Abstract
Purpose
Histone deacetylase inhibitors (HDACis) have been shown to overcome resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) linked to epigenetic changes and epithelial-mesenchymal transition (EMT) state. This randomized phase II study evaluated the outcome of erlotinib with and without the isoform selective HDACi, entinostat.
Patients and Methods
Previously treated patients with stage IIIB/IV non–small-cell lung cancer, no prior EGFR-TKIs, and performance status ≤ 2 were randomly administered erlotinib 150 mg on days 1 through 28 plus entinostat 10 mg orally on days 1 and 15 every 28 days (EE) or erlotinib plus placebo (EP). The primary end point was 4-month progression-free survival (PFS) rate with additional end points including 6-month PFS rate, PFS, and overall survival (OS). Exploratory analyses included EMT- and EGFR-related biomarker analysis on archival tissue.
Results
One hundred thirty-two patients were enrolled (EE, 67; EP, 65). The 4-month PFS rate was comparable for both groups (EE, 18% v EP, 20%; P = .7). In the subset of patients with high E-cadherin levels, OS was longer in the EE group compared with the EP group (9.4 v 5.4 months; hazard ratio, 0.35; 95% CI, 0.13 to 0.92; P = .03) with a corresponding trend toward increased PFS. The adverse event (AE) profile was acceptable, with rash, fatigue, diarrhea, and nausea the most common AEs in both groups.
Conclusion
Erlotinib combined with entinostat did not improve the outcomes of patients in the overall study population when compared with erlotinib monotherapy. High E-cadherin expression levels at time of diagnosis indicate an increased sensitivity to HDACi/EGFR-TKI inhibition providing the basis for a biomarker-driven validation study.
INTRODUCTION
Despite the more recent successful development of therapies targeting oncogenic drivers of non–small-cell lung cancer (NSCLC), the outcome for patients with advanced NSCLC remains dismal with a 5-year survival rate of 1% for stage IV disease (40% of newly diagnosed patients). Both de novo and acquired resistance to targeted therapies limit the duration of their clinical benefit. Erlotinib is an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) approved for the treatment of second- and third-line advanced NSCLC and in the maintenance setting.1,2 Resistance to EGFR-TKI is multifactorial and involves genetic and epigenetic mechanisms that provide opportunities for therapeutic intervention. We and others previously showed that activity of EGFR-TKIs correlates with markers of epithelial-mesenchymal transition status such that higher E-cadherin (epithelial) levels indicate sensitivity; whereas higher vimentin and ZEB-1 levels (both mesenchymal) indicate resistance.3–5 Introduction of E-cadherin or induction of endogenous E-cadherin expression in EGFR-TKI–resistant NSCLC cells sensitized cells to EGFR-TKIs.6 Combining EGFR-TKIs with approaches that lead to induced E-cadherin levels and an epithelial cell phenotype may be expected to delay and/or prevent the emergence of EGFR-TKI drug tolerance or resistance. Preclinical data indicate that entinostat can delay as well as reverse resistance to EGFR-TKI therapy in NSCLC by inhibiting epigenetic modifications leading to drug tolerance as well as reverting the cancer cell phenotype from a resistant mesenchymal to a sensitive epithelial one.6,7
Based on these findings, a randomized, placebo-controlled, phase II study of erlotinib with and without entinostat in patients with advanced stage NSCLC whose disease progressed on prior treatment was carried out. Exploratory analysis was planned to evaluate the relationship between levels of E-cadherin expression in patients' diagnostic samples with clinical outcome and investigational treatment.
PATIENTS AND METHODS
Patients
Patients older than 18 years were eligible if they were histologically or cytologically confirmed to have stage IIIB or stage IV NSCLC, had received one or two previous chemotherapy or chemoradiotherapy regimens for advanced NSCLC and their disease had progressed based on radiologic evidence, had at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors criteria, and their disease had an Eastern Cooperative Oncology Group performance status rating of ≤ 2. Exclusion criteria included previous stem-cell transplantation; clinical evidence of CNS metastases that were untreated or unstable; previous treatment with a histone deacetylase inhibitor or EGFR-TKI; concurrent anticancer therapy; or any other coexisting malignancies or malignancies diagnosed within the last 5 years other than basal cell carcinoma, squamous cell skin carcinoma, papillary thyroid cancer, carcinoma in situ of the bladder, or cervical cancer in situ. All patients provided written, informed consent, and approval for the study was obtained from independent ethics committees.
