Abstract
Cholinergic and dopaminergic mechanisms within the mesolimbic dopamine system are suggested to play a role in the manifestation of depression and anxiety-related disorders. However, despite the fact that cholinergic mechanisms in the ventral tegmental area (VTA) highly regulate dopamine activity, the role of VTA cholinergic mechanisms in depression-related behaviors is relatively unknown. Here we sought to determine whether enhancing cholinergic tone in the VTA would alter depression and anxiety-related behavior in the forced swim test (FST), elevated plus maze (EPM) and sucrose preference test (SPT). Adult Sprague Dawley male rats received VTA infusion of the acetylcholinesterase inhibitor, physostigmine (0, 1, 2 μg/side), immediately prior to the FST, EPM, or SPT. Physostigmine administration increased immobility time in the FST, decreased time spent on open arms in the EPM, and decreased sucrose preference. In a separate cohort of rats, we also examined whether activation of VTA muscarinic receptors was sufficient to alter behavior in the FST and EPM. Similar to physostigmine, VTA infusion of the muscarinic receptor agonist, pilocarpine (0, 3, 30 μg/side), increased immobility time in the FST and decreased time spent on open arms in the EPM. These data suggest that enhanced VTA cholinergic tone promotes pro-depressive and anxiogenic-like effects and demonstrate that specific activation of VTA muscarinic receptors is also sufficient to induce pro-depressive and anxiogenic responses. Together, these findings reveal a novel role of VTA cholinergic, and specifically muscarinic receptor, mechanisms in mediating responses to stress and anxiety.
Keywords: acetylcholine, depression, anxiety, muscarinic receptor, mesolimbic dopamine system, ventral tegmental area
Introduction
Depression is a stress-related mood disorder that affects approximately 20% of adults in the United States [19, 20]. Individuals diagnosed with major depressive disorder (MDD) experience various symptoms including anhedonia, fatigue, significant changes in appetite or weight, and thoughts or attempts of suicide [3]. In addition, anxiety is highly comorbid with depression, experienced by over 50 % of individuals diagnosed with depression [19, 27]. Dysfunction of the mesolimbic dopamine (DA) system is associated with both major depressive disorder (MDD) and generalized anxiety disorder (GAD) in humans [15, 25, 33]. Dopaminergic mechanisms also mediate depression and anxiety-related behavior in preclinical rodent models [16, 25]. In addition to the dopaminergic system, the cholinergic system is also known to powerfully modulate depression symptomology. Specifically, manipulations that increase brain cholinergic tone induce or exacerbate depressive-like symptoms while administration of cholinergic receptor antagonists induces antidepressant-like effects [1, 9, 17, 18, 23]. Thus, while the etiology of depression is still unknown, current evidence strongly suggests that dopaminergic and cholinergic mechanisms likely play an important role in the pathogenesis of depression. Indeed, preclinical examinations of cholinergic mechanisms in depression have highlighted roles for cholinergic activity in several dopaminergic brain regions – including the prefrontal cortex (PFC) [24, 36], the nucleus accumbens (NAc) [26, 29], and the hippocampus [23]. However, the role of ventral tegmental area (VTA) cholinergic activity in depression and anxiety related behavior has not been extensively examined to date, despite the fact that DA cell bodies are localized in the VTA.
Within the mesolimbic system, recent studies in rodents have revealed a causal role for phasic DA activity in the VTA to NAc pathway in mediating both pro-depressive and antidepressant-like behavioral responses to stress [6, 15, 35]. These findings strongly suggest that mechanisms that regulate phasic DA activity could also play a critical role in mediating responses to stress. Indeed, findings from our laboratory and others have shown that VTA cholinergic mechanisms regulate both phasic DA cell firing and downstream phasic DA release in the NAc [14, 22, 38]. Furthermore, our recent findings revealed a novel role for cholinergic activity in the VTA in mediating both pro-depressive and antidepressant-like behavioral responses in the forced swim test (FST) [2]. However, it is unclear whether VTA cholinergic mechanisms also regulate other behavioral phenotypes associated with depression and anxiety. In the current study, our objective was to determine whether increasing cholinergic tone in the VTA of Sprague-Dawley rats would alter stress, anxiety, and anhedonia-related behaviors as measured in the FST, elevated plus maze (EPM), and sucrose preference test (SPT). Given our previous results showing the ability of muscarinic acetylcholine receptor (mAChR) blockade to reverse the pro-depressive effects of enhanced VTA cholinergic activity in the FST [2], we also examined whether direct VTA mAChR activation was sufficient to alter behavioral responses in the FST and EPM. Together, our findings provide novel insight into the role of VTA cholinergic, and specifically mAChR-mediated, mechanisms in response to stress and anxiety.
