Abstract
Early hearing loss leads to crossmodal plasticity in regions of the cerebrum that are dominated by acoustical processing in hearing subjects. Until recently, little has been known of the connectional basis of this phenomenon. One region whose crossmodal properties are well-established is the auditory field of the anterior ectosylvian sulcus (FAES) in the cat, where neurons are normally responsive to acoustic stimulation and its deactivation leads to the behavioral loss of accurate orienting toward auditory stimuli. However, in early-deaf cats, visual responsiveness predominates in the FAES and its deactivation blocks accurate orienting behavior toward visual stimuli. For such crossmodal reorganization to occur, it has been presumed that novel inputs or increased projections from non-auditory cortical areas must be generated, or that existing non-auditory connections were ‘unmasked.’ These possibilities were tested using tracer injections into the FAES of adult cats deafened early in life (and hearing controls), followed by light microscopy to localize retrogradely labeled neurons. Surprisingly, the distribution of cortical and thalamic afferents to the FAES was very similar among early-deaf and hearing animals. No new visual projection sources were identified and visual cortical connections to the FAES were comparable in projection proportions. These results support an alternate theory for the connectional basis for cross-modal plasticity that involves enhanced local branching of existing projection terminals that originate in non-auditory as well as auditory cortices.
Keywords: Hearing loss, Visual cortex, Somatosensory cortex, Thalamus, Sensory substitution
1. Introduction
Individuals who experience profound sensory loss early in life often exhibit dramatic functional neurological changes that lead to perceptual and behavioral improvements in the remaining senses. Regarded as ‘adaptive’ or ‘compensatory plasticity,’ these behavioral effects have been reported for early-blind or early-deaf humans for a variety of sensory tasks (for review, see Merabet and Pascual-Leone, 2010; Frasnelli et al., 2011). In a broader context, the phenomenon where the representation of a damaged or lost sensory modality is replaced by the remaining, intact modalities is termed ‘crossmodal plasticity’ and this functional effect has been confirmed in experimental animals. In a seminal series of experiments on compensatory plasticity, visually-deprived cats demonstrated auditory localization behaviors which exceeded that present in normally-sighted controls. Furthermore, a region of normally visual cortex not only showed auditory crossmodal plasticity in visually deprived animals, but also contained auditory neurons with supranormal localization sensitivities (e.g., Rauschecker and Korte, 1993; Korte and Rauschecker, 1993).
Compared to the volume of studies of vision loss, few experimental investigations of the crossmodal effects of early deafness have been conducted, until recently. Congenitally deaf mice have been shown to exhibit both visual and somatosensory responses in the primary auditory (A1) area, as well as an expanded representation of the primary visual area (Hunt et al., 2006). In early-deaf ferrets, auditory cortical fields including A1 and the anterior auditory field (AAF) exhibited somatosensory-evoked activity (Meredith and Allman, 2012). In congenitally deaf cats, visual crossmodal plasticity has been identified in the dorsal auditory zone (DZ) and the posterior auditory field (PAF; Lomber et al., 2010, 2011), but not in A1 (Kral et al., 2003), while both visual and somatosensory crossmodal reorganization has been demonstrated in the AAF and the auditory field of the anterior ectosylvian sulcus (FAES) of early-deaf cats (Meredith and Lomber, 2011; Meredith et al., 2011).
To date, one of the most comprehensively studied auditory regions to demonstrate crossmodal plasticity is the FAES. In hearing cats, the FAES contains a mixture of auditory (~77%) and non-auditory (~33%; mostly in the form of auditory-visual, and auditory-somatosensory multisensory neurons; Meredith et al., 2011) and many FAES neurons are characterized by sensitivity to acoustic location (Clarey and Irvine, 1990a; Korte and Rauschecker, 1993; Xu et al., 1998; Las et al., 2008) and sound movement (Jiang et al., 2000). Connections from auditory cortical sources dominate inputs to the FAES, especially from areas AAF and DZ (Lee and Winer, 2008) while non-auditory afferents arrive largely from somatosensory area SIV (Meredith et al., 2006) and the visual lateral suprasylvian areas (Clarey and Irvine, 1990b). The FAES is the major source of auditory corticotectal projections (Meredith and Clemo, 1989; Chabot et al., 2013) and, therefore, plays an important role in mediating superior colliculus (SC) function and behaviors (Meredith and Clemo, 1989; Wallace et al., 1993; Malhotra et al., 2004; Meredith et al., 2011). Accordingly, reversible deactivation of the FAES in hearing cats blocks accurate orienting and localization behaviors to auditory stimuli (Malhotra et al., 2004; Meredith et al., 2011). In early-deaf cats, auditory-evoked activity in the FAES is replaced by visual (~70% of neurons) and somatosensory (~30%) responses (Meredith et al., 2011). Although a visuotopic organization was not observed, visual receptive fields displayed complex response properties such as direction and velocity preferences and, collectively, represented the central and contralateral visual field. Ultimately, the crossmodal visual representation in the early-deaf FAES is critical for visuomotor function, since reversible deactivation resulted in the loss of accurate orienting and localization behaviors to contralateral visual cues in early-deaf, but not hearing controls (Meredith et al., 2011). However, little is known about the connectional basis subserving deafness-induced crossmodal plasticity in the FAES.
The mechanisms underlying the phenomenon of crossmodal plasticity have long been the subject of discussion and speculation. In a review, Rauschecker (1995) summarized the logical possibilities that could provide a connectional substrate for the phenomenon: crossmodal plasticity could result from the recruitment of new projections from novel areas, by increased projections from existing sources, or by the ‘unmasking’ of existing crossmodal inputs. The present experiment sought to test these possibilities by making tracer injections into the crossmodally-reorganized FAES of early-deaf cats to identify the distribution and proportional strength of input sources to the region, and comparing these results to data obtained by similar tracer injections made into FAES of hearing animals.
2. Materials and methods
All procedures were performed in compliance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, publication 86-23), the National Research Council’s Guidelines for Care and Use of Mammals in Neuroscience and Behavioral Research (2003) with prior approval by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Also, all procedures were conducted in accord with the Canadian Council on Animal Care’s Guide to the Care and Use of Experimental Animals (Olfert et al., 1993) with prior approval from the University of Western Ontario Animal Use Subcommittee of the University Council on Animal Care.
2.1. Ototoxic procedures
All animals were obtained from pregnant mongrel cats to avoid potential genetic influences on neural connectivity that may be coupled with congenitally deaf lineages. At 6–8 days postnatal (near hearing onset for cats), each animal was deafened using the ototoxic protocol of Xu et al. (1993). Inhalation anesthesia (isofluorane) was used to permit catheterization of the saphenous or jugular vein. A single, subcutaneous dose of kanamycin (300 mg/kg) was then administered followed by the intravenous injection of ethacrinic acid (100 mg/kg). Following recovery, the animals were returned to their mother as quickly as possible where they were housed until they were weaned (~6 weeks postnatal).
2.2. Hearing evaluation
At 4–6 weeks postnatal, treated animals had their hearing tested using standard Auditory Brainstem Responses (ABR, Fig. 1A). Under ketamine (30 mg/kg) and acepromazine (5 mg/kg) anesthesia, a calibrated auditory click (at least 2000 trials each, 0.1 ms square-wave click, rarefaction) delivered through a minispeaker positioned in front of the ear was used as the auditory stimulus. The full range of stimulation intensities was run for one ear before presenting the tests to the other ear. Subdermal recording leads were inserted at sites superior to the mastoid processes of the right and left ears, at a mid-cranial scalp location, and at a mid-back position. Electrical activity recorded by the leads was routed through an amplifier to a computer for signal averaging and storage. Animals with an ABR threshold of >80 dB SPL, like that illustrated in Fig. 1B, were considered profoundly deaf, as defined by the World Health Organization (1991). However, two of the cases showed a partial hearing decrement and the ototoxic procedure was repeated followed by a second ABR test. In these cases, hearing threshold met the criterion of >80 dB SPL hearing threshold before the age of 50 days postnatal, which is before the critical period of auditory maturation in cats (Kral et al., 2005; Kral, 2013). Treated animals were raised until maturity (>6 months of age) when data collection occurred. All ototoxically treated animals failed to startle or react to loud sounds, nor could they be aroused from sleep without tactile stimulation. In addition, mature animals with normal ABRs (hearing threshold ~15 dB SPL; see Fig. 1A), were used as hearing controls.
Fig. 1.
