This study demonstrates that treatment with isobutylmethylxanthine is an efficient method of expanding muscle progenitor cells, while preserving regenerative potential, before transplantation. Phosphodiesterase inhibitor treatment improves myoblast culture conditions in such a way as to reinvigorate myoblast transplantation as a viable therapeutic approach to halt muscle wasting in Duchenne muscular dystrophy.
Keywords: CCAAT-enhancer-binding protein-β, Phosphodiesterase inhibitors, Stem cell transplantation, Muscular dystrophies, Pax7 transcription factor, Skeletal muscle satellite cells
Abstract
Duchenne muscular dystrophy (DMD), caused by mutations in the dystrophin gene, is the most common muscular dystrophy. Characterized by rounds of muscle degeneration and regeneration, DMD features progressive muscle wasting and is fatal. One approach for treatment is transplantation of muscle progenitor cells to repair and restore dystrophin expression to damaged muscle. However, the success of this approach has been limited by difficulties in isolating large numbers of myogenic progenitors with strong regenerative potential, poor engraftment, poor survival of donor cells, and limited migration in the diseased muscle. We demonstrate that induction of the transcription factor CCAAT/enhancer-binding protein β (C/EBPβ) using the cyclic adenosine monophosphate phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) results in enhanced myoblast expansion in culture and increased satellite cell marker expression. When equal numbers of IBMX-treated cells were transplanted into dystrophic muscle, they contributed to muscle repair more efficiently than did vehicle-treated cells and engrafted into the satellite cell niche in higher numbers, demonstrating improved cell migration from the site of injury and enhanced survival after transplantation. Thus, pharmacologic stimulation of C/EBPβ expression reprograms myoblasts to a more stem cell-like state, promotes expansion in culture, and improves engraftment such that better transplantation outcomes are achieved.
Significance
Duchenne muscular dystrophy is a genetic disorder for which no cure exists. One therapeutic approach is transplantation of myogenic progenitors to restore dystrophin to damaged muscle, but this approach is limited by poor engraftment of cultured myoblasts. Transient upregulation of CCAAT/enhancer-binding protein β in primary myoblasts using the phosphodiesterase isobutylmethylxanthine (IBMX) increases satellite cell marker expression in cultured myoblasts, improves their migration, and increases their survival after transplantation. When transplanted into C57BL/10ScSn-mdx/J mice , IBMX-treated myoblasts restored dystrophin expression and were able to occupy the satellite cell niche more efficiently than controls. A myoblast culture approach that reprograms myoblasts to a more primitive state, resulting in improved transplantation outcomes and reinvigorating research into myoblast transplantation as a viable therapeutic approach, is described.
Introduction
Postnatal growth and repair of skeletal muscle depend on muscle precursor cells, termed satellite cells [1, 2]. These normally quiescent cells activate upon injury, re-enter the cell cycle, upregulate the myogenic factor MyoD, differentiate, and fuse to give rise to new myofibers or to repair damaged ones [3]. Satellite cells also have the ability to self-renew to repopulate the satellite cell niche [4]. Pax7 is the canonical marker of satellite cells; however, satellite cells appear to exist as a heterogeneous population expressing different cell surface markers, including the cell adhesion protein M-cadherin; the chemokine receptor CXCR4; and the heparan sulfate proteoglycans, syndecan-3 and -4 [4–8]. A small subpopulation of satellite cells, the satellite side population, are characterized by the expression of ABCG2 and Sca-1 and their ability to exclude Hoechst dye 33342 [9].
Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy, affecting 1 in 3,500 male births, and is caused by spontaneous or inherited mutations in the dystrophin gene [10, 11]. The absence of dystrophin leads to membrane fragility and degeneration of the muscle fiber [12]. To date, no cure for DMD exists, underscoring the need to identify the mechanisms controlling myogenic potential, migration in the host musculature, and repopulation of the satellite cell niche. One promising approach for treatment of DMD is transplantation of satellite cells or muscle progenitor cells expressing dystrophin into damaged muscle.
The use of satellite cell-derived myoblasts as a therapeutic approach for DMD has been limited by failure of the transplanted cells to migrate from the site of injection, low donor cell survival rates, insufficient cell fusion, and poor repopulation of the stem cell niche, necessary for sustained repair and long-term cure [13]. Adding to the challenge of cell-based therapies, large numbers of satellite cells are difficult to isolate from muscle biopsy specimens, necessitating their expansion in culture, which, in turn, reduces their regenerative potential [14]. Thus, to be a viable treatment for DMD, a suitable progenitor cell must be isolated and expanded without loss of stem cell properties, permitting efficient repair, homing to the satellite cell niche, and self-renewal for sustained therapeutic benefit.
The transcription factor CCAAT/enhancer-binding protein β (C/EBPβ), is an important regulator of mesenchymal stem cell fate and is upregulated in muscle wasting, such as sarcopenia [15]. Our work has shown that in healthy muscle, C/EBPβ expression is localized to Pax7+ satellite cells and its expression is downregulated during myogenesis in parallel with Pax7 [16, 17]. Forced expression of C/EBPβ in myoblasts stimulates Pax7 expression, reduces MyoD protein expression, and inhibits myogenesis. As such, through stimulation of Pax7 and inhibition of MyoD, C/EBPβ maintains satellite cells in an undifferentiated state [16].
Phosphodiesterases are a class of enzymes responsible for controlling the cellular concentration of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by cleaving their phosphodiester bond to yield 5′-cyclic nucleotides (5′ AMP or 5′-GMP, respectively) [18, 19]. Inhibitors of phosphodiesterases (PDEi) are a class of drugs widely used for various pharmacological properties, including anti-inflammatory, vasodilator, and antithrombotic functions [20–22]. Preclinical studies have demonstrated that phosphodiesterase 5A (PDE5A) inhibitors (sildenafil and tadalafil), which prevent the breakdown of cGMP, protect skeletal muscle of C57BL/10ScSn-mdx/J mice (mdx mice) from contraction-induced injury [23] and restore blood supply to their muscles after exercise [24]. Currently, PDE5A inhibitors are in clinical trials.
Isobutylmethylxanthine is a methyl-xanthine nonselective PDEi that increases cellular cAMP and cGMP levels. Increased intracellular cAMP levels lead to activation of protein kinase A (PKA) and the phosphorylation of cAMP response element-binding protein (CREB) [25–28]. Phosphorylated CREB stimulates C/EBPβ expression by binding to the cAMP response elements (CRE) in the Cebpb promoter [26]. IBMX has been extensively used to transiently stimulate C/EBPβ expression in preadipocytes in culture to induce adipogenesis [26–29]. In this study, we cultured satellite cell-derived myoblasts in IBMX with the goal of transiently stimulating C/EBPβ expression to stimulate Pax7 expression, reduce MyoD expression, and improve the suitability of these cells before transplantation into dystrophic muscle. We demonstrate that treatment of cultured myoblasts with IBMX for 5 days increases their proliferation in culture and the expression of satellite cell markers associated with efficient engraftment, improves cell survival upon transplantation, and enhances migration of myoblasts in a C/EBPβ-dependent manner. Treatment of myoblasts with low doses of IBMX was sufficient to significantly improve their ability to repair dystrophic muscle and to engraft into the satellite cell niche. IBMX treatment should be considered as a simple yet effective method to reprogram myoblasts to a more stem cell-like state that correlates with better transplantation outcomes for muscle degenerative disorders.
Materials and Methods
Mice and Animal Care
C57BL/6-Tg(human ubiquitin C–green fluorescent protein [GFP])30Scha/J mice (GFP mice) aged 6 weeks, C57BL/6J mice aged 6 weeks, and mdx mice aged 4 weeks were obtained from the Jackson Laboratory. A mouse bearing a C/EBPβ-floxed allele had been created previously [30], and homozygous progeny (C/EBPβfl/fl) were crossed with mice bearing the Pax7-CreERtm allele [31] to generate wild-type (WT; C/EBPβfl/flPax7CreER−/−) and conditional knockout (cKO; C/EBPβ−/−Pax7CreER-/+) animals as previously described [16]. To induce CreERtm activity, WT and cKO animals were subjected to daily intraperitoneal injections of tamoxifen (3 mg/40 g; Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com) for 5 days. All animals were housed in a controlled facility (22°C with 30% relative humidity on a 12-hour light/dark cycle) and provided with food and water ad libitum. Animals were bred and handled as recommended by the guidelines established by the University of Ottawa Animal Care Service and the Canadian Council on Animal Care.
