Human adipose-derived stem cells (hASCs) were obtained from subcutaneous adipose tissue of healthy, adult, female donors (lean and obese) undergoing abdominal plastic surgery. The differences observed in proliferation, migration, and differentiation capacity in obese hASCs, compared with those of lean donors, at two different oxygen conditions, occurred in parallel with changes in cell surface markers, both under basal conditions and during differentiation. Obesity appears to be an important determinant of stem cell function independent of oxygen tension.
Keywords: Adipogenic differentiation, Immunophenotype, Human mesenchymal stem cells, Obesity, Plasticity
Abstract
Adipose tissue is a major source of mesenchymal stem cells (MSCs), which possess a variety of properties that make them ideal candidates for regenerative and immunomodulatory therapies. Here, we compared the immunophenotypic profile of human adipose-derived stem cells (hASCs) from lean and obese individuals, and explored its relationship with the apparent altered plasticity of hASCs. We also hypothesized that persistent hypoxia treatment of cultured hASCs may be necessary but not sufficient to drive significant changes in mature adipocytes. hASCs were obtained from subcutaneous adipose tissue of healthy, adult, female donors undergoing abdominal plastic surgery: lean (n = 8; body mass index [BMI]: 23 ± 1 kg/m2) and obese (n = 8; BMI: 35 ± 5 kg/m2). Cell surface marker expression, proliferation and migration capacity, and adipogenic differentiation potential of cultured hASCs at two different oxygen conditions were studied. Compared with lean-derived hASCs, obese-derived hASCs demonstrated increased proliferation and migration capacity but decreased lipid droplet accumulation, correlating with a higher expression of human leukocyte antigen (HLA)-II and cluster of differentiation (CD) 106 and lower expression of CD29. Of interest, adipogenic differentiation modified CD106, CD49b, HLA-ABC surface protein expression, which was dependent on the donor’s BMI. Additionally, low oxygen tension increased proliferation and migration of lean but not obese hASCs, which correlated with an altered CD36 and CD49b immunophenotypic profile. In summary, the differences observed in proliferation, migration, and differentiation capacity in obese hASCs occurred in parallel with changes in cell surface markers, both under basal conditions and during differentiation. Therefore, obesity is an important determinant of stem cell function independent of oxygen tension.
Significance
The obesity-related hypoxic environment may have latent effects on human adipose tissue-derived mesenchymal stem cells (hASCs) with potential consequences in mature cells. This study explores the immunophenotypic profile of hASCs obtained from lean and obese individuals and its potential relationship with the altered plasticity of hASCs observed in obesity. In this context, an altered pattern of cell surface marker expression in obese-derived hASCs in both undifferentiated and differentiated stages is demonstrated. Differences in proliferation, migration, and differentiation capacity of hASCs from obese adipose tissue correlated with alterations in cell surface expression. Remarkably, altered plasticity observed in obese-derived hASCs was maintained in the absence of hypoxia, suggesting that these cells might be obesity conditioned.
Introduction
White adipose tissue (WAT) is conceivably the most plastic organ in the body, capable of considerable expansion and retraction in response to chronic deregulation in the energy balance. Adipose tissue expansion leads to obesity and contributes to obesity-related insulin resistance and its consequent morbidities. Human adipose tissue-derived mesenchymal stem cells (hASCs) are progenitor cells that are part of a structural component of the stroma surrounding mature adipocytes and maintain tissue homeostasis by regulating the number of mature adipose cells [1, 2].
Increasing evidence indicates that the homeostatic environment as well as the specific tissue source can influence the characteristics of hASCs, chiefly with regard to proliferation, migration, apoptotic susceptibility, and lipolytic capacity. In addition, the differentiation potential of isolated progenitor cells to adipogenic and osteogenic lineages has been found to be dependent on anatomical origin (subcutaneous, omental pericardic, or thymic) [3, 4]. There is no clear explanation for these distinctions, but local and systemic factors may help explain some of them. For example, low oxygen tension has dramatic effects on hASC physiology and impacts adipose tissue functionality. Accordingly, adipogenic differentiation of hASCs appears to be promoted by “high” in vitro oxygen tension (21%), whereas proliferation is enhanced at lower oxygen levels (5%) [5]. Analogously, low oxygen tension alters the physiological characteristics of embryonic, hematopoietic, and neuronal stem cells [6, 7]. Reduced adipose tissue oxygen partial pressure is a hallmark of obesity, presumably because adipose tissue vascularization is insufficient to maintain oxygen levels in expanding tissue, with potential implications for tissue function. Indeed, previous work showed that obese-derived hASCs are precommitted toward adipocyte differentiation with an upregulated inflammatory gene expression associated with their loss of stem cellness [8].
We hypothesized that this predisposition combined with a hostile environment mediated by hypoxia could have latent effects on hASCs with potential consequences in mature cells. Because the immunophenotype features of hASCs have been shown to be dependent on the adipose tissue source [9] and distinct stem cell immunophenotypes might impact adipogenic differentiation [10], changes in the immunophenotype profile in the context of an obese phenotype may result in dysfunctional behavior.
We show here that the antigen surface profile in undifferentiated hASCs and during adipogenic differentiation differs according to body mass index (BMI). Hypoxia has an equivalent effect on the plasticity of hASCs from healthy and obese donors; however, these effects do not correlate with the different immunophenotypic profiles detected in lean and obese hASCs. Overall, our results demonstrate that obesity determines the stem cell population in adipose tissue, and persistent hypoxia is necessary but not sufficient to orchestrate significant functional changes in mature adipocytes.
Materials and Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) high-glucose/Ham’s F12 medium (F12; 1:1), phosphate-buffered saline (PBS), trypsin-EDTA, and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, MA, https://www.thermofisher.com). Hanks’ Balanced Salt Solution (HBSS) and penicillin/streptomycin were purchased from PAA Laboratories (Pasching, Austria, http://www3.gehealthcare.com). Collagenase, type 1, was purchased from Worthington Biochemical Corp. (Lakewood, NJ, http://www.worthington-biochem.com). Alexa Fluor 488 annexin V/Dead Cell Apoptosis Kit and Vybrant CFDA SE Cell Tracer Kit for flow cytometry were purchased from Invitrogen (Thermo Fisher Scientific). Bovine serum albumin (BSA), mitomycin C, sodium azide, dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), biotin, human insulin solution, pantothenate acid, Oil Red, paraformaldehyde, isopropanol, and Nile Red were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Rosiglitazone was purchased from Cayman Chemical (Ann Arbor, MI, https://www.caymanchem.com). Monoclonal antibodies for flow cytometry were purchased from BD (Franklin Lakes, NJ, http://www.bd.com), Beckman Coulter (Brea, CA, https://www.beckmancoulter.com), and e-Bioscience (San Diego, CA, http://www.ebioscience.com).
Study Subjects
Subcutaneous adipose tissue from healthy female donors undergoing abdominal plastic surgery (n = 8 lean, BMI 23 ± 1 kg/m2; n = 8 obese, BMI 35 ± 5 kg/m2) was used in the study. Donors were classified as lean or obese based on BMI following World Health Organization criteria [11]. All participants gave their informed consent and the study was reviewed and approved by the Ethics and Research Committee of University Hospital Joan XXIII from Tarragona, Spain. All samples were part of an adipose-tissue biobank collection from our institution, ensuring the traceability and storage conditions of all samples.
hASC Isolation and Cell Culture
hASCs were isolated following published protocols [2, 12]. Briefly, washed lipoaspirate tissues were digested with collagenase in PBS-0.2% BSA at 37°C for 1 hour with agitation, followed by centrifugation for 5 minutes at 300g at room temperature (RT). Collected stromal vascular cells were cultured in stromal culture medium (SCM) consisting of DMEM/F12, 10% FBS, and 1% antibiotics (penicillin and streptomycin) at 37°C, 5% CO2 overnight. hASCs primary cultures at passage 0 were grown to 90% confluence and then harvested with trypsin-EDTA, and aliquots of 1 × 106 cells were cryopreserved in liquid nitrogen until required [10]. To thaw for use, each vial of hASCs was removed from liquid nitrogen and rapidly thawed at 37°C in a water bath. Thawed cells were transferred to a 15-ml disposable centrifuge tube containing 10 volumes of the required growth medium, followed by centrifugation at 300g at RT for 5 minutes. The cell pellet was resuspended in fresh SCM and transferred to a culture vessel at 5 × 103 cells/cm2. Cells were maintained at 37°C in a humidified tissue culture incubator containing 5% CO2 under high (21%) or low (5%) oxygen concentration. Medium was changed twice weekly and passaging was done at near confluence (>80%). Cells were washed in PBS and detached with trypsin for immunophenotypic analysis by flow cytometry, migration and proliferation studies, or directly induced for adipogenic differentiation.
