Human amniotic epithelial cells possess four unique properties: immune privilege, differentiation ability, an immunomodulatory effect, and a lysosome-rich character. These properties make this cell type a strong candidate for serving as an alternative cell source in patients who require hepatocyte transplantation.
Summary
Stem cell-based therapies hold the potential to alleviate the burden of many serious diseases, including those of the liver. Among the different types of stem cells, human placenta-derived stem cells are potentially one of the most clinically applicable stem cells because of their tissue-specific advantages. They are a readily available cell source that can be procured in a noninvasive manner, and there are few ethical concerns regarding their use. Recent studies have demonstrated that the amniotic epithelium contains stem cells that possess four unique and advantageous properties; human amniotic epithelial cells (hAECs) have low immunogenicity, secrete several immune regulatory molecules, possess the potential to differentiate into all three germ layers, and contain abundant lysosomes allowing them to secrete lysosomal enzymes. This perspective article provides an overview of the beneficial properties of hAECs and proposes a rational strategy for translating placental stem cells toward clinical application for various liver diseases.
Significance
This article provides an overview of the beneficial properties of one type of human placental stem cell and proposes a rational strategy for translating placental stem cells toward clinical application for various liver diseases.
Introduction
Recent advances in stem cell research have raised the hopes for many patients who suffer from various diseases and injuries to different organs, including the liver. As there are many etiologies for liver disease, it is a reasonable approach to identify a suitable stem cell type to rationally treat each disease based on the disease mechanism. This review focuses on a promising placental stem cell type, the human amniotic epithelial stem cell, and its potential clinical use for a variety of liver diseases.
Placenta Tissue-Specific Advantages
Several issues prohibit the immediate clinical application of many stem cell types. Human placentae are routinely discarded after birth, which largely obviates ethical concerns and with approximately 4 million birth in the United States per year, the tissue is readily available. Placental stem cells carry many source-specific advantages as compared with embryonic stem cells, induced pluripotent stem cells, and mesenchymal stem cells. Among these placental stem cells, amniotic epithelial cells are abundant and possess both pluripotent stem cell and mesenchymal stem cell-like characteristics [1, 2]. Despite their pluripotent stem cell-like differentiation potential, human amniotic epithelial cells (hAECs) are not considered tumorigenic because they do not form teratomas when injected into immunodeficient animals [1, 3]. In addition to these research studies, human amnion has been transplanted into volunteers' forearms to test immunogenicity. No serious adverse events, including tumor formation, were reported [4]. The nontumorigenicity of hAEC may be due to the relatively stable genetic status of these cells. The global DNA methylation and histone acetylation statuses of hAEC are intermediate between pluripotent stem cells and somatic cells, which could explain the dichotomous property of hAEC that possess both stem cell-like plasticity (differentiation capability) and genetic stability (nontumorigenic) [5].
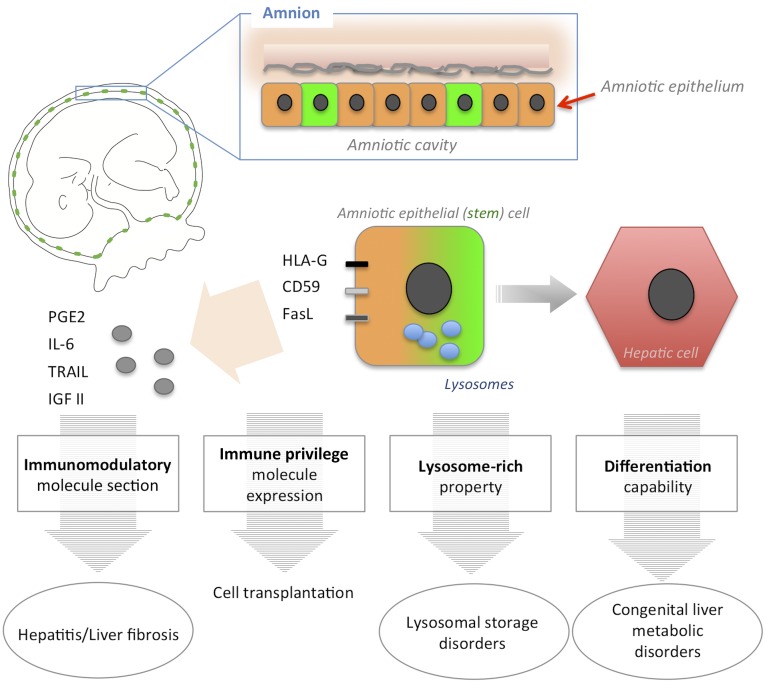
Here, we summarize four unique properties of hAECs in addition to the placenta tissue-specific advantages, which make them advantageous for clinical use: (a) low immunogenicity, (b) immunomodulation, (c) multipotency, and (d) richness in lysosomes (Fig. 1).
