Abstract
This study investigated the effects of the AMPA/Kainate receptor antagonist, NBQX, on cue-elicited cocaine-seeking behavior and concomitant changes in Fos protein expression. After cocaine self-administration training, rats underwent 24 days of abstinence during which they were exposed daily either to the self-administration environment with response-contingent cues previously paired with cocaine infusions available (Extinction group) or to an alternate environment (No Extinction group). Subsequently, rats were tested for cocaine-seeking behavior (i.e., operant responses without cocaine reinforcement) elicited by the cocaine-associated cues after pretreatment with either vehicle or NBQX (10 mg/kg, IP). NBQX markedly attenuated cue-elicited cocaine-seeking behavior relative to vehicle pretreatment in the No Extinction group and also decreased cue-elicited Fos protein expression in a region-specific manner in the anterior cingulate and orbitofrontal cortices, basolateral amygdala, nucleus accumbens core, and dorsal caudate-putamen, suggesting involvement of AMPA glutamate systems in specific subregions of the neuronal circuitry activated by cocaine cues.
Keywords: drug craving, conditioning, immediate early gene, incentive motivation, addiction
1. Introduction
The treatment of drug dependence is challenging because of the chronic nature of the disorder and the high susceptibility to relapse, even after prolonged periods of abstinence (O’Brien et al., 1993). Although the triggers preceding relapse are varied and relapse itself encompasses a multifaceted problem (O’Brien, 2003), cues previously associated with the drug (e.g., paraphernalia or the context of drug administration) often elicit intense craving that may contribute to relapse in abstinent drug users (Childress et al., 1993; Ehrman et al., 1992; Sinha et al., 2000; Wallace, 1989). In animals, exposure to drug-associated cues elicits drug-seeking behavior (Bossert et al., 2005; Shaham et al., 2003; Weiss, 2005), which is considered a valid model of cue-elicited craving (Epstein et al., 2006; Markou et al., 1993).
Evidence suggests glutamate mechanisms contribute to drug-seeking behavior triggered by cocaine-associated cues (Kalivas & McFarland, 2003; Tzschentke & Schmidt, 2003). In particular, AMPA/Kainate (KA) glutamate receptor activation is critical because antagonism of these receptors attenuates cocaine-seeking behavior maintained by cues under a second-order schedule of reinforcement (Backstrom & Hyytia, 2003), as well as reinstatement of extinguished cocaine-seeking behavior by cocaine-paired cues (Backstrom & Hyytia, 2006). Although the neuroanatomical specificity of these effects has not been fully investigated, the nucleus accumbens is likely involved because infusion of an AMPA/KA receptor antagonist into the nucleus accumbens core decreases cue-elicited cocaine-seeking behavior (Backstrom & Hyytia, 2007; Di Ciano & Everitt, 2001). Furthermore, glutamate levels are increased in this region after exposure to cocaine-associated stimuli (Hotsenpiller et al., 2001) and infusions of AMPA into the nucleus accumbens reinstate cocaine-seeking behavior (Cornish et al., 1999; Suto et al., 2004). Additionally, AMPA/KA receptor blockade in the dorsal striatum attenuates cocaine-seeking behavior maintained under a second-order schedule of reinforcement (Vanderschuren et al., 2005).
Research using expression of the immediate early gene (IEG) product, Fos, as a marker of neuronal activation has implicated a neural circuit involving the nucleus accumbens, the anterior cingulate and prelimbic subregions of the prefrontal cortex, hippocampal formation, and the basolateral amygdala in the incentive motivational effects of cocaine cues (Ciccocioppo et al., 2001; Crawford et al., 1995; Franklin & Druhan, 2000; Neisewander et al., 2000). Moreover, we have recently demonstrated that a proportion of neurons exhibiting cue-elicited Fos expression in each of these regions coexpress AMPA receptor subunits, suggesting that an action of glutamate at AMPA receptors may in part drive cue-elicited Fos expression (Zavala et al., 2007). In the present study, we examined the effects of antagonizing AMPA/KA receptors with 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) on cue-elicited cocaine-seeking behavior and concomitant changes in Fos protein expression in limbic and cortical regions activated by cocaine cues. The dose of NBQX was determined based on an initial experiment demonstrating no effect on locomotion.
2. Experimental Procedures
2.1. Animals
Male Sprague Dawley rats weighing 200–225 g were housed individually in a climate-controlled colony with a 12-h reverse light/dark cycle (lights off at 6:00 AM). Rats were acclimated to handling for 5–7 days. Care of the animals was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and all procedures were approved by the IACUC at Arizona State University.
2.2. Drugs
Cocaine hydrochloride (RTI International, Research Triangle Park, NC) was dissolved in bacteriostatic saline and filtered through a 0.2 μm filter. NBQX (Tocris Bioscience, Ellisville, MO) was prepared fresh daily, dissolved in double-distilled water and administered at a volume of 1 ml/kg.
2.3. Experiment 1: Effects of NBQX on locomotion
Locomotion was assessed in Plexiglas chambers (24 × 45 × 20 cm high) with two photobeams 32 cm apart and 4 cm above a bar grid floor. Crosses were recorded for 60 min as the number of times the photobeams were broken consecutively. Rats were pretreated with either vehicle (1 ml/kg; n = 11) or NBQX (10 mg/kg, IP; n = 11) 20 min prior to testing.
2.4. Experiment 2: Effects of NBQX on cue-elicited cocaine seeking and Fos expression
2.4.1. Surgery
Catheters were implanted intravenously as described by Zavala et al. (2007). Rats were anesthetized with sodium pentobarbital (50 mg/kg, IP, Abbott Laboratories, Chicago, IL) with atropine sulfate (10 mg/kg, IP; Sigma, St. Louis, MO) pretreatment to facilitate respiration. The catheters ran subcutaneously along the neck, exited through an incision across the skull, and were secured to the top of the skull using dental acrylic and anchor screws. To ensure catheter patency, a 0.1 ml solution of bacteriostatic saline containing heparin sodium (70 USP U/ml; Baxter Healthcare Corporation, Deerfield, IL) and ticarcillin disodium (20 mg/ml; GlaxoSmithKline Research Triangle Park, NC) was administered daily. For the first four days after surgery, the solution also contained urokinase (66.67 mg/m; ImaRX Therapeutics, Inc., Tucson, AZ). l). Catheter patency was confirmed by infusing 0.3 ml methohexital sodium (16.67 mg/ml, IV; Sigma), which produces brief anesthetic effects only when administered IV.