Immunophenotyping
hASCs (2 × 105 cells) were incubated with a panel of primary antibodies, as previously described [11]; the antibodies were specifically chosen based on the known profile of bone marrow (BM)-derived MSCs. Cells were incubated for 20 minutes at RT (protected from light) with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated antibodies to human cluster of differentiation (CD) 36, CD49b, CD147, HLA-abc, and HLA-class II (HLA II); PeCy5-conjugated antibodies to human CD90, CD29, CD106, CD105, CD73, and CD34; PeCy7-conjugated antibodies to human CD45 and CD117; and anti-allophycocyanin (APC)-conjugated antibodies to human CD184 and CD44. Finally, hASCs were suspended in 400 μl of HBSS without calcium and magnesium, and the vital dye Hoechst 33342 (Ho342; 40 µg/ml final concentration) was added to discriminate nucleated cells. Samples were kept in the dark at 4°C until analysis. Data were collected using a MoFlo analyzer (Beckman Coulter) equipped with three lasers operating at 351, 488, and 633 nm. Nile Red fluorescence was measured using a 630/30-nm band-pass filter. Surface markers were detected using FITC (530/30-nm band-pass filter), PeCy5 (560/40-nm band-pass filter), PeCy7 (600/30-nm band-pass filter), and APC (650/50-nm band-pass filter) channels. Gating was based on forward scatter and side scatter dot plots. A side scatter versus Ho342 dot-plot was used to discriminate nucleated cells from debris. At least 20,000 events were analyzed in each run.
Hypoxia Treatment
Although oxygen tension in adipose tissue depends on blood flow, it varies typically between 3% and 11% [13]. An oxygen concentration of 5% was chosen as optimal for inducing changes in gene expression as described in BM-derived MSCs [14, 15]. hASCs from 3 lean and 3 obese donors were thawed in SCM at 5% O2 and maintained at this oxygen tension throughout the experiment. Adipogenic differentiation potential was analyzed immediately after cells were removed from the hypoxia incubator.
Cell Proliferation
Proliferation was quantified with the Vybrant carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE) Cell Tracer Kit (Thermo Fisher Scientific), which provides a versatile and well-retained cell tracing reagent. Cells were detached from culture plates, washed in cold PBS, and gently resuspended in prewarmed (37°C) PBS containing 2 µM CFDA-SE at approximately 1 × 106 cells/ml. Following incubation for 15 minutes at 37°C, cells were collected by centrifugation, resuspended in fresh prewarmed medium, and incubated for a further 30 minutes to ensure complete modification of the probe. After a final wash, CFDA-CE fluorescence was measured with the FC 500 Series flow cytometer (Beckman Coulter) using 488-nm excitation. The approximate excitation and emission peaks of the product after hydrolysis were 492 nm and 517 nm, respectively. At least 20,000 events were analyzed in each run.
Cell Migration
A migration assay was performed using the Transwell system (8-μm pore polycarbonate membrane) as previously described [16, 17]. Briefly, the lower chamber wells were filled with DMEM with 0.1% BSA and without FBS. DMEM plus 30% FBS was used as a positive control of migration. hASCs from lean or obese individuals (8 × 104 cells in 0.2 ml) were added to the upper well of the chamber and incubated for 24 hours at 37°C in a humidified atmosphere at 5% or 21% O2. Cells that migrated to the lower chamber were collected and counted using an automatic cell counter (Bio-Rad TC10; Bio-Rad Laboratories, Hercules, CA, http://www.bio-rad.com). Cells in the lower side of the Transwell membrane were fixed with 4% glutaraldehyde for 2 hours and stained overnight with 2% toluidine blue. Transwells were then rinsed with water and the upper membrane side was cleaned with a cotton swab to eliminate nonmigratory cells. Finally, the membrane was cut and placed onto a slide. We counted the number of cells on the lower side in five different, randomly selected ×10 fields using a bright-field microscope (Zeiss Axio Vert.A1; Zeiss, Oberkochen, Germany, http://www.zeiss.com). At least three independent experiments were performed using hASCs from different lean and obese individuals.
Adipogenic Differentiation
To derive mature adipocytes, hASCs from lean and obese individuals were induced to differentiate according to published protocols [10]. Briefly, confluent hASCs were cultured for 3 days in adipogenic induction medium containing DMEM/F12 supplemented with 10% FBS, 1% penicillin/streptomycin, 1 mM dexamethasone, 500 mM IBMX, 33 mM biotin, 5 mM rosiglitazone, 100 nM insulin, and 17 mM pantothenate. Cultures were replenished every 3 days with maintenance medium (supplemented medium without IBMX and rosiglitazone) for 2 weeks, after which differentiation potential was assessed. To do this, cells were washed with PBS, fixed with 2% paraformaldehyde for 30 minutes, and washed again with deionized water. Adipogenic differentiation was assessed by Oil Red O staining (0.3% Oil Red O in isopropanol) for 1 hour at room temperature, after which cells were washed and visualized with a microscope (Zeiss Axio Vert.A1).
Quantification of Lipid Droplets
Measurement of intracellular triacylglycerol was performed by Nile Red staining essentially as described by Greenspan et al. [18]. A working solution was prepared immediately before use by dilution of the stock solution (100 µg/ml Nile Red in dimethyl sulfoxide) in PBS to give a final concentration of 0.05 μg/ml. Following trypsin detachment, the cells were washed once with ice-cold PBS and Nile Red was added. The cells were incubated for 15 minutes at RT, centrifuged, and washed once with HBSS without calcium or magnesium. Subsequently, the cells were resuspended in PBS-1% BSA and 5% sodium azide, and fluorescence was measured as described under Immunoblot Analysis. Accumulation of lipid droplets was also visualized by staining the cells with Oil Red O. Cells were fixed in formalin solution 10% (Sigma-Aldrich) for 30 minutes and stained with Oil Red O for 45 minutes. After staining, dishes were washed and photographed (Zeiss Primovert; Zeiss).
Gene Expression Analysis
Total RNA was isolated from adipose tissue or cells using the RNeasy Lipid Tissue Midi Kit (Qiagen Science, Hilden, Germany, https://www.qiagen.com). RNA quantity was measured at 260 nm and purity was assessed by the OD260/OD280 ratio. One microgram of RNA was transcribed to cDNA with random primers using the Reverse Transcription System (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Quantitative gene expression was evaluated by real-time PCR (qPCR) on a 7900HT Fast Real-Time PCR System using TaqManR Gene Expression Assay (Applied Biosystems) for quantifying human adiponectin (APM) (Hs 00605917_m1) and peroxisome proliferator-activated receptor γ (PPARγ) (Hs00234592_m1). Results were calculated using the comparative threshold cycle (Ct) method and expressed relative to the expression of the housekeeping genes cyclophilin 1A (PPIA) (Hs 04194521_s1) and 18S (Hs03928985).