Methods
Animals
Adult male Sprague Dawley rats (250–280g) were obtained from Charles Rivers Laboratories (Wilmington, MA, USA). Rats were group housed (2 to 3 per cage) and allowed to acclimatize to the housing facility for 1 week prior to surgical procedures. Animals were housed in a temperature-controlled room (22–24°C) under 12-h light/dark cycle (lights on at 7:00AM and lights off at 7:00 PM). Food and water were available ad libitum in home cages.
Surgery
Prior to surgery, rats were anesthetized with ketamine HCL (100 mg/kg, i.p., Sigma Aldrich, USA) and xylazine (10 mg/kg, i.p., Sigma Aldrich, USA). Rats were then placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) for intra-cranial cannula implantation. Coordinates were obtained from the rat brain atlas [28] with anteroposterior (AP), mediolateral (ML), and dorsoventral (DV) positions referenced from Bregma. A bilateral cannula apparatus with cannula spaced 1 mm apart (Plastics One, Roanoke, VA, USA) was placed 1 mm above the VTA (AP-5.2 mm, ML ±0.5 mm, DL −7.0 mm from dura) and secured to the skull using screws (Gexpro, High Point, NC) and dental cement (Dentsply, Milford, DE, USA). After surgery, rats were singly housed and allowed to recover for 5–7 days before behavioral testing. Animal protocols were approved by Yale University Institutional Animal Care and Use Committee (IACUC) and performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Drug Administration
In preparation for brain-region specific drug delivery, bilateral internal cannula containing the drug were inserted into the guide cannula and extended 1 mm beyond the guide cannula to target the VTA (−8.0 mm from dura). Drugs were delivered in a 0.5 μL total volume over 1 min via a micro-infusion pump (New Era Pump Systems, Farmingdale, NY) and syringe (25 gauge, Hamilton Syringe, Reno, NE, USA). After the 1 min drug infusion, the internal cannulae were left in place for an additional 1 min to allow for complete diffusion of drug into the brain tissue. Physostigmine (0, 1, or 2 μg, VWR, Bridgeport, NJ), an acetylcholinesterase inhibitor, and pilocarpine (0, 3, or 30 μg, Sigma Aldrich, St. Louis, MO), a selective mAChR agonist, were each dissolved in 0.9% saline and infused at behaviorally relevant doses that we and others have previously used in the VTA [2, 21, 42]. VTA infusion of vehicle or drug was performed immediately prior to the sucrose preference, elevated plus maze, forced swim or locomotor test session.
Forced Swim Test
The forced swim test (FST) was performed similar to protocols previously described by others [11, 12]. During the pre-test, no pharmacological manipulation was given and behavior was not recorded. In the pre-test, a new cohort of rats naïve to any behavioral test rats were individually placed into a clear polypropylene, cylindrical water tank (diameter 30 cm; height 60 cm; water depth > 40 cm; water temperature between 23 and 26 °C) for 15 min, to establish immobility for the subsequent test. The FST test session occurred during the second swim session, which took place 24 h after the pre-test. Rats were placed into the water tank immediately after intra-VTA drug infusion and immobility was scored from min 1 through min 6 (time: 1:00–6:00, 5 min total) of the 10 min test session. Each rat was randomly assigned to a specific drug administration group. FST was recorded by video camera and immobility was defined as an interruption of swimming behavior, when rats showed a lack of hind paw paddling. Thus, scoring of immobility time started when rats assumed a passive floating position, using only minimal movements required to keep their heads above water. For FST analysis, each test session was quantified by stopwatch by an experimenter blind to the treatment condition. At the end of the test session, rats were dried with a clean towel and monitored for 30 min in their home cage.
Sucrose Preference Test
The sucrose preference test was performed after a habituation period, where a new cohort of rats naïve to any behavioral test animals were given access to 1% sucrose solution (Sigma, St Louis, MO, USA) for 48 h in their home cage in place of the standard water bottle. Normal ad lib access to chow was maintained throughout habituation period. On test day, rats first underwent 4 h of water deprivation and were then given 1 h access to two identical bottles, one filled with 1% sucrose solution and the other with water. Sucrose and water consumption was determined by measuring the change in weight of bottles. The sucrose preference was defined as the ratio of the weight of sucrose vs water consumed during the 1h test. The relative consumption of 1% sucrose vs. water was calculated by weighing the bottles before and after 1 h test duration. Access to water bottles and chow was resumed immediately after the test.
