Abstract
More than half of all subjects with chronic heart failure are older adults with preserved ejection fraction (HFpEF). Effective therapy for this condition is yet to be delineated by clinical trials suggesting that a greater understanding of underlying biologic mechanisms is needed, especially for the purpose of clinical intervention and future clinical trials. Amyloid infiltration of the myocardial is an underappreciated contributing factor to HFpEF that is often caused by misfolded monomers or oligomers of the protein transthyretin. While previously called senile cardiac amyloidosis and traditionally requiring endomyocardial biopsy for diagnosis, advances in our pathophysiologic understanding of this condition, coupled with nuclear imaging techniques using bone isotopes that can non-invasively diagnose this condition and the development of potential therapies, have collected resulted in a renewed interest in this previously considered “rare” condition. This reviewer focuses on the re-emergence of nuclear cardiology using pyrophosphate agents which hold promise for early, non-invasive identification of affected individuals.
Keywords: Cardiac Amyloid, Aging, Technetium pyrophosphate
Heart failure is among the most common cause of hospitalization among older adults in the US with annual mortality rates of 15–50%.1 The incidence and prevalence of heart failure are strikingly age-dependent, with prevalence rates in adults > 80 years approaching 10% and mortality rates increasing with advancing age. More than 50% of heart failure patients have heart failure with a preserved ejection fraction (HFpEF). The hospitalization rate continues to rise among subjects with HFpEF as compared to a flattening in those with systolic heart failure2. Large scale clinical trials3–7, have unfortunately not demonstrated efficacy of any specific therapy for the population with HFpEF. Identifying distinct subgroups of patients with HFpEF is vital, because the mechanism(s) of disease, prognosis and optimal treatment can differ between groups. Thus, it is important to search for underlying mechanisms and prognostic factors in HFpEF, especially for the purpose of clinical intervention and future clinical trials. With an increasing burden of disease and the lack of efficacy observed in recently conducted clinical trials, a greater understanding of biologic mechanisms that contribute to diastolic dysfunction and the genesis of HFpEF is warranted.
Numerous biologic mechanisms have been implicated in the genesis of myocardial stiffness8–10 including intrinsic cardiomyocyte stiffness9,11 related to abnormal calcium homeostasis12, the cytoskeleton (e.g. microtubules and intermediate filaments13,14 or titin15,16) as well as abnormalities in the extracellular matrix related to collagen and elastin17–20. Amyloid infiltration in the extracellular matrix markedly alters myocardial stiffness resulting in upward and leftward shifts in the end diastolic pressure volume relation21, is associated with most severe forms of diastolic dysfunction by Doppler imaging22 and in-vitro length-tension experiments demonstrate increased diastolic force compared to controls.23 These data suggest that amyloid infiltration is a mechanism underlying HFpEF.
The diagnosis of transthyretin (TTR) cardiac amyloidosis is difficult to make on clinical grounds alone as congestive heart failure, atrial arrhythmia, and conduction abnormalities are all non-specific disease manifestations and are otherwise common in older persons.24 Classically, the gold standard for diagnosis endomyocardial biopsy, which is not only costly (~$5,400 including costs for pathologic interpretation) but also requires technical expertise for its performance and pathological evaluation. While a few presentations are more suggestive of the underlying restrictive physiology including marked right-sided heart failure with increasing abdominal girth, early satiety, and lower extremity edema, as well as the development of relative hypotension in a person with longstanding hypertension, none of these findings have sufficient sensitivity or specificity to establish the diagnosis. Thus, additional diagnostic testing is always required.
The most commonly ordered studies are the electrocardiogram and two-dimensional transthoracic echocardiogram. Classic ECG findings in patients with cardiac amyloidosis include low QRS voltage, pseudo-infarction patterns, conduction abnormalities including bundle branch block and hemi-block, and rhythm disturbances such as atrial fibrillation. However, the classic finding of low voltage is a late phase development in transthyretin cardiac amyloidosis and a minority of subjects with biopsy proven disease has low voltage based on typical definitions involving standard 12 lead electrocardiograms.25 Indeed, up to 15% of patients have electrocardiographic evidence of left ventricular hypertrophy.25 More useful is to calculate the voltage to mass ratio from the electrocardiogram and echocardiogram, though in practice this is rarely performed. In reality, however, electrocardiographic findings lack both sensitivity and specificity for persons with biopsy-proven cardiac amyloidosis. The combination of low voltage and pseudo-infarct patterns are seen in only a minority of patients and low voltage can be seen in many other conditions including obesity, chronic obstructive pulmonary disease, pericardial effusion, and hypothyroidism.