Study Design and Treatment
Patients were randomly assigned in a blinded 1:1 ratio to receive erlotinib (150 mg/d orally) with entinostat (10 mg orally) or matching placebo administered on days 1 and 15 of a 28-day cycle. The randomization schedule was prepared using blocks of size 4 and was stratified according to the patients' smoking status at the time of enrollment (current or previous smokers v never smokers). Patients could receive up to six cycles of therapy. Treatment could be discontinued early in the event of unacceptable or intolerable toxicity, evidence of progressive disease, or patient withdrawal. Before unblinding at the time of progression, patients who received erlotinib plus placebo and whose disease had progressed were given the option of crossing over to combination therapy (erlotinib plus entinostat) in an open-label extension. Patients in the active arm whose disease did not progress after six cycles of treatment had the option of continuing the combination therapy in an open-labeled extension of the protocol. Dose interruptions of entinostat of up to 14 days were allowed. The dose of erlotinib could be adjusted according to prescribing guidelines.
Efficacy
The primary efficacy end point was progression-free survival (PFS) at 4 months. Secondary end points were PFS at 6 months and overall best objective response. Planned exploratory end points were PFS, overall survival (OS), and biologic correlates including E-cadherin protein expression, Kirsten rat sarcoma viral oncogene homolog (KRAS), and EGFR gene mutation rates. Objective tumor response was assessed every 8 weeks until radiologic evidence of progression using Response Evaluation Criteria in Solid Tumors criteria (Version 1.0) was reported. Adverse events (AEs) per the Common Terminology Criteria for Adverse Events of the National Cancer Institute (version 3.0) were assessed at each every-other-week visit.
Molecular Analysis
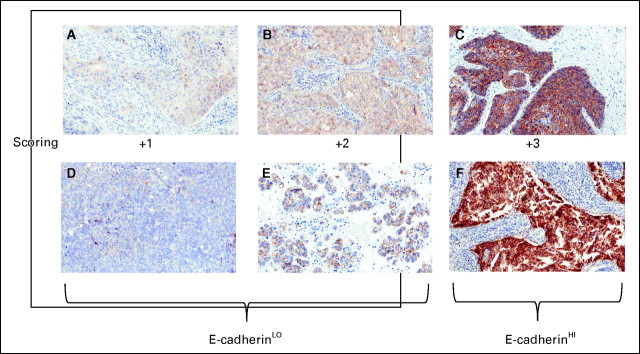
Formalin-fixed, paraffin-embedded tumor specimens taken at the time of diagnosis were collected from patients enrolled onto the trial. Gene sequencing was carried out at Transgenomic (Kansas), E-cadherin immunohistochemistry at Targeted Molecular Diagnostics (now Quintiles, Chicago, IL) with independent slide review at University of Colorado, and EGFR FISH at University of Colorado. Samples harboring EGFR exon 19 deletions and/or L858R mutations were classified as EGFR mutation–positive. Samples with KRAS mutations in codons 12 and 13 were classified as KRAS mutation–positive. Samples with E-cadherin immunohistochemistry staining intensity scores of 0, +1 (< 50% of the cells have complete circumferential membrane staining at a low intensity), and +2 (≥ 50% of the cells have complete circumferential membrane staining at a moderate intensity) were classified as E-cadherinLO. Those with +3 (> 90% of the cells have complete circumferential membrane staining at a high intensity) were classified as E-cadherinHI. Independent hybrid scoring (H-score) algorithm (0-300) defined as intensity score (0-3) % times % positive cells (0-100) provided additional classification of samples. H-scores were determined for each sample, pooled independently of treatment arm, and a median score of 150 was derived. An H-score of at least 150 was assigned to E-cadherinH-LO and an H-score of more than 150 was assigned to E-cadherinH-HI. EGFR FISH positivity was assigned to samples with high polysomy or gene amplification as defined previously.8
Statistical Analysis
The efficacy analysis was performed in accordance with the intention-to-treat principle. All patients randomly assigned in the study were included in the efficacy analysis according to their randomized treatment group assignment, irrespective of the actual receipt of such treatment. Additionally, follow-up of individual patients was to continue until resolution of each efficacy end point, even if study treatment was modified or discontinued. All patients who received at least one dose of study treatment were considered evaluable for safety analysis.
The sample size was determined assuming that the 4-month PFS rate would be 35% for erlotinib monotherapy and 55% for erlotinib plus entinostat. With a sample size of 55 patients per treatment group, the study had 80% power to detect such a difference in the 4-month PFS rate with two-sample χ2 test and one-sided significance level of 10%. The 4-month PFS rate was estimated based on the proportion of all randomly assigned patients who were alive without documented disease progression at 4 months after the randomization date. For this analysis, disease progression was based on radiologic and/or clinical evaluation. An approximate two-sided 95% CI was constructed for the true 4-month PFS rate. The primary inferential comparison between treatment groups was made using the Cochrane-Mantel-Haenszel χ2 test, stratified by the randomization stratification factor for smoking status. The 6-month PFS rate was calculated similarly.
