This article reports a novel differentiation method using a small-molecule WNT inhibitor, WNT-C59 (C59), to efficiently induce human anterior cortex. C59 efficiently induced CTIP2+/COUP-TF1− cells, which are characteristic of the cells found in the anterior cortex. These results raise the possibility of C59 contributing to cell replacement therapy for motor neuron diseases or insults.
Keywords: Neural differentiation, Wnt signaling, Cortical motor neuron, Cell transplantation, Pluripotent stem cells
Abstract
The recapitulation of human neural development in a controlled, defined manner from pluripotent stem cells (PSCs) has considerable potential for studies of human neural development, circuit formation and function, and the construction of in vitro models of neurological diseases. The inhibition of Wnt signaling, often by the recombinant protein DKK1, is important for the induction of cortical neurons. Here, we report a novel differentiation method using a small-molecule WNT inhibitor, WNT-C59 (C59), to efficiently induce human anterior cortex. We compared two types of small molecules, C59 and XAV939 (XAV), as substitutes for DKK1 to induce cortical neurons from PSCs in serum-free embryoid body-like aggregate culture. DKK1 and XAV inhibited only the canonical pathway of Wnt signaling, whereas C59 inhibited both the canonical and noncanonical pathways. C59 efficiently induced CTIP2+/COUP-TF1− cells, which are characteristic of the cells found in the anterior cortex. In addition, when grafted into the cortex of adult mice, the C59-induced cells showed abundant axonal fiber extension toward the spinal cord. These results raise the possibility of C59 contributing to cell replacement therapy for motor neuron diseases or insults.
Significance
For a cell therapy against damaged corticospinal tract caused by neurodegenerative diseases or insults, cortical motor neurons are needed. Currently, their induction from pluripotent stem cells is considered very promising; however, an efficient protocol to induce motor neurons is not available. For efficient induction of anterior cortex, where motor neurons are located, various WNT inhibitors were investigated. It was found that one of them could induce anterior cortical cells efficiently. In addition, when grafted into the cortex of adult mice, the induced cells showed more abundant axonal fiber extension toward spinal cord. These results raise the possibility that this inhibitor contributes to a cell-replacement therapy for motor neuron diseases or insults.
Introduction
Movement depends on signaling pathways originating from the motor cortex. The mammalian motor cortex has a six-layered structure. Layer V includes cortical motor neurons (CMNs) that have a pyramidal shape and extend axons to innervate motor neurons in the spinal cord [1]. The degeneration of CMNs causes motor neuron diseases including amyotrophic lateral sclerosis (ALS) and hereditary spastic paraplegia [2]. A loss or deterioration of motor functions can also occur upon stroke or brain injury that causes the death of CMNs.
Cell replacement therapy is gaining considerable attention to treat motor dysfunctions. Fetal frontal cortex cells transplanted into the frontal region of neonatal [3] or adult [4] mice were found to extend axons to the spinal cord. These findings suggest that the transplantation of fetal frontal cortex, which includes CMN progenitor cells, is able to reconstitute the corticospinal pathway. However, human fetal brains are not a feasible source of donor cells. Alternative donor cells include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). These PSCs can be unlimitedly expanded and give rise to a wide variety of differentiated cells, making them a promising cell source for treating intractable neurological disorders, including Parkinson’s disease [5–7] and ALS [8]. In fact, recent studies have shown that mouse [9] or human [10] ESC-derived neurons extend long axons from the motor cortex to the pyramidal tract in neonate mice. However, the efficiency to induce CMNs from ESCs or iPSCs is very low.
In order to induce cortical neurons from mouse or human ESCs/iPSCs, serum-free embryoid body-like aggregates (SFEB) [11–13] or monolayer [9, 10, 14–17] protocols are used. CMNs are located in the frontal/motor area of the anterior cortex, and the inhibition of Wnt signaling is important to induce this area. WNTs contribute to the proper patterning of neural structures along the anterior-posterior axis [18]. In these methods, a recombinant protein, DKK1, is used as the WNT inhibitor. However, DKK1 is produced in animal cells and Escherichia coli, raising the possibility of infection or immune rejection due to cross-species contamination when the induced cells are transplanted to humans. Therefore, an alternative WNT inhibitor to induce CMNs is required for clinical application.
In the present study, we investigated the effects of two small compounds as substitute WNT inhibitors in an induction protocol for cortical neurons from human iPSCs: WNT-C59 (C59) and XAV939 (XAV) (supplemental online Fig. 1). C59 inhibits the activity of porcupine (PORCN), which is a membrane-bound O-acyltransferase required for palmitoylation, secretion, and the biological activity of WNTs. The inhibition of PORCN by C59 prevents both canonical and noncanonical Wnt-mediated signaling [19]. XAV inhibits tankyrase 1 and 2, thus stabilizing axin and stimulating β-catenin degradation [20], whereas DKK1 inhibits WNT binding to its receptor, LRP5/6 [21]. In other words, XAV and DKK1 inhibit only canonical Wnt signaling. We induced cortical neurons from human iPSCs in the presence of DKK1, XAV, or C59 and found that C59 induced frontal cortical neurons expressing a marker for layer V (CTIP2+/COUP-TF1−) most efficiently. This result suggests that C59 can be used to induce CMNs or their progenitors from human iPSCs.
