Abstract
Strategies to enhance, suppress, or qualitatively shape the immune response are of importance for diverse biomedical applications, such as the development of new vaccines, treatments for autoimmune diseases and allergies, strategies for regenerative medicine, and immunotherapies for cancer. However, the intricate cellular and molecular signals regulating the immune system are major hurdles to predictably manipulating the immune response and developing safe and effective therapies. To meet this challenge, biomaterials are being developed that control how, where, and when immune cells are stimulated in vivo, and that can finely control their differentiation in vitro. We review recent advances in the field of biomaterials for immunomodulation, focusing particularly on designing biomaterials to provide controlled immunostimulation, targeting drugs and vaccines to lymphoid organs, and serving as scaffolds to organize immune cells and emulate lymphoid tissues. These ongoing efforts highlight the many ways in which biomaterials can be brought to bear to engineer the immune system.
Keywords: immunoengineering, vaccination, immunotherapy
1. INTRODUCTION
The immune system plays a critical part in the health of organisms, from invertebrates to humans, and can be either a cure or cause of disease. Traditionally, the immune system has been viewed by biomedical engineers as an adversary to the effective design of biomaterials, as an organizer of the host response that shortens the lifespan and function of implants or the mediator of rapid clearance of systemically administered drug carriers. However, interest is increasingly focusing on engineering biomaterials [in the form of solid implants, hydrogels, microparticles, or nanoparticles (NPs)] to rationally control the immune system by enhancing or suppressing immune reactions in an antigen-specific or -nonspecific manner to treat disease or overcome adverse immune situations. In addition, as in other areas of cell biology, biomaterials can be designed as tools to control tissue, cell, and molecular interactions that regulate immune cells in order to shed new light on the functioning of the immune system. This new area of immune engineering using biomaterials is generating promising new strategies for vaccination, cancer immunotherapy, treatment of autoimmune disorders, and establishing tolerance to organ transplants. In this review, we discuss some of the important directions that scientists have pursued in using biomaterials to direct the functions of the immune system.
2. EFFECTS OF BIOMATERIALS ON IMMUNE CELLS, AND IMMUNOMODULATION USING PARTICULATES, PROTEIN CHEMISTRY, CONTROLLED RELEASE, AND BIOMIMICRY
Research into bio-inspired materials and endogenous mechanisms of immune stimulation have shown tantalizing results for developing efficacious vaccines and therapies for curing diseases. In this section, we discuss immune-engineering approaches utilizing biomimetic presentation, cell targeting, the controlled release of immunomodulators, and the properties of endogenous materials to stimulate or suppress the immune response. The body of work presented here serves as an introduction to these topics and seeks only to emphasize recent developments in these fields and the key advancements that have shown these approaches to be of possible use in clinical therapies.
2.1. Protein-Based Approaches for Effective Immunization
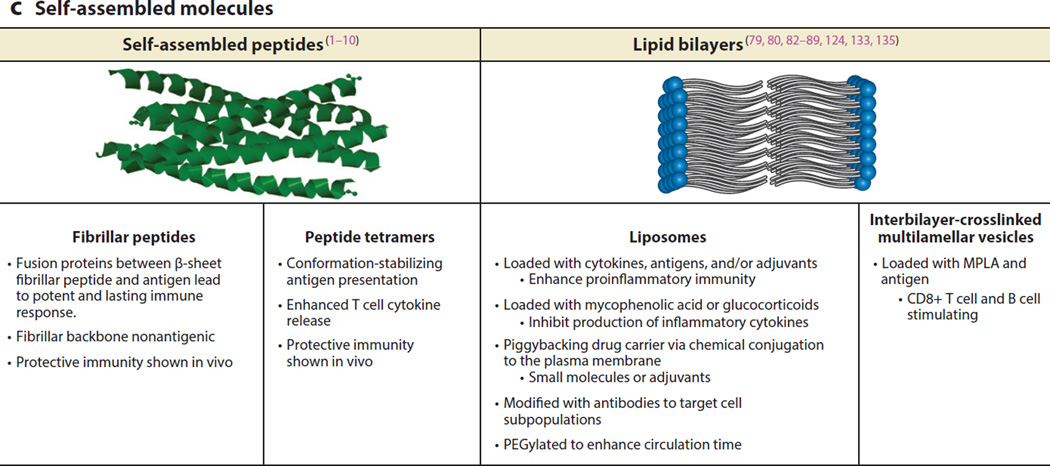
The biomimetic presentation of synthetic antigens via self-assembled fibrillar peptides without the aid of adjuvants has been shown to elicit strong long-lasting antibody responses to vaccines and immunotherapies (1). Collier and coworkers (2) showed that a fusion protein of a self-assembling β-sheet fibrillar peptide, Q11, and the antigenic ovalbumin (OVA) peptide OVA323--339, maintained anti-OVA antibody responses in mice for more than 1 year. They also showed that the sustained immune response relied on CD4+ T cells, and that the Q11 peptide by itself was nonimmunogenic, even when delivered in Complete Freund’s Adjuvant. It was later shown that the multivalent fibrillar conformation of the presented fusion protein was critical to sustaining the immune response (3). Further, durable antibody responses against a malaria epitope (4) and high-affinity B cell responses (without systemic inflammation) (5) have also been shown utilizing these epitopes. Finally, using supramolecular assemblies, a variety of structurally complex proteins have also been shown to retain their structure and function (6, 7). Thus, from these studies it is evident that fibrillar self-assembled proteins, although not inflammatory on their own, are potent adjuvants for long-term adaptive immunity (3).
The engineered self-assembled peptide approach has been shown to be effective in generating long-term immunity against several model antigens including OVA, green fluorescent protein, and malaria peptides (1, 2, 4, 6, 8). In the last two years, these constructs have also been shown to induce, in dendritic cells (DCs), high expression of the proinflammatory activation markers CD80 and CD86, and also to induce antigen-specific differentiation of T cells into T follicular helper cells and B cells into germinal center cells (5). This activation led to the production of high-titer, high-affinity immunoglobulin G (IgG) antibodies in vivo that crossreacted with the native protein antigen and were neutralizing in an in vitro influenza hemagglutination inhibition assay to a greater extent than was induced by alum and to an equal extent with Complete Freund’s Adjuvant (5).
Another interesting self-assembly approach that aimed to overcome the low immunogenicity of the highly conserved ectodomain of the influenza matrix protein 2 (M2e) was to form protein nanoclusters of self-assembled conformation-stabilized M2e tetramers (tM2e) (10). Intranasal vaccination of mice with the protein nanoclusters resulted in 100-fold higher levels of serum M2e–specific IgG when compared with mice immunized with soluble tM2e. Furthermore, activation of antigen-specific T cell cytokine release was observed, and the adoptive transfer of serum from immunized mice conferred complete protection against lethal challenge with homo- as well as heterosubtypic viruses, likely through the delivered antigen-specific antibodies in the transferred serum. Interestingly, incorporating Toll-like receptor (TLR)-9 ligands (CpG oligonucleotides) as an adjuvant did not provide additional benefit to the protein nanoclusters. Taken together these studies are examples of the innovative use of the principles of protein chemistry to develop protein-based vaccines that consist of both the antigen against which the immune response is directed plus the adjuvant or immunomodulatory ability of the formulation, without the need for an exogenous biomaterial. Thus, these approaches are unique in their elegant simplicity and they exemplify the use of protein-based biomaterials that have multifunctionality.
2.2. Effects of Biomaterials on Dendritic Cells
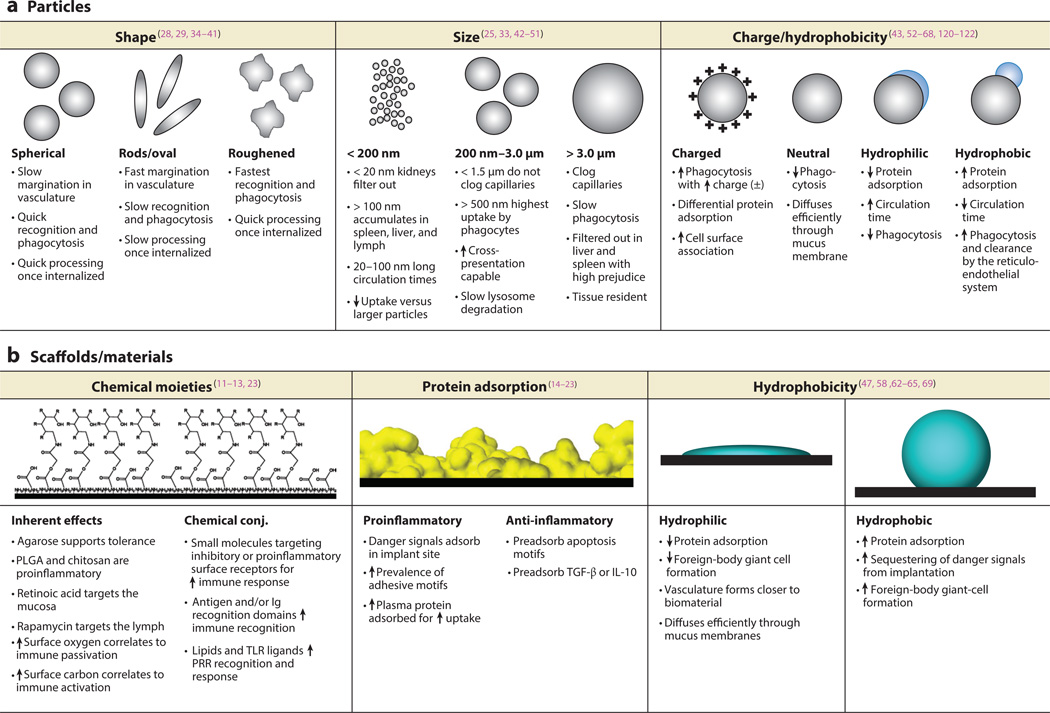
Traditionally, vaccine-delivery and tissue-engineering approaches have used biomaterials as vehicles for delivery or as support structures. Thus, it is important to understand how biomaterials affect key cells that direct immune responses, specifically DCs. Furthermore, elucidating how key properties of biomaterials---such as surface chemistry, surface energy, surface topology, and the size and shape of a material---influence responses by DCs offers opportunities to use the biomaterial component of a device for immunomodulation. Babensee and coworkers (11, 12) have shown a differential effect on the phenotype of DCs derived from human peripheral blood that depends on the biomaterial used to treat them in vitro. Treating DCs with alginate and agarose films did not stimulate DCs above that of DCs cultured on tissue culture polystyrene controls; however, treating DCs with poly(lactic-co-glycolic) acid (PLGA) or chitosan films supported DC maturation, which was shown by increased surface expression of the proinflammatory markers CD80, CD86, CD83, HLA-DQ, and CD44; allostimulatory capacity; and a variety of released proinflammatory cytokines (11, 13). Mixed lymphocyte reactions showed that DCs treated with PLGA and chitosan films supported higher levels of T cell proliferation when compared with those of untreated, immature DCs (iDCs); also, DCs treated with hyaluronic-acid films induced lower levels of T cell proliferation when compared with iDCs. Recently, it was shown that culturing DCs pretreated with these different biomaterials affected the phenotype and polarization of co-cultured autologous T cells.(12) Interestingly, treatment of DCs with agarose films that had an added model antigen (OVA) induced the expansion of CD4+CD25+Foxp3+ T regulatory cells (Tregs) in autologous DC--T cell cocultures. Furthermore, in this coculture, agarose treatment induced the release of interleukin (IL)-12p70 and IL-10 at higher levels when compared with treating DCs with other biomaterial films and OVA, suggesting, respectively, Th1 and Th2 polarization. Treatment with PLGA film and OVA induced in DCs the release of interferon-γ (IFN-γ) at higher levels when compared with those observed for cocultures with iDCs or DCs treated with all other biomaterial films (12). In total, these results indicate that to intelligently design vaccines and clinical therapies, the biocompatibility criterion should include an analysis of the immunomodulatory characteristics of the material.
A complete understanding of the mechanisms by which DCs recognize and respond to biomaterials remains to be elucidated. Likely mechanisms include receptor-mediated processes known to be important in host responses to biomaterials, which are directed through biomaterial-adsorbed cell-adhesive proteins, and complement-activation fragments using receptors on leukocytes, such as integrins and complement receptors. However, additional interactions can be considered, as suggested by the mechanisms by which DCs recognize pathogens and sense tissue damage or danger. Such mechanisms include pattern-recognition receptors such as TLRs and C-type lectin receptors. Many endogenous TLR ligands are proteins known to adsorb to biomaterials, such as fibrinogen and fibronectin (14); also, such proteins are glycosylated (15), suggesting the potential for responses mediated by C-type lectin receptors. Both the adsorption of complement-activation fragments and other danger signals to biomaterials have been implicated in the response of DCs to biomaterials (16). Recent research using MyD88- and Tlr-knockout mice demonstrated that DCs use TLR2, TLR4, and TLR6 for their responses to a diverse set of biomaterials (17). Also recently, β2 integrins have been shown to play a part in mediating DC adhesion and response to biomaterials (18). These studies implicate extracellular matrix proteins adsorbed to biomaterial surfaces as being integral to DC response to these proteins. DCs cultured on collagen or vitronectin substrates released higher levels of IL-12p40, but DCs cultured on albumin- or serum-coated plates generated higher amounts of IL-10 (19). These results imply that intelligently engineered biomaterials may guide the presentation, orientation, or conformation of adsorbed proteins in such a way that the combination products in which they are used will induce either DC tolerance or activation to codelivered antigen.