For this phase II study, PFS and OS were defined as exploratory efficacy end points. Both end points were measured relative to the date of randomization. The duration of PFS was right-censored for patients who met one of the following conditions: (1) no baseline disease assessments; (2) nonprotocol systemic anticancer treatment started before documentation of disease progression; (3) death or disease progression after more than one missed disease assessment visit; or (4) alive and did not have documentation of disease progression before the data analysis cutoff date. For such patients, PFS was right-censored according to standard convention. The outcomes for both end points were summarized descriptively using the Kaplan-Meier method. Comparison between groups was made using the stratified log-rank test. Hazard ratios for treatment groups were estimated using a stratified Cox proportional hazards model.
The efficacy analyses described in the Efficacy section for the overall study population were also performed for subgroups of patients defined by baseline expression levels for selected investigational biomarkers of response, such as E-cadherin expression. The P values reported from these analyses were not adjusted for multiple testing. As such, the causation for any observed difference between treatment groups for PFS and/or OS within a subgroup should not be attributed to the study treatment.
RESULTS
Patient Characteristics
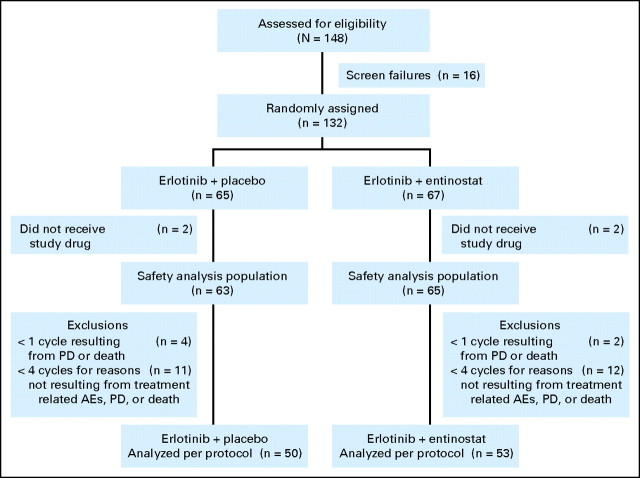
The study was conducted at 30 US Oncology study sites; 23 of which enrolled at least one patient. Enrollment began in September 2008 and was completed in July 2009. One hundred thirty-two patients were randomly assigned; 65 patients were assigned to the erlotinib-placebo (EP) arm and 67 patients to the erlotinib-entinostat (EE) arm (Fig 1). With the exception of four patients (two in each treatment group), all patients received at least one dose of study treatment. Table 1 lists baseline characteristics of all eligible patients. Treatment groups were well balanced for baseline factors evaluated with slightly more female, adenocarcinoma, and second-line–treated patients in the EE arm. Most patients were male, current or previous smokers, and had a histologic diagnosis of adenocarcinoma. In both treatment groups, patients received a median of two cycles of study treatment, which was discontinued in the majority of cases because of disease progression. At documented disease progression within six cycles of treatment, patients randomly assigned to the EP arm were eligible to cross over for treatment with erlotinib and entinostat. Sixteen patients opted to cross over. Their best overall response was five patients (31%) with stable disease of more than 60 days; two patients (13%) with stable disease for less than 60 days; four patients (25%) with progressive disease, and five patients (31%) who were not evaluable. Patients treated with erlotinib and entinostat who did not have progressive disease after six cycles of treatment (n = 8) were eligible to continue treatment on an open-label extension of the protocol.
Fig 1.
CONSORT diagram describing numbers and treatment of non–small-cell lung cancer patients enrolled in ENCORE–401 (Entinostat Combinations Overcoming Resistance–401). AE, adverse event; PD, progressive disease.
Table 1.
