Abstract
Mitochondrial dysfunctions are recognized as major factors for various diseases including cancer, cardiovascular diseases, diabetes, neurological disorders, and a group of diseases so called “mitochondrial dysfunction related diseases”. One of the major hurdles to gain therapeutic efficiency in diseases where the targets are located in the mitochondria is the accessibility of the targets in this compartmentalized organelle that imposes barriers towards internalization of ions and molecules. Over the time, different tools and techniques were developed to improve therapeutic index for mitochondria acting drugs. Nanotechnology has unfolded as one of the logical and encouraging tools for delivery of therapeutics in controlled and targeted manner simultaneously reducing side effects from drug overdose. Tailor-made nanomedicine based therapeutics can be an excellent tool in the toolbox for diseases associated with mitochondrial dysfunctions. In this review, we present an extensive coverage of possible therapeutic targets in different compartments of mitochondria for cancer, cardiovascular, and mitochondrial dysfunction related diseases.
Graphical abstract
1. Introduction
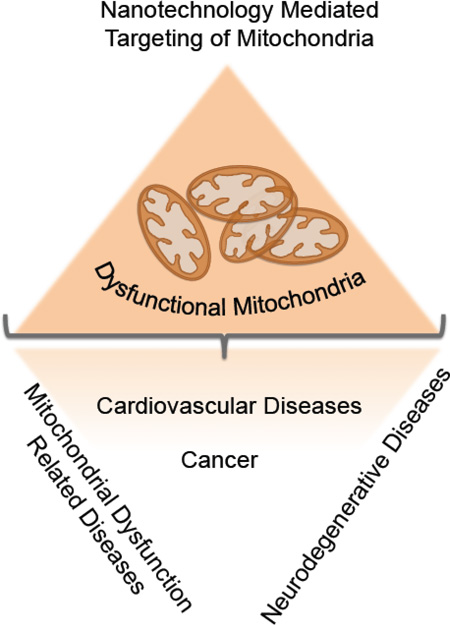
Mitochondria are recognized as the most intriguing energy producing organelle of the eukaryotic cells, which produce adenosine triphosphate (ATP) via oxidative phosphorylation (OXPHOS) (1–3). The name mitochondrion was derived from two Greek words “mitos” for “thread” and “chondrion” for “granule” (2). The complex structural morphology of mitochondria was first visualized in 1952 by high resolution electron microscopy studies (4). In addition to participation in OXPHOS, these organelles store various ions and proteins to manage oxidative stress and cell mortality. Mitochondrial DNA (mtDNA) built of 16569 base pairs encode for various proteins, RNAs, and the close proximity to the reactive oxygen species (ROS) make these genome highly susceptible to mutations in comparison to extent of mutations found in the nuclear DNA (nDNA). In addition to the complex structure, mitochondrial network also maintains high levels of dynamic processes to keep the balance between fusion and fission processes. Another layer of complexity is added on these organelles due to the presence of unusual phospholipid cardiolipin (CL), which contributes largely for the array of mitochondrial processes. Thus, this closed packed complex yet dynamic mitochondria maintain cellular mortality events by controlling vital cellular reactions, such as, energy production, redox balance, Ca2+ levels, initiation of apoptosis, and homeostasis of biomolecules in the cytosol. Even minor modifications of any of these parameters can disrupt cellular processes causing mitochondrial dysfunction and excessive mutation of mtDNA brings additional possibility of multiple mitochondrial diseases (5). A list of mitochondrial dysfunction related diseases are depicted in Figure 1. Thus, therapeutic modulation by attacking different mitochondrial targets can provide better management of diseases where mitochondrial dysfunctions are involved (6). However, the complex structure of the mitochondria designed by the nature to maintain important cellular functions imposes quite a challenge to engineer technologies which can navigate to this organelle for accessing different targets.
Figure 1.
Contribution of mitochondrial dysfunctions in progression of various diseases.
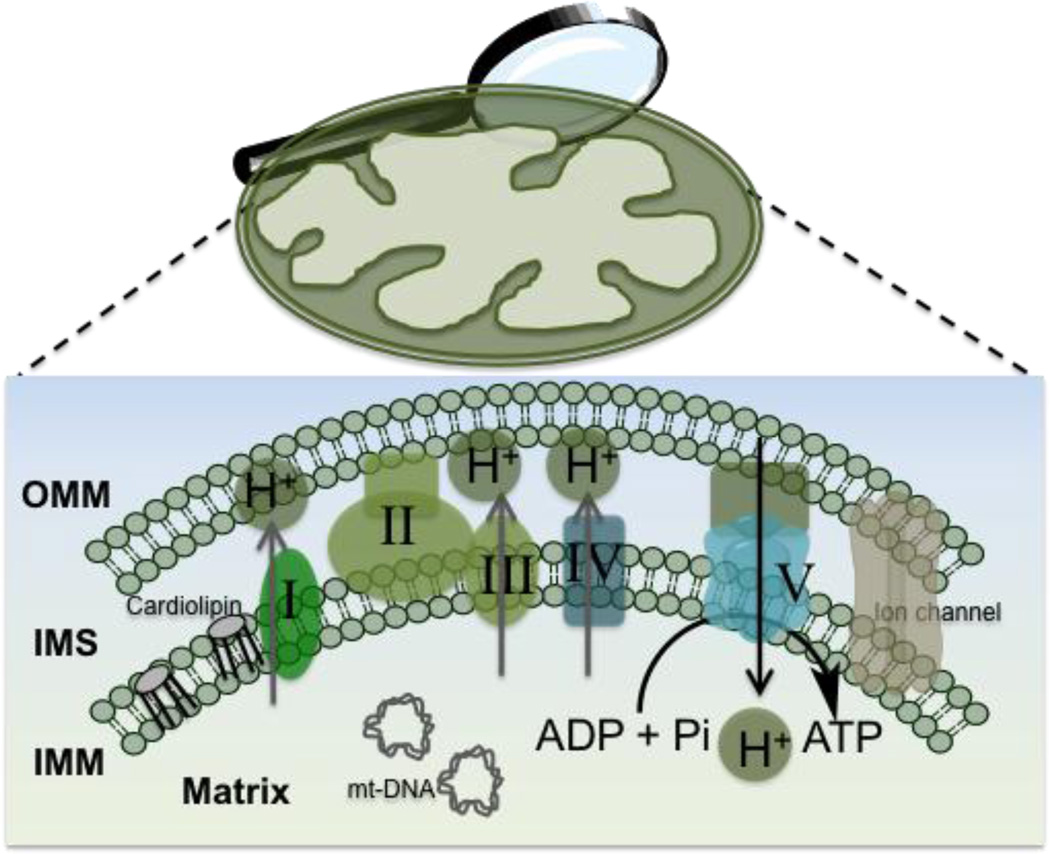
A mitochondrion consists of four major compartments: the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM), and mitochondrial matrix (MM). The OMM contains 8–10% of the total proteins, mostly protein translocators, pore forming proteins, and fission and fusion proteins of mitochondria encoded by nucleus and synthesized at the cytosolic ribosomes. Based on the size, small low molecular weight compounds usually participate in diffusion to cross the OMM, high molecular weight large molecular entities use protein translocators and pores to cross this membrane which has much similarities with the cell membranes. IMM is densely packed with complex structure with unusually high protein to phospholipid ratio providing restrictive environment for entrance of chemical entities into the matrix. Furthermore, the negative mitochondrial membrane potential (Δψm) of ~−160 to −180 mV that prevails across the membranes imposes additional level of difficulty for foreign molecules to cross into the matrix (7). A schematic view of different compartments in the mitochondria is depicted in Figure 2.
Figure 2.
Schematic overview of different parts of mitochondrion.
We recently published a short review on mitochondria targeted technologies for cancer therapeutics (8). In this review, we highlight mitochondrial targets and strategies to use nanotechnology tools for therapeutic interventions of cancer, as well as cardiovascular disease (CVD) and mitochondrial dysfunction related diseases.
2. Mitochondrial Targets for Cancer
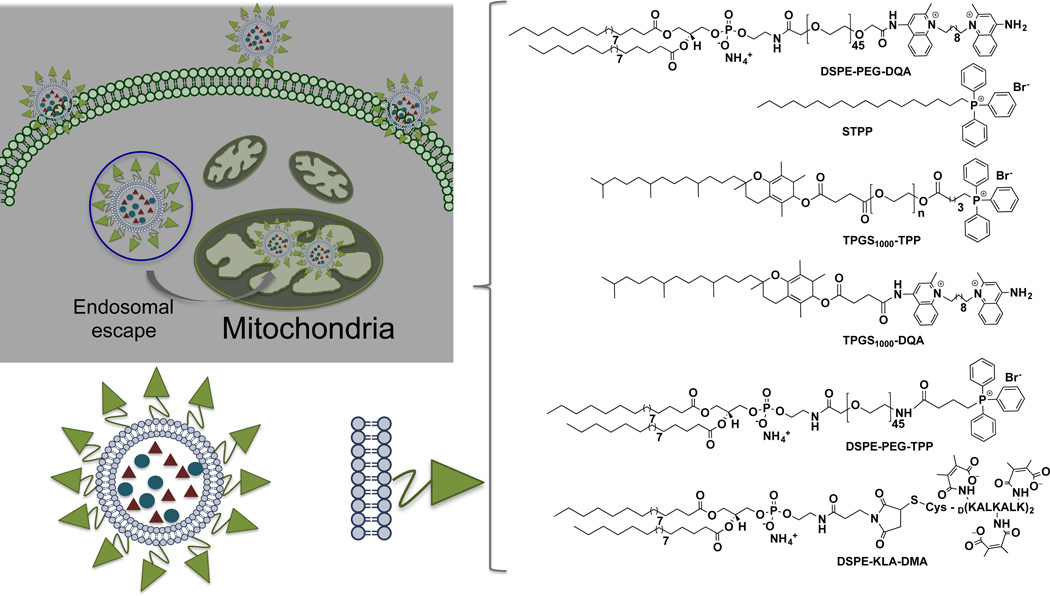
Mitochondria of cancer cells acquire altered characteristics, such as hampered OXPHOS and enhanced rates of glycolysis, hyperpolarized mitochondria, changes in the expression of permeability transition pore complex (PTPC) components (9–12). Skulachev and coworkers showed that certain organic cations can penetrate the mitochondrial membrane and these cations can be used to deliver therapeutics inside the mitochondrial matrix upon covalent conjugation (13, 14). Thereafter, several mitochondria targeted therapeutics were developed where triphenylphosphonium (TPP) cation was used as a mitochondria-targeting ligand (15–17). These molecules have delocalized cationic charge over high hydrophobic surface area to penetrate the IMM of mitochondria. A few other low molecular weight molecules are known in the literature to target mitochondria, but upon in vivo administration small molecular entities often display poor pharmacokinetic (PK) and undesirable biodistribution (bioD). Additionally, low molecular weight small molecules often loose biological activity upon covalent modification with functionalities essential for insertion of mitochondria targeting moieties. Over the last few decades, nanotechnology made significant impact for generation of drug delivery systems to improve bioD and PK profiles of therapeutics (18–23). However, these efforts are limited to the cellular level and there is substantial lack of progress for development of nanodelivery platforms for subcellular targeting, such as, mitochondria-targeted drug delivery systems. A summary of therapeutic delivery vehicles known to date for mitochondrial association of therapeutics and the specific targets in the mitochondria is represented in Table 1. A number of non-polymeric delivery systems targeted to the mitochondria are graphically shown in Figure 3.
Table 1.
Mitochondria targeted molecules, delivery vehicles, and their targets in the mitochondria.