Materials and Methods
Maintenance and Differentiation Culture of Human ESCs/iPSCs
Human iPSCs (836B1) were maintained on SNL feeder cells inactivated by mitomycin C (Wako Pure Chemical Industries, Ltd., Osaka, Japan, http://www.wako-chem.co.jp) in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 medium (F12) (Wako) supplemented with 20% (vol/vol) Knockout Serum Replacement (KSR; Invitrogen, Tokyo, Japan, http://www.thermofisher.com), 0.1 mM nonessential amino acids (NEAA; Invitrogen), 2 mM l-glutamine (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 0.1 mM 2-mercaptoethanol (Wako), and 5 ng/ml basic fibroblast growth factor (bFGF; Invitrogen). For the induction of cortical neurons using a serum-free floating culture of embryoid body-like aggregates with quick reaggregation (SFEBq), feeder cells were removed with CTK (0.25% [wt/vol] trypsin [Invitrogen] and 0.1% [wt/vol] collagenase IV [Gibco, Tokyo, Japan, http://www.thermofisher.com] in PBS [Wako] containing 20% KSR [Invitrogen] and 1 mM CaCl2 [Wako]), and PSCs were dissociated into single cells by using Accumax (Innovative Cell Technologies, San Diego, CA, http://www.accutase.com). The cells were counted, and 9,000 cells per well were aggregated in a low cell binding, Lipidure coated 96-well plate (NOF Corp., Tokyo, Japan, https://www.nof.co.jp). The differentiation medium contained DMEM/F12 supplemented with 15% (vol/vol) KSR, 0.1 mM NEAA, 2 mM l-glutamine (Invitrogen), and 0.1 mM 2-mercaptoethanol (Wako). From day 0 to 3, 50 µM Y-27632 (Wako) was added to the differentiation medium to increase cell viability. From day 0 to day 6, 10 ng/ml bFGF (Invitrogen) was added to the medium to increase cell viability and the induction of ectodermal fate [22]. To induce forebrain fate, the medium was supplemented with WNT inhibitor (500 ng/ml human recombinant DKK1 [R&D Systems Inc., Minneapolis, MN, https://www.rndsystems.com], 0.5 µM XAV939 [Stemgent, Lexington, MA, https://www.stemgent.com], or 10 nM WNT-C59 [Cellagen Technology, San Diego, CA, http://www.cellagentech.com]) along with 10 µM SB431542 (TGF-β/activin/nodal inhibitor; Sigma-Aldrich) and 0.1 µM LDN193189 (BMP inhibitor; Stemgent). On day 18, cell aggregates were transferred to a 24-well culture plate coated with 50 µg/ml poly-l-ornithine (Sigma-Aldrich), 5 µg/ml laminin (BD Biosciences, Heidelberg, Germany, http://www.bdbiosciences.com), and 5 µg/ml fibronectin (Sigma-Aldrich) in Neurobasal Medium (Invitrogen) supplemented with 2 mM l-glutamine (Invitrogen), 2% (vol/vol) B27 (without vitamin A; Invitrogen), and 10 units/ml penicillin and streptomycin (Invitrogen). Medium was changed every 4 or 5 days.
Transplantation
Animals were cared for and handled according to the Guidelines for Animal Experiments of Kyoto University and the Guide for the Care and Use of Laboratory Animals of the Institute for Laboratory Animal Research (Washington, DC, http://dels.nas.edu/ilar). Six- to 8-week-old adult NOD/ShiJic-scid JcI (NOD-SCID) male mice (CLEA Japan Inc., Tokyo, Japan, http://www.clea-japan.com) were anesthetized with isoflurane (Mylan Inc., Tokyo, Japan, http://www.mylan.co.jp), and cell transplantation was performed with stereotactic injection of one aggregate (approximately 3 × 105 cells) on differentiation day 46 in 2 µl of PBS (Wako) through a blunt-end 26-gauge Hamilton syringe needle (Hamilton, Reno, NV, http://www.hamiltoncompany.com) into the right side of the cortex (from the bregma: A, +0.5 to +2.5; L, −0.5 to −2.5; V, −1.0). Six months after transplantation, the animals were perfused transcardially with 4% paraformaldehyde (PFA; Wako). The brains and spinal cords were fixed with 4% PFA overnight, transferred to 30% (wt/vol) sucrose (Nacalai Tesque, Kyoto, Japan, http://www.nacalai.co.jp) in PBS, and preserved at 4°C. They were then embedded with O.C.T. Compound (Sakura Finetek, Torrance, CA, http://www.sakura-americas.com), cut with a cryostat (Leica Biosystems, Buffalo Grove, IL, http://www.leicabiosystems.com) at 30-µm thickness and preserved in antifreeze (30% [vol/vol] glycerol [Nacalai Tesque], 30% [vol/vol] ethylene glycol [Wako], and 40% [vol/vol] 0.1 M PBS) at −30°C until use.
Immunostaining
On days 18, 32, and 46 from the day of neural induction, individual aggregates were fixed in 4% PFA (Wako) and washed with and preserved in PBS at 4°C until use. The aggregates were embedded with O.C.T. Compound (Sakura Finetek), cryosectioned at 10 µm using Cryostat (Leica), and attached to an MAS-coated glass slide (Matsunami, Osaka, Japan, http://www.matsunami-glass.co.jp). Cryosections (aggregates and brains) were blocked in 5% (vol/vol) normal donkey serum (NDS; EMD Millipore, Billerica, MA, http://www.millipore.com)/0.3% (vol/vol) Triton X-100 (Sigma)/PBS for 1 h at room temperature. Primary antibodies were diluted in 1% NDS/0.3% Triton X-100/PBS and incubated overnight at 4°C. The samples were then washed in 0.05% (vol/vol) Tween 20 (Nacalai Tesque)/PBS and incubated with secondary antibodies in 1% NDS/0.3% Triton X-100/PBS for 2 h at room temperature, followed by washing in 0.05% Tween 20/PBS and incubation with 4′,6-diamidino-2-phenylindole (Invitrogen) for 10 min at room temperature. Finally, the samples were washed in 0.05% Tween 20/PBS and mounted with antiphotobleaching mounting medium (2.4 g of Mowiol [Calbiochem, Billerica, MA, http://www.merckmillipore.com], 6 ml of glycerol [Wako], 6 ml of distilled water, 12 ml of 200 mM Tris-HCl [Nacalai Tesque], and 0.66 g of 1.4-diazabicycle[2.2.2]octane [Wako]).
The primary antibodies used were as follows: anti-β-catenin (1:500; Abcam, Cambridge, MA, http://www.abcam.com), anti-PAX6 (1:500; BD Biosciences), anti-MAP2ab (1:2000; Sigma-Aldrich), anti-β-tubulin (TUJ1; 1:2,000; Covance, Princeton, NJ, http://www.covance.com), anti-SOX1 (1:1,000; R&D Systems), anti-SOX2 (1:500; R&D Systems), anti-CTIP2 (1:1,000; Abcam), anti-COUP-TF1 (1:1,000; Perseus Proteomics Inc., Tokyo, Japan, http://www.ppmx.com), anti-SP8 (1:1,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), anti-BRN2 (1:1,000; Santa Cruz), anti-TBR1 (1:1,000; Millipore), anti-SATB2 (1:200; Abcam), anti-γ-aminobutyric acid (GABA; 1:1,000; Sigma-Aldrich), anti-tyrosine hydroxylase (1:1,000; Millipore), anti-vesicular glutamate transporter 1 (anti-VGLUT1; 1:1,000; Synaptic Systems, Goettingen, Germany, http://www.sysy.com), and anti-human neural cell adhesion molecule (NCAM; 1:1,000; Santa Cruz). Alexa fluorescent-conjugated antibodies (1:500; Invitrogen) were used as secondary antibodies. Stained cells and brains were imaged with a confocal fluorescence microscope (Olympus Corp., Tokyo, Japan, http://www.olympus.co.jp) and Biorevo (Keyence Inc., Osaka, Japan, http://www.keyence.co.jp).