Demographics and Baseline Disease Characteristics (all patients randomly assigned)
| Parameter/Statistic | Erlotinib + Placebo (n = 65) |
Erlotinib + Entinostat (n = 67) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 67 | 66 | ||
| < 65 | 27 | 42 | 27 | 40 |
| ≥ 65 | 38 | 58 | 40 | 60 |
| Sex | ||||
| Male | 43 | 66 | 39 | 58 |
| Female | 22 | 34 | 28 | 42 |
| Race | ||||
| White | 55 | 85 | 55 | 82 |
| Asian | 2 | 3 | 1 | 2 |
| Black/African American | 6 | 9 | 5 | 8 |
| Other | 2 | 3 | 6 | 9 |
| ECOG performance status | ||||
| 0 | 22 | 34 | 29 | 43 |
| 1 | 34 | 52 | 30 | 45 |
| 2 | 9 | 14 | 8 | 12 |
| Pathologic subtype | ||||
| Adenocarcinoma | 28 | 43 | 39 | 58 |
| Squamous cell carcinoma | 21 | 32 | 18 | 27 |
| Other | 16 | 25 | 10 | 15 |
| Treatment setting for prior therapy* | ||||
| One prior therapy | 64 | 99 | 66 | 99 |
| More than one prior therapy | 27 | 42 | 33 | 48 |
| Time since initial diagnosis | ||||
| Median | 1.0 | 1.0 | ||
| < 1 year | 29 | 45 | 32 | 48 |
| ≥ 1 year | 36 | 55 | 35 | 52 |
| Best response to regimen received before study | ||||
| Complete or partial response | 21 | 32 | 14 | 21 |
| Stable disease | 12 | 19 | 18 | 27 |
| Progressive disease | 32 | 49 | 35 | 52 |
| Smoking status | ||||
| Ever | 54 | 83 | 56 | 84 |
| Never | 11 | 17 | 11 | 16 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Patients may be counted in more than one row.
Efficacy
In the intent-to-treat analysis of the overall study population, the primary end point of a PFS rate at 4 months did not significantly differ between the EP and EE treatment groups (20% v 18%; respectively; Table 2). Secondary end points of a 6-month PFS rate, PFS (Fig 2A), overall survival (OS; Fig 2B), and objective response rate were likewise not significantly different.
Table 2.
Summary of Clinical Outcomes (all patients randomly assigned)
| Treatment Arm | Full Analysis Set–ITT |
Per Protocol Set |
||
|---|---|---|---|---|
| Erlotinib + Placebo (%; n = 65) | Erlotinib + Entinostat (%; n = 67) | Erlotinib + Placebo (%; n = 50) | Erlotinib + Entinostat (%; n = 53) | |
| 4-mo PFS rate | 20 | 17.9 | 24 | 20.8 |
| Odds ratio* | 0.84 | 0.72 | ||
| 95% CI | 0.34 to 2.04 | 0.27 to 1.89 | ||
| P, CMH stratified test | .70 | .51 | ||
| 6-mo PFS rate | 10.8 | 11.9 | 14 | 15.1 |
| Odds ratio* | 1.06 | 0.89 | ||
| 95% CI | 0.34 to 3.27 | 0.28 to 2.84 | ||
| P, CMH stratified test | .92 | .85 | ||
| PFS, months | ||||
| Median | 1.88 | 1.97 | 1.94 | 2.34 |
| Hazard ratio† | 0.99 | 1.07 | ||
| 95% CI | 0.68 to 1.44 | 0.7 to 1.64 | ||
| P, stratified log-rank test | .98 | .73 | ||
| Objective response rate | 9.2 | 3.0 | 12 | 3.8 |
| Odds ratio* | 0.31 | 0.30 | ||
| 95% CI | 0.06 to 1.54 | 0.06 to 1.45 | ||
| P, CMH stratified test | .13 | .11 | ||
| Overall survival, months | ||||
| Median | 6.7 | 8.9 | 7.7 | 9.3 |
| Hazard ratio† | 0.85 | 0.89 | ||
| 95% CI | 0.59 to 1.23 | 0.58 to 1.36 | ||
| P, stratified log-rank test | .39 | .59 | ||
NOTE. Hazard ratios and odds ratios adjusted for patient smoking status (randomization stratification).
Abbreviations: CMH, Cochrane-Mantel-Haenszel; ITT, intention to treat; mo, month; PFS, progression-free survival.
Odds ratio < 1 implies event less likely in entinostat group.
Hazard ratio < 1 implies effect greater in entinostat group.
Fig 2.
