Cardiac cell-based regenerative therapies include application of a cell suspension and the implantation of an in vitro engineered tissue construct to the damaged area of the heart. Both strategies have their advantages and challenges. This review discusses the current state of the art in myocardial regeneration, the challenges to success, and the future direction of the field.
Summary
Cardiovascular diseases account for the majority of deaths globally and are a significant drain on economic resources. Although heart transplants and left-ventricle assist devices are the solution for some, the best chance for many patients who suffer because of a myocardial infarction, heart failure, or a congenital heart disease may be cell-based regenerative therapies. Such therapies can be divided into two categories: the application of a cell suspension and the implantation of an in vitro engineered tissue construct to the damaged area of the heart. Both strategies have their advantages and challenges, and in this review, we discuss the current state of the art in myocardial regeneration, the challenges to success, and the future direction of the field.
Significance
This article outlines the advantages and limitations of the cell injection and patch approaches to cardiac regenerative therapy. If the field is to move forward, some fundamental questions require answers, including the limitations to the use of animal models for human cell-transplantation studies; the best way to measure success in terms of functional improvements, histological integration, electrical coupling, and arrhythmias; and where the cells should be applied for maximal benefit—the epicardium or the myocardium.
Introduction
Cardiovascular disease (CVD) remains a leading cause of death globally and a major health-care burden [1]. Myocardial infarction (MI) is a major cause of CVD death. MI results from insufficient blood supply to the heart muscle and can cause the death of 1 billion cells on average [2]. The heart is capable of limited endogenous regeneration [3, 4], but it is insufficient to repopulate the myocardium with cardiomyocytes (CMs) postinjury. Consequently, the ventricle undergoes pathological remodeling (i.e., wall thinning and chamber dilatation), which reduces contractile function and often results in heart failure.
The application of biomaterials alone to the myocardium can reduce adverse post-MI remodeling [5], but it is not a long-term regenerative solution because the millions of lost cells are not replaced. Hence, it is generally agreed that to restore contractile function, CMs need to be applied to the ventricle [6].
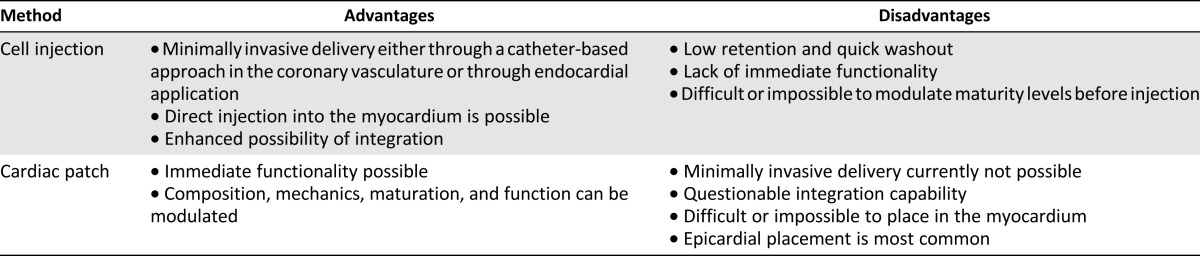
In this review, we outline the sources of CMs available for cardiac regenerative therapy approaches; the advantages and disadvantages of the two primary regenerative strategies, direct cardiac cell injection and cardiac patch implantation (Table 1); and the challenges to the field and its direction moving forward.
Table 1.
A comparison of cell replacement therapy methods
Cardiomyocyte Sources
Human CMs are a limited resource. Adult CMs are considered terminally differentiated and proliferate minimally [3, 4]; they also cannot be cultured indefinitely. As such, adult CMs cannot be expanded to sufficient numbers from cardiac biopsy specimens, and this has motivated the investigation into alternative CM sources. A variety of cell types have been studied, including various adult stem cells and progenitors [7].
