Highlights
-
•
Telomere length is hypothesised to be a biological marker of ageing.
-
•
In LBC1936 and LBC1921 telomere length declines with age.
-
•
Telomere length change did not predict change in cognitive and physical abilities.
Keywords: Telomeres, Cognition, Cognitive ageing, Physical ageing
Abstract
Telomere length is hypothesised to be a biological marker of both cognitive and physical ageing. Here we measure telomere length, and cognitive and physical abilities at mean ages 70, 73 and 76 years in the Lothian Birth Cohort 1936 (LBC1936), and at mean ages 79, 87, 90 and 92 years in the Lothian Birth Cohort 1921 (LBC1921). We investigate whether telomere length change predicts change in cognitive and physical abilities. In LBC1936 telomere length decreased by an average of 65 base pairs per year and in LBC1921 by 69 base pairs per year. However, change in telomere length did not predict change in cognitive or physical abilities. This study shows that, although cognitive ability, walking speed, lung function and grip strength all decline with age, they do so independently of telomere length shortening.
1. Introduction
Determining the biological factors that influence both cognitive and physical decline in later life is an important challenge facing researchers today (Blazer et al., 2015, den Ouden et al., 2011). Telomeres are nucleo-protein complexes at the end of eukaryotic chromosomes. They protect the ends of chromosomes, but shorten each time a somatic cell replicates (Harley et al., 1990, Lindsey et al., 1991). Environmental factors also contribute to accelerated decline in telomere length. These include low socio-economic status, smoking, oxidative stress, and psychological stress (Valdes et al., 2005, von Zglinicki, 2002, Robertson et al., 2013). Telomere length decreases with age and a systematic review determined that the correlation between telomere length and chronological age is about −0.3 (Muezzinler et al., 2013). Leukocyte telomere length has previously been associated with a number of traits and diseases in older age including cognitive abilities (Harris et al., 2006, Harris et al., 2012, Yaffe et al., 2009, Der et al., 2012, Ma et al., 2013), dementia (Grodstein et al., 2008, Martin-Ruiz et al., 2006, Panossian et al., 2003, von Zglinicki et al., 2000), physical health (Gardner et al., 2013, Woo et al., 2014, Masi et al., 2014, Baylis et al., 2014) and obesity (Valdes et al., 2005, Njajou et al., 2012), and has been hypothesised to be a biological marker of ageing (von Zglinicki and Martin-Ruiz, 2005). However, a systematic review concluded that current results were equivocal and that more studies, including longitudinal studies, were required that assessed telomere length and ageing-related functional measures (Mather et al., 2011). Longitudinal studies have the potential to measure age-related decline in telomere length, and cognitive and physical abilities more accurately than cross-sectional studies and also allow the investigation of the change of multiple variables in parallel with each other.
There are many studies that show lower childhood cognitive ability is associated with poorer health and more illness in adulthood and older age, and to earlier mortality from all causes and from several specific causes, such as cardiovascular disease (Deary et al., 2010). Early life IQ has previously been associated with telomere length in midlife (Schaefer et al., 2015). The mechanism of the childhood cognition-illness/death association is not understood, but it is possible that telomeres might provide a biomarker of how lifestyle has affected the body.
We previously reported mostly-null cross-sectional associations between telomere length and cognitive function, walking speed, lung function, and grip strength in the Lothian Birth Cohorts of 1921 and 1936 (LBC1921 and LBC1936) (Harris et al., 2006, Harris et al., 2012). More recently, we showed that the same cognitive and physical abilities decline on average between ages 70 and 76 years in LBC1936 (Marioni et al., 2015). Here, we report longitudinal analyses investigating whether decline in telomere length predicts cognitive and physical decline in the Lothian Birth Cohorts. We also investigate whether baseline telomere length influences subsequent decline in cognitive and physical abilities. Finally, we test whether cognitive ability measured in childhood is related to telomere length decline in later life.