Biomaterials have been shown to have an adjuvant effect that enhances the immunogenicity of antigens. Adsorption of OVA to phagocytosable microparticles, or onto PLGA scaffolds, has been found to support an antibody-mediated immune response that was maintained for longer than 16 weeks and was seen to be predominantly of a “Th2” type,, as indicated by IgG1 antibody titers (20). In an experiment comparing two formulations that used equivalent amounts of polymer, antigen loading, and release rates, it was found that delivery of antigen by an implanted PLGA scaffold induced an elevated antigen-specific humoral response with greater longevity than did an injection of microparticles, (21). It was hypothesized that danger signals released from tissue damage during surgery caused the enhanced immune response to antigen released from the implanted scaffolds. This was supported by the fact that HMGB1, a potent danger signal, was found in higher concentrations in exudates from subcutaneously implanted PLGA scaffolds with incorporated OVA (22). This result suggests the possible role of an adjuvant effect caused by danger signals induced by the biomaterial. Supporting the differential effects observed in vitro of PLGA and agarose in inducing, respectively, the maturation of DCs or not, PLGA scaffolds delivering the model antigen, OVA, acted as adjuvants in the humoral OVA-specific immune response, but agarose delivery of OVA did not (23). Hence, the selection of biomaterials can differentially affect the adaptive immune response to a co-delivered antigen. Furthermore, the in vitro assessment of the effects of biomaterials on DC maturation has been validated as predicting the in vivo adjuvant effect of the biomaterial with the co-delivered antigen.
2.3. Nano- or Microparticle Engineering for Immune Outcomes
Nano- or microparticle engineering can be used to affect many particle characteristics to achieve a desired immune effect, including particle-surface chemistry, size, and shape. Particle shape plays a significant part in extracellular trafficking, recognition by immune cells, and in the intracellular processing of particles (24). Due to physiological differences in processing, the shapes of nano- and microparticles have been shown to have roles in targeting drug delivery for cancer therapies (25), vaccine development (16, 26), and in chronic inflammation (27). The hypothesized mechanisms for how particle shape influences immune phenotype include how the particle interacts with the endothelium (28--30), spleen (31), liver (32), and lymphatic system (33). For example, spherical particles in vessels do not deviate from their laminar-stream motion unless perturbed externally (34). Conversely, ovoid and asymmetrical particles tumble, spin, and break their streamline in the vasculature and, thus, tend to marginate toward the walls of blood vessels (28, 29). This difference in flow behavior could be utilized to deliver endothelial therapies or to better transport particles in the bloodstream.
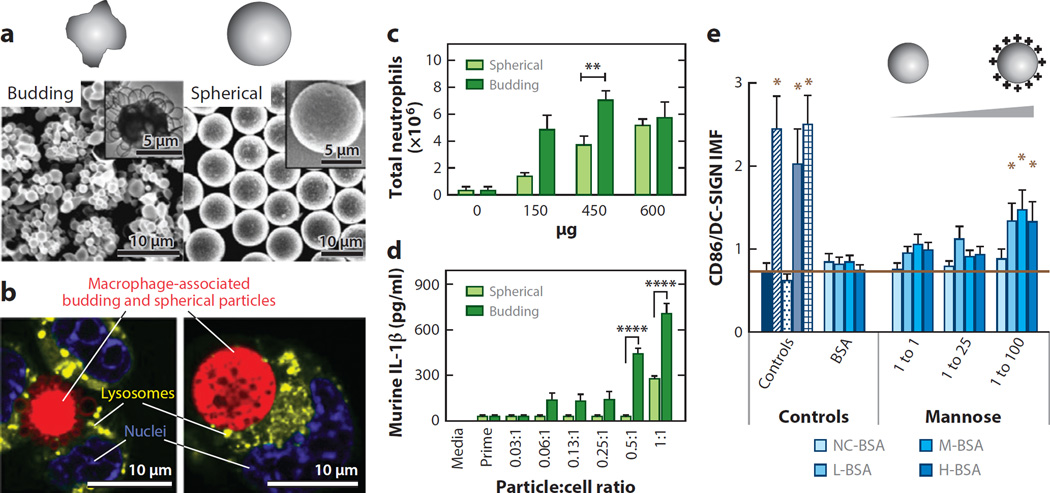
The clearance of particles can also be drastically affected by particle shape. The spleen filters particulates of 200 nm or smaller from the bloodstream; thus, if particles are rod or ovoid in shape and have a diameter or width that is less than 200 nm but a length of several microns they could pass through the spleen (33). Additionally, the extent of phagocytosis has been shown to depend heavily on particle shape, with longer, thinner (worm-like) morphologies leading to phagocytosis that is 50 times lower than that of spherical particles(35). Further study has shown that elliptical disk particles avoid phagocytosis in a manner that depends on their orientation (36). Particles with a high aspect ratio have been shown to be internalized more slowly than those with a low aspect ratio (37--39). Studies have shown that elongated particles are trafficked toward the nucleus and are oriented tangentially when compared with spherical particles, which are trafficked quickly and exhibit hexagonal packing in the cell (38, 40). Additionally, budding, or highly textured, microparticles have been shown to activate neutrophils significantly more than smooth particles (41) (Figure 2 a–b). Further, budding particles were more readily phagocytosed than smooth particles and induced more lipid-raft recruitment to the phagosome. Budding particles have also been found to induce stronger IL-1β secretion than smooth particles through activation of the NLRP3 inflammasome (41).
Figure 2.
Nano- or microparticle engineering for immune outcomes. (a) Scanning electron microscope and transmission electron microscope (inset) images of budding and spherical polystyrene-b-poly(ethylene oxide) microparticles. (b) Confocal microscopy images of macrophage-associated (red) budding and spherical particles, (green) lysosomes, (blue) nuclei. (c--d) Particle-induced neutrophil recruitment (c) and interleukin (IL)-1β cytokine secretion (d) depends on surface curvature. (e) Dendritic cell (DC) inflammatory maturation factor (IMF) levels in response to glycoconjugate adsorbed to wells with different surface properties. The molar ratio of thiolated glycan to bovine serum albumin (BSA) is indicated. The isoelectric point (pI) of glycoconjugates of BSAwas scaled from a pI of ~4.0 to ~10.0 using ethylenediamine (EDA). NC-BSA: no EDA added; L-BSA: 0.05 M EDA, low pI; M-BSA: 0.15 M EDA, medium pI; H-BSA: 0.90 M EDA, high pI. (Panels a--d adapted with permission from 41; panel e adapted with permission from 52.)
Intravenously injectable microparticle therapies must use particle sizes that are smaller than 1.5 µm in order for the particles to not clog capillaries (37, 42). Additionally, particle size has been shown to drastically alter the circulation times of injected therapies (25) and to alter how particles are transported through the body---i.e., through the lymphatic or vascular system (43). In general, small particles are less likely to be taken up by macrophages than large ones due to the fact that the proper geometric configuration for efficient complement activation can be achieved less easily on the more highly curved surfaces of the smaller particles than on the surfaces of larger ones (43--45). For optimal circulation times it has been found that NPs should have diameters between 20 and 100 nm (46, 47). At sizes smaller than 20 nm, NPs are filtered out by the kidney (48); at sizes larger than 100 nm, particles begin to be sequestered by sinusoids in the spleen and fenestrae of the liver (49). Particles larger than approximately 1.0 µm tend to aggregate under physiological conditions and be retained mechanically by capillaries, resulting in rapid uptake by tissue-resident phagocytic cells and high accumulation in the liver, spleen, and, to a lesser extent, in the bone marrow (50). Thus, when the particle size falls between 20 nm and 1 µm, clearance mechanisms are minimized, and circulation time is greatly prolonged (43). Intracellularly, particle size has been shown to mediate the efficiency of cross presentation of exogenous antigens by DCs (51). Phagocytosed 50 nm particles with antigen bound to them were found to be shuttled to an acidic environment within 30 min of phagocytosis, and the antigen was inefficiently cross presented. Antigen bound to 500 nm and 3 µm beads was found not to be trafficked to an acidic environment in the cell and, thus, had increased efficiency in peptide processing and cross presentation onto MHC (major histocompatibility complex) class I molecules. The effect of this was stimulation of a cytotoxic CD8+ T cell response (rather than the default CD4+ T response for exogenously delivered antigen), which is often needed for viral infections, antitumor immunity, or surveillance (51). Thus, the size of antigen carriers also has a critical role in biodistribution, cellular uptake, and the intracellular trafficking of antigen for determining the type of immune response (humoral or cytotoxic).
Surface chemistry, such as the charge of particles, also has a significant role in the intracellular and extracellular processing of particles. Recently, Babensee and coworkers (52) showed that the charge of a carrier can enhance DC maturation in response to glycoconjugates (Figure 2e). In this study, it was shown that the conjugate charge induced the second largest increase in proinflammatory DC maturation, falling behind only ligand density in potency (52). It has also been shown that the surface charge of NPs influences the adsorption of opsonins (and other plasma proteins), leading to macrophage recognition, which is followed by phagocytosis and elimination (53). Despite conflicts in the literature about how charge directly affects circulation times and processing by cells, a general consensus has been reached that the absolute value of the zeta potential may be the most important factor for the phagocytosis of NPs (43). Virtually all reports have found that macrophage uptake increases as the surface charge increases (that is, either a positive or negative zeta potential) (54, 55). Further, NPs that are neutral at physiological pH avoid uptake by phagocytic cells, thereby exhibiting markedly delayed blood clearance (56--58). Chemical composition at the surface of NPs also alters the quantity of protein adsorbed, the kinetics of protein adsorption, and cellular trafficking (59, 60). These factors have critical roles in the physiological filtering of the particle to the lymph, spleen, or liver, or toward targeted moieties. The adsorbed biolayer further promotes or suppresses adhesion of the NPs to cell membranes, thereby influencing cellular uptake and processing, and the ultimate fate of the particle.
Another aspect of particle-surface chemistry is hydrophobicity, which is a key factor in opsonization. Hydrophobic particles in the body are preferentially coated by plasma proteins (e.g., immunoglobulin, complement, albumin) and then cleared by the reticuloendothelial system (61). Thus, most particulate-based systems have relied on hydrophilic coatings or modifications to reduce this passive mechanism of adsorption and clearance. The most popular modification is to coat particles with polyethylene glycol (PEG) to increase the surface hydrophilicity of the particle and thus reduce nonspecific protein adsorbance (58, 62, 63). On the surface of NPs, PEG forms a dense network of hydrophilic chains that act as a steric barrier to plasma proteins and macrophages (63--65). PEGylated NPs have been shown to effectively reduce macrophage uptake in vitro and prolong circulation half-life in vivo, decrease the accumulation of NPs in the liver, and be cleared mainly by the spleen after a long residence in blood (66--68). The mechanism of the PEG coating on NPs and its effect on opsonization, biodistribution, and tumor-targeted delivery have been reviewed in many papers and, thus, are not further expounded upon here (69, 47).
Additionally, it has recently been shown that the modality of surface presentation of identical agents can alter the immune response. For example, glycan--bovine serum albumin constructs displayed from the surface of beads, from flat-well surfaces, or delivered in a range of soluble concentrations have been shown to differentially enhance DC maturation (71). Glycoconjugates presented from a nonphagocytosable flat-well surface showed the highest amount of DC maturation, and soluble conjugates showed the lowest. Interestingly, presentation of conjugates on phagocytosable beads showed an intermediate level of DC activation when compared with a soluble or nonphagocytosable presentation. These interactions were found to be receptor-specific and able to be inhibited by blocking antibodies. Further, chitin microparticle preparations in a phagocytosable size (1--10 µm), but not in soluble or nonphagocytosable sizes (40--100 µm), have been shown to induce activation of mitogen-activated protein kinase (MAPK) within macrophages in a manner dependent on TLR2, and to eventually induce high activation of M1 macrophages (72). It has also been shown that particulate antigens, but not soluble proteins, mediate follicular DC activation of B cells, and that virus-like particles, and not their constitutive proteins, are efficiently transported to murine splenic follicular DCs (73). Taken together, these studies indicate that it is not only the material, surface interface, charge, and other biophysical properties of materials that can mediate the immune response but also the form in which these materials or constructs are delivered that can drive the immune response toward a desired outcome.
2.4. Controlled Release of Immunostimulatory Molecules
Controlling the release of immunostimulatory molecules (TLR ligands, cytokines, or chemokines) from particulate or scaffold biomaterial systems to enhance immune outcomes holds immense promise for future therapeutics. The use of liposomes, polymer NPs, and various hydrogel formulations combined with TLR ligands, cytokines, and chemokines has shown that the immune response can be directed toward a proinflammatory Th1, Th2, cytotoxic, or B cell-stimulating response, or a combination of these.