Western Blot Analysis
The differentiated aggregates on day 18 were harvested and homogenized in lysis buffer (Sigma-Aldrich). The samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, http://www.bio-rad.com) and transferred to polyvinylidene difluoride membranes (GE Healthcare Life Sciences (GE), Piscataway, NJ, http://www.gelifesciences.com). The membranes were blocked with 5% (wt/vol) skim milk (BD Biosciences) and incubated with anti-β-catenin (1:2,500; Abcam), phosphorylated PKC (pPKC; 1:1,000; Cell Signaling, Beverly, MA, http://www.cellsignal.com) and anti-β-Actin (1:20,000; Sigma-Aldrich) at 4°C overnight. Then, the membranes were stained with horseradish peroxidase-conjugated secondary antibody (1:5,000; GE). The antibodies were detected with an enhanced chemiluminescence detection system (GE) and observed with ImageQuant LAS4000 (GE). The band intensity was determined by using ImageQuant TL (GE).
Polymerase Chain Reaction
Total RNA was purified from samples at various time points by using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany, http://www.qiagen.com). More than 50 ng of total RNA were reverse-transcribed by using SuperScript III Reverse Transcriptase and Oligo-dT (Invitrogen). Quantitative polymerase chain reactions (qPCRs) were carried out with SYBR Premix Ex Taq and the Thermal Cycler Dice Real Time System (TaKaRa Bio Inc., Shiga, Japan, http://www.takara-bio.co.jp). The data were assessed by using a delta-Ct method and normalized by GAPDH expression. Reverse-transcription PCR was carried out with Ex Taq (TaKaRa). The PCR products were separated by electrophoresis on an agarose gel (Sigma-Aldrich) and detected under UV illumination (AMZ system science, Osaka, Japan, http://www.amzsystemscience.com). The primers used for PCR are listed in Table 1.
Table 1.
Primers used for PCR
Statistical Analysis
Values are expressed as means ± SEM. All statistical analyses were performed by using GraphPad PRISM software (Version 6; GraphPad Software Inc., La Jolla, CA, http://www.graphpad.com). For comparison of three or four groups, the statistical significance of differences was determined by one-way analysis of variance followed by Tukey’s post hoc test. For comparison of two groups, unpaired t test was used. Probability values less than 5% were considered statistically significant.
Results
C59 Efficiently Induced Anterior Cortex From Human iPSCs
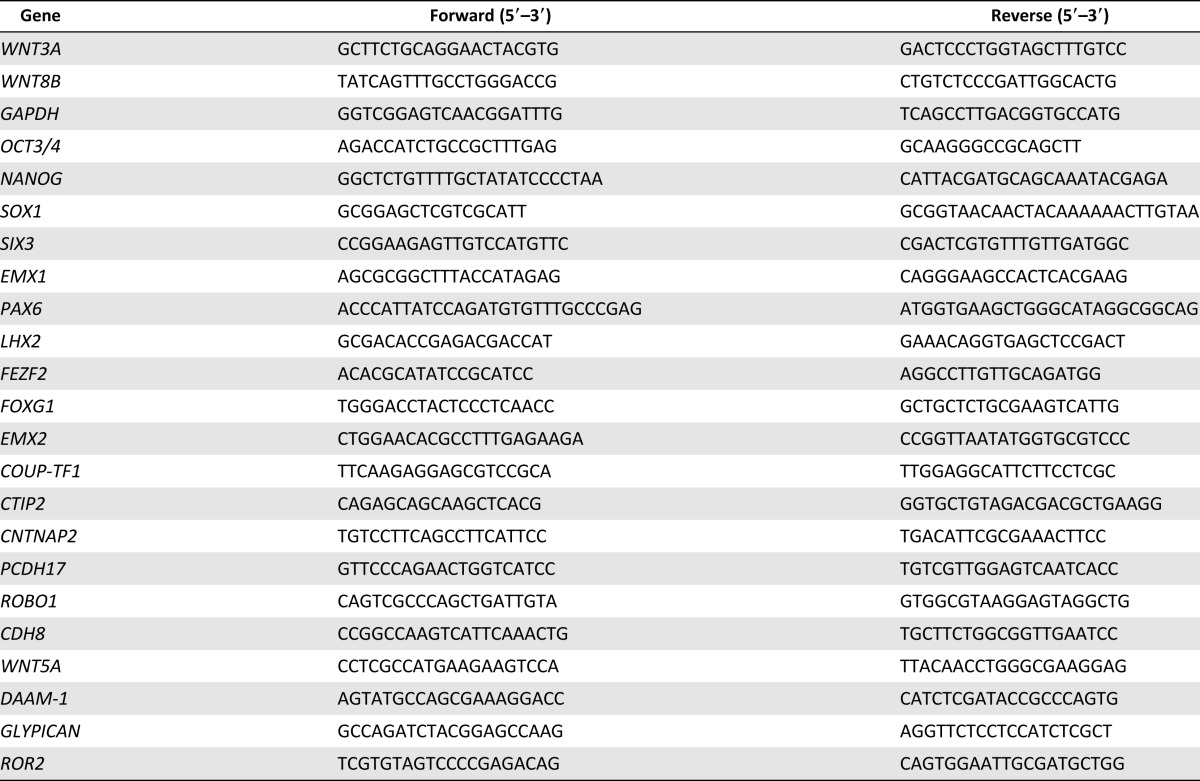
We tried to generate dorsal telencephalon from human iPSCs (836B1) based on an SFEBq method [11–13] with dual SMAD inhibition (SB431542 and LDN193189) [23, 24] (Fig. 1A). We chose this method to induce frontal neurons [13], because differentiation by monolayer culture tends to induce posterior cortex [10, 17]. We started differentiation in the presence of either dimethyl sulfoxide (shown as control in the figures), DKK1, XAV, or C59, and continued the culture until day 18.
Figure 1.