Immunoblot Analysis
Cells were lysed and homogenized in radioimmunoprecipitation assay buffer containing protease inhibitor cocktail (Sigma-Aldrich), and the protein concentration was determined with the BCA Protein Assay Kit (Thermo Fisher Scientific, Boston, MA). Equal amounts of total protein were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels, transferred to Immobilon membranes (EMD Millipore, Billerica, MA, https://www.emdmillipore.com) and blocked [19]. Immunoreactive bands were visualized with SuperSignal West Femto chemiluminescent substrate (Thermo Fisher Scientific) and images were captured using the VersaDoc imaging system and Quantity One software (Bio-Rad).
Statistical Analysis
Results are expressed as mean ± SD. Comparisons between groups were performed with Student’s t test. Significance was determined as p < .05. Flow cytometry data were analyzed using FlowJo Software (Tree Star, Ashland, OR, http://www.flowjo.com) and statistical significance was tested with the unpaired Student’s t test.
Results
Characterization of hASCs From Lean and Obese Individuals
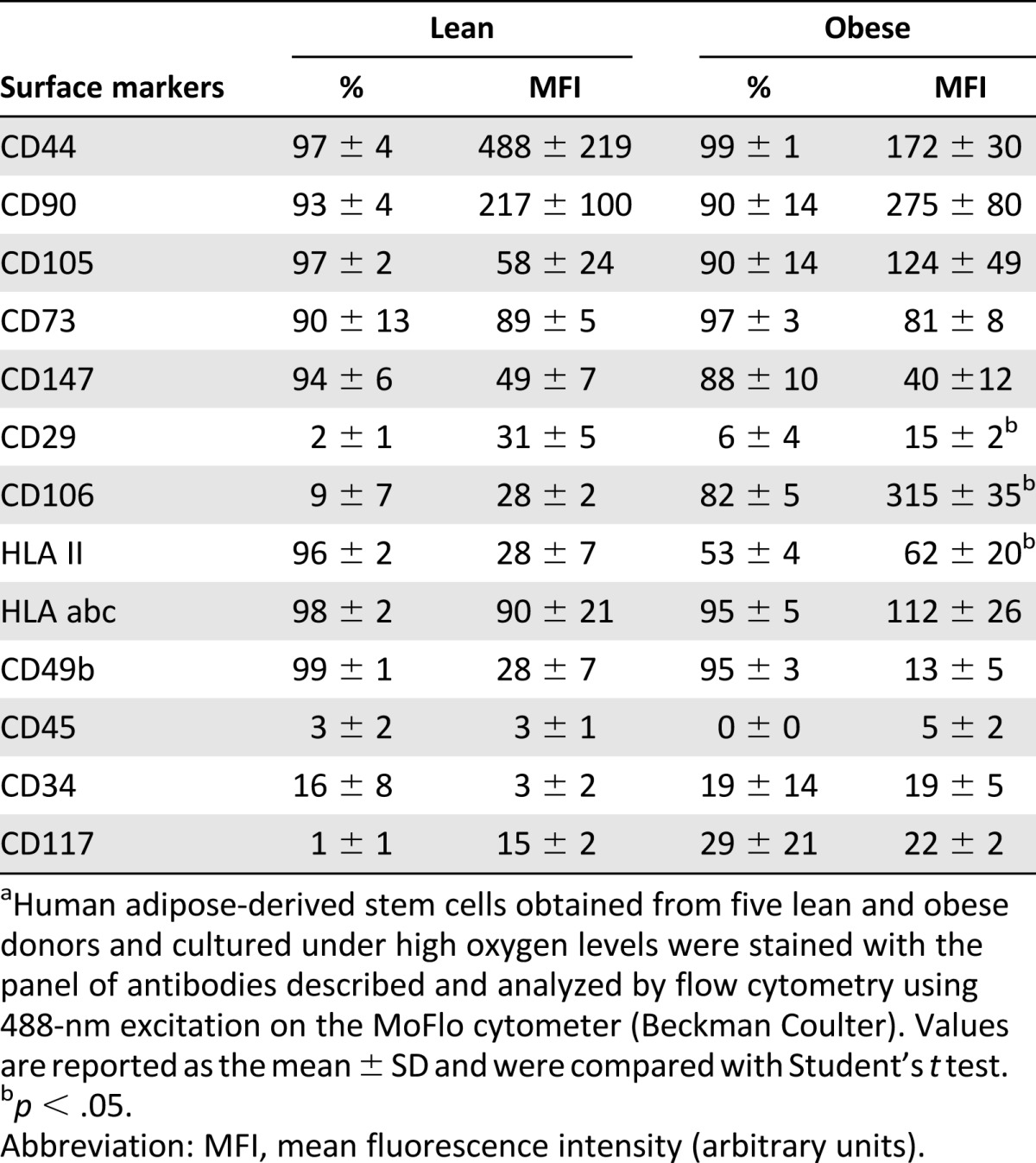
Isolated hASCs were prepared and stained according to the protocol described by Pachón-Peña et al. [10] and following the reagent manufacturer’s recommendations. Flow cytometry analysis of cell marker expression revealed that undifferentiated hASCs showed minimal functional and quantitative criteria as established by the International Society of Cell Therapy and the International Federation for Adipose Therapeutics and Science. hASCs were positive for the expression of typical surface antigens, including CD90 (Thy-1 antigen), CD29 (integrin β 1), CD44 (hyaluronic acid receptor), CD106 (vascular cell adhesion molecule 1, VCAM-1), CD73 (ecto-5′-nucleotidase), CD36 (glycoprotein IV, GPIV), CD147 (neurothelin), CD49b (integrin α2), human leukocyte antigen (HLA) class I -abc and HLA-major histocompatibility complex class II antigen (HLA-II), and negative for the expression of CD34 (single-chain transmembrane glycoprotein), CD45 (leukocyte common antigen), and CD117 (transmembrane tyrosine-kinase receptor) (Table 1). This expression profile has been described in previous studies and recognizes a specific hASC population [20–22]. All experiments were performed with these cells at passages 3–7.
Table 1.