Preparation and Culture of Primary Myoblasts and C2C12 Myoblasts
Satellite cell-derived myoblasts were isolated as described elsewhere [16, 32]. Briefly, hindlimb muscles of adult (aged 6–8 weeks) C57BL/6, GFP, WT, or cKO mice were dissected and digested with collagenase/Dispase (Roche Diagnostics, Risch-Rotkreuz, Switzerland, http://www.roche.com) and the muscle slurry was filtered through a 70-μm cell strainer to remove undigested muscle. Cells were washed with serum-free media and enriched for myoblasts by selective plating. Primary myoblasts were grown on matrigel-coated plates in growth media (Dulbecco’s modified Eagle’s medium [DMEM] containing 20% fetal bovine serum [FBS], 10% horse serum [HS]) supplemented with basic fibroblast growth factor (10 ng/ml) and hepatocyte growth factor (2 ng/ml) (Peprotech, Rocky Hill, NJ, https://www.peprotech.com). For in vitro and transplantation studies, IBMX (Sigma-Aldrich) was used at a concentration of 30 μM or 300 μM and potassium hydroxide (0.35 N) was used as the vehicle control. When required, 10 μM bromodeoxyuridine (BrdU; Sigma-Aldrich) was added 6 hours before fixation. For differentiation, primary myoblasts were induced to differentiate (DM; DMEM containing 2% FBS, 10% HS) in the presence or absence of IBMX for 2 days.
Cell Growth Curve
Myoblasts cultured under growth conditions for up to 5 days in the absence or presence of IBMX were subjected to crystal violet staining (0.03%) at days 0, 1, 3, and 5. The following day, the dye was eluted with 1% SDS and read in a 96-well plate by a SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com) at 570 nm. Cell number was expressed as the optical density at 570 nm.
Scratch Wound Assay
In vitro migration assays were performed by disruption of confluent myoblast monolayers as previously described [33]. IBMX-treated myoblasts were trypsinized and replated at equal densities. The next day, confluent myoblasts were treated with mitomycin C (50 μg/ml; Sigma-Aldrich) for 2 hours before the monolayer was wounded with a sterile pipette tip. Cells were washed twice with phosphate-buffered saline (PBS) and switched to DM in the absence or presence of AMD3100 (10 μM; Sigma-Aldrich). Pictures were taken at 0 hours and 6 hours. The percentage of wound closure was calculated as follows: (width of 0-hour wound gap − width of 6-hour wound gap)/width of 0-hour wound gap × 100%.
Western Analysis
Whole cell extracts were resolved on a 12% SDS-polyacrylamide gel electrophoresis gel and after transfer to polyvinylidene fluoride membrane, probed with specific antibodies: C/EBPβ (C-19; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com/), C/EBPβ (1H7; ThermoFisher Scientific, Waltham, MA, https://www.thermofisher.com), C/EBPβ (E299; Abcam, Cambridge, U.K., http://www.abcam.com/), Pax7-c (Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA, http://dshb.biology.uiowa.edu/), MyoD (5.8A; Santa Cruz Biotechnology), myosin heavy chain (MF-20; DSHB) and cyclophilin B (Abcam). Chemiluminescence was detected with the Luminescent Image Analyzer LAS-4000 (Fujifilm Life Science, Stamford, CT, http://www.fujimed.com).
Real-Time Quantitative Polymerase Chain Reaction
Total RNA was isolated from cultured myoblast using the RNeasy kit (Qiagen, Valencia, CA, https://www.qiagen.com), treated with DNase (Ambion, ThermoFisher Scientific) and reverse transcribed by using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, http://www.bio-rad.com) following manufacturer’s protocols. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed by using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories) on an Mx3005p thermocycler (Stratagene, Agilent Technologies, Santa Clara, http://www.agilent.com). Primer sequences can be found in supplemental online Table 1.
Myoblast Transplantation
Muscle regeneration in mdx mice aged 5 weeks was induced by injecting 30 μl of 10 μM cardiotoxin (CTX; Sigma-Aldrich) into the midbelly of both tibialis anterior (TA) muscles. The next day, 105 vehicle- or IBMX-treated GFP myoblasts were injected into the right and left TA of the CTX-injured TA muscle, respectively. Mice were sacrificed 2 months after transplantation, and the TAs were collected, embedded, in optical cutting temperature compound, flash frozen in isopentane cooled by liquid nitrogen, and sectioned (8 μm thick) for GFP fluorescence and immunohistochemistry. To assess survival of transplanted cells, IBMX-treated WT and cKO myoblasts were stained with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (Sigma-Aldrich) and injected into the TA of CTX-injured mdx mice.
Immunostaining and Antibodies
PFA-fixed myoblasts were permeabilized in PBS containing 0.3% Triton X-100 and 10% goat serum (Cedarlane, Burlington, Ontario, Canada, https://www.cedarlanelabs.com). Cryosections were thawed at room temperature, fixed in 4% PFA, and permeabilized with PBS containing 0.5% Triton X-100 and blocked in PBS containing 0.1% Triton X-100, 5% donkey serum (Cedarlane). For Pax7 staining, sections were processed for antigen retrieval at 95°C for 20 minutes in citrate buffer before permeabilization. Blocking was followed by incubation in primary antibody solution overnight at 4°C. Primary antibodies used in this study were as follows: MF-20 (DSHB), Pax7-c (DSHB), MyoD (C-20; Santa Cruz Biotechnology), GFP (Abcam), BrdU (biotin; Abcam), Ki67 (Abcam), and dystrophin (Abcam). Detection was accomplished with secondary antibodies conjugated to a fluorescent dye (Cy3, Alexa 488, or Alexa Fluor 647; all from Jackson ImmunoResearch, West Grove, PA, https://www.jacksonimmuno.com/). In vivo apoptosis was measured by using the In Situ Cell Death Detection Kit, TMR red (Roche) as per manufacturer’s protocol. The staining was completed by counterstaining the nuclei with 4′,6-diamidino-2-phenylindole (0.5 μg/ml).
Flow Cytometry
Satellite-cell derived myoblasts pretreated with vehicle or 30 μM IBMX were detached with cell dissociation buffer (Invitrogen, ThermoFisher Scientific, Carlsbad, CA). Cells were stained with human M-cadherin (R&D Systems, Minneapolis, MN, https://www.rndsystems.com) on ice for 30 minutes, followed by incubation for 30 minutes with sheep IgG allophycocyanin-conjugated secondary antibody, fluorescein isothiocyanate rat anti-mouse CD184 (CXCR4; BD Biosciences, Franklin Lakes, NJ, https://www.bdbiosciences.com) and PE mouse anti-human CD338 (ABCG2; BD Biosciences). Cells were analyzed in a CyAn ADP analyzer, and data were analyzed using Kaluza flow analysis software (both from Beckman Coulter, Brea, CA, https://www.beckmancoulter.com).
Statistical Analysis
Statistical analysis was performed by using Prism 5.0 software (GraphPad Software, La Jolla, CA, http://www.graphpad.com). A two-tailed or one-tailed Student’s t test was performed for comparing a single experimental condition to the control condition. One-way analysis of variance followed by the Tukey post hoc test was used for comparing three or more experimental variables. All experiments are representative of a minimum of three biological replicates and are presented as the mean ± SEM. In the figures, significance is indicated as n.s., not significant; ∗, p < .05; ∗∗, p < .01; or ∗∗∗, p < .001.