Cerebral cortex differentiation and expression analyses. (A): Schematic diagram of the culture procedure for cortical neuron differentiation. (B): Western blotting analysis on day 18. Each WNT inhibitor inhibited Wnt/β-catenin-dependent signaling during the SFEBq differentiation procedure, but C59 also inhibited Wnt/β-catenin independent signaling. (C, D): Time course analyses of the expression of WNT factors show characteristics of a rostral-low and caudal-high gradient. (E): Gene expression analyses of various differentiation markers. The expressions of undifferentiation markers, OCT3/4 and NANOG, were decreased, while the expressions of telencephalon markers, SIX3, EMX1, PAX6, and LHX2, and of the specification marker of corticospinal motor neurons, FEZF2, were increased. (F–H): Time course analyses of the expressions of markers for the telencephalic region. Differences were considered significant by ANOVA and Tukey’s test. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001; ∗∗∗∗, p < .0001 (n = 4). Abbreviations: bFGF, basic fibroblast growth factor; C59, WNT-C59; DMEM, Dulbecco’s modified Eagle’s medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KSR, Knockout Serum Replacement; OFL, ornithine-fibronectin-laminin; pPKC, phosphorylated PKC; SFEBq, serum-free floating culture of embryoid body-like aggregates with quick reaggregation; XAV, XAV939.
First, we confirmed the effects of the WNT inhibitors on human iPSCs. Analyses of the aggregates by Western blot and immunostaining using a β-catenin antibody revealed that all inhibitors suppressed β-catenin stabilization and nuclear transition on day 18 (Fig. 1B and supplemental online Fig. 2), indicating that they inhibited canonical Wnt signaling. However, only C59 suppressed pPKC, indicating that it also inhibited noncanonical Wnt signaling, as reported previously [19]. Neuronal cells in the developing brain secrete several WNTs [25]. Therefore, we examined the expression of WNTs by the iPSC-derived neuronal cells. Quantitative PCR analyses revealed that C59 suppressed the expression of WNT3A and WNT8B, which are expressed in a caudal-high gradient of the cortex [26] (Fig. 1C, 1D).
Next, we examined the expression of various differentiation markers until day 18 (Fig. 1E). The expressions of two undifferentiated markers (OCT3/4 and NANOG) were decreased as differentiation progressed. In contrast, the expression of an ectodermal marker (SOX1), a telencephalon marker (SIX3), and dorsal telencephalon markers (EMX1, PAX6, and LHX2) increased with the progression. The expression of FEZF2, which is required for the birth and specification of CMNs [27], also increased. Markers for endoderm (SOX17), mesoderm (BRACHYURY), midbrain (EN1), and hindbrain and spinal cord (HOXB4) were not detected (data not shown). These results indicated that dorsal telencephalon was induced by this protocol. As a next step, we examined the expressions of an anterior marker (FOXG1) [28] and two posterior markers (EMX2 and COUP-TF1) [29]. Quantitative PCR analyses revealed that the expression of FOXG1 was increased in the presence of C59, and that of EXM2 and COUP-TF1 was suppressed by either C59 or XAV (Fig. 1F–1H). These results suggested that C59 induced anterior cortex efficiently compared with DKK1 or XAV.
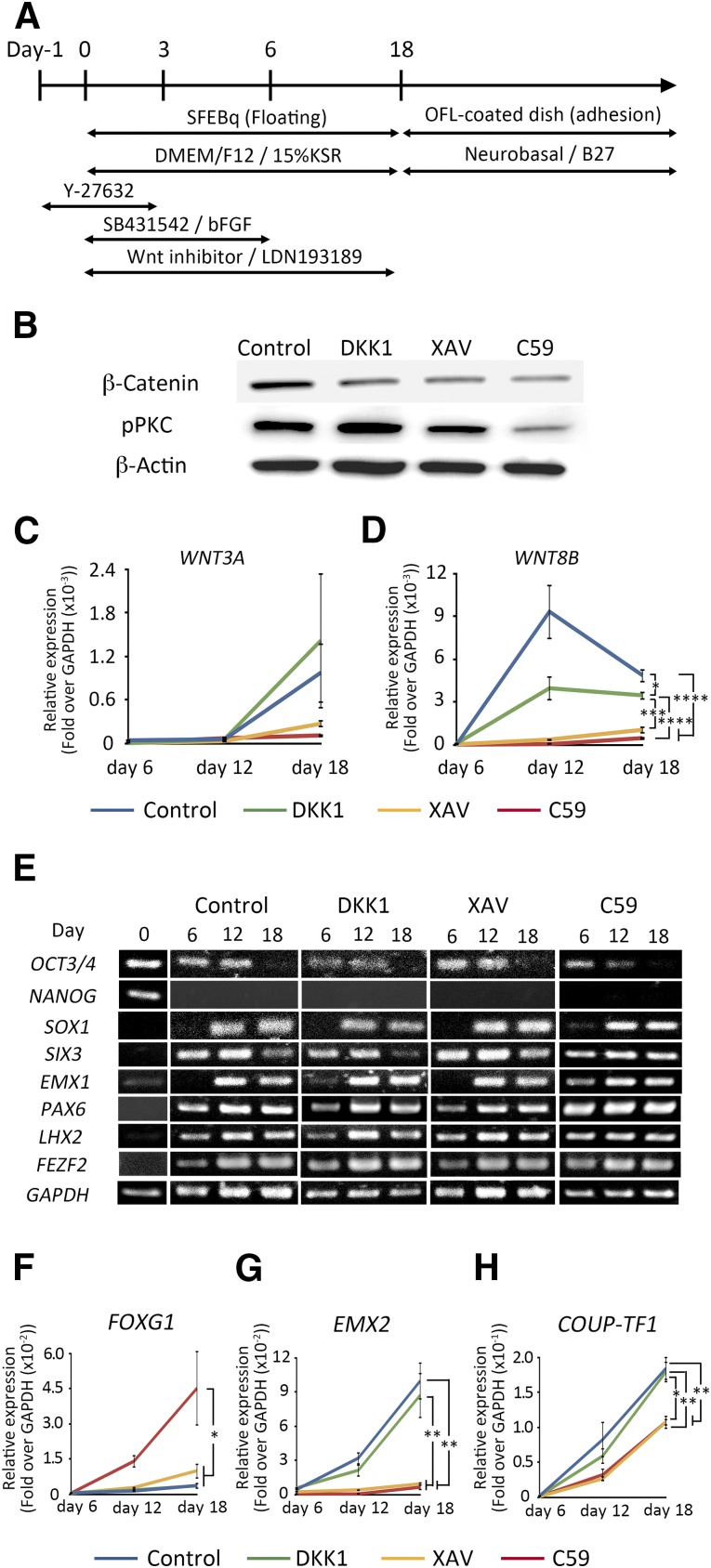
We confirmed this finding by immunocytochemistry of the induced cell aggregates. The aggregates were attached onto ornithine-fibronectin-laminin-coated dishes on day 18 and incubated in neurobasal/B27 medium until day 32. Rosette-like structures were formed in the aggregates, and two postmitotic neuronal makers, MAP2ab and TUJ1, were expressed outside the structures (Fig. 2A). The aggregates expressed PAX6, SOX1 (Fig. 2B), and SOX2 (supplemental online Fig. 3), which are markers for neuroepithelium or radial glia, indicating that early neural cells formed rosette-like structures and migrated out of them to become postmitotic neurons. Because human ESC-derived neural cells spontaneously acquire an intracortical dorsocaudal-ventrorostral polarity during differentiation [13], we next examined the expression of a rostral marker, SP8, and a caudal marker, COUP-TF1, in the aggregates. Immunostaining revealed that C59 induced more SP8+/COUP-TF1− cells (34.3 ± 1.73%) than did DKK1 (0.34 ± 0.12%) or XAV (2.83 ± 0.94%) (Fig. 2C, 2D). These results suggested again that C59 induced anterior cortex efficiently compared with DKK1 or XAV.