| Therapeutics | Targeting groups (in bold face) on nanodelivery vehicles |
Target and its location |
|---|---|---|
| 3-Bromopyruvate | Au-TPP NPs (24) | HK-II in OMM |
| ClPhIQ Acid | PAMAM-ClPhIQ Acid (25) | TSPO and OMM |
| Lonidamine (LND) | DSPE-PEG-DQA (26) PLGA-b-PEG-TPP(27) |
PTP, HK-II inhibitor, OMM, IMM and MM |
| PTX | Liposomes-Rh123 (28), TPGS-1000-TPP (29), PEI-PEG-TPP (30) Liposomes-KLA (31) |
Unknown in MM * |
| Curcumin | PLGA-b-PEG-TPP (27), DQAsomes-DQA (32) | Antioxidant in MM and IMS |
| DNase I, PI, RNA | (DF-)MITO-Porter (33–35) | mtDNA in MM |
| 2, 4-DNP | PLGA-b-PEG-TPP (27) | Protonphore in IMM and matrix |
| α-TOS | PLGA-b-PEG-TPP (27) | BCl-2 protein inhibitor and antioxidant in OMM, IMM and MM |
| DNA and other therapeutic genes | PAMAM-G-5-TPP (30, 36) and DQAsomes-DQA (37, 38) | mtDNA in MM |
| DOX | PEI-TPP (39), Liposomes-STPP (40) | Complex I of ETC in IMM |
| Pt(II) and Pt(IV) drugs and prodrugs | MWCNTs-Rh-123 (41) PLGA-PEG-TPP (42) |
mtDNA in MM |
| Ceramide | Liposomes-STPP (43) | Apoptosis inducer, IMS, IMM and MM |
| Sclareol | Liposomes-STPP (44) | Apoptosis inducer, IMS, IMM and MM |
| Resveratrol | DSPE-PEG-DQA (36) | Antioxidant in MM and IMS |
| Daunorubicin and amlodipine | Liposomes-DQA (45, 46) | Apoptosis inducer, IMS, IMM and MM |
| CCI-779 | Liposomes-Zwitterionic oligopeptide (47) | mTOR in IMS and matrix |
| CoQ10 | PCL-TPP (48) | ETC in MM |
| Topotecan hydrochloride | TPGS1000-DQA (49) | Topoisomerase I inhibitor and MM |
TPP=Triphenylphosphonium, HK-II=Hexokinase-II, OMM=outer mitochondria membrane, ClPhIQ acid =1-(2-chlorophenyl)isoquinoline-3-carboxylic acid, PAMAM=Polyamidoamine, TSPO=Translocator protein, DSPE-PEG-DQA=Polyethylene glycol-distearoylphosphatidylethanolamine-dequalinium, PLGA-b-PEG-TPP=Poly(lactic-co-glycolic acid)-polyethylene glycol-triphenylphosphonium, PTP=Permeability transition pore, IMM=Inner mitochondrial membrane, PTX=Paclitaxel, TPGS-1000-TPP=Tocopheryl polyethylene glycol 1000 succinate-triphenylphosphonium, PEI-PEG-TPP = Poly(ethylene imine)-polyethylene glycol-triphenylphosphonium, KLA= D[KLAKLAK]2, 2, 4-DNP = 2, 4-dinitrophenol, IMS = Intermembrane space, α-TOS = α-tocopheryl succinate, BCl2= B-cell lymphoma 2, DOX = Doxorubicin, STPP = Stearyl triphenylphosphonium, MWCNTs = Multi-walled carbon nanotubes, Rh123 = Rhodamine 123, ETC=electron transport chain, mTOR =mammalian target of rapamycin, CoQ10 = Coenzyme Q10, PCL = polycaprolactone, CCI-779 = Cell cycle inhibitor-779
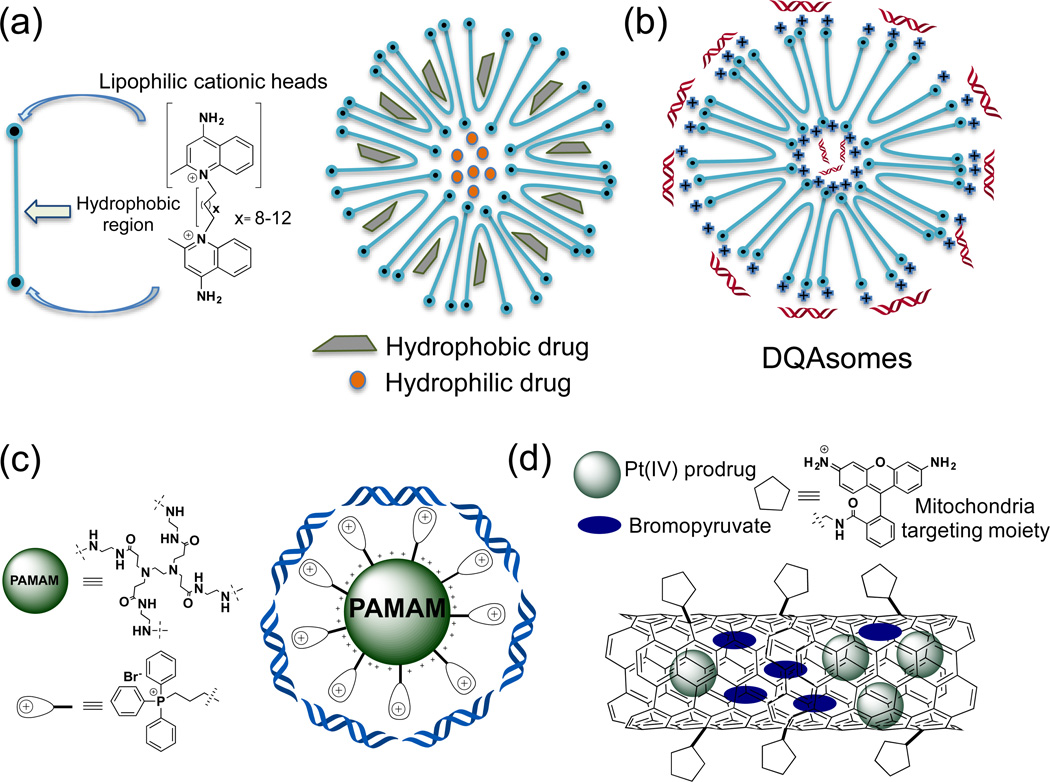
Figure 3.
Schematic diagrams for the different mitochondria targeted scaffolds derived by amphiphilic DQA cations, PAMAM and carbon nanotubes. (a) Structure of amphiphilic dequalinium (DQA) cation and its drug entrapped micellar nanostructures; (b) DQA based nanomicelles for gene delivery to mitochondria; (c) TPP anchored PAMAM based dendrimers for gene delivery to mitochondria; (d) Rh110 functionalized carbon nanotubes based mitochondria delivery of dual drugs.
2.1. Targets for cancer in the OMM
The OMM imposes only weak barrier compared to IMM in navigation of foreign molecules to the mitochondria. The OMM contains voltage-dependent anion channels (VDAC) (50). These protein-based channels non-specifically transport ions, nucleotides, and other molecules to the IMS. The VDAC is known to mediate apoptosis by releasing cytochrome c (cyt c) and also provides a platform for interaction of pro- and anti-apoptotic members of B-cell lymphoma 2 (BCl2) family as well as hexokinase I (HK-I) and HK-II that are overexpressed in various cancer types (51–56). These proteins participate in rapid growth of cancer cells and provide apoptosis suppressive properties. VDAC also contributes to enhanced cholesterol contents in cancer cells compared to normal cells. Thus, targeting VDAC in OMM can be important for treatment of cancer.
In cancer cells, both HK-I and HK-II are tightly bound to the VDAC at the OMM (57, 58). This HK-VDAC interaction deviates pyruvate towards lactate production and results in preventing cellular apoptosis. Thus, interfering HK binding to VDAC either by HK inhibitors or by using molecules that compete with VDAC for HK binding can result in novel therapeutics for cancer. Lonidamine (LND), 2-deoxyglucose, and 3-bromopyruvate (3-BP) are commonly used drugs for inhibition of HK (57, 59). Efficacious delivery of these molecules can boost the therapeutic potency by several folds using NP based scaffolds. Mitochondria-targeted gold nanoparticles (AuNPs) functionalized with 3-BP and lipophilic TPP cations with delocalized positive charge to specifically target the ΔΨm, were recently developed for delivery of 3-BP to the mitochondria (24). These targeted NPs carrying 3-BP demonstrated enhanced ability to modulate cancer cell metabolism by inhibiting glycolysis as well as demolishing OXPHOS process as compared to 3-BP formulated in a non-targeted NP or free 3-BP. Further, the anticancer activity of targeted AuNPs containing 3-BP was improved upon laser irradiation by exciting the surface plasmon resonance band of AuNPs and thereby utilizing a combinatory effect of chemo and photothermal therapies.
The translocator protein (TSPO) is a transmembrane protein primarily associated to the OMM (60). Higher expression of TSPO was found in aggressive and metastatic prostate and breast cancers (61, 62), and thus this protein can be considered as a target for cancer therapy. A G(4)-polyamidoamine (PAMAM) scaffold using 1-(2-chlorophenyl)isoquinoline-3-carboxylic acid (ClPhIQ acid) as a TSPO inhibitor and lissamine (Liss) as an imaging agent was constructed (25). This construct was evaluated for its theranostic property in C6 rat glioma and MDA-MB-231 human breast cancer cells. The bright and localized cellular fluorescence was observed with ClPhIQ-PAMAM-Liss construct and it exclusively co-localized with the Mitotracker green supporting association with the mitochondria. Thus, these PAMAM based synthetic dendrimers that internalized into the cell and target the mitochondria can be used for delivery of mitochondria-acting therapeutics.
A liposome-based carrier system, MITO-Porter, was developed to deliver macromolecules such as green fluorescence protein (GFP) (63). This liposome based system delivered GFP to the mitochondria using a fusion pathway.
The BCl-2 family is comprised of both pro- and anti-apoptotic proteins located in the OMM that regulate apoptosis by ascertaining mitochondrial outer membrane permeabilization (MOMP). Many tumors express anti-apoptotic BCl-2 protein to circumvent apoptosis. Selective delivery of inhibitors and peptides that bind to the active site of BCl-2 would lead to a better therapeutic option to evade tumor growth (64, 65). The BCl-2 homology 3 (BH3) protein preferentially binds to BCl-2 through its homology domain and neutralizes its activity to induce apoptosis (66, 67). Small molecules mimicking the topology of BH3, referred as BH3 mimics, can bind and deactivate the function of pro-apoptotic BCl-2 and serve as promising drug candidates (68–70). BH3 mimetic small molecules, ABT-737, ABT-263, gossypol, AT-101, and alpha-tocopheryl succinate (α-TOS) along with other chemotherapeutics, show promising selectivity in targeting pro-apoptotic proteins and increased efficacy (71, 72). However, clinical outcomes show that these drugs have severe dose dependent adverse effects such as thrombocytopenia (73). Nanotechnology-based targeted delivery might find applications to reduce the toxicity and increase therapeutic efficacy of these molecules.
2.2. Targets for Cancer in the IMM and MM
The IMM is composed of convoluted invaginations termed as ‘cristae’ with high surface area. The presence of unusual phospholipid, CL and high protein to lipid ratio in the IMM allow topological alterations for reorganization of the membrane to deliver multiple functions under tightly regulated signaling cascade. IMM comprises of critical membrane proteins and enzymes, including those from electron transport chain (ETC), ions, and metabolite transporters such as the adenine nucleotide translocase (ANT), the mitochondrial calcium uniporter (MCU), and ATP synthase essential for the survival of cell. MM composed of a variety of soluble proteins, enzymes of Kreb’s and fatty acid cycles, biomolecules, ions, ribosomes, and mtDNA. ETC in the IMM generates a proton gradient across the IMM to drive ATP production by ATP synthase. Cancer cells are characterized with their different phenotypes in terms of the expression of various biomolecules and proteins. Modulating their phenotypic characteristics by means of inhibiting and/or activating certain pathways can lead to effective therapeutic regimens for cancer treatment.
The PTPC forms as a super channel that comprises VDAC/ANT complex. This super channel allows transport of variety of small molecules including ATP and adenosine diphosphate (ADP). Compounds that modulate the VDAC/ANT complex channel can serve as potent candidate. One such candidate is LND that has ability to initiate apoptosis in cancer cells. In the recent past, delivery of therapeutic genes for the expression of various pro-apoptotic proteins, mtDNA damaging agents, antioxidants, small molecules, and anti-apoptotic protein inhibitors was achieved for the effective mitochondrial targeted treatment strategies in cancer.
Despite the vast understanding of mtDNA-derived defects, both at the genetic and biochemical proportions, there is no effective treatment modality in hand due to the lack of approaches that can provide effective mitochondrial gene delivery. Torchilin and coworkers designed a mitochondria-specific transfection vector based on delocalized lipophilic cation 1,1′-decamethylene bis(4-aminoquinaldiniumchloride) or dequalinium (DQA) that shows mitochondria association properties (74). Its amphiphilic nature promotes liposome-like structures popularly known as DQAsomes with positively charged surface. DQAsomes expeditiously bind and shield DNA and their liposomal structures can encapsulate small molecules. Binding of DNA to these liposomes was confirmed by competitive binding of SYBR™ dye. Complete loss of DNA-dye complex fluorescence was observed upon increasing the molar equivalents of DQAsomes in a DNA-dye mixture precludes the SYBR™ dye from binding to the DNA (74). These liposomes deliver DNA in the form of ‘DQAplexes’ and are able to transfect cells in vitro with efficiency comparable to lipofectamine (37). Upon interaction of these ‘DQAplexes’ with intact mitochondria results in the release of DNA cargo from the DQAsomes (38). The utility of DQAsomes for entrapment of small molecules was extended as one of the first mitochondria drug delivery vehicles to deliver drugs in their pristine form (75, 76). Paclitaxel loaded DQAsomes were demonstrated to retard the growth of human colon cancer in nude mice (75, 77, 78). Lu and coworkers have synthesized DQA appended polyethylene glycol-distearoylphosphatidylethanolamine (DQA-PEG2000-DSPE) conjugate to prepare liposomes for delivery of resveratrol in the mitochondria (36). The DQA appended liposomes significantly increased the uptake and mitochondrial association properties. These liposomes induced apoptosis both in non-resistant and resistant cancer cells and demonstrated subsequent release of cyt c. Significant antitumor activity of these liposomes were demonstrated both in tumor spheroids model as well as in xenograft model of cisplatin resistant A549/CDDP cells in nude mice. Augmented anticancer efficacy was shown when mitochondria targeting resveratrol liposomes were co-administered with chemotherapeutic ‘vinorelbine’ loaded liposomes (36). This approach was further extended to efficacious co-delivery of LND and epirubicin using similar mitochondrial targeted DQA appended liposomes (26). Chemotherapeutic agent daunorubicin and an inducer of apoptosis amlodipine were incorporated in the DQA functionalized liposomes for their delivery in the mitochondria. These liposomes selectively induced mitochondria mediated apoptosis and exhibited significant tumor growth reduction in vivo (45, 46). DQA was also used with TPGS1000, known as vitamin E polyethylene glycol succinate, to construct mitochondria targeting liposomes for the delivery of chemotherapeutic drug, topotecan hydrochloride a topoisomerase I inhibitor. These mitochondria targeted topotecan entrapped liposomes demonstrated higher efficacy for treatment of chemo-resistant cancer and showed inhibition of metastases in vivo (49).
Another class of hyper-branched synthetic molecules, dendrimers, which can be modified to install multiple drugs and targeting moieties, were demonstrated to be effective as nanocarrier systems for delivery of various pharmaceutical agents to the mitochondria Torchilin and coworkers described development of a mitochondria-targeted G(5)-D-Ac-TPP scaffold where mitochondria targeting was achieved using covalent conjugation of TPP with the surface functionalities of the dendrimer and remaining free NH2 were acetylated for nonspecific binding. Cellular and subcellular compartmentalization studies with FITC coupled G(5)-D-FITC-Ac-TPP showed effective mitochondrial localization and this formulation was found to be non-toxic to cells (30). Thus, G(5)-D-Ac-TPP scaffold can be used as a nontoxic mitochondria-targeted nanocarrier for delivery of therapeutics either by loading in the dendrimer core through electrostatic interaction or by covalent conjugation on the surface. Subsequently, Cheng and coworkers utilized the G5-TPP dendrimer scaffold to deliver nucleic acids to mammalian cell mitochondria (79). These TPP conjugated PAMAM polymeric gene vectors demonstrated targeting aspects towards mitochondria resulting in better transfection efficacy in HeLa and COS-7 cells. Transfection efficiency of these scaffolds was shown to be greater than the commercially available transfection reagent SuperFect and comparable with Lipofectamine 2000. The increase in the transfection ability of G5-TPP dendrimers to the mitochondria was correlated to DNA binding/unpacking ability, efficient endosomal escape, and better serum resistance (79). Further investigation and systematic studies are needed to explore the utility of these scaffolds for co-delivery of therapeutic genes along with anticancer drugs.