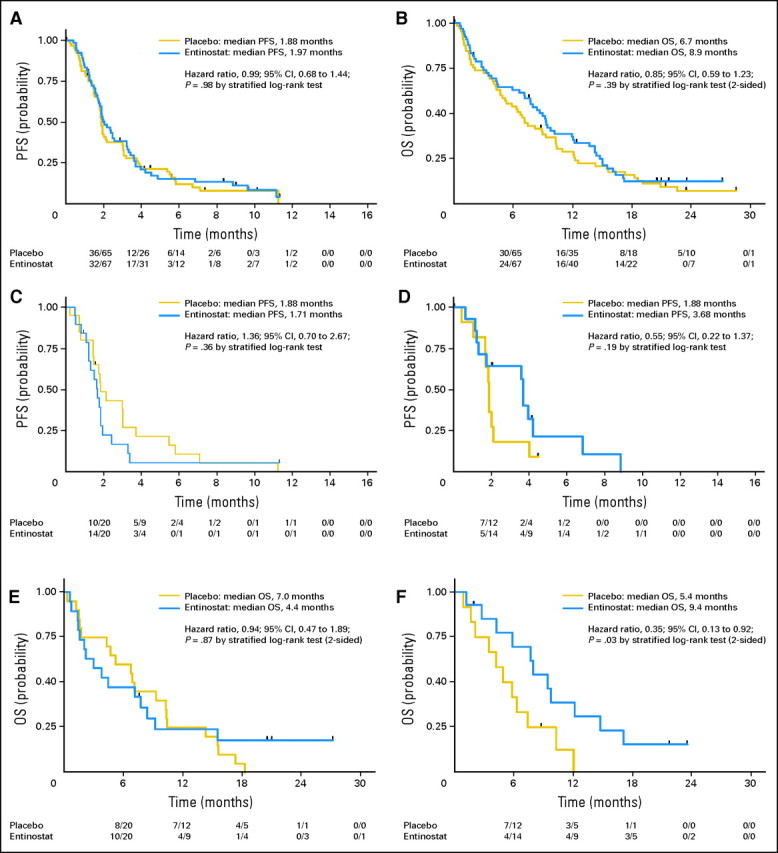
Kaplan-Meier estimates of (A) progression-free survival (PFS) in the full, randomly assigned patient population; (B) overall survival (OS) in the full, randomly assigned patient population; (C) PFS in a subset of E-cadherinLO (immunohistochemistry [IHC], 0, +1, +2) patients; (D) PFS in a subset of E-cadherinHI (IHC, +3) patients; (E) OS in E-cadherinLO (IHC, 0, +1, +2) patients; and (F) OS in E-cadherinHI (IHC, +3) patients.
Safety
Safety was assessed in all patients who received at least one dose of study treatment (n = 128). The majority of reported AEs were rated by the investigator as Common Terminology Criteria for Adverse Events grade 1 or 2 (mild or moderate severity) with rash, fatigue, diarrhea, and nausea being the most common in both groups. There was a higher incidence of AEs considered related to blinded study drug (EE, 90.8% v EP, 81%); however, the percentage of patients with a serious AE (EE, 49.2% v EP, 46%) or with an AE leading to treatment discontinuation (EE, 43.1% v EP, 42.9%) were similar between groups. A higher incidence of grade 3 and 4 AEs (Table 3) were seen in the EE arm than in the EP arm (76.9% v 58.7%, respectively), with fatigue, rash, dyspnea, hypophosphatemia, and pleural effusion being the most common. There were 28 deaths on study (EE, 12 patients; EP, 16 patients), the majority of which were as a result of progressive disease. Only one death (as a result of pneumonia in the EE group) was felt by the investigator to be possibly related to the study drug (erlotinib).
Table 3.
Adverse Events
| Adverse Event | Erlotinib + Placebo (n = 63) |
Erlotinib + Entinostat (n = 65) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Fatigue | 32 | 51 | 9 | 14 | 1 | 2 | 38 | 58 | 13 | 20 | ||
| Rash | 35 | 56 | 3 | 5 | 33 | 51 | 7 | 11 | ||||
| Dyspnea | 11 | 17 | 2 | 3 | 20 | 31 | 6 | 9 | ||||
| Hypophosphatemia | 3 | 5 | 2 | 3 | 9 | 14 | 4 | 6 | 1 | 2 | ||
| Pleural effusion | 4 | 6 | 2 | 3 | 1 | 2 | 7 | 11 | 4 | 6 | 1 | 2 |
| Asthenia | 7 | 11 | 2 | 3 | 11 | 17 | 4 | 6 | ||||
| Anemia | 7 | 11 | 0 | 0 | 14 | 22 | 3 | 5 | ||||
| Dehydration | 5 | 8 | 0 | 0 | 10 | 15 | 3 | 5 | ||||
| Dermatitis acneiform | 12 | 19 | 3 | 5 | 10 | 15 | 3 | 5 | ||||
| Hypokalemia | 7 | 11 | 0 | 0 | 6 | 9 | 2 | 3 | 1 | 2 | ||
| Pneumonia | 3 | 5 | 2 | 3 | 6 | 9 | 2 | 3 | 1 | 2 | ||
| Syncope | 1 | 2 | 0 | 0 | 3 | 5 | 3 | 5 | ||||
| Diarrhea | 30 | 48 | 4 | 6 | 37 | 57 | 2 | 3 | ||||
| Hypoxia | 4 | 6 | 3 | 5 | 3 | 5 | 0 | 0 | 1 | 2 | ||
| Abdominal pain | 7 | 11 | 4 | 6 | 2 | 3 | 0 | 0 | ||||
Molecular Analysis
Biomarker results in baseline patient diagnostic samples are summarized in Table 4. In those patients with evaluable samples, the treatment arms were balanced with respect to EGFR mutations, KRAS mutations, and E-cadherin expression, whereas some imbalance in EGFR FISH was observed (EE, 60% positive v EP, 38% positive). E-cadherin levels were determined in 66 of 132 patients (50% yield determined by tissue availability and sample quality) with 26 (40%) of 66 patients expressing high levels (+3) of E-cadherin by immunohistochemistry (IHC) score and 30 (48%) of 63 patients expressing high levels (> 150) by H-score (Appendix Fig A1, online only). There was a strong association between the H-score and IHC scoring methods (P < .0001).