Figure 2.
Characteristics of the internal structure of the induced aggregates on day 32. (A): Each WNT inhibitor induced the neuronal markers MAP2ab and TUJ1 except in the rosette-like region. (B): Rosette-like structures expressed the neuroepithelium markers PAX6 and SOX1. (C): Rosette-like structures also expressed the rostral marker SP8 and the caudal marker COUP-TF1. (D): The percentage of SP8+/COUP-TF1− cells with respect to total cells was quantified in the sections. Differences were considered significant by ANOVA and Tukey’s test (n = 3). ∗∗∗∗, p < .0001. Scale bars = 50 μm. Abbreviations: ANOVA, analysis of variance; C59, WNT-C59; XAV, XAV939.
C59 Induces Neurons in Layer V of Anterior Cortex
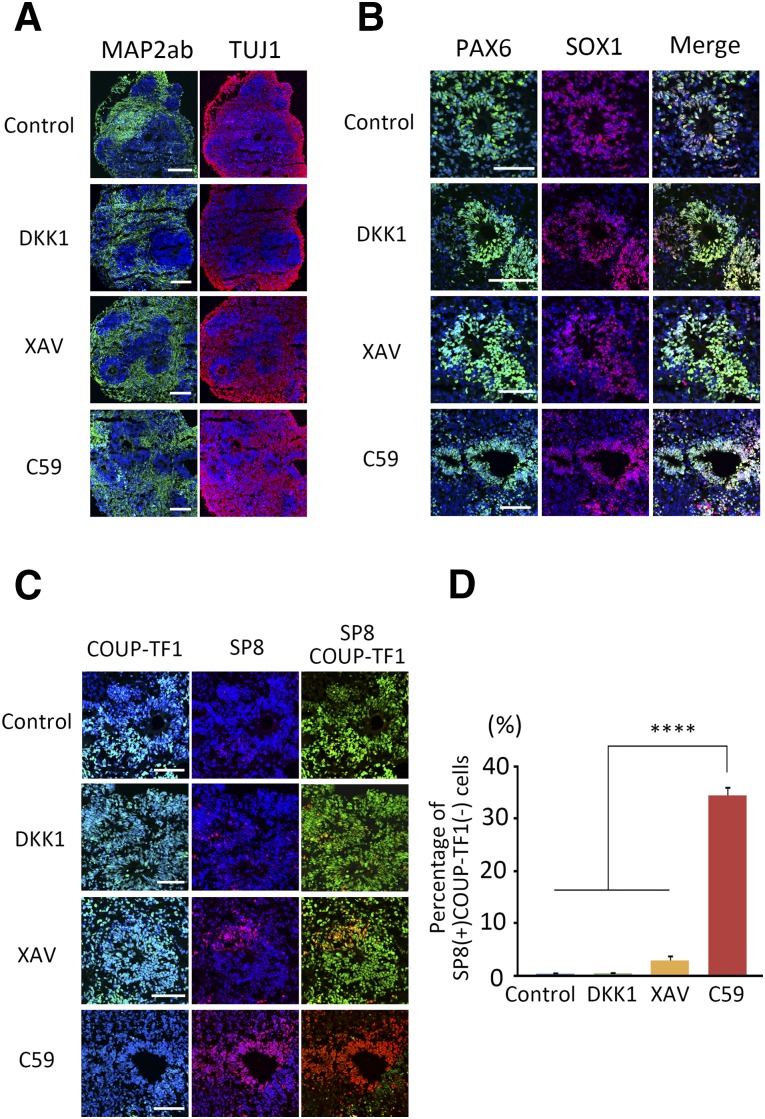
The cerebral cortex forms six layers as it develops [30], and several factors are selectively expressed in these layers. BRN2 is expressed in the ventricular and subventricular zones and upper layers II–IV [31]. The transcription factor TBR1 is expressed in multiple types of layers V–VI neurons [32]. The transcription factor CTIP2 is related to subcerebral projection neurons in layer V and is essential for generating CMNs [33]. Finally, the transcription factor SATB2 is expressed in layers II–IV and promotes callosal projection neuron identity [34].
Along with being a marker for posterior cortex, COUP-TF1 is a negative regulator of CMN differentiation [35]. Because a specific marker for CMN has not been identified, we regarded CTIP2+/COUP-TF1− cells as an indicator of CMN cells. Aggregates on day 46 showed layer formation, as identified by the different expression patterns of TBR1, CTIP2, and SATB2 (supplemental online Fig. 4A) and of BRN2 and CTIP2 (Fig. 3A). All inhibitors induced layers VI and V, but the number of layer IV SATB2+ cells was very small (supplemental online Fig. 4B, 4C). Importantly, C59 induced more CTIP2+ cells compared with control or other WNT inhibitors (Fig. 3B). C59 also induced more CTIP2+/COUP-TF1− cells (30.7% ± 9.20%) compared with DKK1 (6.15% ± 2.74%) or XAV (4.91% ± 2.73%) (Fig. 3C). Moreover, another WNT inhibitor, LGK-794, which has the same function as C59, induced cells with similar characteristics as those differentiated using C59 (supplemental online Fig. 4D–4F). Other iPSC lines (1147F1 and 1231A3) and an ESC line (Kh-1) gave consistent results (supplemental online Fig. 5). Overall, these results suggest that C59 efficiently induced the cells to differentiate into CMNs compared with DKK1 or XAV.
Figure 3.