One strategy aimed at promoting endogenous cell proliferation involves modulating posttranslational regulation. MicroRNAs (miRNAs) are short, single-stranded, noncoding RNAs that bind and inhibit the translation of target messenger RNAs. In recent years, miRNAs have been identified that promote endogenous adult CM [8] and cardiac progenitor cell (CPC) [9] proliferation. Moreover, these miRNAs were shown to reduce infarct size and improve cardiac function in a mouse MI model [8]. Conversely, other miRNAs, such as the miR-15 family, have been identified to promote cell cycle withdrawal [10, 11]. As such, inhibitors of this category of miRNA, such as small hairpin RNA, have been proposed as a means of increasing the number of mitotic CMs [12], and it has been demonstrated that inhibiting the miR-15 family can reduce infarct size and cardiac remodeling, and improve cardiac function in a mouse MI model [11].
Endogenous CPCs have also been investigated as an alternative therapeutic cardiac cell source. Cardiosphere-derived cells (CDCs), obtained from primary adult heart cultures, are heart-derived multipotent stem cells [13] thought to function primarily through indirect mechanisms [14]. CDCs have been used clinically in the phase I study CADUCEUS (Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction), wherein patients injected with autologous CDCs 1.5–3 months post-MI had decreased scar size and increased viable myocardium (i.e., regeneration); however, no global functional improvements were observed [15].
Recently, the focus has shifted to human-induced pluripotent stem cells (hiPSCs). Human iPSCs are biologically similar to human embryonic stem cells (hESCs), the archetype for pluripotency, but sidestep the ethical and political issues surrounding human embryo use [16]. Furthermore, hiPSCs robustly differentiate into patient-specific [17], bona fide CMs [18, 19], which suggests unlimited numbers of autologous CMs can be produced for cell therapy, and treatment can be provided without immunosuppression.
Initially, iPSCs were generated using retroviral transduction [20]. Although effective, this approach risked oncogenic transformation due to the random insertion of the reprogramming factors. Safer, nonintegrating methods have since been developed (e.g., Sendai virus, mRNA or miRNA, and direct protein delivery) [16]. Research is also ongoing to determine if “naïve” hiPSCs (an earlier pluripotency state than the conventional “primed” hiPSCs [21]) may be more amenable to genomic modifications [22] and/or differentiation into somatic tissues [23, 24].
The most efficient cardiac differentiation protocols replicate lineage commitment pathways [18]. Current protocols have high efficiencies capable of generating highly-enriched CM populations. Early work involved applying stage-specific growth factors to embryoid bodies [25–28]. Monolayer-based protocols followed, using activin A and bone morphogenetic protein 4 [29, 30]; and, most recently, growth factor proteins were replaced by small molecules [31].
However, the resultant hPSC-CMs obtained from these protocols are more akin to human fetal CMs, based on gene expression [32], electrophysiology [33], and morphology [34] than adult CMs. Currently, it is not known what the implications, as they pertain to cardiac regenerative therapy, may be that hPSC-CMs are smaller, less electrically excitable [35, 36], have a lower sensitivity to adrenergic stimulation [37, 38], and have impaired excitation-contraction coupling [39, 40] relative to adult CMs. Although it would be presumed that more mature CMs would provide a functional advantage upon injection and/or implantation, it is possible that less-mature CMs might be better equipped to contend with the hostile environment of the infarcted myocardium.
Another major difficulty is that despite these methodological advances, generating patient-specific iPSC-derived CMs (iPSC-CMs) still requires 2–3 months from biopsy to CMs, and may present a potential risk of teratoma formation due to residual pluripotent cells [41]. Consequently, direct reprogramming strategies are being explored. Proof-of-concept mouse studies show somatic cells can be directly converted to CMs [42–44]. Recently, direct reprogramming of human fibroblasts was also achieved [45]. However, current induced CM (iCM) protocols have low efficiencies compared with iPSC-CM protocols (5%–13% vs. 90%–95%) [16]. Also, iCMs are not fully characterized. Some reports suggest they are similar to ESC-CMs [46, 47], whereas others report they are less mature than hiPSC-CMs [48]. Regardless, transdifferentiation could revolutionize regenerative medicine by eliminating the risk of teratomas, and by directly reprogramming fibroblasts in the postinfarct scar into functional myocardium in vivo.
