Endothelial progenitor cells (EPCs) are currently being studied as candidate cell sources for revascularization strategies. Widespread clinical acceptance of EPCs for clinical therapies remains hampered by several challenges. The challenges and issues surrounding the use of EPCs and the current strategies being developed to improve the harvest efficiency and functionality of EPCs for application in regenerative medicine are discussed.
Keywords: Endothelial cell, Clinical translation, Hematopoietic cells, Hematopoietic progenitors, Tissue-specific stem cells, Tissue regeneration, Stem/progenitor cell
Abstract
Endothelial progenitor cells (EPCs) are currently being studied as candidate cell sources for revascularization strategies. Significant advances have been made in understanding the biology of EPCs, and preclinical studies have demonstrated the vasculogenic, angiogenic, and beneficial paracrine effects of transplanted EPCs in the treatment of ischemic diseases. Despite these promising results, widespread clinical acceptance of EPCs for clinical therapies remains hampered by several challenges. The present study provides a concise summary of the different EPC populations being studied for ischemic therapies and their known roles in the healing of ischemic tissues. The challenges and issues surrounding the use of EPCs and the current strategies being developed to improve the harvest efficiency and functionality of EPCs for application in regenerative medicine are discussed.
Significance
Endothelial progenitor cells (EPCs) have immense clinical value for cardiovascular therapies. The present study provides a concise description of the EPC subpopulations being evaluated for clinical applications. The current major lines of investigation involving preclinical and clinical evaluations of EPCs are discussed, and significant gaps limiting the translation of EPCs are highlighted. The present report could be useful for clinicians and clinical researchers with interests in ischemic therapy and for basic scientists working in the related fields of tissue engineering and regenerative medicine.
Introduction
The term “endothelial progenitor cells” (EPCs) might be fundamentally used to refer to populations of cells that are capable of differentiation into mature endothelial cells (ECs), with purported physiological roles in angiogenesis (the sprouting of new blood vessels from existing ones) and vasculogenesis (de novo formation of vascular networks) [1]. These features make EPC populations valuable cellular candidates or therapeutic targets in regenerative medicine, with several strategies being developed to use them, including direct cellular transplantation and tissue engineering approaches. Efforts to translate these efforts to the clinic have, however, been hampered by several issues, including controversies over the identity and functions of EPCs, the limited numbers of EPCs, and their clinical potency. In the present report, we begin with a description of EPC populations, leading to an overview of clinical strategies that have been developed to use EPCs in regenerative medicine. The factors limiting the use of EPCs and the current research themes to resolve these issues are also discussed.
Identification and Characterization of EPC Populations
The discovery of endothelial progenitor cells has been credited to Asahara et al. for identifying a hematopoietic population in adult peripheral blood capable of eliciting postnatal vasculogenesis [2]. Subsequent studies suggested that EPC numbers could be used in clinics as a biomarker of cardiovascular disease [3], an important line of investigation that continues today [4]. In the context of regenerative medicine, it is the capacity for vascular regeneration and the potential for ischemic therapy for which EPCs are most valued. However, significant controversies exist over the identity and roles of EPCs in vascular repair. Thus, a brief discourse on the major EPC populations reported in published studies is necessary to facilitate further discussion. During the past two decades, the term “EPC” has been used to describe a burgeoning range of cell types defined by their isolation and culture methods, as well as the ontological sources, ranging from fetal trophoblastic tissue to adult bone marrow. A detailed discussion of the myriad EPCs used in studies is beyond the scope of the present report, and the reader is directed to excellent articles on this topic [4, 5]. We briefly describe two major categories: hematopoietic EPCs and nonhematopoietic EPCs, which differ largely in their ontological origins and isolation methods. It is important to note, however, that it would not be possible to delineate a “superior” cell source for vascular regenerative therapies. Rather, the differences in the isolation and identification of these populations [6] and the potential different contributions to neovasculogenesis [7] should be recognized.
Hematopoietic EPCs
Asahara et al. postulated that EPCs could be isolated from a hematopoietic source and demonstrated that CD34+ cells from peripheral blood can contribute to neovascularization and ischemic rescue after injection into an animal model of peripheral limb ischemia [2]. Similarly, CD133, another hematopoietic stem cell marker, can be targeted to derive less mature progenitor populations [8]. Cell sorting on CD34 and/or CD133 thus emerged as a strategy to derive populations enriched in circulating EPCs (cEPCs), and methods to characterize and derive endothelial cells from such populations have been extensively described [9, 10]. Numerous clinical trials have since been conducted to study the use of cEPC-enriched populations for the treatment of ischemic conditions, including acute myocardial infarction and critical limb ischemia [11]. However, questions remain regarding the precise definition of a bona fide cEPC. Initial studies suggested EPCs exhibited a CD34+/CD133+/VEGFR2+ phenotype [12], a view supported by clinical observations of correlations between this phenotype and cardiovascular conditions [13]. This remains the most commonly recognized profile for cEPCs, despite other studies suggesting the use of other markers, including CD45, CD105, CD106, CD117, CD144, acetylated low-density lipoprotein uptake, and aldehyde dehydrogenase activity [5]. It was thus striking when clonal cultures of CD34+/CD133+/VEGFR2+ cells were found to only be capable of differentiating into hematopoietic, and not endothelial, lineages, leading to suggestions that these cells were nonangioblastic hematopoietic progenitors, which support angiogenesis through paracrine effects [14]. In contrast, the nonhematopoietic CD34+/CD45− fraction was found in the same study to generate adherent endothelial cells, which were capable of forming networked, vessel-like structures when cultured on Matrigel (BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com), indicative of the presence of endothelial lineages in this population. Significant debate on the cEPC theory ensued, with proponents [15] arguing against the method used and the interpretation of results by Case et al. [14]. This has been, in large part, resolved by the development of highly defined assays to induce colony formation from cEPCs, with clonogenic assays performed to demonstrate the ability of CD133+ cells to differentiate into both hematopoietic and endothelial lineages [9, 16]. Interestingly, it has been observed that CD34− cells are capable of augmenting in vitro vascular network formation and vascularization events in vivo [17], providing some basis for the argument that CD133+/CD34+ cells are bona fide EPCs but require the presence of auxiliary cells in the CD34− fraction to potentiate vasculogenesis.