Comparison of CTIP2-expressing cells on day 46. (A): Immunocytochemistry for layer and region markers on day 46 using the ventricular and subventricular zone marker BRN2 (white), caudal marker COUP-TF1 (green) and layer V marker CTIP2 (red). (B, C): Percentages of CTIP2 (B) and CTIP2+/COUP-TF1− (C) cells were investigated for each WNT inhibitor. Differences were considered significant by ANOVA and Tukey’s test (n = 3). ∗, p < .05; ∗∗, p < .01. Scale bars = 50 μm. Abbreviations: ANOVA, analysis of variance; C59, WNT-C59; DAPI, 4′,6-diamidino-2-phenylindole; XAV, XAV939.
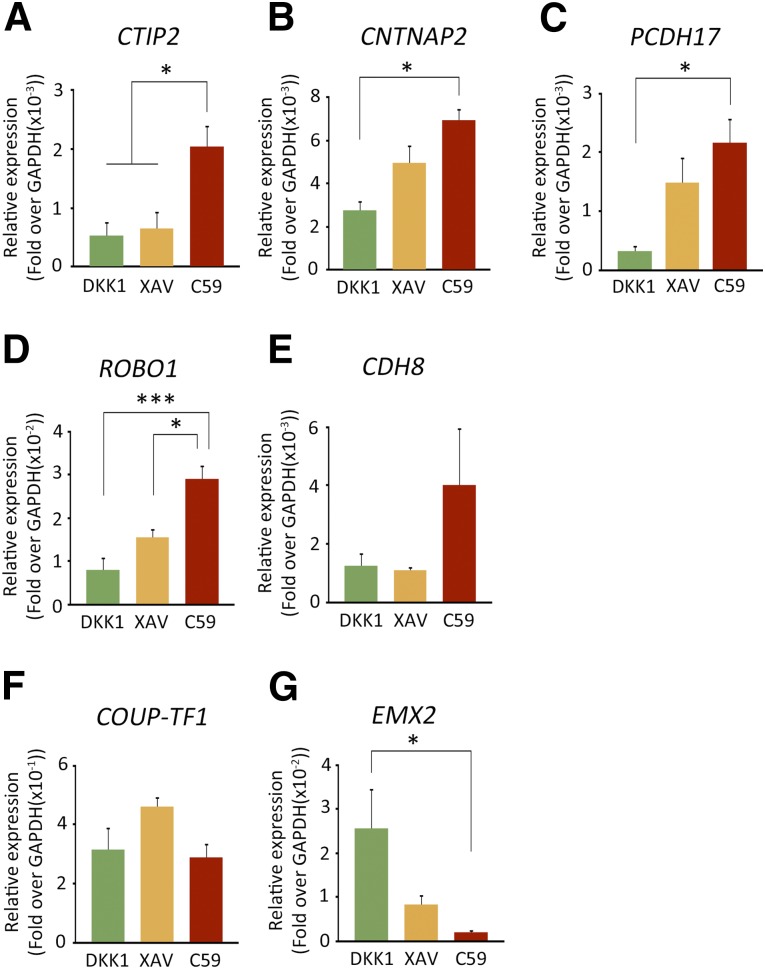
In order to confirm the rostral to caudal identity of the human iPSC-derived cells, we continued to culture the aggregates until day 60 and performed qPCR analyses to investigate the expression of a subset of differentially regulated genes during early human neocortical development (8–12 postconceptional weeks) [36]. Genes exhibiting a rostral-high to caudal-low gradient included CTIP2, CNTNAP2, PCDH17, ROBO1, and Cadherin8 (CDH8). On the other hand, genes exhibiting a caudal-high to rostral-low gradient contained COUP-TF1 and EMX2. qPCR analyses revealed that neural aggregates differentiated with C59 contained more cells with rostral identity compared with DKK1 or XAV (Fig. 4).
Figure 4.
Expressions of region markers in differentiated cells on day 60. The differentiated cells were analyzed by qPCR for rostral-high expression genes: CTIP2 (A), CNTNAP2 (B), PCDH17 (C), ROBO1 (D), and CDH8 (E); and for caudal-high expression genes COUP-TF1 (F) and EMX2 (G). Differences were considered significant by ANOVA and Tukey’s test. ∗, p < .05; ∗∗∗, p < .001 (n = 5). Abbreviations: ANOVA, analysis of variance; C59, WNT-C59; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; qPCR, quantitative polymerase chain reaction; XAV, XAV939.
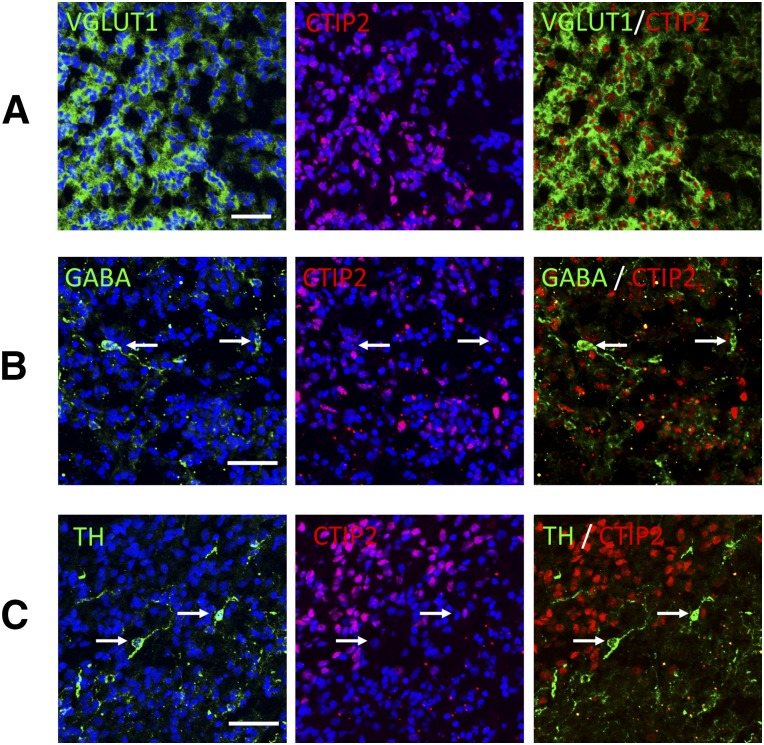
Excitatory projection neurons transmit signals to other cortical regions and express VGLUT, whereas inhibitory interneurons regulate local circuitry and express GABA. These neurons have distinct origins and developmental programs [37]. We investigated neuronal cell types in the C59-induced neural cells on day 46. The majority was VGLUT1+ cells (65.9% ± 2.8%; Fig. 5A), and almost all CTIP2+ cells expressed VGLUT1. Among the other types of neurons identified, 1.20% ± 0.9% were GABAergic (Fig. 5B), and 1.18% ± 0.3% were dopaminergic neurons (Fig. 5C). Serotonergic and cholinergic neurons were not detected. Because CMNs are in layer V and also excitatory neurons, namely glutamatergic [38], it is likely that the iPSC-derived neurons treated with C59 contained CMNs or their progenitors.