Results
IBMX Upregulates C/EBPβ Expression and Reversibly Inhibits Myogenesis
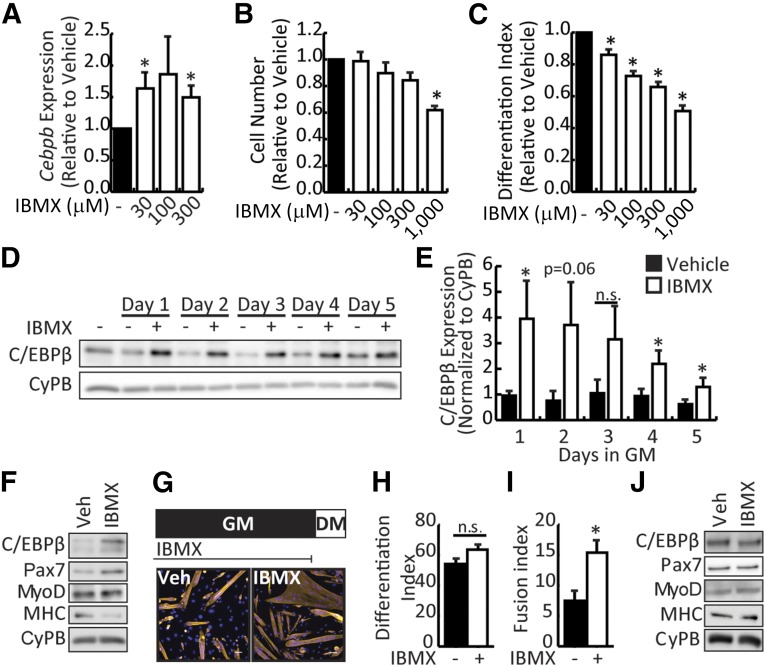
C/EBPβ maintains myoblasts in an undifferentiated state, and thus genetic overexpression of this factor is of no use for cell therapy purposes [16]. Given that C/EBPβ expression can be induced by increased cAMP signaling via CREB [26], the phosphodiesterase inhibitor IBMX was used to induce C/EBPβ expression in primary mouse myoblasts. Primary myoblasts isolated from C57BL/6 mouse hindlimb were treated with increasing doses of IBMX (from 30 to 300 μM) for 24 hours in growth medium. As seen in preadipocyte cultures, treatment of myoblasts with as low as 30 μM IBMX induced Cebpb mRNA expression (Fig. 1A). Low doses of IBMX (30–300 μM) did not affect cell numbers after a 2-day treatment, suggesting that these doses were not toxic to the myoblasts (Fig. 1B). However, 1,000 μM IBMX significantly reduced cell numbers, suggesting toxicity at this dose. Consistent with previous reports, higher Cebpb expression correlated with a decrease in the efficiency of differentiation that was dose dependent, with significant reductions in the differentiation index observed for all doses tested (Fig. 1C).
Figure 1.
IBMX stimulates expression of C/EBPβ in myoblasts. (A): Cebpb expression in primary myoblast cultured in 0, 30, or 300 μM IBMX for 24 hours in GM. (B): Cell numbers after culture of primary myoblasts in IBMX under growth conditions for 2 days. (C): Differentiation index (number of nuclei in MyHC+ cells/total nuclei) of primary myoblasts cultured for 2 days in differentiation medium in the absence or presence of IBMX. (D): Representative Western blot of C/EBPβ expression in primary myoblasts treated with 30 μM IBMX for up to 5 days in GM. CyPB is a loading control. (E): Quantification of C/EBPβ expression normalized to cyclophilin B. (F): Western analysis of myogenic marker expression in primary myoblasts cultured in vehicle or IBMX for 5 days under growth conditions and then induced to DM for 2 days in the continual absence or presence of IBMX. (G): Primary myoblasts were cultured in vehicle or IBMX for 5 days in GM, harvested, replated, and induced to DM in the absence of IBMX (top). Cells were fixed and stained for myosin heavy chain (yellow) and 4′,6-diamidino-2-phenylindole (blue). Representative pictures are shown (bottom). Scale bar = 50 μm. MyHC+ nuclei were counted to assess (H) differentiation index (as in C) and (I) fusion index (number of nuclei in MyHC+ cells/number of myotubes) for cells treated and differentiated as in (G). (J): Western analysis of myogenic marker expression from myoblasts cultured as in (G). All data are presented as mean ± SEM (n = 3; ∗, p < .05). Abbreviations: C/EBPβ, CCAAT/enhancer-binding protein β, CyPB, cyclophilin B; DM, differentiate; GM, growth medium; IBMX, isobutylmethylxanthine; n.s., not significant; Veh, vehicle.
C/EBPβ protein expression was verified after a 30-μM IBMX treatment in primary myoblasts as a time course from 1 to 5 days in growth medium (Fig. 1D, 1E). C/EBPβ expression was stimulated at all time points tested, with higher variability in the response noted after 2 and 3 days of treatment. This finding suggests that longer treatment periods were advantageous to fully stimulate C/EBPβ expression in myoblasts. Indeed, treatment of differentiating myoblasts with IBMX at 30 μM not only inhibited myogenesis but also stimulated expression of Pax7, a C/EBPβ target gene, as well as inhibited myosin heavy chain (MyHC) expression, without affecting MyoD expression (Fig. 1F; supplemental online Fig. 1A). Given that high C/EBPβ levels correlate with reductions in MyoD protein expression [16, 17], the stable MyoD expression in the presence of IBMX was surprising and suggested that IBMX can maintain MyoD levels independent of its effects on C/EBPβ expression. Indeed, treatment with forskolin (an activator of adenylyl cyclase) upregulates MyoD expression in isolated primary myoblasts [34].
To determine whether the effects of IBMX on myogenesis were reversible, primary myoblasts were cultured in growth medium in the presence of 30 μM IBMX for 5 days and replated at equal density for differentiation in the absence of the compound for 2 days. Myoblasts grown in the presence of IBMX showed effective differentiation similar to that of vehicle-treated myoblasts upon withdrawal (Fig. 1G, 1H) but produced fibers that were significantly larger (two-fold) than those in vehicle-treated cultures (Fig. 1G, 1I). After removal of IBMX, protein levels of C/EBPβ and Pax7 returned to levels similar to those in vehicle-treated myoblasts (Fig. 1J; supplemental online Fig. 1B).