Cell Injection
The most common cell replacement therapy is the direct injection of cell suspensions using catheters or open chest surgery [49]. Clinical trials to test safety and efficacy have been conducted for various adult stem cell and progenitor populations [16], including bone marrow mesenchymal stem cells (MSCs), peripheral mononuclear cells (PMCs), and resident cardiac cells. Notably, these cell types have no intrinsic ability to produce large numbers of CMs. Rather, it is thought they secrete paracrine factors that act directly to induce beneficial effects or indirectly by altering gene expression in the surrounding myocardium [50]. In fact, most cardiac cell injection studies achieved beneficial effects without long-term cell persistence [50]. Examples of identified paracrine factors include vascular endothelial growth factor [51], thymosin β4 [52], and stromal-derived factor [53]. Recently, a secretome analysis of human bone marrow cells identified a novel cardioprotective and angiogenic paracrine factor, myeloid-derived growth factor [54].
Overall, these trials have shown varying degrees of clinical benefit [49]. A meta-analysis of 10 intracoronary cell injection trials using BMCs and PMCs showed modest but significant functional improvements in patients treated within 14 days of an acute MI [55]. In general, the clinical trial results to date have been moderate, which has spawned debates on what constitutes a clinically meaningful improvement and how success should be measured. While these debates continue, new clinical trials are being initiated. In a recent proof-of-principle clinical trial, single-cell, hESC-derived CPCs were embedded in a fibrin gel and implanted onto the epicardium of patients with severe heart failure by positioning the cell-fibrin mixture beneath the pericardial flap [56]. There are also ongoing efforts to gain approval for hPSC-CM clinical trials [16, 57].
Improved outcomes from cell injection therapies may result from the combined delivery of cells and paracrine factors and/or cell suspensions that contain multiple cell types (e.g., a CM source and endothelial cells [ECs]) to promote vascularization [58]. To date, the single-pronged approach has yielded results below the benchmark of true regeneration. Conversely, a multipronged synergistic approach could promote engraftment and vascularization, and ultimately, functional improvements.
Whether transplanted cells can functionally integrate with the host myocardium remains an open question. The results with hESC-CMs are inconclusive, partly because of the limitations of the animal models used for assessment. Human ESC-CMs injected into infarcted rodent hearts showed functional improvements at 1 month [59, 60] but not 3 months [60]; and graft-host electrical coupling was reported in both rat [61] and guinea pig [62] (Fig. 1Aa, Ab) models. However, small animals have faster beat rates than humans (e.g., human CMs: 60–120 bpm; rodent: 350–600 bpm; guinea pig: 200–250 bpm [62]), and although hESC-CMs can beat at 6 Hz (360 bpm) under electrical field stimulation [63], prolonged (>2 weeks) conditioning at this rate may result in a pathological phenotype and cell death. Large-animal models, therefore, are considered more suitable for hPSC-CM integration studies because of the overlap in graft and host beat rates [6]. Limited integration was observed in pigs (55–120 bpm) transplanted with hESC-CMs [64] (Fig. 1Ac); and although integration was evident in a nonhuman primate model (100-130bpm), transient but serious arrhythmias were observed following cell application [6] (Fig. 1Ad). These results may very well illustrate another important limitation of animal models—species-to-species differences in ion channel expression [65], which may hinder electrical coupling.
Figure 1.