Elevated Plus Maze
The elevated plus maze test consists of placing rats that were naïve to any behavioral test in a plus-shaped maze with two open and two enclosed arms for a 5 min session. Each arm had an open roof and was elevated 40–70 cm from the floor. The time spent in the open arms and number of crosses into open arms was subsequently quantified using a stopwatch. Time spent in the open arm is defined as >50% of the rat’s body has crossed the threshold of the defined open arm zone of the EPM. Animals were returned to their home cage at the end of the 5 min session.
Locomotor Activity
Automated activity monitors (Digiscan animal activity monitor; Omnitech Electronics, Columbus OH) were used to assess locomotor activity. The monitors were equipped with two parallel rows of 16 sensors spaced 2.5 cm apart and were controlled by a computer using Micromax software (Omnitech Electronics). VTA drug infusions were performed immediately prior to the test – consistent with the FST, SPT, and EPM experimental design. Photobeam breaks were recorded, quantified and analyzed in 5 min bins.
Statistics
Time spent immobile in FST, sucrose preference in SPT, and time spent in open arms in the EPM were analyzed using a one-way analysis of variance (ANOVA), performed with GraphPad Prism 6 (Graph Pad Software, San Diego, CA). If the ANOVA revealed a significant main effect, a Tukey post hoc analysis was performed to compare between specific drug-administration groups. Locomotor activity was analyzed using a two-way repeated measures analysis of variance (ANOVA), with time as the repeated measure.
Histological Verification
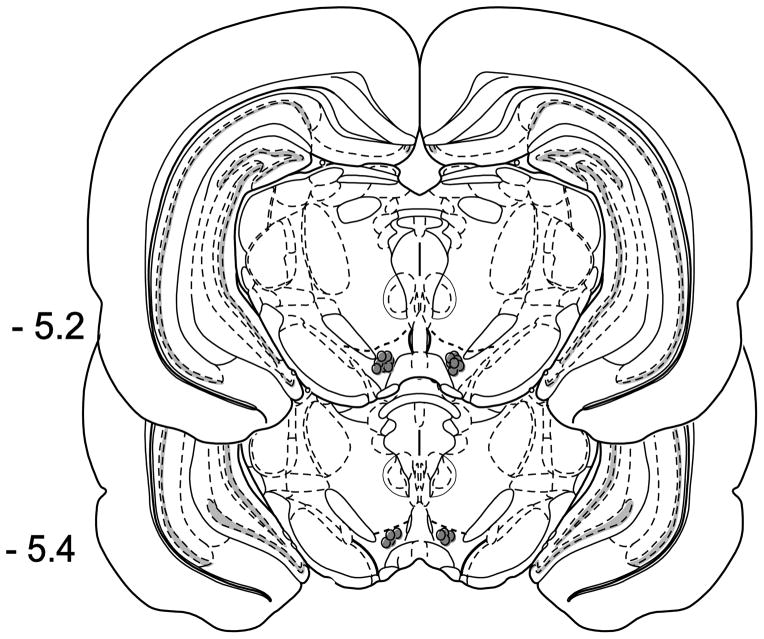
At the end of all behavioral experiments, rats were given a lethal dose of pentobarbital (150 mg/kg i.p.). Chicago blue dye was infused into the VTA (0.5 μl volume), rats were sacrificed, and brains were removed and sliced into 1 mm sections using a brain matrix for histological verification of dye localization. Any subjects with misplaced cannula were excluded from the subsequent statistical analyses of the behavioral data (Fig. 1).
Fig. 1.
Histological verification of cannula placements. Filled circles indicate representative placements for the physostigmine and pilocarpine experiments.