IBMX Promotes Expansion of Myoblasts In Vitro
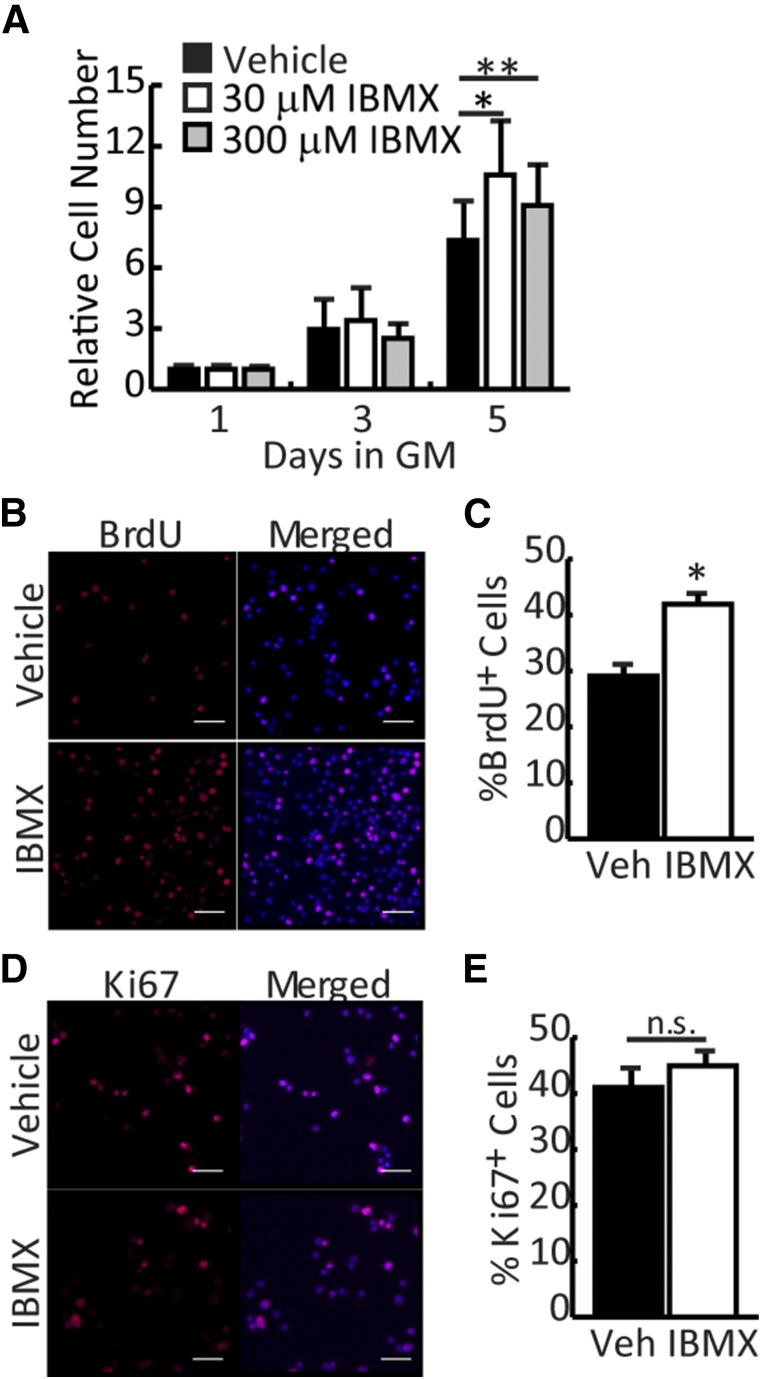
For myoblast transplantation protocols to be successful, isolated myoblasts must be expanded in culture without losing regenerative potential. To determine whether IBMX could support the expansion of freshly isolated myoblasts in culture, cell number was assessed by crystal violet staining at different time points during growth. Although numbers were equivalent between vehicle- and IBMX-treated cells after 3 days in culture, cell number increased significantly for IBMX-treated cultures on day 5 regardless of dose (Fig. 2A). To determine whether the growth kinetics of IBMX-treated cultures were changing, we assessed cell proliferation using BrdU incorporation and Ki67 staining. After a 6-hour pulse, IBMX-treated myoblasts (30 μM) had significantly increased BrdU incorporation (42%) compared with vehicle-treated myoblasts (29%) (Fig. 2B, 2C) indicating more cells in S-phase during the pulse. However, the number of Ki67+ cells did not change significantly with IBMX treatment (Fig. 2D, 2E), suggesting that IBMX promotes expansion of myogenic precursors by reducing the transit time through the cell cycle (BrdU+ cells) without affecting the percentage of cells in the growth fraction (Ki67+ cells).
Figure 2.
IBMX promotes expansion of myoblasts in vitro. (A): Vehicle- and IBMX-treated myoblasts (30 and 300 μM) were stained with crystal violet (absorbance at 570 nm) to assess cell number at days 1, 3, and 5 of treatment. (B): Myoblasts in GM were cultured for 5 days with vehicle or IBMX, after which they were pulsed with BrdU for 6 hours, fixed, and stained for BrdU (red) and and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Representative pictures are shown. Scale bar = 50 μM. (C): BrdU+ cells were counted and represented as the percentage of total nuclei. (D): Myoblasts were treated as in B and were fixed and stained for Ki67 (red) and DAPI (blue). Representative pictures are shown. Scale bar = 50 μm. (E): Ki67+ cells were counted and represented as the percentage of total nuclei. All data are presented as mean ± SEM (n = 4; ∗∗, p < .01; ∗, p < .05). Abbreviations: BrdU, bromodeoxyuridine; GM, growth medium; IBMX, isobutylmethylxanthine; n.s., not significant; Veh, vehicle.
Stimulation of Satellite Cell Marker Expression by IBMX Is C/EBPβ-Dependent
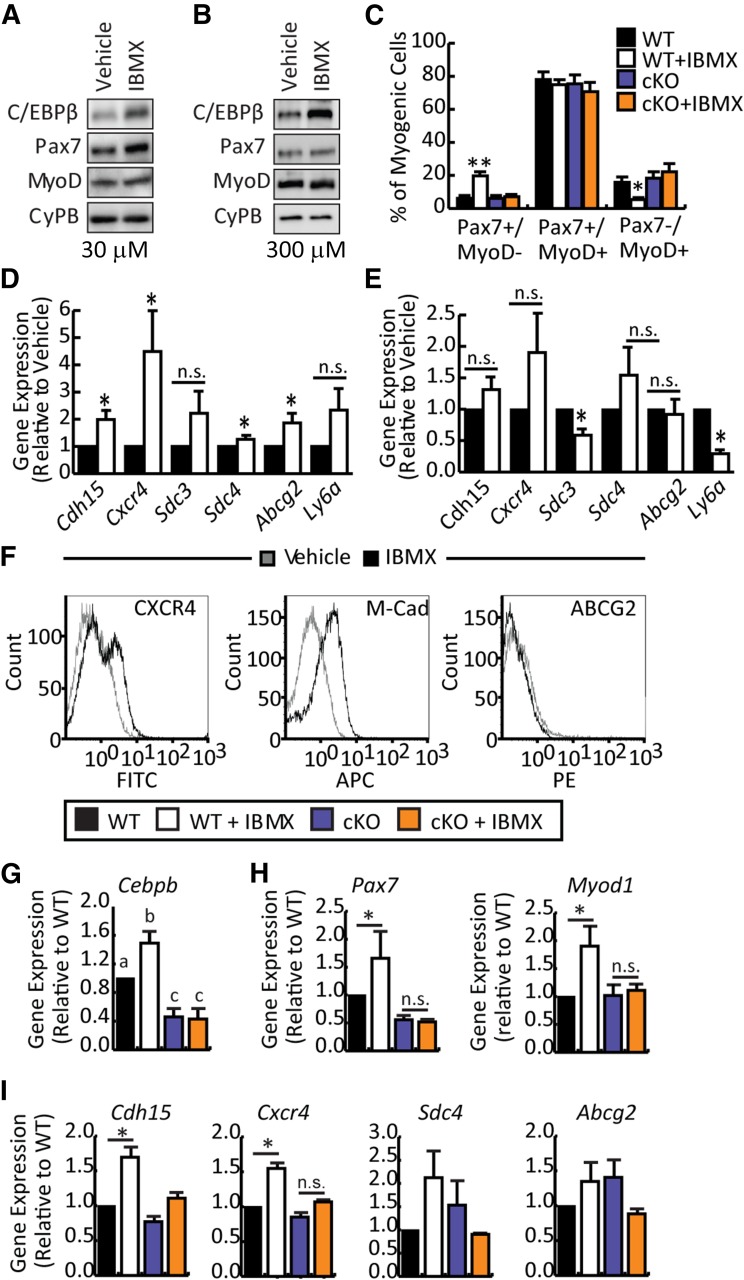
Ectopic expression of C/EBPβ potently inhibits myogenesis and promotes Pax7 expression [16]. To examine the molecular profile of IBMX-treated myoblasts, freshly isolated myoblasts were cultured in vehicle or IBMX for 5 days in growth media at doses of 30 μM and 300 μM (Fig. 3A, 3B). The lower IBMX dose increased Pax7 protein expression by 2.5-fold (Fig. 3A; supplemental online Fig. 2A) whereas 300 μM only minimally increased Pax7 expression by approximately 1.5-fold (Fig. 3B; supplemental online Fig. 2B). MyoD protein levels were unaffected at both doses, consistent with observations in differentiation medium (Fig. 3A, 3B; supplemental online Fig. 2). Given that the MyoD protein levels were unchanged with IBMX treatment, we examined the proportion of cells that were differentiating (Pax7−/MyoD+), proliferating (Pax7+/MyoD+), and self-renewing (Pax7+/MyoD−) in growth medium [35]. In vehicle-treated WT cultures, we noted that most cells coexpressed Pax7 and MyoD (∼70%–78%). Approximately 15% of the culture expressed only MyoD, whereas approximately 6% were positive for only Pax7 expression (Fig. 3C). IBMX treatment significantly increased the proportion of reserve cells (Pax7+/MyoD−) in the culture to approximately 20%, while decreasing the number of differentiating cells (Pax7−/MyoD+) to ∼5%, without affecting the double-positive population size. These results suggest that IBMX can maintain the Pax7+/MyoD− state more efficiently than can standard culture conditions, thereby increasing the population of cells desirable for transplantation.