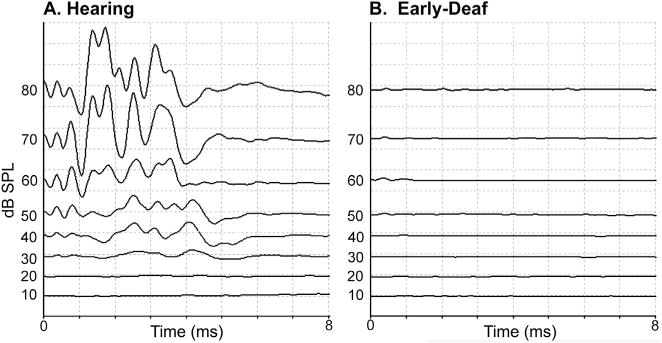
Auditory brainstem responses (ABR) conducted on one ear of (A) a hearing animal and (B) an early-deaf animal. In each panel, the waveforms are arranged according to stimulation intensity, from highest (80 dB SPL – top) to lowest (10 dB SPL – bottom). Stimuli consisted of clicks (0.1 ms, rarefication, 4000 repetitions) presented through a minispeaker positioned directly in front of the external acoustic meatus. For the hearing animal, hearing threshold occurred at ~20 dB SPL while no acoustically-induced activity was observed in the early-deaf animal at any stimulation intensity.
2.3. Neuroanatomical procedures
Adult cats were anesthetized (sodium pentobarbital, 30 mg/kg i.v.) and their heads were secured in a stereotaxic frame. Under aseptic conditions, a unilateral craniotomy and durotomy was made to expose the AES cortex, which is known to exhibit variable positions and configurations on the lateral surface of the cortical hemisphere (Clemo and Stein, 1983, 1985). An electrode carrier was used to support the syringe (Hamilton 5 μl; 31 gauge needle) containing the tracer biotinylated dextran amine (BDA; 10 kMW, lysine fixable 10% in PBS, or a 50/50 mix of 10 kMW and 3k MW BDA, 10% in PBS). The carrier was angled 53–60° (from vertical) with 35–40° cant (anterior-to-posterior from the coronal plane) and the needle tip was inserted at a point 0.8–1.5 mm anterior to the vertical limb of the AES to a depth of 5.25–5.7 mm. The tracer was ejected at a rate of ~1.5 μl/min) until 0.7–1.3 μl was expressed. After the injection was complete and the needle was retracted, the exposed cortical surface was packed with gelfoam, the incision was sutured closed, and standard postoperative analgesia (buprenorphine), thermal and fluid support) was provided. Injections involving hearing controls were derived from archived data from a published study of FAES connections with SIV (Meredith et al., 2006).
2.4. Histological processing
After a 7–10 day post-injection period for tracer transport, the animals were deeply anesthetized (40 mg/kg, i.v.) and perfused transcardially with heparinized saline followed by fixative (4.0% paraformaldehyde). The brain was exposed, blocked stereotaxically, removed and cryoprotected (25% sucrose in 0.1 M phosphate buffer). Coronal sections (50 μm thick) were cut using a freezing microtome and collected serially from the coronal sulcus of the cortex to the anterior border of the inferior colliculus in the midbrain. A series of sections (at 250–300 μm interval) was then processed for visualization of BDA after the protocol of Veenman et al. (1992) with heavy metal intensification. Reacted sections were mounted on treated slides, dehydrated and coverslipped without counterstain. Our lab has used these methods successfully in other published studies of cortical connectivity (Kok et al., 2013; Allman et al., 2009; Meredith and Allman, 2012).
2.5. Data analysis
Neuronal labeling was visualized using a light microscope (Nikon Eclipse-600) that was also equipped with a PC-driven digitizing stage controlled by Neurolucida software (MBF Biosciences, Williston VT) for plotting the data. Using this device, a calibrated tracing was made of each tissue section that included its outline, the border between gray and white matter, labeled neurons, and the injection site (when appropriate). The injection site was defined as the large aggregate of densely labeled cell bodies and neuropil at the end of the injection needle track. With regard to the injection site, it is important to note that the FAES is located deep within the wall and fundus of a sulcus that is known to demonstrate different cortical arrangements in different animals (Clemo and Stein, 1983, 1985). Hence, tracer injections could not be visually guided (as for gyral injections) nor was standard stereotaxy effective. Instead, injections that met the anatomical criteria for targeting the FAES (as described by Meredith and Clemo, 1989) could only be confirmed in the post-processed tissue. As a consequence, although numerous cases were attempted, only seven were appropriately confined to the FAES to be included in the present study (4 hearing cats, female, 2.7–4 kg; and 3 deaf, 2 female, 3–3.5 kg). Of these hearing cats, one had an incomplete cortical series and was used for only thalamocortical connections; another had an incomplete thalamocortical series and was used only for corticocortical connections. All others were used for both cortical and thalamic evaluations. After processing, light microscopy revealed BDA-labeled neurons that were sharply dark throughout their soma and, sometimes, dendrites. Only labeled neuronal somas were scored/marked. Labeled neurons were plotted at 200 × magnification and Neurolucida kept a count of the numbers of neurons marked in relation to tissue outlines and cytoarchitectonic borders. In this manner, an entire series of sections were plotted at regular intervals (250–300 μm) through the brain for each case. The summed number of identified cortical neurons was regarded as the “total” cortical projection, and the numbers of neurons localized in each functional area were normalized as a percentage of that total. A similar normalization was conducted separately for the thalamocortical connections. For comparison purposes, these normalization procedures are the same as those used for other published investigations of crossmodal connectivity (Kok et al., 2013; Chabot et al., 2015; Wong et al., 2015; see also Cappe et al., 2009).
For purposes of visual display and comparison, plots of tissue containing data were converted to a graphic format using a graphics program. Because it was not possible to conduct cytoarchitectonic assays on the archived tissue, the sulcal and gyral patterns defined by the stereotaxic atlas of the cat brain (Reinoso-Suárez, 1961), updated by more recent studies (Avendaño et al., 1988; Bowman and Olson, 1988; Clascá et al., 1997; Clemo and Meredith, 2004; Clemo et al., 2007; Lee and Winer, 2008; Lomber and Malhotra, 2008; Lomber and Payne, 2004; Mellott et al., 2010; Meredith, 2004; Meredith and Clemo, 1989; Mucke et al., 1982; Payne, 1993; Reinoso-Suárez,1961; Ribaupierre,1997; Rosenquist, 1985; Updyke, 1986; van der Gucht et al., 2001) were used to determine the borders of the cortical functional areas. The relative proportion of cortical projections from each area to the FAES was normalized as a percentage of the total projection from the cases in which the entire rostral-caudal series of cortical sections was available (BDA66, 71, 29 hearing; BDA65, 68, W87 early deaf). Thalamic tissue sections containing neurons labeled from the FAES were plotted in the same fashion. Using a graphics program, thalamic sections were brought into register with the thalamic map (Huang et al., 1999) and the location of labeled thalamocortical neurons was tabulated by region and compared (average ± standard error; non-parametric Wilcoxon rank sum test) between treatment groups. The relative proportion of thalamic projections to the FAES was normalized as a percentage of the total projection from the cases in which the entire rostral-caudal series of thalamic sections was available (BDA66, 71, 69 hearing; BDA65, 68, W87). A list of abbreviations for the names of the cortical and thalamic functional subdivisions is provided in Table 1.
Table 1.
List of abbreviations.