In parallel with these efforts, EPCs were also observed to share many common characteristics with monocytic cells [18]. These cells were conventionally selected for their ability to adhere to tissue culture surfaces, leading to the term “early EPCs” (eEPCs). The attached cells demonstrate the ability to uptake lectin and acetylated low-density lipoproteins and express monocytic surface markers, including CD14 [19]. eEPCs have been suggested to derive from monocytes distinct from the CD34− cEPCs [20]. The exact lineage of these cells has been confounded by contaminant monocytes possibly imparting monocyte-like characteristics to the actual EPCs [21], or the EPCs acquiring endothelial-like characteristics secondary to culture in vascular endothelial growth factor (VEGF)-rich conditions [20]. Regardless of lineage, eEPCs play primarily supportive roles in angiogenesis vascular repair without differentiating themselves into functional endothelial cells [22]. Angiogenic factors secreted by eEPCs include CXCL12, CXCL1, and VEGF, with migration inhibitory factor, a potent cytokine known to induce endothelial and smooth muscle differentiation, the most prominent in the early and late stages of the ischemic event [23]. This has led to calls for a change in nomenclature to circulating angiogenic cells to better reflect their major capacity to induce angiogenesis and vascular sprouting, rather than in the direct formation of nascent blood vessels.
Nonhematopoietic EPCs
In contrast to the hematopoietic EPC, EPCs have been demonstrated to derive from nonhematopoietic tissue, presumably from vessel walls [24, 25]. Termed “endothelial colony forming cells” (ECFCs) or “outgrowth endothelial cells” (OECs/EOCs) for their ability to form colonies of endothelial outgrowths under permissive conditions, ECFCs are most commonly isolated by plating blood-derived mononuclear cells on collagen-coated substrates in endothelial-supportive media [26]. Endothelial outgrowths can be observed to emerge following extended culture, and these cells are capable of rapid amplification, stably generating endothelial progeny with potent vasculogenic properties [27]. It is of interest that the cells derived from such long-term cultures more readily generate mature endothelial progeny in vitro and have also been observed to physically contribute to vasculogenesis [28]. In contrast, it has generally been recognized that hematopoietic EPCs, and eEPCs, in particular, potentiate angiogenesis through the secretion of cytokines [18, 29].
Thus, isolated ECFCs actually represent a heterogeneous mix of progenitors and terminally differentiated endothelial cells with varying proliferative potential, and the lack of surface markers to definitively isolate vasculogenic progenitor populations have contributed to the lack of enthusiasm for translating the use of these cells to the clinic. Proposed profiles for the identification of ECFC-initiating cells include CD146+/CD45−/CD133−, which would be in line with the hypothesis that these EPCs originate from vessel walls rather than the bone marrow [30]. More recently, a CD45−/CD34+/CD31low profile was used to prospectively isolate cells from term placental tissues, which generated pure endothelial populations in culture [25]. Selection on such stringent profiles, however, has been known to yield extremely low yields and, thus, be nonviable for therapeutic use [4].
Clinical Application of EPCs in Regenerative Medicine
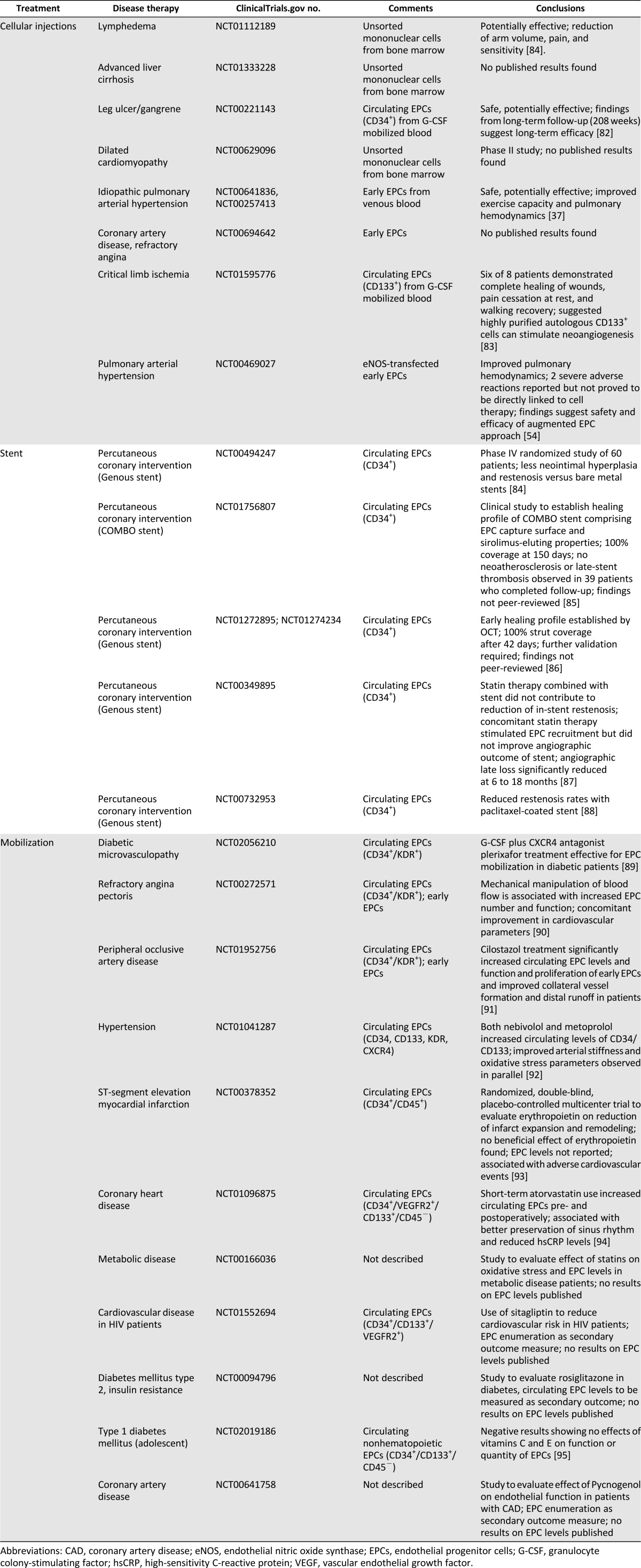
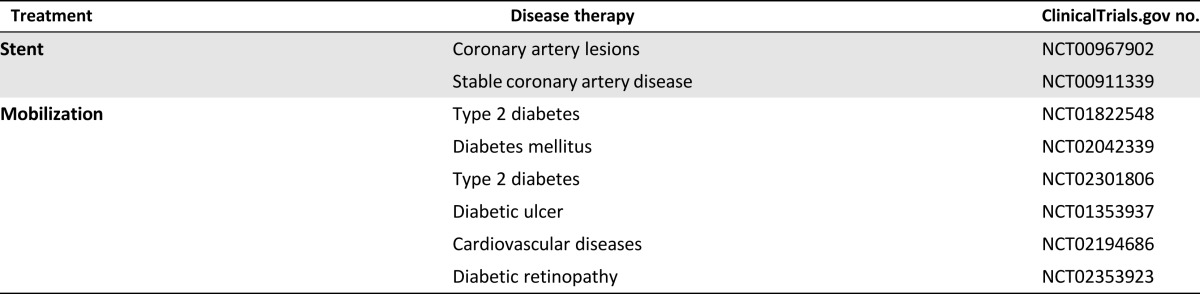