As with the electrocardiogram, classic echocardiographic patterns of cardiac amyloidosis do exist, but are neither sensitive nor specific. Persons with cardiac amyloidosis are more likely to have thickened ventricular walls and refractile myocardium. However, in the early phases of the disease many persons with amyloid will have normal or just slightly elevated echocardiographic wall thickness, and only a minority will have characteristic granular echogenicity. Data using bone isotopes (discussed below) have demonstrated that myocardial tracer uptake occurs before manifest echocardiographic findings indicative of transthyretin cardiac amyloid demonstrating the enhanced sensitivity of this approach in comparison to echocardiography.26–29
Additional modalities can be used to further increase diagnostic accuracy. Sub-endocardial myocytes are longitudinally oriented and are particularly susceptible to damage in amyloidosis, resulting in early impairment in longitudinal contraction not appreciated on standard two-dimensional echocardiography when evaluating global indices such as ejection fraction. More advanced echocardiographic techniques such as tissue Doppler imaging as well as strain and strain rate measurements, which assess cardiac systolic function predominantly by assessing contraction of the heart along its short-axis, can be useful. Both strain and strain rate techniques can show characteristic impairments, especially the pattern of apical sparring can enhance the suspicion for cardiac amyloidosis30 but their utility in early diagnosis is not been carefully studied.
Cardiac magnetic resonance imaging can also be used to identify cardiac amyloid. Intravenous gadolinium contrast accumulates within amyloid infiltrated myocardium. As a result, the combination of myocardial late gadolinium enhancement and altered gadolinium blood pool kinetics can suggest the presence of amyloid and localize it within the heart. Reports from small single center studies, suggest that sensitivity is reasonably high (~85%) 31–34 and that while specific patterns are more suggestive of cardiac amyloid (e.g. sub-endocardial enhancement), the patterns in cardiac amyloid can be quite heterogeneous,35,36 thus the specificity for identifying cardiac amyloid is reduced. Additionally, neither of the commonly employed imaging techniques (echocardiography or magnetic resonance imaging) can distinguish primary light chain from transthyretin cardiac amyloid.
Literature shows transthyretin cardiac amyloid is underdiagnosed and much more common than previously thought.24,25,37,38 Transthyretin cardiac amyloidosis predominately afflicts older adults in their seventh and eighth decade of life. One form of the disease, previously called senile cardiac amyloidosis is caused by deposits of monomer, oligomers of wild type transthyretin (ATTRwt) and does not have a genetic basis.24,39 The other form, called familial amyloid cardiomyopathy is caused by mutations in the transthyretin gene; is inherited in an autosomal dominant fashion and has an age dependent penetrance. Cardiac involvement in transthyretin cardiac amyloid is also known as familial amyloid cardiomyopathy when associated with variant transthyretin or senile systemic amyloidosis when associated with wild-type transthyretin. Although there are more than 20 mutations which have been associated with cardiac involvement, one mutation, V122I (substitution of isoleucine for valine at position 122), has been reported with high frequency (prevalence of 3.4% to 3.9%40,41) in African-Americans. In the over-60 cohort the prevalence was almost five times that in African Americans of similar age participating in the Cardiovascular Health Study (10±3% vs. 2±0.5%; p < 0.01). In the elderly, wild-type or normal transthyretin may become structurally unstable resulting in deposition of amyloid fibrils primarily in heart tissue, leading to diastolic dysfunction, restrictive cardiomyopathy and HF.42–45 The frequency of transthyretin amyloid deposition in cardiac ventricles reported from autopsy studies in patients >80 range from 1.8% to 25%46,47, with a rate of clinical cardiac disease pre-mortem of 34%.46 Data among HFpEF subjects who were average age of 74 years at diagnosis shows 21% had amyloid deposits on autopsy performed at an average age of 76 years. Of HFpEF patients ≥75 years at heart failure diagnosis, 32% had amyloid deposition vs. 8% of patients < 75 years at heart failure diagnosis (p=0.002). Only 20% of the patients with amyloid on autopsy had a pre-morbid diagnosis made.48