| Cortical regions: | |
|---|---|
| Area A1 | Primary auditory cortex |
| Area A2 | Second auditory cortex |
| Area 1 | Primary somatosensory cortex |
| Area 3 | |
| Area 3a | |
| Area 3b | |
| Area 4s | |
| Area 4f | |
| Area 5 | |
| Area 5b | |
| Area 6a | |
| Area 6i | |
| Area 7 | Parietal cortex |
| Area 7m | Parietal cortex, medial |
| Area 17 | Primary visual cortex |
| Area 18 | Secondary visual cortex |
| Area 19 | Third visual cortex |
| Area 20a | |
| Area 20b | |
| Area 21a | |
| Area 21b | |
| Area 35 | |
| Area 36 | |
| AAF | Anterior Auditory field |
| AEV | Anterior Ectosylvian Visual area |
| AID | Agranular Insular-dorsal |
| AIV | Agranular Insular-ventral |
| ALG | Anterior Lateral gyrus visual area |
| ALLS | Anterolateral Lateral Suprasylvian visual area |
| AMLS | Anteromedial Lateral Suprasylvian visual area |
| CgA | Cingulate gyrus, anterior |
| CgP | Cingulate gyrus, posterior |
| CVa | Cingulate visual area |
| DLS | Dorsal Lateral Suprasylvian visual area |
| dPE | Dorsal Posterior Ectosylvian auditory area |
| DZ | Dorsal Zone of auditory cortex |
| FAES | Auditory field of the Anterior Ectosylvian sulcus |
| G | Primary gustatory cortex |
| GI | Granular insular area |
| IN | Insular auditory area |
| IL | Infra Limbic area |
| iPE | Intermediate Posterior Ectosylvian auditory area |
| ME | Medial Entorhinal area |
| MZ | Multisensory zone of rostral suprasylvian sulcus |
| PI | Parainsular area |
| PL | Prelimbic area |
| PLLS | Posterolateral Lateral Suprasylvian visual area |
| PMLS | Posteromedial Lateral Suprasylvian visual area |
| PS | Posterior Suprasylvian visual area |
| RS | Retrosplenial area |
| S2 | Second somatosensory cortex |
| S2m | Second somatosensory cortex, medial |
| S3 | Third somatosensory cortex |
| S4 | Fourth somatosensory cortex |
| S5 | Fifth somatosensory cortex |
| SVA | Splenial Visual area |
| TE | Temporal auditory area |
| VAF | Ventral Auditory field |
| VLS | Ventral Lateral Suprasylvian visual area |
| vPAF | Ventral Posterior auditory field |
| vPE | Ventral Posterior Ectosylvian auditory field |
|
| |
| Thalamic nuclei: | |
|
| |
| D | Dorsal nucleus of medial geniculate body |
| DD | Deep dorsal nucleus of medial geniculate body |
| DS | Dorsal superficial nucleus of medial geniculate body |
| FF | fields of Forel |
| LD | Lateral dorsal nucleus |
| LGN | Lateral geniculate nucleus |
| LMN | Lateral mesencephalic nucleus |
| LP | Lateral posterior nucleus |
| MDBd | Dorsal division of medial geniculate body |
| MGBm | Medial division of medial geniculate body |
| MGBv | Ventral division of medial geniculate body |
| POl | Posterior area of thalamus, lateral region |
| POm | Posterior area of thalamus, medial region |
| Pul | Pulvinar |
| SGl | Suprageniculate nucleus, lateral part |
| SGm | Suprageniculate nucleus, medial part |
| V | Ventral division of the medial geniculate body |
| Vb | Ventrobasalcomplex |
3. Results
3.1. Injection sites
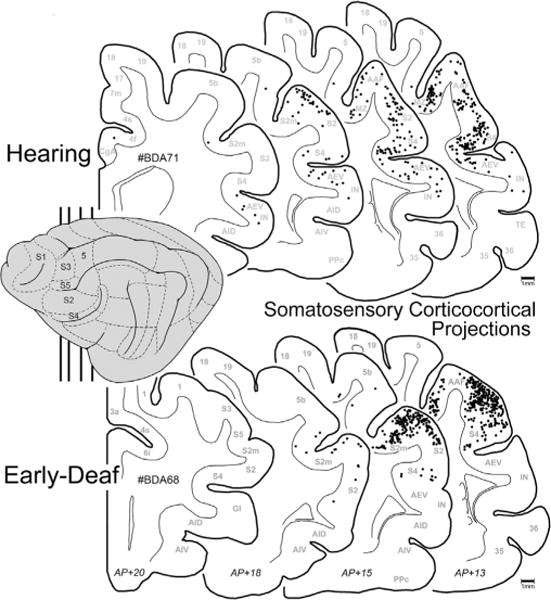
As noted in earlier studies of the anterior ectosylvian region (Clemo and Stein, 1983, 1985; Meredith and Clemo, 1989), this sulcal cortex is highly variable from animal to animal, and sometimes may even fail to invaginate under the middle ectosylvian gyrus (which becomes apparent only after tissue processing). Another complication for examination of this region is that the FAES resides in close proximity to, and shares a common border with, the visual area of the ectosylvian sulcus (AEV) as well as the fourth somatosensory (S4) area. These combined factors severely limited the success of tracer injections that were confined within the defined dimensions of the FAES (described by Meredith and Clemo, 1989 for hearing cats; Wong et al., 2014 for early-deaf cats) to seven cases (4 hearing cats, 3 deaf) whose injection sites are photographically documented in Fig. 2. As this figure shows, the injection sites occupy essentially the same full-thickness portion of the medial bank of the sulcus. These injection locations also correspond with electrophysiological studies of the FAES in hearing animals (Meredith and Clemo, 1989; Las et al., 2008; Meredith et al., 2006; Meredith and Allman, 2009), where ~80% of the neurons showed auditory responses (Meredith et al., 2011) as well as in early-deaf animals, where ~70% of the neurons exhibited visual activity (Meredith et al., 2011). Furthermore, in two of the early-deaf animals (#BDA65, 68) used in the present study, electrophysiological recordings in the opposite hemisphere demonstrated both visual and somatosensory crossmodal plasticity in the FAES (reported in Meredith et al., 2011). Representative examples of labeled cortical and thalamic neurons from hearing and early-deaf animals are shown in Fig. 3.
Fig. 2.
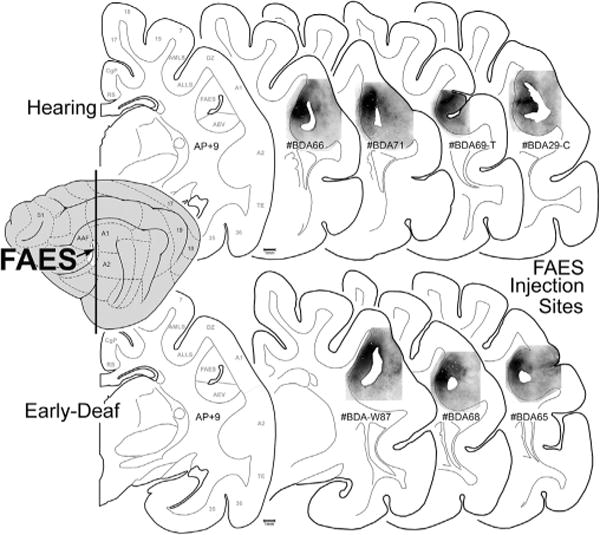
FAES tracer injection site summary. The lateral view of the cat cortex (left) indicates the location of the FAES (colored white at arrow) in relation to other cortical fields. The vertical line represents the approximate anterior-posterior (A–P) level of each of the coronal sections (to the right). The coronal section labeled AP + 9 is derived from Reinoso-Suárez (1961), with the functional subdivisions delimited by the gray lines (for abbreviation definitions see abbreviation table). Individual coronal sections illustrate the location of BDA tracer injection (blackened area) for the hearing (top row) and for the early-deaf cats (bottom-row) within the upper, medial bank of the sulcus corresponding to the position of the FAES region. Hearing case labeled #BDA69-T was comprised of an entire rostral-caudal series through the thalamus; hearing case labeled #BDA29-C was constituted by an entire rostral-caudal series through the cortex. All other cases had the full series of sections for both cortex and thalamus.
Fig. 3.
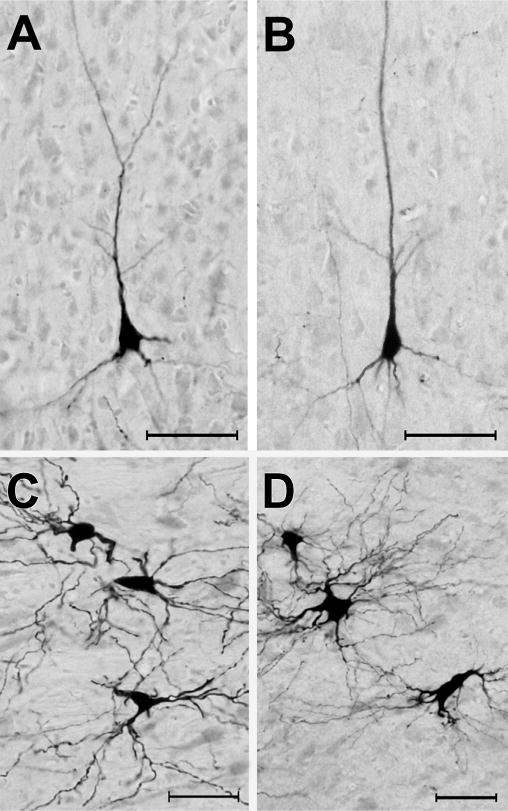
Photomicrographic examples of neurons retrogradely labeled by tracer (BDA) injection into the FAES. Panels A–B show BDA-labeled pyramidal neurons from area DZ in hearing (A) and early-deaf (B) animals (pial surface is toward the top). Panels C–D illustrated BDA-labeled neurons from the medial division of the MGB from hearing (C) and early-deaf animals. All scale bars = 50 μm.