Figure 1.
Four unique therapeutic properties of human amniotic epithelial cells. Amniotic epithelium is the innermost layer of the placenta facing the amniotic cavity. Some amniotic epithelial cells express human embryonic stem cell surface markers, such as stage-specific embryonic antigen (SSEA)-3, SSEA-4, tumor rejection antigen 1 (TRA1)-60, and TRA1-81 (depicted in green). Human amniotic epithelial cells possesses four unique properties: immune privilege, differentiation ability, an immunomodulatory effect, and a lysosome-rich character. These properties can be harnessed to treat various liver diseases, such as lysosomal storage disorders, congenital metabolic disorders, and liver fibrosis. Abbreviations: FasL, Fas ligand; HLA-G2, human leukocyte antigen G2; IGF II, insulin-like growth factor II; IL-6, interleukin-6; PGE2, prostaglandin E2; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Immune Privilege
hAECs express low major histocompatibility complex (MHC) class I and lack MHC class II antigens on their surfaces [6, 7], resulting in a low immunogenic profile upon transplantation [8]. Akle et al. reported that an immunotype-mismatched human amniotic membrane did not elicit the host immune system when transplanted under a volunteers’ skin [4]. In contrast to these polymorphic MHC antigen expressions, the hAEC expresses nonpolymorphic, nonclassic human leukocyte antigen G (HLA-G), which is thought to protect the fetus from rejection by maternal natural killer (NK) cells [9, 10]. HLA-G expression increases during the pregnancy and doubles in a term amnion compared with a preterm amnion [11].
The expression of other immunoinhibitory molecules, including CD59 and Fas ligand (FasL), on the hAEC surface has also been reported [12–14]. The CD59 molecule regulates complement-mediated cell lysis by preventing C9 polymerization, a process required for the formation of the complement membrane attack complex. Fas-Fas ligand binding is one of the fundamental immunoregulatory systems in many immune-privilege sites. At the feto-maternal interface (placenta), Fas ligand-expressing cells induce the apoptosis of infiltrating lymphocytes and limit leukocyte trafficking between the mother and the fetus. These anti-T-cell, anti-NK-cell, and complement immune regulatory systems provide an advantage to the hAEC for allogeneic and even xenogeneic transplantation. Kubo et al. transplanted human amnion to both the rat limbal area and under the kidney capsule and found that the xenogeneic (human to rat) immune responses were significantly mitigated [15]. In addition, all HLA-G, CD59, and FasL are secreted from hAECs into amniotic fluid [16, 17]. These soluble factors provide immune tolerance not only to the transplanted hAECs but also to neighboring cells.
Immunomodulatory Effect
hAECs appear to use wider-ranging immunomodulation mechanisms compared with other mesenchymal stem cell types. They secrete soluble HLA-G, which binds to the CD158D and CD8 receptors on NK and T cells and triggers apoptosis [18–22]. Mixed lymphocyte reaction (MLR) assays revealed that hAECs inhibited 66%–93% of lymphocyte activation in a dose-dependent manner [23]. In addition to these soluble forms of cell surface molecules, hAECs secrete other immunosuppressive factors, including prostaglandin E2 (PGE2), insulin-like growth factor II, platelet-derived growth factor, transforming growth factor-β2 (TGF-β2), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [6, 17, 24–28]. hAECs also release anti-inflammatory cytokines and proteins, including interleukin (IL)-1 receptor antagonist protein; tissue inhibitors of metalloproteinase1-1, -2, -3, -4; and IL-10 [29, 30]. These hAEC-derived soluble factors suppress proinflammatory cytokines, regulate macrophage recruitment, and inhibit the chemotactic activity of neutrophils and macrophages [17, 31].