2.4.2. Self-administration
Self-administration took place in chambers (20 × 28 × 20 cm) contained inside a sound-attenuating chamber and equipped with two levers mounted on the front wall (Med Associates, St. Albans, VT), a cue light above one lever, a tone generator (500 Hz, 10 db above background), and a house light mounted on the top center of the back wall. The lever with the cue light was designated as the active lever and the other one as the inactive lever. Each chamber was contained inside a sound-attenuating chamber. Infusion pumps were located outside the chambers and contained syringes attached via Tygon tubing to liquid swivels (Instech, Plymouth Meeting, PA). The outlet of the swivels was fastened to the catheters via Tygon tubing that ran through a metal spring leash (Plastics One).
After a 5–7 days of recovery from surgery, rats were randomly divided into groups that were either trained to press a lever reinforced by cocaine-HCl infusions (0.75 mg/kg/0.1 ml, IV; Cocaine group; n = 40) or given yoked saline infusions (Saline group; n = 17). Training occurred daily for 2 h, at the same time of day across 24 consecutive days. Schedule completions by a Cocaine rat on the active lever resulted in simultaneous presentation of the tone, cue light, and house light, followed one second later by a cocaine infusion. Saline-yoked rats were simultaneously presented the same stimulus complex and received a saline infusion contingent upon responses of their Cocaine rat counterpart. Upon completion of the 6-s infusion, the cue light, tone, and infusion pump were inactivated simultaneously. The house light remained on for an additional 20-s signaling a timeout period during which lever presses had no scheduled consequences. Responses on the inactive lever were recorded but had no scheduled consequences. Based on individual performance, rats progressed from a fixed ratio (FR) 1 schedule to a variable ratio (VR) 2, VR 3, VR 5 and finally to a VR 10 schedule of reinforcement which was chosen because it engenders robust responding during reinstatement (Acosta et al., 2008). Lever presses by Saline rats had no programmed consequences. Two days prior to self-administration training, rats were restricted to 16 g of food/day to facilitate acquisition (Carroll et al., 1981). The rats remained food-restricted until a criterion of 7 infusions/h was achieved across 2 days, after which rations were increased gradually to ad libitum access for the remainder of the experiment. Saline rats received the same food restriction as their Cocaine rat counterpart. Rats were maintained on a VR 10 schedule of reinforcement with no food restrictions for at least the last 5 days of training.
2.4.3. Extinction
Upon completing self-administration training, rats were further assigned to Extinction or No Extinction conditions counterbalanced for total number of cocaine infusions obtained during self-administration. Saline rats were assigned to the same condition as their Cocaine rat counterpart. Rats in the Cocaine-Extinction group (n = 20) received 24 daily 2-h extinction sessions during which response-contingent presentations of the cues previously paired with cocaine infusions were available on an FR 1 schedule. The infusion pumps were activated during extinction training, however, no infusions were delivered. Extinction training was designed to decrease the incentive motivational and/or conditioned reinforcing effects of the cocaine-associated cues. The Saline-Extinction group (n = 9) was presented with the cues contingent upon the responses of their Cocaine rat counterpart. Rats in the Cocaine-No Extinction (n = 20) and Saline-No Extinction (n = 8) groups were placed in gray plastic holding cages of equal size as the operant conditioning chambers, but with different bedding and visual cues. The No Extinction groups were run at the same time as the Extinction groups, although the holding cages were located in a separate room from where self-administration had occurred.
2.4.4. Cocaine-seeking behavior
Testing occurred the day after the last extinction session. Prior to the test day, the Cocaine-Extinction and Cocaine-No Extinction groups were further assigned to receive a pretreatment of vehicle (n = 10) or NBQX (10 mg/kg, IP; n = 10), with assignment counterbalanced for total number of cocaine infusions obtained during cocaine self-administration. Saline rats were assigned to the same condition as their Cocaine rat counterpart (n = 4–5). To control for injection stress, all rats were given injections of vehicle 20 min prior to extinction sessions on each of the 3 days prior to testing.
On the test day, all rats received their drug pretreatment 20 min prior to placement back into their self-administration environment. Cocaine groups received response-contingent presentations of the cues previously paired with cocaine infusions on an FR 1 schedule, except that no fluid was infused upon onset of the pump. Saline groups were presented with the same cues contingent upon the responses of their Cocaine rat counterpart. Lever presses by Saline groups produced no scheduled consequences. Testing lasted 90 min, during which responses by all rats on both the active (i.e., cocaine-seeking behavior) and inactive lever were recorded. A 90 min test session was chosen because this time frame coincides with the peak levels of Fos expression (Herdegen & Leah, 1998; Nestler, 2004) and is consistent with our previous reports that have examined cue-elicited Fos expression (Neisewander et al., 2000; Zavala et al., 2007).
2.4.5. Tissue preparation
All chemicals used in the procedures described subsequently were obtained from Sigma (St. Louis, MO) unless otherwise indicated. Immediately after the test for cocaine-seeking behavior, rats were deeply anesthetized using sodium pentobarbital (100 mg/kg, IP) and within 4–6 min intracardially perfused with ice cold 0.1 M phosphate buffered saline (PBS), pH 7.4, followed by ice cold 4% paraformaldehyde (PFA) dissolved in 0.1 M PBS, pH 7.4. Brains were then removed and post-fixed in PFA for 24 h and stored in 30% sucrose at 4° C. Serial brain sections (40 μm) were then cut using a freezing microtome from different levels corresponding to +3.2, +1.6, −2.56, and −5.6 mm from bregma (Paxinos & Watson, 1998). Sections were stored in a cryoprotectant solution containing 0.02 M PBS (pH 7.2), 30% sucrose, 10% polyvinyl pyrrolidone and 30% ethylene glycol at −20° C until processed for immunolabeling.