Although historically difficult to diagnose and traditionally requiring endomyocardial biopsy, the diagnosis of transthyretin cardiac amyloidosis has become easier in recent, specifically with realization that bone isotopes26–29,49–59 can identify transthyretin cardiac amyloid early in the course of disease (e.g. before echocardiographic manifestations)26,29 and have a very high sensitivity and specificity for distinguishing transthyretin cardiac amyloid (both mutant and wild type) from light chain cardiac amyloid and other types of cardiomyopathy that mimic amyloid (e.g. hypertrophic cardiomyopathy).26,29,55,56,60 Differentiating transthyretin from light chain cardiac amyloid has important prognostic, management, counseling and therapeutic implications. The prognosis of light chain cardiac amyloid (median survival of ~4–6 months in the setting of concomitant heart failure and without treatment) is significantly different from transthyretin cardiac amyloidosis (median survival of ~70–75 months from initial manifestations). These nuclear medicine techniques that employ bone isotopes have the potential to dramatically alter the outcomes of patients with transthyretin cardiac amyloidosis by making early diagnosis a reality and because targeted pharmacotherapies are designed to prevent transthyretin misfolding and amyloid deposition and prevent disease progression but not remove amyloid.52,53,60,61
Irrespective of amyloid type, care must be taken to avoid potentially toxic therapies commonly used in patients with atrial fibrillation and congestive heart failure such as digoxin, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers, and beta blockers. Dihydropyridine calcium channel blockers also bind amyloid fibrils and can exert potentially deleterious negative inotropic effects and result in high degree atrioventricular block and shock. Thus dihydropyridine calcium channel blockers should not be used in patients with cardiac amyloidosis, but are a standard of care for patients with hypertrophic cardiomyopathy, making the distinction clinically important. Additionally, digoxin use has been questioned. Both ACE inhibitors and angiotensin receptor blockers may induce hypotension, as angiotensin blockade can significantly reduce vascular tone in the setting of concomitant sympathetic dysfunction due to TTR deposition. Beta blockers may have undesirable negative inotropic and chronotropic effects. As patients with cardiac amyloidosis have a restrictive cardiomyopathy with a relatively fixed stroke volume, augmentation of cardiac output relies disproportionately on increased heart rate. This normal heart rate response is frequently impaired with normal aging and concomitant amyloid and may be further exacerbated by beta blockade. Accordingly, ACE inhibitors, angiotensin receptor blockers, and beta blockers which form the standard of care for heart failure patients can be poorly tolerated and potentially dangerous in patients with cardiac amyloidosis and thus should be used with extreme caution.
With regard to counseling, transthyretin cardiac amyloidosis, as previously described, can be caused a mutation in the transthyretin gene which is inherited in an autosomal dominant fashion or in the setting of a normal transthyretin gene sequence (e.g. wild type TTR). Nuclear scanning with bone isotopes identify both forms of transthyretin cardiac amyloid with high sensitivity and specificity, thereby facilitating further genetic testing and if positive counseling of family members regarding their risk of inheritance. Differentiation of light chain from transthyretin cardiac amyloidosis remains challenging and misdiagnosis is associated with potential for significant harm. In light chain cardiac amyloid, the fibrils are composed of immunoglobulin light chains that are produced by a clonal population of plasma cells in the bone marrow. Treatment involves chemotherapeutic agents targeted at the plasma cell. Such treatment would be inappropriate and harmful to subjects to transthyretin amyloid. Currently, the gold standard for definitive diagnosis is endomyocardial biopsy coupled with either immunohistochemistry or in cases in which this is inconclusive, mass spectroscopy. This technique is well suited for diagnosing cardiac amyloidosis, as amyloid deposits are usually deposited diffusely throughout the myocardium. Unfortunately, these diagnostic requirements are typically performed only in specialized centers with particular expertise, do not provide sufficient information about the extent or distribution of cardiac amyloidosis, disease progression, or response to treatment, and in practice can lead to delayed care. Additionally, many older adults are reluctant to undergo invasive procedures. Thus, there is an important role for non-invasive, accurate, highly reproducible method to diagnose transthyretin cardiac amyloid and technetium pyrophosphate scanning has been demonstrated to meet these requirements.