Results
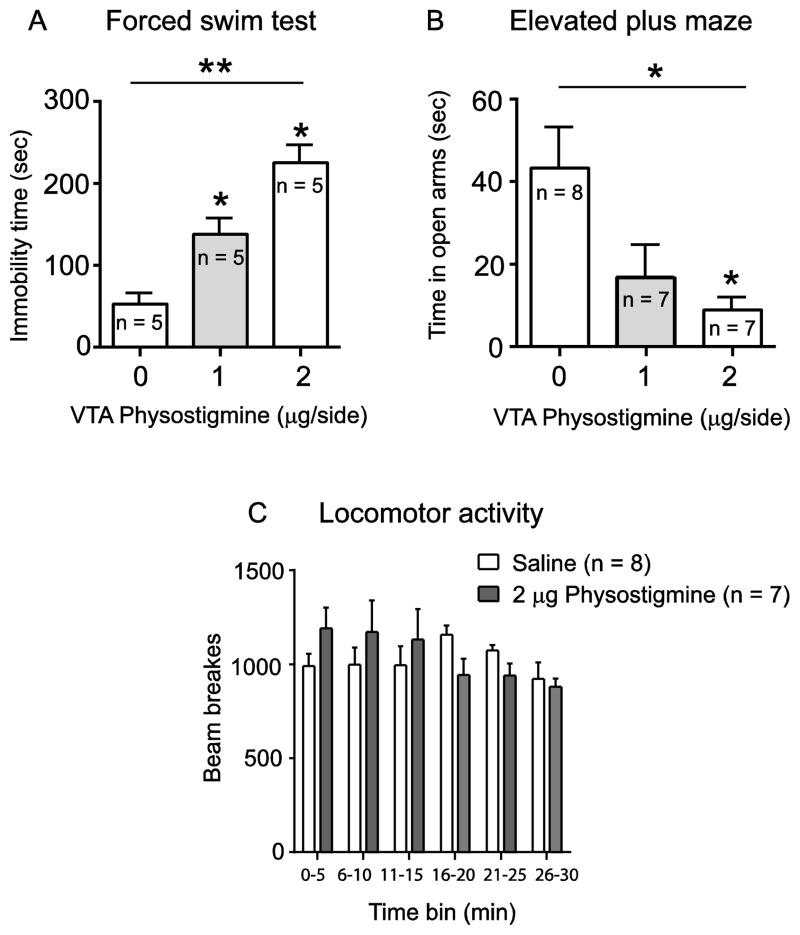
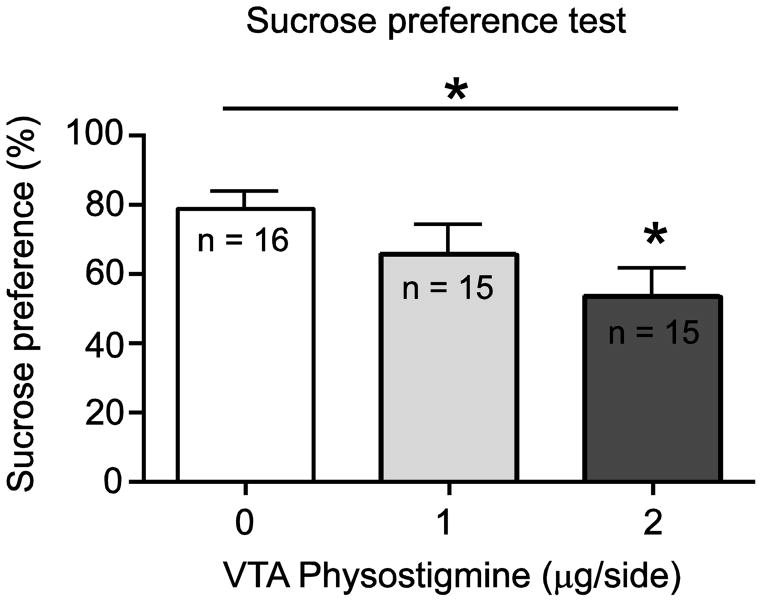
In order to determine whether enhancing cholinergic tone in the VTA would alter stress or anxiety-related behaviors, we administered physostigmine (0, 1, 2 μg/side) into the VTA of adult male Sprague Dawley rats and observed their behavioral responses in the FST and EPM (Fig. 2). Consistent with our previous findings, VTA physostigmine increased immobility in the FST, as revealed by a significant main effect of drug treatment (F(2,12) = 25.04; p < 0.01, Fig. 2A). Post hoc analysis revealed that both doses of physostigmine significantly increased immobility time compared to vehicle (p < 0.05). In a separate cohort, VTA physostigmine infusion also altered behavioral responding in the EPM, as revealed by a significant main effect of drug treatment (F(2,19) = 5.744; p < 0.05, Fig. 2B). Post hoc analysis revealed that the 2 μg/side dose significantly reduced time spent in the open arms of the EPM compared to saline treated rats (p < 0.05). We also observed that the number of entries in the open arm positively correlated with time spent on open arm for each treatment group (data not shown). Moreover, no significant differences in locomotor activity (F 5,65 = 1.210; p = 0.3145, Fig. 2C) were observed after physostigmine administration; suggesting that the effects of physostigmine were not due to non-specific locomotor effects. In addition, we have previously shown that the pro-depressive effect of physostigmine is site-specific, as administering physostigmine to a site 2mm dorsal to VTA led to no significant differences in FST immobility time compared to vehicle treated animals [2]. Next, to determine whether an increase in VTA cholinergic tone mediates anhedonia-related behavior, physostigmine (0, 1, 2 μg/side) was administered into the VTA and the sucrose preference was determined using the SPT. One way ANOVA revealed a significant main effect of drug treatment (F (2,43) = 3.228; p < 0.05, Fig. 3) in the SPT. Post hoc analysis also revealed that the 2 μg/side dose of physostigmine significantly decreased sucrose preference compared to saline treated rats (p < 0.05).