Immunophenotypic profile of undifferentiated human adipose-derived stem cells isolated from lean and obese individualsa
Obesity Disturbs hASCs Plasticity and Immunophenotypic Profile
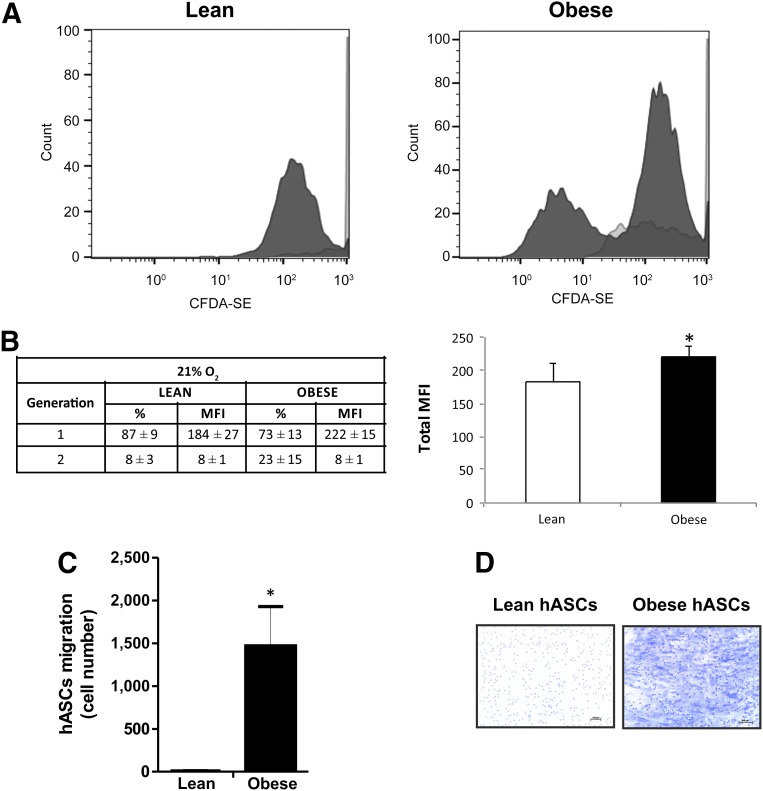
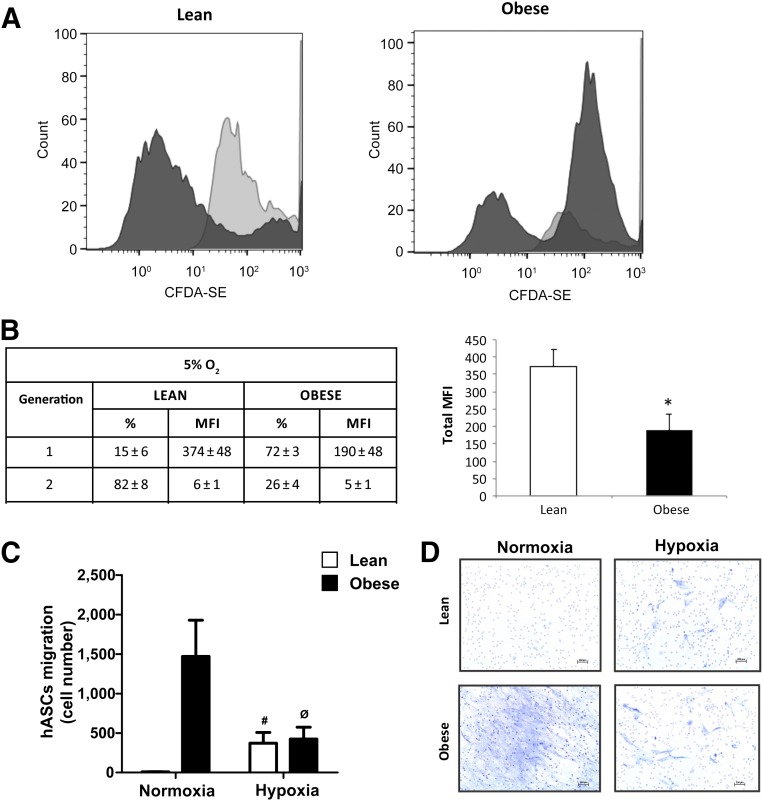
Previous results demonstrated that the metabolic phenotype of CD34+ MSCs isolated from obese adipose tissue significantly differed from equivalent cells isolated from lean adipose tissue [23]. To question whether an obese environment might similarly affect the plasticity of isolated hASCs, we evaluated proliferation, migration, and differentiation capacity of hASCs obtained from lean and obese individuals. To study the proliferative capacity, we used the Vybrant CFDA-SE Kit to evaluate the number of labeled cells during 72 hours. We found that proliferation of obese-isolated hASCs was significantly greater than hASCs isolated from lean individuals (total mean fluorescence intensity [MFI] 230 ± 16 and 192 ± 28 [arbitrary units], respectively; p < .05) (Fig. 1A, 1B).
Figure 1.
Proliferation and migration capacity of hASCs under high oxygen. (A): hASCs isolated from lean and obese individuals were cultured in growth medium at high (21%) oxygen levels. hASCs were treated with 2 µM CFDA-SE for 30 minutes and detected according to the recommended staining protocol. Cell division results in sequential halving of CFDA-SE fluorescence, resulting in a cellular fluorescence histogram in which the peaks represent successive generations. (B): Successive generation data expressed in percent and MFI values. (C): Quantification of migration capacity of hASCs isolated from lean and obese individuals after 24 hours. (D): Representative toluidine blue-stained cells (magnification, ×10). Values are reported as the mean ± SD of hASCs isolated from eight independent, lean and eight obese individuals. ∗, p < .05. Abbreviations: CFDA-SE, carboxy-fluorescein diacetate, succinimidyl ester; hASC, human adipose-derived stem cell; MFI, mean fluorescence intensity (arbitrary units).
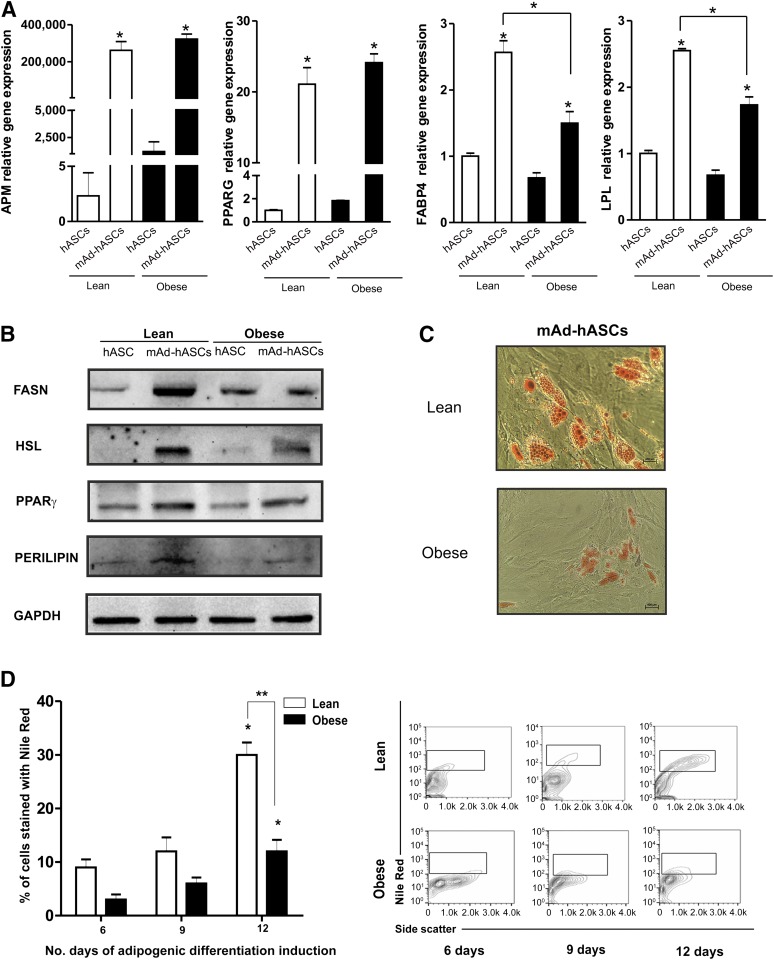
Next, we evaluated migration of hASCs using Transwell assays. Obese hASCs showed significantly greater basal migration capacity than lean hASCs (p < .05) (Fig. 1C, 1D). We then induced adipogenic differentiation by culturing hASCs in defined medium. After 10 days in culture, mature adipocytes derived from hASCs from lean and obese individuals (mAd-hASCs) showed a similar gene upregulation of adipogenic markers such as adiponectin (APM) and PPARγ, as compared with equivalent noninduced cultures. However, a decrease in the expression of genes related to lipid metabolism, such as fatty acid binding protein 4 and lipoprotein lipase, was detected in mAd-hASCs derived from obese donors as compared with those from lean donors (p < .05) (Fig. 2A). Protein expression analysis confirmed that although a comparable level of PPARγ protein was detected between obese and lean mAd-hASCs, obese cells showed a decrease in markers of lipid accumulation including fatty acid synthase (FASN), hormone sensitive lipase (HSL), and PERILIPIN compared with equivalent lean mAd-hASCs (Fig. 2B). Accordingly, lipid droplet formation evaluated by Oil Red O staining (Fig. 2C) and Nile Red staining (p < .05) (Fig. 2D) was significantly greater in lean-derived mAd-hASCs compared with obese-derived mAd-hASCs after 12 days of differentiation. Collectively, these results reveal that hASCs isolated from an obese environment have an increased proliferative and migration capacity but an impaired intracellular lipid accumulation.