2. Materials and methods
2.1. Lothian Birth Cohort 1936 (LBC1936)
LBC1936 consists of 1091 (548 men and 543 women) surviving members of the Scottish Mental Survey of 1947 (Scottish Council for Research in Education, 1949). At approximately age 11 years most took a valid mental ability test, the Moray House Test version 12 (MHT). At a mean age of 69.5 years (SD 0.8) they were recruited to a study to determine influences on cognitive ageing (Deary et al., 2007, Deary et al., 2012a). They underwent a series of cognitive and physical tests. Two further waves of cognitive and physical tests have occurred at mean ages 73 and 76 years. DNA was extracted from peripheral blood leukocytes at ages 70, 73 and 76 years from which telomere length was measured. Cognitive tests taken at each of the three waves included six Wechsler Adult Intelligence Scale-IIIUK (WAIS-III) (Wechsler, 1998) non-verbal subtests (matrix reasoning, letter number sequencing, block design, symbol search, digit symbol, and digit span backward). From these six cognitive tests a general fluid cognitive factor (gf) was derived. The scores from the first unrotated component of a principal components analysis were extracted and labelled as gf. This component explained 52% of the variance, with individual test loadings ranging between 0.65 and 0.72. Physical trait measures included time taken to walk six metres at normal pace, grip strength measured with a Jamar Hydraulic Hand Dynamometer (all subjects had three trials with the dominant hand; the best of the three trials was used), and forced expiratory volume from the lungs in one second (FEV1) measured using a microspirometer (the best of the three trials was used).
2.2. Lothian Birth Cohort 1921 (LBC1921)
LBC1921 consists of 550 (234 men and 316 women) surviving members of the Scottish Mental Survey of 1932 (Scottish Council for Research in Education, 1933). At approximately age 11 years most took a valid mental ability test, the MHT. At a mean age of 79.1 years (SD 0.6) they were recruited to a study to determine influences on cognitive ageing (Deary et al., 2004, Deary et al., 2012b). They underwent a series of cognitive and physical tests. Four further waves of cognitive and physical tests have occurred at mean ages 83, 87, 90 and 92 years. DNA was extracted from peripheral blood leukocytes at ages 79, 87, 90 and 92 years from which telomere length was measured. Cognitive tests taken at each of these four waves included Raven’s Progressive Matrices (Raven et al., 1977), Verbal Fluency (Lezak, 1995) and Logical Memory (Wechsler, 1987). From these three cognitive tests a general fluid cognitive factor (gf) was derived using principal component analysis. The scores from the first unrotated component were extracted and labelled as gf. This component explained 53% of the variance, with individual test loadings ranging between 0.65 and 0.73. Physical trait measures included time taken to walk six metres at normal pace, grip strength measured with a Jamar Hydraulic Hand Dynamometer (all subjects had three trials with the dominant hand; the best of the three trials was used) and forced expiratory volume from the lungs in one second (FEV1) measured using a microspirometer (the best of the three trials was used).
Ethics permission for the LBC1936 was obtained from the Multi-Centre Research Ethics Committee for Scotland (Wave 1: MREC/01/0/56), the Lothian Research Ethics Committee (Wave 1: LREC/2003/2/29), and the Scotland A Research Ethics Committee (Waves 2 and 3: 07/MRE00/58). Ethics permission for the LBC1921 was obtained from the Lothian Research Ethics Committee (Wave 1: LREC/1998/4/183; Wave 3:1702/98/4/183) and the Scotland A Research Ethics Committee (Waves 4 and 5:10/S1103/6). All persons gave their informed consent prior to their inclusion in the study.
2.3. Telomere length measurement
DNA was extracted from whole blood by standard procedures at the Wellcome Trust Clinical Research Facility Genetics Core at the Western General Hospital, Edinburgh. Telomere length was measured using a quantitative real-time polymerase chain reaction (PCR) assay (Martin-Ruiz et al., 2004). The intra-assay coefficient of variation was 2.7% and the inter-assay coefficient of variation was 5.1%. Four internal control DNA samples were run within each plate to generate absolute telomere lengths and to correct for plate to plate variation. These internal controls are cell lines of known absolute telomere length, 6.9 kb, 4.03 kb, 2.0 kb and 1.32 kb respectively, whose relative ratio values (telomere starting quantity/glyceraldehyde 3-phosphate dehydrogenase starting quantity) were used to generate a regression line by which values of relative telomere length for the actual samples were converted into absolute telomere lengths. The correlation between relative telomere length and absolute telomere length was 0.8. Measurements were performed in quadruplicate and the mean of the measurements used. PCRs were performed on an Applied Biosystems (Pleasonton, CA, USA) 7900HT Fast Real Time PCR machine.