The immunomodulatory effects of hAECs have been demonstrated in disease-specific settings. Human hepatic stellate cell-line (LX-2) cells have been used as an in vitro model to demonstrate the immunomodulatory (antifibrotic) effects of hAECs. Hodge et al. demonstrated that the hAEC culture supernatant suppressed TGF-β1-induced αSMA gene upregulation [32]. They speculated that this antifibrotic effect was mediated by unidentified secreted factors, and they tested possible candidates, including the soluble forms of HLA-G1, PGE2, bone morphogenetic protein-7, IL-10, FasL, and TRAIL. No single factor was sufficient to reproduce the antifibrotic effect of the hAEC culture supernatant [32], which suggested that it is more likely a synergetic effect of multiple factors or a yet-to-be-identified antifibrotic factor. In rodent lung injury models, hAECs also demonstrate immune modulation. Tan et al. showed that transplanted hAECs mediated lung repair by modulating macrophage recruitment and polarization [33]. Further studies revealed that hAECs induced differentiation and proliferation of regulatory T cells that switched macrophages from M1 to M2 phenotype [34]. Although these reports are focused on lung repair, the findings indicate a systemic mechanism of immunomodulation mediated by hAECs.
Differentiation Capability
In vitro studies have shown the differentiation potential of hAECs. Miki et al. induced differentiation to all three germ layers by using exogenous growth factor stimulation protocols [1]. Under each differentiation condition, unfractionated primary hAECs acquired the lineage-specific marker genes and protein expression associated with the ectoderm (neural lineage), endoderm (pancreatic and hepatic lineages), or mesoderm (cardiac lineage). Some of the neural lineage marker genes were expressed before the induction of differentiation. Ilancheran et al. also demonstrated hAEC differentiation into neural, hepatic, pancreatic, cardiac, and mesenchymal lineages [3]. They also observed that primary hAECs stain positive for the antineural marker antibodies: nestin, microtubule-associated protein-2 (MAP2), and glial fibrillary acidic protein. Although a quantitative analysis of the neural marker-positive cells in primary hAECs has not been performed, immunofluorescent images indicate that most primary hAECs express these neural markers. On the other hand, flow cytometric analyses indicate that primary hAECs contain stem cell marker-positive cells with ratios ranging from 5% to 50% (stage-specific embryonic antigen [SSEA]-3 and tumor rejection antigen 1 (TRA1)-60, 5%; TRA1-81, 10%; and SSEA-4, 50%). These data indicate that most primary hAECs are committed or precommitted to the ectodermal lineage, with some of the cells retaining stem cell characteristics at different developmental stages. Three possibilities may explain the origin of endoderm and mesoderm lineage-committed cells: (a) A small population of stem cell marker-positive cells differentiate toward the two lineages, (b) the ectoderm lineage-committed cells transdifferentiate into other lineages, or (c) a small number of other lineage-committed cells also exist in the primary hAECs.