NPs are designed to act as effective carriers of adjuvant compounds in order to safely provide the inflammatory signals necessary for triggering immunity to coadministered antigens. Most classical adjuvants, such as alum, activate the NLRP3 inflammasome. Activation of the inflammasome requires the signaling of both TLRs and NLRP3 (74, 75) in antigen-presenting cells (APCs). Antigen-loaded PLGA NPs have been constructed to conjugate lipopolysaccharide (LPS) (76), CpG oligonucleotides (77), or poly(inosinic:cytidylic acid) (78) to their surface to stimulate TLRs while simultaneously engaging the NLRP3 receptors. It has been found that these particles produce potent CD4+ and CD8+ responses from T cells and that these responses can be used to promote potent immune responses to model antigens, e.g., OVA (78), and clinically relevant vaccines to murine West Nile encephalitis (76, 77). Mice inoculated with these NPs were found to have high amounts of systemic IL-1β, produce large and sustained amounts of antigen-specific IgG, and were found to have a nearly 100% protection rate when challenged with the pathogen (76, 77). However, these compounds are not ideal for clinical translation due to the pleiotropic effects and toxicity of LPS or CpG oligonucleotides. Other TLR ligands, such as MPLA for TLR4, have been included in clinical vaccine formulations (co-delivered with alum) (81).
Another approach to enhancing vaccination has been to use lipid bilayer particles to deliver antigens and adjuvants together to APCs (79, 80). Moon et al. (80) have shown that interbilayer-crosslinked multilamellar vesicles formed by crosslinking headgroups of adjacent lipid bilayers within multilamellar vesicles can form potent vaccines. These multilayered vesicles, utilizing monophosphoryl lipid A (MPLA) as an adjuvant, loaded with a model antigen (OVA) elicited robust antibody titers that were 1,000 times greater than those elicited by simple liposomes, and they triggered steadily increasing humoral and CD8 T cell responses with antigen-specific T cells expanding to a peak of nearly 30% of the total CD8 cell population (80).
In the absence of TLR ligands, the delivery of encapsulated proinflammatory cytokines, such as IFN-γ (82), IL-1α (83), IL-2 (84), and others (85--87), along with the antigen of interest, has also been pursued as a means of stimulating the immune system. Studies have generally showed improved performance over that of particles not bearing cytokines. For example, polycyanoacrylate NPs loaded with tumor necrosis factor-α (TNF-α) showed a 78% reduction over particles not bearing TNF-α in terms of tumor expansion when the particles were coadministered with tumor antigen. However, cytokine-loaded particles have shown little clinical efficacy as monotherapies (88), and, thus, more potent combinatorial approaches have recently been pursued.
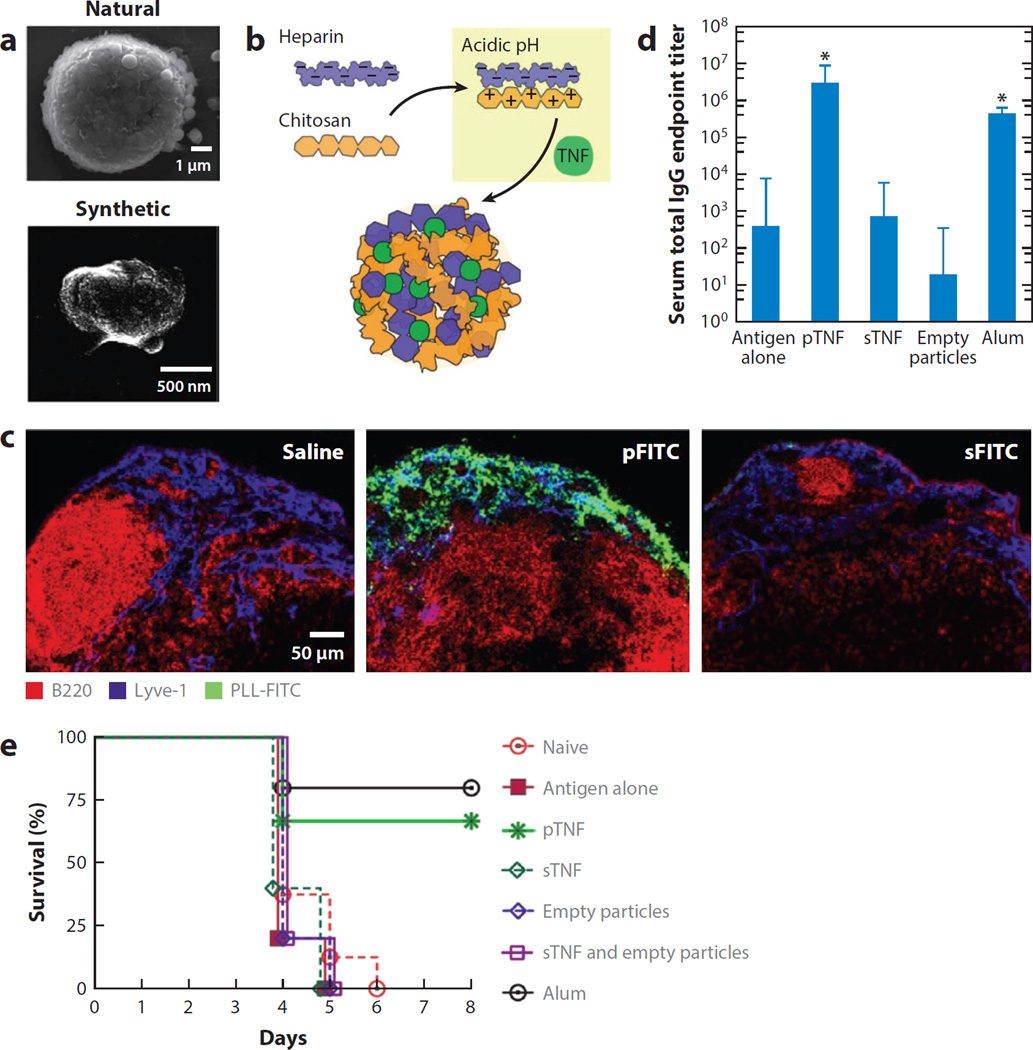
During natural infections, mast cells deliver inflammatory cytokines to lymph nodes (LNs) via the release of inflammatory granules, NP complexes of heparin, cytokines, and other factors, which target these stimuli to the draining LNs. In an elegant biomimetic strategy, St. John et al. (89) packaged inflammatory cytokines (TNF-α or IL-12) in polyelectrolyte NPs mimicking mast cell granules (Figure 3). These synthetic granules were engineered by complexing heparin (the key cytokine-binding component of native mast cell granules) and chitosan together with cytokine cargos (Figure 3a and b); these granules effectively targeted minute quantities of cytokines to LNs following injection (Figure 3c). When used as a delivery vehicle for TNF-α during vaccination of mice with hemagglutinin from the influenza virus, these particles enhanced adaptive immune responses and increased the survival of mice during lethal challenge without toxicity (Figure 3d and e).
Figure 3.
Synthetic mast cell (MC) granules for targeted vaccination. (a) Scanning electron microscope micrograph of an activated rat peritoneal MC (natural) and a synthetic particle consisting of heparin and chitosan (synthetic). (b) Diagram demonstrating the modeling of synthetic particles after MC granules, where chitosan, made positively charged under acidic conditions, is substituted for MC proteases, enabling inflammatory mediators to be entrapped within a similar matrix structure containing heparin. (c) Lymph node (LN) sections after injection of saline, particles containing poly-l-lysine conjugated to the fluorochrome fluorescein isothiocyanate (PLL-FITC) (pFITC, green) or soluble PLL-FITC (sFITC, green). These LNs were isolated 45 minutes post-injection, sectioned, and stained for B cells (B220, red) and LN sinuses (Lyve-1, blue). (d) Day 21 geometric mean titers for total immunoglobulin (Ig) G after vaccination with haemagglutinin in combination with the designated adjuvants, with a boost at day 14. (e) Synthetic particles containing tumor necrosis factor (TNF) (pTNF) or the positive control, alum, increased the survival of mice challenged with influenza significantly over naive mice, antigenalone controls or soluble TNF (sTNF). (Adapted with permission from 89.)
2.5. Delivering Anti-Inflammatory Immunomodulators
The studies above discuss proinflammatory immunomodulation of the immune system. However, in the treatment of autoimmune diseases caused by disorders of immune regulation, such as multiple sclerosis, psoriasis, rheumatoid arthritis, and type 1 diabetes, an ideal therapy should induce long-term durable antigen-specific T cell tolerance or immune suppression. Current therapeutic strategies based on T cell-specific peptides or antibodies cause various side effects, such as cytokine-release syndrome during monoclonal antibody treatment, and anaphylactic responses during peptide infusion, and have shown only marginal efficacy (90--92). Thus, using biomaterials to direct the immune system toward a tolerogenic response has been posited as an ideal vehicle for driving immune tolerance while mitigating unintended side effects. Recently, mycophenolic acid delivered by nanoliposome gels has been shown to significantly increase the survival time of lupus-prone mice even after the mice developed severe renal damage (93). The DCs that internalized gel particles produced a smaller quantity of inflammatory cytokines, such as IFN-γ and IL-12, which was thought to enhance survival in the mice (93). Similarly, in mice liposomes and polymeric dendrimers delivering glucocorticoids have been shown to suppress the production of inflammatory cytokines and reduce physiological indicators of rheumatoid arthritis and multiple sclerosis (94), effects also noted when delivered as plasmids encoding anti-inflammatory proteins (95), or as peptide antigens (96). In another approach, researchers used PLGA NPs loaded with leukemia inhibitory factor, an IL-6 family protein that promotes Treg formulation and immune tolerance (97), to expand Foxp3+CD4+ T cell numbers in a nonhuman primate model in vitro, to downregulate Th17 development, and prolong survival of vascularized heart grafts in mice (98).
Immunoengineers are also harnessing the natural mechanisms induced by apoptotic cells, which often present their antigens in a tolerance-inducing manner (99, 100). Recently, it was discovered that intravenous infusion of apoptotic cells that had been chemically conjugated with an autoantigen of relevance to reverse autoimmune disease were processed in vivo as a natural component of the apoptotic cells (101). To provide a more reliable and reproducible means of achieving the same effect, Miller and colleagues (102) recently demonstrated that intravenous administration of PLGA microparticles (500 nm in diameter) conjugated with encephalitogenic peptides targeted these antigens to splenic marginal-zone macrophages normally involved in the clearance of apoptotic cells, leading to successful induction of long-term T cell tolerance in mice with relapsing experimental autoimmune encephalomyelitis. Another approach has been to design NPs displaying peptide--MHC complexes of the targeted autoantigens, which can bind directly to T cells in vivo. Tsai et al. (103) showed that, unexpectedly, NPs coated with diabetes-related antigen and MHC complexes expanded cognate regulatory T cells in diabetic mice and suppressed the development of type 1 diabetes in prediabetic mice.
Combinatorial delivery approaches aimed at suppressing T cell activation and enhancing Treg differentiation have also recently been employed. Combinations of transforming growth factor (TGF)-β1, IL-2, and either rapamycin (104) or retinoic acid (89) have been shown to induce a Treg phenotype, and to suppress naive T cell proliferation and function, and are capable of inducing Foxp3+ Tregs in human cells. Both rapamycin and retinoic acid have been found to produce Tregs with similar functionality but with drastically different expression levels of CCR7, CCR9, and CCR10 (105), leading to significantly different migratory patterns and distributions in vivo (104). T cells treated with retinoic acid were found to home to mucosal tissues, and T cells treated with rapamycin homed toward lymphoid tissues. Interestingly, this homing led to differences in histological colitis scores in a Powrie murine model of T cell-mediated colitis: with rapamycin, Treg-treated mice were better able to reverse colitis than were Treg-treated mice with retinoic acid (104). Another combinatorial approach has been to co-deliver antigen with 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), a small tolerance-inducing molecule used to suppress the immune response against the co-delivered antigen (106). In this study, the delivery of the ITE-loaded particles with an epitope from myelin oligodendrocyte glycoprotein caused an expansion of the Foxp3+ Treg compartment and suppressed the development of experimental autoimmune encephalomyelitis, an experimental model of multiple sclerosis (106). Finally, co-delivery of the immunosuppressive factors TGF-β1 and IL-10 in hydrogels has also been shown to deactivate bone marrow DCs and cause a significantly reduced ability for the DCs to stimulate T cells. This approach highlights the ability of intelligently designed biomaterials to orchestrate a downstream immune response by modulating the phenotype of immunomodulatory DCs (107).
3. BIOMATERIALS TARGETING IMMUNE CELLS AND LYMPHOID ORGANS
Engineered biomaterials are being designed and synthesized to target immunomodulators to lymphoid tissues or disease sites to manipulate the immune system for a variety of therapeutic applications, and especially to enhance vaccines. In this section, we discuss the design and application of biomaterials that target lymphatic tissue. This topic is too broad to exhaustively cover in a single section so instead, to provide an overview of directions in the field, we highlight recent examples of biomaterials engineered for vaccine delivery and cell-based immunotherapy.
