Abstract
Sequential expression of the genes of the human beta-globin locus requires the formation of an erythroid-specific chromatin domain spanning > 200 kb. Regulation of this gene family involves both local interactions with proximal cis-acting sequences and long-range interactions with control elements upstream of the locus. To make it possible to analyze the interactions of cis-acting sequences of the human beta-globin locus in their normal spatial and sequence context, we characterized two yeast artificial chromosomes (YACs) 150 and 230 kb in size, containing the entire beta-globin locus. We have now successfully integrated the 150-kb YAC into the germ line of transgenic mice as a single unrearranged fragment that includes the locus control region, structural genes, and 30 kb of 3' flanking sequences present in the native locus. Expression of the transgenic human beta-globin locus is tissue- and developmental stage-specific and closely follows the pattern of expression of the endogenous mouse beta-globin locus. By using homology-directed recombination in yeast and methods for the purification and transfer of YACs into transgenic mice, it will now be feasible to study the physiological role of cis-acting sequences in specifying an erythroid-specific chromatin domain and directing expression of beta-globin genes during ontogeny.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Chada K., Magram J., Costantini F. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature. 1986 Feb 20;319(6055):685–689. doi: 10.1038/319685a0. [DOI] [PubMed] [Google Scholar]
- Choi T. K., Hollenbach P. W., Pearson B. E., Ueda R. M., Weddell G. N., Kurahara C. G., Woodhouse C. S., Kay R. M., Loring J. F. Transgenic mice containing a human heavy chain immunoglobulin gene fragment cloned in a yeast artificial chromosome. Nat Genet. 1993 Jun;4(2):117–123. doi: 10.1038/ng0693-117. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Constantoulakis P., Josephson B., Mangahas L., Papayannopoulou T., Enver T., Costantini F., Stamatoyannopoulos G. Locus control region-A gamma transgenic mice: a new model for studying the induction of fetal hemoglobin in the adult. Blood. 1991 Mar 15;77(6):1326–1333. [PubMed] [Google Scholar]
- Constantoulakis P., Knitter G., Stamatoyannopoulos G. On the induction of fetal hemoglobin by butyrates: in vivo and in vitro studies with sodium butyrate and comparison of combination treatments with 5-AzaC and AraC. Blood. 1989 Nov 1;74(6):1963–1971. [PubMed] [Google Scholar]
- Curtin P., Pirastu M., Kan Y. W., Gobert-Jones J. A., Stephens A. D., Lehmann H. A distant gene deletion affects beta-globin gene function in an atypical gamma delta beta-thalassemia. J Clin Invest. 1985 Oct;76(4):1554–1558. doi: 10.1172/JCI112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991 Mar 21;350(6315):252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- Driscoll M. C., Dobkin C. S., Alter B. P. Gamma delta beta-thalassemia due to a de novo mutation deleting the 5' beta-globin gene activation-region hypersensitive sites. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7470–7474. doi: 10.1073/pnas.86.19.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enver T., Ebens A. J., Forrester W. C., Stamatoyannopoulos G. The human beta-globin locus activation region alters the developmental fate of a human fetal globin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7033–7037. doi: 10.1073/pnas.86.18.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Epner E., Driscoll M. C., Enver T., Brice M., Papayannopoulou T., Groudine M. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 1990 Oct;4(10):1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P., Pruzina S., Antoniou M., Grosveld F. Each hypersensitive site of the human beta-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev. 1993 Jan;7(1):106–113. doi: 10.1101/gad.7.1.106. [DOI] [PubMed] [Google Scholar]
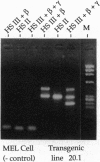
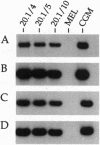
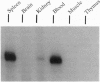
- Gaensler K. M., Burmeister M., Brownstein B. H., Taillon-Miller P., Myers R. M. Physical mapping of yeast artificial chromosomes containing sequences from the human beta-globin gene region. Genomics. 1991 Aug;10(4):976–984. doi: 10.1016/0888-7543(91)90188-k. [DOI] [PubMed] [Google Scholar]
- Gnirke A., Huxley C. Transfer of the human HPRT and GART genes from yeast to mammalian cells by microinjection of YAC DNA. Somat Cell Mol Genet. 1991 Nov;17(6):573–580. doi: 10.1007/BF01233622. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Vidal M., Kaeda J., Luzzatto L., Greaves D. R., Grosveld F. High-level, erythroid-specific expression of the human alpha-globin gene in transgenic mice and the production of human hemoglobin in murine erythrocytes. Genes Dev. 1989 Oct;3(10):1572–1581. doi: 10.1101/gad.3.10.1572. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Whyatt D., Fraser P., Yannoutsos N., Greaves D., Dillon N., Grosveld F. Importance of globin gene order for correct developmental expression. Genes Dev. 1991 Aug;5(8):1387–1394. doi: 10.1101/gad.5.8.1387. [DOI] [PubMed] [Google Scholar]
- Ikuta T., Kan Y. W. In vivo protein-DNA interactions at the beta-globin gene locus. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10188–10192. doi: 10.1073/pnas.88.22.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Moore A. L., Green L. L., Vergara G. J., Maynard-Currie C. E., Austin H. A., Klapholz S. Germ-line transmission and expression of a human-derived yeast artificial chromosome. Nature. 1993 Mar 18;362(6417):255–258. doi: 10.1038/362255a0. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Vanin E., deLange T., Flavell R. A., Grosveld F. G. Beta-globin gene inactivation by DNA translocation in gamma beta-thalassaemia. Nature. 1983 Dec 15;306(5944):662–666. doi: 10.1038/306662a0. [DOI] [PubMed] [Google Scholar]