Host-graft electrical coupling in animal hearts transplanted with hESC-CMs. (A): Rat. (Aa): Merged image indicating location of fluorescently labeled graft. (Ab): Electrical activation mapping at 4 Hz (240 bpm) shows slight conduction delay at graft site. (Ac–Ad): Isochronal electrical activation maps without (Ac) and with (Ad) fluorescent image overlay to indicate graft location. Maps show the interval (in ms) between the stimulus pulse and the local fluorescence increase. A slight conduction delay is evident at the graft site. Reproduced from [61] with permission. (B): Guinea pig. (Ba): Calcium mapping of a cryoinjured heart at 3 Hz (180 bpm). Top: Traces of fluorescent intensity versus time for grafts located in the border zone (1, blue) or cryoinjury zone (2, red) relative to host ECG (black) indicate coupling. Bottom: Calcium isochronal activation map showing the interval (in ms) between the stimulus pulse and the local fluorescence increase. Graft in border zone (1) shows uniform rapid activation, whereas graft in cryoinjury zone (2) shows gradual activation from edge to edge. (Bb): Calcium mapping of uninjured heart at 3 Hz (180 bpm). Top: Trace of fluorescent intensity versus time for graft relative to host ECG indicates coupling. Bottom: Calcium isochronal activation map for graft shows rapid uniform activation. Reproduced from [62] with permission. (C): Pig. (Ca, Cb): Electrical activation maps of the junctional (left) and new ventricular ectopic (right) rhythms shown from the anteroposterior (Ca) and left lateral (Cb) view. These images show that the earliest electrical activation (red) during the junctional rhythm was shifted to the graft area during the ventricular ectopic rhythm. Reproduced from [64] with permission. (D): Nonhuman primate. (Da): Heart diagram denoting regions shown in (Db–Dd). (Db): Image of fluorescently labeled graft sites denoted by the red and blue rectangles. (Dc): Graft during diastole. (Dd): Graft during systole. (De–Dh): Calcium mapping at different rates for the graft sites indicated in (Db). Traces of fluorescence intensity versus time relative to host ECG indicate coupling. Reproduced from [6] with permission. Abbreviations: AU, arbitrary units; Fl, fluorescence.
Alternatively, the moderate success of cell injection therapy could be attributed to the relative immaturity of the transplanted cells [6]. Electrical conduction and coupling (e.g., ion channel currents, densities, and kinetics [66]), as well as contractile rate and force, are all developmentally regulated and distinctively different in hPSC-CMs and human adult CMs [67]. Long-term culture has induced some degree of maturation [66], but extending the timeline from biopsy to implantation is not desirable, and the degree of maturation achieved by extended culture is insufficient. The injection of isolated cells also cannot provide the tissue-level connections and organization needed for immediate functionality. The ability of the graft to function upon implantation and/or its nearer resemblance to adult tissue are considered possible solutions to adverse remodeling and rapid host-graft integration.
Cardiac Patch
Cardiac patches have been investigated as a possible solution to cell injection challenges. Cell injection strategies have a maximal engraftment efficiency of 10% because of rapid wash out, ejection, and death of the injected cells [68, 69]. Cell requirements, therefore, are massive (e.g., 1 billion CMs for a sizable graft in a monkey) [6]. Cell retention and survival has been improved by injecting cells with hydrogels that rapidly gel in situ [69]. In vitro cultured cardiac patches provide even better delivery precision and retention of transplanted material. Through in vitro cultivation, the size, shape, and functional properties of cardiac patches can also be tailored; and bioactive peptides and growth factors (e.g., paracrine factors) can be released in a controlled and targeted manner.
Engineered cardiac tissues (ECTs) are designed to manipulate the cell microenvironment to facilitate cell assembly into functional tissues with adult-like morphological, functional, and mechanical properties. Various synthetic [poly(glycerol sebacate), polyglycolic acid, poly-l-lactic acid] and natural (alginate, collagen, chitosan, decellularized heart) biomaterials have been used as hydrogels and scaffolds [69, 70]), and advances in microfabrication and nanotechnology have enabled the generation of highly anisotropic tissues [71–74]. The majority of three-dimensional (3D) ECTs have used rat CMs [68, 70]; however, human ECTs have recently emerged [67]. Improved structural and functional properties of 3D ECTs and enhanced survival in vivo have been demonstrated through strategic cocultures with fibroblasts (or MSCs [75]) and CMs, likely because of the matrix deposition and paracrine signaling activity of the nonmyocytes [68]. Similarly, coculture with ECs in 3D ECTs has proved beneficial, demonstrating increased vascularization and CM proliferation, and host vasculature anastomosis [68, 75]. Several key maturation features have also been improved using ECT-based strategies (e.g., electrical field stimulation, mechanical stretching [cyclic, static or incremental] and topographical alignment cues [67]).