Similar approach was extended by Tsiourvas and coworkers to deliver doxorubicin (DOX) using lipophilic decyl-TPP functionalized poly(ethylene imine) hyperbranched polymer (PEI-TPP) to the cancer cell mitochondria (39). The resulting nanoassembly, PEI-DOX-TPP, with ~100 nm mean diameter was shown to be effective at sub-micromolar concentration to deliver DOX to the mitochondria.
Suitably functionalized liposomes with cellular and subcellular-targeting moieties can provide an alternative therapeutic avenue for site directed drug delivery. A number of liposome-based mitochondria targeted delivery vehicles are summarized in Figure 4. Weissig and coworkers demonstrated the utility of liposomes in mitochondria targeting via appropriate surface modification with mitochondriotropic residues (80). Mitochondriotropic liposomes were prepared with stearyl TPP (STPP) bromide as the targeting ligand. Intracellular localization of these liposomes was demonstrated by using 0.5 mol% phospholipid conjugated rhodamine-PE dye along with other phospholipids and STPP as the targeting ligand. These liposomes were found to demonstrate mitochondrial association properties in BT-20 human breast cancer cells. Weissig and coworkers also used STPP liposomes to deliver ceramides to the mitochondria to induce apoptosis with improved metabolic toxicity as compared to the non-targeted delivery systems (43). These mitochondria-targeted ceramide delivery systems were further evaluated to exhibit antitumor activity in vivo. Biodistribution of STPP liposome was found to be similar to that of non-targeted analogues. In another example, water insoluble anticancer drug, sclareol, was delivered using STPP based mitochondria targeted liposomes to improved therapeutic index (44). A dual targeting approach was adopted by Murthy and coworkers to deliver DOX in cancerous cells followed by association with the mitochondria using STPP and folic acid (FA) functionalized liposomes (40). These DOX containing FA-STPP based liposomes exhibited greater cytotoxicity compared to non-targeted and simple targeted liposomal formulations depicting synergistic effects of FA and STPP.
Figure 4.
Schematic diagrams for the TPP functionalized liposomes constructed based on different phospholipids bearing mitochondria targeted agents. Structure of mitochondria targeted liposome and demonstration of its endosomal escape capability to finally reach mitochondria matrix, and chemical structures of mitochondria targeted molecules and lipids used for the preparation of liposomes to target mitochondria.
D-α-tocopheryl polyethylene glycol 1000 succinate-TPP (TPGS1000-TPP) cation was used as a mitochondria targeting ligand that was incorporated in the liposomal membrane to deliver paclitaxel (PTX) (29). This selective mitochondrial delivery of PTX induced apoptosis in resistant cancer cells (29). Similar strategy was employed to incorporate rhodamine-123 (Rh123) as a mitochondria targeted ligand on the liposomes for efficacious delivery of PTX. These Rh123 functionalized liposomes were shown to have higher cellular uptake with increased accumulation in the mitochondria as evaluated by fluorescence microscopy and FACS analysis. These Rh123 functionalized liposomes showed an increase in the potency of PTX as compared to its free drug (28). In another report, polyethylene glycol-phosphatidylethanolamine (PEG-PE) based liposomes were used to deliver PTX (81). These TPP appended liposomes were shown to have less toxicity compared to the earlier reported STPP based liposomes. Significant increase in the cytotoxicity and anti-tumor efficacy of PTX-loaded TPP-PEG-liposomes were observed compared to unmodified PTX-loaded liposomes both in vitro and in vivo. However, systematic in vitro and further in vivo studies are needed to prove the exact behavior and potential of these liposomes as therapeutics. A multistage pH responsive break down property based zwitterionic oligopeptide appended liposome was prepared to remove surface barriers for exposing the cationic species on the surface to direct therapeutics to the mitochondria (47). HHG2C18 consists of two amino acid groups from glutamic acid and histidine, hydrophilic block of pH-cleavable group hexahydrobenzoic amide, and hydrophobic block of two stearyl chains, to mimic natural phospholipids. The surface charge of HHG2C18-L is negative under physiological conditions (pH 7.4), whereas, at the tumor site surface charge gets reverses to positive in presence of slightly acidic milieu to increase tumor cellular uptake. Owing to the presence of imidazole of histidine, these nano-constructs facilitate proton sponge effects to escape endosome and lysosomes for subsequent localization in the mitochondria. A specific inhibitor CCI-779, of mammalian target of rapamycin (mTOR), a serine/ threonine protein kinase that mediates cell growth and cell proliferation was incorporated in these nano constructs to block mitochondrial mTOR. Significant potency enhancement of mitochondria targeted CCI-779/HHG2C18-L constructs was observed both in vitro and in vivo. However, the complex nature of these nano-constructs with less versatility, limits their use as potent drug delivery agents.
Recently a liposomal construct possessing extracellular pH responsive and mitochondrial targeting properties was used for efficacious delivery of PTX (31). The peptide D[KLAKLAK]2 (KLA) amines were shielded by 2, 3-dimethylmaleic anhydride (DMA) and further combined with DSPE to yield DSPE-KLA-DMA (DKD). The DKD bearing liposomes were able to reverse their surface charge in the acidic tumor extracellular pH (~6.8) from negative to positive to preferentially accumulate in the mitochondria. These liposomes demonstrated enhanced efficacy in treating A549 cells and A549/Taxol resistant cells in vitro as well as in vivo.
Polymeric NPs derived from biocompatible and biodegradable components are explicatively used as alternative delivery agents to the liposomes (23, 82). Owing to their amenable variations in hydrophobic and hydrophilic blocks composition, these polymers can be used to encapsulate both hydrophilic and hydrophobic therapeutics via emulsion or nanoprecipitation methods, respectively (83). A sustained and controlled drug release profile with overall higher stability and non-immunogenic properties of polymeric NPs as compared to the conventional liposomes have the ability to increase the potency of the delivered therapeutics (84, 85). A class of biodegradable polyesters, such as, poly(glyocolic acid) (PGA), poly(lactic acid) (PLA), and their copolymer poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL) are used frequently to build hydrophobic corona of NPs whereas the FDA approved PEG is used to make hydrophilic shell on the NP core (86). The hydrophobic corona provides stability, PEG allows for longer retention time and immune system escape within the body. Further, the PEG blocks can be attached with the targeting moieties to enhance uptake. These desirable qualities along with the broad scope for chemical modification of polymers make polymeric NPs versatile delivery systems for mitochondrial delivery.
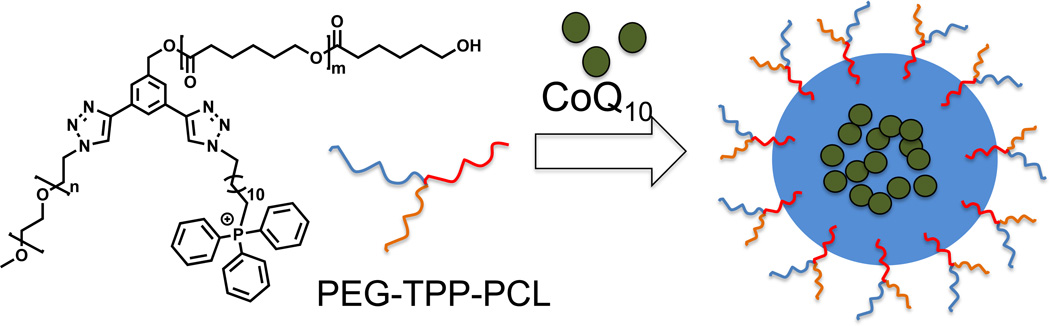
A recent example demonstrated use of mitochondria-targeted polymer based nano-delivery system to deliver coenzyme Q10 (CoQ10) to combat oxidative stress (48). These NPs were constructed from a miktoarm polymer of formula ABC where A = PEG; B = TPP, and C = PCL (Figure 5). The resultant micelles had high drug loading with small critical micellar concentration. Inefficient localization of the NPs inside the mitochondria were observed in confocal microscopy studies due to the disadvantage lies in its structural design with TPP moieties near the hydrophobic PCL (48).
Figure 5.
Triblock star polymer containing PEG, PCL, TPP and CoQ10 loaded mitochondria targeted NPs.
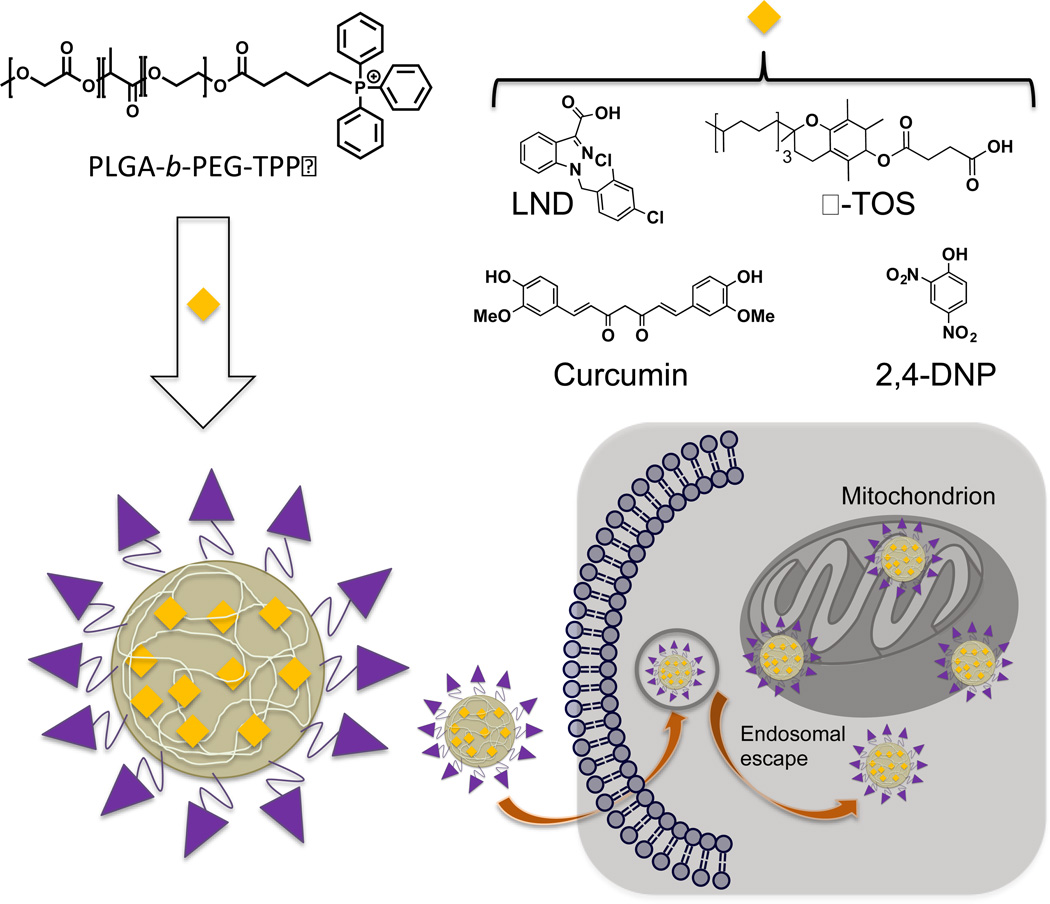
Our group has developed a TPP terminated PLGA-b-PEG based block copolymer to target mitochondria (Figure 6). Upon self-assembly, amphiphilic block copolymer composed of PLGA forms a core and PEG as a corona with mitochondria targeting TPP on the surface (21, 27, 42, 87). The TPP moieties on the surface of the NPs provide cumulative positive charge delocalized over a large hydrophobic phenyl rings making these NPs ideal system for penetration in the lipid membranes. Further these NPs take the advantage of Δψm that exists across the IMM to get associated with the mitochondria. These PLGA-based NPs were fine-tuned for their size and surface charge to provide better mitochondrial association using an engineered polymer blending technology (27). We found that an optimum size of <100 nm and a positive zeta potential of >22 mV is needed for efficient mitochondrial affinity. Owing to the unique biological properties, such as, non-toxicity and non-immunogenicity along with the facile cellular and subcellular uptake, and controlled release of the payload allow the use of these scaffolds as novel drug delivery vehicles (Figure 6). The presence of unique topology with lipophilic cations on the NP surface allow for endosomal escape. Cellular and subcellular distribution was assessed through the quantitative measurement of the Cd from the CdSe quantum dot (QD) loaded targeted and non-targeted NPs. Fluorescence microscopy imaging of targeted and non-targeted NP treated cells indicated significantly higher association of targeted NPs with the mitochondria of cells whereas the non-targeted NPs were mainly found scattered in the cytosol. The versatility of these NPs was further demonstrated through delivering a variety of mitochondria-acting therapeutics, such as, curcumin, mitochondrial decoupler 2,4-dinitrophenol (2,4-DNP) as an anti-obesity drug, LND, and α-TOS. A significant increase in the potency was observed by directing these therapeutics to the mitochondria.
Figure 6.
Schematic presentation of engineered drug loaded polymeric NP decorated with TPP cations on the surface to target mitochondria.
After successful demonstration of mitochondria directed delivery of the above mentioned therapeutics, recently we used these biocompatible polymeric NPs to deliver a cisplatin prodrug to the mitochondria to access mtDNA which lack NER machinery (42). Directing DNA damaging agents such as cisplatin to the mitochondria can potentially address the problems associated with conventional platinum-based compounds (88, 89). For this purpose, a hydrophobic mitochondria-targeted cisplatin prodrug, Platin-M, was synthesized using a strain promoted azide-alkyne cycloaddition reaction (90). Platin-M was loaded in PLGA-b-PEG-NPs for controlled release of cisplatin in the MM. Cisplatin released from Platin-M in the mitochondria can attack mtDNA to form Pt-DNA adducts and induce cell death. Significantly higher activity of Platin-M and its targeted-NPs in cisplatin-resistant cells was observed. Thus, a precise strategy for mitochondria targeting of cisplatin for chemoresistance cancers using a unique dual-targeting approach is an alternative route for cisplatin-based therapy (42). Recently, this technology was studied in a large animal model by demonstrating the safety and toxicity of mitochondria-targeted NPs loaded with Platin-M (91). This study also highlights the ability to manufacture the mitochondria targeted NPs in large scale.