Figure 3.
IBMX-mediated stimulation of satellite cell marker expression and migration is dependent on C/EBPβ. (A, B): Primary myoblasts were cultured in vehicle or IBMX (30 and 300 μM) for 5 days and analyzed by Western blot for C/EBPβ, Pax7, and MyoD protein expression. Cyclophilin B was a loading control. (C): Percentage of quiescent (Pax7+/MyoD−), proliferating (Pax7+/MyoD+), and differentiating (Pax7−/MyoD+) cells as determined by immunocytochemistry in primary myoblast cultures treated for 5 days in GM with 30 μM IBMX or vehicle. (D): Real-time quantitative polymerase chain reaction of satellite cell markers Cdh15, Cxcr4, Sdc3, Sdc4, Abcg2, and Ly6a in primary myoblasts cultured for 5 days in GM with 30 μM IBMX or vehicle. (E): Satellite cell marker expression in primary myoblasts cultured in GM for 5 days in the absence or presence of 300 μM IBMX. (F): Flow cytometry histograms of CXCR4,M-Cad, and ABCG2 expression on vehicle and IBMX-treated myoblasts. (G): Cebpb expression in myoblasts isolated from C/EBPβ cKO or WT non-Cre-expressing floxed littermates treated with vehicle or 30 μM IBMX for 5 days in GM. (H): Myogenic markers expression (Pax7 and Myod1) in myoblasts cultured as in (G). (I): Satellite cell marker expression (Cdh15, Cxcr4, Sdc4, and Abcg2) in myoblasts cultured as in (G). Data are presented as mean ± SEM (n ≥ 3; ∗∗, p < .01; ∗, p < .05; means with different letters have a p value <.05, and means indicated with the same letter are not statistically significantly different). Abbreviations: APC, allophycocyanin; C/EBPβ, CCAAT/enhancer-binding protein β; cKO, conditional knockout; CyPB, cyclophilin B; FITC, fluorescein isothiocyanate; IBMX, isobutylmethylxanthine; M-Cad, m-cadherin; n.s., not significant; WT, wild-type.
Because Pax7 is a target of C/EBPβ [16], we examined the effect of IBMX on the different states in C/EBPβ-null myoblasts (Fig. 3C), isolated from a cKO (C/EBPβ−/−Pax7CreER+/−), in which C/EBPβ is knocked down in Pax7+ cells following activation of the CreER recombinase with tamoxifen. In vehicle-treated C/EBPβ-null cultures, the number of differentiating (∼18%), proliferating (∼75%), and quiescent (∼6%) cells were similar to those in vehicle-treated wild-type cultures. However, in cells lacking C/EBPβ, IBMX treatment did not increase the Pax7+/MyoD− population, suggesting that the increase in Pax7+ cells by IBMX depends on C/EBPβ expression.
Other than Pax7, expression of additional stem cell markers is also correlated with better transplantation outcomes. After a 5-day treatment with 30 μM IBMX, the expression of satellite cell and side population markers was evaluated. IBMX treatment significantly increased Cdh15 (2-fold), Cxcr4 (4-fold), Sdc4 (1.5-fold), and Abcg2 (2-fold) expression (Fig. 3D). Although Sdc3 and Ly6a expression trended toward an increase in the presence of IBMX, this failed to reach statistical significance (Fig. 3D). A higher dose of IBMX (300 μM), although able to promote C/EBPβ expression, failed to stimulate satellite cell marker expression significantly and negatively affected Sdc3 and Ly6a expression (Fig. 3E). As such, the 30-μM dose was selected for further investigation. To confirm that mRNA expression correlated with increased protein expression of these markers, CXCR4, M-cadherin, and ABCG2 expression was quantified by using flow cytometry (Fig. 3F). Both CXCR4 and M-cadherin expression was increased with IBMX treatment as compared with vehicle-treated control, whereas ABCG2 was not appreciably increased. Taken together, these results suggest that IBMX stimulates the expression of a subset of stem cell markers in a C/EBPβ-dependent fashion and promotes a gene expression profile similar to that of undifferentiated myogenic progenitors.
IBMX treatment is expected to, through an increase in cellular cAMP levels, influence multiple pathways in addition to upregulating the expression of C/EBPβ. To determine whether C/EBPβ is required for the upregulation of satellite cell markers in IBMX-treated myoblasts, primary myoblasts from cKO mice or their WT (C/EBPβfl/flPax7CreER−/−) non-Cre-expressing floxed littermates were treated with IBMX or vehicle in growth medium, and satellite cell marker expression was examined by RT-qPCR. Cebpb excision was approximately 60% in cKO myoblasts and was not further stimulated by IBMX treatment (Fig. 3G). Pax7 stimulation by IBMX was dependent on the expression of C/EBPβ, as Pax7 expression was reduced in cKO myoblasts and was not further upregulated by IBMX in these cells (Fig. 3H), consistent with our previous findings [16]. IBMX also induced Myod1 expression in WT cells but not in C/EBPβ-deficient cells, indicating that C/EBPβ is required for this effect (Fig. 3H). For the stem cell markers, the induction of Cdh15, Cxcr4, and Sdc4 by IBMX was C/EBPβ-dependent because their induction was lost in cKO cells (Fig. 3I). IBMX-mediated stimulation of Abcg2 expression was not dependent on C/EBPβ and was highly variable in this genetic background (Fig. 3I).
IBMX-Treated Myoblasts Repair Dystrophic Muscle and Occupy the Host Satellite Cell Niche With Greater Efficiency
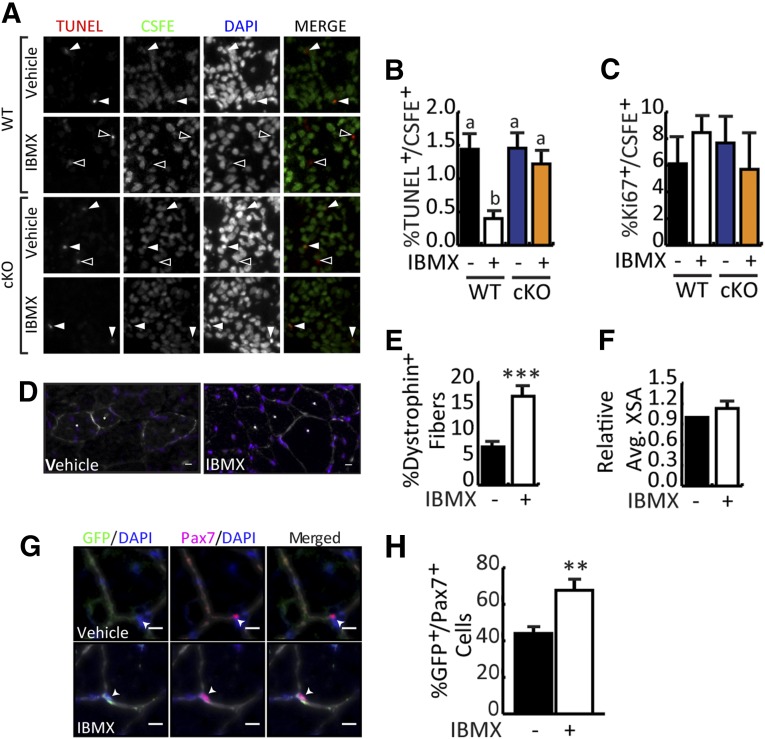
Given the improved molecular profile of IBMX-treated cells, we postulated that IBMX-treated cells would engraft more efficiently into dystrophic muscle, as has been observed for muscle stem cells [9, 36]. Interestingly, C/EBPβ is a potent prosurvival factor that can both directly inhibit caspases through a XEXD box as well as inhibit p53 activity [37, 38], and high Pax7 expression supports myoblast survival [39]. Equal numbers of WT or cKO myoblasts cultured with vehicle or IBMX for 5 days under growth conditions were stained with the vital dye carboxyfluorescein succinimidyl ester (CSFE) before transplantation into CTX-injured TA muscles of mdx mice. Twenty-four hours after grafting, muscle was harvested to quantify donor cell survival and proliferation (Fig. 4A–4C). By using immunohistochemistry, the percentage of terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL)+/CSFE+ cells was scored; despite no measurable difference in apoptosis rates between vehicle-treated WT and cKO donor cells, treatment of WT cells with IBMX significantly reduced the percentage of TUNEL+ cells by 75% as compared with vehicle-treated controls (Fig. 4A, 4B). The protective effect of IBMX was lost, however, when myoblasts lacking C/EBPβ were transplanted, suggesting that the enhanced survival is mediated by C/EBPβ (Fig. 4B). Despite enhanced expansion in culture, culture of isolated myoblasts with IBMX did not improve their proliferation once injected into host muscle (Fig. 4C), nor was proliferation adversely affected by loss of C/EBPβ.