Proof-of-concept studies demonstrated that patches of neonatal rat CMs implanted onto infarcted hearts of syngeneic rats could integrate with the host heart, induce functional improvements, and electrically couple without delay or evidence of arrhythmias [76]. The beneficial outcomes of cardiac patch therapy have been extensively demonstrated in small-animal models [75, 77, 78] and are currently being tested in large-animal models [58].
An important obstacle to clinical application is the need for thicker cardiac patches. A recent study demonstrated transplanted tissue sections >400 µm thick have poor survival due to diffusion limitations [79]. The most effective method of producing thick ECTs will likely involve an embedded engineered vasculature that enables immediate and direct anastomosis with the host vasculature. As mentioned, coculture with ECs promotes vascularization of ECTs, but thick tissues will require a higher density of branched vessels than this strategy can provide, given the high metabolic activity of CMs. Various vascularization strategies have been investigated (e.g., chemically modifying scaffolds [80], embedding microvessel fragments [80], and 3D printing scaffolds with sacrificial molds that form vessel-like structures [81, 82]).
One notable strategy to produce thicker tissues involves stacking layers of cardiac tissue. A scaffold-free approach was developed wherein cultured CM monolayers are released as intact cell sheets from a temperature-sensitive culture surface [83], and ECT scaffolds were attached together by a series of loops and hooks, inspired by the mechanisms of Velcro (Velcro Industries, Manchester, NH, http://www.velcro.com) and plant burr attachment [84]. Either of these stacking methods suggests the possibility of integrating an engineered EC layer amid the CM layers, which could promote vascularization and enable thicker tissues to be formed.
Implantation Location
It remains to be determined where cells or cardiac patches should be implanted for optimal therapeutic benefit. The ventricular wall is comprised of the epicardium, the outer epithelial muscle lining; the myocardium, the muscle tissue; and the endocardium, the inner endothelial muscle lining (Fig. 2A). Proponents of intramyocardial placement point to evidence of integration therein with cell injections, and, in a recent comparative study, better graft-host electrical integration was observed with intramyocardial delivery than epicardial placement [85].
Figure 2.
Cardiac regenerative therapy options. (A): The wall of each heart chamber is comprised of three layers: endocardium, myocardium, and epicardium. Reproduced from [92] with permission. (B): Various injection locations for cardiac cell injection therapy. (C): Various cardiac patch strategies and epicardial patch placement. (B, C): Reproduced from [93] with permission. Abbreviations: AV, aortic valve, MV, mitral valve, PV, pulmonary valve, TV, tricuspid valve.
However, direct injection of a cell suspension into the myocardium (Fig. 2B) is inherently limited by the very small volume of free space available because of the high cell density in the myocardium (108 cells per cm3 [86]). This space becomes more restricted following injury such as MI when dead cells are replaced by a dense collagenous scar. Conversely, the epicardial surface can readily accommodate a cardiac patch of clinically relevant size (Fig. 2C). Epicardial cardiac patch placement was found to induce functional improvement post-MI [58, 76], and a recent clinical trial was also initiated wherein hESC-derived CPCs in a fibrin hydrogel were applied during open chest surgery onto the epicardium [56]. Moreover, the epicardium is considered a safe location for patch deployment relative to the endocardium, where the risk of embolization and thrombosis is high (Fig. 2).