3.1. Vaccine Delivery
Subunit vaccines consisting of recombinant protein or polysaccharide antigens delivered in tandem with molecular adjuvants using synthetic delivery systems could be attractive alternatives to live viral or bacterial vaccines, by combining low toxicity with an absence of antivector immunity. Rapid progress has been made in designing and synthesizing novel biomaterials, nanomaterials in particular, to carry both antigen and adjuvant, protect them from degradation in vivo, and deliver them to targeted lymphoid tissues, including LNs and mucosa-associated lymphoid tissues (e.g., lymphoid organs of the airways, gastrointestinal tract, and reproductive tract).
The LN is the anatomic location where naive T cells make contact with APC partners such as DCs (108), and the LNs contain a much higher concentration of DCs than peripheral tissues do. In addition, humoral immunity is initiated by B cells in LNs; B cells take up antigen directly in LN follicles to generate protective antibodies (109). Thus, both T cell and antibody responses require antigen and adjuvant to be delivered to LNs to efficiently induce primary immune responses.
Vaccines are typically injected intramuscularly or subcutaneously and must then reach LNs by draining through lymphatic vessels. A key factor regulating this convective transport is the size of the vaccine’s components because the extracellular matrix of connective tissues traps large particles. The role of particle size in LN targeting was elegantly demonstrated by the laboratories of Hubbell, Swartz and coworkers (110--112), which synthesized oxidation-sensitive poly(propylene sulfide) NPs (PPS NPs) of well-defined sizes with covalently conjugated antigens to define the optimal particle sizes required for targeting vaccine antigens to DCs in LNs. These studies showed that 20 nm PPS NPs were more readily taken up into the lymphatics and retained in draining LNs for longer periods (up to 120 hours), compared to larger particles 100 nm in size. Moreover, up to 40--50% of resident LN DCs internalized 20 nm NPs. When conjugated with OVA, 25-nm polyhydroxylated-OVA NPs elicited similar levels of both cellular and humoral immunity as OVA injected with LPS, which is a very strong adjuvant compound with unacceptable toxicity for vaccine use. Similar conclusions about the optimal solid NP sizes for maximal LN uptake have been drawn by other studies using polystyrene NPs as model vaccine carriers (113, 114). Beyond tissue-level transport and delivery to LNs, particle size also has a role in determining the efficiency of uptake by DCs, with submicron-sized particles taken up by DCs more efficiently than larger particles (115).
Mucosa-associated lymphoid tissues---that is, lymphoid tissues draining mucosal surfaces---are another important target for vaccine delivery. Vaccination through mucosal surfaces, such as the airways or reproductive tract, offers the possibility of needle-free administration, and mucosal immunity may be key for optimal protection against pathogens that invade through mucosal surfaces (116). However, mucosal surfaces present a series of barriers to vaccine absorption: vaccines are diluted in mucosal secretions, captured in mucus gels, attacked by proteases and nucleases, and excluded by epithelial barriers (116, 117). Using the same PPS NP system described above, Nembrini et al. (118) showed that pulmonary administration of NPs can deliver antigen very effectively to DCs in the pulmonary parenchyma, promote highly efficient cross presentation, and induce potent protective mucosal and systemic CD8+ T cell immunity. When this NP system was applied to deliver a tuberculosis antigen (Ag85B) adjuvanted with CpG oligonucleotides, pulmonary vaccination led to enhanced induction of antigen-specific polyfunctional Th1 and Th17 responses, and protective efficacy against aerosolized Mycobacterium tuberculosis challenge when compared with soluble Ag85B with CpG or with the same formulation administered intradermally (119). The effective mucosal uptake of these PPS NP vaccines seems to agree well with work done to define the properties of particles required for mucus penetration. A series of detailed studies by the Hanes laboratory (120--122) has shown that particles with sizes less than 500 nm, dense PEGylation, and lacking overt charge are required for efficient diffusion through mucus. Efficient vaccination via NP vaccines in the lungs may also be facilitated in part by active uptake by macrophages and DCs that constitutively sample antigens across the mucosal barrier (123). Li et al. (124) showed that coadministration into the lungs of antigen-loaded lipid nanocapsules of approximately 200 nm in diameter together with TLR agonist adjuvants led to dramatically elevated antigen delivery to lung-draining LNs (leading to 100% protection against pulmonary vaccinia virus challenge) when compared with the same vaccine components administered in soluble form (0% protection for the soluble vaccine). The accessibility of reproductive tract mucosa may enable additional strategies for vaccine delivery. For example, Kuo-Haller et al. (125) demonstrated that poly(ethylene-co-vinyl acetate) disks designed for insertion into vaginal mucosa could release protein vaccines for up to 2 weeks, leading to substantially enhanced mucosal IgG and IgA responses when compared with traditional parenteral vaccination.
3.2. Hitchhiking on Lymphocytes
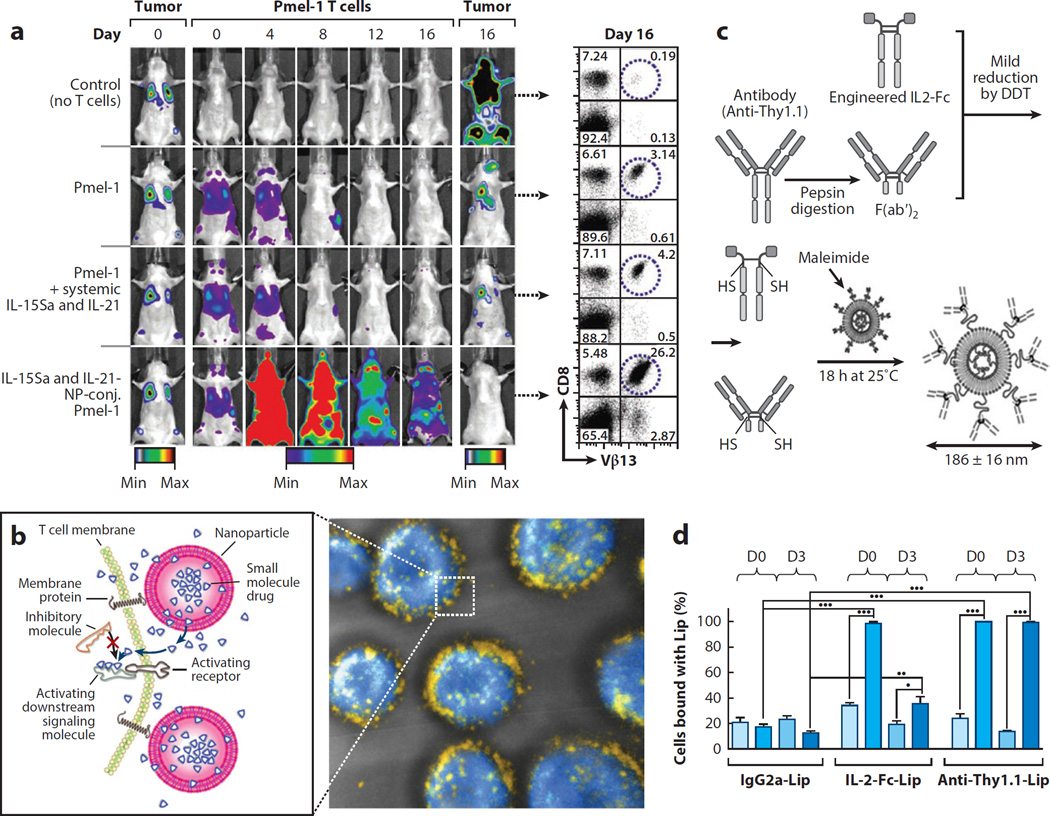
Immunotherapy treatments aim to stimulate or augment a patient’s own immune system to attack cancer or infectious disease (126, 127). One of the most promising immunotherapy strategies undergoing clinical testing to treat cancer is adoptive cell therapy (ACT), in which ex vivo expanded, autologous antigen-specific T cells are infused into patients with advanced cancers or viral infections (128--130). However, a limitation of this approach is the decline in function of transplanted T cells after infusion, particularly in the setting of solid cancers where tumors actively suppress T cell effector functions. In order to maintain high numbers of functional tumor-specific cytotoxic T lymphocytes (CTLs), immunostimulatory agents, such as interleukin cytokines, are often coadministered with transferred cells. However, these adjuvant agents often exhibit severe dose-limiting toxicities (131, 132). To enhance the efficacy of adjuvant drugs during ACT while avoiding systemic toxicity, Stephan et al. (133) developed a novel strategy of piggybacking drug carriers loaded with supporting agents directly onto the T cells themselves. Adjuvant agent-releasing lipid NPs were chemically conjugated to the plasma membrane of donor cells, enabling continuous pseudoautocrine stimulation of transferred T cells in vivo (Figure 4a). In a model of ACT for cancer, marked enhancements in tumor elimination were achieved using this strategy (Figure 4a). Using the same strategy it was further shown that small-molecule drugs can be efficiently delivered into the T cell synapse, enhancing their therapeutic efficacy (134) (Figure 4b). In a related strategy aiming to alter the differentiation fate of CD4+ T cells, the Fahmy group (98) reported on the design of NPs loaded with leukemia inhibitory factor or IL-6 and designed specifically to bind to helper T cells through functionalization with anti-CD4 antibodies. When mixed with donor lymphocytes just prior to infusion, these particles bound to CD4+ T cells and modulated their differentiation to promote tissue-graft survival or promote an increase in Tregs (98).
Figure 4.
Hitchhiking on lymphocytes. (a) Tumor-specific Pmel-1 T cells conjugated with interleukin-15 superagonist (IL-15Sa) and IL-21-releasing nanoparticles (NPs) robustly proliferate in vivo and eradicate established B16 murine melanomas. Dual, longitudinal, in vivo bioluminescence imaging shows the growth of Gaussia luciferase--expressing B16F10 tumors and click beetle red luciferase--expressing T cells. Flow cytometry plots show the frequencies of Vβ13+CD8+ tumor-specific T cells recovered from pooled lymph nodes of representative mice 16 days after T cell transfer. (b, left) Schematic view of strategy to modulate T cell responses via nanoparticle conjugation to membrane proteins: Surface-conjugated drug-loaded nanoparticles slowly release their cargo compounds, which locally permeate the plasma membrane and block molecules in the cytosol that dampen T cell activation. (Right) 3D reconstruction of confocal microscopy images showing CD8+ effector T cells (carboxyfluorescein succinimidyl ester stain shown in blue) immediately after conjugation with fluorescent, multilamellar lipid vesicles (yellow). (c) Schematic of T cell--targeted immunoliposome preparation. IL-2-Fc and anti-Thy1.1 F(ab′)2 were mildly reduced by dithiothreitol (DTT) to expose hinge region free thiols (-SH) for reaction with the liposome maleimide functional headgroups. (d) Quantification of percentages of endogenous or transferred T cells labeled by day 0 or day 3 liposome (Lip) injections in the blood. (Panel a adapted with permission from 133; panel b adapted with permission from 134; panels c and d adapted with permission from 135.)
The ex vivo manipulation of cells is a clinically laborious and expensive therapy, but another alternative is to target supporting agents to T cells directly in vivo. Zheng et al. (135) demonstrated the possibility of direct in vivo targeting using cytokines or cell-specific antibodies as ligands to target PEGylated liposomes to therapeutic T cells, thus enabling repeated stimulation of ACT T cells (Figure 4c and d). The high efficiency of targeted NP binding to T cells as they recirculate in the blood suggests that this could be a straightforward strategy for modulating the function of immune cells.
4. BUILDING LYMPHOID ORGAN STRUCTURES
4.1. Engineered Lymphoid Tissues
Cells of the immune system are generated in primary lymphoid organs (the thymus and bone marrow) and maintained or activated in secondary lymphoid organs (the spleen, LNs, and Peyer’s patches). Much as in other applications in regenerative medicine, biomaterials have been explored as scaffolds to create synthetic lymphoid organs in vivo and as platforms to generate functional lymphoid tissues in vitro (136, 137). In vivo synthetic lymphoid organs can be used as platforms to dissect critical cues in lymphoid tissue organogenesis or neogenesis, or to provide enhanced immune function in humanized mouse models, where the native murine lymphoid tissue exhibits structural or organizational defects due to the immunodeficient background of the animals during development, as well as incomplete crosstalk among mouse and human cytokines and receptors (138). In vitro models of lymphoid tissue can also be used for fundamental studies, and, further, could provide an important nonanimal model-based solution to preclinical toxicity testing of pharmaceutical agents and act as a screening tool for vaccine or drug development.