Regardless of the lineage commitment of primary hAECs, the plasticity of the hAECs has attracted many researchers trying to identify an appropriate stem cell type for clinical translation. In several studies, researchers have induced hAEC differentiation into specific cell types, including hepatocytes. Takashima et al. detected albumin (ALB) and α-1 antitrypsin (A1AT) mRNA expression in primary hAECs and successfully induced α-fetoprotein and transthyretin with oncostatin M or hepatocyte growth factor supplementation [35]. However, late-phase hepatic differentiation markers, including ornithine transcarbamylase (OTC) and glucose 6-phosphatase (G6Pase), were not induced under these culture conditions [35]. Marongiu et al. cultured hAECs on porcine liver-derived extracellular matrices and successfully induced expression of ALB, A1AT, cytochrome P450s, and asialoglycoprotein receptor 1 [36]. The report also demonstrated in vivo hepatic differentiation of unfractionated primary hAECs into mature hepatocytes upon transplantation into immunodeficient (severe combined immunodeficiency/beige) mouse livers. Six months after transplantation, hAEC engraftment was confirmed by the detection of human DNA. The terminal hepatic differentiation of hAECs was determined by quantitative real-time polymerase chain reaction for nine cytochrome P450s, five metabolic enzymes, two plasma proteins, five hepatocyte-specific transcription factors, and three transporter genes expressions. Late-phase hepatic differentiation markers, including OTC and G6Pase, were expressed in the engrafted hAECs at a level similar to that of human adult hepatocytes. These data indicate that hAECs can differentiate into fully functional hepatocytes under appropriate conditions, such as those present in the mouse liver environment.
Lysosome-Rich Characteristics
In addition to their unique immunological and developmental properties, hAECs are lysosome-rich, with relatively high levels of lysosomal enzyme activity. Recent findings suggest this high lysosome activity is related to the active autophagy process that occurs during placentation [37]. In 1991, Scaggiante et al. reported that hAECs released substantial amounts of some lysosomal hydrolases, including sphingomyelinase, N-acetyl-β-glucosaminidase, α-fucosidase, β-glucuronidase, α-mannosidase, and arylsulfatase [38]. These observations point to the potential usefulness of hAECs for the treatment of patients with lysosomal storage diseases.
Cell Therapy Approaches and Target Diseases
Human hepatocyte transplantations have demonstrated therapeutic efficacy in many congenital metabolic diseases and in decompensated liver cirrhosis. This cell replacement approach aims to compensate for the missing or impaired hepatic enzyme functions. The properties of hAECs make them an attractive alternative cell source for hepatocyte transplantation. Therapeutic applications for liver diseases can be categorized in three strategic approaches. One approach takes advantage of the rich-lysosome contents of the hAECs to treat patients with lysosome storage disorders as a novel type of enzyme replacement therapy. Second, the differentiation potential of hAECs to generate functional hepatic cells can be used to compensate for multiple causes of metabolic dysfunction. Finally, the immunomodulatory property of hAECs can be applied to treat various liver injuries, such as inflammation and fibrosis.
Numerous preclinical studies have already demonstrated the promising therapeutic value of these unique properties of hAECs. Here, we categorize and summarize some of these landmark studies.
Lysosomal Storage Disorders
A consequence of a single enzyme deficiency, lysosomal storage disorders (LSDs) result in the accumulation of cellular materials within lysosomes. There are more than 50 known LSDs, but the current standard of treatment is mainly supportive care and symptomatic treatment. Bone marrow transplantation has produced some success in treating certain types of LSDs. Primary hAECs contain 17 lysosomal enzymes, and the activities of 7 of these enzymes are higher than in hematopoietic cells. In several clinical trials in the 1980s, surgeons subcutaneously implanted amniotic membrane or hAECs into LSD patients [39–42]. The clinical outcomes were varied, probably because of patient selection (age, clinical advancement, and target disease). Consistent with recent findings from clinical bone marrow transplantation, amniotic membrane or hAEC transplantations showed some efficacy in treating mucopolysaccharidoses and Niemann-Pick disease [39, 41, 43]. Recently, the therapeutic efficacy of hAEC transplantation has been validated in murine disease models [44, 45]. These findings suggest that hAEC transplantation for LSDs should be revisited with the latest technologies and evidence-based patient selection.