In spite of the ongoing controversy over EPC identity, the clinical potential of EPCs in vascular regenerative applications cannot be overlooked [5, 6], with currently more than 150 interventional studies registered at ClinicalTrials.gov. The disease conditions being investigated include ischemic diseases, such as myocardial infarction and peripheral vascular disease (Tables 1, 2). From the completed and ongoing trials, three major applications targeting EPCs can be identified: (a) cellular injections for ischemic conditions; (b) EPC-capture stents; and (c) EPC mobilization therapies.
Table 1.
Completed interventional trials involving endothelial progenitor cells listed on ClinicalTrials.gov
Table 2.
Ongoing interventional trials involving endothelial progenitor cells listed on ClinicalTrials.gov
Cellular Injections
EPCs as a candidate cell source for therapy offer many attractive characteristics, including (a) ready accessibility from peripheral blood; (b) potent angiogenic and vasculogenic effects; and (c) the stability of the lineage and a reduced risk of tumorigenicity. These features led to many studies on their possible utility for therapeutic neovascularization, for which hematopoietic EPCs have been largely favored in such applications because of their ease of harvest, with minimal manipulations and culture periods [31].
In a murine model of peripheral limb ischemia induced by femoral artery ligation, cEPC injections were shown to significantly improve tissue perfusion and were associated with increased limb salvage rates (58.8% in the cEPC group vs. 7.1% in the control group) [32]. Data from that and other similarly themed studies led to significant optimism for the use of cEPCs for the treatment of ischemic conditions and the initiation of phase I/II clinical trials involving cEPC injections into ischemic myocardia [33]. The results from the primary endpoints suggested the safety of cellular injections, which was borne out by further evidence from randomized, placebo-controlled trials [34, 35]. The administration of unfractionated bone marrow, however, was unable to rescue ischemia in critical limb ischemia studies, suggesting the EPC fraction is responsible for the therapeutic effects [36]. Aside from cEPCs, eEPCs have also been evaluated in the clinic. In a randomized, controlled study of idiopathic pulmonary arterial hypertension, intravenous infusion of autologous eEPCs resulted in improved pulmonary hemodynamics, without severe adverse effects [37]. Taken together, these results provide cautious optimism for EPCs as a cellular candidate for regenerative therapies, and more data from ongoing trials will be useful in establishing the safety profile of EPC therapy. However, questions remain regarding the best route of administration for safety and efficacy. In treatment of peripheral arterial disease (PAD), a meta-analysis conducted of 108 studies involving cellular therapies for the treatment of PAD suggested intramuscular and intra-arterial injections were equally well-tolerated, with the former presenting improved clinical outcomes [39]. Thus, although intra-arterial delivery provides the advantage of improved distribution, in particular, to “occult” and inaccessible sites, the inefficiency of homing has curtailed such approaches, and direct injections into the injured tissue remain preferred [11]. In their study, Franz et al. demonstrated the safety and efficacy of a “dual-administration” approach, in which intramuscular cell injections were supplemented with intra-arterial cellular injections to improve distribution to the distal vasculature [39]. Other approaches include strategies to improve stem cell homing through gene therapy or local injections of homing factors (reviewed by Herrmann et al. [40]).