- Kitchen H., Brett I. Embryonic and fetal hemoglobin in animals. Ann N Y Acad Sci. 1974 Nov 29;241(0):653–671. doi: 10.1111/j.1749-6632.1974.tb21921.x. [DOI] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Wrighton N., Hurst J., Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986 Jul 4;46(1):89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- Krakowsky J. M., Panke E. S., Lee R. F., McNeish J., Potter S. S., Lingrel J. B. Analysis of possible repressor elements in the 5'-flanking region of the human beta-globin gene. DNA. 1989 Dec;8(10):715–721. doi: 10.1089/dna.1989.8.715. [DOI] [PubMed] [Google Scholar]
- Lloyd J. A., Krakowsky J. M., Crable S. C., Lingrel J. B. Human gamma- to beta-globin gene switching using a mini construct in transgenic mice. Mol Cell Biol. 1992 Apr;12(4):1561–1567. doi: 10.1128/mcb.12.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S., Smithies O. A Chinese G gamma + (A gamma delta beta)zero thalassemia deletion: comparison to other deletions in the human beta-globin gene cluster and sequence analysis of the breakpoints. Nucleic Acids Res. 1985 Sep 25;13(18):6559–6575. doi: 10.1093/nar/13.18.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magram J., Chada K., Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature. 1985 May 23;315(6017):338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- Morley B. J., Abbott C. A., Sharpe J. A., Lida J., Chan-Thomas P. S., Wood W. G. A single beta-globin locus control region element (5' hypersensitive site 2) is sufficient for developmental regulation of human globin genes in transgenic mice. Mol Cell Biol. 1992 May;12(5):2057–2066. doi: 10.1128/mcb.12.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Pevny L., Rothstein R., Costantini F. Transfer of a yeast artificial chromosome carrying human DNA from Saccharomyces cerevisiae into mammalian cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5109–5113. doi: 10.1073/pnas.87.13.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Modification and transfer into an embryonal carcinoma cell line of a 360-kilobase human-derived yeast artificial chromosome. Mol Cell Biol. 1990 Aug;10(8):4163–4169. doi: 10.1128/mcb.10.8.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine S. P., Ginder G. D., Faller D. V., Dover G. H., Ikuta T., Witkowska H. E., Cai S. P., Vichinsky E. P., Olivieri N. F. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993 Jan 14;328(2):81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- Perrine S. P., Miller B. A., Faller D. V., Cohen R. A., Vichinsky E. P., Hurst D., Lubin B. H., Papayannopoulou T. Sodium butyrate enhances fetal globin gene expression in erythroid progenitors of patients with Hb SS and beta thalassemia. Blood. 1989 Jul;74(1):454–459. [PubMed] [Google Scholar]
- Peterson K. R., Clegg C. H., Huxley C., Josephson B. M., Haugen H. S., Furukawa T., Stamatoyannopoulos G. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7593–7597. doi: 10.1073/pnas.90.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Enver T., Nakamoto B., Josephson B., Papayannopoulou T., Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990 Nov 23;250(4984):1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- Reitman M., Lee E., Westphal H., Felsenfeld G. An enhancer/locus control region is not sufficient to open chromatin. Mol Cell Biol. 1993 Jul;13(7):3990–3998. doi: 10.1128/mcb.13.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J. H., Morten J. E., Anand R. Targeted integration of neomycin into yeast artificial chromosomes (YACs) for transfection into mammalian cells. Nucleic Acids Res. 1992 Jun 25;20(12):2971–2976. doi: 10.1093/nar/20.12.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A., Beermann F., Thies E., Montoliu L., Kelsey G., Schütz G. Transgenic mice generated by pronuclear injection of a yeast artificial chromosome. Nucleic Acids Res. 1992 Jun 25;20(12):3073–3077. doi: 10.1093/nar/20.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A., Montoliu L., Kelsey G., Schütz G. A yeast artificial chromosome covering the tyrosinase gene confers copy number-dependent expression in transgenic mice. Nature. 1993 Mar 18;362(6417):258–261. doi: 10.1038/362258a0. [DOI] [PubMed] [Google Scholar]
- Shih D. M., Wall R. J., Shapiro S. G. Developmentally regulated and erythroid-specific expression of the human embryonic beta-globin gene in transgenic mice. Nucleic Acids Res. 1990 Sep 25;18(18):5465–5472. doi: 10.1093/nar/18.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss W. M., Dausman J., Beard C., Johnson C., Lawrence J. B., Jaenisch R. Germ line transmission of a yeast artificial chromosome spanning the murine alpha 1(I) collagen locus. Science. 1993 Mar 26;259(5103):1904–1907. doi: 10.1126/science.8096090. [DOI] [PubMed] [Google Scholar]
- Strauss W. M., Jaenisch R. Molecular complementation of a collagen mutation in mammalian cells using yeast artificial chromosomes. EMBO J. 1992 Feb;11(2):417–422. doi: 10.1002/j.1460-2075.1992.tb05070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouboulis J., Dillon N., Grosveld F. Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev. 1992 Oct;6(10):1857–1864. doi: 10.1101/gad.6.10.1857. [DOI] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]