Figure 4.
IBMX-treated myoblasts contribute to repair of dystrophic muscle and occupy the host satellite cell niche. (A): Donor myoblasts isolated from WT and cKO mice were cultured for 5 days in the absence or presence of IBMX. Equal cell numbers were stained with vital dye CSFE and transplanted into cardiotoxin (CTX)-injured tibialis anterior (TA) muscle of C57BL/10ScSn-mdx/J mice (mdx mice). Twenty-four hours after transplantation, TA muscles were collected to assess donor cell apoptosis and proliferation. Representative images of TUNEL- and CSFE-stained muscle after transplantation with vehicle or IBMX-treated myoblasts. Solid arrows indicate TUNEL+/CSFE+ cells. Open arrowheads indicate TUNEL+ cells that are not from the donor. DAPI staining reveals all nuclei. (B): Quantification of the percentage of TUNEL+/CSFE+ cells from (A). (C): Quantification of the percentage of Ki67+/CSFE+ cells of stained muscles as in (A). (D): Myoblasts isolated from GFP mice were cultured in vehicle or IBMX for 5 days in GM. Myoblasts were then harvested and 105 cells were transplanted into CTX-injured TA muscles of mdx mice. Two months after transplantation, TA muscles were harvested for assessment of donor cell engraftment. Dystrophin staining (white) of TA muscle of mdx mice 2 months after transplantation. Representative images are shown. Scale bar = 10 μm. (E): Quantification of dystrophin+ fibers in TA engrafted with vehicle- or IBMX-treated myoblasts. (F): Mean cross-sectional area of dystrophin+ fibers, shown relative to control. (G): Representative images of TA sections stained with GFP (green), Pax7 (pink), laminin (white), and DAPI (blue). Scale bar = 10 μm. (H): Quantification of GFP+/Pax7+ cells after transplantation. All data are presented as mean ± SEM (n = 6; ∗∗, p < .01; ∗∗∗, p < .001; means with different letters have a p value <.05, and means indicated with the same letter are not statistically significantly different). Abbreviations: Avg., average; cKO, conditional knockout; CSFE, carboxyfluorescein succinimidyl ester; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; IBMX, isobutylmethylxanthine; TUNEL, terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling; WT, wild-type.
For long-term study of engraftment, donor cells were isolated from GFP mice, cultured in the absence or presence of IBMX, transplanted into CTX-injured TA muscle of mdx mice, and allowed to engraft for 2 months. The number of dystrophin+ fibers in the entire cross-section of the TA was assessed to determine the myogenic potential of transplanted myoblasts. IBMX-treated myoblasts produced more dystrophin+ (∼17%) fibers compared with vehicle-treated myoblasts (∼7%) (Fig. 4D, 4E) suggesting that IBMX-treated myoblasts are better at repairing dystrophic muscle than are vehicle-treated myoblasts. Despite enhanced fusion of IBMX-treated myoblasts in culture (Fig. 1I), the cross-sectional area of dystrophin+ fibers was not different in the muscle that received IBMX-treated donor cells as compared with vehicle-treated cells (Fig. 4F). Nonetheless, quantification of sublaminar GFP+/Pax7+ cells revealed that IBMX-treated myoblasts made up 68% of the satellite cell population found in the niche, whereas vehicle-treated myoblasts contributed only 43%, indicating that IBMX-treated myoblasts are more efficient at engrafting into the satellite cell niche (Fig. 4G, 4H). Indeed, niche engraftment is necessary for participation of donor cells in multiple rounds of repair, and therefore long-term repair. Taken together, these results indicate that IBMX-treatment of myoblasts during expansion leads to better engraftment and restoration of dystrophin expression to dystrophic muscles.
IBMX Enhances Myoblast Migration Through Induction of CXCR4
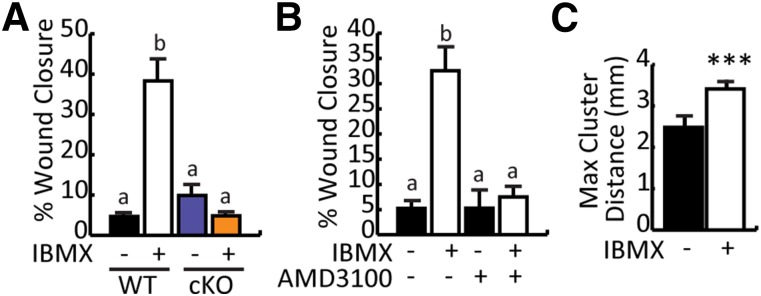
CXCR4 is required for stromal cell-derived factor-1 (SDF-1) induced myoblast migration [40] and improves myogenic progenitor extravasation and engraftment into dystrophic muscle [41]. Because Cxcr4 expression was upregulated by IBMX in a C/EBPβ-dependent manner, we examined whether IBMX improved migration of myoblasts in vitro. Confluent monolayers of vehicle or IBMX-treated myoblasts were scratched and pictures were taken at 0 hours and 6 hours after induction to differentiate in the presence of mitomycin C, to inhibit proliferation, and to determine the extent of wound closure. Myoblasts that were pretreated with IBMX showed increased wound closure (∼35%) compared with vehicle-treated myoblasts (∼5%) (Fig. 5A). In the absence of C/EBPβ (cKO), migration was equivalent to WT controls and was not further stimulated by IBMX (Fig. 5A), suggesting that the improved migration of IBMX-treated cells requires C/EBPβ.
Figure 5.
IBMX-enhanced cellular migration is dependent on CCAAT/enhancer-binding protein β and mediated by CXCR4. (A): Vehicle- and IBMX-treated WT and cKO myoblasts were harvested, replated, treated with mitomycin C, scratched, and switched to differentiate. Pictures were taken at 0 hours and 6 hours. Percentage wound closure was calculated as follows: (width of 0-hour wound gap – width of 6-hour wound gap)/width of 0-hour wound gap × 100%. (B): Scratch wound assay on WT myoblasts precultured in the presence of IBMX or vehicle as in (A) and monitored for wound closure in the presence or absence of AMD3100. (C): Maximum cluster distance of dystrophin+ fibers 2 months after transplantation of IBMX or vehicle-treated myoblasts into cardiotoxin-injured C57BL/10ScSn-mdx/J mice muscle. All data are presented as mean ± SEM (n ≥ 4; ∗∗∗, p < .001; means with different letters are statistically significantly different from one another; and means with the same letter are not statistically significantly different). Abbreviations: cKO, conditional knockout; IBMX, isobutylmethylxanthine; Max, maximum; WT, wild-type.