The safety and efficacy notwithstanding, a major limitation on the feasibility of the approach lies in the insufficiency of the cellular numbers for therapy. Extrapolating from animal studies, an estimated 12 liters of blood would be required to generate sufficient EPCs for the effective treatment of ischemia in an average adult patient [11]. This inadequacy is exacerbated by the compromised EPC quantity and quality in patients with cardiovascular and metabolic disorders [11]. In their clinical study, Losordo et al. addressed this issue by supplementing patients with cytokines to mobilize EPCs from the bone marrow into circulation before harvesting the CD34+ progenitors. However, this protocol might be associated with mobilizing committed hematopoietic precursors and not EPCs per se [15]. Additionally, some concern exists over cardiac enzyme elevations arising from the cell mobilization regimen [34].
Recent research efforts have thus turned to cell isolation and expansion methods. Wadajkar et al. described the use of growth factor-loaded, antibody-conjugated magnetic microparticles for the one-step capture and in situ culture of EPCs on microparticles that can be scaled up with bioreactor cultures [41]. Such platforms, with minimized manipulations, facilitate upscaling and the ease of transition into the clinic. Additionally, the immunoselection method can be applied to other sources of EPCs; white adipose tissue, for example, has been shown to be an accessible source of EPCs [42]. Alternatively, EPCs can be retrieved from cryogenically preserved cord blood for autologous use [43]. Fetal tissues have demonstrated significant advantages over their adult counterparts, including faster proliferation rates and expansion capacities [26]. Aside from cord blood, other perinatal tissue, such as the placenta, can be exploited as a source of primitive EPCs. Postulating a perivascular niche for EPCs, Patel et al. performed cell selection on a CD34+/CD45−/CD31low profile [25]. Placental tissue is highly vascularized and angiogenically dynamic and a single term-placenta was shown to yield 27 times as much ECFCs as a single unit of cord blood. Culture expansion protocols have been developed to expand harvested populations after isolation. These have included extended culture in defined cytokine-rich environments, which were shown to induce up to 1,468-fold cEPC expansion [16, 44]. The results from these and other similarly themed studies suggest that expanded cells retain their potency in the rescue of murine hind limb ischemia [9, 32]. Thus, protocols to effectively derive and expand ECFCs under xeno-free conditions have also been developed, which might provide a cost-effective method to prepare ECFCs for clinical applications [45]. Excessive expansion, however, has been associated with replicative senescence and impaired capacities of ECFCs for vascular repair [46]. Also, in light of the lack of adequate markers, the potency of the injected cells remains impossible to predict. This uncertainty has been compounded by the potentially impaired functionality of EPCs in diseased patients [47]. EPC function, for example, is known to be compromised by impaired glucose metabolism at multiple stages [48].
Aside from the quantity, the enhancement of efficacy and bioactivity presents another possibility to improve EPC therapies. Strategies for ex vivo priming include the use of stromal-derived factor-1 to elicit surface expression of integrin α-4 and α-M, as well as matrix metalloproteinase-2 secretion, leading to improved homing to ischemic sites [49]. More recently, Bouchentouf et al. described the addition of cytokines to suspension blood bags, which served to prime the mononuclear cells toward an angiogenic phenotype [50]. When these primed cells were injected into murine models of myocardial infarction, cardiac function was improved and angiogenesis enhanced, suggesting the efficacy of this approach. The study, however, did not detail the fate of the cells after injection, and the main mechanism for repair might not have been revascularization but other paracrine effects. Additionally, the blood was obtained from healthy volunteers, and it remains unclear whether EPC-compromised patients would respond similarly. Other possible strategies to improve EPC functionality include augmentation of angiogenic genes, such as ACE2 [51] and IL10 [52]. EPCs modified with VEGF, for example, were shown to restore erectile function in diabetic rats after intrapenile injection. Another related application is the use of EPCs as a delivery vehicle for ex vivo gene transfer applications. With their ability to incorporate into host vasculature, and the resultant constant proximity to circulatory blood, genetically engineered EPCs could be used to deliver therapeutic factors directly into the circulation [53]. Additionally, the therapeutic genes can be placed under the control of inducible promoters, such that the factors can be released on demand. In the recently concluded pulmonary hypertension and angiogenic cell therapy (PHACeT) trial, endothelial nitric oxide synthase (eNOS)-transfected eEPCs were systemically administered to patients with pulmonary arterial hypertension (PAH) [54]. EPC injections have previously been shown to stimulate endothelial repair and ameliorate PAH conditions [55], and in the PHACeT trial, the use of eNOS-augmented eEPCs was expected to have increased vasodilatory and vasoregenerative effects. Modest improvements in quality of life measures were observed in patients following treatment, although these could not be sustained, and the group was unable to ascertain the safety or efficacy of this approach. Although severe adverse reactions were observed in 2 of 7 patients (one death and one case of sepsis), causal links to the therapy were deemed unlikely, and the hemodynamic parameters throughout the cell administration and follow-up periods suggested the feasibility and safety of gene-augmented EPC injection therapies. In the context of cellular injections, it is perhaps interesting to note that EPC injections might have applications beyond revascularization. As a case in point, there is currently an increasing trend to study the use of EPCs for orthopedic applications [42]. Peripheral CD34+ cells have been shown to home to fracture sites in a rat model of nonunion fracture [56]. On engraftment, these cells induced significant revascularization and contributed significantly to osteogenic repair of the fracture. These observations likely resulted in part from the osteopotentiating effects of endothelial cells, which secrete bone-inductive factors such as bone morphogenetic protein and transforming growth factor-β1 [57]. Ongoing research being undertaken by our group and others include optimizing of in vitro culture conditions to improve the vasculogenic and osteogenic properties of EPCs [58]. Aside from bone, such mutually synergistic effects are being studied for the engineering of a wide variety of tissues [59].