Radiolabelled phosphate derivatives, initially developed as bone seeking tracers, including [99mTc]-diphosphonate, can localize amyloid within the heart. Several phosphate derivatives tagged with [99mTc] including [99mTc]-pyrophosphate ([99mTc]-PYP), [99mTc]- methylene diphosphonate ([99mTc]-MDP), [99mTc]-hydroxy methylene diphosphonate ([99mTc]-HPD), and [99mTc]-3,3-diphosphono-1,2-propanodicarboxylic acid ([99mTc]-DPD) have all been shown to effectively identify TTR cardiac amyloid.26,29,33,50,51,55–57,60
Of all the bone seeking tracers, [99mTc]-DPD has been the most widely employed in the context as tracer for transthyretin cardiac amyloidosis. Currently, this isotope is not approved by the Food and Drug Administration and therefore is not available for clinical use in the United States, whereas it is widely adopted in Europe. [99mTc]-DPD imaging has been shown to be 100% sensitive and 88% specific for transthyretin cardiac amyloid and with a high negative predictive value (100%) for excluding light chain cardiac amyloid and a positive predictive value of 88% for transthyretin cardiac amyloid.26,33,50,55 [99mTc]-DPD imaging is now widely used in Europe, with more than 2,000 scans having been performed to date in major amyloid centers in London, Germany, Italy and the Netherlands. Among subjects who are carriers of the trTTR mutations 99mTc-DPD uptake occurs before clear echocardiographic and electrocardiographic phenotype and in asymptomatic elderly people with echocardiographic and biopsy proven wild type transthyretin related cardiomyopathy.29 Additionally, studies have shown that the heart tracer retention (calculated as heart-to whole body ratio) is related to the severity of cardiac amyloid deposition as expressed by interventricular wall thickness, left ventricular systolic/diastolic dysfunction and is of prognostic values in that 99mTc-DPD myocardial uptake is predictive of major adverse cardiac events.26 In a recent study of a large number of subjects, the high sensitivity of [99mTc]-DPD scintigraphy in detecting transthyretin related cardiac amyloidosis was confirmed.50
[99mTc]-PYP is available in the United States and is approved as an imaging agent for myocardial infarction. A number of a case reports since 1980 demonstrated myocardial uptake of [99mTc]-PYP in amyloid patients. More recently data has demonstrated a significant utility of this tracer similar to that reported for other bone tracers. Using a quantitative method, a quantitative analysis of myocardial uptake, defined as the ratio of myocardial mean counts to ventricular cavity mean counts, was found to have a sensitivity of 84.6% and specificity of 94.5% for distinguishing cardiac amyloidosis from non-amyloid causes of heart failure. In another study, 99mTc-PYP SPECT in 45 subjects with biopsy proven amyloidosis was highly sensitive (97%) and specific (100%) for distinguishing transthyretin from light chain cardiac amyloid.56 A larger series of pooled data using 99mTc-PYP scanning form Columbia University Medical Center, Mayo Clinic and Boston University among 128 subjects (72±9 years, range 34- 89 years, 84% male) in which 88 (68% had transthyretin cardiac amyloid, 58 with wild type transthyretin and 30 with mutant transthyretin), 24 (18.8% had light chain cardiac amyloid) and 16 (13.3% were controls) the sensitivity was 90%, specificity of 90%, PPV 95% and NPV of 80%.
In summary, labelled diphosphonates play an important role in the typing of amyloidosis and in diagnosing heart involvement in patients with transthyretin cardiac amyloidosis with confidence. Cardiac involvement in transthyretin patients may be diagnosed earlier with bone scintigraphy in transthyretin patients compared to echocardiography. Given the high sensitivity and specificity of these techniques, their ability to differentiate subjects with either heart failure and a preserved ejection fraction or light chain cardiac amyloidosis from those with transthyretin cardiac amyloidosis and the non-invasive nature of this approach and lower cost compared to the gold standard, endomyocardial biopsy, clinicians may find this testing most useful for their most vulnerable older adult patients with HFpEF and the possibility of underlying amyloid.