To determine whether the enhanced migration after treatment with IBMX was mediated through CXCR4, the scratch wound assay was repeated in the presence of AMD3100, a nonpeptide antagonist of CXCR4. In the absence of AMD3100, IBMX treatment stimulated wound closure when compared with vehicle-treated controls; however, this effect was lost in the presence of AMD3100, suggesting that the enhanced migration is mediated by the C/EBPβ-dependent stimulation of CXCR4 expression (Fig. 5B).
Transplantation of dystrophin+ myoblasts can form clusters of dystrophin-expressing fibers in dystrophin-deficient muscle. Thus, to assess cell migration in vivo, we measured the maximum distance (in millimeters) between dystrophin+ clusters in TA muscles of mdx mice transplanted with vehicle or IBMX-treated donor cells 2 months after grafting (Fig. 5C). Muscles that received IBMX-treated cells had a ∼40% increase in the mean cluster distance, indicating that they were better able to migrate away from the site of grafting than vehicle-treated controls.
Discussion
The use of myoblast transplantation for the treatment of muscular dystrophies has been limited by both poor engraftment and lack of significant satellite cell niche repopulation, necessary for long-term regeneration and complete restoration of dystrophin expression. Although primary myoblasts are poorly suited for transplantation, other, more rare populations, such as the satellite side population cells and CSM4B (CD45−Sca-1−Mac-1−CXCR4+β1-integrin+) cells engraft with greater efficiency [9, 36]. However, these populations are difficult to isolate in high numbers and lose their regenerative potential with in vitro expansion, precluding their therapeutic usage.
Our work has identified the bzip transcription factor C/EBPβ as an important negative regulator of myogenesis. Consistent with this, C/EBPβ expression is highest in satellite cells and decreases in concert with Pax7 during early differentiation [16]. Because maintenance of the satellite cell undifferentiated state during myoblast culture is desirable for transplantation, and myoblasts are easy to isolate in high numbers, we used the phosphodiesterase inhibitor IBMX to stimulate the expression of satellite cell markers on myoblasts.
Contrary to what we expected, MyoD protein levels remained unchanged in the presence of the high C/EBPβ found in IBMX-treated cells, an effect that may represent the equilibrium between the negative effects of C/EBPβ on MyoD protein expression and the positive effects of increased cAMP signaling [34]. Despite the unchanged MyoD protein levels across the whole population, IBMX treatment did significantly reduce the Pax7−/MyoD+ population while concomitantly increasing the Pax7+/MyoD− population, confirming that IBMX imposes a restraint on differentiation through both Pax7 and MyoD. These results suggest that the IBMX-mediated increase in MyoD expression is not homogeneous in the population but rather restricted to a subset of the treated population. Further isolation of the MyoD− population could further enhance transplantation outcomes.
Withdrawal from IBMX restored the differentiation of treated cells allowing for efficient contribution to repair. Interestingly, cells that were pretreated with IBMX made myotubes twice as large as vehicle-treated myoblasts. This result correlates with a previous study in which the mouse myogenic cell clone Ric10 was treated with different cAMP stimulating reagents, such as IBMX and forskolin [42]. Increased PKA-cAMP signaling after forskolin treatment increased myotube-myotube fusion, resulting in extra large myotubes, designated “myosheet,” similar to that seen in IBMX-treated myoblasts in our study (Fig. 1G, 1I) [42]. However, IBMX treatment did not produce larger dystrophin+ fibers after transplantation, and, given that high C/EBPβ levels correlate with decreased fusion whereas loss of C/EBPβ promotes hyperfusion in culture [16], it is unlikely that this effect of IBMX is dependent on C/EBPβ.
Clinical trials of myoblast transplantation in patients with Duchenne muscular dystrophy have been disappointing because of the rapid cell death of myoblasts within hours of transplantation [43–45]. Many approaches have been investigated to enhance myoblast survival upon transplantation, including hypoxia preconditioning; heat shock; and coinjection with small molecules (dextran sulfate), biomaterials (fibrin gel), or macrophages [46–50]. Interestingly, C/EBPβ is a potent prosurvival factor [37, 38] and is upregulated in several tumors, demonstrating its role as an important mediator of cell survival during tumorigenesis [30, 38]. Thus, in addition to improving stem cell marker expression in myoblasts, IBMX treatment, through C/EBPβ, appears to also improve transplantation outcomes through increased myoblast survival after grafting. Indeed, IBMX-treated donor cells were less apoptotic than vehicle-treated cells after transplantation into injured muscle, and this effect was dependent on C/EBPβ expression. Recently, Brg1, a component of the Swi/Snf chromatin remodeling complex, was shown to be required for maintaining viability in myoblasts through regulation of Pax7 expression [51]. Pax7 is required for maintenance of the satellite cell pool as deletion of Pax7 triggers cell cycle abnormalities characterized by an extended G2/M phase, and a progressive loss of muscle precursors to cell death [39]. Thus Pax7 regulation by C/EBPβ could provide a mechanism for improved survival in IBMX-treated cells [16]. C/EBPβ is not, however, required for the development of muscle embryonically, or the specification of Pax7+ cells, because both of these are normal in C/EBPβ KO mice, suggesting that C/EBPβ may be a more important regulator of Pax7 expression in the postnatal animal.
IBMX also stimulated CXCR4 mRNA and protein expression in a C/EBPβ-dependent manner. CXCR4 contributes to the migration of cells to dystrophic tissues that express high levels of SDF-1 and is required for myoblast migration [40, 41, 52]. CXCR4 is also a known target of C/EBPβ in breast cancer cells [53]. Consistent with this, IBMX-treated myoblasts had significantly enhanced migration under differentiation conditions that was not seen in C/EBPβ-deficient myoblasts, suggesting that the increased migratory ability induced by IBMX is dependent on increased C/EBPβ expression. Further, inhibition of CXCR4 abolished the effect of IBMX on migration. In vivo, cells treated with IBMX migrated further in the injured host muscle than vehicle-treated cells, suggesting that the enhanced repair and engraftment could be due, at least in part to better movement through the injured muscle tissue. Because CXCR4 expression in muscle progenitor cells increases extravasation into skeletal muscle after intravenous and intra-arterial transplantation in mdx5cv mice, IBMX treatment of myoblasts may allow for improved intra-arterial delivery of graft cells [41].
Conclusion
In this study, we demonstrate that treatment with IBMX is an efficient method of expanding muscle progenitor cells, while preserving regenerative potential, before transplantation. The regulation of C/EBPβ expression by IBMX leads to the upregulation of several satellite cell markers and improves cell migration and cell survival, resulting in better engraftment into dystrophic muscle. In addition to identifying C/EBPβ-dependent effects on myoblasts following IBMX treatment, it is clear that treatment with IBMX has several beneficial effects on myoblasts favoring better transplantation outcomes that are independent of C/EBPβ expression and should be further investigated. Moreover, the pharmacological reprogramming of myoblasts with IBMX reverts them to a more primitive state, but not into a cell population that has been thoroughly defined in vivo. This hybrid profile merits further analysis to better understand the molecular mechanisms that preserve the undifferentiated state of muscle stem cells and their self-renewal properties. Together, the molecular profile of myoblasts treated with IBMX improved their engraftment into injured muscle and resulted in their persistence in the satellite cell niche, suggesting that long-term sustained repair is possible with this approach. Our research demonstrates that phosphodiesterase inhibitor treatment improves myoblast culture conditions in such a way as to reinvigorate myoblast transplantation as a viable therapeutic approach to halt muscle wasting in DMD.
Supplementary Material
Acknowledgments
This work was supported by grants from the Muscular Dystrophy Association to N.W.-B.; N.L.-T. is supported by an Ontario Graduate Scholarship. The Pax7-CreER mouse was kindly provided by Dr. Charles Keller at the Oregon Health & Science University (Portland, OR), and the Cebpbfl/fl mouse was a kind gift from Dr. Esta Sterneck at the Center for Cancer Research at the National Institutes of Health. We thank François Marchildon, Dr. Lynn Megeney, Dr. Catherine Tsilfidis, and Dr. Lisheng Wang for their input.