2.4.6. Immunocytochemistry
Sections from rats in each group were processed simultaneously for Fos protein expression. Initially, sections were washed extensively in 0.02 M PBS (7 times for 10 min), and incubated for 1 h in 0.4% Triton X-100 in 0.02 M PBS containing 1.5% normal goat serum (Vector laboratories, Burlingame, CA). Sections were then incubated for 48 h at 4° C with the anti-c-fos rabbit polyclonal primary antibody (1:20,000; Santa Cruz Biotechnology, Santa Cruz, CA, sc-52) containing 0.02 M PBS, 0.4% Triton X-100, and 1.5% normal goat serum. The tissue was then washed in 0.02 M PBS (5 times for 5 min) and incubated for 1 h in 0.02 M PBS containing biotinylated horse anti-goat antibody IgG (1:400; Vector Laboratories), 0.4% Triton X-100, and 1% normal goat serum. The tissue was then given additional washes in 0.02 M PBS (3 times for 10 min) and incubated for 1 h in avidin-biotinylated horse-radish peroxidase complex (ABC Elite kit, Vector Laboratories) diluted in 0.02 M PBS and 0.4% Triton X-100. The sections were then washed three times in 0.05 M Tris buffer, pH 7.6 and incubated in 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB) containing 2.5% nickel ammonium sulfate and 0.005% hydrogen peroxidase for 5–7 min. The reaction was terminated by rinsing the tissue in 0.02 M PBS (4 times for 10 min). Finally, sections were mounted onto slides coated with gelatin chrom-alum, dried, dehydrated, and coverslipped.
2.4.7. Immunoreactivity analysis
Regions analyzed were located at four different levels: +3.2 mm from bregma, which contained the prelimbic cortex (PrL), infralimbic cortex (IL), and orbitofrontal cortex (OF); +1.6 mm from bregma, which contained the Cg2 region of the anterior cingulate cortex (ACg), dorsal caudate-putamen (dCPu), nucleus accumbens shell (NAcS), and nucleus accumbens core (NAcC); −2.56 mm from bregma, which contained the motor cortex (MC), central amygdala (CeA), and basolateral amygdala (BlA); −5.6 mm from bregma, which contained the ventral subiculum (VSub) and ventral tegmental area (VTA). The rostral-caudal extent of each region was sampled from each hemisphere in three different tissue sections. Fos immunoreactivity was examined using a Nikon Eclipse E600 (Nikon Instruments, Melville, NY) microscope. For the NAcS and VSub, the sample area for each measure was 0.065 mm2 and for the dCPu the sample area was 0.26 mm2 and 12 samples were counted (i.e., 2 sample areas within the region/2 hemispheres/3 sections). For all other regions the sample area was 0.26 mm2 and there were a total of 6 sample areas counted (i.e., 1 sample area/2 hemispheres/3 sections). The counts from all of the sample areas from a given region were averaged to provide a mean number of immunoreactive cells per sample area. Fos immunoreactivity was identified as a blue-black oval shaped nucleus by an observer blind to treatment conditions using Image tool (Version 3.0, University of Texas Health Sciences Center, San Antonio, TX).
2.5. Statistical Analysis
Locomotion was analyzed using analysis of variance (ANOVA) with drug (Vehicle vs. NBQX) as a between-subjects factor and time as a repeated measure. We have shown previously that Saline rats assigned to Extinction versus No Extinction conditions do not differ in any behavioral or neurochemical dependent measure (Neisewander et al., 2000; Zavala et al., 2007), indicating that their history of exposure to different environments during the 24-day extinction phase has no bearing on cocaine seeking or Fos expression. We confirmed this to be the case in the present study. Thus, data from these conditions were combined in further analyses (i.e., Saline group). Separate ANOVAs were used to analyze total reinforcers, active, and inactive lever responses during self-administration, extinction and the test day, as well as the number of Fos positive immunoreactive cells with group (Saline, Cocaine-Extinction, vs. Cocaine-No Extinction) and test drug (Vehicle vs. NBQX) as between subjects factors, and day as a repeated measure for self-administration and extinction data and time as a repeated measure for test day responses. Sources of main effects and interactions were further analyzed using tests of simple effects and Tukey tests. To determine the association between Fos expression and motor behavior, the correlation between number of Fos-labeled cells and lever presses on the test day within brain areas where significant effects were found was determined using Pearson product-moment correlation (r).
3.0. Results
3.1. Effects of NBQX on locomotion
Figure 1 presents the effects of NBQX pretreatment on locomotor activity. Spontaneous locomotion was not affected, evident as a lack of a significant main effect of drug (F1,20 = 0.19, P=0.89) or drug by time interaction (F5,100 = 0.80, P=0.80).
Figure 1.
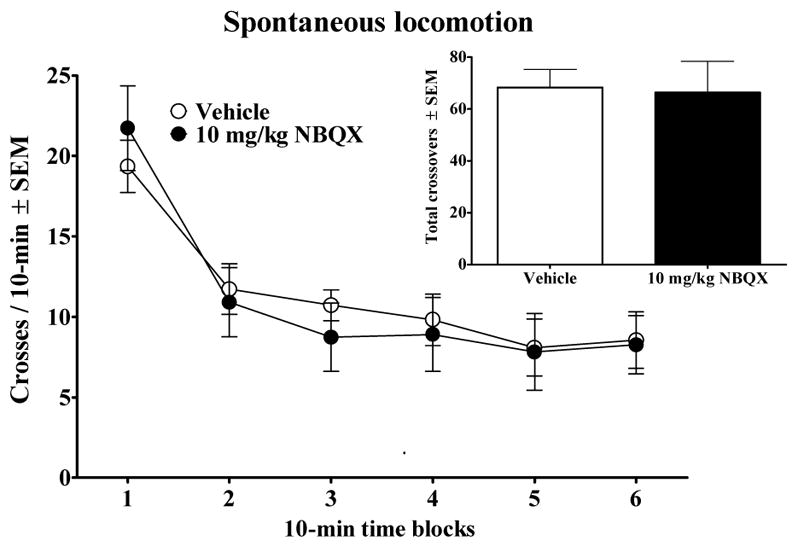
Effects of Vehicle (n = 11) or 10 mg/kg, IP NBQX (n = 11) pretreatment on spontaneous locomotor activity. Rats were injected with their pretreatment 20 min prior to being placed into the locomotor activity chambers.
3.2. Cocaine self-administration
Rats exhibited a mean (±SEM) total cocaine intake of 367.91 ± 20.82 mg/kg, IV throughout the 24 days of training. Cocaine intake was stable (variation of < 10%) during the last 7 days of training and did not vary between Cocaine groups (F1,36 = 0.42, P=0.52). The rats self-administered a mean (±SEM) of 22.87 ± 1.41 infusions/day which amounted to 17.15 ± 1.05 mg/kg/day of cocaine. Rats exhibited a preference for the active lever during the last 7 days of training (Wilcoxen signed rank test, z = −3.97, P<0.001) with a mean (±SEM) number of responses on the active and inactive lever of 208.98 ± 15.17 and 81.70 ± 39.53/2 h, respectively. The high variability of responding on the inactive lever was due to a few rats that exhibited stereotypic behavior on the inactive lever.