A major criticism of epicardial placement is that epicardial cells are not CMs; thus, they can physically separate the graft and host myocardium and hinder electrical coupling. During development, epicardial-derived cells robustly support myocardial growth by providing progenitor cells and mitogens [87, 88]. Postnatally, epicardial cells migrate into the myocardium, especially after injury, to give rise to both ECs and smooth muscle cells of new blood vessels and possibly functional CMs [87, 88]. Given the beneficial role of the epicardium during development and following injury, the way forward may be to harness its power by introducing bioactive peptides into a cardiac patch to mobilize epicardial cells and eliminate the graft-host tissue barrier. For example, thymosin β4 promotes the migration of epicardial cells into the myocardium, and induces both coronary vascularization and CM survival [88]; and follistatin-like 1 was shown to promote integration, limit fibrosis, and improve cardiac function, CM proliferation, and vascularization, when applied via an acellular epicardial patch, but not when it was transgenically overexpressed [87].
Conclusion
If we are to see a meaningful advancement in cardiac cell replacement therapy, a strategy that is suitable for cell delivery in a large number of patients is needed. The vast cell numbers required and the problems with long-term persistence and integration hamper cell injection therapy. Although it is possible that maturation could be induced in the context of single-cell suspensions by manipulating endogenous expression levels [89–91] of key proteins or treating cells with exogenous chemical signals (e.g., growth factors, hormones), these strategies typically induce a higher degree of maturation in the context of 3D ECTs. On the other hand, cardiac patch therapy requires open chest surgery, which excludes a large number of patients who would benefit from such therapy; the risk of a thrombotic event also could deter patients with thrombotic/embolic predispositions. Additionally, standardized protocols for ECT generation and maturation will be required before clinical translation is possible. Given the current state of the art, it seems most likely that a true regenerative therapy will come in the form of a thick, vascularized, adult-like, functional ECT and/or a patch that secretes bioactive molecules to mobilize epicardial cells, delivered by a minimally invasive method to the epicardial surface of the heart. There are obviously numerous challenges that need to be met before this can become a reality, but with the rapid advancements over the last decade, it is only a matter of time before the solutions are found.
Acknowledgments
This work is funded by the Heart and Stoke Foundation (GIA T6946), Canadian Institutes of Health Research Operating Grant MOP-126027, Natural Sciences and Engineering Research Council of Canada Discovery Grant RGPIN 326982-10, and National Institutes of Health Grant 2R01 HL076485. M.R. is supported by a Canada Research Chair (Tier 2) and the Steacie Fellowship.
Author Contributions
N.T.F. and M.R.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
M.R. has compensated intellectual property rights, is a compensated consultant for TARA Biosystems, and has stock options in TARA Biosystems. The other author indicated no potential conflicts of interest.
References
- 1.Mendis S, Davis S, Norrving B. Organizational update: The World Health Organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46:e121–e122. doi: 10.1161/STROKEAHA.115.008097. [DOI] [PubMed] [Google Scholar]
- 2.Reinecke H, Minami E, Zhu WZ, et al. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tous E, Purcell B, Ifkovits JL, et al. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011;4:528–542. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 6.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawad H, Ali NN, Lyon AR, et al. Myocardial tissue engineering: A review. J Tissue Eng Regen Med. 2007;1:327–342. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 8.Eulalio A, Mano M, Dal Ferro M, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 9.Sirish P, López JE, Li N, et al. MicroRNA profiling predicts a variance in the proliferative potential of cardiac progenitor cells derived from neonatal and adult murine hearts. J Mol Cell Cardiol. 2012;52:264–272. doi: 10.1016/j.yjmcc.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porrello ER, Johnson BA, Aurora AB, et al. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hullinger TG, Montgomery RL, Seto AG, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert AD, Diecke S, Chen IY, et al. Reprogramming and transdifferentiation for cardiovascular development and regenerative medicine: Where do we stand? EMBO Mol Med. 2015;7:1090–1103. doi: 10.15252/emmm.201504395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 23.Honda A, Hatori M, Hirose M, et al. Naive-like conversion overcomes the limited differentiation capacity of induced pluripotent stem cells. J Biol Chem. 2013;288:26157–26166. doi: 10.1074/jbc.M113.502492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rais Y, Zviran A, Geula S, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 25.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mummery C, van der Heyden MA, de Boer TP, et al. Cardiomyocytes from human and mouse embryonic stem cells. Methods Mol Med. 2007;140:249–272. doi: 10.1007/978-1-59745-443-8_14. [DOI] [PubMed] [Google Scholar]