3.2. Visual cortical projections
Given that early deafness functionally reorganizes the FAES largely as a visual region (Meredith et al., 2011), it would be expected that connections to the FAES from visual cortical areas would either emerge de novo, or that the number of projections from visual areas with established connections would become substantially enhanced. This notion was tested by examining the visual cortical areas for retrogradely labeled neurons following tracer injection into the FAES. As shown in Fig. 4 (and by Clarey and Irvine, 1990b), visual cortical areas that access the FAES in a hearing animal include the neighboring AEV area, the ALLS and, to a lesser extent, the PLLS. A few labeled neurons are also identified in the AMLS and PMLS regions, but connections with areas 17/18/19 or other posterior visual areas (such as DLS) are rarely observed. The visual cortical areas that project to the FAES in an early-deaf animal show essentially the same distribution of areal labeling as seen in the hearing animals, as depicted in Fig. 4. When labeled neurons found in visual cortical areas from hearing and early-deaf groups are quantitatively compared, as graphed in Fig. 5, these analyses indicate that novel visual areas are not recruited as projection sources to the crossmodally-reorganized FAES. In addition, extensive projection changes among the existing connections also do not occur, since 18% of the total projection to FAES arises in visual areas of hearing animals, closely corresponding to the 19% observed for the early-deaf cases. Regarding the connectivity of specific visual regions, inputs from neighboring visual AEV represent an average 6.4% of the total projection to the FAES in the hearing but 9.5% in the early-deaf group (range of differences in sample = 1.2–5.3%). On the other hand, other visual regions such as the AMLS and ALLS show slight reductions in their projection strengths in early-deaf animals. Given that visually-responsive neurons increase in proportion from 25% to ~70% in the FAES of early-deaf animals (Meredith et al., 2011), neither novel visual projections nor changes in existing projection sources sufficiently account for the proportional increase observed functionally.
Fig. 4.
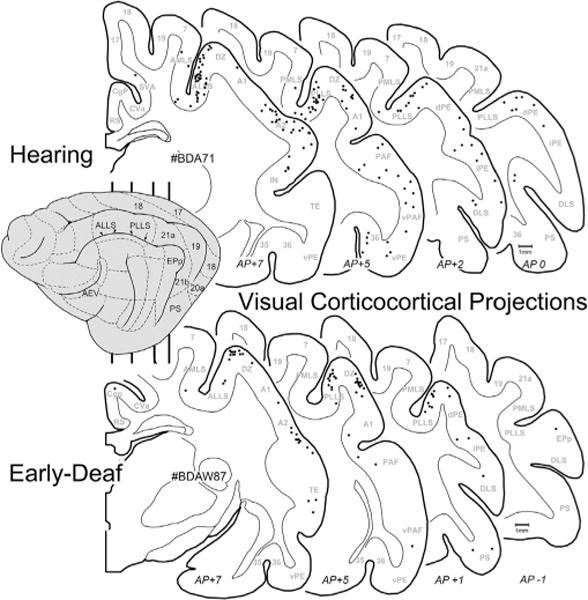
Visual corticocortical projections to FAES. On the lateral view of the cat cortex (left) the major visual regions are depicted (for abbreviations, see abbreviation table), and the vertical lines indicate the approximate levels from which the depicted coronal sections were taken (approximate anterior-posterior levels listed at bottom). Sections through the cortex of a hearing (top; case BDA71) and an early-deaf (bottom; case BDAW87) animal are outlined with the grey–white border and subcortical nuclei plotted; each dot represents one retrogradely labeled neuron from the FAES injection. Note that the most consistently labeled of the visual cortical areas were the ALLS/PLLS regions for both hearing and deaf cases.
Fig. 5.
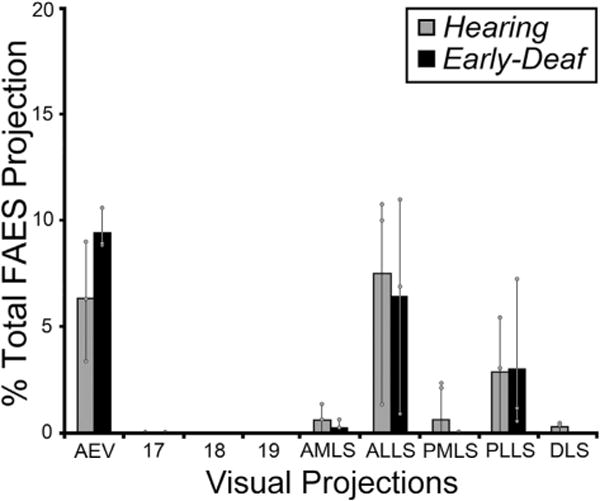
The data from all visual cortical areas from all subjects indicate that the majority of inputs to FAES consistently arose from the same visual cortical areas for both hearing (grey bars) and early-deaf (black bars) animals. Bars represent the average proportion of the total corticocortical projection; the thin vertical line through the bars connects the range of values from the individual cases (grey dots). There were no instances in which novel projections from visual cortical areas were apparent in early-deaf animals that were not also present in hearing cats. Note that most existing connections exhibited similar proportions under the different hearing conditions. See Table 1 for list of abbreviations.
3.3. Somatosensory cortical projections
Early deafness also induces an increase in the somatosensory activation of auditory cortices in experimental animals (Meredith and Lomber, 2011; Meredith et al., 2011; Meredith and Allman, 2012) and humans (Levanen et al., 1998; Auer et al., 2007; Karns et al., 2012). In the feline FAES, somatosensory responsiveness changes from 7% of neurons in hearing animals to 34% in the early-deaf (Meredith et al., 2011). Therefore, it might be expected that projections from somatosensory cortical areas would either emerge de novo or existing connections would be proportionally increased in early-deaf animals. As illustrated in Fig. 6 (and described in Meredith et al., 2006), somatosensory cortical areas that project to the FAES in a hearing animal include the nearby sulcal area S4 as well as both the gyral and sulcal portions of S2, while few if any labeled neurons are identified in other somatosensory regions (S1, S3, S5). In this same figure, somatosensory cortical projections to the FAES in an early-deaf animal almost exclusively arise from areas S4 and S2/S2m. As quantified for all animals in Fig. 7, it is evident that novel somatosensory projections of substantial size (e.g., >2%) are not induced in the deaf animals. Projection increases among the existing somatosensory connections do not occur either, since 41% of the total cortical projection to FAES in hearing animals arise from somatosensory areas, compared with 37% in the early-deaf cases. Furthermore, existing connections from areas S2 and S4 remain the major somatosensory projection sources (hearing avg. = 36%; early deaf avg. = 27% of total projection) (see Fig. 7), while the area MZ (which is a bimodal auditory-somatosensory region in hearing animals; hearing avg. = 2%) shows an increase to 9% of total projections to FAES in the early deaf. Thus, within the early-deaf FAES, enhanced somatosensory representation does not appear to be derived from increased inputs from traditionally defined somatosensory cortical sources.
Fig. 6.
Somatosensory corticocortical projections to FAES. On the lateral view of the cat cortex (left) the major somatosensory regions are depicted and the vertical lines indicate the approximate levels from which the coronal sections were taken (approximate AP levels listed at bottom). Sections through the cortex of a hearing (top; case BDA71) and an early-deaf (bottom; case BDA68) cat are outlined with the grey–white border illustrated; each dot represents one retrogradely labeled neuron from the FAES injection. Note that the most densely labeled somatosensory areas were S4, S2 and S2m regions for both the hearing and the deaf cases.
Fig. 7.
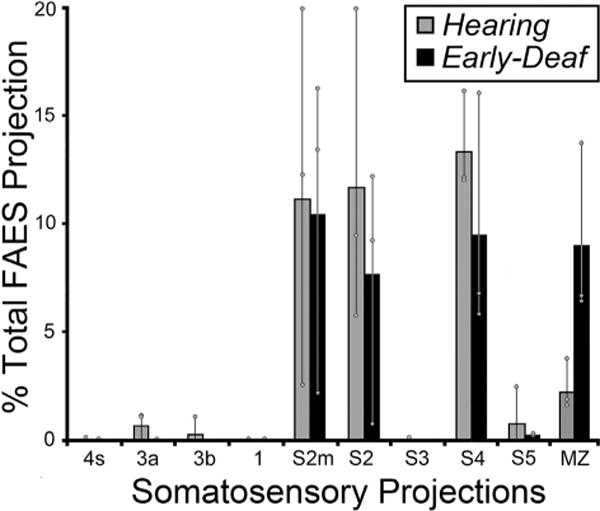
The data from all somatosensory cortical areas from all subjects indicate that the majority of inputs to FAES consistently arose from the same somatosensory cortical areas for both hearing (grey bars) and early-deaf (black bars) animals. New projection sources were not evident while most existing connections exhibited similar proportions under the different hearing conditions. Bars represent the average proportion of the total corticocortical projection; the thin vertical line through the bars connects the range of values from the individual cases (grey dots). See Table 1 for list of abbreviations.