Author Contributions
N.L.-T.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.F.: collection and assembly of data, final approval of manuscript; N.W.-B.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCullagh KJ, Perlingeiro RCR. Coaxing stem cells for skeletal muscle repair. Adv Drug Deliv Rev. 2015;84:198–207. doi: 10.1016/j.addr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motohashi N, Asakura A. Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Biol. 2014;2:1–21. doi: 10.3389/fcell.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seale P, Ishibashi J, Scimè A, et al. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2:E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernig A, Bone M, Irintchev A, et al. M-cadherin is a reliable marker of quiescent satellite cells in mouse skeletal muscle. Basic Appl Myol. 2004;14:161–168. [Google Scholar]
- 7.Cornelison DD, Filla MS, Stanley HM, et al. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak MZ, Majka M, Kucia M, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka KK, Hall JK, Troy AA, et al. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray PN, Belfall B, Duff C, et al. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985;318:672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- 11.Rahimov F, Kunkel LM. The cell biology of disease: Cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol. 2013;201:499–510. doi: 10.1083/jcb.201212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allikian MJ, McNally EM. Processing and assembly of the dystrophin glycoprotein complex. Traffic. 2007;8:177–183. doi: 10.1111/j.1600-0854.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 13.Skuk D, Roy B, Goulet M, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther. 2004;9:475–482. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Montarras D, Morgan J, Collins C et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005;309:2064–2067. [DOI] [PubMed]
- 15.Giresi PG, Stevenson EJ, Theilhaber J, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21:253–263. doi: 10.1152/physiolgenomics.00249.2004. [DOI] [PubMed] [Google Scholar]
- 16.Marchildon F, Lala N, Li G, et al. CCAAT/enhancer binding protein beta is expressed in satellite cells and controls myogenesis. Stem Cells. 2012;30:2619–2630. doi: 10.1002/stem.1248. [DOI] [PubMed] [Google Scholar]
- 17.Fu D, Lala-Tabbert N, Lee H, et al. Mdm2 promotes myogenesis through the ubiquitination and degradation of CCAAT/enhancer-binding protein β. J Biol Chem. 2015;290:10200–10207. doi: 10.1074/jbc.M115.638577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azevedo MF, Faucz FR, Bimpaki E, et al. Clinical and molecular genetics of the phosphodiesterases (PDEs) Endocr Rev. 2014;35:195–233. doi: 10.1210/er.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurice DH, Ke H, Ahmad F, et al. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov. 2014;13:290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahimi R, Ghiasi S, Azimi H, et al. A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine. 2010;49:123–129. doi: 10.1016/j.cyto.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Nieder C, Zimmermann FB, Adam M, et al. The role of pentoxifylline as a modifier of radiation therapy. Cancer Treat Rev. 2005;31:448–455. doi: 10.1016/j.ctrv.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Rendell M, Bamisedun O. Skin blood flow and current perception in pentoxifylline-treated diabetic neuropathy. Angiology. 1992;43:843–851. doi: 10.1177/000331979204301007. [DOI] [PubMed] [Google Scholar]
- 23.Asai A, Sahani N, Kaneki M, et al. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goethe R, Basler T, Phi-van L. Regulation of C/EBPbeta mRNA expression and C/EBPbeta promoter activity by protein kinases A and C in a myelomonocytic cell line (HD11) Inflamm Res. 2007;56:274–281. doi: 10.1007/s00011-007-6170-y. [DOI] [PubMed] [Google Scholar]
- 26.Niehof M, Manns MP, Trautwein C. CREB controls LAP/C/EBP beta transcription. Mol Cell Biol. 1997;17:3600–3613. doi: 10.1128/mcb.17.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J-W, Klemm DJ, Vinson C, et al. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J Biol Chem. 2004;279:4471–4478. doi: 10.1074/jbc.M311327200. [DOI] [PubMed] [Google Scholar]
- 28.Lechner S, Mitterberger MC, Mattesich M, et al. Role of C/EBPβ-LAP and C/EBPβ-LIP in early adipogenic differentiation of human white adipose-derived progenitors and at later stages in immature adipocytes. Differentiation. 2013;85:20–31. doi: 10.1016/j.diff.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 30.Sterneck E, Zhu S, Ramirez A, et al. Conditional ablation of C/EBP beta demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene. 2006;25:1272–1276. doi: 10.1038/sj.onc.1209144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishijo K, Hosoyama T, Bjornson CRR, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Megeney LA, Kablar B, Garrett K, et al. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- 33.Goetsch KP, Niesler CU. Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal Biochem. 2011;411:158–160. doi: 10.1016/j.ab.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Tabebordbar M, Iovino S, et al. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell. 2013;155:909–921. doi: 10.1016/j.cell.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zammit PS, Golding JP, Nagata Y, et al. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerletti M, Jurga S, Witczak CA, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buck M, Poli V, Hunter T, et al. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–816. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 38.Yoon K, Zhu S, Ewing SJ, et al. Decreased survival of C/EBP beta-deficient keratinocytes is due to aberrant regulation of p53 levels and function. Oncogene. 2007;26:360–367. doi: 10.1038/sj.onc.1209797. [DOI] [PubMed] [Google Scholar]
- 39.Relaix F, Montarras D, Zaffran S, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brzoska E, Kowalewska M, Markowska-Zagrajek A, et al. Sdf-1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol Cell. 2012;104:722–737. doi: 10.1111/boc.201200022. [DOI] [PubMed] [Google Scholar]
- 41.Perez AL, Bachrach E, Illigens BMW, et al. CXCR4 enhances engraftment of muscle progenitor cells. Muscle Nerve. 2009;40:562–572. doi: 10.1002/mus.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukai A, Hashimoto N. Localized cyclic AMP-dependent protein kinase activity is required for myogenic cell fusion. Exp Cell Res. 2008;314:387–397. doi: 10.1016/j.yexcr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Briggs D, Morgan JE. Recent progress in satellite cell/myoblast engraftment -- relevance for therapy. FEBS J. 2013;280:4281–4293. doi: 10.1111/febs.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Y, Maley M, Beilharz M, et al. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Huard J, Acsadi G, Jani A, et al. Gene transfer into skeletal muscles by isogenic myoblasts. Hum Gene Ther. 1994;5:949–958. doi: 10.1089/hum.1994.5.8-949. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Wen Y, Bi P, et al. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012;139:2857–2865. doi: 10.1242/dev.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, Smolenski RT, Jayakumar J, et al. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102(suppl 3):III216–III221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 48.Laumonier T, Pradier A, Hoffmeyer P, et al. Low molecular weight dextran sulfate binds to human myoblasts and improves their survival after transplantation in mice. Cell Transplant. 2013;22:1213–1226. doi: 10.3727/096368912X657224. [DOI] [PubMed] [Google Scholar]
- 49.Gerard C, Forest MA, Beauregard G, et al. Fibrin gel improves the survival of transplanted myoblasts. Cell Transplant. 2012;21:127–137. doi: 10.3727/096368911X576018. [DOI] [PubMed] [Google Scholar]
- 50.Lesault P-F, Theret M, Magnan M, et al. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS One. 2012;7:e46698. doi: 10.1371/journal.pone.0046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padilla-Benavides T, Nasipak BT, Imbalzano AN. Brg1 controls the expression of Pax7 to promote viability and proliferation of mouse primary myoblasts. J Cell Physiol. 2015;230:2990–2997. doi: 10.1002/jcp.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pescatori M, Broccolini A, Minetti C, et al. Gene expression profiling in the early phases of DMD: A constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007;21:1210–1226. doi: 10.1096/fj.06-7285com. [DOI] [PubMed] [Google Scholar]
- 53.Park B-H, Kook S, Lee S, et al. An isoform of C/EBPβ, LIP, regulates expression of the chemokine receptor CXCR4 and modulates breast cancer cell migration. J Biol Chem. 2013;288:28656–28667. doi: 10.1074/jbc.M113.509505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.