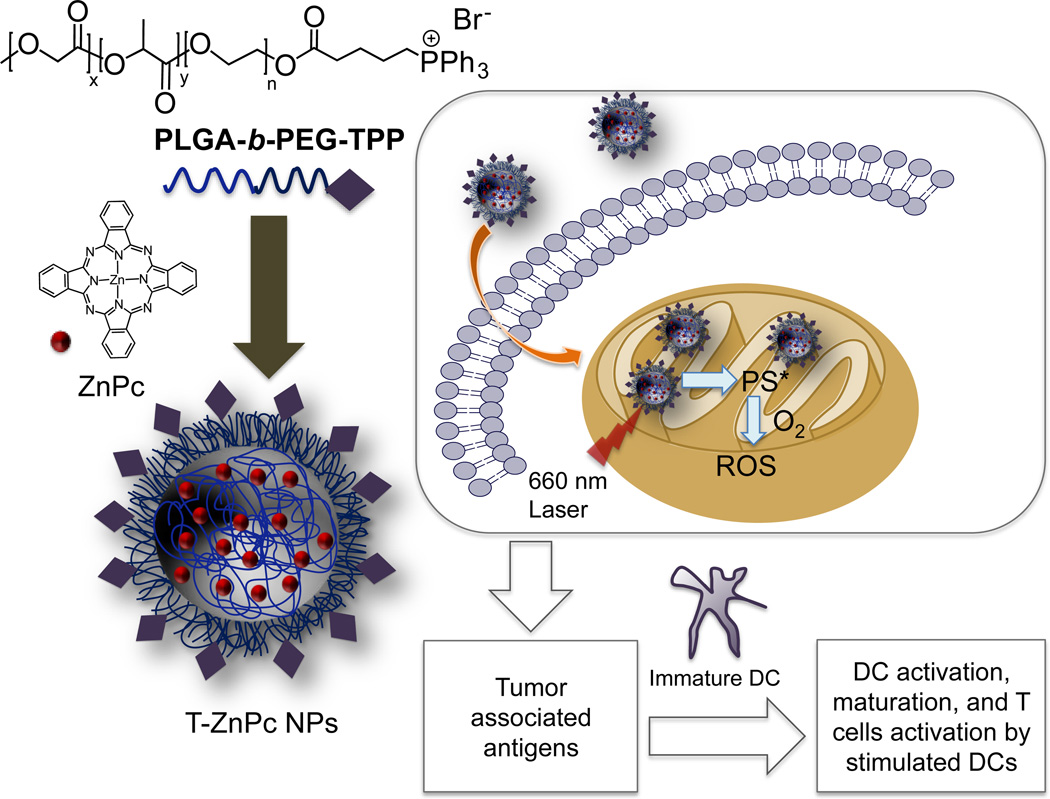
These biocompatible polymeric NPs were also used in mitochondria targeted photodynamic therapy (PDT). PDT has emerged as an anticancer tool that involves the use of a photosensitizer (PS) that is excited with the aid of light for production of singlet oxygen that damages cellular components and promote apoptosis. Zinc phthalocyanine (ZnPc) was entrapped inside the NPs of PLGA-b-PEG-TPP polymer to act on mitochondria upon irradiation (87, 92, 93). Efficacious delivery of ZnPc to the mitochondria produced singlet oxygen upon radiation locally inside the mitochondria to induce apoptosis and increased the potency. Tumor associated antigens (TAAs) generated from the treatment of breast cancer MCF-7 cells with targeted NPs loaded with ZnPc upon light irradiation activated dendritic cells (DCs) to generate high levels of interferon-gamma, a crucial cytokine considered as a product of T and natural killer cells (Figure 7). This noteworthy ex vivo DC stimulation ability of the TAAs generated from mitochondria-targeted PDT opens up the possibility of using mitochondria targeted light activated PS as possible vaccine for cancer.
Figure 7.
Schematic diagram for the construction of mitochondria targeted ZnPc-loaded NPs for PDT using a 660 nm laser light to produce ROS to causes cell death and subsequent activation of DCs using TAAs.
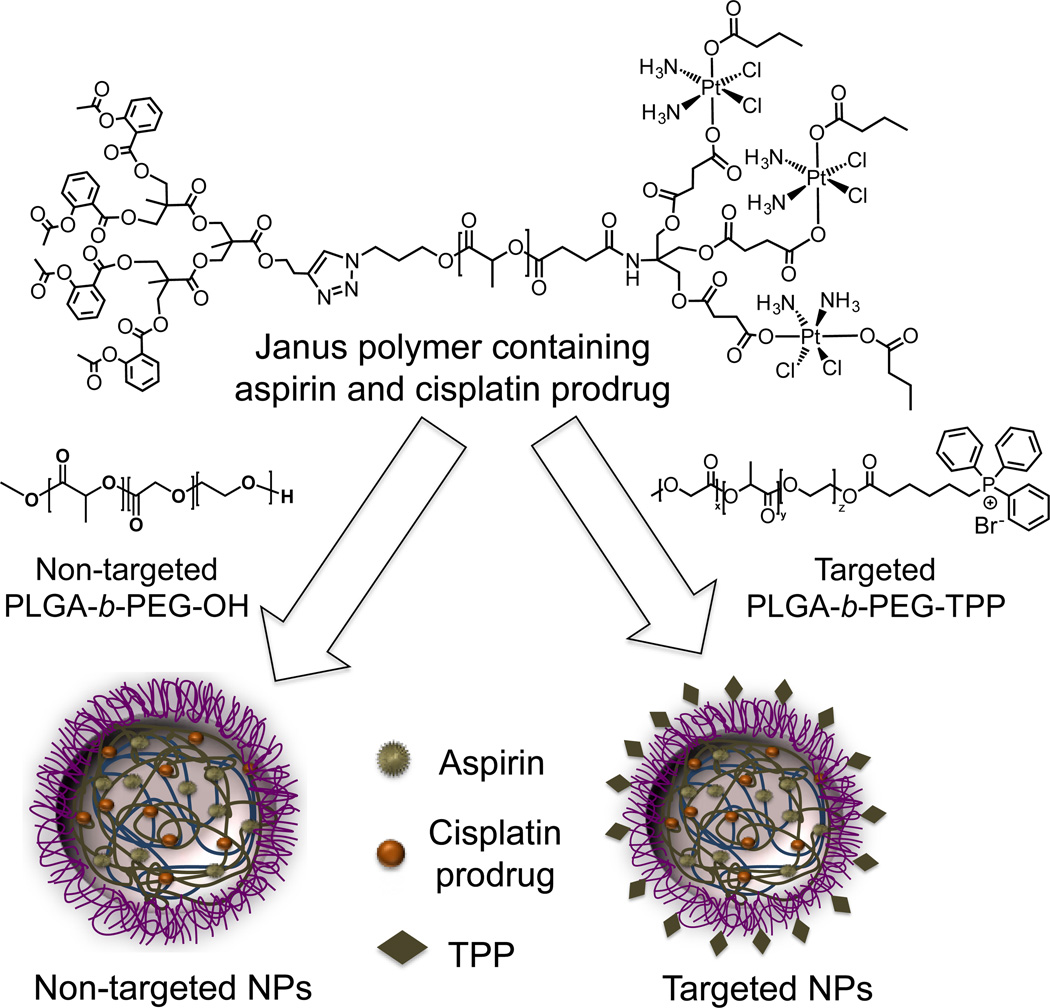
Very recently, this mitochondria targeted polymer was used to deliver a combination of cisplatin and aspirin in the mitochondria of prostate cancer cells (94) (Figure 8). We developed a Janus polymer containing a precisely controlled ratio of cisplatin prodrug and aspirin. The NPs obtained by blending this polymer with PLGA-b-PEG-OH demonstrated low cellular uptake profile. However, the blended NPs resulting from mitochondria-targeted PLGA-b-PEG-TPP and Janus polymer showed enhanced cellular uptake. We also demonstrated the use of this polymer in delivering anti-inflammatory agents such as aspirin for diseases where inflammation mediated processes are involved. Our recent work showed the use of this polymer to reduce inflammation in vivo using an aspirin analogue as an anti-inflammatory drug (95).
Figure 8.
Delivery of a combination of aspirin and a cisplatin prodrug using blended NPs constructed from a Janus polymer containing both the therapeutics and mitochondria targeted PLGA-b-PEG-TPP.
3. Mitochondrial Targets for Cardiovascular Diseases (CVD)
Heart diseases which include atherosclerosis, coronary heart disease (CHD), stroke, myocardial ischemia and reperfusion (IR) injury, myocardialinfarction, cardiomyopathy (CM), and chronic heart failure (HF), are the leading cause of mortality and morbidity worldwide (96). Most of these diseases are associated with cardiac cell death by apoptosis or necrosis, where mitochondria play an essential role (97–99). Necrosis is mainly triggered by opening of the mitochondrial permeability transition pore (mPTP) which results in swelling of the mitochondria and OMM rupture (100). ROS such as superoxide anions and hydroxyl radicals cause oxidation of proteins, membrane phospholipids and mtDNA, release of cyt c and apoptotic inducing factor (AIF), ultimately leading to apoptosis (101).
Atherosclerosis mainly contributes to CVD-related mortality and acute coronary syndrome (102–106). Many studies suggested that mitochondrial K+ channels have an important role in cardioprotection. The mechanism of cardioprotection, though unclear, may involve mild uncoupling of the mitochondrial membrane potential causing Ca2+ overload and ROS generation. Mild swelling resulting from influx of K+ into the mitochondria may also regulate the PT pore (107). Mitochondrial potassium sensitive ATP channel (mKATP) also plays critical role in cardioprotection. Complex II is related to mKATP in such a way that complex II inhibitors open mKATP, and mKATP openers inhibit complex II (108). A possible mechanism of cardioprotection could be the washout of the inhibitor that inhibits ROS generation and Ca2+ overload allowing a gradual reintroduction of the electron flux.
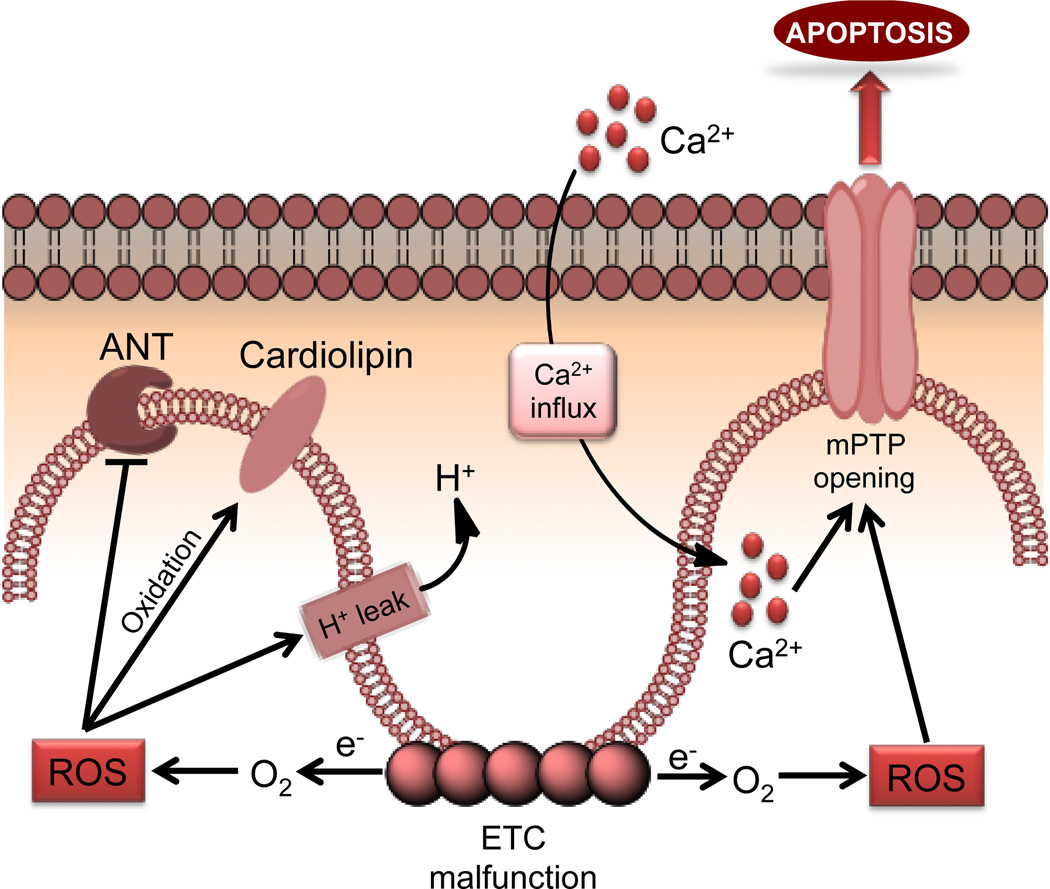
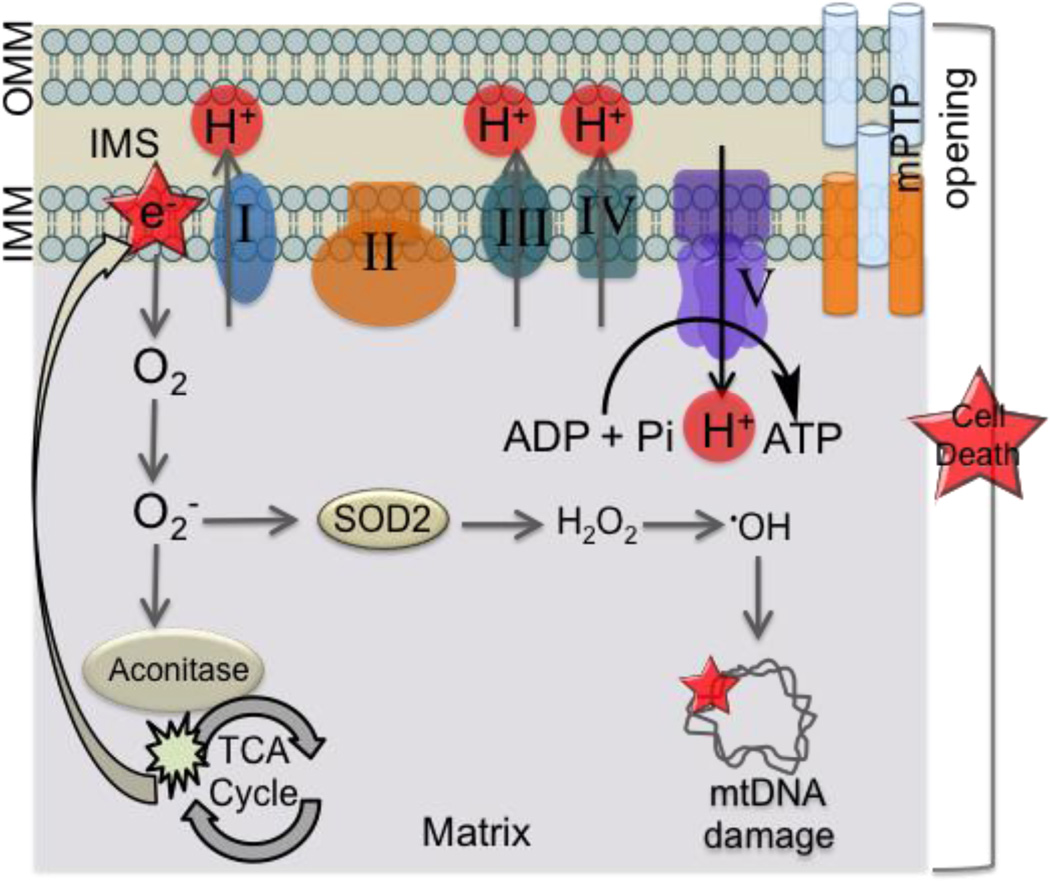
Cardiomyopathy is characterized by decreased cardiac function (107, 109–111). CM is the precursor to heart failure and acute CM is mainly caused due to myocardial ischemia. Cardiac remodeling and hypertrophy are often caused by permanent occlusion. The reperfusion phase of IR injury particularly injures mitochondria the most. Mitochondrial derangements that occur during the post-IR phase in the heart includes symptoms such as: (i) inhibition of respiratory complexes (112) and ANT (113), (ii) higher proton leak of the inner membrane (114), (iii) oxidation of CL and other associated membrane protein dysfunctions (115), (iv) excessive ROS generation (116), (v) opening of mPTP and consequent release of proteins such as cyt c (117), (vi) nucleotide depletion (118), and (vii) mitochondrial Ca2+ overload (Figure 9). Inefficient metabolic processes such as these lower the ATP and phosphocreatine levels and decreases the metabolic reserve and flexibility.