Congenital Liver Metabolic Disorders
The hepatic differentiation potential of hAECs suggests usefulness in cell therapies for congenital liver metabolic disorders (CLMD). Approximately 1 in 1,500 children is born with a metabolic disorder, and many of these critical inborn errors of metabolic or synthetic processes principally involve the liver. Current therapy for CLMDs consists of life-long dietary restriction with or without supplementation of amino acids. Liver organ transplantation can improve outcomes; however, the invasiveness of this procedure limits its use until the disease becomes life-threatening. An alternative to organ transplantation, hepatocyte transplantation (HTx), is considered a reasonable approach to treating these diseases. HTx is a less invasive procedure with lower associated morbidity, fewer complications, and significantly reduced recovery time and costs. More than 50 cases of clinical HTx have been reported and have demonstrated therapeutic efficacy [46, 47]. The rationale for this cell replacement approach is that the diseased hepatocytes are, in most cases, lacking only one enzymatic activity and are otherwise functional. Many CLMDs consist of various phenotypes, and the clinical data indicate that patients with low levels of functioning enzymes (mild phenotype) survive and can live a regular life.
This finding suggests that partial cell replacement could compensate for the genetically lacking enzyme function. For example, an infusion of normal hepatocytes equivalent to 5% of the parenchymal mass achieved a medium-term reduction in serum bilirubin in a patient with Crigler-Najjar syndrome [48]. An OTC-deficient child who received 1.9 × 109 hepatocytes had a subsequent normalization of plasma ammonia and glutamine levels on a normal diet without phenylbutyrate/phenylacetate therapy [49]. These cases clearly demonstrate that CLMD can be effectively treated via partial cell replacement therapy.
Because the goal of this approach is to compensate for a single hepatic enzyme function, the therapeutic cell does not need to be a fully functional hepatocyte. Rather, it only needs to possess the missing enzyme function. Currently, a shortage of donor hepatocytes prohibits the extensive clinical application of HTx, making hAEC-derived hepatic cells an attractive alternative source for clinical HTx. One of the most astonishing preclinical studies was conducted in a mouse model of maple syrup urine disease [50, 51]. While all control (untreated) animals died before 28 days of age, 9 of 11 animals (82%) that underwent transplantation with primary hAECs survived more than 100 days, demonstrated normal weight gain and activity, and showed no gross symptoms of the disease. Although engrafted and differentiated hAECs in the recipient liver were not directly identified by histologic assessment, approximately 5% of the DNA in the recipient mouse livers was of human origin. This preclinical study indicates that the hAEC transplantation does not necessarily require in vitro cell expansion or differentiation before transplantation, supporting the possibility of protocols requiring minimal cell manipulation for future clinical translation [52].
Liver Fibrosis
Most liver diseases are caused by a variety of liver cell injuries and, regardless of the cause, progress from inflammation (hepatitis) to fibrosis. The pathogenic mechanisms of inflammation and fibrosis can both be addressed by the immunomodulatory (anti-inflammation and antifibrotic) properties of hAECs. As described in the section titled "Immunomodulatory Effect," an MLR assay demonstrated that hAECs directly attenuate the lymphocyte response and hAEC-conditioned medium is able to suppress hepatic stellate cell activation [32].
Several animal models of liver cirrhosis, using chemically or surgically induced liver fibrosis, have demonstrated the therapeutic efficacy of hAECs. Parolini’s group applied the human amnion membrane as a patch on the liver surface of bile duct ligation model rats [53, 54]. Despite the xenogeneic conditions (human-rat), the human amnion membrane patch modulated the severity and progression of fibrosis. Manuelpillai et al. demonstrated that systemic hAEC transplantation improved carbon tetrachloride-induced mouse liver fibrosis [55, 56]. The xenogeneic (human-mouse) cell transplantation induced a significant reduction of activated hepatic stellate cells in the host liver. The monocyte chemoattractant protein-1 level was also decreased, and M2 macrophage-associated genes, including YM-1, IL-10, and CD206, were upregulated in the hAEC-transplanted mouse liver. These observations indicate that the hAEC-mediated antifibrotic action might be a synergetic effect of both soluble factors and cell-to-cell interactions.