3.4. Auditory cortical projections
Early deafness obviously eliminates patterned acoustic activation in all auditory cortical areas. Given the activity-dependent mechanisms for development and maintenance of synaptic connections, it might be expected that projections received by the FAES from other auditory cortices would be lost or substantially reduced in early-deaf animals. This notion was tested by examining the auditory cortical areas for retrogradely labeled neurons (excluding FAES self-labeling) following tracer injection into the FAES. As shown in Fig. 8 (and described in Lee and Winer, 2008), auditory cortical projections in hearing animals largely arise from nearby AAF (includes both gyral and sulcal aspects) and area A2, with consistent but substantially fewer projections originating in areas A1, DZ, and PAF. Few if any labeled neurons are observed in the other auditory cortical subregions. Fig. 8 also shows projections to FAES of early-deaf animals from AAF, A1, A2, DZ, PAF and MZ where it can be seen that the two groups (hearing, early-deaf) exhibit basically the same distribution of retrogradely labeled neurons. As is summarized quantitatively in Fig. 9, wholesale loss of auditory cortical connectivity to the FAES is not observed in the early-deaf. Instead, projections from auditory cortical regions to the FAES are largely the same for hearing (avg. = 36% of total corticocortical projections) and early-deaf animals (avg. = 38%). For both the hearing and early-deaf groups, the major auditory cortical regional sources are from the AAF and A2. Although the trend for these projections is one of reduction (hearing AAF = 11% vs. early-deaf AAF = 9.5%; hearing A2 = 14% vs. early-deaf A2 = 12%), these values are within their respective range of variation (see Fig. 8). In addition, other auditory cortical areas such as DZ and A1 show small increases in projection strength in early-deaf cases. Thus, evidence from early-deafened FAES indicates that the sources of auditory cortical inputs are neither eliminated nor are they consistently reduced.
Fig. 8.
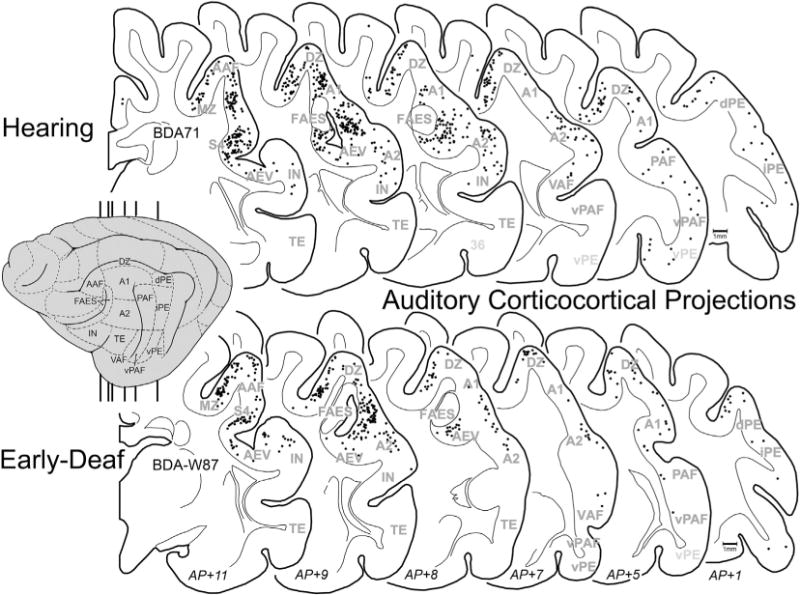
Auditory corticocortical projections to FAES. On the lateral view of the cat cortex (gray, left) the major auditory regions are depicted (for abbreviations, see Abbreviation table), and the vertical lines indicate the approximate levels from which the coronal sections were taken (approximate AP levels listed at bottom). Sections through the cortex of a hearing (top; case BDA71) and an early-deaf (bottom; case BDA-W87) cats are outlined with the grey–white border and subcortical nuclei depicted; each dot represents one retrogradely labeled neuron from the FAES injection. Note that the most densely labeled auditory areas were AAF, A2, A1, DZ and MZ regions for both the hearing and the deaf cases. Outlined clear area in FAES region represents injection site.
Fig. 9.
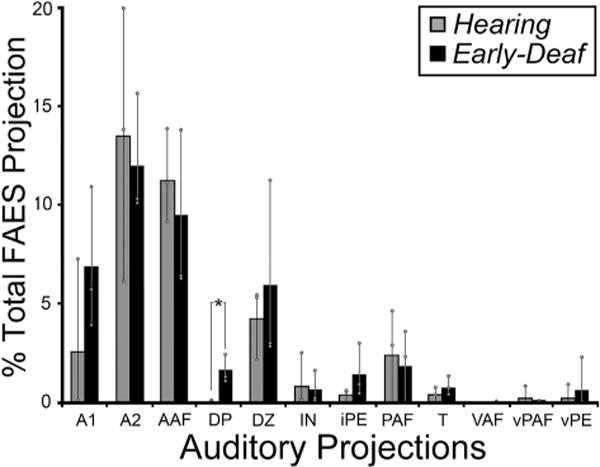
Data from all auditory cortical areas from all subjects indicate that the majority of inputs to FAES consistently arose from the same auditory cortical areas for both hearing (grey bars) and early-deaf (black bars) animals. New projection sources were not evident and most existing connections exhibited similar proportions under the different hearing conditions; only the projection from DP showed a statistically significant change (asterisk; Wilcoxon; p < 0.049). Bars represent the average proportion of the total corticocortical projection; the thin vertical line through the bars connects the range of values from the individual cases (grey dots). See Table 1 for list of abbreviations.
3.5. Thalamocortical connections
Deafness abolishes patterned acoustic activation of the auditory thalamus. Therefore, because activity-dependent mechanisms are known to promote and maintain synaptic thalamocortical connections, it might be expected that projections to FAES from thalamic auditory regions would be lost or substantially reduced in early-deaf animals, possibly accompanied by compensatory increases in non-auditory (e.g., Vb, LGN) and/or non-specific/multisensory thalamic projections. This idea was tested by examining the thalamus for retrogradely labeled neurons following tracer injection into the FAES of hearing and early-deaf cats. As shown in Fig. 10, thalamic projections from a hearing animal largely arise from the medial and ventral subdivisions of the medial geniculate body, with a much smaller proportion arising from the dorsal divisions. Also, a small proportion of FAES inputs originate from multisensory regions of the suprageniculate and posterior thalamic nuclei. In an early-deaf animal, a large proportion of labeled neurons are found within the medial and ventral divisions of the medial geniculate body, while a few neurons are scattered across its dorsal division, the suprageniculate and posterior nuclei, as shown in Fig. 10. Thus, comparison of individual normal and deaf cases reveal a close similarity of thalamic inputs. The thalamo-cortical data from all hearing and early-deaf animals is compiled and summarized in Fig. 11. This group data demonstrates that the same thalamic areas target the FAES in both hearing and early-deaf animals, and in largely the same proportions.
Fig. 10.
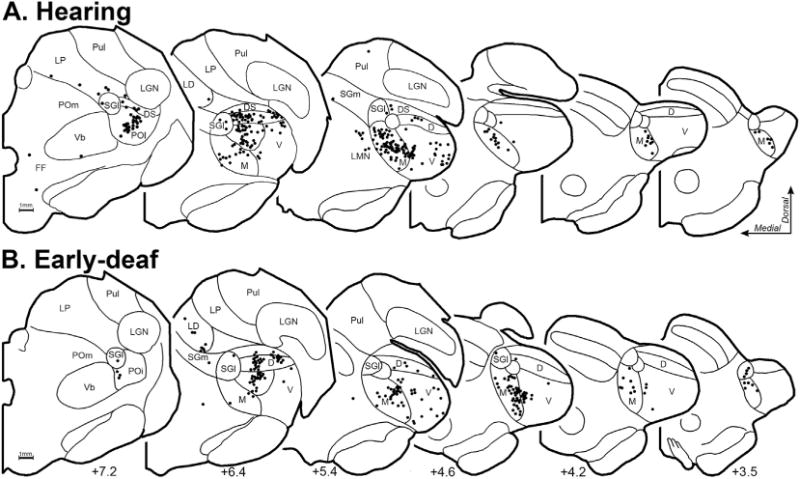
Thalamocortical neurons that project to FAES in hearing (A-top row; case BDA66) and early-deaf cats (B-bottom row; case BDA65). Depicted are coronal half-sections through the anterior- (left) posterior (right) extent of the thalamus, with the A–P position indicated at bottom. Cytoarchitectural features (thin black contours) were plotted and identified according to the criteria of Huang et al. (1999) for the cat thalamus. Each labeled neuron is indicated by a single, black dot. Note that neurons labeled from the FAES largely arise from the medial aspect of auditory thalamus in hearing as well as early-deaf animals. Abbreviations are defined in abbreviation table.
Fig. 11.