Table 4.
Baseline Biomarker Status (all patients randomly assigned)
| Biomarker | Erlotinib +Placebo (n = 65) |
Erlotinib + Entinostat (n = 67) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| EGFR mutation* | ||||
| Total tested | 36 | 55 | 46 | 69 |
| Exon 19+ | 3 | 8 | 3 | 7 |
| EGFR FISH* | ||||
| Total tested | 29 | 45 | 30 | 45 |
| Positive | 11 | 38 | 18 | 60 |
| Negative | 18 | 62 | 12 | 40 |
| KRAS mutation* | ||||
| Total tested | 34 | 52 | 44 | 66 |
| KRAS mutation | 7 | 21 | 4 | 9 |
| E-cadherin* | ||||
| Total tested | 32 | 49 | 34 | 51 |
| 0 | 2 | 6 | 0 | 0 |
| 1+ | 6 | 19 | 5 | 15 |
| 2+ | 12 | 38 | 15 | 44 |
| 3+ | 12 | 38 | 14 | 41 |
Abbreviations: EGFR, epidermal growth factor receptor; FISH, fluorescent in situ hybridization; KRAS, Kirsten rat sarcoma viral oncogene homolog.
Percentages computed from total No. of patients tested.
Patient characteristics for the E-cadherinHI subset were balanced with respect to sex, histology, smoking status, and KRAS. Imbalances were observed with respect to age (median, 77 years in the EP arm v 66 years in the EE arm), progressive disease on therapy before study (EP, 50% v EE, 64%), EGFR mutations (exon 19, 0% in EP arm v 21% in EE arm), and EGFR FISH (EP, 17% positive v EE, 64% positive). Three patients in the E-cadherinHI EP subset also had subsequent entinostat treatment as part of the cross-over option.
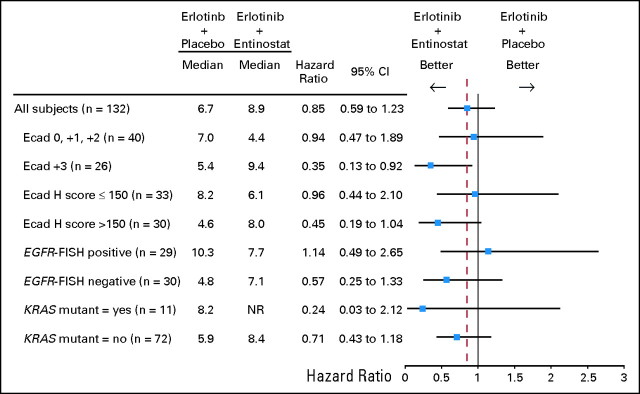
Clinical outcome by biomarker status, key patient characteristics, and by comparison to overall study results is summarized in Figure 3. In the subset of E-cadherinHI patients, OS was longer in the EE group compared with the EP group (median, 9.4 v 5.4 months; hazard ratio [HR], 0.35; 95% CI, 0.13 to 0.92; P = .03; Fig 2F). Correspondingly, a trend toward increased PFS within this subset was also observed for EE-treated patients (median, 3.7 v 1.9 months; HR, 0.55; 95% CI, 0.22 to 1.37; P = .19; Fig 2D). In the E-cadherinLO subset, no significant differences were detected between the EE and EP groups for OS (Fig 2E) and PFS (Fig 2C), though a trend toward a slightly worse outcome within the EE group was noted (Fig 3). Of the 14 E-cadherinHI patients treated with the EE combo, three were positive for EGFR-activating mutations whereas none were detected in EP-treated group. After excluding the three patients with activating EGFR mutations from the comparison of OS outcome between the EE and EP groups, the HR was improved to 0.31 (P = .03) and OS improved to 12.2 months for the EE group. Similarly, excluding cross-over patients from the comparison of OS outcome between the EE and EP groups would result in an HR improvement favorable to the EE group.
Fig 3.