Fig. 2.
Effect of intra-VTA physostigmine (0, 1, 2 μg/side) on FST, EPM, and locomotor activity. Physostigmine increased immobility time in the FST (A), decreased time spent on open arms of EPM (B), and had no effect on locomotor activity (C). Data are expressed as mean ± S.E.M.; (* p < 0.05, ** p < 0.01).
Fig. 3.
Effect of intra-VTA physostigmine (0, 1, 2 μg/side) on SPT. Physostigmine decreased sucrose preference. Data expressed as mean ± S.E.M.; (* p < 0.05).
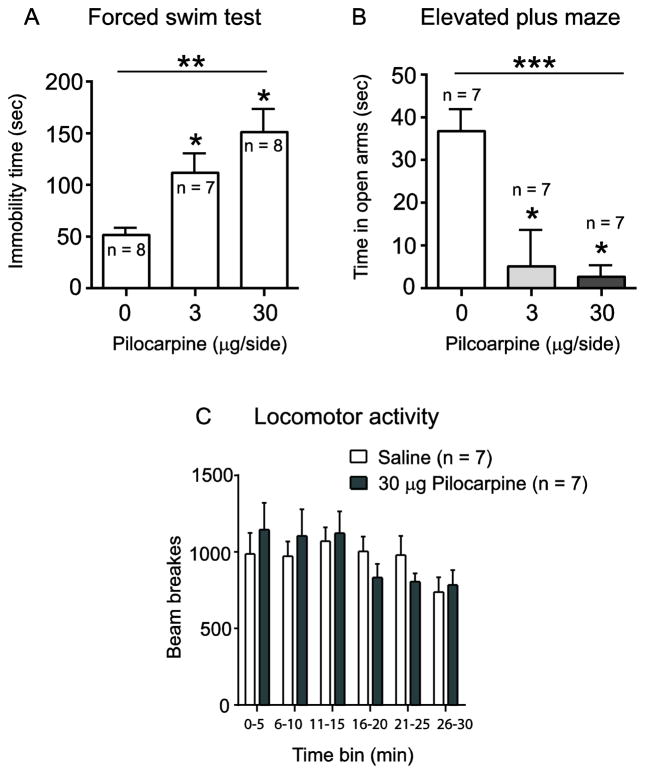
We have previously shown that blockade of VTA mAChRs reverses the pro-depressive effects of VTA physostigmine in the FST [2]. Here, we wanted to determine if selective activation of mAChRs in the VTA was sufficient to alter behavioral responses in the FST and EPM – the two behavioral measures that were most sensitive to VTA physostigmine. Thus, pilocarpine (0, 3, and 30 μg/side), a selective muscarinic agonist, was administered into the VTA and behavioral responses in the FST and EPM were observed (Fig. 4). One-way ANOVA showed a significant main effect of drug in the FST (F (2,20) = 9.654; p < 0.01, Fig. 4A). Post hoc comparison revealed that pilocarpine, at both the 3 μg/side and 30 μg/side doses, increased immobility time compared to the saline group (p< 0.05, Fig. 4A). Pilocarpine administered into the VTA also altered behavioral responding in the EPM, as shown by a significant main effect of drug (F (2,18) = 31.41, p < 0.001, Fig. 4B). Post hoc analysis revealed that both doses of pilocarpine significantly reduced time spent on open arms of the EPM compared to saline (p < 0.05, Fig. 4B). We also observed that the number of entries in the open arm for each treatment group positively correlated with time spent on open arm of corresponding drug treatment (data is not shown). Further, the effect of pilocarpine on FST and EPM was not due to non-specific locomotor effects as VTA drug infusion of pilocarpine (30 μg/side) led to no significant differences in locomotor activity (F(5,60) = 2.06; p = 0.0828, Fig. 4C). Taken together, these results demonstrate that selective mAChR activation in the VTA is sufficient to promote pro-depressive and anxiogenic-like responses in the FST and EPM, similar to the effects of VTA physostigmine.