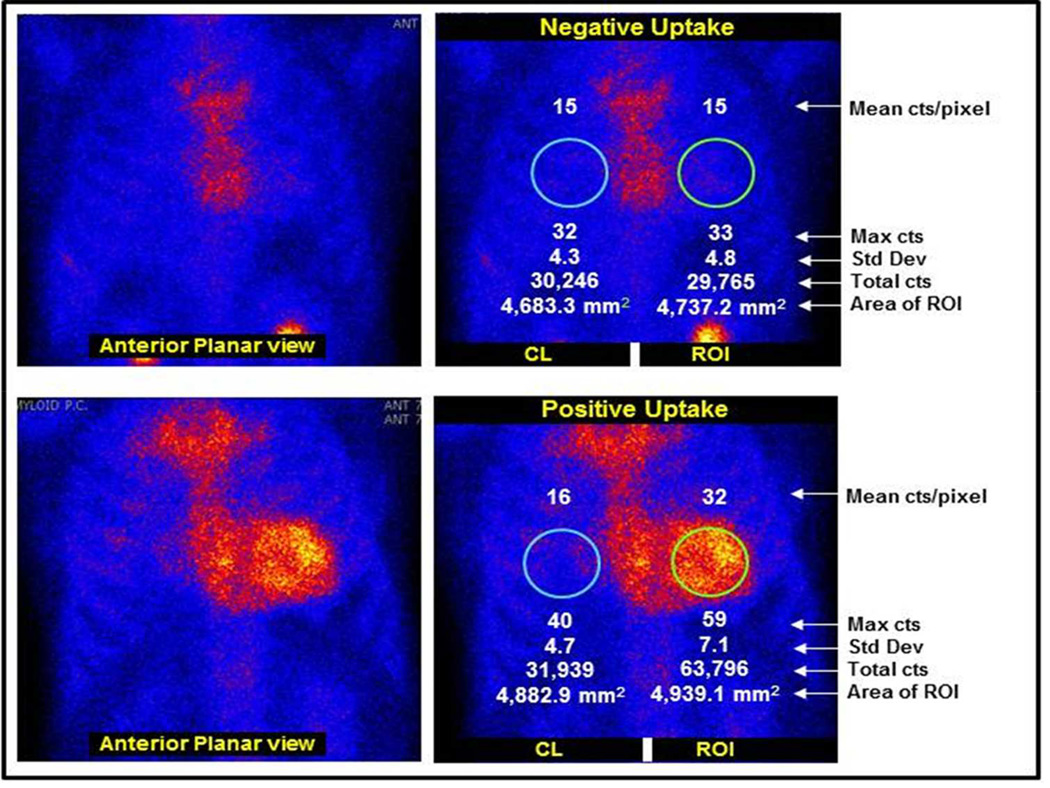
Figure.
Tc-PYP99 myocardial planar scanning in a patient with biopsy proven AL amyloid (upper panel) with sternal but no myocardial uptake and in a patient with biopsy proven TTR cardiac amyloid (lower panel) in which there is diffuse myocardial uptake.
Summary of Benefits of Bone Scintigraphy for TTR Cardiac Amyloidosis.
High sensitivity and specificity of bone scintigraphy for establishing the present of transthyretin (TTR) not light chain (AL) cardiac amyloidosis.
Safe, accurate and potentially cost effective approach which contrasts to current approaches that rely on invasive, potentially risky, not widely available and costly methodology (endomyocardial biopsy) and pathologic evaluation that is confounded by uncertain diagnostic methods (immunohistochemistry) requiring specialized expertise.
Provides for early and accurate diagnosis (e.g. distinguishing transthyretin from light chain cardiac amyloidosis) in order to establish prognosis and ensure appropriate treatment and avoid inappropriate therapy.
Clinical Significance.
Amyloid infiltration of the heart is an under-appreciated cause of HFpEF
Transthyretin (TTR) cardiac amyloid can be due to either mutations, called familial amyloid cardiomyopathy (FAC), inherited autosomal dominantly or from wild type TTR (formerly called senile cardiac amyloidosis)
Cardiac imaging with bone scintigraphy has a high sensitivity and specificity for establishing the presence of TTR not AL cardiac amyloidosis.
Acknowledgments
Funding: Source: Dr. Maurer is supported by a K24 award (AG036778-05) from the NIA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Maurer has been a paid consultant to Pfizer, Inc, Alnylam Pharmaceuticals, Inc. Prothena, Inc and ISIS Pharmaceuticals. His institution (Columbia University Medical Center) has received funding for the conduct of clinical trials from Pfizer, Inc. and Alnylam Pharmaceuticals.