T cells differentiate from hematopoietic precursors in the thymus. Strategies to generate functional human T cells using in vitro mimics of the thymus have been pursued as models to enhance understanding of the fundamental biology of T cell differentiation and also as potential platforms for generating large numbers of therapeutic lymphocytes for treatments such as ACT in cancer. Pioneering work by the Scadden laboratory (139) first showed that porous 3D tantalum-coated carbon sponges could be used as a supporting scaffold for thymic stromal cells. In these studies, the addition of human hematopoietic precursor cells to scaffolds harboring murine thymic stroma led to the differentiation of nearly 80% of the seeded human cells into CD3+ T cells, a 2--3-fold increase in the frequency of T cell differentiation when compared with the use of 2D in vitro cultures. The lymphocytes that were generated expressed diverse T cell receptor-α and -β chains and responded appropriately to mitogenic stimuli in vitro. In pursuit of strategies to generate T cells in the absence of xenogeneic stromal cells, Taqvi et al. (140) reported using synthetic microspheres functionalized with recombinant notch ligands, which mimic key signals provided by stromal cells during thymocyte development. This approach may be able to be combined with other biomaterials-based approaches to create fully synthetic thymic microenvironments.
Other groups have focused on engineering secondary lymphoid organs that could support functional immune responses in vivo or provide a model of human lymphoid tissue in vitro. One approach that is useful in the setting of vaccination is to use biomaterials to engineer the microenvironment of native LNs by injecting immunomodulating biomaterials intranodally---that is, directly into native lymphoid tissue. Jewell et al. (78) showed that biodegradable microparticles injected into peripheral LNs could disperse throughout the T-and B zones of the tissue and release inflammatory stimuli to alter the activation state of DCs for approximately 1 week, thus significantly enhancing the response to vaccination. Alternatively, an exciting challenge would be to use biomaterials to support the organogenesis of de novo lymphoid organs. The development of secondary lymphoid organs depends on a complex set of orchestrated cellular and cytokine and chemokine cues, but, interestingly, lymphoid tissue neogenesis can also be triggered by ectopic expression or a single lymphoid cytokine or chemokine (141, 142). Building on such fundamental observations, Suematsu & Watanabe (143) showed that the implantation of macroporous collagen scaffolds seeded with lymphoid stromal cell lines engineered to express the key instructive cytokine lymphotoxin-α could lead to the spontaneous generation of complete lymphoid tissues in mice. These engineered tissues showed segregated accumulations of T cells and B cells in separate zones reminiscent of native LNs, as well as generation of the specialized endothelium of LNs (high endothelial venules) (143). Importantly, these engineered lymphoid organs were functional for T cell and B cell activation and could support antigen-specific immune responses in immunodeficient animals (143--145).
In vitro LN constructs generated from murine or all-human cell preparations have also been pursued. Giese et al. (146, 147) employed agarose gels or nonwoven polyamide fiber scaffolds as matrices for human T and B cells, and DCs that mimic the extremely high cell densities present in lymphoid tissues. These constructs have shown the ability to support antibody responses to licensed vaccines and physiologically relevant alterations in cytokine production in response to immunosuppressive drugs (146). Higbee et al. (148) developed a modular in vitro system termed MIMIC (Modular Immune In vitro Construct) with separate in vitro scaffolds representing peripheral tissues and LNs, and that aimed to predict the responses of cultured human leukocytes to vaccine administration or other biologicals. The lymphoid tissue module of this system was able to predict antibody responses measured in the blood of immunized individuals following tetanus vaccination.
The vigorous migration of lymphocytes is also a key part of LN physiology (149). To this end, composite scaffolds comprising ordered, macroporous PEG hydrogels infiltrated with fibrillar collagen matrix have been shown to support physiological lymphocyte migration between DCs adherent to the macroporous PEG support (150, 151). Chemokines promoting the robust migration of lymphocytes scanning for antigen in LNs are produced by the LN stroma (152). Tomei et al. (153) demonstrated that murine fibroblastic reticular cells, the stromal cells lining T cell areas of LNs, are induced to produce the key lymphoid chemokine CCL21 by exposure to 3D fluid flow. These various strategies, which aimed to recapitulate critical features of the LN microenvironment, have both provided new insights into the physiology of lymphoid tissue and shown progress toward developing in vitro models of de novo immune reactions that might be used for screening vaccine or drug formulations.
4.2. Biomaterials as Vaccination Nodes for Immunization
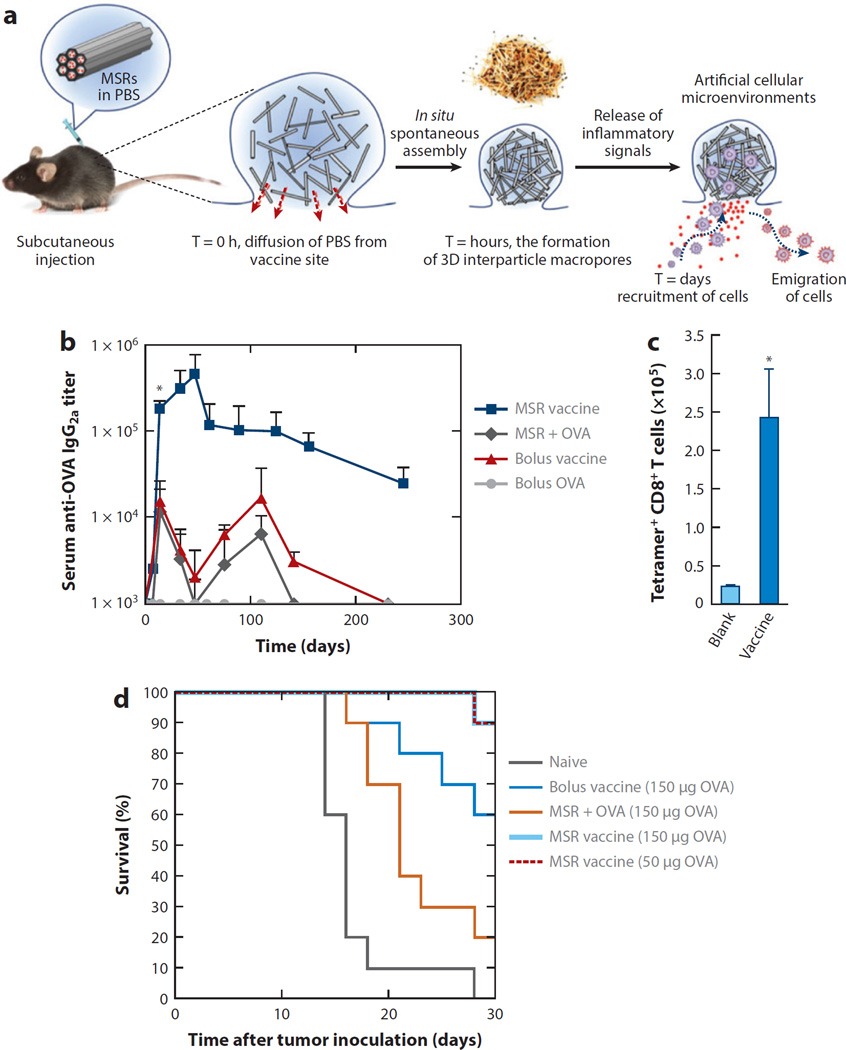
The engineering strategies for biomaterials discussed above focus on the cells and tissue functions of primary lymphoid organs (where immune cells mature) and secondary lymphoid organs (where immune cells are activated) during immune responses. However, biomaterials can also be employed to shape events happening outside the lymphoid organs during the earliest stages of a nascent immune response. In these cases, investigators have pursued strategies using biomaterials as matrices to promote the attraction of APCs or their precursors, APC differentiation and activation, and loading with antigen to promote the immune response. Ali et al. (154, 155) developed an implantable PLGA sponge scaffold designed to regulate each of these steps through the sequential release of scaffold-embedded granulocyte--macrophage colony-stimulating factor (GM-CSF), CpG oligonucleotides complexed with polyethyleneimine, and tumor lysate. GM-CSF is a chemoattractant and differentiation factor for DC precursors. In these studies, scaffolds were designed to release this cytokine during 1--2 weeks to recruit and differentiate DCs in the scaffold. CpG and tumor lysate were largely retained in the scaffolds during this same time frame, allowing cells recruited to the implant to be activated (by the inflammatory CpG signal) and loaded with antigen (the tumor lysate). The activated and antigen-loaded cells then migrated to the draining LNs, leading to efficient priming of antitumor T cells (155). Interestingly, injections of microparticles releasing the same vaccine components were not effective when compared with the scaffold-based vaccine, but why the 3D scaffold structure may be important for this response remains to be determined. When compared with implants carrying only one factor or the other, GM-CSF- and CpG-loaded scaffolds elicited the highest accumulation of plasmacytoid DCs and CD8+ DCs (important for crosspresentation of antigen to CD8+ T cells), which correlated with greatly enhanced protection in a murine model of therapeutic vaccination for melanoma (154). Based on these impressive preclinical results, this scaffold-based vaccine is being rapidly moved forward into a small clinical trial of preventing melanoma at the Wyss Institute. (https://clinicaltrials.gov/ct2/show/NCT01753089). Building on these results, Mooney and coworkers further developed injectable pore-forming 3D scaffolds that spontaneously assemble in vivo from mesoporous silica rods (MSRs) as an implant to shape the host immune cell response (Figure 5a) (156). When loaded with OVA, GM-CSF and CpG-ODN, this MSR-scaffold-based vaccine elicited potent immune serum antibody responses, and cytotoxic T cell responses that enhanced protection against OVA-expressing tumors (Figure 5b–d).
Figure 5.
Injectable and spontaneously assembling scaffolds consisting of mesoporous silica rods (MSRs) modulate immune cells in vivo and increase vaccine efficacy. (a) A schematic representation of in vivo spontaneous assembly of MSRs and recruitment of host cells for maturation. PBS: phosphate-buffered saline. (b) Enzyme-linked immunosorbent assay (ELISA) analysis of serum ovalbumin (OVA)- specific immunoglobulin (Ig) G2a titers after immunization with, respectively, soluble components of the vaccine (bolus vaccine), MSRs loaded with OVA, or MSR vaccine. (c) Number of tetramer+ CD8+ T cells in spleen 7 days after vaccination with blank MSR (labeled Blank) or complete MSR vaccine (labeled Vaccine). (d) Survival rate after subcutaneous injection of various vaccine formulations 10 days before EG.7-OVA tumor inoculation. (Adapted with permission from 156.)
As noted above, Singh et al. (157) have also designed injectable PEG-based hydrogels carrying the DC attractant CCL20, and PLGA microspheres carrying antigen-encoding DNA and small interfering RNA against the suppressive cytokine IL-10. These composite gels attracted DCs, induced priming of antigen-specific T cells, and increased survival in a prophylactic model of vaccination against lymphoma (158). The synthetic immune priming center-based particles showed 45% more CD8+ CTL response and 53% stronger CD4+ CTL activity than naked DNA vaccine in mice, and tumor-challenged mice had a 40% survival rate (twice that of those receiving the naked DNA vaccine) (158). Similarly, mice inoculated with a different antigen (hepatitis-B surface antigen) were shown to significantly switch their immune phenotype toward a Th1 response as evidenced by an increase in IFN-γ production and a decrease in IL-4 production by CD4+ T cells. This further led to enhanced antiviral CTL activity and higher murine survival (159).
Park et al. employed a multifunctional depot strategy with nanogels that simultaneously release tolerance inhibitors and pro-immunity cytokines (160) . To enhance the efficacy of tumor therapy, nanoliposomal polymeric gels were loaded with a TGF-β inhibitor and IL-2. These nanogels significantly delayed tumor growth, increased the survival of tumor-bearing mice to 100%, and increased the activity of natural killer cells and infiltration of intratumoral-activated CD8+ T cells (160). Another strategy for inducing immune responses against cancer relies on using synthetic matrices to surround an accessible tumor with immunostimulatory factors designed to attract and activate immune effectors to the tumor, with the goal of turning a tumor into its own vaccine. Hori et al. (161--163) developed self-gelling alginate solutions composed of soluble alginate chains mixed with immunomodulatory factors (cytokines and adjuvants) and calcium-releasing alginate microspheres. When mixed and injected peritumorally in a mouse model of melanoma, calcium exchange from the microspheres led to gelation of alginate around established tumors. Peritumoral alginate matrices releasing a superagonist form of the potent immunostimulatory cytokine IL-15 (IL-15SA) concentrated the cytokine in the tumor during several days, whereas simple intratumoral injection of the same dose of IL-15 led to rapid clearance of the cytokine. This prolonged exposure to IL-15SA led to greatly increased levels of CD8+ T cells and reduced levels of Tregs in tumors, and cured approximately 60% of mice bearing B16F10 melanoma tumors (161). Altogether these approaches demonstrate that biomaterials can controllably orchestrate sequential steps leading to the induction of an optimal immune response, thus greatly enhancing the potency of vaccines and cancer immunotherapies.
5. SYNTHETIC ANTIGEN-PRESENTING CELLS
Bioconjugation approaches offer opportunities to control display parameters such as the form of presentation of immunomodulatory ligands, controlling the release of antigens, or controlling delivery of soluble immunomodulators. Such approaches have allowed complex immune synapses to be stripped down not only to elucidate their key components but also to engineer functionality through the properties of the materials used or display parameters. These approaches are supported by reports showing that the intensity of the T cell stimulus depends on the density of the bound receptors that are in contact with a surface (164, 165). The presence of high-density clusters has also been shown to accelerate T cell activation (164, 165). Further, in the LNs, APCs are thought to concentrate the presentation of T cell stimuli to enable effective immune responses, a process that is mimicked in the synthetic system (166). Using this idea, several immunomodulatory approaches have been attempted including carbon nanotube display of T cell agonists, multivalent polymeric presentation of immune stimulants, and artificial APCs designed for ex vivo and in vivo applications.