Figure 2.
Adipogenic differentiation capacity of hASCs. (A): Expression of APM and PPARγ mRNA was analyzed by real-time polymerase chain reaction in hASCs and differentiated mAd-hASCs from the same lean and obese individuals. Results are given as mean ± SD from three independent experiments performed in duplicate. ∗, p < .05 versus undifferentiated hASCs. (B): Protein expression of FASN, HSL, PPARγ, and PERILIPIN was analyzed by Western blotting under the same conditions as in A. A representative immunoblot is shown. (C): Representative cytograms of intracellular lipid enrichment in hASCs from lean and obese individuals cultured at 21% O2 (magnification, ×20) (D): Quantification of Nile Red staining of hASCs from lean and obese individuals cultured for 6, 9, and 12 days in adipogenic differentiation medium at 21% O2 and stained with Nile Red (0.05 μg/ml). ∗, p < .01 for 12 days versus 6 and 9 days; ∗∗, p < .01 for hASCs from lean versus obese individuals at 12 days of adipogenic differentiation. Abbreviations: APM, adiponectin; FASN, fatty acid synthase; hASC, human adipose-derived stem cell; HSL, hormone sensitive lipase; mAD-hASCs, mature adipocytes derived from human adipose-derived stem cells from lean and obese individuals; PPAR, peroxisome proliferator-activated receptor; PERILIPIN, lipid droplet-associated protein.
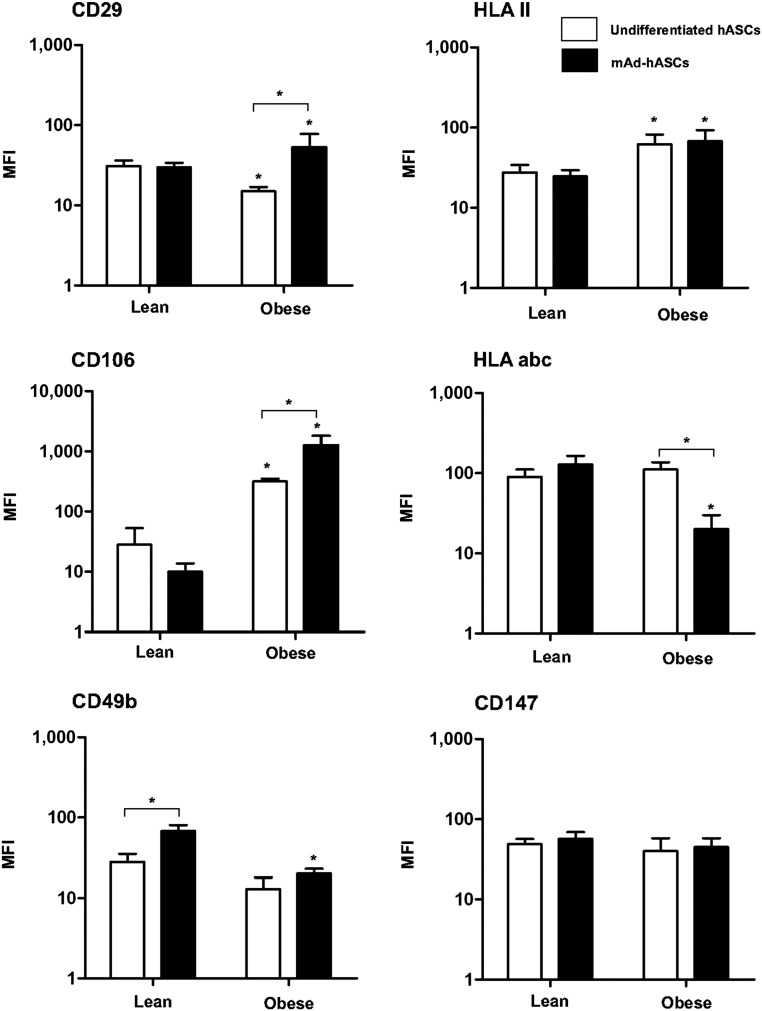
It is recognized that variations in MSC phenotypes are intimately associated with cell-specific and CD marker profiles [24, 25]. To question whether an obese environment modifies the immunophenotype of hASCs, we evaluated surface antigen expression by flow cytometry in undifferentiated hASCs and mAd-hASCs from lean and obese individuals. We found that marker expression was dependent on the differentiated status of the cells, but also on the clinical phenotype of the source tissue. Accordingly, expression of HLA-II and CD106 was significantly increased in obese-isolated undifferentiated hASCs relative to cells isolated from lean individuals, whereas expression of CD29 was significantly decreased (Fig. 3). Also, we observed decreased CD106 expression during differentiation of lean hASCs, but this was increased in differentiated obese hASCs. Furthermore, CD49b was significantly upregulated in mAd-hASCs from lean but not obese individuals compared with undifferentiated cells, and HLA-ABC expression was downregulated in obese but not lean hASCs during differentiation (Fig. 3).
Figure 3.
Immunophenotypic profile of undifferentiated and differentiated hASCs isolated from lean and obese individuals cultured at 21% O2. Undifferentiated hASCs (white bars) and mAd-hASCs (black bars) were stained with the panel of antibodies described and analyzed by flow cytometry using a MoFlo analyzer (Beckman Coulter) equipped with 3 lasers operating at 351, 488, and 633 nm. Surface markers were detected using fluorescein isothiocyanate (530/30-nm band-pass filter), PeCy5 (560/40-nm band-pass filter), PeCy7 (600/30-nm band-pass filter), and APC (650/50-nm band-pass filter) channels. Gating was based on forward scatter and side scatter dot plots. A side-scatter versus Ho342 dot plot was used to discriminate nucleated cells from debris. Values are expressed in MFI and reported as the mean ± SD of hASCs from eight independent lean and eight obese individuals. ∗, p < .05. Abbreviations: hASC, human adipose-derived stem cell; mAD-hASC, mature adipocytes derived from human adipose-derived stem cells from lean and obese individuals; MFI, mean fluorescence intensity (arbitrary units).