2.4. Statistical analyses
Linear mixed models were used to determine if telomere length and cognitive and physical abilities changed over time. One individual with chronic lymphocytic leukaemia was removed from the LBC1936 analyses. Covariates included age (centred at the minimum value) as the time scale, sex, for telomere length white blood cell counts (lymphocyte, basophil, neutrophil, eosinophil and monocyte) and for physical abilities height. Individual participant number was included as a random effect. Baseline telomere length was added as a fixed effect interaction with age to test if it predicted decline in cognitive and physical abilities. Age 11 MHT score (corrected for age at time of testing and sex) was then added as a fixed effect interaction with age to test if it predicted decline in telomere length. In LBC1921, linear regression was used to determine if age 11 MHT score was associated with telomere length at age 79 years.
Linear mixed models were then used to investigate if telomere length change predicted change in cognitive and physical abilities. Again covariates included age (centred at the minimum value) as the time scale, sex, white cell counts and for physical abilities height. Individual participant number was included as a random effect. Linear mixed models were performed in R using the lme4 and lmerTest packages (Kuznetsova et al., 2013, Bates et al., 2013).
3. Results
Descriptive statistics for telomere length, general fluid cognitive ability (gf), time taken to walk six metres, forced expiratory volume in one second (FEV1) and grip strength for LBC1936 waves 1 (age ∼70 years), 2 (age ∼73 years) and 3 (age ∼79 years) are shown in Table 1, and for LBC1921 waves 1 (age ∼79 years), 3 (age ∼87 years), 4 (age ∼90 years) and 5 (age ∼92 years) are shown in Table 2.
Table 1.
Summary descriptive data for LBC1936. gf = general cognitive ability, FEV1 = forced expiratory volume in one second.
| Age 70 |
Age 73 |
Age 76 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All |
Age 73 completers |
Age 76 completers |
All |
Age 76 completers |
All |
|||||||
| N | Mean (SD, range) |
N | Mean (SD, range) |
N | Mean (SD, range) |
N | Mean (SD, range) |
N | Mean (SD, range) |
N | Mean (SD, range) |
|
| Age (years) | 1091 | 69.5 (0.8, 67.6–71.3) |
866 | 69.5 (0.8, 67.6–71.3) |
697 | 69.5 (0.8, 67.6–71.3) |
866 | 72.5 (0.7, 70.9–74.2) |
697 | 72.5 (0.7, 70.9–74.2) |
697 | 76.2 (0.7, 74.6–77.7) |
| Telomere length (kb) | 1070 | 4.2 (0.6, 2.7–7.1) |
855 | 4.2 (0.6, 2.7–7.1) |
691 | 4.2 (0.6, 2.7–7.1) |
844 | 4.0 (0.7, 1.9–9.4) |
678 | 4.0 (0.7, 1.9–9.4) |
689 | 3.7 (0.7, 1.9–7.2) |
| gf | 1072 | 0.04 (1.0, −3.5–3.0) |
853 | 0.12 (1.0, −3.5–3.0) |
687 | 0.19 (1.0, −3.5–3.0) |
856 | 0.02 (0.98, −3.4–3.1) |
690 | 0.11 (0.96, −2.7–3.1) |
668 | −0.08 (1.0, −3.0–3.1) |
| 6 m walk time (s) | 1085 | 3.9 (1.2, 1.1–14.7) |
863 | 3.8 (1.1, 1.1–14.7) |
695 | 3.7 (1.0, 1.1–14.7) |
860 | 4.4 (1.3, 1.6–16.0) |
692 | 4.2 (1.1, 1.6–16.0) |
692 | 4.7 (0.5, 1.5–15.3) |
| FEV1 (L) | 1085 | 2.4 (0.7, 0.5–5.1) |
863 | 2.4 (0.7, 0.7–5.1) |
695 | 2.4 (0.7, 0.7–5.1) |
856 | 2.3 (0.7, 0.4–5.2) |
692 | 2.3 (0.7, 0.4–5.2) |
690 | 2.1 (0.6, 0.6–4.1) |
| Grip strength (Kg) | 1086 | 29.6 (10.2, 6.0–60.0) |
864 | 30.1 (10.0, 6.0–60.0) |
696 | 30.4 (10.1, 6.0–60.0) |
865 | 29.1 (9.5, 2.0–59.0) |
696 | 29.4 (9.5, 2.0–59.0) |
691 | 27.9 (9.6, 1.0–55.0) |
Table 2.