Conclusion
hAECs possess four unique properties: immune privilege, differentiation ability, an immunomodulatory effect, and a lysosome-rich character. These properties make this cell type a strong candidate for serving as an alternative cell source in patients who require hepatocyte transplantation. Before the clinical use of hAECs, the appropriate cell dose and timing of transplantation must be defined with stringent evaluation. As of today, there are no registered clinical trials with the aim of treating liver disease using hAECs. However, more than 30 ongoing mesenchymal stem cell clinical trials are being conducted in the setting of liver diseases. All of these trials are designed to identify an immunomodulatory effect of the mesenchymal stem cells in liver cirrhosis (73.5%) or organ/islet transplantation (26.5%). One clinical trial is using transplanted hAECs to treat patients with persistent corneal epithelial defect (NCT00344708). Although the expected therapeutic mechanism of hAEC transplantation is not related to liver disease, this clinical trial endorses the safety of the use of hAEC for future clinical trials in the setting of liver diseases. On the basis of the trend of these clinical trials, once a therapeutic mechanism and logically suitable target diseases are clarified, the clinical application of hAECs for liver diseases is a feasible approach.
Acknowledgments
I thank Dr. Brendan Grubbs for his insightful critiques and Cristy M. Lytal and Jennifer Izumi Divine for their editorial support during preparation of the manuscript. This work was supported by California Institute for Regenerative Medicine Grant TR3-05488.
Disclosure of Potential Conflicts of Interest
T.M. owns stock in Stemnion, Inc.
References
- 1.Miki T, Lehmann T, Cai H, et al. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 2.Miki T. Amnion-derived stem cells: In quest of clinical applications. Stem Cell Res Ther. 2011;2:25. doi: 10.1186/scrt66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilancheran S, Michalska A, Peh G, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 4.Akle CA, Adinolfi M, Welsh KI, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 5.Easley CA, 4th, Miki T, Castro CA, et al. Human amniotic epithelial cells are reprogrammed more efficiently by induced pluripotency than adult fibroblasts. Cell Reprogram. 2012;14:193–203. doi: 10.1089/cell.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori J, Wang M, Kamiya K, et al. Immunological characteristics of amniotic epithelium. Cornea. 2006;25(suppl 1):S53–S58. doi: 10.1097/01.ico.0000247214.31757.5c. [DOI] [PubMed] [Google Scholar]
- 7.Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 8.Hammer A, Hutter H, Blaschitz A, et al. Amnion epithelial cells, in contrast to trophoblast cells, express all classical HLA class I molecules together with HLA-G. Am J Reprod Immunol. 1997;37:161–171. doi: 10.1111/j.1600-0897.1997.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre S, Adrian F, Moreau P, et al. Modulation of HLA-G expression in human thymic and amniotic epithelial cells. Hum Immunol. 2000;61:1095–1101. doi: 10.1016/s0198-8859(00)00192-0. [DOI] [PubMed] [Google Scholar]
- 10.Pazmany L, Mandelboim O, Valés-Gómez M, et al. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science. 1996;274:792–795. doi: 10.1126/science.274.5288.792. [DOI] [PubMed] [Google Scholar]
- 11.Lim R, Chan ST, Tan JL, et al. Preterm human amnion epithelial cells have limited reparative potential. Placenta. 2013;34:486–492. doi: 10.1016/j.placenta.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Rooney IA, Morgan BP. Characterization of the membrane attack complex inhibitory protein CD59 antigen on human amniotic cells and in amniotic fluid. Immunology. 1992;76:541–547. [PMC free article] [PubMed] [Google Scholar]
- 13.Runic R, Lockwood CJ, Ma Y, et al. Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J Clin Endocrinol Metab. 1996;81:3119–3122. doi: 10.1210/jcem.81.8.8768884. [DOI] [PubMed] [Google Scholar]