Figure 9.
Changes in mitochondrial functional pathways associated with cardiomyopathy.
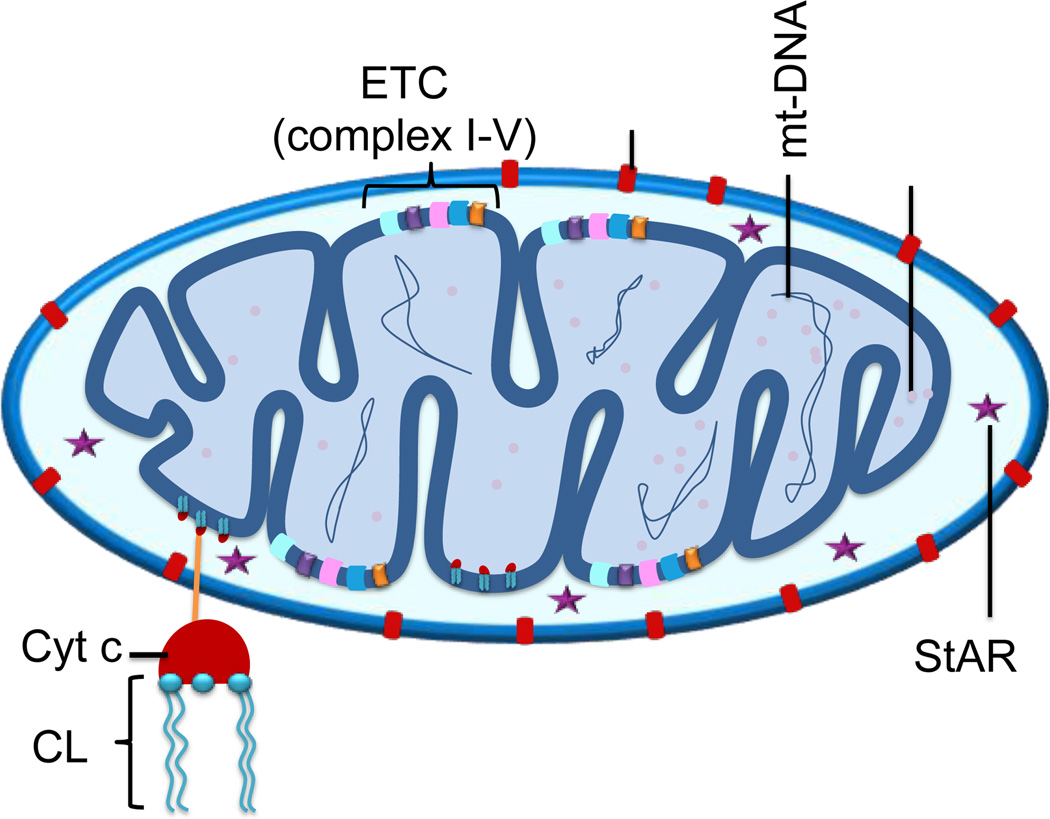
Here, we highlight the potential targets in the mitochondria for therapeutic interventions of CVDs (Figure 10).
Figure 10.
Possible targets located in the mitochondrion for CVDs. ETC-electron transport chain, VDAC-voltage dependent anion channel, mtDNA-mitochondrial DNA, StAR-steroidogenic acute regulatory protein, CL-cardiolipin, cyt c-cytochrome c.
3.1. Targets for CVD in the OMM
OMM separates the IMS from the cytosol. It is relatively permeable to solutes with mass up to 500 Da due to the VDAC protein porin (119). OMM regulates the metabolites, ions, and information exchange between mitochondria and cytosol, in which VDAC plays a critical role. Kay et. al. reported that the functional state of OMM is a sensitive indicator for ischemic damage of mitochondria (120). VDAC controls uptake of Ca2+ from cytosol to the mitochondria and the release of ROS from the mitochondria to the cytosol (121). The Ca2+ homeostasis is indispensable for cardiac function. The loss of Ca2+ homeostasis causes contractile dysfunction and arrhythmias in failing myocardium, leading to HF (122–124). ROS (e.g. superoxide) are closely related to pathological conditions, such as inflammation, IR injury, and drug metabolism (125–127). VDAC is a rational target in attenuating HR and IR by inhibiting cardiomyocyte apoptosis. Salnikov. et al. reported activity of gold nanoparticles (AuNPs) upon the OMM of ventricular cells and isolated cardiac mitochondria of rats (128). They demonstrated that 3 nm but not 6 nm particles could penetrate the OMM and accumulate in IMS via VDAC. The inhibitors of VDAC such as König polyanion and 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid can prevent this permeabilization activity. VDAC closes during the apoptosis cascade and inhibits the release of ATP or uptake of ADP and respiratory substrates from the cytosol to mitochondrion (119). The VDAC inhibitors, such as BH4 derivative peptides (129, 130), ruthenium red (RuR) (131), ruthenium amine binuclear complex (Ru360) (132), fluoxetine (133), and modulators of Ca2+ regulation can be potential therapeutic agents for heart-related disease caused by accelerated cardiac cell apoptosis.
3.2. Targets for CVD in the IMS
Redox control in the IMS contributes to various pathways in the mitochondria, including apoptotic signaling, assembly of respiratory chain components, antioxidant activation (134). Loss of redox control by ROS may lead to initial apoptotic cascades and dysfunction of respiratory and energetic systems. Cardiomyopathy and HF may be caused by the ROS in this compartment in cardiac cells. Mitochondrial cholesterol transporter, steroidogenic acute regulatory protein (StAR) binds to cholesterol mainly at the OMM and shuttling cholesterol from OMM to IMM for steroid biosynthesis (135, 136). This transportation process is the rate limiting step for steroidogenesis and its disruption may lead to atherosclerosis (137, 138). Therefore, the ROS in IMS and StAR may be used as targets for cardiomyopathy and atherosclerosis. Several NP-based delivery systems show promising approaches to cardiomyopathy, ischemic stroke, and HF (139–141). Cerium and yttrium oxide NPs are known to show direct antioxidants properties to protect cells from a variety of oxidative stress (142). For example, Niu et. al. reported efficiency of CeO2 NPs in inhibition of progressive cardiac dysfunction and remodeling using an ischemic cardiomyopathy murine model, in which CeO2 NPs acted as effective free radical scavengers to block oxidative stress (143). In their studies, CeO2 NPs were intravenously injected via tail vein into 5-week old monocyte chemoattractant protein (MCP)-1 mice. The CeO2 NP treatment showed inhibition of progressive left ventricular dilatation and dysfunction. Quantitative analysis of anti-3-nitrotyrosine antibody indicated that CeO2 treatment interrupted peroxynitrite, a marker of oxidative stress, formation in the myocardium of MCP-1 mice. The CeO2 NPs also demonstrated attenuated levels of NOx in terms of total nitrated protein in the serum, indicating reduced myocardial oxidative stress. They proposed that CeO2 NPs inhibited the expression of endoplasmic reticulum stress-associated genes at mRNA and protein levels, attenuating apoptosis in the myocardium.
3.3. Targets for CVD in the IMM
Mitochondrial electron transport is a major subcellular source of ROS in the failing myocardium because of the extreme abundance of mitochondria in cardiac myocytes (101). The regulation of CL plays significant roles in optimal activity of mitochondrial enzymes, cholesterol translocation by activating the mitochondrial cholesterol side chain cleavage, energy metabolism, and apoptosis (144). The alteration of CL is associated with CVD. It was reported that the loss of CL in the heart is correlated to the development of HF (145). Oxidation of CL results in decrease of OXPHOS and HF on reperfusion, which could be hampered or attenuated by melatonin (146). The preservation of CL contents demonstrated to have less alteration of mitochondrial complex I and III, reduced oxidative damage, and enhanced function of heart. CL is susceptable to peroxidative attack due to the presence of unsaturated double bonds and the proximity to ETC proteins. The ROS induced peroxidation of CL results in dissociation of cyt c from IMM, causing apoptosis (147) which is associated with heart ischemia (148). The oxidized CL (oxCL) produces natural occurring antibodies, which allows to act in the first line defense to invade pathogens during CVD (149). Thus, CL can be a possible target for the application of nanotechnology tools to combat CVD.
The ATP generation is essential for maintaining cardiac function. Mitochondrial respiratory and ATP synthase activity are implemented in the decrease of cardiomyocytes in human and dog with chronic HF (122, 150). HF can be regulated by F1F0-ATPase in the IMM. The F1F0-ATPase normally promotes ATP synthesis, but it hydrolyzes ATP under ischemic conditions. Excessive ATP hydrolysis decreases the cardiac energy reserve leading to HF (151). Grover et al. reported selective F1F0-ATPase inhibitor BMS-199264 with significant cardio-protective profile by reducing ATP hydrolysis (152). Thus, the F1F0-ATPase can be a target for nanotechnology-based tools against HF and IR.
The mPTP inhibitors, such as cyclosporin-A (CsA) (153, 154), cyclophilin-D (155), cinnamic anilides (156), and antioxidants are known to prevent the mPTP opening. Yin et al. reported a NP system containing CsA which when used in combination with adipose-derived stem cell (ASCs) in a swine model of myocardial infarction showed therapeutic benefits (157).
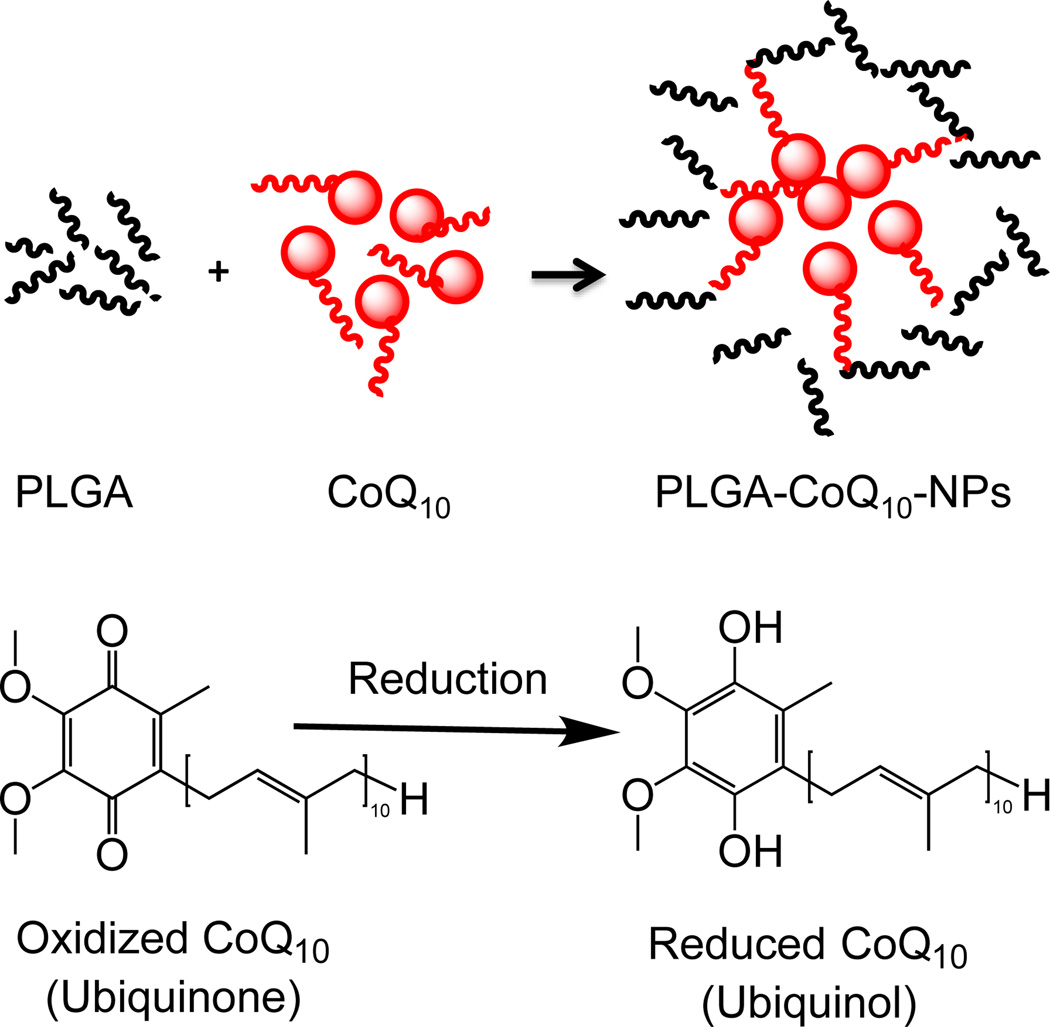
CoQ10 has attracted great interest in the clinical studies for myocardial function and heart failure (158, 159). CoQ10 effectively counteracts oxidative damage in IMM and improves mitochondrial efficiency, making myocardium more tolerated to hypoxia/reoxygenation stress (160, 161). PLGA-CoQ10 NPs of <100 nm were successfully prepared by an emulsion-diffusion-evaporation method, which demonstrated enhanced oral bioavailability and anti-inflammatory activity compared to free CoQ10 (Figure 11) (162). Recently, Yamada et al. reported CoQ10 delivery using MITO-Porter, a liposomal delivery system, to target mitochondria for preventing IR injury (163). The CoQ10-MITO-Porter was intravenously injected in the hepatic IR injury mice model. The MITO-Porter was in vivo confirmed to accumulate in the mitochondria of the liver. The fusogenic properties of MITO-Porter make it possible for CoQ10 to target IMM. The delivery of CoQ10 to liver mitochondria inhibits the IR injury by preventing the ROS levels. Therefore, delivery of CoQ10 to the mitochondrial using NP-based platforms can be promising therapeutic method for CVD.