T cells have been stimulated ex vivo using single-walled carbon nanotube (SWNT) bundles presenting antibody stimuli (167). Studies have shown that SWNTs displaying a T cell-stimulating antibody (anti-CD3) induce potent activation of T cells due to the high density of antibody that is displayed from the surfaces of these complexes. Another approach utilized SWNTs to present MHC-1 via adsorbing neutrAvidin to the SWNT and then take biotinylated peptide-specific MHC-1 and display it from the SWNT. Using this approach, significant CD8+ T cell activation was seen along with an enhanced antigen-specific T cell response that was more than threefold higher than the soluble control in similar conditions (168). A different multimeric approach using poly(amido amine) dendrimers has been shown in a variety of studies to enhance immunity with increasing multivalency (169--173). For example, Fahmy et al (171) showed that plate-bound anti-CD3 immobilized by poly(amido amine) dendrimers and co-delivered with doxorubicin, a potent antimitogenic drug, was able to significantly bind and inhibit T cell proliferation.
Artificial immune-stimulating systems are an emerging technology that aims to induce therapeutic immunity without the need for autologous APCs. Steenblock et al. (174) loaded PLGA microparticles with IL-2 and then adsorbed anti-CD3 to the surface. They showed that CD8+ T cells were highly proliferative in response to the scaffold’s sustained release of IL-2, whereas the increase in CD4+ T cells was limited by apoptosis. They found that synaptic accumulation of IL-2 in the early stages of activation was important for activation, and that slow and sustained release of IL-2 was critical for expansion of the T cell population, whereas an initial short-lived burst of cytokines (on the order of hours) played no role in expansion of the population (174). In a mouse tumor model, a single injection at day 10 after tumor implantation of loaded particles releasing IL-2 and displaying anti-CD3 and anti-CD28 significantly delayed the kinetics of tumor growth (175). A final approach to immune stimulation, based on immobilization of surface ligands, has been developed by Roy and coworkers (140). They differentiated stem cells into immunocompetent T cells using beads displaying synthetic notch ligands.
All of the approaches described above used either in vivo expansion and stimulation or ex vivo training and expansion of mature antigen-specific T cells. However, these approaches are not viable in patients for whom autologous T cells are not available (such as patients with lymphoma or those who are immune-deficient). For such patients, robust and reproducible in vitro generation of functional, transplantable T cells from embryonic stem or adult stem cells will be necessary (176). With this in mind, Roy and coworkers (140) functionalized magnetic microbeads with the notch ligand DLL4 using streptavidin--biotin binding and antibody--antigen coupling. Thy1.2+ early T cells were successfully generated from mouse bone marrow hematopoietic stem cells using DLL4 functionalized beads. In a similar approach, stem cells were differentiated into antigen-specific CD8+ human T cells using DLL1-coated plates and cytomegalovirus or human leukocyte antigen (HLA) A*201 tetramers loaded with influenza A virus epitopes (176). The resultant T cell population was a polyclonal population that was specific for cytomegalovirus or influenza; additionally, these cells were found to exhibit cytolytic functionality against infected cells and to have high IFN-γ production and Granzyme B secretion (176).
6. SYSTEMS APPROACHES
Systems biology combines high-content multiplexed measurements with statistical and computational modeling to elucidate biological functions and interrelationships at various scales. The temporal and spatial complexity of the events that lead to proinflammatory or tolerogenic immune responses have been increasingly found to involve complex signaling interactions that occur both intra- and intercellularly (177). Many important insights have emerged from studies that have focused on a single level of the hierarchy [for example, TLR signaling pathways in DCs (178), or paracrine cytokine signaling by T cells (179)], but such insights cannot offer a picture of cause and effect when immune cells are presented with complex biomaterial stimuli. This is due, in large part, to overlapping and competing signaling from membrane and intercellular receptors (180). As such, a systems biology approach to immunology has been proposed to help elucidate the intricate behavior of the immune system in response to biomaterials. Three different scales of immune response have been considered in reference to biomaterials: DNA and RNA expression, surface-marker expression and signaling, and secreted cytokines and chemokines.
Gene expression or RNA microarrays, or both, have recently been used to analyze the effects of a range of stimuli from biomaterials including carbon nanotubes (181, 182), substrates (183), and particulate biomaterials (184); foreign-body reactions have also been analyzed (185). Although these approaches utilize a method of analysis known as shot-gun genomics to determine the immune response to various biomaterials, many insights have been gained into the underlying mechanisms of what is happening in cells. For example Aldinucci et al. (182) showed that when DCs were placed on multiwalled carbon nanotubes the nanotubes affected DC gene regulation for functions such as focal adhesion, adherent junctions, actin organization, and the ability of DCs to properly reorganize the cytoskeleton upon LPS recognition. These findings have implications for the immune response noted by Fadel et al. (167, 168); and these results led researchers to study the behavior of the adherent DCs that were not properly rearranging their cytoskeleton and had decreases in endocytic processes (182).
Although DNA- and RNA-expression microarrays provide an excellent way to determine what is happening to a cell in terms of transcription, it has been confirmed many times and in multiple models that transcriptional activity does not necessarily directly relate to translational activity, which does not necessarily lead to functional protein expression or secretion. Thus, high-throughput strategies that assess functional outcomes (such as protein expression or secretion) have been developed. A high-throughput tool for assessing surface markers has been developed by Kou & Babensee (186); it looked at the fold change in CD86 (proinflammatory) over DC-SIGN (constitutively expressed) surface markers on DCs to assess proinflammatory DC activation. This tool was then used to assess DC activation on a library of titanium surfaces (187), as well as to elucidate which molecular properties of a library of polymethacrylates (188) were associated with DC activation. Thus, with no a priori knowledge of a material’s properties and of immune activation, the researchers were able to develop a model that showed that biomaterial surface chemistry was most important in determining induced DC phenotypes. Specifically, surface oxygen content was associated with iDCs, and increased surface carbon was associated with enhanced DC maturation (187, 188). In these studies, secreted cytokines were also measured using a fairly high-throughput multiplexed cytokine secretion assay and a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). However, this method becomes prohibitively expensive for larger libraries of materials. Thus, Garcia-Cordero et al. (189) recently introduced, characterized, and applied a microfluidic technology platform for low-cost high-throughput quantitation of soluble proteins in various sample matrices. This platform was applied in the context of systems vaccinology to assess the synergistic production of inflammatory cytokines from DCs stimulated with 10 different adjuvants that target TLRs. With this system, they were able to measure in a mouse model six different cytokines simultaneously at four different time points in response to MPLA plus Gardiquimod (InvivoGen, San Diego, CA) and MPLA plus CpG-B in samples from bronchoalveolar lavage and in blood serum. Garcia-Cordero et al. found that their results agreed with the industry-standard ELISA (enzyme-linked immunosorbent assay), used 1,000 times less reagent, and could multiplex simultaneous measurements to a much higher degree than other assays. Reducing the necessary sample volume to a few nanoliters was key to decreasing the cost of reagents by more than three orders of magnitude and to being able to assess other high-throughput assay outputs, such as those developed by Kou et al.(186–189) Anderson and colleagues (190) have provided another important approach to combining surface-characterized polymer microarrays in a high-throughput manner to elucidate the relationships between surface structure and function that drive cellular interactions. All of the systems biology approaches described above utilized statistical modeling (principle component analysis (187), partial least squares regression (188), cluster analysis (18), or pathway signatures analysis (182)) to glean insights from massive data sets. It is necessary to combine rich quantitative experimental data and knowledge of biochemical or cellular parameters in order to gain knowledge from these data sets. The predicted outcomes from the models must then be tested at the bench to examine the strength of the underlying model. The models or questions that can be generated from computational analysis of large data sets can be used to inform experimental designs because it is from the accumulated data sets that correlations or predictions can be made. Furthermore, independently produced data sets that have some overlapping measurements can be combined mathematically to further expand the questions that can be addressed. It has been found that much more complete and informative models of immunological processes than those that are formulated purely by hypothesis-driven research or represented by simplified cartoons in reviews can be made by using iterative cycles of model building, simulation, prediction, experiment, and model refinement. This field of systems approaches to immunology and biomaterials is in its infancy, and it holds the potential to drive the development of the next generation of biomaterials and to foster a deeper understanding of underlying biological process and interactions between biomaterials and immune cells.
7. OUTLOOK AND CHALLENGES
As illustrated by the diverse examples provided here, the application of biomaterials as immunomodulating therapeutics and as model systems for studying fundamental immunology shows great promise. Rapid advances in this relatively new area of biomaterials research have been enabled by an integration of lessons learned during the past 15 years in other areas, such as drug delivery and regenerative medicine. The demonstration that biomaterials can be used to amplify or suppress immune responses has already been applied to models of infectious disease, cancer, and autoimmunity. However, a number of key challenges and areas for future work are ripe to be addressed.
One area where more can be done using biomaterials for immune modulation is in using synthetic materials as tools to dissect the rule set governing immune responses in vivo. For example, various attempts to determine how the kinetic pattern of exposure to antigen and inflammatory signals influences the induction of immunity during vaccination have been pursued using common tools in the vaccinologist’s toolbox, such as the administration of DNA vaccines to achieve prolonged antigen expression, or the administration of viral vectors with short versus long times to immune clearance. But such approaches are inherently constrained by the immune response itself, which directly governs the pattern of vaccine kinetics that are obtained through the elimination of antigen-expressing cells. Biomaterials that provide a defined pattern of antigen--adjuvant availability independent of ongoing inflammation or effector responses are already being designed (191, 192), and systematic studies of how parameters---such as the duration, magnitude, and temporal pattern of vaccine exposure---affect the magnitude, quality, and durability of immune memory are needed. A first step in this direction was taken by Pulendran and coworkers (193), who compared trivalent inactivated influenza vaccine and live, attenuated influenza vaccine in humans and mice. The trivalent vaccine induced higher antibody titers and more plasmablasts than the live, attenuated vaccine did. In humans given the trivalent vaccine, expression of the kinase CaMKIV at day 3 after vaccination was inversely correlated with later antibody titers and, thus, could be used as a predictive metric for vaccine efficacy (193). More recently, genomic analysis of the human immune response to the influenza vaccine showed that 20 genes exhibit a significant correlation with transcriptional immune responses and with downstream antibody responses (194). These results showed that variation at the level of genes involved in membrane trafficking and antigen processing significantly influences the human response to influenza vaccination, and that the transcriptional response could be used as a correlate for downstream vaccine efficacy. More such studies could provide valuable guidance to the vaccine field at large, irrespective of their individual potential for translation directly into viable vaccine platforms.
A second area for future work, which is closely linked to the first, is to better define how synthetic biomaterials intrinsically modulate innate immunity and adaptive responses. Recent studies focusing on how classic adjuvants, such as alum and other foreign materials, interact with the immune system likely have relevance to determining how other synthetic biomaterials may stimulate host molecular sensors of danger and damage (195--197). Such insights could open up new avenues for developing safe and inexpensive strategies to modulate host immunity in vaccines and immunotherapies.
Finally, the overarching goal of most biomaterials research is to develop new materials that can be translated to clinical use. As with the development of any new type of biomedical intervention, this is a costly process with a high rate of failure, and it often requires a transfer of basic research findings to commercial entities capable of carrying out the necessary development and manufacturing scale-up to prepare clinical-grade materials. However, academic collaborations between biomaterials researchers and clinicians may provide a second route for clinical translation, as illustrated by the ongoing Wyss Institute-funded Phase I trial of implantable vaccine scaffolds (discussed in Section 4). Such translational efforts will be aided by the recognition of key areas of need and opportunity. For example, cancer immunotherapy, after more than 20 years of preclinical and clinical studies that showed much promise but little clinical success, has now begun to show clear clinical results, and multiple ongoing trials of immunomodulatory drugs have demonstrated that the immune system can attack and destroy metastatic cancer even in heavily pretreated patients with advanced cancer (198--200). Biomaterials-based immunotherapies that can further enhance the efficacy or reduce the toxicity of these immunomodulatory strategies will be well positioned to capitalize on the renewed sense of promise in the field of cancer immunotherapy (201).
As highlighted in this review, much of the recent work in immune-stimulating biomaterials has focused on developing novel vaccine strategies and cancer immunotherapies, but many opportunities remain beyond these applications, in which the ability of biomaterials to regulate the immune system can have an impact on both basic immunology and disease therapy. Although most vaccine studies have addressed infectious diseases and cancer, much less attention has focused on vaccination strategies promoting tolerance, which could have an impact on autoimmune diseases such as multiple sclerosis. Biomaterials designed to promote tolerance may also have a role to play in solving problems in transplant rejection, and debilitating, chronic graft-versus-host disease. Biomaterials should also have an expanded role as tools for modeling or mimicking the human immune system to improve our understanding of human immunity. For example, biomaterials approaches from regenerative medicine may have an important part to play in creating improved human tissue microenvironments in humanized mouse models of infectious disease and cancer to allow a bridge to be built between the utility of small animal models and the predictive power of human clinical studies.