Dr. Maurer drafted the manuscript and had control over all aspects of the review.
References
- 1.Miller LW, Missov ED. Epidemiology of heart failure. Cardiol Clin. 2001;19:547–555. doi: 10.1016/s0733-8651(05)70242-3. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. The New England journal of medicine. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. The New England journal of medicine. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 8.van Heerebeek L, Borbely A, Niessen HW, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 9.Borbely A, van der Velden J, Papp Z, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 10.Bronzwaer JG, Paulus WJ. Matrix, cytoskeleton, or myofilaments: which one to blame for diastolic left ventricular dysfunction? Prog Cardiovasc Dis. 2005;47:276–284. doi: 10.1016/j.pcad.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 12.Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:715–724. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- 13.Zile MR, Richardson K, Cowles MK, et al. Constitutive properties of adult mammalian cardiac muscle cells. Circulation. 1998;98:567–579. doi: 10.1161/01.cir.98.6.567. [DOI] [PubMed] [Google Scholar]
- 14.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137–2145. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borbely A, Falcao-Pires I, van Heerebeek L, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55:1418–1424. doi: 10.1161/HYPERTENSIONAHA.109.149112. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw AD, Baicu CF, Rentz TJ, et al. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borbely A, Papp Z, Edes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009;61:139–145. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 21.Bhuiyan T, Helmke S, Patel AR, et al. Pressure-Volume Relationships in Patients with Transthyretin (ATTR) Cardiac Amyloidosis Secondary to V122I Mutations and Wild Type TTR: TRACS (Transthyretin Cardiac Amyloid Study) Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.109.910455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavine SJ. Genesis of the restrictive filling pattern: pericardial constraint or myocardial restraint. J Am Soc Echocardiogr. 2004;17:152–160. doi: 10.1016/j.echo.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Petre RE, Quaile MP, Wendt K, et al. Regionally heterogeneous tissue mechanics in cardiac amyloidosis. Amyloid. 2005;12:246–250. doi: 10.1080/13506120500386824. [DOI] [PubMed] [Google Scholar]
- 24.Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. Journal of the American Geriatrics Society. 2012;60:765–774. doi: 10.1111/j.1532-5415.2011.03868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. The American journal of cardiology. 2014;114:1089–1093. doi: 10.1016/j.amjcard.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4:659–670. doi: 10.1016/j.jcmg.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Bauer R, Dikow N, Brauer A, et al. The "Wagshurst study": p.Val40Ile transthyretin gene variant causes late-onset cardiomyopathy. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2014;21:267–275. doi: 10.3109/13506129.2014.967846. [DOI] [PubMed] [Google Scholar]
- 28.Aljaroudi WA, Desai MY, Tang WH, Phelan D, Cerqueira MD, Jaber WA. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: state of the art review and focus on emerging nuclear techniques. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2014;21:271–283. doi: 10.1007/s12350-013-9800-5. [DOI] [PubMed] [Google Scholar]
- 29.Glaudemans AW, van Rheenen RW, van den Berg MP, et al. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2014;21:35–44. doi: 10.3109/13506129.2013.871250. [DOI] [PubMed] [Google Scholar]
- 30.Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–1849. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 31.Ruberg FL, Appelbaum E, Davidoff R, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. The American journal of cardiology. 2009;103:544–549. doi: 10.1016/j.amjcard.2008.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. Journal of the American College of Cardiology. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 33.Perugini E, Rapezzi C, Piva T, et al. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart. 2006;92:343–349. doi: 10.1136/hrt.2005.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 35.vanden Driesen RI, Slaughter RE, Strugnell WE. MR findings in cardiac amyloidosis. AJR American journal of roentgenology. 2006;186:1682–1685. doi: 10.2214/AJR.04.0871. [DOI] [PubMed] [Google Scholar]