Figure 11.
Schematic diagram of CoQ10-NPs and its reduction process.
The lipophilic benzoquinone based electron carrier, idebenone (IDB), is a synthetic analogue of CoQ10. IDB acts as a free radical scavenging molecule and can thus function as an antioxidant (164). It is insoluble in water and shows limited bioavailability after oral administration. To obtain enhanced solubility and increased bioavailability of IDB, NPs were constructed using various stabilizers including hydrophilic polymers, amphiphilic polymers, and surfactants such as iabrasol, Brij 35, Pluronic F-68, Tween 80, sodium lauryl sulphate and hydroxypropyl methylcellulose (165). Doxorubicin induced cardiotoxicity characterized by decreased body weight and heart weight was supplemented by these NPs (50 mg/kg) resulting improved body and heart weight.
3.4. Targets for CVD in the MM
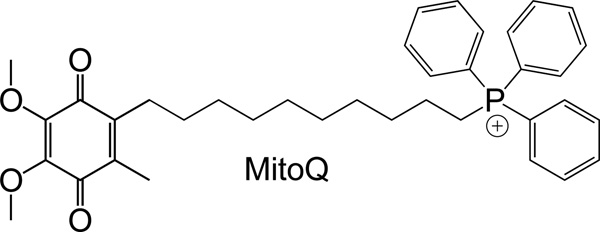
MitoQ is one of the antioxidants that works in the MM with its ability to accumulate in the mitochondrial matrix. It was designed to mimic the endogenous antioxidant CoQ10 and has been intensively studied as a mitochondria-targeted antioxidant to decrease ROS and oxidative damage (Figure 12) (17, 166–172). Bennett and co-workers reported antioxidative property of MitoQ in atherosclerosis and metabolic syndrome models (173). In their studies, MitoQ was orally administered for 14 weeks in fat-fed apolipoprotein knock out (ApoE−/−) and ataxia telangiectasia mutated (ATM)+/−/ApoE−/− mice models. MitoQ treated ATM+/+/ApoE−/− and ATM+/−/ApoE−/− mice showed reduced serum levels of total cholesterol, triglyceride, and low-density lipoprotein (LDL). Additionally, MitoQ treated animals demonstrated decreased plaque numbers and proliferation, and reduced mtDNA damage in the liver. The metabolic changes in mice with MitoQ may be attributed to the less oxidative damage via redox signaling pathways.
Figure 12.
Structure of MitoQ.
Recently, Yu et. al. reported that the mtDNA damage promotes atherosclerosis and plaque vulnerability (174). The mtDNA defects led to decreased expressions of respiratory complexes and reduced mitochondrial respiration in vascular smooth muscle cells, monocyte/macrophages, and other organs. Apoptosis and inhibition of cell proliferation along with the storage of adipose tissue promoted atherosclerosis. Furthermore, mutation of mitochondrial tRNA and/or mtDNA are associated with CHD and cardiomyopathy (175–177). Cardiac aging in mice is correlated to mtDNA mutations and deletions (178). Hence, gene therapy using NP platforms for mtDNA and/or mtRNA (179) might be effective methods to inhibit CVD.
Park et al. developed a H2O2-responsive anti-oxidant polymer, polyoxalate containing vanillyl alcohol (PVAX), with abilities to rapidly scavenge H2O2 and release vanillyl alcohol with anti-oxidant, anti-inflammatory, and antiapoptotic properties (141). They demonstrated that these nanoparticles exerted anti-oxidant and anti-inflammatory activities. Using DOX-induced cardiac and hepatotoxicity models, this work demonstrated that administration of PVAX NPs significantly inhibited ROS production and pro-inflammatory signals in the liver and heart of DOX-treated mice.
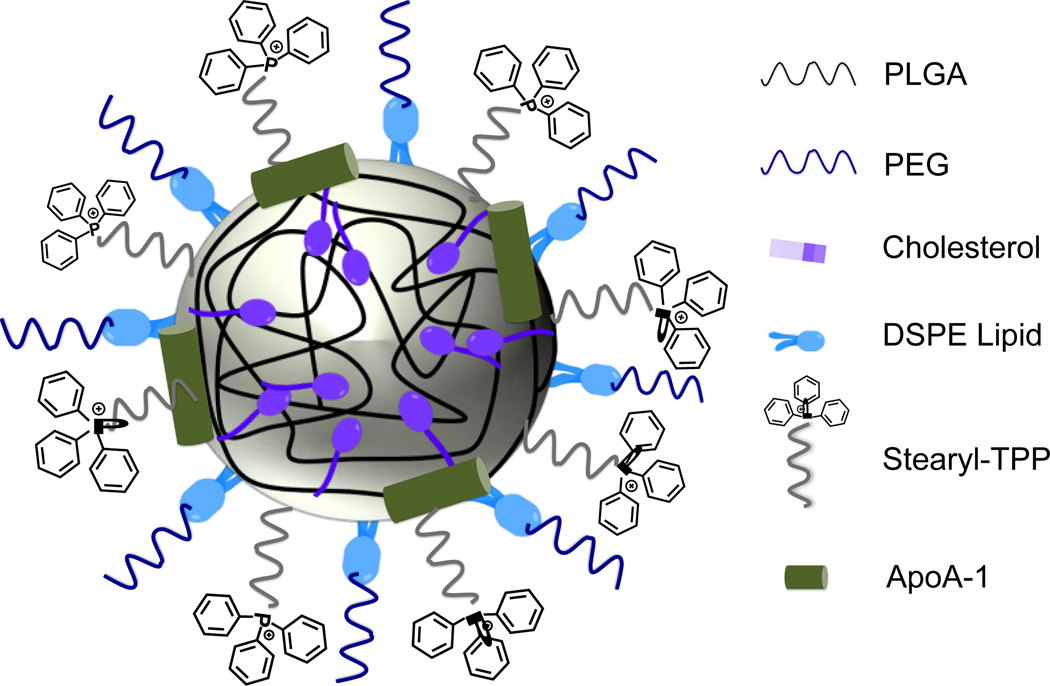
High density lipoprotein (HDL) based delivery vehicles are used for drugs and imaging agents (180). However, reconstituted HDL-based delivery systems often face the compatibility issues. Recently, our lab developed a mitochondria targeted completely synthetic HDL mimicking NP for atherosclerosis (181). This HDL mimic consists a core of biodegradable PLGA and cholesteryl oleate, a phospholipid coating layer ornamented with TPP cations for mitochondria targeting, and this lipid layer was further decorated with apolipoprotein A-I (apoA-I) mimetic L-4F peptide (Figure 13). Targeted NP-treated cells indicated the presence of the NPs mainly in the MM and the IMS; small amounts of NPs were found in the IMM or the OMM. In vivo studies in Sprague-Dawley rat indicated that the targeted HDL synthetic NPs were majorly distributed in the heart within 24 h. The low dose of NPs (10 mg/kg) showed reduced lipid profile levels of cholesterol and triglyceride. This platform is promising for the plaque imaging and therapy of CVD.
Figure 13.
Schematic diagram of the mitochondria-targeted biodegradable synthetic HDL with various components.
4. Targets for Mitochondrial Dysfunction Related Diseases
Absence of effective DNA repair machinery in the mitochondria enhances the impacts of radical-induced mtDNA damage. At birth, all mtDNA copies in an individual are identical and this is genetically termed as homoplasmy. Ramification of mitochondria with a mixture of mutant and wild type mtDNA known by the genetic term “heteroplasmy” result in several diseases (182, 183). When the weightage of the mutant mtDNA exceeds a critical threshold level, cells show the abnormalities in the mitochondrial respiratory chain and this event is know as the threshold effect (184). Events associated with initial electron leak further increase the extent of electron leakage resulting in a vicious cycle. The leaked electrons and the radicals formed alter the integrity of the mitochondrial membrane. These events together cause further dysfunctions resulting in disruption of cellular energy production and aged cells. Thus, any abnormality in the mitochondria can hamper tissue-specific activities performed by this important organelle and result in mitochondrial dysfunction related disorders. A number of mitochondrial dysfunction related diseases are discussed below.
4.1. Autism Spectrum Disorders
Autism spectrum disorders (ASD) comprise of a set of neurodevelopmental ataxia that is recognized by behavioral changes including difficulties in communication and social intercommunication and repetitive behaviors. Nearly two decades ago, Coleman and Blass suggested that patients with ASD may exhibit abnormal carbohydrate metabolism (185). ASD was also related to the abnormality in the brain bioenergetics with impaired mitochondrial functions (186). A more recent controlled study suggested that mitochondrial dysfunctions may be present in up to 80% of children with ASD (187). Studies also suggested that mitochondrial dysfunctions and oxidative stress are closely associated. For example, a decrease in the mitochondrial function along with a simultaneous increase in biomarkers of oxidative DNA damage and decreased superoxide dismutase 2 (SOD2) activity were found in temporal lobe brain samples (BA 21) (188). In another study, markers associated with oxidative stress in brain tissue were observed alongside reduced ETC complex activities in the autistic children (189). A higher level of oxidative damage to mitochondrial proteins is also observed in the autistic patients. The oxidative stress markers are also related with the functioning of TCA enzyme, aconitase, and GSH/GSSG in the cerebellum and the temporal lobe (190).
4.2. Mitochondrial Dystrophy
Muscular dystrophies (MD) comprise of a heterogeneous group of genetic diseases identified by gradual degeneration and weakness of skeletal muscle. Among the several types of MD that exist, Duchenne muscular dystrophy (DMD) and Becker Muscular Dystrophy (BMD) are the two most commonly found ones (191). Both of these are caused by the mutations to the dystrophin gene that is located on the p arm of the X chromosome (192). Dystrophin protein is responsible for contraction of the myofibrils and membrane stability. Ca2+ dysregulation is implicated in MD. Since mitochondria can function as a low affinity, high capacity Ca2+ buffer system, Ca2+ dysregulation promotes Ca2+ overload of mitochondria and thus mitochondrial dysfunction. Lack of dystrophin is believed to cause membrane fragility and give rise to micro-tears in the sarcolemma. Consequently, an abnormal Ca2+ influx and mitochondrial Ca2+ overload triggers the mitochondrial permeability transition via the formation of large pore proteins spanning both inner and outer mitochondrial membranes.
Mitochondria are the main source of intracellular ROS. At low Ca2+ concentrations, the mPTP gets activated and as a result of the partial mitochondrial depolarization there is a decrease in ROS production. At high Ca2+ concentrations, there is a loss of cyt c and progressive gating of electron flow via the ETC and the mitochondria rapidly transitions to a non-equilibrium state and as a consequence of which the mPTP gets activated and the ROS levels increase (193). Studies have suggested that there is substantial decrease in the expressions of mitochondrial complexes I, III, IV and V (194). Increased levels of cyclophilin D results in an increase in sensitivity of Ca2+ induced activation of the mega channel/mPTP (195). Mdx mice, a model of DMD, when treated with the cyclophilin inhibitor Debio-025 showed reductions in mitochondrial swelling and the disease associated necrotic manifestations.
Antisense therapy involving the use of antisense oligonucleotides (AONs) is a powerful tool for introducing post-transcriptional modifications and thereby regulating disease related target genes (196). However, naked AONs suffer limited bioavailability, poor cell trafficking, and endosomal entrapment (197). Polymeric NPs composed of PEG, poly(isobutyl cyanoacrylate) (PIBCA), PLGA are used in a number of examples to deliver AONs (196). Inorganic nanomaterials such as gold, silver, silica, calcium phosphate based carriers are also used to design hybrid systems to deliver AONs (196). Recently, rapamycin NPs have shown to target defective autophagy in MD (198). These agents do not have mitochondria specific therapeutic targets and are thus beyond the scope of this article.
4.3. Mitochondrial Dysfunctions in Epilepsy
Epilepsy is typically characterized by recurrent seizures, which consists of synchronized discharges of a large groups of neuronal cells that disrupt the normal functioning of the brain (199). Mitochondrial dysfunction could be one of the potential causes of epileptic seizures (199). A number of factors which get altered during epileptogenesis are shown in Figure 14. Evidences also indicate that epilepsy may be a consequence of free radicals, oxidative stress and mitochondrial dysfunction (200). Severe impairment in activity of the respiratory chain complex I was observed (201). Superoxide radicals are produced in considerable amounts during inhibition of the respiratory chain that can overload the endogenous ROS scavenging mechanisms resulting in oxidative damage to mitochondrial proteins, phospholipids and DNA. Also, recent evidences show that there is a direct relation between mitochondrial oxidative stress and DNA damage that occur during the various phases of epileptogenesis (202).