- 14.Harirah HM, Donia SE, Parkash V, et al. Localization of the Fas-Fas ligand system in human fetal membranes. J Reprod Med. 2002;47:611–616. [PubMed] [Google Scholar]
- 15.Kubo M, Sonoda Y, Muramatsu R, et al. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–1546. [PubMed] [Google Scholar]
- 16.Hsu CD, Hong SF, Harirah H, et al. Amniotic fluid soluble fas levels in intra-amniotic infection. Obstet Gynecol. 2000;95:667–670. doi: 10.1016/s0029-7844(99)00664-x. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Niederkorn JY, Neelam SS, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:900–907. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 18.Sanders SK, Giblin PA, Kavathas P. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class 1 molecule on cytotrophoblasts. J Exp Med. 1991;174:737–740. doi: 10.1084/jem.174.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(suppl):S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Banas RA, Trumpower C, Bentlejewski C, et al. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008;69:321–328. doi: 10.1016/j.humimm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Rebmann V, Pfeiffer K, Pässler M, et al. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53:14–22. doi: 10.1034/j.1399-0039.1999.530102.x. [DOI] [PubMed] [Google Scholar]
- 22.Robert-Gangneux F, Gangneux JP, Vu N, et al. High level of soluble HLA-G in amniotic fluid is correlated with congenital transmission of Toxoplasma gondii. Clin Immunol. 2011;138:129–134. doi: 10.1016/j.clim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya K, Wang M, Uchida S, et al. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005;80:671–679. doi: 10.1016/j.exer.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Wichayacoop T, Briksawan P, Tuntivanich P, et al. Anti-inflammatory effects of topical supernatant from human amniotic membrane cell culture on canine deep corneal ulcer after human amniotic membrane transplantation. Vet Ophthalmol. 2009;12:28–35. doi: 10.1111/j.1463-5224.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu YH, Vaghjiani V, Tee JY, et al. Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One. 2012;7:e35758. doi: 10.1371/journal.pone.0035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar LK, Reiny R, Yamamoto SY, et al. Relaxin causes proliferation of human amniotic epithelium by stimulation of insulin-like growth factor-II. Am J Obstet Gynecol. 2003;188:234–241. doi: 10.1067/mob.2003.80. [DOI] [PubMed] [Google Scholar]
- 28.Steed DL, Trumpower C, Duffy D, et al. Amnion-derived cellular cytokine solution: A physiological combination of cytokines for wound healing. Eplasty. 2008;8:e18. [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Y, Ma DH, Hwang DG, et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Koh JW, Shin YJ, Oh JY, et al. The expression of TIMPs in cryo-preserved and freeze-dried amniotic membrane. Curr Eye Res. 2007;32:611–616. doi: 10.1080/02713680701459441. [DOI] [PubMed] [Google Scholar]
- 31.Franz MG, Payne WG, Xing L, et al. The use of amnion-derived cellular cytokine solution to improve healing in acute and chronic wound models. Eplasty. 2008;8:e21. [PMC free article] [PubMed] [Google Scholar]
- 32.Hodge A, Lourensz D, Vaghjiani V, et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy. 2014;16:1132–1144. doi: 10.1016/j.jcyt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Tan JL, Chan ST, Wallace EM, et al. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 2014;23:319–218. doi: 10.3727/096368912X661409. [DOI] [PubMed] [Google Scholar]
- 34.Tan JL, Chan ST, Lo CY, et al. Amnion cell-mediated immune modulation following bleomycin challenge: Controlling the regulatory T cell response. Stem Cell Res Ther. 2015;6:8. doi: 10.1186/scrt542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takashima S, Ise H, Zhao P, et al. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct Funct. 2004;29:73–84. doi: 10.1247/csf.29.73. [DOI] [PubMed] [Google Scholar]