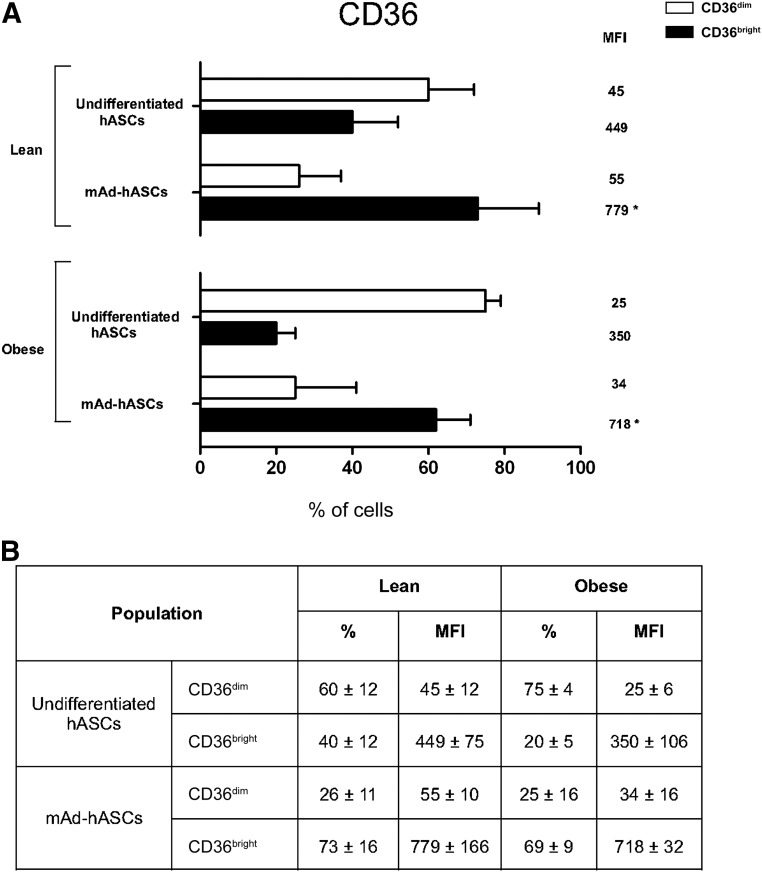
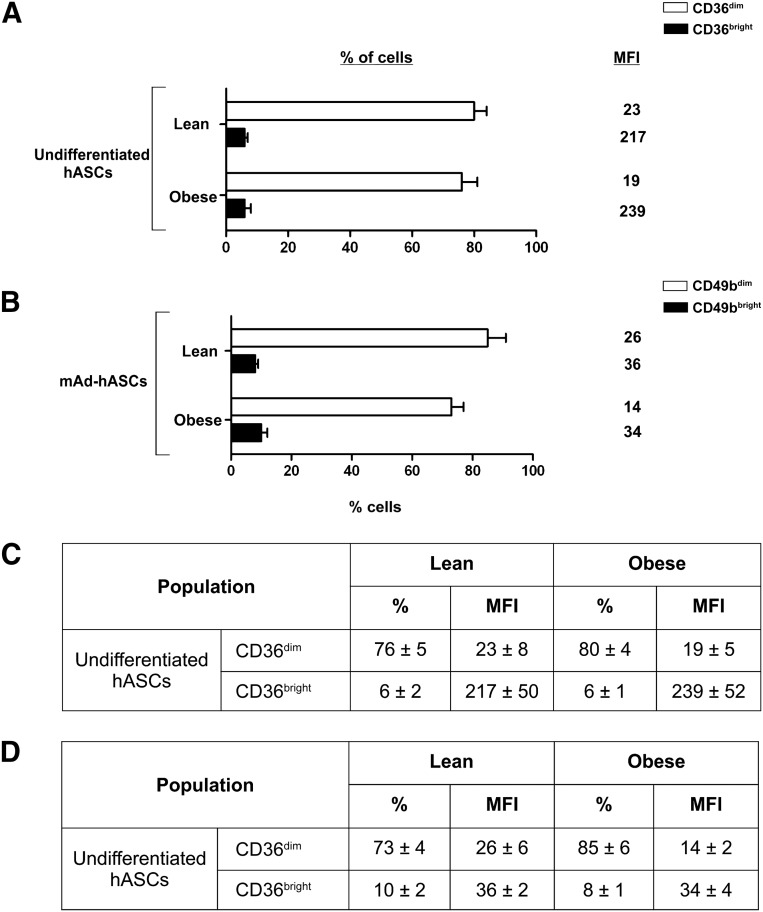
Remarkably, quantitative flow cytometric analysis revealed two distinct populations of undifferentiated and mAd-hASCs from lean individuals based on expression of CD36 (CD36dim/CD36bright) (Fig. 4A, 4B). Regardless of this profile, high expression of CD36 in mAd-hASCs from both lean and obese individuals was detected (73% ± 16% vs. 69% ± 9% in cell percentage; total MFI: 779 ± 166 vs. 718 ± 2, respectively). Collectively, these data reveal that the altered plasticity observed in hASCs from an obese environment is paralleled by changes in cell surface markers, both in undifferentiated and differentiated cultures.
Figure 4.
Membrane CD36 expression on undifferentiated and differentiated hASCs cultured at 21% O2. (A): Quantification of CD36 expression in hASCs and mAd-hASCs from lean and obese individuals cultured at 21% O2. (B): Data expressed in percentage of cells and MFI indicating the presence of two populations of CD36+ with CD36dim/CD36bright. Values are reported as the mean ± SD of hASCs from eight independent lean and eight obese individuals. ∗, p < .05. Abbreviations: hASC, human adipose-derived stem cell; mAD-hASC, mature adipocytes derived from human adipose-derived stem cells from lean and obese individuals; MFI, mean fluorescence intensity (arbitrary units).
Hypoxia-Related Plasticity Dysfunction in hASCs Correlates With an Altered Immunophenotypic Profile
Adipose tissue hypoxia is potentially implicated in adipocyte dysfunction [26, 27]. Accordingly, hASCs isolated from obese patients display a gene expression profile associated with a loss of “stem cellness” and a commitment to adipocyte differentiation [28, 29]. This predisposition, combined with a hypoxic environment, may affect hASCs with consequences for mature adipocytes.
We first evaluated whether low in vitro oxygen culture conditions (5% O2), which mimics the physiological oxygen tension in adipose tissue in vivo [30–32], impacted proliferation. hASCs isolated from lean individuals exhibited a higher proliferative capacity at 5% O2 compared with normal oxygen tension (total MFI: 380 ± 49 vs. 192 ± 28, respectively; p < .05) (compare Fig. 1B and Fig. 5B). In contrast, proliferation of hASCs isolated from obese individuals remained unchanged (total MFI: 195 ± 49 vs. 230 ± 16) (compare Fig. 1B and 5B). We also found an increased migration capacity of lean hASCs after 24 hours of incubation at 5% O2 (Fig. 5C, 5D) as well as an increase in the quantity of apoptotic cells (data not shown), whereas the migration capacity of obese hASCs was decreased under the same conditions (Fig. 5C, 5D).
Figure 5.
Proliferation and migration capacity of hASCs under low oxygen. (A): hASCs isolated from lean and obese individuals were cultured in growth medium at 5% O2. hASCs were treated with 2 µM CFDA-SE as described in Figure 1 legend. (B): Successive generation data are expressed in percent and MFI values. Values are reported as the mean ± SD of hASCs from three independent lean and three obese individuals. ∗, p < .05 versus lean. (C): Migration capacity of hASCs isolated from lean and obese individuals at 21% O2 and after 24-hour exposure to 5% O2. Values are reported as the mean ± SD of hASCs from three independent lean and three obese individuals. #, p < .01 versus lean under normoxic conditions; ∅, p < .01 versus obese under normoxic conditions. (D): Representative toluidine blue-stained cells (magnification, ×10). Abbreviations: CFDA-SE, carboxy-fluorescein diacetate, succinimidyl ester; hASC, human adipose-derived stem cell; MFI, mean fluorescence intensity (arbitrary units).
Next, we analyzed the effects of low oxygen for intracellular lipid formation. Although culture at 5% O2 attenuated lipid droplet formation in both lean and obese mAd-hASCs compared with 21% O2, accumulation of lipids in lean-derived mAd-hASCs was greater than in obese-derived mAd-hASCs after 12 days of adipogenic differentiation (26% ± 14% and 6% ± 3%, respectively).
We then measured surface marker expression during differentiation in low oxygen. Because of the low number of adipogenic-differentiated hASCs from obese individuals, global surface marker expression analysis during differentiation of obese hASCs at 5% O2 was not possible. As described, data from flow cytometry revealed two populations of undifferentiated and mAd-hASCs from lean individuals with distinct expression of CD36+ (CD36dim/CD36bright) under normal oxygen levels (Fig. 4). Strikingly, the relative proportion of these subsets varied at low oxygen levels. Accordingly, a decrease in the percentage of undifferentiated hASCs from lean and obese individuals (85% and 70% in the CD36bright population at 5% O2, respectively) was observed, as well as a decrease in the mean fluorescence intensity (2-fold and 1.4-fold in lean and obese subjects, respectively) (Fig. 6A, 6C). Interestingly, among CD49+ cells, two populations with different phenotypes (CD49bdim/CD49bright) were also identified at low oxygen levels both in lean and obese populations (Fig. 6B, 6D). Collectively, our data uncover a distinguishing immunophenotypic profile in hASCs in response to low oxygen levels, which also associates with the donor BMI.
Figure 6.