- 27.Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Paige SL, Osugi T, Afanasiev OK, et al. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Klos M, Wilson GF, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Synnergren J, Améen C, Jansson A, et al. Global transcriptional profiling reveals similarities and differences between human stem cell-derived cardiomyocyte clusters and heart tissue. Physiol Genomics. 2012;44:245–258. doi: 10.1152/physiolgenomics.00118.2011. [DOI] [PubMed] [Google Scholar]
- 33.Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 34.Robertson C, Tran DD, George SC. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31:829–837. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hara T, Virág L, Varró A, et al. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLOS Comput Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillekamp F, Haustein M, Khalil M, et al. Contractile properties of early human embryonic stem cell-derived cardiomyocytes: Beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy. Stem Cells Dev. 2012;21:2111–2121. doi: 10.1089/scd.2011.0312. [DOI] [PubMed] [Google Scholar]
- 38.Schaaf S, Shibamiya A, Mewe M, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon E, Kong CW, Li RA. Human pluripotent stem cell-based approaches for myocardial repair: From the electrophysiological perspective. Mol Pharm. 2011;8:1495–1504. doi: 10.1021/mp2002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itzhaki I, Schiller J, Beyar R, et al. Calcium handling in embryonic stem cell-derived cardiac myocytes: Of mice and men. Ann N Y Acad Sci. 2006;1080:207–215. doi: 10.1196/annals.1380.017. [DOI] [PubMed] [Google Scholar]
- 41.Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Efe JA, Hilcove S, Kim J, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 44.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Islas JF, Liu Y, Weng KC, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109:13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu JD, Stone NR, Liu L, et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam YJ, Lubczyk C, Bhakta M, et al. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development. 2014;141:4267–4278. doi: 10.1242/dev.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wada R, Muraoka N, Inagawa K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013;110:12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 51.Zangi L, Lui KO, von Gise A, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smart N, Bollini S, Dubé KN, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung ES, Miller L, Patel AN, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: The STOP-HF randomized Phase II trial. Eur Heart J. 2015;36:2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korf-Klingebiel M, Reboll MR, Klede S, et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med. 2015;21:140–149. doi: 10.1038/nm.3778. [DOI] [PubMed] [Google Scholar]
- 55.Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 56.Menasché P, Vanneaux V, Hagège A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 57.Prowse AB, Timmins NE, Yau TM, et al. Transforming the promise of pluripotent stem cell-derived cardiomyocytes to a therapy: challenges and solutions for clinical trials. Can J Cardiol. 2014;30:1335–1349. doi: 10.1016/j.cjca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Ye L, Chang YH, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells [published correction appears in Cell Stem Cell 2015 Jan 8;16:102] Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 60.van Laake LW, Passier R, Doevendans PA, et al. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 61.Gepstein L, Ding C, Rahmutula D, et al. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies [published correction appears in Stem Cells 2011 Sep;29(9):1475] Stem Cells. 2010;28:2151–2161. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- 62.Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nunes SS, Miklas JW, Liu J, et al. Biowire: A platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 65.Schram G, Pourrier M, Melnyk P, et al. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–950. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 66.Yang X, Pabon L, Murry CE. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feric NT, Radisic M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv Drug Deliv Rev. 2016;96:110–134. doi: 10.1016/j.addr.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: State of the art. Circ Res. 2014;114:354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- 69.Sun X, Nunes SS. Overview of hydrogel-based strategies for application in cardiac tissue regeneration. Biomed Mater. 2015;10:034005. doi: 10.1088/1748-6041/10/3/034005. [DOI] [PubMed] [Google Scholar]
