mixtures in relation to climate and management factors. GCB Bioenergy 2: 16–25. 10.1111/j.1757-1707.2010.01035.x [DOI] [Google Scholar]
- 55.Kaye JP, Hart SC (1997) Competition for nitrogen between plants and soil microorganisms. Trends Ecol Evol 12: 139–143. 10.1016/S0169-5347(97)01001-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All data have been submitted to Dryad, with the following DOI: http://dx.doi.org/10.5061/dryad.m717n