Summary descriptive data for LBC1921. gf = general cognitive ability, FEV1 = forced expiratory volume in one second.
| Age 79 |
Age 87 |
Age 90 |
Age 92 |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All |
Age 87 completers |
Age 90 completers |
Age 92 completers |
All |
Age 90 completers |
Age 92 completers |
All |
Age 92 completers |
All |
|||||||||||
| N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | N | Mean (SD, range) | |
| Age (yrs) | 550 | 79.1 (0.6, 77.7–80.6) | 233 | 79.1 (0.6, 77.8–80.6) | 128 | 79.1 (0.6, 77.8–80.6) | 59 | 79.1 (0.6, 77.9–80.1) | 235 | 86.6 (0.4, 85.7–87.5) | 129 | 86.6 (0.4, 85.7–87.5) | 59 | 86.6 (0.4, 85.7–87.4) | 129 | 90.1 (0.1, 89.2–90.8) | 59 | 90.1 (0.1, 89.9–90.4) | 59 | 92.1 (0.3, 91.3–92.7) |
| Tel length (kb) | 497 | 4.1 (0.4, 1.9–5.6) | 186 | 4.1 (0.4, 1.9–5.6) | 99 | 4.2 (0.4, 3.3–5.6) | 45 | 4.1 (0.4, 3.3–5.3) | 146 | 4.2 (0.6, 1.9–5.8) | 91 | 4.2 (0.5, 1.9–5.3) | 45 | 4.1 (0.6, 1.9–5.3) | 92 | 3.2 (0.7, 1.4–5.3) | 57 | 3.1 (0.6, 1.7–4.6) | 58 | 2.9 (0.5, 1.0–4.2) |
| gf | 538 | 0.07 (0.9, −2.6–2.9) | 231 | 0.34 (0.9, −1.8–2.9) | 128 | 0.45 (0.9, −1.7–2.9) | 59 | 0.43 (0.9, −1.7–2.4) | 202 | -0.08 (1.1, −3.2–3.3) | 126 | 0.21 (0.9, −2.3–2.1) | 59 | 0.33 (0.8, −1.5–1.9) | 118 | -0.15 (1.1, −2.3–2.3) | 57 | 0.04 (1.0, −2.0–2.2) | 54 | -0.12 (1.0, −1.9–2.4) |
| 6 m walk time (s) | 541 | 4.7 (1.9, 1.8–27) | 232 | 4.4 (1.4, 2.3–12.1) | 128 | 4.3 (1.3, 2.5–9.9) | 59 | 4.3 (1.3, 2.5–9.9) | 192 | 6.7 (5.1, 2.8–56.1) | 121 | 5.9 (2.9, 2.8–22.5) | 59 | 5.7 (2.8, 2.9–22.5) | 97 | 7.2 (4.8, 3.4–46.4) | 58 | 6.5 (2.9, 3.4–19.1) | 57 | 7.7 (2.7, 3.6–13.9) |
| FEV1 (L) | 544 | 1.9 (0.6, 0.5–3.9) | 233 | 2.0 (0.6, 0.6–3.7) | 128 | 2.0 (0.6, 0.7–3.3) | 59 | 2.1 (0.5, 0.9–3.1) | 206 | 1.8 (0.6, 0.5–3.9) | 129 | 1.8 (0.6, 0.5–3.9) | 59 | 1.8 (0.5, 0.9–2.9) | 128 | 1.7 (0.5, 0.5–3.2) | 59 | 1.7 (0.5, 0.7–2.9) | 59 | 1.5 (0.5, 0.6–2.6) |
| Grip strength (Kg) | 544 | 26.5 (9.1, 4.0–59.0) | 233 | 28.0 (9.6, 11.0–59.0) | 128 | 28.7 (9.3, 14.0–53.0) | 59 | 28.5 (9.6, 15.0–51.0) | 205 | 21.5 (8.8, 0.0–44.0) | 127 | 22.3 (8.9, 1.0–44.0) | 57 | 23.0 (9.1, 6.0–44.0) | 128 | 21.0 (8.3, 6.0–46.0) | 59 | 21.6 (7.8, 6.0–45.0) | 59 | 18.7 (8.9, 1.0–42.0) |
In LBC1936, mean telomere length decreased with age. In LBC1921, mean telomere length remained relatively stable between ages 79 and 87 years and then decreased with age. In both cohorts gf, FEV1 and grip strength all decreased with age and time taken to walk six metres increased. Mean age, telomere length and FEV1 did not differ between all individuals who participated in a particular wave of testing and those who returned for later waves of testing. Individuals who returned for further waves of testing generally had a slightly higher gf, a faster walk time and a stronger grip strength on the first occasion of testing.