- 36.Wassmuth R, Abdel-Aty H, Bohl S, Schulz-Menger J. Prognostic impact of T2-weighted CMR imaging for cardiac amyloidosis. European radiology. 2011;21:1643–1650. doi: 10.1007/s00330-011-2109-3. [DOI] [PubMed] [Google Scholar]
- 37.Castano A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart failure reviews. 2014 doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Current medical research and opinion. 2013;29:63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 39.Damy T, Mohty D, Deux JF, et al. [Senile systemic amyloidosis: definition, diagnosis, why thinking about?] Presse medicale. 2013;42:1003–1014. doi: 10.1016/j.lpm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson DR, Gorevic PD, Buxbaum JN. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet. 1990;47:127–136. [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 42.Pages RA, Robbins J, Edelhoch H. Binding of thyroxine and thyroxine analogs to human serum prealbumin. Biochemistry. 1973;12:2773–2779. doi: 10.1021/bi00738a034. [DOI] [PubMed] [Google Scholar]
- 43.Saraiva MJ. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum Mutat. 2001;17:493–503. doi: 10.1002/humu.1132. [DOI] [PubMed] [Google Scholar]
- 44.Quintas A, Vaz DC, Cardoso I, Saraiva MJ, Brito RM. Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J Biol Chem. 2001;276:27207–27213. doi: 10.1074/jbc.M101024200. [DOI] [PubMed] [Google Scholar]
- 45.Hammarstrom P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A. 2002;99(Suppl 4):16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa PP, Figueira AS, Bravo FR. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978;75:4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 48.Mirzoyev SAEW, Mohammed SF, Donovan SL, Roger VL, Grogan D, Redfield MM. Cardiac Amyloid Deposition is Common in Elderly Patients with Heart Failure and Preserved Ejection Fraction. Circulation. 2010;122 [Google Scholar]
- 49.Wadhwa SS, Nour R. Tc-99m HDP uptake in cardiac amyloidosis. Clinical nuclear medicine. 1999;24:156–158. doi: 10.1097/00003072-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Hutt DF, Quigley AM, Page J, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. European heart journal cardiovascular Imaging. 2014;15:1289–1298. doi: 10.1093/ehjci/jeu107. [DOI] [PubMed] [Google Scholar]
- 51.Longhi S, Guidalotti PL, Quarta CC, et al. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovascular imaging. 2014;7:531–532. doi: 10.1016/j.jcmg.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Kristen AV, Scherer K, Buss S, et al. Noninvasive risk stratification of patients with transthyretin amyloidosis. JACC Cardiovascular imaging. 2014;7:502–510. doi: 10.1016/j.jcmg.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Sachchithanantham S, Wechalekar AD. Imaging in systemic amyloidosis. British medical bulletin. 2013;107:41–56. doi: 10.1093/bmb/ldt021. [DOI] [PubMed] [Google Scholar]
- 54.Kristen AV, Haufe S, Schonland SO, et al. Skeletal scintigraphy indicates disease severity of cardiac involvement in patients with senile systemic amyloidosis. International journal of cardiology. 2013;164:179–184. doi: 10.1016/j.ijcard.2011.06.123. [DOI] [PubMed] [Google Scholar]
- 55.Rapezzi C, Guidalotti P, Salvi F, Riva L, Perugini E. Usefulness of 99mTc-DPD scintigraphy in cardiac amyloidosis. Journal of the American College of Cardiology. 2008;51:1509–1510. doi: 10.1016/j.jacc.2007.12.038. author reply 10. [DOI] [PubMed] [Google Scholar]
- 56.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castano A, Bokhari S, Brannagan TH, 3rd, Wynn J, Maurer MS. Technetium pyrophosphate myocardial uptake and peripheral neuropathy in a rare variant of familial transthyretin (TTR) amyloidosis (Ser23Asn): a case report and literature review. Amyloid. 2012;19:41–46. doi: 10.3109/13506129.2011.638682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hongo M, Hirayama J, Fujii T, et al. Early identification of amyloid heart disease by technetium-99m-pyrophosphate scintigraphy: a study with familial amyloid polyneuropathy. American heart journal. 1987;113:654–662. doi: 10.1016/0002-8703(87)90703-4. [DOI] [PubMed] [Google Scholar]
- 59.Wizenberg TA, Muz J, Sohn YH, Samlowski W, Weissler AM. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. American heart journal. 1982;103:468–473. doi: 10.1016/0002-8703(82)90331-3. [DOI] [PubMed] [Google Scholar]
- 60.Bokhari S, Shahzad R, Castano A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol. 2014;21:175–184. doi: 10.1007/s12350-013-9803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Archives of cardiovascular diseases. 2013;106:528–540. doi: 10.1016/j.acvd.2013.06.051. [DOI] [PubMed] [Google Scholar]