Forest plot of median overall survival and associated hazard ratios by biomarker status. Ecad, E-cadherin; EGFR, epidermal growth factor receptor; FISH, fluorescent in situ hybridization; KRAS, Kirsten rat sarcoma viral oncogene homolog.
DISCUSSION
Our prospective, randomized phase II study of patients with NSCLC who previously experienced chemotherapy failure was based on preclinical findings that demonstrated entinostat combined with erlotinib enhances the effect of erlotinib alone and can reverse resistance to EGFR-TKI through epigenetic modulation of tumor phenotype.6 Our study demonstrated an acceptable safety profile with the entinostat-erlotinib combination but did not demonstrate improved efficacy as measured by PFS, objective response rate, or OS in the intention-to-treat population. In the subgroup of patients whose tumors strongly expressed E-cadherin, both PFS and OS were longer in the group that received the combination therapy. The prolonged survival observation in the E-cadherinHI subset treated with the entinostat-erlotinib combination may be as a result of the delay and/or prevention of emergent erlotinib resistant clones that would drive disease progression.
Identifying biomarkers that would predict the activity of a treatment for cancer is a mainstay of clinical research in oncology. Examples of biomarkers that align with the mechanism of drug activity are seen in various areas in oncology, such as estrogen receptors and HER2-neu in breast cancer9,10 and EGFR mutation11 and EML4-ALK gene rearrangement in NSCLC.12
In our study, patients with high levels of E-cadherin benefited the most from the EE combination, with a median OS of 9.4 months compared with 5.4 months in EP-treated patients (HR = 0.35; P = .03) or 4.4 months in EE-treated patients with low E-cadherin expression. Evaluation of median OS for the E-cadherinHI group excluding EGFR mutants favored EE, suggesting that the imbalance in EGFR mutations was not a contributing factor to the survival advantage observed in the EE E-cadherinHI group. EGFR FISH analysis identified nine of 14 EE E-cadherinHI patients as EGFR FISH–positive, whereas only two of 12 were positive in the EP E-cadherinHI group. EGFR copy number may therefore be a contributing factor to the OS advantage in the EE-treated E-cadherinHI group. However, three of the four patients with greatest OS in the EE E-cadherinHI group were EGFR FISH–negative, indicating that EGFR copy number alone may not be a factor. KRAS mutations were evenly distributed with two patients in the EE group and two patients in the EP group. Additional baseline characteristics such as performance status, number of previous chemotherapy treatments, and histology may also influence the outcome of the E-cadherinHI group, however their impact remains unclear.
From previous studies4 one might have expected that patients treated with erlotinib alone and expressed high E-cadherin would have had a longer survival compared with patients with low E-cadherin expression. In our study, there was no apparent benefit to patients with high E-cadherin levels treated with erlotinib alone. E-cadherin assay methodology, the cutoffs used, and patient characteristics may be among the factors that account for the lack of effect observed in our study's erlotinib-alone arm.
In our study, IHC was used to identify the subgroup of patients who had clinically benefited from the combined therapy and +3 was used as a cutoff to identify the good outcome group with an HR of 0.35. As approximately 40% of patients with evaluable tissue from the time of initial diagnosis exhibited +3 E-cadherin expression, the proportion of NSCLC patients that could potentially benefit from the combination is relatively high and includes both squamous and nonsquamous histology. In our study, the reproducibility of the IHC assessment was studied at two independent laboratories with two scoring algorithms and was found to be highly reproducible. Thus, our study opens up the potential of using E-cadherin IHC as a valuable predictive test. Further standardization and refinements of the E-cadherin protein expression assay as well as determination of E-cadherin status at the time of treatment will help define the subgroups with differential outcome to EE treatment.
The role of biomarker selection for molecular targeted therapy in patients with advanced NSCLC has been demonstrated in other recently reported trials. For patients with the EML4-ALK gene rearrangement based on FISH assay (3% to 4% of unselected patients), treatment with crizotinib (Pfizer, New York, NY) has demonstrated a prolonged PFS compared with historic controls.13 And in the randomized study of erlotinib with and without MetMAb (Genentech/Roche, San Francisco, CA), patients with high expression of C-MET protein by IHC showed improved clinical benefit by increasing PFS and OS.14 In all the mentioned studies for patients with advanced NSCLC, including the entinostat-erlotinib study, a subset of patients was identified with significant clinical benefit to the targeted therapies. Future clinical biomarker studies need to address eventual overlap between the different biomarkers to assign the right group of patients to the right therapy.
The combined therapy of erlotinib and entinostat in patients with advanced NSCLC who previously experienced chemotherapy treatment failure did not result in improved clinical outcome in the unselected patient population. Planned biomarker analysis identified a subset of patients with high E-cadherin expression levels that had significantly longer OS in the group treated with the combination. Given the acceptable safety profile of the combination, these results warrant a confirmatory study to establish the role of epigenetic therapy in targeting EGFR-TKI resistance.