3.3. Cocaine-seeking behavior during extinction training
Figure 2 presents the effects of extinction training on active and inactive lever responding. Responses on the active lever across extinction were significantly greater in the Cocaine-Extinction group compared to the Saline-Extinction group on the first day of extinction, as indicated by a significant group × day interaction (F23,460 = 11.23, P<0.001) and a test of simple effects (P<0.05). Response rates declined across days in the former group, such that there were no group differences by the end of extinction. Responses on the inactive lever did not vary between groups. Moreover, there were no differences in responding among groups when pretreatment condition on the test day was included in the analysis.
Figure 2.
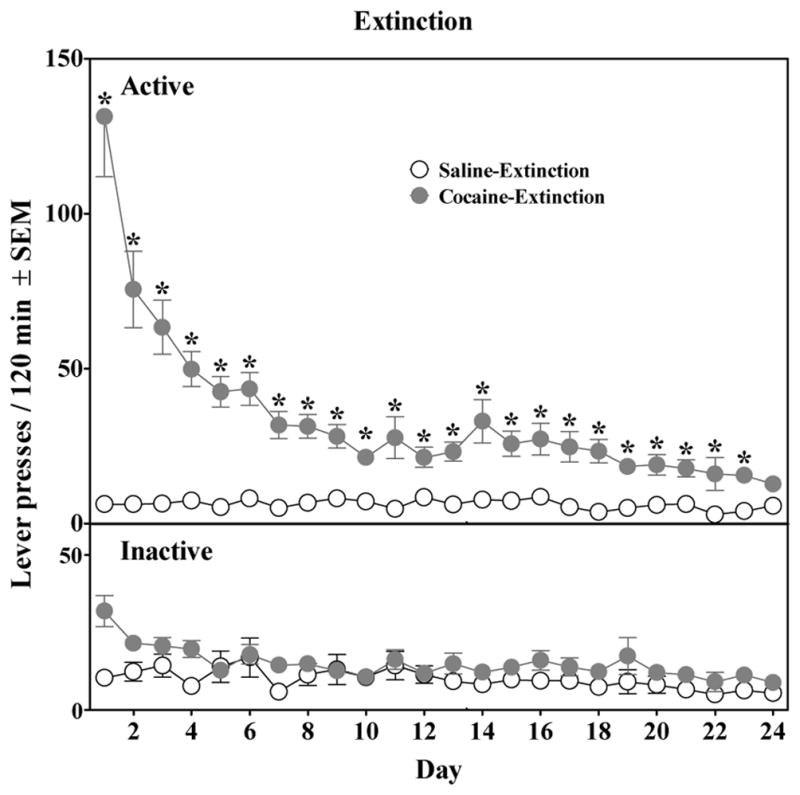
Effect of extinction training on active (top) and inactive (bottom) lever presses in rats with a history of cocaine self-administration [Cocaine-Extinction (n = 20)] or saline-yoked administration [Saline-Extinction (n = 9)]. * represents a difference from the Saline-Extinction group (P<0.05).
3.4. Effects of NBQX on cocaine-seeking behavior
The effect of NBQX pretreatment on cocaine-seeking behavior on the test day is presented in Figure 3. The results indicated that rats in the Cocaine-No Extinction Vehicle group exhibited significantly more responses on the active lever than rats in all other groups, as indicated by a significant group × test drug × time interaction (F16,408 = 5.16, P<0.001) and tests of simple effects (P<0.05). This increase in active lever responses in the Cocaine-No Extinction group pretreated with Vehicle was evident across the first 50 min of testing (Tukey tests, P<0.05). Moreover, rats in the Cocaine-No Extinction group pretreated with NBQX exhibited significantly less active lever responses compared to those pretreated with vehicle, and also failed to exhibit a significant increase in responding compared to all four control groups. Lever presses among the four control groups did not differ on the test day. Additionally, the Cocaine-No Extinction Vehicle group exhibited greater presses on the inactive lever compared to all other groups during the second 10-min time block, evident by a significant group × test drug × time interaction (F16,408 = 5.24, P<0.001) and tests of simple effects and subsequent Tukey test, (P<0.05). Inactive lever presses did not vary among the other groups.
Figure 3.
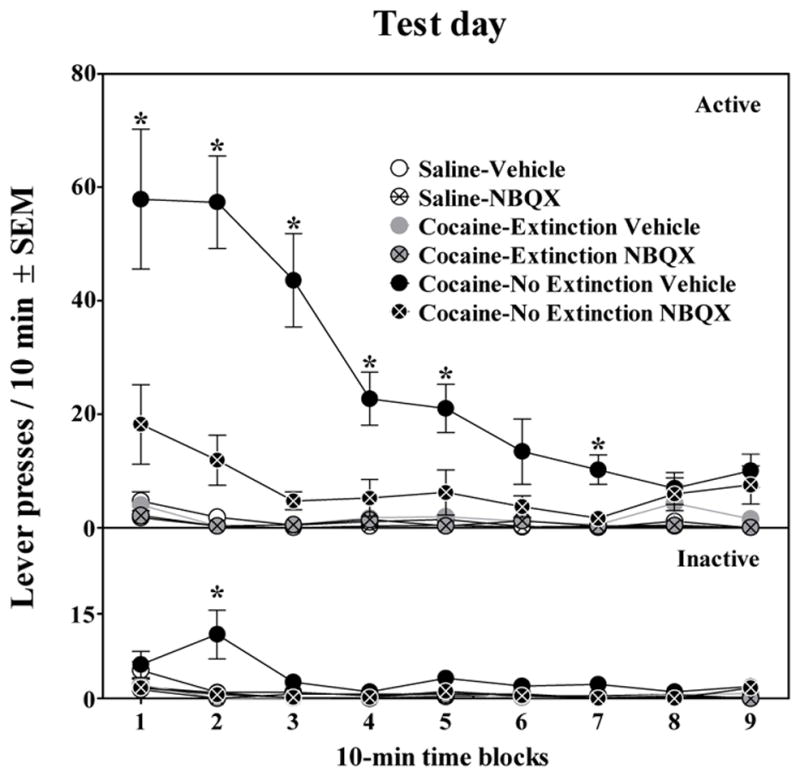
Lever presses on the active and inactive levers across the 90-min test session. Rats in the Saline, Cocaine-Extinction, and Cocaine-No Extinction groups were pretreated with either Vehicle (n = 8–10) or 10 mg/kg, IP NBQX (n = 9–10) 20 min prior to being placed into the self-administration environment. *represents a difference from all other groups (Tukey test, P<0.05).