Membrane CD36 and CD49b expression in undifferentiated hASCs cultured under low oxygen. (A, B): Quantification of CD36 (A) and (B) CD49b expression in undifferentiated hASCs from lean and obese individuals at 5% O2. (C, D): Data expressed in percentage of cells and MFI values indicating the presence of two populations of CD36+ and (D) CD49B+ cells, with dim/bright MFI with respect to percentage of cells at 21% O2. Values are reported as the mean ± SD of hASCs from three independent lean and three obese individuals. ∗, p < .05. Abbreviations: hASC, human adipose-derived stem cell; mAD-hASC, mature adipocytes derived from human adipose-derived stem cells from lean and obese individuals; MFI, mean fluorescence intensity (arbitrary units).
Discussion
Adipose tissue is a major source of MSCs, which possess an assortment of features that make them suitable for regenerative and immunomodulatory applications [33]. hASCs are affected by changing environmental conditions that result in an adaptation of their properties to a particular function. Here, we demonstrate that compared with a lean environment, adipose tissue from obese individuals contains a dysfunctional population of hASCs that can be identified by a unique immunophenotypic profile and aberrant differentiation.
Our results reveal that obese hASCs have a high proliferation capacity, significantly higher basal migration, and reduced lipid droplet formation relative to lean hASCs. This altered plasticity of obese hASCs is paralleled by changes in cell surface marker expression, under basal conditions and also during differentiation. Hypoxia disturbs the plasticity of lean hASCs in a manner similar to the behavior observed in obese hASCs; however, notwithstanding the changes in surface marker expression detected under low oxygen levels, the immunophenotypic profile of obese hASCs is not mimicked in lean hASCs by hypoxia. These results point to the presence of a different population of hASCs as a consequence of the obese state and not only of environmental hypoxia. Consistent with previous studies [34–36], our data indicate that low oxygen levels maintain hASCs in their undifferentiated state and decrease their differentiation potential to adipocytes. In accordance with these findings, other studies have shown that high donor BMI results in a potentially compromised osteogenic capacity in vitro [37]. Low oxygen levels, as observed in adipose tissue from obese subjects, may account for the immunophenotypic changes in these cells, triggering functional differences.
Despite the relevance of cell surface marker expression as a tool to identify and track stem cells, including hASCs, no studies have been published that comprehensively examine surface marker expression in hASCs from lean and obese individuals. This is particularly relevant when considering that these cells are proposed as a promising tool for regenerative therapies, and differences in surface antigen expression may define a specific profile in both the undifferentiated and in the mature states. These observations may allow an understanding of how these cells might function in regenerative medicine applications.
Surface marker expression is routinely determined by flow cytometry to classify cell type, but it should be noted that an MSC cannot be classified based solely on a single marker protein. Moreover, consecutive single-parameter measurements for determination of an expression pattern of various stem cell markers may suffer from artifacts due to heterogeneous cell populations. Consequently, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy have proposed three minimal criteria to define human MSCs [19]. We have defined hASCs according to these criteria [20–22]. Other methods have been frequently used to identify, isolate, and characterize human and murine ASCs from adipose tissue [23, 38]. Thus, previous studies have reported that human and murine WAT is a rich reservoir of CD45-CD34+ ASCs, with a high basal migration capacity in lean-derived, but not obese-derived, ASCs [23, 39]. The discrepancies between our data and those of other authors regarding migratory capacity may be due to differences in the type of hASCs isolated, because CD34+ cells used in these earlier reports represent the classical phenotype of endothelial progenitor cells (EPCs). These EPCs differentiate into endothelial cells and might contribute to vessel formation. Indeed, they express typical surface markers (CD34+/KDR+/CD45−) attributed to true circulating EPCs derived from bone marrow [40] but do not represent the niche of hASCs that generate mature adipocytes.
A striking finding of our work was the increased migratory capacity of hASCs from obese individuals. Interestingly, these cells show an upregulation of CD106 and HLA II surface cell markers in undifferentiated cells and, although we acknowledge that no mechanistic conclusions can be reached from these observations, both proteins are involved in migration and immunomodulatory events. We show that CD49b, a transmembrane glycoprotein that noncovalently associates with CD29 (integrin β1) to form the integrin α2β1 complex known as very late activation antigen 2 (VLA-2), was upregulated in normal oxygen levels in mAd-hASCs. CD49b is recognized as a collagen receptor that plays a critical role in the establishment of resting memory CD4 T cells of the BM and the transmigration of effector cells into the BM through the sinusoidal endothelium [41, 42]. Although CD49b has been described to be involved in generation of T-helper cell memory, where and how CD49b works during the immune response remains unclear. Additionally, several studies have reported that MSCs exert their immunomodulatory activities through direct cell-cell contact and/or secretion of soluble factors [43]. Cell-cell adhesion mediated by CD106 is known to be critical for T-cell activation and leukocyte recruitment to the site of inflammation, and, therefore, plays an important role in evoking effective immune responses. Moreover, CD106 is critical for MSC-mediated immunosuppression [44] and for the binding of hematopoietic progenitor cells [45]. Our data lead us to speculate that increased expression of HLA II/CD106 in a subpopulation of undifferentiated and differentiated hASCs, together with upregulated expression of CD49b in differentiated hASCs from obese individuals, could be related to the powerful immunosuppressive activity of hASCs. Further experiments are required to elucidate the downstream mechanisms and potential applications of these findings.
The significance of the two populations of cells with distinct CD36 expression remains to be investigated. CD36 has been described to regulate the expression of genes involved in lipid metabolism and has been also shown to be essential in the regulation of adipocyte differentiation and cellular lipid uptake [46]. Our data showing modulation of CD49b/CD36 expression at low oxygen levels and high expression of CD36 on differentiated hASCs are consistent with previous studies demonstrating a key role for this receptor in modulation of adipocyte transcriptional regulators, such as PPARγ, and adipogenic differentiation [46–49]. Further experiments will be required to determine the significance of these distinct populations, especially in relation to CD49b in the context of low oxygen tension. Other α2 integrins are regulated during osteogenic stimulation [50] and have also been frequently associated with enhanced osteogenic differentiation capacity at low oxygen levels [51]. We show here that hypoxia regulates CD49b expression, and the modest percentage of undifferentiated hASCs cells with high levels of surface CD49b might predict a higher osteogenic differentiation capacity of these cells in low oxygen. Additional studies to evaluate the potential relationship between cell surface markers and the osteogenic versus adipogenic potential of hASCs should be considered.
Conclusion
Our data demonstrate that proliferation, migration, and differentiation capacities, as well as the immunophenotypic profile of hASCs, are modulated by the microenvironment within adipose tissue. In the obese context, low oxygen levels and chronic low-grade inflammation are considered key microenvironmental factors that might determine commitment and plasticity of progenitor cells within adipose tissue. We have observed that hypoxia disturbs cell plasticity in undifferentiated and also in adipogenic-differentiated hASCs isolated from obese individuals, and these changes are paralleled by modulation of the immunophenotypic profile. The altered behavior observed in obese hASCs independently of oxygen levels, however, points to additional environmental factors as a potential origin of the characteristic dysfunction of hASCs observed in obesity.
Our results highlight the importance of examining stem cells within their physiologic environment to better understand their functional properties, which may be invaluable for developing novel cytotherapeutic strategies with possible clinical impact. Additional studies will be required to further assess the characteristics of these cells and to elucidate their true physiological nature.
Acknowledgments
This study was supported by grants from the Spanish Ministry of Economy and Competitiveness (PI11/0085, PI14/00228 to J.V. and SAF2012-36186 to S.F.-V.). The Spanish Biomedical Research Center in Diabetes and Associated Metabolic Disorders (CB07708/0012) is an initiative of the Instituto de Salud Carlos III. G.P.-P. received grant support from the Ministerio de Salud Carlos III (Programa contrato de perfeccionamiento postdoctoral Sara Borrell CD10/00285). S.F.-V. acknowledges support from the Miguel Servet tenure track program (CP10/00438) from the Fondo de Investigación Sanitaria, cofinanced by the European Regional Development Fund. C.S. acknowledges support from Ramón y Cajal Spanish program from the Ministerio de Educación y Ciencia (RYC2013-13186). We thank Dr. Kenneth McCreath for helpful comments on the manuscript.