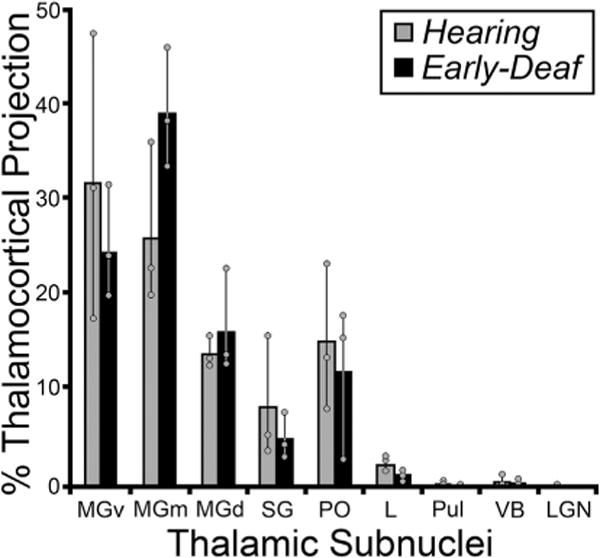
Summary of thalamic projections to the FAES in hearing (gray bars) and early-deaf (black bars) cats. Essentially, thalamic sub-nuclei that project to FAES in hearing animals, in particular the medial, dorsal and ventral regions of the auditory Medial Geniculate Nucleus (MGm, MGd, MGv) are maintained in early-deafened animals, and at similar proportions (no statistically significant changes were identified; Wilcoxon, p > 0.05). Also, non-auditory nuclei (e.g., VB, LGN) that did not strongly connect to the FAES in hearing animals did not reveal novel connections in early-deaf cases. Bars represent the average proportion of the total thalamococortical projection; the thin vertical line through the bars connects the range of values from the individual cases (grey dots). See Table 1 for list of abbreviations.
4. Discussion
Until very recently, the mechanisms underlying crossmodal plasticity have received more speculation than empirical examination. As proposed by Rauschecker (1995) and reiterated by numerous publications and reviews, when activation from a major sensory system is lost or damaged, crossmodal replacements might result from enhanced ingrowth of new projections, or from increased projections from existing sources, or from the ‘unmasking’ of existing inputs that were otherwise silent. The first two of these possible mechanisms subserving crossmodal plasticity were directly addressed in the present study.
4.1. FAES crossmodal plasticity: novel versus increased non-auditory cortical projections?
In the early-deaf FAES, the proportion of visually responsive neurons increased ~260%, and somatosensory-responsive neurons increased ~500%, while auditory activation was reduced to zero (Meredith et al., 2011). However, as summarized in Fig. 12, the present study observed that the pattern and proportion of neurons projecting to FAES from visual, somatosensory or auditory cortices of early-deaf animals is largely (within ± <5%) the same as observed in hearing animals. Furthermore, in the early-deaf, the proportion of neurons projecting to FAES from visual and somatosensory thalamic regions remained extremely low, while the proportion projecting from auditory thalamus was minimally affected. Given this general lack of connectional change after early deafness, it might seem that crossmodal plasticity failed to occur. However, this is very unlikely because identical methodologies were employed to induce the functional demonstrations of crossmodal plasticity in several different auditory cortical regions (Meredith et al., 2011; Meredith and Lomber, 2011; Meredith and Allman, 2012, 2015) as well as within the FAES of the opposite hemisphere of two of the animals used in the present study (Meredith et al., 2011). Furthermore, these same ototoxic procedures were effective in recently published studies of crossmodal plasticity where connectional effects were demonstrated (Kok et al., 2013; Wong et al., 2015). Therefore, when considering the crossmodal plasticity found in the FAES, novel non-auditory projection sources were not observed and the minor proportional changes in areal sources of inputs seem wholly insufficient to account for the massive functional reorganization of the region.
Fig. 12.
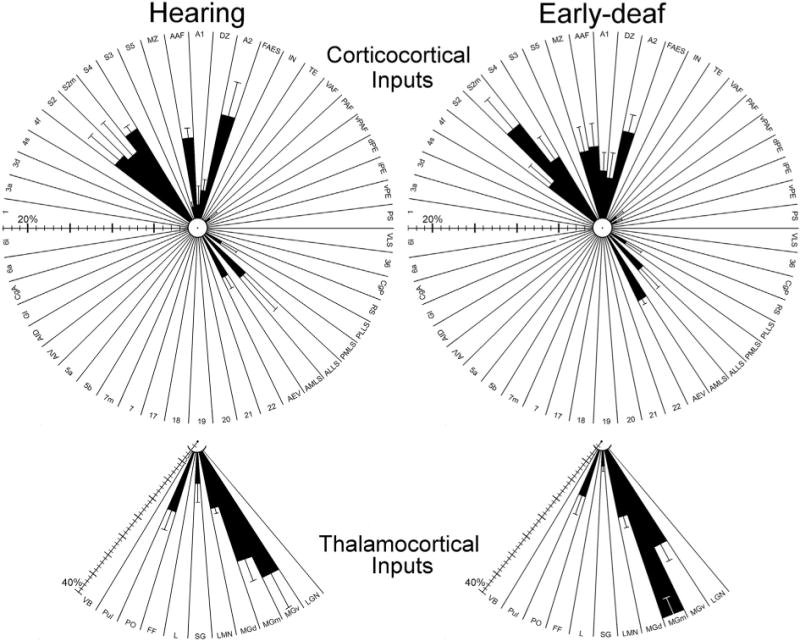
Summary of cortical (top) and thalamic (bottom) connections with FAES in hearing (left) and early-deaf (right) animals. Shown are results (black bars = mean ± se) for every examined cortical and thalamic area (abbreviations defined in abbreviation list); regions with <1% of total projection could not be effectively plotted. Note the scale bars for cortical and thalamic connections are different, since they are based on totals from different populations of neurons. These radial-plots of the major results reveal a ‘footprint’ of the patterns and proportions of connections with FAES that are very similar for hearing and early-deaf animals alike. Hence, crossmodal plasticity in the FAES following deafness cannot be explained by changes in sources of afferent projections.
4.2. Hearing loss and inputs to A1
Although the crossmodal status of A1 is unresolved for congenitally deaf cats (Kral et al., 2003), crossmodal effects have been observed in A1 of deaf subjects in other species (Levanen et al., 1998; Finney et al., 2001; Auer et al., 2007; Allman et al., 2009; Meredith and Allman, 2012; Karns et al., 2012; Cardin et al., 2013) as well as in A1 of hearing cats (Krueger-Firster et al., 2015). A striking lack of connectional reorganization has been observed in primary auditory cortex for both early-deaf (Chabot et al., 2015) and congenitally deaf cats (Barone et al., 2013) where afferent projections from other auditory cortices to A1 were very similar for hearing and for deaf animals. Specifically, only 3.1% difference was observed between the total proportion of auditory cortical connections to A1 in hearing (46.5%) and early-deaf animals (49.6%; Fig. 13 in Chabot et al., 2015). Similarly, the proportions of afferents to A1 from visual cortices were nearly the same for hearing and early-deaf animals (hearing = 10.8%; early-deaf = 9.8%; Chabot et al., 2015). Likewise, Stanton and Harrison (2000) did not observe changes in thalamocortical projections to A1 in early-deaf cats. Thus, for both A1 and FAES, their afferent connectional rules appear to be conserved in reaction to hearing loss: established connections are proportionally maintained while novel non-auditory projections were not recruited.
4.3. Neural development and onset of hearing loss
Given that these (FAES, A1) hierarchically dissimilar auditory cortical regions both reveal few connectional changes following deafness, it seems plausible that the functional insult to the auditory system may have occurred too late in development to generate different connectional effects. Permanent, thalamo-cortical sensory circuitry is known to begin establishing connections in cortex near prenatal day E50 in the cat (Johnson and Casagrande, 1993; Hermann et al., 1994). Thus, thalamo-cortical connections are well established before the onset of deafening (30 DPN) in the experimental, early-deaf studies. In fact, none of the auditory cortical regions examined so far (A1, AAF, DZ, FAES) revealed thalamo-cortical connectional changes that paralleled their functional reorganization after deafness (see also Stanton and Harrison, 2000; Meredith and Allman, 2012), supporting the notion that auditory thalamocortical connections are developmentally established prior to the onset or influence of acoustically-evoked activity (Johnson and Casagrande, 1993; Hermann et al., 1994). However, horizontal cortico-cortical connections initiate their development at a later developmental date, which is just after birth (Callaway and Katz, 1990), and continue to be refined through the critical period of postnatal development. Within this time frame, Cornwell et al. (1984) used 4 day old cats to demonstrate that the basic, adult pattern of auditory (and visual) cortical projections is present at that time. In addition, in animals that experience no patterned auditory activity at any developmental point, the A1 of congenitally deaf cats revealed a very similar cortico-cortical connectional pattern as that observed for the early-deaf A1. These observations suggest that the developmental stage of onset of hearing loss exhibits little effect on cortico-cortical sources of inputs to A1.