Fig. 4.
Effect of intra-VTA pilocarpine (0, 3, 30 μg/side) on FST, EPM, and locomotor activity. Pilocarpine increased immobility time in the FST (A), decreased time spent on the open arms of EPM (B), and had no effect on locomotor activity (C). Data expressed as mean ± S.E.M.; (* p < 0.05, ** p < 0.01, *** p < 0.001).
Discussion
To our knowledge, the present study is the first to examine the role of VTA cholinergic, and specifically mAChR, mechanisms in stress and anxiety-related preclinical behavioral models. Here we found that enhancing VTA cholinergic tone, with physostigmine, promoted pro-depressive-like, anxiogenic, and anhedonic-like behavioral responses in the FST, EPM, and SPT, respectively. Moreover, selective activation of VTA mAChRs was sufficient to increase immobility time in the FST and decrease open arm time in the EPM, which is similarly suggestive of pro-depressive and anxiogenic responses. Together, these results reveal a novel role for VTA mAChR activity in mediating responses to stress and anxiety.
Cholinergic mechanisms in the mesolimbic dopamine system are known to modulate depression and anxiety-related behaviors [5, 24, 29, 37]. In addition, cholinergic mechanisms regulate phasic dopamine activity in the VTA to NAc pathway [13, 32, 38] that is known to causally mediate susceptibility to stress and anxiety [6, 35]. However, depression and anxiety-related cholinergic investigations in the VTA to NAc pathway have focused primarily on the NAc [5, 29, 37] and have not included the VTA. While the effect of acetylcholinesterase inhibition in the NAc has not been directly examined in stress and anxiety-related behaviors, evidence from several studies suggests that cholinergic activity in the NAc promotes a pro-depressive phenotype, similar to what we observed with enhanced VTA cholinergic activity. Specifically, pharmacological blockade of M2 mAChR autoreceptors in the NAc increases ACh levels in the NAc and induces a pro-depressive-like response in the FST [5]. In addition, direct NAc infusion of corticotropin releasing factor (CRF), which robustly increases NAc ACh levels, also increases immobility time in the FST, decreases open arm time in the EPM, decrease center time in the open field test, and decreases sucrose preference [7]. Thus, enhanced ACh in the NAc is strongly associated with pro-depressive and anxiogenic responses across several behavioral paradigms. In contrast, decreased NAc ACh is associated with fluoxetine-induced antidepressant effects in the FST [5]. This association between decreased NAc ACh activity and antidepressant-like behavioral effects is consistent with our previous VTA investigations, where we found that pharmacological blockade of VTA AChRs induces an antidepressant-like response in the FST [2]. Thus, the current evidence suggests the enhancing cholinergic activity in either the VTA or NAc is sufficient to promote pro-depressive-like responses while decreasing cholinergic activity, in contrast, produces antidepressant-like effects.
As indicated earlier, cholinergic mechanisms in both the VTA and NAc modulate dopaminergic activity [13, 32, 38] that causally mediates susceptibility to stress and anxiety [6, 35]. While we have demonstrated that increasing VTA cholinergic tone induces depressive-like and anxiogenic-like behavior, previous work has shown that VTA AChR activation is sufficient to promote DA burst firing patterns [41] that are known to facilitate DA release in the NAc [34]. Similarly in the NAc, the ability of CRF to increase NAc ACh levels and to promote pro-depressive behavioral effects is accompanied by a significant increase in NAc DA levels [7]. Specifically, Chen et al. reported that direct NAc infusion of corticotropin releasing factor (CRF) robustly increased NAc ACh levels and increased immobility time in the FST, decreased open arm time in the EPM, decreased center time in the open field test, decreased sucrose preference, and showed significant increase in NAc DA levels [7]. Our findings compliment these previous studies and implicate the potential role of the VTA mAChRs in modulating DA activity to induce pro-depressive behaviors.