Author Contributions
G.P.-P., J.P., S.F.-V., and J.V.: conception and design, collection and/or assembly of data, manuscript writing; C.S., M.E., and X.D.: collection and/or assembly of data, manuscript writing; W.O.-O., R.S., and F.J.T.: collection and/or assembly of data.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Gimble JM, Bunnell BA, Frazier T, et al. Adipose-derived stromal/stem cells: A primer. Organogenesis. 2013;9:3–10. doi: 10.4161/org.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimble JM, Guilak F, Nuttall ME, et al. In vitro differentiation potential of mesenchymal stem cells. Transfus Med Hemother. 2008;35:228–238. doi: 10.1159/000124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwen KA, Priewe AC, Winnefeld M, et al. Gluteal and abdominal subcutaneous adipose tissue depots as stroma cell source: Gluteal cells display increased adipogenic and osteogenic differentiation potentials. Exp Dermatol. 2014;23:395–400. doi: 10.1111/exd.12406. [DOI] [PubMed] [Google Scholar]
- 4.Lin RZ, Moreno-Luna R, Zhou B, et al. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15:443–455. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Fujita M, Tanaka Y, et al. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. BioRes Open Access. 2013;2:199–205. doi: 10.1089/biores.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Z, Zhang W, Jiang S, et al. Effect of oxygen tensions on the proliferation and angiogenesis of endometriosis heterograft in severe combined immunodeficiency mice. Fertil Steril. 2014;101:568–576. doi: 10.1016/j.fertnstert.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Oñate B, Vilahur G, Camino-López S, et al. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics. 2013;14:625. doi: 10.1186/1471-2164-14-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah F, Li J, Dietrich M, et al. Comparison of stromal/stem cells isolated from human omental and subcutaneous adipose depots: Differentiation and immunophenotypic characterization. Cells Tissues Organs. 2014;200:204–11. doi: 10.1159/000430088. [DOI] [PubMed] [Google Scholar]
- 10.Pachón-Peña G, Yu G, Tucker A, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva, Switzerland: World Health Organization, 2000. [PubMed]
- 12.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goossens GH, Blaak EE. Adipose tissue oxygen tension: Implications for chronic metabolic and inflammatory diseases. Curr Opin Clin Nutr Metab Care. 2012;15:539–546. doi: 10.1097/MCO.0b013e328358fa87. [DOI] [PubMed] [Google Scholar]
- 14.Wu EH, Li HS, Zhao T, et al. [Effect of hypoxia on the gene profile of human bone marrow-derived mesenchymal stem cells] Sheng Li Xue Bao. 2007;59:27–32. [PubMed] [Google Scholar]
- 15.Hu L, Li DP, Zhang ZJ, et al. [Role of bone marrow mesenchymal stem cells in restoring the functions of degenerative nucleus pulposus cells] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2014;36:25–32. doi: 10.3881/j.issn.1000-503X.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Jin D, Tuo Y, et al. [Calcitonin gene-related peptide promoting migration of rat bone marrow mesenchymal stem cells and stimulating expression of vascular endothelial growth factor] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:1371–1376. [PubMed] [Google Scholar]
- 17.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan P, Mayer EP, Fowler SD. Nile red: A selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceperuelo-Mallafré V, Duran X, Pachón G, et al. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J Clin Endocrinol Metab. 2014;99:E908–E919. doi: 10.1210/jc.2013-3350. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez LM, Bernal A, San Martín N, et al. Metabolic rescue of obese adipose-derived stem cells by Lin28/Let7 pathway. Diabetes. 2013;62:2368–2379. doi: 10.2337/db12-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerlin L, Donnenberg VS, Rubin JP, et al. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013;83:134–140. doi: 10.1002/cyto.a.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calkoen FG, Vervat C, van Pel M, et al. Despite differential gene expression profiles pediatric MDS derived mesenchymal stromal cells display functionality in vitro. Stem Cell Res (Amst) 2015;14:198–210. doi: 10.1016/j.scr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Wood IS, de Heredia FP, Wang B, et al. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 27.O’Rourke RW, White AE, Metcalf MD, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy OT, Perugini RA, Nicoloro SM, et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Rel Dis. 2011;7:60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeGirolamo KM, Courtemanche DJ, Hill WD, et al. Use of safety scalpels and other safety practices to reduce sharps injury in the operating room: what is the evidence? Can J Surg. 2013;56:263–269. doi: 10.1503/cjs.003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Holzwarth C, Vaegler M, Gieseke F, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 33.Melief SM, Zwaginga JJ, Fibbe WE, et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Translational Medicine. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller ZA, Schiele NR, Sims JK, et al. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem Cell Res Ther. 2013;4:79. doi: 10.1186/scrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JH, Nguyen MP, Lee D, et al. Hypoxia-induced endothelial progenitor cell function is blunted in angiotensinogen knockout mice. Mol Cells. 2014;37:487–496. doi: 10.14348/molcells.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fotia C, Massa A, Boriani F, et al. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology. 2015;67:1073–1084. doi: 10.1007/s10616-014-9731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frazier TP, Gimble JM, Devay JW, et al. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol. 2013;14:34. doi: 10.1186/1471-2121-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hager G, Holnthoner W, Wolbank S, et al. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy. 2013;15:1426–1435. doi: 10.1016/j.jcyt.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Padura I, Gregato G, Marighetti P, et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res. 2012;72:325–334. doi: 10.1158/0008-5472.CAN-11-1739. [DOI] [PubMed] [Google Scholar]
- 40.Graziani F, Leone AM, Basile E, et al. Endothelial progenitor cells in morbid obesity. Circ J. 2014;78:977–985. doi: 10.1253/circj.cj-13-0976. [DOI] [PubMed] [Google Scholar]
- 41.Hanazawa A, Löhning M, Radbruch A, et al. CD49b/CD69-dependent generation of resting T helper cell memory. Front Immunol. 2013;4:183. doi: 10.3389/fimmu.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanazawa A, Hayashizaki K, Shinoda K, et al. CD49b-dependent establishment of T helper cell memory. Immunol Cell Biol. 2013;91:524–531. doi: 10.1038/icb.2013.36. [DOI] [PubMed] [Google Scholar]
- 43.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 44.Kokovay E, Wang Y, Kusek G, et al. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell. 2012;11:220–230. doi: 10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Ren M, Yan L, Shang CZ, et al. Effects of sodium butyrate on the differentiation of pancreatic and hepatic progenitor cells from mouse embryonic stem cells. J Cell Biochem. 2010;109:236–244. doi: 10.1002/jcb.22401. [DOI] [PubMed] [Google Scholar]
- 46.Motojima K, Passilly P, Peters JM, et al. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 47.Christiaens V, Van Hul M, Lijnen HR, et al. CD36 promotes adipocyte differentiation and adipogenesis. Biochim Biophys Acta. 2012;1820:949–956. doi: 10.1016/j.bbagen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Erener S, Hesse M, Kostadinova R, et al. Poly(ADP-ribose)polymerase-1 (PARP1) controls adipogenic gene expression and adipocyte function. Mol Endocrinol. 2012;26:79–86. doi: 10.1210/me.2011-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011;16:236–257. doi: 10.2478/s11658-011-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popov C, Radic T, Haasters F, et al. Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binder B, Sagun J, Leach J. Reduced serum and hypoxic culture conditions enhance the osteogenic potential of human mesenchymal stem cells. Stem Cell Rev. 2015;11:387–393. doi: 10.1007/s12015-014-9555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]