Capture Stents
Another major research topic centered on EPCs in regenerative medicine revolves around the use of “capture” stents for cardiovascular applications, which sequester EPCs from the circulation to promote endothelialization of the denuded luminal surface. Capture is effected by immobilized antibodies on the stent surface, typically against CD34. The regenerated endothelia are then suggested to reduce risk of restenosis and stent thrombosis and to eliminate the need for prolonged anticoagulative regimens, problems that continue to plague existing stent designs [60]. Additionally, antibody conjugation has been shown to passivate the surface, reducing platelet adhesion and the coagulative effects to improve hemocompatibility measures [61].
Randomized clinical trials conducted on the EPC capture stents have shown them to be safe, and postmarketing surveillance has yielded no evidence to suggest increased risks of adverse cardiac events from use. Compared with drug-eluting stents (DESs), they have been associated with higher in-stent late loss and target vessel failure but a reduced incidence of late-stent thrombosis [62]. The results from the endothelial progenitor cells capture stent in the treatment of acute ST-segment elevation myocardial infarction trial does suggest an increased risk of stent thrombosis [62], although this finding is currently under dispute. More recently, OrbusNeich has produced the “COMBO,” a new-generation EPC capture stent, which elutes sirolimus for 180 days, thus combining the efficacy of DESs with the longer term improved safety profile of “bioengineered stents” [63]. A prospective, multicenter, randomized clinical trial is underway to compare the COMBO stent against current DESs (Clinicaltrials.gov identifier, NCT00967902).
Tied to the controversy on EPC identity, questions remain regarding the choice of CD34 as an appropriate capture target. To meet the need for rapid endothelialization, it has been argued that late EPCs or even circulating ECs should be specifically targeted instead, and surfaces coated with antibodies against CD309 [64] or CD144 [65] have been associated with improved endothelialization outcomes. Extending this theme further, Chen et al. modified the capture surface further to facilitate transfection of the captured cells [66]. In their study, they demonstrated the local transfection of captured CD133-expressing cells with small interfering RNA against adenosine kinase. This resulted in upregulation of adenosine and, hence, improved EPC functionality. Thus, a clearer definition of the surface antigens for capture and elucidation of major signaling networks in the differentiation and functionality of EPCs might provide for more rational designs of EPC capture and postcapture modifications. In the context of the present report, the discussion on EPC capture surfaces for in vivo endothelialization can be further extended to vascular tissue engineering [67]. Regeneration of the luminal surface remains a critical issue in vascular tissue engineering, with most efforts centered on “preseeding” the luminal surfaces with endothelial cells before implantation [68]; such in vitro endothelialization methods are, however, labor- and cost-intensive and, thus, impractical. Much research activity is now centered on adopting, developing, and improving EPC capture technologies for vascular grafts and has been discussed in detail in a recent review [69].
Mobilization Treatments
Exogenous mobilization of circulating EPCs was first proposed using cytokine therapy as a means to mirror endogenous mobilization by ischemic tissue [70]. The elevated EPC numbers in circulation were then thought to increased EPC homing to, and augmentation of, neovascularization in ischemic sites. In contrast to cellular injections, this process is more readily translatable, because the need for external manipulations is eliminated, and the drugs used in the process typically have well-established safety protocols. Additionally, it is particularly useful for the treatment of systemic conditions or conditions involving inaccessible tissue sites. For example, EPC mobilization is being studied for use in treating deep vein thrombosis, in which EPCs are thought to home to thrombotic sites, resolve clots, form new vasculature, and exert protective effects in the prevention of clot recurrence [71]. Similar to the above-mentioned studies on cellular infusions for orthopedic applications, mobilization of EPCs has also been shown to have beneficial effects for fracture healing [72]. Common mobilizing agents include those used for hematopoietic stem cell manipulation in oncology, including chemokines, growth factors, and cytokines [73]. These typically operate on the basis that EPCs reside in the hematopoietic fraction and that hematopoietic mobilization would, in turn, release EPCs into circulation. For example, granulocyte colony-stimulating factor (G-CSF) is currently used clinically to stimulate bone marrow production of granulocytes for the treatment of neutropenia. In vivo, G-CSF elicits release of matrix metalloproteinases and other enzymes from neutrophils, resulting in modification of the hematopoietic niche and subsequent release of hematopoietic precursors. This process has been shown to increase circulating CD34+ cell numbers and has been associated with increased arteriogenesis in patients with coronary artery disease [74]. The identity of these CD34+ cells and their roles in the remodeling process, however, remain unclear. In their meta-analysis, Fadini et al. suggested G-CSF monotherapy might have limited effects in patients with peripheral arterial disease and would thus fail to improve similar endpoints against cellular injection therapies [38]. Compared with drugs that target the hematopoietic fraction, vasomodulatory drugs have been explored in more targeted efforts. Statins, for example, are commonly prescribed to reduce the risk of cardiovascular events, and atorvastatin has recently been found to elevate CD34+/CD133+/KDR+ levels in patients with heart failure [75]. The extent of EPC-mediated vascular repair via statin activation remains unclear, however, although in vitro studies have indicated increased viability and delayed senescence of EPCs with atorvastatin supplementation [76]. Other agents being studied to increase circulating EPC numbers include nonpharmacological interventions, such as physical activity [77] and diet [78]. In all such applications, however, it should be noted that the precise role of EPCs in the resolution of disease and/or achieving clinical outcomes is difficult to delineate, because they are typically measured as a biomarker and evaluated in correlational studies. Thus, no clinical data exist to conclusively demonstrate mobilization of EPCs as the primary mechanism of cure. Another limitation of the approach is the nonspecificity of the treatment, in that mobilized EPCs might not traffic adequately to the disease site. Additionally, patients with chronic vascular diseases are unlikely to benefit from such therapies owing to underlying EPC dysfunction and senescence. As previously alluded to, glucose metabolism influences EPC function on multiple levels, including homing and differentiation into functional vessels. Thus, EPC mobilization therapies for diabetic patients might be well-served by concurrent or pretreatment with antidiabetic agents [79] and homing factors [80].