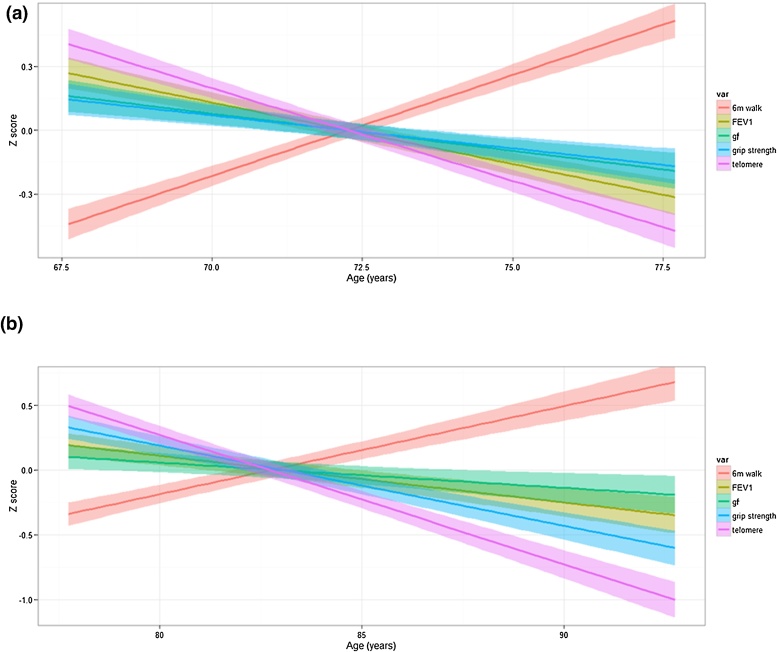
Mean trajectory plots for change in telomere length, gf, six metre walk time, FEV1, and grip strength for LBC1936 and LBC1921 are shown in Fig. 1.
Fig. 1.
Mean trajectory plots for change in telomere length, general cognitive ability (gf) six metre walk time, forced expiratory volume in 1 s (FEV1), and grip strength for (a) LBC1936 and (b) LBC1921.
In LBC1936, a linear mixed model indicated that telomere length decreased by 64.8 base pairs (bp) per year (p < 2 × 10−16), which is 1.5% of the mean telomere length at age 70 years. Telomeres were 177.9 bp longer in males than females (p = 4.66 × 10−7). Telomere length decreased with increasing lymphocyte cell count (p = 9.5 × 10−4), but was not associated with any other white blood cell count. As previously shown27, gf decreased by 0.05 standard deviations per year, 6 m walk time increased by 0.15 s per year, FEV1 decreased by 0.05 L per year, and grip strength decreased by 0.04 kg per year (all p values < 2 × 10−16). There was no evidence to suggest that baseline telomere length was associated with trajectory of decline in cognitive and physical abilities (all p-values > 0.2). Age 11 Moray House Test (MHT) score was not linked to differences in change in telomere length (p = 0.88).
In LBC1921, a linear mixed model indicated that telomere length decreased by 69.3 bp per year (p < 2 × 10−16), which is 1.7% of the mean telomere length at age 79 years. Telomeres were 256.9 bp longer in males than females (p = 1.2 × 10−8). Telomere length was not associated with white blood cell counts (all p values > 0.05). gf decreased by 0.05 standard deviations per year, 6 m walk time increased by 0.27 s per year, FEV1 decreased by 0.03 L per year, and grip strength decreased by 0.74 kg per year (all p values < 2 × 10−16). Baseline telomere length was not associated with decline in cognitive or physical abilities (all p-values > 0.1). Age 11 Moray House Test (MHT) score was linked to the amount of telomere length change such that, for a standard deviation increase in age 11 cognitive ability score, there was a 9.7 bp greater decrease in telomere length per year (p = 0.044). Age 11 MHT score was not associated with telomere length at age 79 years (p = 0.79).
In LBC1936 and LBC1921 there was no evidence to suggest that differences in telomere length change correlated with differences in change in cognitive or physical abilities (all p-values > 0.1) (Table 3).
Table 3.