3.5. Cocaine-seeking behavior across abstinence periods
Rats in the Cocaine-No Extinction Vehicle group demonstrated a time-dependent increase in cocaine-seeking behavior, as this group had a greater number of responses after a 24-day abstinence period, compared to the rats in the Cocaine-Extinction Vehicle group that had a 1-day abstinence period [(t28 = 4.26, P<0.001)]. Specifically, rats in the Cocaine-No Extinction Vehicle group had a mean (±SEM) number of responses/90 min of 243.10 ± 25.87, compared to the rats in the Cocaine-Extinction Vehicle group that had a mean (±SEM) response rate of 117.10 ± 16.46.
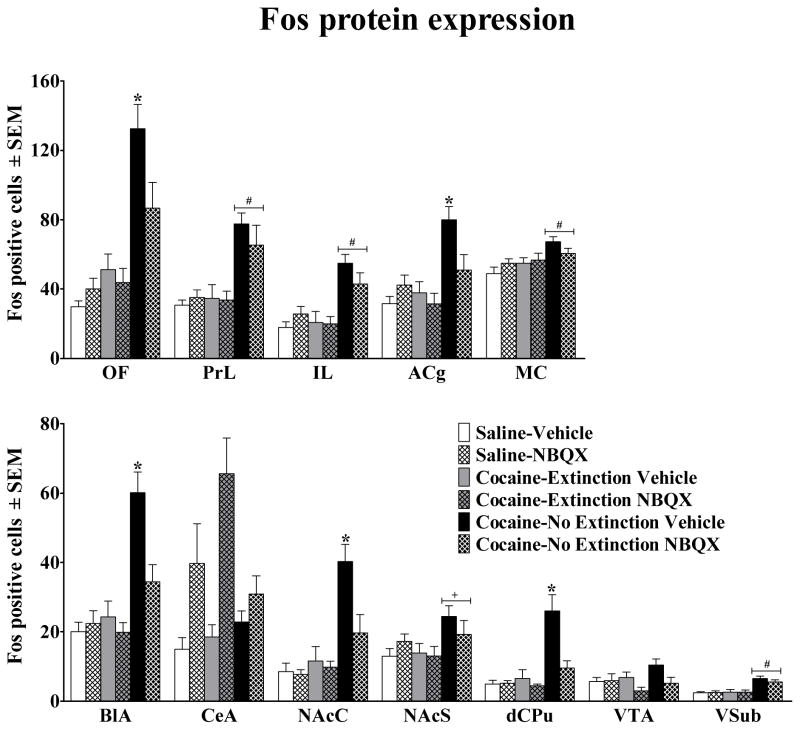
3.6. Effects of NBQX on Fos expression
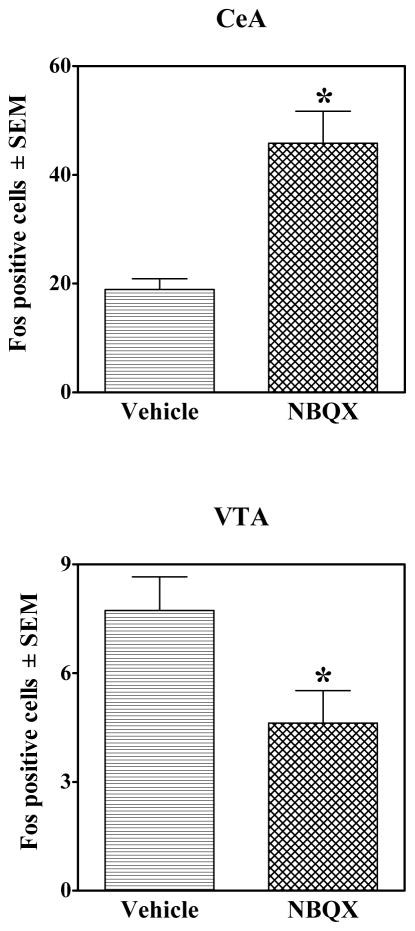
Figure 4 illustrates the specific brain regions analyzed and the size and location of the sampling areas. Figure 5 illustrates representative sample regions analyzed at a magnification of 20× and Figure 6 presents the results of the Fos protein analyses. Exposure to the cocaine-associated cues on the test day increased Fos protein expression in a region-specific manner in the Cocaine-No Extinction group relative to all other groups in all regions except the CeA and VTA, evident as a significant main effect of group (F2,51 = 4.57–34.73, P<0.05) and subsequent Tukey tests (P<0.05). Importantly, NBQX pretreatment selectively decreased cue-elicited Fos protein expression in the Cocaine-No Extinction NBQX group in the ACg, OF, BlA, NAcC, and dCPu, as indicated by a significant decrease in Fos protein expression in the Cocaine-No Extinction NBQX group relative to the Cocaine-No Extinction Vehicle group [group × test drug interaction (F2,51 = 3.70–6.43, P<0.05) and subsequent Tukey tests, (P<0.05)]. NBQX alone failed to alter Fos protein expression, except in the CeA and VTA in which there was an increase and decrease, respectively, in Fos expression in NBQX-treated animals regardless of conditioning history (see Figure 7), indicated by a significant main effect of test drug pretreatment (F2,51 = 5.46–22.88, P<0.05).
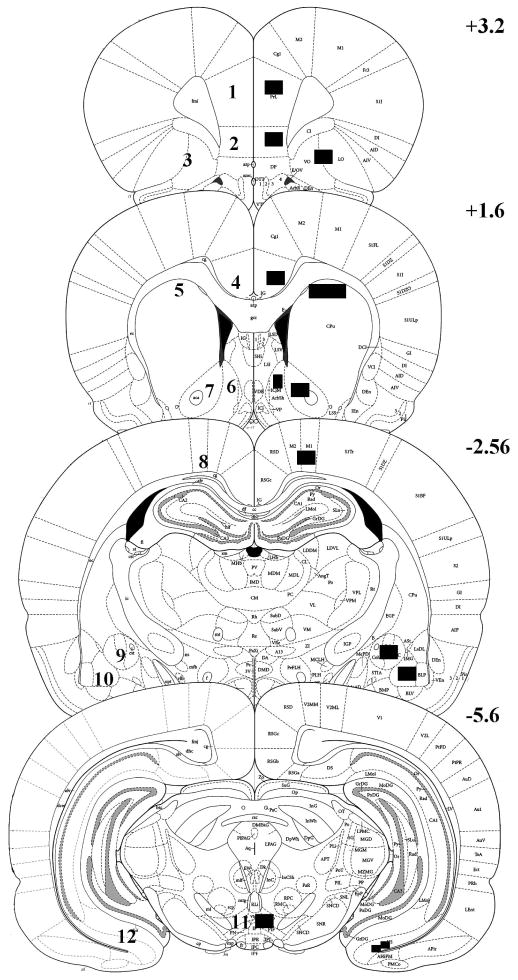
Figure 4.