Conclusion
Endothelial progenitor cells are important therapeutic targets in the field of regenerative medicine, with potential utility, not just in cardiology and cardiovascular therapies, but also in other tissue engineering applications. Significant gaps lie in our understanding of EPC biology, however, and continued research is required to understand the identity and roles of EPCs in health and disease. These efforts will provide valuable data to guide our efforts toward the rational design and engineering of cellular therapeutic agents.
Acknowledgments
J.K.Y.C. received salary support from the Ministry of Health’s National Medical Research Council (Grant NMRC/CSA/043/2012), Singapore. M.S.K.C. received funding support from the National Medical Research Council Cooperative Basic Research Grant (Grant BNIG12nov009) and the Ministry of Education Academic Research Fund (Grant RGT09/13).
Author Contributions
M.S.K.C., W.K.N., and J.K.Y.C.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Sukmawati D, Tanaka R. Introduction to next generation of endothelial progenitor cell therapy: A promise in vascular medicine. Am J Transl Res. 2015;7:411–421. [PMC free article] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 4.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol. 2014;229:10–16. doi: 10.1002/jcp.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoder MC. Endothelial progenitor cell: A blood cell by many other names may serve similar functions. J Mol Med (Berl) 2013;91:285–295. doi: 10.1007/s00109-013-1002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni D, Bassetti B, Gambini E, et al. The CD133+ cell as advanced medicinal product for myocardial and limb ischemia. Stem Cells Dev. 2014;23:2403–2421. doi: 10.1089/scd.2014.0111. [DOI] [PubMed] [Google Scholar]
- 9.Masuda H, Alev C, Akimaru H, et al. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res. 2011;109:20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 10.Ahrens I, Domeij H, Topcic D, et al. Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS One. 2011;6:e23210. doi: 10.1371/journal.pone.0023210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 12.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 13.Boilson BA, Kiernan TJ, Harbuzariu A, et al. Circulating CD34+ cell subsets in patients with coronary endothelial dysfunction. Nat Clin Pract Cardiovasc Med. 2008;5:489–496. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Avogaro A, Agostini C. Critical assessment of putative endothelial progenitor phenotypes. Exp Hematol. 2007;35:1479–1481. doi: 10.1016/j.exphem.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Masuda H, Iwasaki H, Kawamoto A, et al. Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Cells Translational Medicine. 2012;1:160–171. doi: 10.5966/sctm.2011-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Lee SH, Yoo SY, et al. CD34 hybrid cells promote endothelial colony-forming cell bioactivity and therapeutic potential for ischemic diseases. Arterioscler Thromb Vasc Biol. 2013;33:1622–1634. doi: 10.1161/ATVBAHA.112.301052. [DOI] [PubMed] [Google Scholar]
- 18.Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 19.Urbich C, Heeschen C, Aicher A, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 20.Medina RJ, O’Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prokopi M, Pula G, Mayr U, et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 22.Krenning G, van der Strate BW, Schipper M, et al. CD34+ cells augment endothelial cell differentiation of CD14+ endothelial progenitor cells in vitro. J Cell Mol Med. 2009;13(8B):2521–2533. doi: 10.1111/j.1582-4934.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanzler I, Tuchscheerer N, Steffens G, et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res Cardiol. 2013;108:310. doi: 10.1007/s00395-012-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram DA, Mead LE, Moore DB, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 25.Patel J, Seppanen E, Chong MSK, et al. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Translational Medicine. 2013;2:839–847. doi: 10.5966/sctm.2013-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 27.Melero-Martin JM, Khan ZA, Picard A, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 28.Yoon C-H, Hur J, Park K-W, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 29.Heil M, Ziegelhoeffer T, Mees B, et al. A different outlook on the role of bone marrow stem cells in vascular growth: Bone marrow delivers software not hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 30.Mund JA, Estes ML, Yoder MC, et al. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1045–1053. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye L, Poh K-K. Enhancing endothelial progenitor cell for clinical use. World J Stem Cells. 2015;7:894–898. doi: 10.4252/wjsc.v7.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 34.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Cui J, Peng W, et al. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology. 2010;117:140–147. doi: 10.1159/000320217. [DOI] [PubMed] [Google Scholar]
- 36.Teraa M, Sprengers RW, Schutgens RE, et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: The randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015;131:851–860. doi: 10.1161/CIRCULATIONAHA.114.012913. [DOI] [PubMed] [Google Scholar]