In contrast to enhanced cholinergic activity, VTA AChR blockade which is sufficient to decrease phasic DA release in the NAc [32, 38] also induces antidepressant-like responses in the FST [2]. Together, these data show that VTA and NAc cholinergic mechanisms that enhance NAc DA are also sufficient to induce pro-depressive-like and anxiogenic-like behavioral phenotypes. A more recent study revealed NAc cholinergic effects that are in apparent contrast to previous NAc investigations. Specifically, Warner-Schmidt and colleagues found that optogenetic inhibition of NAc cholinergic neurons produced pro-depressive-like effects in the FST, SPT and tail suspension test [38]. However, the apparent ability of both increased NAc ACh levels and decreased NAc cholinergic activity to facilitate pro-depressive effects may be due to the complex mechanisms by which NAc ACh regulates dopaminergic activity. Tonic ACh activity in the NAc is known to inhibit DA release due to short term, synaptic depression and subsequent AChR inhibition removes this depression and allows for subsequent, enhanced DA release [8, 13, 41]. Thus, one potential mechanistic explanation for the pro-depressive effects in the Warner-Schmidt study is that optogenetic inhibition of NAc cholinergic neurons removed the short term DA-depression to facilitate an increase in DA levels, similar to the effects of enhancing ACh tone in the VTA or NAc, thus promoting a pro-depressive behavioral phenotype. However, this hypothesis has not been directly tested to date. Thus, additional work is needed to further elucidate the mechanisms through which cholinergic activity in the VTA to NAc pathway system mediates responses to stress and anxiety.
Concerning subtype specific VTA AChR mechanisms, we previously found that VTA mAChR blockade reversed the pro-depressive effects of VTA physostigmine in the FST [2]. Several studies had previously demonstrated a role of VTA mAChRs in multiple behaviors, including feeding behavior [30, 31], conditioned reinforcement [39] and cue-induced drug-seeking [32, 42]. However, our previous FST investigation was the first to suggest a role for VTA mAChR mechanisms in stress-related behavior. Here, our findings demonstrate that specific activation of VTA mAChRs is sufficient to promote pro-depressive and anxiogenic-like responses in the FST and EPM. These new mAChR findings are consistent with what one would predict based on previous examinations of VTA mechanisms in stress and anxiety. Specifically, recent work has shown that VTA hyperactivity is associated with general anxiety disorder (GAD) in humans [4] – consistent with our mechanistic hypothesis for the ability of mAChR activation to promote anxiogenic responses through enhanced VTA DA activity. Our VTA findings are also consistent with what has been observed with the downstream mAChR target, extracellular regulated signaling kinase (ERK). Specifically, Iniguez and colleagues found that overexpression of VTA ERK, which is activated downstream of VTA mAChRs, produces an anxiogenic-like responses in the EPM and a pro-depressive-like effect in the FST [16] – similar to our observed effect of VTA mAChR activation. These findings, together with our results, suggest that future VTA mAChR investigations are likely to reveal novel mechanisms that robustly mediate responses to stress and anxiety.
In closing, our findings provide novel understanding of the ability of VTA mAChR mechanisms to robustly modulate behavioral responses to stress and anxiety. These results have important clinical implications, especially in light of the evidence demonstrating rapid antidepressant effects following systemic administration of the mAChR antagonist, scopolamine [10, 24, 40]. Based on our findings, we suggest that additional VTA mAChR examination may provide important, new understanding of the neurobiological mechanisms that underlie stress and anxiety-related disorders.
Highlights.
We examined VTA acetylcholine effects in stress, anxiety and anhedonia behaviors
Enhancing VTA cholinergic tone produced pro-depressive and anxiogenic-like effects.
VTA muscarinic AChR activation induced pro-depressive and anxiogenic-like behavior.