Figure 5.
Differentiated cells produced mainly glutamatergic projection neurons on day 46. iPSC line 836B1 on day 46 expressed glutamatergic neurons (A), GABAergic neurons (B), and dopaminergic neurons (C). Arrows indicate neuronal marker+ CTIP2− cells. Scale bar = 50 μm. Abbreviations: iPSC, induced pluripotent stem cell; TH, tyrosine hydroxylase; VGLUT1, vesicular glutamate transporter 1.
C59-Induced Aggregates Extended Axonal Fibers From the Cerebral Cortex to the Pyramidal Decussation in Adult Mice
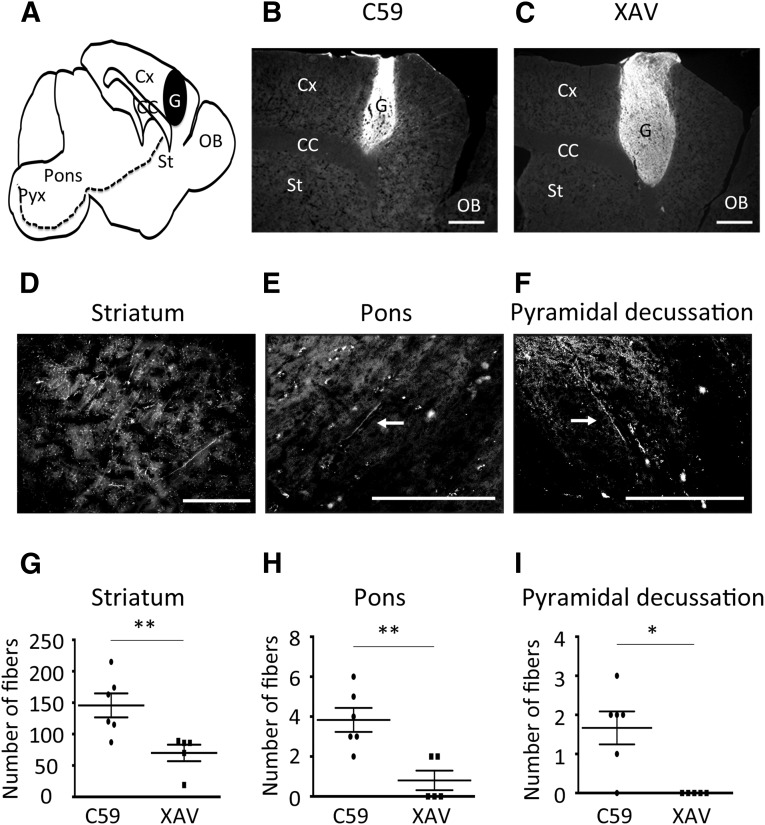
In order to investigate whether the induced aggregates included CMNs or their progenitors, we transplanted one aggregate (3 × 105 cells in 2 µl of PBS) into the cortex of NOD-SCID adult mice. Six months after transplantation, the grafts and neurites were analyzed by immunostaining for human-specific NCAM in sagittal sections (Fig. 6A). The grafts were detected in all mice (6/6 in C59 and 5/5 in XAV; Fig. 6B, 6C). Then, we counted the number of human NCAM+ fibers from the graft to the pyramidal decussation (to measure the width, we covered the transplanted side of the brain from the midline to the whole graft, with 7 slices at 300-µm intervals per animal). We found more axonal fibers at the striatum (Fig. 6D, 6G), pons (Fig. 6E, 6H), and pyramidal decussation (Fig. 6F, 6I) in the case of grafts derived from C59-induced aggregates compared with those from XAV-induced ones. These fibers were found to have extended along the tract in the contralateral spinal cord (supplemental online Fig. 6). These results suggest that the aggregates differentiated under C59 treatment contained more CMNs or their progenitors.
Figure 6.
In vivo analyses of the graft at 6 months after transplantation. (A): Sagittal section of the brain. Neurites derived from the graft extended toward the spinal cord through the corticospinal tract (dotted line). (B, C): Six months after transplantation, the graft survived in the host. (D–F): The neurites derived from the graft could be detected at the striatum (D), pons (E), and pyramidal decussation (F). (G, H): The number of fibers was counted in each region (C59, n = 6; XAV, n = 5). Differences were considered significant by unpaired t test. ∗, p < .05; ∗∗, p < .01. Scale bars = 500 µm (B, C) and 200 µm (D–F). Abbreviations: C59, WNT-C59; CC, corpus callosum; Cx, cortex; G, graft; OB, olfactory bulb; Pyx, pyramidal decussation; St, striatum; XAV, XAV939.
Discussion
The induction of CMNs from ESCs or iPSCs hold promise as cell therapy against damaged corticospinal tracts caused by neurodegenerative diseases or insults. However, neither an efficient protocol to induce CMNs nor a specific marker for CMNs is available. In order to improve efficient induction of anterior cortex, where CMNs are located, we compared two small WNT inhibitors, C59 and XAV, during the neuronal differentiation of human iPSCs. Intriguingly, C59 induced more CTIP2+/COUP-TF1− cells compared with XAV or DKK1, a common WNT inhibitor. In addition, when grafted into the cortex of adult mice, C59-induced cells showed more abundant axonal fiber extensions toward the spinal cord. These results suggest that C59 induced CMNs or their progenitors efficiently.