- 37.Wang X-X, Zhang F-R, Shang Y-P, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: A pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Franz RW, Shah KJ, Pin RH, et al. Autologous bone marrow mononuclear cell implantation therapy is an effective limb salvage strategy for patients with severe peripheral arterial disease. J Vasc Surg. 2015;62:673–680. doi: 10.1016/j.jvs.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann M, Verrier S, Alini M. Strategies to stimulate mobilization and homing of endogenous stem and progenitor cells for bone tissue repair. Front Bioeng Biotechnol. 2015;3:79. doi: 10.3389/fbioe.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadajkar AS, Santimano S, Tang L, et al. Magnetic-based multi-layer microparticles for endothelial progenitor cell isolation, enrichment, and detachment. Biomaterials. 2014;35:654–663. doi: 10.1016/j.biomaterials.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin RZ, Moreno-Luna R, Muñoz-Hernandez R, et al. Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis. 2013;16:735–744. doi: 10.1007/s10456-013-9350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin R-Z, Dreyzin A, Aamodt K, et al. Functional endothelial progenitor cells from cryopreserved umbilical cord blood. Cell Transplant. 2011;20:515–522. doi: 10.3727/096368910X532729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O E, Lee BH, Ahn HY, et al. Efficient nonadhesive ex vivo expansion of early endothelial progenitor cells derived from CD34+ human cord blood fraction for effective therapeutic vascularization. FASEB J. 2011;25:159–169. doi: 10.1096/fj.10-162040. [DOI] [PubMed] [Google Scholar]
- 45.Reinisch A, Hofmann NA, Obenauf AC, et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina RJ, O’Neill CL, O’Doherty TM, et al. Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function. Stem Cells. 2013;31:1657–1668. doi: 10.1002/stem.1414. [DOI] [PubMed] [Google Scholar]
- 47.Kuliszewski MA, Ward MR, Kowalewski JW, et al. A direct comparison of endothelial progenitor cell dysfunction in rat metabolic syndrome and diabetes. Atherosclerosis. 2013;226:58–66. doi: 10.1016/j.atherosclerosis.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 48.Yiu KH, Tse HF. Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function. Arterioscler Thromb Vasc Biol. 2014;34:1136–1143. doi: 10.1161/ATVBAHA.114.302192. [DOI] [PubMed] [Google Scholar]
- 49.Zemani F, Silvestre JS, Fauvel-Lafeve F, et al. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 50.Bouchentouf M, Forner K, Cuerquis J, et al. A novel and simplified method of culture of human blood-derived early endothelial progenitor cells for the treatment of ischemic vascular disease. Cell Transplant. 2011;20:1431–1443. doi: 10.3727/096368910X557164. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Xiao X, Chen S, et al. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61:681–689. doi: 10.1161/HYPERTENSIONAHA.111.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Chen Q, Zhang Z, et al. Interleukin-10 overexpression improves the function of endothelial progenitor cells stimulated with TNF-α through the activation of the STAT3 signaling pathway. Int J Mol Med. 2015;35:471–477. doi: 10.3892/ijmm.2014.2034. [DOI] [PubMed] [Google Scholar]
- 53.Lin RZ, Dreyzin A, Aamodt K, et al. Induction of erythropoiesis using human vascular networks genetically engineered for controlled erythropoietin release. Blood. 2011;118:5420–5428. doi: 10.1182/blood-2011-08-372946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Granton J, Langleben D, Kutryk MB, et al. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: The PHACeT trial. Circ Res. 2015;117:645–654. doi: 10.1161/CIRCRESAHA.114.305951. [DOI] [PubMed] [Google Scholar]
- 55.Yang JX, Pan YY, Zhao YY, et al. Endothelial progenitor cell-based therapy for pulmonary arterial hypertension. Cell Transplant. 2013;22:1325–1336. doi: 10.3727/096368912X659899. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto T, Kawamoto A, Kuroda R, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Teoh S-H, Chong MSK, et al. Vasculogenic and osteogenesis-enhancing potential of human umbilical cord blood endothelial colony-forming cells. Stem Cells. 2012;30:1911–1924. doi: 10.1002/stem.1164. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Teoh SH, Chong MS, et al. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng Part A. 2013;19:893–904. doi: 10.1089/ten.TEA.2012.0187. [DOI] [PubMed] [Google Scholar]
- 59.Critser PJ, Voytik-Harbin SL, Yoder MC. Isolating and defining cells to engineer human blood vessels. Cell Prolif. 2011;44(suppl 1):15–21. doi: 10.1111/j.1365-2184.2010.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sethi R, Lee CH. Endothelial progenitor cell capture stent: Safety and effectiveness. J Interv Cardiol. 2012;25:493–500. doi: 10.1111/j.1540-8183.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 61.Chong MS, Teoh SH, Teo EY, et al. Beyond cell capture: Antibody conjugation improves hemocompatibility for vascular tissue engineering applications. Tissue Eng Part A. 2010;16:2485–2495. doi: 10.1089/ten.TEA.2009.0680. [DOI] [PubMed] [Google Scholar]
- 62.Beijk MAM, Klomp M, van Geloven N, et al. Two-year follow-up of the Genous™ endothelial progenitor cell capturing stent versus the Taxus Liberté stent in patients with de novo coronary artery lesions with a high-risk of restenosis: A randomized, single-center, pilot study. Catheter Cardiovasc Interv. 2011;78:189–195. doi: 10.1002/ccd.23143. [DOI] [PubMed] [Google Scholar]
- 63.Granada JF, Inami S, Aboodi MS, et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv. 2010;3:257–266. doi: 10.1161/CIRCINTERVENTIONS.109.919936. [DOI] [PubMed] [Google Scholar]
- 64.Markway BD, McCarty OJ, Marzec UM, et al. Capture of flowing endothelial cells using surface-immobilized anti-kinase insert domain receptor antibody. Tissue Eng Part C Methods. 2008;14:97–105. doi: 10.1089/ten.tec.2007.0300. [DOI] [PubMed] [Google Scholar]
- 65.Lee JM, Choe W, Kim BK, et al. Comparison of endothelialization and neointimal formation with stents coated with antibodies against CD34 and vascular endothelial-cadherin. Biomaterials. 2012;33:8917–8927. doi: 10.1016/j.biomaterials.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 66.Chen W, Zeng W, Sun J, et al. Construction of an aptamer-SiRNA chimera-modified tissue-engineered blood vessel for cell-type-specific capture and delivery. ACS Nano. 2015;9:6069–6076. doi: 10.1021/acsnano.5b01203. [DOI] [PubMed] [Google Scholar]