- 36.Marongiu F, Gramignoli R, Dorko K, et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53:1719–1729. doi: 10.1002/hep.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito S, Nakashima A. Review: The role of autophagy in extravillous trophoblast function under hypoxia. Placenta. 2013;34(suppl):S79–S84. doi: 10.1016/j.placenta.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 38.Scaggiante B, Comelli M, Romeo D. Secretion of lysosomal hydrolases by cultured human amnion epithelial cells. Exp Cell Res. 1991;195:194–198. doi: 10.1016/0014-4827(91)90516-w. [DOI] [PubMed] [Google Scholar]
- 39.Scaggiante B, Pineschi A, Sustersich M, et al. Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation. 1987;44:59–61. doi: 10.1097/00007890-198707000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Yeager AM, Singer HS, Buck JR, et al. A therapeutic trial of amniotic epithelial cell implantation in patients with lysosomal storage diseases. Am J Med Genet. 1985;22:347–355. doi: 10.1002/ajmg.1320220219. [DOI] [PubMed] [Google Scholar]
- 41.Akle C, McColl I, Dean M, et al. Transplantation of amniotic epithelial membranes in patients with mucopolysaccharidoses. Exp Clin Immunogenet. 1985;2:43–48. [PubMed] [Google Scholar]
- 42.Sakuragawa N, Yoshikawa H, Sasaki M. Amniotic tissue transplantation: Clinical and biochemical evaluations for some lysosomal storage diseases. Brain Dev. 1992;14:7–11. doi: 10.1016/s0387-7604(12)80272-5. [DOI] [PubMed] [Google Scholar]
- 43.Bembi B, Comelli M, Scaggiante B, et al. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am J Med Genet. 1992;44:527–533. doi: 10.1002/ajmg.1320440430. [DOI] [PubMed] [Google Scholar]
- 44.Hong S-B, Seo M-S, Park S-B, et al. Therapeutic effects of human amniotic epithelial stem cells in Niemann-Pick type C1 mice. Cytotherapy. 2012;14:630–638. doi: 10.3109/14653249.2012.663485. [DOI] [PubMed] [Google Scholar]
- 45.Kosuga M, Sasaki K, Tanabe A, et al. Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice. Mol Ther. 2001;3:139–148. doi: 10.1006/mthe.2000.0234. [DOI] [PubMed] [Google Scholar]
- 46.Strom SC, Bruzzone P, Cai H, et al. Hepatocyte transplantation: Clinical experience and potential for future use. Cell Transplant. 2006;15(suppl 1):S105–S110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 47.Dhawan A, Puppi J, Hughes RD, et al. Human hepatocyte transplantation: Current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–298. doi: 10.1038/nrgastro.2010.44. [DOI] [PubMed] [Google Scholar]
- 48.Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 49.Mitry RR, Dhawan A, Hughes RD, et al. One liver, three recipients: Segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77:1614–1616. doi: 10.1097/01.tp.0000122224.98318.19. [DOI] [PubMed] [Google Scholar]
- 50.Skvorak KJ, Dorko K, Marongiu F, et al. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology. 2013;57:1017–1023. doi: 10.1002/hep.26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skvorak KJ, Dorko K, Marongiu F, et al. Improved amino acid, bioenergetic metabolite and neurotransmitter profiles following human amnion epithelial cell transplant in intermediate maple syrup urine disease mice. Mol Genet Metab. 2013;109:132–138. doi: 10.1016/j.ymgme.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Strom SC, Skvorak K, Gramignoli R, et al. Translation of amnion stem cells to the clinic. Stem Cells Dev. 2013;22(suppl 1):96–102. doi: 10.1089/scd.2013.0391. [DOI] [PubMed] [Google Scholar]
- 53.Ricci E, Vanosi G, Lindenmair A, et al. Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank. 2013;14:475–488. doi: 10.1007/s10561-012-9337-x. [DOI] [PubMed] [Google Scholar]
- 54.Sant’Anna LB, Cargnoni A, Ressel L, et al. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant. 2011;20:441–453. doi: 10.3727/096368910X522252. [DOI] [PubMed] [Google Scholar]
- 55.Manuelpillai U, Tchongue J, Lourensz D, et al. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl₄-treated mice. Cell Transplant. 2010;19:1157–1168. doi: 10.3727/096368910X504496. [DOI] [PubMed] [Google Scholar]
- 56.Manuelpillai U, Lourensz D, Vaghjiani V, et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One. 2012;7:e38631. doi: 10.1371/journal.pone.0038631. [DOI] [PMC free article] [PubMed] [Google Scholar]