Schematic representation of coronal sections of the rat brain (+3.2, +1.6, −2.56, and −5.6 mm from Bregma) adapted from Paxinos and Watson (1998) illustrating regions analyzed for Fos protein expression. Numbers in the sections represent the regions analyzed as follows: 1) prelimbic cortex (PrL); 2) infralimbic cortex (IL); 3) orbitofrontal cortex (OF); 4) Cg2 region of the anterior cingulate cortex (ACg); 5) dorsal caudate-putamen (dCPu); 6) nucleus accumbens shell (NAcS); 7) nucleus accumbens core (NAcC); 8) motor cortex (MC); 9) central amygdala (CeA); 10) basolateral amygdala (BlA); 11) ventral tegmental area (VTA); 12) ventral subiculum (VSub). For each brain region, the location and the size of the subregion measured within the region is indicated.
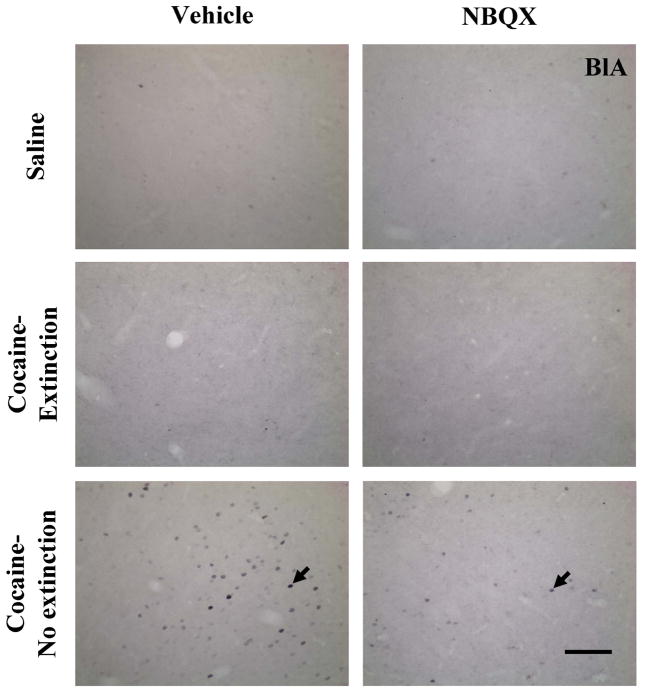
Figure 5.
Photomicrographs of coronal sections taken at 20× magnification demonstrating labeling in the basolateral amygdala (BlA) for Fos-immunoreactivity (black arrows) in representative rats in the Saline, Cocaine-Extinction, and Cocaine-No Extinction groups pretreated with Vehicle or NBQX. Scale bar is equal to 100 μm.
Figure 6.
Number of Fos-immunoreactive labeled cells (±SEM) in cortical, striatal, and limbic regions of rats following the test for cocaine-seeking behavior (see Figure 4 caption for definition of brain region abbreviations and Figure 3 caption for explanation of group labels). For all regions the number of Fos positive cells are presented per 0.26 mm2, except for the he NAcS and VSub in which the number of Fos positive cells are presented per 0.065 mm2. *represents a difference from all other groups, Tukey HSD test (P<0.05). #represents a difference from Saline and Cocaine-Extinction groups, main effect of group and Tukey HSD test (P<0.05). +represents a difference from the Cocaine-Extinction groups, main effect of group and Tukey HSD (P<0.05).
Figure 7.
Number of Fos-immunoreactive labeled cells (±SEM) in the CeA (top graph) and VTA (bottom graph) of rats following systemic administration of Vehicle or NBQX (10 mg/kg, IP) pretreatment on the test day. The data are averaged across the Saline, Cocaine-Extinction, and Cocaine-No Extinction conditions. *represents a main effect of test drug (P<0.05).
3.7. Correlation between responding and Fos expression
In areas where significant increases in Fos expression were observed by the Cocaine-No Extinction group, the correlation between number of Fos-labeled cells and lever presses on the test day was examined to determine the association between motor responding and Fos expression. There was no relationship between responding and expression of Fos, evident by the lack of significant correlations in any of the regions examined (P>0.05).
4.0. Discussion
This study is the first to identify subregions of the neural circuitry activated by cocaine-conditioned cues in which the resulting Fos expression is mediated, at least in part, via AMPA receptor signaling pathways. Cue-conditioned neuronal activation was evident among vehicle-pretreated groups as an increase in Fos expression in the Cocaine-No Extinction group relative to Cocaine-Extinction and Saline-Yoked control groups. Consistent with our previous research (Neisewander et al., 2000; Zavala et al., 2007), this pattern of Fos expression across groups was evident in the BlA, NAc, dCPu, and subregions of the prefrontal cortex. Rats in the Cocaine-No Extinction group pretreated with the AMPA/KA receptor antagonist NBQX exhibited an attenuation of cue-elicited cocaine-seeking behavior relative to those pretreated with Vehicle (see Figure 3), consistent with previous research (Backstrom & Hyytia, 2003; 2006). Importantly, within the Cocaine-No Extinction groups, concomitant decreases in Fos expression were evident with NBQX compared to vehicle pretreatment in the OF and ACg regions of the prefrontal cortex, BlA, dCPu, and NAcC (see Figure 6). The findings suggest that cue-elicited Fos expression in these regions involves AMPA receptor stimulation.