Effect of change in telomere length on change in cognitive and physical abilities. gf = general cognitive ability, FEV1 = forced expiratory volume in one second.
| LBC1936 |
LBC1921 |
|||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | P | Beta | 95% CI | P | |
| gf | −1.7 × 10−3 | −8 × 10−3–0.01 | 0.74 | 0.0011 | −0.02–0.02 | 0.91 |
| 6 m walk time (s) | 6.2 × 10−3 | −0.02–0.03 | 0.59 | 0.010 | −0.06–0.08 | 0.78 |
| FEV1 (L) | 2.0 × 10−3 | −4 × 10−3–8 × 10−3 | 0.50 | 0.0029 | −4 × 10−3–0.01 | 0.43 |
| Grip strength (Kg) | −0.03 | −0.1–0.07 | 0.47 | −0.035 | −0.1–0.07 | 0.53 |
4. Discussion
This study indicates that, in both LBC1936 and LBC1921, mean telomere length decreased by ∼65 bp per year, which is just under 2% of the mean telomere length at baseline. This is slightly higher than that reported for other longitudinal studies, which ranged from 32 to 46 bp per year (Muezzinler et al., 2013). Cognitive and physical abilities also decreased during this period. Telomere length at baseline was not associated with decline in cognitive or physical abilities between the ages of 70 and 76 (LBC1936), or 79 and 92 (LBC1921) years. In LBC1921 childhood cognitive ability was linked to the amount of telomere length change such that, individuals with a higher childhood cognitive ability underwent a greater decrease in telomere length per year in later life. The rate of decrease in telomere length did not correlate with the rate of decrease in cognitive and physical abilities in either cohort.
As far as we aware this is the first longitudinal study, measuring at least three time points, to investigate if telomere length decline is associated with cognitive and physical decline. A recent meta-analysis based on two time points also found little evidence for telomere length decline as biomarker for physical decline (Gardner et al., 2013). Our results largely agree with previously published cross-sectional findings that telomere length does not associate with cognitive and physical ability (Yaffe et al., 2009, Harris et al., 2012, Bendix et al., 2011). The results confirm the conclusions from a number of previous papers that telomere length is not informative as a biomarker for multiple dimensions of age-related risks including cognitive decline, multi-morbidity and mortality (Cawthon et al., 2003, Martin-Ruiz et al., 2005, Martin-Ruiz et al., 2011). In LBC1936 and LBC1921, telomere length was longer in males than in females, which contradicts many previous studies (Gardner et al., 2014). However, this may reflect the fact that life-expectancy of women is higher than men. Due to the older-age range of the Lothian Birth Cohorts, the men are typically much healthier than those of a similar age in the general population, whereas the women may be more representative of women of a similar age in the general population (Harris et al., 2012). Also a meta-analysis study looking at different methods of measuring telomere length concluded that only the Southern blot method generates results where women have longer telomeres than men (Gardner et al., 2014). Interestingly, mean telomere length at age 79 years in LBC1921 (4.1 kb) was longer than mean telomere age at age 76 years in LBC1936 (3.7 kb). This may be due to the selection of relatively healthier participants into a study at age 79 years (Deary et al., 2012a) compared to those aged 76 years who were already involved in a study. However, the physical ability data does not support this theory e.g., mean grip strength at age 79 years in LBC1921 (26.5 kg) was less than mean grip strength in LBC1936 at age 76 years (27.9 kg). Also, a recent study showed that although there is a negative correlation between age and telomere length up to age 75 years, after 75 years the correlation becomes positive (Lapham et al., 2015).
In LBC1936 higher lymphocyte count was associated with shorter telomeres, indicating that white blood cell distribution may be a predictor of telomere length, as shown previously (Glei et al., 2015). In LBC1921, age 11 cognitive ability was linked to telomere length change such that individuals with a higher Moray House Test score at age 11 years showed a greater decline in telomere length in later life. This was not due to individuals with higher age 11 cognitive ability scores having longer telomeres at age 79 years. Age 11 cognitive ability scores did not influence telomere length change in LBC1936 and the significant result in LBC1921 may be due to type 1 error. Therefore, this finding needs replicating in another study before being considered further.