Appendix
Fig A1.
(A-F) Immunohistochemistry analysis of E-cadherin expression in archival tissue samples from patients enrolled onto ENCORE-401 (Entinostat Combinations Overcoming Resistance–401). Two examples of each assigned immunohistochemistry score are provided with designations of +1 and +2 as E-cadherinLO and +3 as E-cadherinHI.
Footnotes
See accompanying article on page 2280
Supported by Syndax Pharmaceuticals, Waltham, MA.
Presented at the American Society of Clinical Oncology/International Association for the Study of Lung Cancer/American Society for Radiation Oncology Multidisciplinary Conference in Thoracic Oncology, Chicago, IL, December 9, 2010, and the 15th World Conference on Lung Cancer, Amsterdam, the Netherlands, July 3, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00602030.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Marcus A. Neubauer, US Oncology Network (C) Consultant or Advisory Role: Samir E. Witta, Syndax Pharmaceuticals (C); Alexander I. Spira, GLG Research (C); Paul A. Bunn Jr, Syndax Pharmaceuticals (C), OSI Pharmaceuticals (C), Genentech (C), Amgen (C), Bristol-Myers Squibb (C), ImClone Systems (C), GlaxoSmithKline (C), Eisai (C), Daiichi Sanyko (C), sanofi-aventis (C), Boehringer Ingelheim (C), AstraZeneca (C); Fred R. Hirsch, Genentech (C), ImClone Systems (C), Celgene (C), Ventana Medical Systems (C), Bristol-Myers Squibb (C), Boehringer Ingelheim (C), OSI Pharmaceuticals (C) Stock Ownership: Alexander I. Spira, Eli Lilly Honoraria: None Research Funding: Fred R. Hirsch, Ventana Medical Systems, Celgene, OSI Pharmaceuticals, Syndax Pharmaceuticals, ImClone Systems Expert Testimony: None Other Remuneration: Samir E. Witta, Syndax Pharmaceuticals
AUTHOR CONTRIBUTIONS
Conception and design: Samir E. Witta, Paul A. Bunn Jr, Fred R. Hirsch
Collection and assembly of data: Marcus A. Neubauer, Alexander I. Spira, Robert L. Ruxer, Marileila Varella-Garcia, Fred R. Hirsch
Data analysis and interpretation: Robert M. Jotte, Katrik Konduri, Marileila Varella-Garcia, Paul A. Bunn Jr, Fred R. Hirsch
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Cappuzzo F Ciuleanu T Stelmakh L, etal: Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study Lancet Oncol 11:521–529,2010 [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA Rodrigues Pereira J Ciuleanu T, etal: Erlotinib in previously treated non-small-cell lung cancer N Engl J Med 353:123–132,2005 [DOI] [PubMed] [Google Scholar]
- 3.Coldren CD Helfrich BA Witta SE, etal: Baseline gene expression predicts sensitivity to gefitinib in non-small cell lung cancer cell lines Mol Cancer Res 4:521–528,2006 [DOI] [PubMed] [Google Scholar]
- 4.Yauch RL Januario T Eberhard DA, etal: Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients Clin Cancer Res 11:8686–8698,2005 [DOI] [PubMed] [Google Scholar]
- 5.Thomson S Buck E Petti F, etal: Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition Cancer Res 65:9455–9462,2005 [DOI] [PubMed] [Google Scholar]
- 6.Witta SE Gemmill RM Hirsch FR, etal: Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines Cancer Res 66:944–950,2006 [DOI] [PubMed] [Google Scholar]
- 7.Sharma SV Lee DY Li B, etal: A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations Cell 141:69–80,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuzzo F Hirsch FR Rossi E, etal: Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer J Natl Cancer Inst 97:643–655,2005 [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart MJ: New developments in hormone receptor-positive disease Oncologist 15:18–28,2010. suppl 5 [DOI] [PubMed] [Google Scholar]
- 10.Lin SX Chen J Mazumdar M, etal: Molecular therapy of breast cancer: Progress and future directions Nat Rev Endocrinol 6:485–493,2010 [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Chmielecki J: Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer Nat Rev Cancer 10:760–774,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber DE, Minna JD: ALK inhibition for non-small cell lung cancer: From discovery to therapy in record time Cancer Cell 18:548–551,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak EL Bang YJ Camidge DR, etal: Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer N Engl J Med 363:1693–1703,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spigel DR Ervin TJ Ramlau R, etal: FInal efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC J Clin Oncol 29:477s,2011. suppl 15 abstr 7505 [Google Scholar]