Figure 14.
Changes of mitochondrial properties during the progression of epileptogenesis.
A wide range of liposomes, polymeric nanoparticles, solid nanoparticles and metallic NPs are employed to deliver a number of anti-epileptic drugs (AEDs) (203). Liposomes were used to deliver AEDs such as gamma-aminobutyric acid and phenytoin to show suppressed epileptic seizures (203). Polymeric NPs with advantages of higher stability, controlled drug release, and higher circulatory half-life were also used to deliver AEDs such as thyrotropin-releasing hormone, clonazepam (204), ethosuximide (205), valproate (206), loperamaide (207), phenytoin (205, 208), and carbamazepine (205).
As discussed before, ROS is well known etiological agent in epilepsy. Hidekatsu et al. successfully prolonged the anticonvulsant effect in amygdaloid-kindled rats by the use of a superoxide dismutase-liposomal formulation (209). Kizelsztein et al. observed controlled release of tempamine, a potential antioxidant, using a PEGylated liposomes in an animal model (210). β-Carotene is an antioxidant known for its epileptic convulsions. Bioavailability and stability of polysorbate-80-coated β-carotene nanoparticles for epileptic convulsions in mice models was found to be higher when compared to unmodified β-carotene (49).
4.4. Amyotrophic Lateral Sclerosis
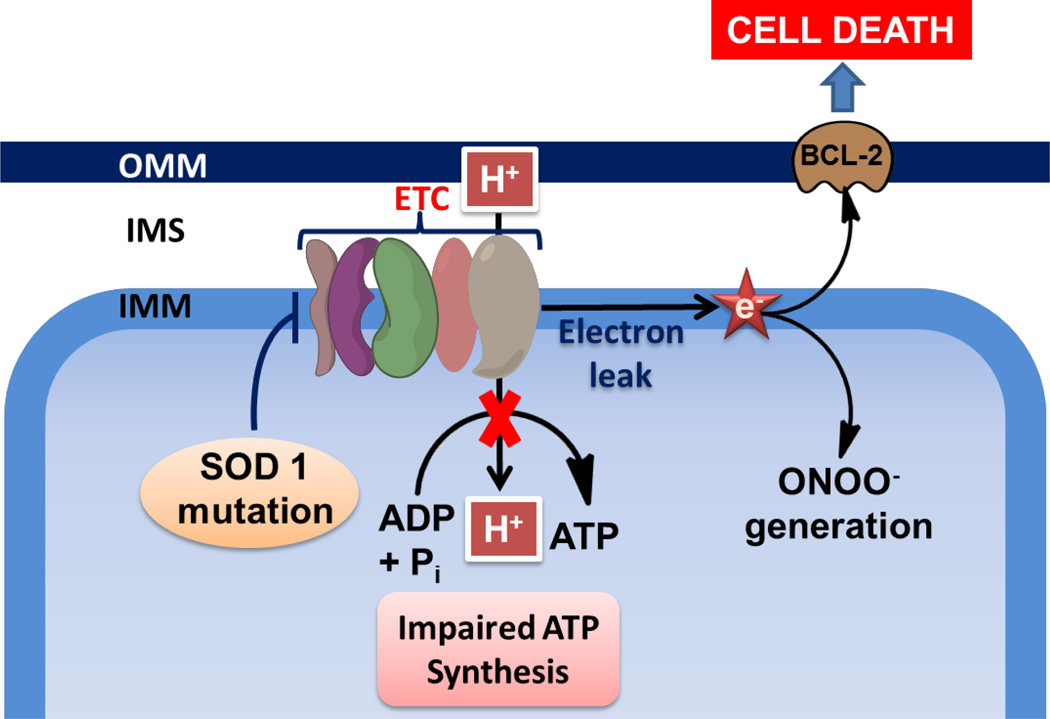
Amyotrophic lateral sclerosis (ALS) arises from selective degeneration of the cortical motor neurons and anterior horn cells of the spinal cord. In this disease, a mutation of superoxide dismutase 1 (SOD1) causes a defect in the overall motor neuron dynamics, subsequently affecting the transport of mitochondria within neuronal cells (211). Evidence suggests that increased levels of SOD1 expression directly influence up-regulation of more than 50 mitochondrial proteins, including mitofusin 2 (Mfn2). This indicates that, in addition to destruction of ROS, SOD1also acts as a feedback regulator of membrane potential within mitochondria (211). The changes associated with mitochondria in ALS are presented in Figure 15. Mutation in the SOD1 protein leads to misfolding, which directs its translocation into the mitochondrial intermembrane space causing aggregation. The mutant SOD1 protein is also the causative agent for cell death in ALS models of disease (212).
Figure 15.
Mitochondrial changes during ALS.
Therapeutic approaches for ALS therapy include, vaccines targeting the epitopes of misfolded protein, adaptive immune neuroprotection, stem cell therapy as well as recent emerging applications of nanotechnology in neuroscience (213). The nascent approach of nanotechnology in this field have focused in part on regulating ROS production, since several results suggest that the loss of major neuronal portion in ALS is mainly due to the damages caused by ROS production. Recently PLGA-NPs carrying SOD1 to neurons was reported to provide protection against hydrogen peroxide-induced oxidative stress in human neurons (214). SOD-like mimicking activity was observed with carboxyfullerenes which demonstrated increased reactivity to superoxide radicals and a high cell permeability, and exhibited neuroprotective ability in vitro (215). In another study, a reactive product of lipid peroxidation, known as acrolein, was identified in the motor neurons of patients with ALS as well as in the brains of patients with Alzheimer’s diseases. In the same study, mesoporous silica NPs loaded with hydralazine and functionalized with PEG were able to rescue acrolein-mediated apoptosis in vitro (216). AuNPs modified with SOD1 monomer and utilizing colorimetric detection system was developed for diagnosis of ALS (217). Thus, nanomedicine offers the potential to improve effective diagnosis and treatment of ALS.
5. Mitochondrial Targets for Neurological Diseases
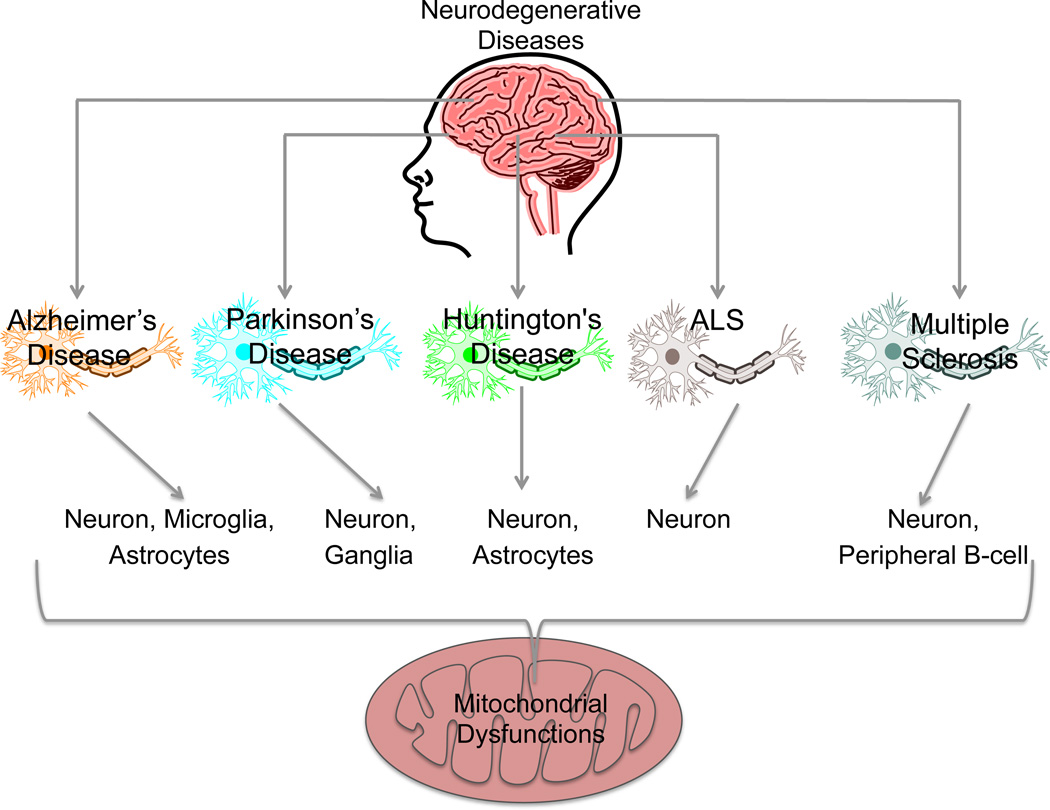
Mitochondrial dysfunctions leads to severe neurological disorders (218). A number of diseases where mitochondrial dysfunctions play significant roles along with the cell type that suffer from these dysfunctions are depicted in Figure 16. Neurons are highly dependent on OXPHOS as they do not have the ability to multiply and regenerate. Thus, any dysfunction and communication gap can cause severe defects in neuronal functions (219). Oxidative stress localized in the mitochondria, disrupts calcium homeostasis as well as apoptosis, contributing to neurological diseases. These diseases include Alzheimer's disease (AD), Parkinson's, and Huntington's diseases, stroke, ALS, and psychiatric disorders (220). Defects in mitochondrial gene are also the major cause of syndromes which include: chronic progressive external ophthalmoplegia; Kearns-Sayre syndrome of chronic progressive external ophthalmoplegia, retinitis pigmentosa, sensory-neural deafness and cardiac conduction disturbances, Leber's hereditary optic neuropathy, syndrome of mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes, myoclonic epilepsy with ragged red fibers, and neuropathy with ataxia and retinitis pigmentosa (221).
Figure 16.
Mitochondrial dysfunctions in different neurodegenerative diseases along with the cell types that suffer from mitochondrial dysfunctions.
Current research assessments of mitochondrial biogenesis, mitophagy, and dynamics suggest that mitochondrion as an organelle, exhibits movement as part of neuroprotective strategies, both in case of neural disease and injuries (222). The application of nanotechnology in design and development of therapeutic materials for the treatment neurodegenerative diseases in particular where mitochondria is the target is at the preliminary stages of development. Despite this, recently several impressive body of research and technologies were developed, however this particular area is beyond the scope of the current review.
6. Mitochondrial Nanomedicine: The Future
As the mitochondria play central roles in life and death processes of cells, their abnormalities are responsible for various diseases. Nanotechnology-based toolbox can provide unique approach to target the mitochondria of diseased cells to provide “precision medicine” for better management of these diseases. The recent developing NP-based strategies have shown great promise for targeting specific sites of organ, tissue, or cells; thus, the ability to use nanotechnology may provide unexplored therapeutic efficacies when targeted to subcellular organelle such as the mitochondria. We must acknowledge that the use of nanotechnology in targeting such a complex and dynamic organelle as mitochondria can be a difficult task to achieve; however, the early advances as shown in this review is promising and encouraging. However, careful examinations of targeting properties of nanomaterials need to be critically explored to take advantage of these unique materials and their properties for subcellular targeting. Most of the reported works mainly focus on evaluation of targeting abilities of nanomaterials by fluorescence imaging. Mitochondrial association properties of such nanodelivery vehicles should be interpreted with caution. One needs to adopt multiple complementary techniques to understand the association properties of delivery vehicles with the mitochondria. Looking ahead, it is clear if carefully executed, nanotechnology based tools can provide novel alternatives for diseases where dysfunctional mitochondria are involved.
Acknowledgments
This work was supported, in whole or in part, by the Department of Defense Prostate Cancer Idea award (W81XWH-12-1-0406); American Heart Association National Scientist Award (14SDG18690009); National Heart, Lung, and Blood Institute of National Institutes of Health (NIH) under award number R56HL121392; National Institute of Neurological Disorders and Stroke of NIH under award number R01NS093314 to S.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: S.D. discloses financial interest in Partikula LLC; Partikula did not support the aforementioned work.