As to why C59 efficiently induced anterior cortex, we consider the following reasons. First, the C59-induced aggregates expressed a higher level of FOXG1 than those induced by DKK1 or XAV (Fig. 1F). FOXG1 represses the expression of WNT and BMP [26], which are expressed in the cortical hem, an embryonic organizer of the hippocampus [12]. Second, C59 induced more SP8-positive cells (Fig. 2D). SP8 is required for specification of the rostral cortical identity [39]. It is induced by Fgf signaling and subsequently maintains the expression of Fgf8 within the rostral cortical signaling center. FGF8 signaling also promotes the expansion of the rostral telencephalon [26]. Third, C59 tended to repress the expression of WNT3A and WNT8B compared with XAV (Fig. 1C, D), although the value was not statistically significant. It has been reported that these WNTs cause a caudomedial-high/rostrolateral-low gradient in Wnt-β catenin activity [40]. We also observed the expression of a caudal marker, EMX2 (Fig. 1G), which is consistent with this gradient [29]. Because C59 inhibits both canonical and noncanonical Wnt signaling, whereas DKK1 and XAV inhibit only canonical signaling, we propose that a noncanonical pathway may play an important role for the induction of anterior cortex, which has been suggested before C59 inhibits the differentiation of neural stem cells to mature neurons [41]. Planar cell polarity (PCP) signaling, a type of noncanonical Wnt signaling, is involved in embryonic morphogenesis and the development of the nervous system by coordinating convergent extension (CE) movements [42, 43]. CE movements are characterized by medio-lateral and rostro-caudal movements of cells to narrow and elongate the embryo [43]. In day 18 aggregates, several genes involved in CE movements, including WNT5A, DAAM-1, GLYPICAN, and ROR2 [44], were expressed (supplemental online Fig. 7). This finding may indicate that PCP signaling regulates CE movements for rostro-caudal formation of the cortex.
In addition to WNTs, the effects of secreted ligands, such as Hedgehog and Spitz, on signal transduction is regulated by palmitoylation [45]. The inhibition of C59-dependent palmitoylation may be involved in the areal patterning of the induced cells [16].
Some neurons in layer V of the cerebral cortex express CTIP2 [31] and project axons to the spinal cord and the superior colliculus [27, 38]. COUP-TF1 is expressed in the posterior cortex and is involved in neuronal differentiation and areal patterning [46, 47]. CTIP2+/COUP-TF1− cells are located in the motor/frontal region of the cerebral cortex in embryos [35] and project axons to the spinal cord when transplanted into mouse cerebral cortex [4]. Conversely, CTIP2+/COUP-TF1+ cells are located in the occipital region of the cortex and project axons to the superior colliculus [10]. Therefore, we consider the induction of CTIP2+/COUP-TF1− cells as necessary for ESC/iPSC-based clinical application to treat motor neuron diseases or insults.
Conclusion
We found that C59 induced CTIP2+/COUP-TF1− cells from human iPSCs more efficiently than two other WNT inhibitors, DKK1 and XAV. Furthermore, these CTIP2+/COUP-TF1− cells extended axons to the pyramidal decussation. These results raise the possibility that C59 could contribute to cell-replacement therapy for motor neuron diseases or insults.
Supplementary Material
Acknowledgments
We thank Dr. Peter Karagiannis for reading the manuscript. This work was supported by a grant from the Network Program for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development and an Intramural Research Grant for Neurological and Psychiatric Disorders from the National Center of Neurology and Psychiatry.
Author Contributions
M.M.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing; Y.I.: conception and design, provision of study material, manuscript writing; T.O.: provision of study material, data analysis and interpretation; J.T.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.M. has compensated research funding. The other authors indicated no potential conflicts of interest.
References
- 1.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 2.Jara JH, Genç B, Klessner JL, et al. Retrograde labeling, transduction, and genetic targeting allow cellular analysis of corticospinal motor neurons: Implications in health and disease. Front Neuroanat. 2014;8:16. doi: 10.3389/fnana.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebrahimi-Gaillard A, Roger M. Development of spinal cord projections from neocortical transplants heterotopically placed in the neocortex of newborn hosts is highly dependent on the embryonic locus of origin of the graft. J Comp Neurol. 1996;365:129–140. doi: 10.1002/(SICI)1096-9861(19960129)365:1<129::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Gaillard A, Prestoz L, Dumartin B, et al. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- 5.Doi D, Samata B, Katsukawa M, et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi D, Morizane A, Kikuchi T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- 7.Kirkeby A, Grealish S, Wolf DA, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Reports. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 9.Ideguchi M, Palmer TD, Recht LD, et al. Murine embryonic stem cell-derived pyramidal neurons integrate into the cerebral cortex and appropriately project axons to subcortical targets. J Neurosci. 2010;30:894–904. doi: 10.1523/JNEUROSCI.4318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espuny-Camacho I, Michelsen KA, Gall D, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Mariani J, Simonini MV, Palejev D, et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Kadoshima T, Sakaguchi H, Nakano T, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex [published correction appears in Proc Natl Acad Sci USA 2014;111:7498] Proc Natl Acad Sci USA. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroof AM, Keros S, Tyson JA, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Kirwan P, Smith J, et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486, S471. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XJ, Zhang X, Johnson MA, et al. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaspard N, Bouschet T, Hourez R, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 18.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38:148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proffitt KD, Madan B, Ke Z, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 20.Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 21.Semënov MV, Tamai K, Brott BK, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 22.Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- 23.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morizane A, Doi D, Kikuchi T, et al. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89:117–126. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- 25.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 26.Storm EE, Garel S, Borello U, et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 27.Molyneaux BJ, Arlotta P, Hirata T, et al. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Hébert JMG, Fishell G. The genetics of early telencephalon patterning: Some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greig LC, Woodworth MB, Galazo MJ, et al. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugitani Y, Nakai S, Minowa O, et al. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–1765. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molyneaux BJ, Arlotta P, Menezes JR, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 33.Arlotta P, Molyneaux BJ, Chen J, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 34.Leone DP, Srinivasan K, Chen B, et al. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomassy GS, De Leonibus E, Jabaudon D, et al. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci USA. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ip BK, Wappler I, Peters H, et al. Investigating gradients of gene expression involved in early human cortical development. J Anat. 2010;217:300–311. doi: 10.1111/j.1469-7580.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 38.Molnár Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Sahara S, Kawakami Y, Izpisua Belmonte JC, et al. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SM, Tole S, Grove E, et al. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 41.Bengoa-Vergniory N, Gorroño-Etxebarria I, González-Salazar I, et al. A switch from canonical to noncanonical Wnt signaling mediates early differentiation of human neural stem cells. Stem Cells. 2014;32:3196–3208. doi: 10.1002/stem.1807. [DOI] [PubMed] [Google Scholar]
- 42.Clark CE, Nourse CC, Cooper HM. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals. 2012;20:202–220. doi: 10.1159/000332153. [DOI] [PubMed] [Google Scholar]
- 43.Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 44.Wallingford JB. Neural tube closure and neural tube defects: Studies in animal models reveal known knowns and known unknowns. Am J Med Genet C Semin Med Genet. 2005;135C:59–68. doi: 10.1002/ajmg.c.30054. [DOI] [PubMed] [Google Scholar]
- 45.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 46.Faedo A, Tomassy GS, Ruan Y, et al. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18:2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armentano M, Chou SJ, Tomassy GS, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.