The increase in Fos expression observed in the Cocaine-No Extinction groups is not likely due to motor behavior, sensory processing, or changes in signaling from a history of cocaine self-administration. Regarding motor behavior, we have previously demonstrated that increases in Fos after exposure to cocaine-associated cues are evident regardless of whether rats engage in cocaine-seeking behavior during testing (i.e., regardless of whether the lever was present or not (Neisewander et al., 2000). Moreover, in the present study, increased Fos expression in the Cocaine-No Extinction Vehicle-pretreated group did not correlate with lever press responding during the test day in any region examined. Regarding sensory processes, the increases in Fos were not observed in Saline controls that were exposed to the sensory stimuli on the test day. Finally, the Cocaine-Extinction group did not exhibit increases in Fos despite a similar history of cocaine intake as rats in the Cocaine-No Extinction group.
The NBQX-induced decreases in cocaine-seeking behavior and Fos expression were not due to behavioral suppression because we observed no effect on locomotion (see Figure 1), consistent with previous reports (Backstrom & Hyytia, 2006; Karcz-Kubicha & Liljequist, 1995; Mead et al., 1999). Furthermore, the decreases in conditioned Fos expression in the Cocaine-No Extinction group were not due to a general NBQX-induced decrease in Fos since Fos expression was not altered in the NBQX-pretreated Saline or Cocaine-Extinction groups.
NBQX pretreatment did not affect Fos expression in the PrL or IL regions of prefrontal cortex, NAcS, or VSub. The lack of changes in Fos expression in these regions does not exclude a role of AMPA receptors since AMPA signal transduction in these regions may involve molecular pathways that induce other IEGs, such as Arc or Zif268 that are also activated by drug-associated stimuli (Hearing et al., 2008; Koya et al., 2006; Schiltz et al., 2005; Thomas et al., 2003; Zavala et al., 2008). Collectively, the present findings suggest a region-specific involvement of AMPA receptors in cocaine-conditioned c-fos induction.
The mechanism by which AMPA receptors are involved in c-fos induction may involve Ca2+-mediated receptor transduction, given that AMPA receptors are permeable to Ca2+ (Hollmann & Heinemann, 1994) and Ca2+ plays a role in regulation of c-fos gene expression (Hughes & Dragunow, 1995; Poser & Storm, 2001). Alternatively, it is possible that AMPA receptor stimulation alters signaling downstream resulting in c-fos induction.
Imaging studies in both animals (Crawford et al., 1995; Neisewander et al., 2000; Zavala et al., 2007) and humans (Bonson et al., 2002; Childress et al., 1999; Garavan et al., 2000; Kilts et al., 2004; Wexler et al., 2001) have consistently demonstrated activation of the ACg and OF cortices after exposure to cocaine-conditioned stimuli. Moreover, pharmacological inactivation of the OF decreases cue-induced reinstatement (Fuchs et al., 2004b), further supporting a role of this region in cocaine-seeking behavior. The present findings implicate AMPA receptor involvement in these effects. Moreover, our previous study found that AMPA receptor subunits are coexpressed in neurons exhibiting cocaine-conditioned Fos expression in the OF and ACg and that there is an increase in the proportion of Fos-labeled cells that coexpress the AMPA GluR1 subunit in the ACg, suggesting neuroplasticity (Zavala et al., 2007). We hypothesize that AMPA receptor stimulation in these cortical regions is critical for Fos expression associated with cue-elicited cocaine-seeking behavior.
Manipulation of AMPA receptors within the NAcC modulates cocaine-seeking behavior (Cornish et al., 1999; Cornish & Kalivas, 2000; Park et al., 2002; Suto et al., 2004). AMPA/KA receptor antagonism within the NAcC, but not the NAcS, attenuates cue-elicited cocaine-seeking behavior (Backstrom & Hyytia, 2007; Di Ciano & Everitt, 2001; 2004). The present findings are in agreement because systemic NBQX pretreatment decreased cue-elicited Fos in the NAcC, but not in the NAcS. Furthermore, excitotoxic lesion or GABAergic inactivation of the NAcC, but not the NAcS, impairs cue-elicited cocaine-seeking behavior (Fuchs et al., 2004a; Ito et al., 2004). Thus, activation of the NAcC, but not the NAcS, appears necessary for cue-elicited cocaine-seeking behavior and this behavior likely involves AMPA/KA receptor-mediated induction of c-fos.
The dCPu may be critical for the transition from drug use to addiction (Canales, 2005; Everitt & Robbins, 2005). Indeed, human imaging studies show aberrant regulation of dopamine within the striatum in cocaine-dependent patients, and self-reports of cocaine craving are associated with increased activity in this region (Garavan et al., 2000; Volkow et al., 2006). In animals, pharmacological inactivation of the dCPu decreases cue-induced reinstatement (Fuchs et al., 2006). Furthermore, AMPA receptor blockade localized to the dCPu decreases cue-elicited cocaine-seeking behavior (Vanderschuren et al., 2005). The present findings are consistent with the latter report and further suggest that cue-elicited cocaine-seeking behavior involves AMPA-induced Fos signaling in the dCPu.
Neuronal activation studies in animals suggest a role of the BlA in cue-elicited cocaine-seeking behavior (present findings; Ciccocioppo et al., 2001; Neisewander et al., 2000; Zavala et al., 2007), consistent with human imaging studies of cocaine craving (Childress et al., 1999; Grant et al., 1996). The role of the BlA is further supported by anatomical studies demonstrating a decrease in cue-induced reinstatement following lesion or inactivation (Kantak et al., 2002; McLaughlin & See, 2003; Meil & See, 1997), or infusion of dopamine antagonists (Alleweireldt et al., 2006; Di Ciano & Everitt, 2004; See et al., 2001). In contrast, AMPA/KA receptor antagonism in the BlA with either CNQX (See et al., 2001) or LY293558 (Di Ciano & Everitt, 2004) has no effect on cue-elicited cocaine-seeking behavior. In light of these findings, the present results are surprising because NBQX decreased the number of cells expressing conditioned increases in Fos within this region. It is possible Fos expression in the BlA is nonessential for cue-elicited cocaine seeking behavior. Alternatively, more wide spread antagonism of BlA AMPA/KA receptors than that produced by localized infusions may be required to attenuate the behavior. Alternatively, BlA AMPA/KA receptor involvement in cue-elicited cocaine seeking and Fos expression may be more critical with extended abstinence periods prior to testing. Prolonged periods of abstinence from cocaine are associated with increases in motivation for cocaine, which is referred to as the incubation effect (Grimm et al., 2001; Neisewander et al., 2000; Tran-Nguyen et al., 1998). This effect was evident in the present study (i.e., significantly more cocaine seeking in rats experiencing their first extinction session on the test day after 24 days of abstinence compared to those experiencing their first extinction session after one day of abstinence), whereas previous studies examined behavior after only 1–10 days of abstinence. Several neurochemical changes are associated with abstinence-induced increases in cocaine-seeking behavior, including alterations in glutamate receptor subunits (Lu et al., 2003; Tang et al., 2004), dopamine D3 receptors (Neisewander et al., 2004), brain-derived neurotrophic factor (Grimm et al., 2003), and extracellular signal-regulated kinase (Lu et al., 2005b). If incubation effects involve AMPA receptors in the BlA, it is unlikely these receptors contain GluR1 subunits because this protein is elevated to the same degree after 1 or 30 days of withdrawal from cocaine self-administration (Lu et al., 2005a). Further research is needed to investigate the functional significance of the NBQX-induced decreases in Fos observed in the BlA.