Strengths of this study include the longitudinal nature, with measurements at three and four time points of the telomeres and the cognitive and physical abilities in two narrow-age cohorts whose combined age periods range from 70 to 92 years. A further strength is that our absolute values of telomere length were generated using four internal controls which are cell lines of known absolute telomere length, whose relative ratio values were used to generate a regression line by which values of relative telomere length for the actual samples were converted into absolute telomere lengths. This allowed us to accurately correct for plate to plate variations as it is well known that the quantitative real-time PCR assay method is sensitive to efficiency variations between very long or very short telomere amplifications. PCR efficiency is not the same for samples with long telomeres compared to samples with short telomeres. A disadvantage of the study is the relatively short time period between each wave of testing. As with all longitudinal studies, there was attrition, though the statistical method used all the available data. Selection bias due to differential mortality is a common limitation in longitudinal studies. However, in this study baseline telomere length and FEV1 did not differ between individuals who did and did not return for later waves of testing. Individuals who returned for further waves of testing generally had a slightly higher gf, a faster walk time and a stronger grip strength on the first occasion of testing, indicating some selection bias. This may reduce the power of the study to detect associations between telomere length shortening and cognitive and physical decline. A further limitation of the study is that the sample sizes of the cohorts, particularly at later waves, is perhaps not large enough to detect a correlation between telomere length shortening and decline in cognitive and physical abilities. The relative health of the cohorts also reduces the variance of the cognitive and physical phenotypes relative to the general population.
5. Conclusion
We find that, although telomere length, and cognitive and physical abilities all show mean decline with age in LBC1936 from age 70 to 76, and in LBC1921 from age 79 to 92, the shortening of telomeres is independent from the observed decline in cognitive and physical abilities.
Acknowledgements
We thank the cohort participants and team members who contributed to these studies. Phenotype collection in the Lothian Birth Cohort 1936 was supported by Age UK (The Disconnected Mind project). Phenotype collection in the Lothian Birth Cohort 1921 was supported by the UK’s Biotechnology and Biological Sciences Research Council (BBSRC), The Royal Society, and The Chief Scientist Office of the Scottish Government. The work was undertaken by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the BBSRC and Medical Research Council (MRC) is gratefully acknowledged. CMR is supported by the NIHR Biomedical Research Centre at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University.
References
- Blazer D.G., Yaffe K., Karlawish J. Cognitive aging: a report from the Institute of Medicine. JAMA. 2015 doi: 10.1001/jama.2015.4380. [DOI] [PubMed] [Google Scholar]
- den Ouden M.E., Schuurmans M.J., Arts I.E., van der Schouw Y.T. Physical performance characteristics related to disability in older persons: a systematic review. Maturitas. 2011;69:208–219. doi: 10.1016/j.maturitas.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Lindsey J., McGill N.I., Lindsey L.A., Green D.K., Cooke H.J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Valdes A.M., Andrew T., Gardner J.P. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Robertson T., Batty G.D., Der G., Fenton C., Shiels P.G., Benzeval M. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol. Rev. 2013;35:98–111. doi: 10.1093/epirev/mxs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muezzinler A., Zaineddin A.K., Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res. Rev. 2013;12:509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Harris S.E., Deary I.J., MacIntyre A. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci. Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Yaffe K., Lindquist K., Kluse M. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der G., Batty G.D., Benzeval M. Is telomere length a biomarker for aging: cross-sectional evidence from the west of Scotland? PLoS One. 2012;7:e45166. doi: 10.1371/journal.pone.0045166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.E., Martin-Ruiz C., Von Z.T., Starr J.M., Deary I.J. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiol. Aging. 2012;33:1486–1488. doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Ma S.L., Lau E.S., Suen E.W. Telomere length and cognitive function in southern Chinese community-dwelling male elders. Age Ageing. 2013;42:450–455. doi: 10.1093/ageing/aft036. [DOI] [PubMed] [Google Scholar]