Supplementary Material
Figure 1.
(a–c) Overview of interactions of different types and structures of biomaterials with the immune system. Abbreviations: Ig, immunoglobulin; IL-10, interleukin-10; MPLA, monophosphoryl lipid A; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic) acid; PRR, pattern recognition receptor; TGF-β, transforming growth factor-β; TLR, Toll-like receptor; lymph, lymph node; conj., conjugated.
Acknowledgments
D.J.I. gratefully acknowledges grant support from the Bill & Melinda Gates Foundation, the National Institutes of Health (awards AI104715, CA172164, CA174795, and AI095109), the U. S. Army Research Laboratory and the U. S. Army Research Office through the Institute for Soldier Nanotechnologies (contract number W911NF-13-D-0001), and the Ragon Institute of MGH, MIT, and Harvard. L.T. acknowledges the Cancer Research Institute Irvington Postdoctoral Fellowship. J.E.B also acknowledges the National Institutes of Health (awards 1RO1EB004633-01A1 and 1R21EB012339-01A1), and N.A.H. acknowledges the National Institutes of Health Cell and Tissue Engineering Doctoral Training Grant (UL1TR000454).
Footnotes
DISCLOSURE STATEMENT
DJI is a founder and holds equity in Vedantra Pharmaceuticals, which holds a license to patents related to the interbilayer-crosslinked multilamellar vesicles technology discussed in this review.
Contributor Information
Darrell J. Irvine, Email: djirvine@mit.edu.
Julia E. Babensee, Email: julia.babensee@bme.gatech.edu.
LITERATURE CITED
- 1.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem. Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 2.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. PNAS. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–1564. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, et al. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Pompano RR, Santiago FW, Maillat L, Sciammas R, et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudalla GA, Modica JA, Tian YF, Rudra JS, Chong AS, et al. A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv. Healthc. Mater. 2013;2:1114–1119. doi: 10.1002/adhm.201200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung JP, Moyano JV, Collier JH. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr. Biol. (Camb.) 2011;3:185–196. doi: 10.1039/c0ib00112k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder U, Graff A, Buchmeier S, Rigler P, Silvan U, et al. Peptide nanoparticles serve as a powerful platform for the immunogenic display of poorly antigenic actin determinants. J. Mol. Biol. 2009;386:1368–1381. doi: 10.1016/j.jmb.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 9. Deleted in proof. [Google Scholar]
- 10.Wang L, Hess A, Chang TZ, Wang Y-C, Champion JA, et al. Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine. 2014;10:473–482. doi: 10.1016/j.nano.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Babensee JE. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 2012;8:3606–3617. doi: 10.1016/j.actbio.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J, Gerber MH, Babensee JE. Phenotype and polarization of autologous t cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. A. 2014;103:170–184. doi: 10.1002/jbm.a.35150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J. Biomed. Mater. Res. A. 2005;74:503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- 14.Ishii KJ, Coban C, Akira S. Manifold mechanisms of toll-like receptor-ligand recognition. J. Clin. Immunol. 2005;25:511–521. doi: 10.1007/s10875-005-7829-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Hancock WS. Approach to the comprehensive analysis of glycoproteins isolated from human serum using a multi-lectin affinity column. J. Chromatogr. A. 2004;1053:79–88. [PubMed] [Google Scholar]
- 16.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Shokouhi B, Coban C, Hasirci V, Aydin E, Dhanasingh A, et al. The role of multiple toll-like receptor signalling cascades on interactions between biomedical polymers and dendritic cells. Biomaterials. 2010;31:5759–5771. doi: 10.1016/j.biomaterials.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Rogers TH, Babensee JE. The role of integrins in the recognition and response of dendritic cells to biomaterials. Biomaterials. 2011;32:1270–1279. doi: 10.1016/j.biomaterials.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29:4736–4750. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004;25:295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 21.Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005;26:2991–2999. doi: 10.1016/j.biomaterials.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Babensee JE. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008;20:101–108. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Norton LW, Park J, Babensee JE. Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J. Control. Release. 2010;146:341–348. doi: 10.1016/j.jconrel.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo J-W, Doshi N, Mitragotri S. Adaptive micro and nanoparticles: temporal control over carrier properties to facilitate drug delivery. Adv. Drug Deliv. Rev. 2011;63:1247–1256. doi: 10.1016/j.addr.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 26.Kobiasi MA, Chua BY, Tonkin D, Jackson DC, Mainwaring DE. Control of size dispersity of chitosan biopolymer microparticles and nanoparticles to influence vaccine trafficking and cell uptake. J. Biomed. Mater. Res. A. 2012;100:1859–1867. doi: 10.1002/jbm.a.34153. [DOI] [PubMed] [Google Scholar]
- 27.Sieving A, Wu B, Mayton L, Nasser S, Wooley PH. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J. Biomed. Mater. Res. A. 2003;64:457–464. doi: 10.1002/jbm.a.10368. [DOI] [PubMed] [Google Scholar]
- 28.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 29.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann. Biomed. Eng. 2005;33:179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner HR. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc. Res. 1973;5:167–179. doi: 10.1016/0026-2862(73)90069-1. [DOI] [PubMed] [Google Scholar]
- 31.Yoo J-W, Chambers E, Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr. Pharm. Des. 2010;16:2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 32.Ilium L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood clearance and organ deposition of intravenously administered colloidal particles: the effects of particle size, nature and shape. Int. J. Pharm. 1982;12:135–146. [Google Scholar]
- 33.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 35.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin Y, Xia Y. Self-assembly of monodispersed spherical colloids into complex aggregates with well-defined sizes, shapes, and structures. Adv. Mater. 2001;13:267–271. doi: 10.1021/ja011048v. [DOI] [PubMed] [Google Scholar]
- 37.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo J-W, Doshi N, Mitragotri S. Endocytosis and intracellular distribution of PLGA particles in endothelial cells: effect of particle geometry. Macromol. Rapid Commun. 2010;31:142–148. doi: 10.1002/marc.200900592. [DOI] [PubMed] [Google Scholar]
- 39.Zhang K, Fang H, Chen Z, Taylor J-SA, Wooley KL. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjug. Chem. 2008;19:1880–1887. doi: 10.1021/bc800160b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kodali VK, Roos W, Spatz JP, Curtis JE. Cell-assisted assembly of colloidal crystallites. Soft Matter. 2007;3:337–348. doi: 10.1039/b611022n. [DOI] [PubMed] [Google Scholar]
- 41.Vaine CA, Patel MK, Zhu J, Lee E, Finberg RW, et al. Tuning innate immune activation by surface texturing of polymer microparticles: the role of shape in inflammasome activation. J. Immunol. 2013;190:3525–3532. doi: 10.4049/jimmunol.1200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juliano RL, Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 1975;63:651–658. doi: 10.1016/s0006-291x(75)80433-5. [DOI] [PubMed] [Google Scholar]
- 43.Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9:1521–1532. doi: 10.1002/smll.201201390. [DOI] [PubMed] [Google Scholar]
- 44.Vonarbourg A, Passirani C, Saulnier P, Benoit J-P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 45.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 47.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Ren. Physiol. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- 49.Yuan F. Transvascular drug delivery in solid tumors. Semin. Radiat. Oncol. 1998;8:164–175. doi: 10.1016/s1053-4296(98)80042-8. [DOI] [PubMed] [Google Scholar]
- 50.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 51.Tran KK, Shen H. The role of phagosomal pH on the size-dependent efficiency of cross-presentation by dendritic cells. Biomaterials. 2009;30:1356–1362. doi: 10.1016/j.biomaterials.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotaling NA, Cummings RD, Ratner DM, Babensee JE. Molecular factors in dendritic cell responses to adsorbed glycoconjugates. Biomaterials. 2014;35:5862–5874. doi: 10.1016/j.biomaterials.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLOS ONE. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 55.Roser M, Fischer D, Kissel T. Surface-modified biodegradable albumin nano- and microspheres. II: Effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur. J. Pharm. Biopharm. 1998;46:255–263. doi: 10.1016/s0939-6411(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 56.Thiele L, Rothen-Rutishauser B, Jilek S, Wunderli-Allenspach H, Merkle HP, Walter E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J. Control. Release. 2001;76:59–71. doi: 10.1016/s0168-3659(01)00412-6. [DOI] [PubMed] [Google Scholar]
- 57.Xu F, Yuan Y, Shan X, Liu C, Tao X, et al. Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int. J. Pharm. 2009;377:199–206. doi: 10.1016/j.ijpharm.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Xiao K, Li Y, Luo J, Lee JS, Xiao W, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holzapfel V, Musyanovych A, Landfester K, Lorenz MR, Mailänder V. Preparation of fluorescent carboxyl and amino functionalized polystyrene particles by miniemulsion polymerization as markers for cells. Macromol. Chem. Phys. 2005;206:2440–2449. [Google Scholar]
- 60.Musyanovych A, Dausend J, Dass M, Walther P, Mailänder V, Landfester K. Criteria impacting the cellular uptake of nanoparticles: a study emphasizing polymer type and surfactant effects. Acta Biomater. 2011;7:4160–4168. doi: 10.1016/j.actbio.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 61.Kaminskas LM, Boyd BJ. Nanosized drug delivery vectors and the reticuloendothelial system. In: Prokop A, editor. Intracellular Delivery. Netherlands: Springer; 2011. pp. 155–178. [Google Scholar]
- 62.Sheng Y, Liu C, Yuan Y, Tao X, Yang F, et al. Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials. 2009;30:2340–2348. doi: 10.1016/j.biomaterials.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 63.Gref R, Domb A, Quellec P, Blunk T, Müller RH, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22:8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 65.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 66.Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 67.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1995;17:31–48. [Google Scholar]
- 68.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 70.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hotaling NA, Ratner DM, Cummings RD, Babensee JE. Presentation modality of glycoconjugates modulates dendritic cell phenotype. Biomater. Sci. 2014;2:1426–1439. doi: 10.1039/C4BM00138A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shibata Y, Kogiso M, Huang C-J, Nouri-Shirazi M, Mizoguchi E, Dorey CK. Macrophage chitin binding proteins in phagocytosis and M1 activation in response to chitin microparticles. J. Immunol. 2012;188:172–127. [Google Scholar]
- 73.Link A, Zabel F, Schnetzler Y, Titz A, Brombacher F, Bachmann MF. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J. Immunol. 2012;188:3724–3733. doi: 10.4049/jimmunol.1103312. [DOI] [PubMed] [Google Scholar]
- 74.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, et al. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. PNAS. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demento SL, Bonafé N, Cui W, Kaech SM, Caplan MJ, et al. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 2010;185:2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jewell CM, López SCB, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. PNAS. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. PNAS. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sánchez A, Tobío M, González L, Fabra A, Alonso MJ. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-α. Eur. J. Pharm. Sci. 2003;18:221–229. doi: 10.1016/s0928-0987(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 83.Chen L, Apte RN, Cohen S. Characterization of PLGA microspheres for the controlled delivery of IL-1α for tumor immunotherapy. J. Control. Release. 1997;43:261–272. [Google Scholar]
- 84.Hora MS, Rana RK, Nunberg JH, Tice TR, Gilley RM, Hudson ME. Controlled release of interleukin-2 from biodegradable microspheres. Nat. Biotechnol. 1990;8:755–758. doi: 10.1038/nbt0890-755. [DOI] [PubMed] [Google Scholar]
- 85.Li Y-P, Pei Y-Y, Zhou Z-H, Zhang X-Y, Gu Z-H, et al. PEGylated polycyanoacrylate nanoparticles as tumor necrosis factor-α carriers. J. Control. Release. 2001;71:287–296. doi: 10.1016/s0168-3659(01)00235-8. [DOI] [PubMed] [Google Scholar]
- 86.Golumbek PT, Azhari R, Jaffee EM, Levitsky HI, Lazenby A, et al. Controlled release, biodegradable cytokine depots: a new approach in cancer vaccine design. Cancer Res. 1993;53:5841–5844. [PubMed] [Google Scholar]
- 87.Lu L, Stamatas GN, Mikos AG. Controlled release of transforming growth factor β1 from biodegradable polymer microparticles. J. Biomed. Mater. Res. 2000;50:440–451. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 88.Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29:294–306. doi: 10.1016/j.tibtech.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.John AL, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic mast cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 2012;11:250–257. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 91.Freedman MS, Bar-Or A, Oger J, Traboulsee A, Patry D, et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology. 2011;77:1551–1560. doi: 10.1212/WNL.0b013e318233b240. [DOI] [PubMed] [Google Scholar]
- 92.Smith CE, Eagar TN, Strominger JL, Miller SD. Differential induction of IgE-mediated anaphylaxis after soluble versus cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. PNAS. 2005;102:9595–9600. doi: 10.1073/pnas.0504131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, et al. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J. Clin. Investig. 2013;123:1741–1749. doi: 10.1172/JCI65907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schweingruber N, Haine A, Tiede K, Karabinskaya A, van den Brandt J, et al. Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis. J. Immunol. 2011;187:4310–4318. doi: 10.4049/jimmunol.1101604. [DOI] [PubMed] [Google Scholar]