4.4. Activity-dependent crossmodal plasticity
The present results demonstrate a comprehensive lack of connectional changes to crossmodally-reorganized FAES despite its fundamental change in activity. It was expected that activity-dependent mechanisms during development and maturation would promote and maintain connections among co-active visual and somatosensory inputs, while pruning away non-correlated, non-active inputs from auditory regions. This conundrum has led some investigators to suggest that crossmodal plasticity is generated through non-Hebbian mechanisms (Barone et al., 2013). However, an alternate mechanism that has not been considered is that the locus at which crossmodal plasticity occurs is located at the synaptic termination of the afferent projection. This makes logical sense because it is the afferent terminal site, not its distant parent-neuron location (and often within the representation of a different sensory modality), which is best positioned to be influenced by the cessation of patterned auditory activity following hearing loss. This notion is further supported by the observation that dendritic spine density is significantly increased in early deaf FAES (Clemo et al., 2014), specifically on neurons in the laminae that preferentially receive non-auditory inputs (Meredith et al., 2006; Clemo et al., 2014). Presumably, such increases in dendritic spine density are matched by increases in terminal boutons, both of which are the essential elements of a mature synapse. Thus, it is expected that in the deafened FAES, the axons of active (e.g., non-auditory) inputs exhibit more extensive terminal branching, as proposed by Clemo et al. (2014). In this manner, existing non-auditory inputs can carry patterned sensory information broadly to an expanded proportion of synapses in FAES. Therefore, the notion of increased axonal branching after early deafness in the FAES seems to account for many of the known features of the crossmodally reorganized FAES and deserves further examination.
The present study also raises the perplexing issue that auditory cortical connections were not substantially reduced in the early-deaf FAES (see also Kok et al., 2013; Barone et al., 2013; Wong et al., 2015). Again, this seems to contradict the principles of activity-dependent development and pruning of neural connections. But this conflict occurs only if it is assumed that deafened auditory cortex is inactive and no longer conveys patterned sensory information. Indeed, most theories of deafness-induced crossmodal plasticity presume little to no role for the ‘vacated’ auditory cortices (Rauschecker, 1995; Bavelier and Neville, 2002; Dormal and Collignon, 2011). Yet several recent studies have demonstrated that many auditory cortices in the early deaf are robustly active with crossmodal/non-auditory signals. In fact the AAF, which is part of the core auditory cortices of the cat, vigorously exhibits both visual and somatosensory single-unit activity in early-deaf cats (Meredith and Lomber, 2011), and areas DZ and PAF both control crossmodal behaviors in deaf cats (Lomber et al., 2010). Although deaf A1 seems not appear to receive visual inputs in some species (Kral et al., 2003), somatosensory crossmodal plasticity occurs in this region in early-deaf (Meredith and Allman, 2012) and late-deaf ferrets (Allman et al., 2009) and in humans (Levanen et al., 1998; Finney et al., 2001; Auer et al., 2007; Karns et al., 2012). Thus, projections from reorganized ‘auditory’ areas would be expected to relay crossmodal (visual and/or somatosensory) signals to their targets in other ‘auditory’ cortices in the deaf. In the context of the present study, it is important to note that the AAF is the major source of auditory inputs to the FAES in hearing animals, and this projection remains the largest single source of inputs to the FAES among “auditory” cortices in early-deaf animals. Therefore, it should be expected that at least some of the synapses found in the early-deaf FAES carry crossmodal non-auditory signals from ‘auditory’ cortical sources.
5. Conclusion
The observations presented by this work strongly indicate that crossmodal plasticity in the FAES, like other regions of deafened auditory cortex, is subserved by features of existing connections instead of generation projections from novel non-auditory sources. Furthermore, the projections to the FAES occur in essentially similar proportions in both hearing and early-deaf animals, which suggests that connections maintained between ‘auditory’ cortical regions after hearing loss are also likely to play a role in crossmodal plasticity. Ultimately however, no single factor appears to control the development and maintenance of input connections in cross-modally reorganized cortex in the deaf.
Acknowledgments
This work was supported by the National Institutes of Health Grant (NS-39460; MAM) and the Virginia Commonwealth University Presidential Research Initiatives Program (MAM), the Canadian Institutes of Health Research (SGL), and the Natural Sciences and Engineering Research Council of Canada (SGL). We thank S. Ramoa and K. McKee for assistance with data collection, M. Kok for assistance in mapping cortical area functional distributions and M. Kok and C. Wong for reading the manuscript.
Footnotes
Conflicts of interest
The authors have no conflict of interest regarding this work.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design (MAM). Acquisition of data: (MAM, NC, SMC). Analysis and interpretation of data: (MAM; SMC, HRC). Drafting of the manuscript: (MAM). Critical revision of the manuscript for important intellectual content: (MAM, HRC, SGL). Statistical analysis: (MAM). Obtained funding: (MAM, SGL). Administrative, technical, and material support: (MAM, SGL). Study supervision: (MAM, SGL).
References
- Allman B, Keniston LP, Meredith MA. Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci (USA) 2009;106:5925–5930. doi: 10.1073/pnas.0809483106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer ET, Jr, Bernstein LE, Sunkarat W, Singh M. Vibrotactile activation of the auditory cortices in deaf versus hearing adults. Neuroreport. 2007;18:645–648. doi: 10.1097/WNR.0b013e3280d943b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendaño C, Rausell E, Perezaguilar D, Isorna S. Organization of the association cortical afferent connections of area 5: a retrograde tracer study in the cat. J Comp Neurol. 1988;278:1–33. doi: 10.1002/cne.902780102. [DOI] [PubMed] [Google Scholar]
- Barone P, Lacassagne L, Kral A. Reorganization of the connectivity of cortical field DZ in congenitally deaf cat. PLoS One. 2013;2013(8):e60093. doi: 10.1371/journal.pone.0060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Olson CR. Visual and auditory association areas of the cat’s posterior ectosylvian gyrus: thalamic afferents. J Comp Neurol. 1988;272:15–29. doi: 10.1002/cne.902720103. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci. 1990;10:1134–1153. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappe C, Morel A, Barone P, Rouiller EM. The thalamocortical projection systems in primate: an anatomical support for multisensory and sensorimotor interplay. Cereb Cortex. 2009;19:2025–2037. doi: 10.1093/cercor/bhn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin V, Orfanidou E, Ronnberg J, Capek CM, Rudner M, Woll B. Dissociating cognitive and sensory neural plasticity in human superior temporal cortex. Nat Comm. 2013;4:1473. doi: 10.1038/ncomms2463. [DOI] [PubMed] [Google Scholar]
- Chabot N, Mellott JG, Hall AJ, Tichenoff EL, Lomber SG. Cerebral origins of the auditory projection to the superior colliculus of the cat. Hear Res. 2013;300:33–45. doi: 10.1016/j.heares.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Chabot N, Butler BE, Lomber SG. Differential modification of cortical and thalamic projections to cat primary auditory cortex following early- and late-onset deafness. J Comp Neurol. 2015;523:2297–2320. doi: 10.1002/cne.23790. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Irvine DRF. The anterior ectosylvian sulcal auditory field in the cat: I. An electrophysiological study of its relationship to surrounding auditory cortical fields. J Comp Neurol. 1990a;301:289–303. doi: 10.1002/cne.903010211. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Irvine DRF. The anterior ectosylvian sulcal auditory field in the cat: II. A horseradish peroxidase study of its thalamic and cortical connections. J Comp Neurol. 1990b;301:304–324. doi: 10.1002/cne.903010212. [DOI] [PubMed] [Google Scholar]
- Clasca F, Llamas A, Reinoso-Suárez F. Insular cortex and neighboring fields in the cat: a redefinition based on cortical microarchitecture and connections with the thalamus. J Comp Neurol. 1997;384:456–482. doi: 10.1002/(sici)1096-9861(19970804)384:3<456::aid-cne10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Allman BL, Donlan MA, Meredith MA. Sensory and multisensory representations within the cat rostral suprasylvian cortices. J Comp Neurol. 2007;503:110–127. doi: 10.1002/cne.21378. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Lomber SG, Meredith MA. Synaptic basis for crossmodal plasticity: enhanced supragranular dendritic spine density in anterior extosylvian auditory cortex of the early deaf cat. Cereb Cortex 2014. 2014 doi: 10.1093/cercor/bhu225. http://dx.doi.org/10.1093/cercor/bhu225. [DOI] [PMC free article] [PubMed]