The only effects of NBQX regardless of conditioning history were a decrease in Fos in the VTA and an increase in the CeA (see Figure 7). Consistent with the former, in vitro studies of rat VTA slice preparations demonstrate that NBQX decreases activity of dopamine and glutamate neurons (Wang & French, 1993; 1995). The increase in Fos expression in the CeA after acute administration of NBQX likely involves AMPA receptor-mediated disinhibition. Indeed, NBQX enhances Fos expression in the NAc and dopamine agonist-induced expression in the striatum (Dalia & Wallace, 1995; Pollack et al., 2005). These effects are thought to result from AMPA receptor-mediated decreases in GABAergic inhibition (Pollack et al, 2005).
A surprising finding in the present study was the increase in Fos expression in the MC of the Cocaine-No Extinction groups relative to controls, regardless of test day pretreatment (See Figure 6) because we failed to observe such changes previously (Neisewander et al., 2000). This discrepancy is likely due to increased statistical power in the present study, given that the number of Cocaine-No Extinction rats was 20 in the present study compared to 5 in our previous report. Moreover, the area analyzed within the MC was twice as large as that used previously, which may have allowed greater sensitivity to detect changes in Fos expression. It is still unlikely that increases in Fos expression are a result of increased motor behavior (i.e., lever presses), because there was a significant difference between Vehicle- and NBQX-pretreated rats’ lever presses on the test day but no difference in Fos expression in the MC. Interestingly, brain activation in the MC has been reported in response to cocaine-associate cues in cocaine-dependent patients that relapsed but not in those that abstained (Kosten et al., 2006). This finding may reflect thought processes associated with motor processing of habitual cocaine-seeking responses or movements directly involved in drug taking. Collectively, the findings suggest that the increase in Fos expression in the MC may reflect recall rather than expression of cocaine seeking.
In conclusion, the present study expands knowledge of the circuitry underlying cue-elicited motivation for cocaine by indicating a role of AMPA receptors in the OF and ACg regions of prefrontal cortex, BlA, dCPu, and NAcC, but not the PrL and IL regions of prefrontal cortex, NAcS, and VSub. These findings suggest new hypotheses regarding AMPA receptor involvement in the circuitry underlying cocaine seeking. Elucidating the molecular mechanisms involved in activating this circuitry has important implications for developing anti-craving treatments for cocaine dependence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JI, Thiel KJ, Sanabria F, Browning JR, Neisewander JL. Effect of schedule or reinforcement on cue reinstatement of cocaine-seeking behavior. Behavioural Pharmacology. 2008;19:129–136. doi: 10.1097/FBP.0b013e3282f62c89. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Attenuation of cocaine-seeking behaviour by the AMPA/kainate receptor antagonist CNQX in rats. Psychopharmacology (Berl) 2003;166:69–76. doi: 10.1007/s00213-002-1312-y. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacolog. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. European Journal of Pharmacology. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. Journal of Pharmacology and Experimental Therapeutics. 1981;217:241–247. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Research Monograph. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proceedings of the National Academy of Science of the United States of America. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Dalia A, Wallace LJ. Amphetamine induction of c-fos in the nucleus accumbens is not inhibited by glutamate antagonists. Brain Res. 1995;694:299–307. doi: 10.1016/0006-8993(95)00794-q. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. European of Journal of Neuroscience. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004a;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. Journal of Neuroscience. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Science of the United States of America. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. Journal of Neuroscience. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. Journal of Neuroscience. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Liljequist S. Evidence for an anxiogenic action of AMPA receptor antagonists in the plus-maze test. Eur J Pharmacol. 1995;279:171–177. doi: 10.1016/0014-2999(95)00153-c. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. Journal of Neurochemistry. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shaham Y, Hope BT. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. Journal of Neurochemistry. 2005a;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. Journal of Neurochemistry. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nature Neuroscience. 2005b;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Mead AN, Vasilaki A, Spyraki C, Duka T, Stephens DN. AMPA-receptor involvement in c-fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. Eur J Neurosci. 1999;11:4089–4098. doi: 10.1046/j.1460-9568.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behavioural Brain Research. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. Journal of Neuroscience. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology, 47 Suppl. 2004;1:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Research advances in the understanding and treatment of addiction. Am J Addict. 2003;12(Suppl 2):S36–47. [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Developing treatments that address classical conditioning. NIDA Research Monograph. 1993;135:71–91. [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. Journal of Neuroscience. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Pollack AE, St Martin JL, MacPherson AT. Role of NMDA and AMPA glutamate receptors in the induction and the expression of dopamine-mediated sensitization in 6-hydroxydopamine-lesioned rats. Synapse. 2005;56:45–53. doi: 10.1002/syn.20125. [DOI] [PubMed] [Google Scholar]
- Poser S, Storm DR. Role of Ca2+-stimulated adenyl cyclases in LTP and memory formation. Int J Dev Neurosci. 2001;19:387–394. doi: 10.1016/s0736-5748(00)00094-0. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Kelley AE, Landry CF. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. European Journal of Neuroscience. 2005;21:1703–1711. doi: 10.1111/j.1460-9568.2005.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. Journal of Neurochemistry. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Wang T, French ED. Electrophysiological evidence for the existence of NMDA and non-NMDA receptors on rat ventral tegmental dopamine neurons. Synapse. 1993;13:270–277. doi: 10.1002/syn.890130310. [DOI] [PubMed] [Google Scholar]
- Wang T, French ED. NMDA, kainate, and AMPA depolarize nondopamine neurons in the rat ventral tegmentum. Brain Res Bull. 1995;36:39–43. doi: 10.1016/0361-9230(94)00160-3. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]