- Grodstein F., van O.M., Irizarry M.C. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses' health study. PLoS One. 2008;3:e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C., Dickinson H.O., Keys B., Rowan E., Kenny R.A., Von Z.T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Panossian L.A., Porter V.R., Valenzuela H.F. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol. Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T., Serra V., Lorenz M. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab. Invest. 2000;80:1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- Gardner M.P., Martin-Ruiz C., Cooper R. Telomere length and physical performance at older ages: an individual participant meta-analysis. PLoS One. 2013;8:e69526. doi: 10.1371/journal.pone.0069526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J., Yu R., Tang N., Leung J. Telomere length is associated with decline in grip strength in older persons aged 65 years and over. Age (Dordr.) 2014;36:9711. doi: 10.1007/s11357-014-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi S., D'Aiuto F., Martin-Ruiz C. Rate of telomere shortening and cardiovascular damage: a longitudinal study in the 1946 British birth cohort. Eur. Heart J. 2014;35:3296–3303. doi: 10.1093/eurheartj/ehu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis D., Ntani G., Edwards M.H. Inflammation, telomere length, and grip strength: a 10-year longitudinal study. Calcif. Tissue. Int. 2014;95:54–63. doi: 10.1007/s00223-014-9862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njajou O.T., Cawthon R.M., Blackburn E.H. Shorter telomeres are associated with obesity and weight gain in the elderly. Int. J. Obes. (Lond.) 2012;36:1176–1179. doi: 10.1038/ijo.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T., Martin-Ruiz C.M. Telomeres as biomarkers for ageing and age-related diseases. Curr. Mol. Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- Mather K.A., Jorm A.F., Parslow R.A., Christensen H. Is telomere length a biomarker of aging? A review. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Weiss A., Batty G.D. Intelligence and personality as predictors of illness and death: How researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol. Sci. Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- Schaefer J.D., Caspi A., Belsky D.W. Early-life intelligence predicts midlife biological age. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015 doi: 10.1093/geronb/gbv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni R.E., Shah S., McRae A.F. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol. 2015 doi: 10.1093/ije/dyu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scottish Council for Research in Education . University of London Press; London: 1949. The Trend of Scottish Intelligence: a Comparison of the 1947 and 1932 Surveys of the Intelligence of Eleven-Year-Old Pupils. [Google Scholar]
- Deary I.J., Gow A.J., Taylor M.D. The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I.J., Gow A.J., Pattie A., Starr J.M. Cohort profile: the Lothian birth bohorts of 1921 and 1936. Int. J. Epidemiol. 2012;41:1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; London, UK: 1998. WAIS-IIIUK administration and scoring manual. [Google Scholar]
- Scottish Council for Research in Education . University of London Press; London: 1933. The Intelligence of Scottish Children: a National Survey of an Age-Group. [Google Scholar]
- Deary I.J., Whiteman M.C., Starr J.M., Whalley L.J., Fox H.C. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J. Pers. Soc. Psychol. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Yang J., Davies G. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 2012;482:212–215. doi: 10.1038/nature10781. [DOI] [PubMed] [Google Scholar]
- Raven J.C., Court J.H., Raven J. London; H.K Lewis: 1977. Manual for Raven's Progressive Matrices and Vocabulary Scales. [Google Scholar]
- Lezak M. Oxford University Press; Oxford, England: 1995. Neuropsychological Testing. [Google Scholar]
- Wechsler D. Psychological Corporation; New York: 1987. Wechsler Memory Scale-Revised. [Google Scholar]
- Martin-Ruiz C., Saretzki G., Petrie J. Stochastic variation in telomere shortening rate causes heterogeneity of human fibroblast replicative life span. J. Biol. Chem. 2004;279:17826–17833. doi: 10.1074/jbc.M311980200. [DOI] [PubMed] [Google Scholar]
- Kuznetsova, A., Brockhoff, P.B., Christensen, R.H.B., 2013. lmerTest: Tests for random and fixed effects for linear mixed effect. R Package Version 2 0–0.
- Bates, D., Maechler, M., Bolker, B., Walker, S., 2013. lme4: Linear mixed-effects models using Eigen and S4. R Package Version 1 0–4.
- Bendix L., Gade M.M., Staun P.W. Leukocyte telomere length and physical ability among Danish twins age 70+ Mech. Ageing Dev. 2011;132:568–572. doi: 10.1016/j.mad.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R.M., Smith K.R., O'Brien E., Sivatchenko A., Kerber R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C.M., Gussekloo J., van Heemst D., von Zglinicki T., Westendorp R.G. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C., Jagger C., Kingston A. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech. Ageing Dev. 2011;132:496–502. doi: 10.1016/j.mad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Gardner M., Bann D., Wiley L. Gender and telomere length: systematic review and meta-analysis. Exp. Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapham K., Kvale M.N., Lin J. Automated assay of telomere length measurement and informatics for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics. 2015;200:1061–1072. doi: 10.1534/genetics.115.178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei D.A., Goldman N., Weinstein M., Risques R.A. Shorter ends, faster end? Leukocyte telomere length and mortality among older Taiwanese. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1490–1498. doi: 10.1093/gerona/glu191. [DOI] [PMC free article] [PubMed] [Google Scholar]