- 70.Reis LA, Chiu LL, Feric N, et al. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med. 2016;10:11–28. doi: 10.1002/term.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang B, Xiao Y, Hsieh A, Thavandiran N, Radisic M. Micro- and nanotechnology in cardiovascular tissue engineering. Nanotechnology. 2011;22:494003. doi: 10.1088/0957-4484/22/49/494003. [DOI] [PubMed] [Google Scholar]
- 72.Park H, Larson BL, Guillemette MD, et al. The significance of pore microarchitecture in a multi-layered elastomeric scaffold for contractile cardiac muscle constructs. Biomaterials. 2011;32:1856–1864. doi: 10.1016/j.biomaterials.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nawroth JC, Lee H, Feinberg AW, et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat Biotechnol. 2012;30:792–797. doi: 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liau B, Christoforou N, Leong KW, et al. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32:9180–9187. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 77.Naito H, Melnychenko I, Didié M, et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(suppl):I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 78.Sekine H, Shimizu T, Hobo K, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118(suppl):S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 79.Riegler J, Gillich A, Shen Q, et al. Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function. Circulation. 2014;130(suppl 1):S77–S86. doi: 10.1161/CIRCULATIONAHA.113.007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun X, Altalhi W, Nunes SS. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Adv Drug Deliv Rev. 2016;96:183–194. doi: 10.1016/j.addr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Mohanty S, Larsen LB, Trifol J, et al. Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Mater Sci Eng C. 2015;55:569–578. doi: 10.1016/j.msec.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Zhao X, Liu L, Wang J, et al. In vitro vascularization of a combined system based on a 3D printing technique. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1863. (in press) [DOI] [PubMed] [Google Scholar]
- 83.Masuda S, Shimizu T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv Drug Deliv Rev. 2016;96:103–109. doi: 10.1016/j.addr.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Zhang B, Montgomery M, Davenport-Huyer L, et al. Platform technology for scalable assembly of instantaneously functional mosaic tissues. Sci Adv. 2015;1:e1500423. doi: 10.1126/sciadv.1500423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerbin KA, Yang X, Murry CE, et al. Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS One. 2015;10:e0131446. doi: 10.1371/journal.pone.0131446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandarim-de-Lacerda CA, Meirelles Pereira LM. Numerical density of cardiomyocytes in chronic nitric oxide synthesis inhibition. Pathobiology. 2000;68:36–42. doi: 10.1159/000028113. [DOI] [PubMed] [Google Scholar]
- 87.Wei K, Serpooshan V, Hurtado C, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smart N, Bollini S, Dubé KN, et al. Myocardial regeneration: Expanding the repertoire of thymosin β4 in the ischemic heart. Ann N Y Acad Sci. 2012;1269:92–101. doi: 10.1111/j.1749-6632.2012.06708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bett GC, Kaplan AD, Lis A, et al. Electronic “expression” of the inward rectifier in cardiocytes derived from human-induced pluripotent stem cells. Heart Rhythm. 2013;10:1903–1910. doi: 10.1016/j.hrthm.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fu JD, Rushing SN, Lieu DK, et al. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2011;6:e27417. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lieu DK, Fu JD, Chiamvimonvat N, et al. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol. 2013;6:191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin C-J, Lin C-Y, Chen C-H, et al. Partitioning the heart: Mechanisms of cardiac septation and valve development. Development. 2012;139:3277–3299. doi: 10.1242/dev.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnal-Pastor M, Chachques J-C, Monleón Pradas M, et al. Biomaterials for cardiac tissue engineering. In: Andrades JA, editor. Regenerative Medicine and Tissue Engineering. Rijeka, Croatia: InTech; 2013. [Google Scholar]