- 95.Thomas TP, Goonewardena SN, Majoros IJ, Kotlyar A, Cao Z, et al. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheumatol. 2011;63:2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao H, Kiptoo P, Williams TD, Siahaan TJ, Topp EM. Immune response to controlled release of immunomodulating peptides in a murine experimental autoimmune encephalomyelitis (EAE) model. J. Control. Release. 2010;141:145–152. doi: 10.1016/j.jconrel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ. Leukemia inhibitory factor is linked to regulatory transplantation tolerance. Transplantation. 2005;79:726–730. doi: 10.1097/01.tp.0000149324.42994.38. [DOI] [PubMed] [Google Scholar]
- 98.Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Mol. Pharm. 2011;8:143–152. doi: 10.1021/mp100203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 100.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo XR, Pothoven KL, McCarthy D, DeGutes M, Martin A, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. PNAS. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsai SE, Shameli A, Yamanouchi J, Clemente-Casares X, Wang JG, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 104.Jhunjhunwala S, Chen LC, Nichols EE, Thomson AW, Raimondi G, Little SR. All-trans retinoic acid and rapamycin synergize with transforming growth factor-β1 to induce regulatory T cells but confer different migratory capacities. J. Leukoc. Biol. 2013;94:981–989. doi: 10.1189/jlb.0312167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jhunjhunwala S, Balmert SC, Raimondi G, Dons E, Nichols EE, et al. Controlled release formulations of IL-2, TGF-β1 and rapamycin for the induction of regulatory T cells. J. Control. Release. 2012;159:78–84. doi: 10.1016/j.jconrel.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. PNAS. 2012;109:11270–11275. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hume PS, He J, Haskins K, Anseth KS. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials. 2012;33:3615–3625. doi: 10.1016/j.biomaterials.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lammermann T, Sixt M. The microanatomy of T-cell responses. Immunol. Rev. 2008;221:26–43. doi: 10.1111/j.1600-065X.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 109.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 110.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 111.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 112.Rehor A, Hubbell JA, Tirelli N. Oxidation-sensitive polymeric nanoparticles. Langmuir. 2005;21:411–417. doi: 10.1021/la0478043. [DOI] [PubMed] [Google Scholar]
- 113.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 114.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 115.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013;15:85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 117.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 118.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. PNAS. 2011;108:E989–E997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ballester M, Nembrini C, Dhar N, de Titta A, de Piano C, et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine. 2011;29:6959–6966. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 120.Ensign LM, Tang BC, Wang Y-Y, Tse TA, Hoen T, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012;4:138ra79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lai SK, Wang Y-Y, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. PNAS. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J. Exp. Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuo-Haller P, Cu Y, Blum J, Appleton JA, Saltzman WM. Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity. Mol. Pharm. 2010;7:1585–1595. doi: 10.1021/mp100009e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol. Dis. 2008;40:63–67. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Berger C, Berger M, Hackman RC, Gough M, Elliott C, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thompson JA, Lee DJ, Cox WW, Lindgren CG, Collins C, et al. Recombinant interleukin-2 toxicity, pharmacokinetics, and immunomodulatory effects in a phase I trial. Cancer Res. 1987;47:4202–4207. [PubMed] [Google Scholar]
- 133.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Irvine DJ, Stachowiak AN, Hori Y. Lymphoid tissue engineering: invoking lymphoid tissue neogenesis in immunotherapy and models of immunity. Semin. Immunol. 2008;20:137–146. doi: 10.1016/j.smim.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Cupedo T, Stroock A, Coles M. Application of tissue engineering to the immune system: development of artificial lymph nodes. Front. Immunol. 2012;3:1–6. doi: 10.3389/fimmu.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scadden DT, Poznansky MC, Evans RH, Foxall RB, Olszak IT, et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat. Biotechnol. 2000;18:729–734. doi: 10.1038/77288. [DOI] [PubMed] [Google Scholar]
- 140.Taqvi S, Dixit L, Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J. Biomed. Mater. Res. A. 2006;79:689–697. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- 141.Mebius RE. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 142.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 143.Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat. Biotechnol. 2004;22:1539–1545. doi: 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- 144.Tan JKH, Watanabe T. Artificial engineering of secondary lymphoid organs. In: Alt FW, editor. Advances in Immunology. Vol. 105. Amsterdam: Elsevier; 2010. pp. 131–157. [DOI] [PubMed] [Google Scholar]
- 145.Okamoto N, Chihara R, Shimizu C, Nishimoto S, Watanabe T. Artificial lymph nodes induce potent secondary immune responses in naive and immunodeficient mice. J. Clin. Investig. 2007;117:997–1007. doi: 10.1172/JCI30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giese C, Lubitz A, Demmler CD, Reuschel J, Bergner K, Marx U. Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol. 2010;148:38–45. doi: 10.1016/j.jbiotec.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Giese C, Demmler CD, Ammer R, Hartmann S, Lubitz A, et al. A human lymph node in vitro--challenges and progress. Artif. Organs. 2006;30:803–808. doi: 10.1111/j.1525-1594.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 148.Higbee RG, Byers AM, Dhir V, Drake D, Fahlenkamp HG, et al. An immunologic model for rapid vaccine assessment--a clinical trial in a test tube. Altern. Lab. Anim. 2009;37(Suppl. 1):19–27. doi: 10.1177/026119290903701S05. [DOI] [PubMed] [Google Scholar]
- 149.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat. Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 150.Stachowiak AN, Irvine DJ. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. A. 2008;85:815–828. doi: 10.1002/jbm.a.31661. [DOI] [PubMed] [Google Scholar]
- 151.Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv. Mater. 2005;17:399–403. [Google Scholar]
- 152.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 153.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 154.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Deleted in proof. [Google Scholar]
- 157.Singh A, Suri S, Roy K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials. 2009;30:5187–5200. doi: 10.1016/j.biomaterials.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singh A, Qin H, Fernandez I, Wei J, Lin J, et al. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release. 2011;155:184–192. doi: 10.1016/j.jconrel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 159.Singh A, Nie H, Ghosn B, Qin H, Kwak LW, Roy K. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol. Ther. 2008;16:2011–2021. doi: 10.1038/mt.2008.206. [DOI] [PubMed] [Google Scholar]
- 160.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hori Y, Stern PJ, Hynes RO, Irvine DJ. Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials. 2009;30:6757–6767. doi: 10.1016/j.biomaterials.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hori Y, Winans AM, Irvine DJ. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009;5:969–982. doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29:3671–3682. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 164.González PA, Carreño LJ, Coombs D, Mora JE, Palmieri E, et al. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. PNAS. 2005;102:4824–4829. doi: 10.1073/pnas.0500922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Andersen PS, Menné C, Mariuzza RA, Geisler C, Karjalainen K. A response calculus for immobilized T cell receptor ligands. J. Biol. Chem. 2001;276:49125–49132. doi: 10.1074/jbc.M109396200. [DOI] [PubMed] [Google Scholar]
- 166.Anderson AO, Shaw S. Conduit for privileged communications in the lymph node. Immunity. 2005;22:3–5. doi: 10.1016/j.immuni.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 167.Fadel TR, Steenblock ER, Stern E, Li N, Wang X, et al. Enhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuli. Nano Lett. 2008;8:2070–2076. doi: 10.1021/nl080332i. [DOI] [PubMed] [Google Scholar]
- 168.Fadel TR, Li N, Shah S, Look M, Pfefferle LD, et al. Adsorption of multimeric T cell antigens on carbon nanotubes: effect on protein structure and antigen-specific T cell stimulation. Small. 2013;9:666–672. doi: 10.1002/smll.201201684. [DOI] [PubMed] [Google Scholar]
- 169.Sheng K-C, Kalkanidis M, Pouniotis DS, Esparon S, Tang CK, et al. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur. J. Immunol. 2008;38:424–436. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- 170.Pisani MJ, Wheate NJ, Keene FR, Aldrich-Wright JR, Collins JG. Anionic PAMAM dendrimers as drug delivery vehicles for transition metal-based anticancer drugs. J. Inorg. Biochem. 2009;103:373–380. doi: 10.1016/j.jinorgbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 171.Fahmy TM, Schneck JP, Saltzman WM. A nanoscopic multivalent antigen-presenting carrier for sensitive detection and drug delivery to T cells. Nanomedicine Nanotechnol. Biol. Med. 2007;3:75–85. doi: 10.1016/j.nano.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 172.Zhou J, Neff CP, Liu X, Zhang J, Li H, et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011;19:2228–2238. doi: 10.1038/mt.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.García-Vallejo JJ, Ambrosini M, Overbeek A, van Riel WE, Bloem K, et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol. Immunol. 2013;53:387–397. doi: 10.1016/j.molimm.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 174.Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. J. Biol. Chem. 2011;286:34883–34892. doi: 10.1074/jbc.M111.276329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Demento S, Steenblock ER, Fahmy TM. Biomimetic approaches to modulating the T cell immune response with nano- and micro- particles. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009;2009:1161–1166. doi: 10.1109/IEMBS.2009.5332625. [DOI] [PubMed] [Google Scholar]
- 176.Fernandez I, Ooi TP, Roy K. Generation of functional, antigen-specific CD8+ human T cells from cord-blood stem cells using exogenous notch and tetramer-TCR signaling. Stem Cell. 2013;32:93–104. doi: 10.1002/stem.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat. Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 178.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 179.Chueh F-Y, Yu C-L. Engagement of T-cell antigen receptor and CD4/CD8 co-receptors induces prolonged stat activation through autocrine/paracrine stimulation in human primary T cells. Biochem. Biophys. Res. Commun. 2012;426:242–246. doi: 10.1016/j.bbrc.2012.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ng THS, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity: the role of interleukin-10. Front. Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pescatori M, Bedognetti D, Venturelli E, Ménard-Moyon C, Bernardini C, et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials. 2013;34:4395–4403. doi: 10.1016/j.biomaterials.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 182.Aldinucci A, Turco A, Biagioli T, Toma FM, Bani D, et al. Carbon nanotube scaffolds instruct human dendritic cells: modulating immune responses by contacts at the nanoscale. Nano Lett. 2013;13:6098–6105. doi: 10.1021/nl403396e. [DOI] [PubMed] [Google Scholar]
- 183.Forte G, Pagliari S, Ebara M, Uto K, Tam JKV, et al. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng. Part A. 2012;18:1837–1848. doi: 10.1089/ten.TEA.2011.0707. [DOI] [PubMed] [Google Scholar]
- 184.Garrigues GE, Cho DR, Rubash HE, Goldring SR, Herndon JH, Shanbhag AS. Gene expression clustering using self-organizing maps: analysis of the macrophage response to particulate biomaterials. Biomaterials. 2005;26:2933–2945. doi: 10.1016/j.biomaterials.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 185.Mesure L, De Visscher G, Vranken I, Lebacq A, Flameng W. Gene expression study of monocytes/macrophages during early foreign body reaction and identification of potential precursors of myofibroblasts. PLOS ONE. 2010;5:e12949. doi: 10.1371/journal.pone.0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kou PM, Babensee JE. Validation of a high-throughput methodology to assess the effects of biomaterials on dendritic cell phenotype. Acta Biomater. 2010;6:2621–2630. doi: 10.1016/j.actbio.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kou PM, Schwartz Z, Boyan BD, Babensee JE. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater. 2011;7:1354–1363. doi: 10.1016/j.actbio.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kou PM, Pallassana N, Bowden R, Cunningham B, Joy A, et al. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials. 2012;33:1699–1713. doi: 10.1016/j.biomaterials.2011.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garcia-Cordero JL, Nembrini C, Stano A, Hubbell JA, Maerkl SJ. A high-throughput nanoimmunoassay chip applied to large-scale vaccine adjuvant screening. Integr. Biol. (Camb.) 2013;5:650–658. doi: 10.1039/c3ib20263a. [DOI] [PubMed] [Google Scholar]
- 190.Yang J, Mei Y, Hook AL, Taylor M, Urquhart AJ, et al. Polymer surface functionalities that control human embryoid body cell adhesion revealed by high throughput surface characterization of combinatorial material microarrays. Biomaterials. 2010;31:8827–8838. doi: 10.1016/j.biomaterials.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ali OA, Mooney DJ. Immunologically active biomaterials for cancer therapy. Curr. Top. Microbiol. Immunol. 2011;344:279–297. doi: 10.1007/82_2010_69. [DOI] [PubMed] [Google Scholar]
- 192.Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv. Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Franco LM, Bucasas KL, Wells JM, Niño D, Wang X, et al. Integrative genomic analysis of the human immune response to influenza vaccination. eLife. 2013;2:e00299. doi: 10.7554/eLife.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 196.Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 198.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2011;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.