Acknowledgments
This work was supported by grants from the National Institutes of Health T32 MH014276 (KMS), R25 GM104553 (SH) and a supplement to R01 MH093897 (NAA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aboul-Fotouh S. Behavioral effects of nicotinic antagonist mecamylamine in a rat model of depression: prefrontal cortex level of BDNF protein and monoaminergic neurotransmitters. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3745-5. [DOI] [PubMed] [Google Scholar]
- 2.Addy NA, Nunes EJ, Wickham RJ. Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test. Behavioural brain research. 2015;288:54–62. doi: 10.1016/j.bbr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.APA. Diagnostic and statistical manual of mental disorders. 5. Washington, D.C: 2013. [Google Scholar]
- 4.Cha J, Carlson JM, Dedora DJ, Greenberg T, Proudfit GH, Mujica-Parodi LR. Hyper-reactive human ventral tegmental area and aberrant mesocorticolimbic connectivity in overgeneralization of fear in generalized anxiety disorder. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5855–5860. doi: 10.1523/JNEUROSCI.4868-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG. Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience. 2001;104:791–798. doi: 10.1016/s0306-4522(01)00133-6. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends in neurosciences. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC. Neuroimaging studies of mood disorders. Biological psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 10.Drevets WC, Zarate CA, Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biological psychiatry. 2013;73:1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman CH. Models of depression. Vitamins and hormones. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- 12.Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biological psychiatry. 2012;72:258–265. doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. British journal of pharmacology. 2008;153(Suppl 1):S283–297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 15.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iniguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolanos-Guzman CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammel S, Tye KM, Warden MR. Progress in understanding mood disorders: optogenetic dissection of neural circuits. Genes Brain Behav. 2014;13:38–51. doi: 10.1111/gbb.12049. [DOI] [PubMed] [Google Scholar]
- 21.Levin ED, Briggs SJ, Christopher NC, Auman JT. Working memory performance and cholinergic effects in the ventral tegmental area and substantia nigra. Brain research. 1994;657:165–170. doi: 10.1016/0006-8993(94)90964-4. [DOI] [PubMed] [Google Scholar]
- 22.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, Dwyer JM, Fuchikami M, Becker A, Drago F, Duman RS. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiology of disease. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neuroscience and biobehavioral reviews. 2013;37:2015–2025. doi: 10.1016/j.neubiorev.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Pannekoek JN, van der Werff SJ, van Tol MJ, Veltman DJ, Aleman A, Zitman FG, Rombouts SA, van der Wee NJ. Investigating distinct and common abnormalities of resting-state functional connectivity in depression, anxiety, and their comorbid states. Eur Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; London: 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rada P, Colasante C, Skirzewski M, Hernandez L, Hoebel B. Behavioral depression in the swim test causes a biphasic, long-lasting change in accumbens acetylcholine release, with partial compensation by acetylcholinesterase and muscarinic-1 receptors. Neuroscience. 2006;141:67–76. doi: 10.1016/j.neuroscience.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 30.Sharf R, McKelvey J, Ranaldi R. Blockade of muscarinic acetylcholine receptors in the ventral tegmental area prevents acquisition of food-rewarded operant responding in rats. Psychopharmacology (Berl) 2006;186:113–121. doi: 10.1007/s00213-006-0352-0. [DOI] [PubMed] [Google Scholar]
- 31.Sharf R, Ranaldi R. Blockade of muscarinic acetylcholine receptors in the ventral tegmental area disrupts food-related learning in rats. Psychopharmacology (Berl) 2006;184:87–94. doi: 10.1007/s00213-005-0235-9. [DOI] [PubMed] [Google Scholar]
- 32.Solecki W, Wickham RJ, Behrens S, Wang J, Zwerling B, Mason GF, Addy NA. Differential role of ventral tegmental area acetylcholine and N-methyl-D-aspartate receptors in cocaine-seeking. Neuropharmacology. 2013;75:9–18. doi: 10.1016/j.neuropharm.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and biobehavioral reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biological psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibanez-Tallon I, Heintz N, Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA. Ventral tegmental area alpha6beta2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology (Berl) 2013;229:73–82. doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickham RJ, Solecki WB, Nunes EJ, Addy NA. Distinct effects of ventral tegmental area NMDA and acetylcholine receptor blockade on conditioned reinforcement produced by food-associated cues. Neuroscience. 2015;301:384–394. doi: 10.1016/j.neuroscience.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, Heinz BA, Nikolayev A, Tolstikov VV, Anderson WH, Higgs RE, Kuo MS, Felder CC. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. The Journal of pharmacology and experimental therapeutics. 2014;351:448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Liu Y, Chen X. Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat. The Journal of physiology. 2005;568:469–481. doi: 10.1113/jphysiol.2005.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M, Kalivas PW. Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience. 2007;144:1209–1218. doi: 10.1016/j.neuroscience.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]