- 67.Chong MS, Chan J, Choolani M, et al. Development of cell-selective films for layered co-culturing of vascular progenitor cells. Biomaterials. 2009;30:2241–2251. doi: 10.1016/j.biomaterials.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 68.Glynn JJ, Hinds MT. Endothelial outgrowth cells: Function and performance in vascular grafts. Tissue Eng Part B Rev. 2014;20:294–303. doi: 10.1089/ten.teb.2013.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goh ET, Wong E, Farhatnia Y, et al. Accelerating in situ endothelialisation of cardiovascular bypass grafts. Int J Mol Sci. 2015;16:597–627. doi: 10.3390/ijms16010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 71.Li WD, Li XQ. Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.07.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Lee DY, Cho TJ, Kim JA, et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008;42:932–941. doi: 10.1016/j.bone.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: Mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009;68:484–492. doi: 10.1111/j.1365-2125.2009.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier P, Gloekler S, Oezdemir B, et al. G-CSF induced arteriogenesis in humans: Molecular insights into a randomized controlled trial. Curr Vasc Pharmacol. 2013;11:38–46. [PubMed] [Google Scholar]
- 75.Oikonomou E, Siasos G, Zaromitidou M, et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis. 2015;238:159–164. doi: 10.1016/j.atherosclerosis.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 76.António N, Soares A, Fernandes R, et al. Endothelial progenitor cells in diabetic patients with myocardial infarction—Can statins improve their function? Eur J Pharmacol. 2014;741:25–36. doi: 10.1016/j.ejphar.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 77.De Biase C, De Rosa R, Luciano R, et al. Effects of physical activity on endothelial progenitor cells (EPCs) Front Physiol. 2013;4:414. doi: 10.3389/fphys.2013.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang XB, Huang J, Zou JG, et al. Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol. 2007;34:1109–1115. doi: 10.1111/j.1440-1681.2007.04667.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang CH, Ting MK, Verma S, et al. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. Am Heart J. 2006;152:1051.e1–1051.e8. doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 80.Yu JX, Huang XF, Lv WM, et al. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg. 2009;50:608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 81.Maldonado GE, Pérez CA, Covarrubias EE, et al. Autologous stem cells for the treatment of post-mastectomy lymphedema: A pilot study. Cytotherapy. 2011;13:1249–1255. doi: 10.3109/14653249.2011.594791. [DOI] [PubMed] [Google Scholar]
- 82.Kinoshita M, Fujita Y, Katayama M, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012;224:440–445. doi: 10.1016/j.atherosclerosis.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 83.Arici V, Perotti C, Fabrizio C, et al. Autologous immuno magnetically selected CD133+ stem cells in the treatment of no-option critical limb ischemia: Clinical and contrast enhanced ultrasound assessed results in eight patients. J Transl Med. 2015;13:342. doi: 10.1186/s12967-015-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wojakowski W, Pyrlik A, Król M, et al. Circulating endothelial progenitor cells are inversely correlated with in-stent restenosis in patients with non-ST-segment elevation acute coronary syndromes treated with EPC-capture stents (JACK-EPC trial) Minerva Cardioangiol. 2013;61:301–311. [PubMed] [Google Scholar]
- 85.Lee SWL, Lam SCC, Chan KKW, et al. TCT-67 the first establishment of early healing profile, 9-month neointimal growth, and 24 months outcomes of the dual therapy endothelial progenitor cell capturing sirolimus-eluting stent as assessed by longitudinal sequential optical coherence tomography: The EGO-COMBO study. J Am Coll Cardiol. 2013;62:B21–B21. [Google Scholar]
- 86.Lee SW, Lam S, Chan K, et al. Early healing of EPC capturing GENOUS stent by OCT: The EGO-Genous study interim. J Am Coll Cardiol. 2012;59:E388–E388. [Google Scholar]
- 87.den Dekker WK, Houtgraaf JH, Onuma Y, et al. Final results of the HEALING IIB trial to evaluate a bio-engineered CD34 antibody coated stent (Genous™Stent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis. 2011;219:245–252. doi: 10.1016/j.atherosclerosis.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 88.Wöhrle J, Birkemeyer R, Markovic S, et al. Prospective randomised trial evaluating a paclitaxel-coated balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease. Heart. 2011;97:1338–1342. doi: 10.1136/hrt.2011.226563. [DOI] [PubMed] [Google Scholar]
- 89.Fadini GP, Fiala M, Cappellari R, et al. Diabetes limits stem cell mobilization following G-CSF but not plerixafor. Diabetes. 2015;64:2969–2977. doi: 10.2337/db15-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barsheshet A, Hod H, Shechter M, et al. The effects of external counter pulsation therapy on circulating endothelial progenitor cells in patients with angina pectoris. Cardiology. 2008;110:160–166. doi: 10.1159/000111925. [DOI] [PubMed] [Google Scholar]
- 91.Chao T-H, Tseng S-Y, Chen IC, et al. Cilostazol enhances mobilization and proliferation of endothelial progenitor cells and collateral formation by modifying vasculo-angiogenic biomarkers in peripheral arterial disease. Int J Cardiol. 2014;172:e371–e374. doi: 10.1016/j.ijcard.2013.12.295. [DOI] [PubMed] [Google Scholar]
- 92.Hayek SS, Poole JC, Neuman R, et al. Differential effects of nebivolol and metoprolol on arterial stiffness, circulating progenitor cells, and oxidative stress. J Am Soc Hypertens. 2015;9:206–213. doi: 10.1016/j.jash.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 93.Najjar SS, Rao SV, Melloni C, et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: A randomized controlled trial. JAMA. 2011;305:1863–1872. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baran Ç, Durdu S, Dalva K, et al. Effects of preoperative short term use of atorvastatin on endothelial progenitor cells after coronary surgery: A randomized, controlled trial. Stem Cell Rev. 2012;8:963–971. doi: 10.1007/s12015-011-9321-z. [DOI] [PubMed] [Google Scholar]
- 95.Cazeau RM, Huang H, Bauer JA et al. Effect of vitamins C and E on endothelial function in type 1 diabetes mellitus. J Diabetes Res 2016;3271293. [DOI] [PMC free article] [PubMed]