- Clemo HR, Meredith MA. Cortico-cortical relations of cat somatosensory areas SIV and SV. Somatosens Mot Res. 2004;21:199–209. doi: 10.1080/08990220400012505. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Stein BE. Organization of a fourth somatosensory area of cortex in cat. J Neurophysiol. 1983;50:910–925. doi: 10.1152/jn.1983.50.4.910. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Stein BE. Effects of cooling somatosensory cortex on response properties of tactile cells in the superior colliculus. J Neurophysiol. 1985;55:1352–1368. doi: 10.1152/jn.1986.55.6.1352. [DOI] [PubMed] [Google Scholar]
- Cornwell P, Ravizza R, Payne B. Extrinsic visual and auditory cortical connections in the 4-day-old kitten. J Comp Neurol. 1984;229:97–120. doi: 10.1002/cne.902290108. [DOI] [PubMed] [Google Scholar]
- Dormal G, Collignon O. Functional selectivity in sensory deprived cortices. J Neurophysiol. 2011;105:2627–2630. doi: 10.1152/jn.00109.2011. [DOI] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Collignon O, Voss P, Lepore F. Crossmodal plasticity in sensory loss. Prog Brain Res. 2011;191:233–249. doi: 10.1016/B978-0-444-53752-2.00002-3. [DOI] [PubMed] [Google Scholar]
- Hermann K, Antonini A, Shatz CJ. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur J Neurosci. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Huang CL1, Larue DT, Winer JA. GABAergic organization of the cat medial geniculate body. J Comp Neurol. 1999;415:368–392. [PubMed] [Google Scholar]
- Hunt DL, Yamoah EN, Krubitzer L. Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience. 2006;139:1507–1524. doi: 10.1016/j.neuroscience.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lepore F, Poirier P, Guillemot JP. Responses of cells to stationary and moving sound stimuli in the anterior ectosylvian cortex of cats. Hear Res. 2000;139:69–85. doi: 10.1016/s0378-5955(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Casagrande VA. Prenatal development of axon outgrowth and connectivity in the ferret visual system. Vis Neurosci. 1993;10:117–130. doi: 10.1017/s0952523800003266. [DOI] [PubMed] [Google Scholar]
- Karns CM, Dow MW, Neville HJ. Altered cross-modal processing in the primary auditory cortex of congenitally deaf adults: a visual-somatosensory fMRI study with a double-flash illusion. J Neurosci. 2012;32:9626–9638. doi: 10.1523/JNEUROSCI.6488-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok MA, Chabot N, Lomber SG. Cross-modal reorganization of cortical afferents to dorsal auditory cortex following early- and late-onset deafness. J Comp Neurol. 2013;522:654–675. doi: 10.1002/cne.23439. [DOI] [PubMed] [Google Scholar]
- Korte M, Rauschecker JP. Auditory spatial tuning of cortical neurons is sharpened in cats with early blindness. J Neurophysiol. 1993;70:1717–1721. doi: 10.1152/jn.1993.70.4.1717. [DOI] [PubMed] [Google Scholar]
- Kral A. Auditory critical periods: a review from system’s perspective. Neuroscience. 2013;247:117–133. doi: 10.1016/j.neuroscience.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Kral A, Schröder JH, Klinke R, Engel AK. Absence of cross-modal reorganization in the primary auditory cortex of congenitally deaf cats. Exp Brain Res. 2003;153:605–613. doi: 10.1007/s00221-003-1609-z. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15:552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Krueger-Firster J, Kurela LR, Nidiffer AR, Hackett TA, Wallace MT. Differential visual modulation of auditory activity in cat A1 between supra- and infragranular layers. APAN. 2015;2015:39. [Google Scholar]
- Las L, Ayelete-Hashahar S, Nelken I. Functional gradients of auditory sensitivity along the anterior ectosylvian sulcus of the cat. J Neurosci. 2008;28:3657–3667. doi: 10.1523/JNEUROSCI.4539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol. 2008;507:1919–1943. doi: 10.1002/cne.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanen S, Jousmaki V, Hari R. Vibration-induced auditory-cortex activation in a congenitally deaf adult. Curr Biol. 1998;8:869–872. doi: 10.1016/s0960-9822(07)00348-x. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of “what” and “where” processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13:1421–1427. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A. Adaptive crossmodal plasticity in deaf auditory cortex: areal and laminar contributions to supranormal vision in the deaf. Prog Brain Res. 2011;191:251–270. doi: 10.1016/B978-0-444-53752-2.00001-1. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. Cerebral areas mediating visual redirection of gaze: cooling deactivation of fifteen loci in the cat. J Comp Neurol. 2004;474:190–208. doi: 10.1002/cne.20123. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- Mellott JG, van der Gucht E, Lee CC, Carrasco A, Winer JA, Lomber SG. Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32. Hear Res. 2010;267:119–136. doi: 10.1016/j.heares.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA. Corticocortical connectivity of cross-modal circuits. In: Calvert G, Spence C, Stein BE, editors. The Handbook of Multisensory Processes. MIT Press; Cambridge, MA: 2004. pp. 343–355. [Google Scholar]
- Meredith MA, Allman BL. Subthreshold Multisensory Processing in Cat Auditory Cortex NeuroReport 20. 2009:126–131. doi: 10.1097/WNR.0b013e32831d7bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Allman BL. Early hearing-impairment results in crossmodal reorganization of ferret core auditory cortex. Neural Plast. 2012;2012:601591. doi: 10.1155/2012/601591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Allman BL. Single-unit analysis of somatosensory processing in core auditory cortex of hearing ferrets. Eur J Neurosci. 2015;41:686–698. doi: 10.1111/ejn.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR. Auditory cortical projections from the anterior ectosylvian sulcus (Field AES) to the superior colliculus in cat: an anatomical and electrophysiological study. J Comp Neurol. 1989;289:687–707. doi: 10.1002/cne.902890412. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Keniston LR, Dehner LR, Clemo HR. Cross-modal projections from somatosensory area SIV to the auditory field of the anterior ectosylvian sulcus (FAES) in cat: further evidence for subthreshold forms of multisensory processing. Exp Brain Res. 2006;72:472–484. doi: 10.1007/s00221-006-0356-3. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Kryklywy J, McMillan AJ, Malhotra S, Lum-Tai R, Lomber SG. Crossmodal reorganization in the early deaf switches sensory, but not behavioral roles of auditory cortex. Proc Natl Acad Sci (USA) 2011;108:8856–8861. doi: 10.1073/pnas.1018519108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Lomber SG. Somatosensory and visual crossmodal plasticity in the anterior auditory field of early-deaf cats. Hear Res. 2011;280:38–47. doi: 10.1016/j.heares.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Norita M, Benedek G, Creutzfeldt O. Physiologic and anatomic investigation of a visual cortical area situated in the ventral bank of the anterior ectosylvian sulcus of the cat. Exp Brain Res. 1982;46:1–11. doi: 10.1007/BF00238092. [DOI] [PubMed] [Google Scholar]
- Olfert E, Cross BM, McWilliam AA. Guide to the Care and Use of Experimental Animals. Canadian Council on Animal Care 1993 [Google Scholar]
- Payne BR. Evidence for visual cortical area homologs in cat and macaque monkey. Cereb Cortex. 1993;3:1–25. doi: 10.1093/cercor/3.1.1. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Korte M. Auditory compensation for early blindness in cat cerebral cortex. J Neurosci. 1993;13:4538–4548. doi: 10.1523/JNEUROSCI.13-10-04538.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso-Suárez F. Topographischer Hirnatlas der Katz fur experimentale physiologische Untersuchungen (Topographical atlas of the cat brain for experimental-physiological research) Merck; Darmstadt, Germany: 1961. [Google Scholar]
- Ribaupierre F de. Acoustical information processing in the auditory thalamus and cerebral cortex. In: Ehret G, Romand R, editors. The Central Auditory System. Oxford University Press; New York: 1997. [Google Scholar]
- Rosenquist AC. In: Connections of Visual Cortical Areas in the Cat Cerebral Cortex. Peters A, Jones EG, editors. Vol. 3. Plenum Press; NY: 1985. pp. 81–117. [Google Scholar]
- Stanton SG, Harrison RV. Projections from the medial geniculate body to primary auditory cortex in neonatally deafened cats. J Comp Neurol. 2000;426:117–129. doi: 10.1002/1096-9861(20001009)426:1<117::aid-cne8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Updyke BV. Retinotopic organization within the cat’s posterior suprasylvian sulcus and gyrus. J Comp Neurol. 1986;246:265–280. doi: 10.1002/cne.902460210. [DOI] [PubMed] [Google Scholar]
- van der Gucht E, Vandesande F, Arckens L. Neurofilament protein: a selective marker for the architectonic parcellation of the visual cortex in adult cat brain. J Comp Neurol. 2001;441:345–368. doi: 10.1002/cne.1416. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69:1797–1809. doi: 10.1152/jn.1993.69.6.1797. [DOI] [PubMed] [Google Scholar]
- Wong C, Chabot N, Kok MA, Lomber SG. Modified areal cartography in auditory cortex following early and late-onset deafness. Cereb Cortex. 2014;24:1778–1792. doi: 10.1093/cercor/bht026. [DOI] [PubMed] [Google Scholar]
- Wong C, Chabot N, Kok MA, Lomber SG. Amplified somatosensory and visual cortical projections to a core auditory areas, the anterior auditory field, following early- and late-onset deafness. J Comp Neurol. 2015;523:1925–1947. doi: 10.1002/cne.23771. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning. Geneva, CH: 1991. [Google Scholar]
- Xu L, Furukawa S, Middlebrooks JC. Sensitivity to sound-source elevation in nontonotopic auditory cortex. J Neurophysiol. 1998;80:882–894. doi: 10.1152/jn.1998.80.2.882. [DOI] [PubMed] [Google Scholar]
- Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993;70:205–215. doi: 10.1016/0378-5955(93)90159-x. [DOI] [PubMed] [Google Scholar]