References
- 1.Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981;91:227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 3.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 4.Palade GE. The fine structure of mitochondria. Anat Rec. 1952;114:427–451. doi: 10.1002/ar.1091140304. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 7.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathak RK, Kolishetti N, Dhar S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:315–329. doi: 10.1002/wnan.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G. Mitochondria in cancer. Oncogene. 2006;25:4630–4632. doi: 10.1038/sj.onc.1209589. [DOI] [PubMed] [Google Scholar]
- 10.Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Adv Exp Med Biol. 2012;942:287–308. doi: 10.1007/978-94-007-2869-1_13. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan LB, Chandel N. Mitochondria and cancer. Cancer Metab. 2014;2:4. doi: 10.1186/2049-3002-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indran IR, Tufo G, Pervaiz S, Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. BBA-Bioenergetics. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of Coupling of Oxidative Phosphorylation and the Membrane Potential of Mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 14.Skulachev VP, Antonenko YN, Cherepanov DA, Chernyak BV, Izyumov DS, Khailova LS, et al. Prevention of cardiolipin oxidation and fatty acid cycling as two antioxidant mechanisms of cationic derivatives of plastoquinone (SkQs) BBA-Bioenergetics. 2010;1797:878–889. doi: 10.1016/j.bbabio.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Neuzil J, Dong L-F, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13:199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 16.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 17.Tauskela JS. MitoQ--a mitochondria-targeted antioxidant. IDrugs. 2007;10:399–412. [PubMed] [Google Scholar]
- 18.Blanco E, Hsiao A, Ruiz-Esparza GU, Landry MG, Meric-Bernstam F, Ferrari M. Molecular-targeted nanotherapies in cancer: enabling treatment specificity. Mol Oncol. 2011;5:492–503. doi: 10.1016/j.molonc.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol. 2010:55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 20.Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin Drug Deliv. 2015:1–15. doi: 10.1517/17425247.2015.1018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrache S, Pathak RK, Dhar S. Formulation and optimization of mitochondria-targeted polymeric nanoparticles. Methods Mol Biol. 2015;1265:103–112. doi: 10.1007/978-1-4939-2288-8_8. [DOI] [PubMed] [Google Scholar]
- 22.Barry NPE, Sadler PJ. Challenges for Metals in Medicine: How Nanotechnology May Help To Shape the Future. ACS Nano. 2013;7:5654–5659. doi: 10.1021/nn403220e. [DOI] [PubMed] [Google Scholar]
- 23.Marrache S, Pathak RK, Darley KL, Choi JH, Zaver D, Kolishetti N, et al. Nanocarriers for tracking and treating diseases. Curr Med Chem. 2013;20:3500–3514. doi: 10.2174/0929867311320280007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrache S, Dhar S. The energy blocker inside the power house: mitochondria targeted delivery of 3-bromopyruvate. Chem Sci. 2015;6:1832–1845. doi: 10.1039/c4sc01963f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuelson LE, Dukes MJ, Hunt CR, Casey JD, Bornhop DJ. TSPO Targeted Dendrimer Imaging Agent: Synthesis, Characterization, and Cellular Internalization. Bioconjugate Chem. 2009;20:2082–2089. doi: 10.1021/bc9002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Zhang C-X, Wang X-X, Zhang L, Ma X, Zhou J, et al. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials. 2013;34:3366–3380. doi: 10.1016/j.biomaterials.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 27.Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas S, Dodwadkar NS, Sawant RR, Koshkaryev A, Torchilin VP. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. J Drug Target. 2011;19:552–561. doi: 10.3109/1061186X.2010.536983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Zhao W-Y, Ma X, Ju R-J, Li X-Y, Li N, et al. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 2013;34:3626–3638. doi: 10.1016/j.biomaterials.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 30.Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials. 2012;33:4773–4782. doi: 10.1016/j.biomaterials.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang L, Li L, He X, Yi Q, He B, Cao J, et al. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials. 2015;52:126–139. doi: 10.1016/j.biomaterials.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Zupančič Š, Kocbek P, Zariwala MG, Renshaw D, Gul MO, Elsaid Z, et al. Design and development of novel mitochondrial targeted nanocarriers, DQAsomes for curcumin inhalation. Mol Pharm. 2014;11:2334–2345. doi: 10.1021/mp500003q. [DOI] [PubMed] [Google Scholar]
- 33.Yamada Y, Harashima H. Delivery of bioactive molecules to the mitochondrial genome using a membrane-fusing, liposome-based carrier, DF-MITO-Porter. Biomaterials. 2012;33:1589–1595. doi: 10.1016/j.biomaterials.2011.10.082. [DOI] [PubMed] [Google Scholar]
- 34.Yasuzaki Y, Yamada Y, Harashima H. Mitochondrial matrix delivery using MITO-Porter, a liposome-based carrier that specifies fusion with mitochondrial membranes. Biochem Bioph Res Co. 2010;397:181–186. doi: 10.1016/j.bbrc.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa R, Yamada Y, Kawamura E, Harashima H. Mitochondrial delivery of antisense RNA by MITO-Porter results in mitochondrial RNA knockdown, and has a functional impact on mitochondria. Biomaterials. 2015;57:107–115. doi: 10.1016/j.biomaterials.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Wang XX, Li YB, Yao HJ, Ju RJ, Zhang Y, Li RJ, et al. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials. 2011;32:5673–5687. doi: 10.1016/j.biomaterials.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Lyrawati D, Trounson A, Cram D. Expression of GFP in the mitochondrial compartment using DQAsome-mediated delivery of an artificial mini-mitochondrial genome. Pharm Res. 2011;28:2848–2862. doi: 10.1007/s11095-011-0544-0. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Szoka FC. Mechanism of DNA Release from Cationic Liposome/DNA Complexes Used in Cell Transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 39.Theodossiou TA, Sideratou Z, Katsarou ME, Tsiourvas D. Mitochondrial delivery of doxorubicin by triphenylphosphonium-functionalized hyperbranched nanocarriers results in rapid and severe cytotoxicity. Pharm Res. 2013;30:2832–2842. doi: 10.1007/s11095-013-1111-7. [DOI] [PubMed] [Google Scholar]
- 40.Malhi SS, Budhiraja A, Arora S, Chaudhari KR, Nepali K, Kumar R, et al. Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. Int J Pharm. 2012;432:63–74. doi: 10.1016/j.ijpharm.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Yoong SL, Wong BS, Zhou QL, Chin CF, Li J, Venkatesan T, et al. Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials. 2014;35:748–759. doi: 10.1016/j.biomaterials.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 42.Marrache S, Pathak RK, Dhar S. Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc Natl Acad Sci U S A. 2014;111:10444–10449. doi: 10.1073/pnas.1405244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boddapati SV, D‘Souza GGM, Erdogan S, Torchilin VP, Weissig V. Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett. 2008;8:2559–2563. doi: 10.1021/nl801908y. [DOI] [PubMed] [Google Scholar]
- 44.Patel NR, Hatziantoniou S, Georgopoulos A, Demetzos C, Torchilin VP, Weissig V, et al. Mitochondria-targeted liposomes improve the apoptotic and cytotoxic action of sclareol. J Liposome Res. 2010;20:244–249. doi: 10.3109/08982100903347931. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Li R-J, Ying X, Tian W, Yao H-J, Men Y, et al. Targeting therapy with mitosomal daunorubicin plus amlodipine has the potential to circumvent intrinsic resistant breast cancer. Mol Pharm. 2011;8:162–175. doi: 10.1021/mp100249x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Yao H-J, Yu Y, Zhang Y, Li R-J, Ju R-J, et al. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33:565–582. doi: 10.1016/j.biomaterials.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 47.Mo R, Sun Q, Xue J, Li N, Li W, Zhang C, et al. Multistage pH-responsive liposomes for mitochondrial-targeted anticancer drug delivery. Adv Mater. 2012;24:3659–3665. doi: 10.1002/adma.201201498. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A, Soliman GM, Al-Hajaj N, Sharma R, Maysinger D, Kakkar A. Design and evaluation of multifunctional nanocarriers for selective delivery of coenzyme Q10 to mitochondria. Biomacromolecules. 2012;13:239–252. doi: 10.1021/bm201538j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yusuf M, Khan RA, Khan M, Ahmed B. Plausible antioxidant biomechanics and anticonvulsant pharmacological activity of brain-targeted beta-carotene nanoparticles. Int J Nanomedicine. 2012;7:4311–4321. doi: 10.2147/IJN.S34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J Bioenerg Biomembr. 2008;40:199–205. doi: 10.1007/s10863-008-9142-1. [DOI] [PubMed] [Google Scholar]
- 51.Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J Bioenerg Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 52.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 53.Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- 54.Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- 55.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II. Cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Zhang H, Lu W, Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. BBA-Bioenergetics. 2009;1787:553–560. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer's stygian link to the "Warburg Effect" and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Differ. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Austin CJ, Kahlert J, Kassiou M, Rendina LM. The translocator protein (TSPO): a novel target for cancer chemotherapy. Int J Biochem Cell Biol. 2013;45:1212–1216. doi: 10.1016/j.biocel.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Fafalios A, Akhavan A, Parwani AV, Bies RR, McHugh KJ, Pflug BR. Translocator protein blockade reduces prostate tumor growth. Clin Cancer Res. 2009;15:6177–6184. doi: 10.1158/1078-0432.CCR-09-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukherjee S, Das SK. Translocator protein (TSPO) in breast cancer. Curr Mol Med. 2012;12:443–457. [PubMed] [Google Scholar]
- 63.Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, et al. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. BBA-Biomembranes. 2008;1778:423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 65.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Update. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12:1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 68.Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012;109:2766–2771. doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27:S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Q, Wang H-G. Anti-cancer drug discovery and development. Commun Integrative Biol. 2012;5:557–565. doi: 10.4161/cib.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogler M, Dinsdale D, Dyer MJS, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2008;16:360–367. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 74.Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J. DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm Res. 1998;15:334–337. doi: 10.1023/a:1011991307631. [DOI] [PubMed] [Google Scholar]
- 75.Cheng SM, Pabba S, Torchilin VP, Fowle W, Kimpfler A, Schubert R, et al. Towards mitochondria-specific delivery of apoptosis-inducing agents: DQAsomal incorporated paclitaxel. J Drug Deliv Sci Tec. 2005;15:81–86. [Google Scholar]
- 76.Vaidya B, Vyas SP. Transferrin coupled vesicular system for intracellular drug delivery for the treatment of cancer: development and characterization. J Drug Target. 2012;20:372–380. doi: 10.3109/1061186X.2012.662687. [DOI] [PubMed] [Google Scholar]
- 77.D'Souza GG, Cheng SM, Boddapati SV, Horobin RW, Weissig V. Nanocarrier-assisted sub-cellular targeting to the site of mitochondria improves the pro-apoptotic activity of paclitaxel. J Drug Target. 2008;16:578–585. doi: 10.1080/10611860802228855. [DOI] [PubMed] [Google Scholar]
- 78.Cheng S-M, Boddapati SV, D'Souza GGM, Weissig V. DQAsomes as mitochondria-targeted nanocarriers for anti-cancer drugs. In: Amiji MM, editor. Nanotechnology for Cancer Therapy. FL, USA: Taylor & Franics Group; 2007. pp. 787–802. [Google Scholar]
- 79.Wang X, Shao N, Zhang Q, Cheng Y. Mitochondrial targeting dendrimer allows efficient and safe gene delivery. J Mater Chem B. 2014;2:2546–2553. doi: 10.1039/c3tb21348j. [DOI] [PubMed] [Google Scholar]
- 80.Boddapati SV, Tongcharoensirikul P, Hanson RN, D'Souza GG, Torchilin VP, Weissig V. Mitochondriotropic liposomes. J Liposome Res. 2005;15:49–58. doi: 10.1081/lpr-64958. [DOI] [PubMed] [Google Scholar]
- 81.Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release. 2012;159:393–402. doi: 10.1016/j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breunig M, Bauer S, Goepferich A. Polymers and nanoparticles: Intelligent tools for intracellular targeting? Eur J Pharm Biopharm. 2008;68:112–128. doi: 10.1016/j.ejpb.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salvador-Morales C, Zhang L, Langer R, Farokhzad OC. Immunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groups. Biomaterials. 2009;30:2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hines DJ, Kaplan DL. Poly(lactic-co-glycolic) acid-controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. 2013;30:257–276. doi: 10.1615/critrevtherdrugcarriersyst.2013006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marrache S, Tundup S, Harn DA, Dhar S. Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano. 2013;7:7392–7402. doi: 10.1021/nn403158n. [DOI] [PubMed] [Google Scholar]
- 88.Ohta S. Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene. 2006;25:4768–4776. doi: 10.1038/sj.onc.1209602. [DOI] [PubMed] [Google Scholar]
- 89.Wisnovsky Simon P, Wilson Justin J, Radford Robert J, Pereira Mark P, Chan Maria R, Laposa Rebecca R, et al. Targeting Mitochondrial DNA with a Platinum-Based Anticancer Agent. Chem Biol. 2013;20:1323–1328. doi: 10.1016/j.chembiol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pathak RK, McNitt CD, Popik VV, Dhar S. Copper-free click-chemistry platform to functionalize cisplatin prodrugs. Chem Eur J. 2014;20:6861–6865. doi: 10.1002/chem.201402573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feldhaeusser B, Platt SR, Marrache S, Kolishetti N, Pathak RK, Montgomery DJ, et al. Evaluation of nanoparticle delivered cisplatin in beagles. Nanoscale. 2015;7:13822–13830. doi: 10.1039/c5nr03447g. [DOI] [PubMed] [Google Scholar]
- 92.Marrache S, Choi JH, Tundup S, Zaver D, Harn DA, Dhar S. Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr Biol. 2013;5:215–223. doi: 10.1039/c2ib20125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marrache S, Tundup S, Harn DA, Dhar S. Ex vivo generation of functional immune cells by mitochondria-targeted photosensitization of cancer cells. Methods Mol Biol. 2015;1265:113–122. doi: 10.1007/978-1-4939-2288-8_9. [DOI] [PubMed] [Google Scholar]
- 94.Pathak RK, Dhar S. A Nanoparticle Cocktail: Temporal Release of Predefined Drug Combinations. J Am Chem Soc. 2015;137:8324–8327. doi: 10.1021/jacs.5b03078. [DOI] [PubMed] [Google Scholar]
- 95.Kalathil AA, Kumar A, Banik B, Ruiter TA, Pathak RK, Dhar S. New formulation of old aspirin for better delivery. Chem Commun. 2015 doi: 10.1039/c5cc07316b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y, Gustafsson ÅB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–548. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 97.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 98.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 99.Crow MT, Mani K, Nam Y-J, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 100.Ong S-B, Gustafsson ÅB. New roles for mitochondria in cell death in the reperfused myocardium. Cardiovasc Res. 2012;94:190–196. doi: 10.1093/cvr/cvr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 102.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 103.Epstein FH, Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 104.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 105.Oliveira HC, Cosso RG, Alberici LC, Maciel EN, Salerno AG, Dorighello GG, et al. Oxidative stress in atherosclerosis-prone mouse is due to low antioxidant capacity of mitochondria. FASEB J. 2005;19:278–280. doi: 10.1096/fj.04-2095fje. [DOI] [PubMed] [Google Scholar]
- 106.Luthi AJ, Zhang H, Kim D, Giljohann DA, Mirkin CA, Thaxton CS. Tailoring of biomimetic high-density lipoprotein nanostructures changes cholesterol binding and efflux. ACS Nano. 2011;6:276–285. doi: 10.1021/nn2035457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walters AM, Porter GA, Jr, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carvajal K, Moreno-Sanchez R. Heart metabolic disturbances in cardiovascular diseases. Arch Med Res. 2003;34:89–99. doi: 10.1016/S0188-4409(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 110.Gilbert-Barness E. Cardiovascular involvement in metabolic diseases. Pediatr Pathol Mol Med. 2002;21:93–136. doi: 10.1080/15227950252852050. [DOI] [PubMed] [Google Scholar]
- 111.Fosslien E. Review: Mitochondrial medicine--cardiomyopathy caused by defective oxidative phosphorylation. Ann Clin Lab Sci. 2003;33:371–395. [PubMed] [Google Scholar]
- 112.Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- 113.Asimakis GK, Conti VR. Myocardial ischemia: correlation of mitochondrial adenine nucleotide and respiratory function. J Mol Cell Cardiol. 1984;16:439–447. doi: 10.1016/s0022-2828(84)80615-x. [DOI] [PubMed] [Google Scholar]
- 114.Borutaite V, Mildaziene V, Brown GC, Brand MD. Control and kinetic analysis of ischemia-damaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischemia? Biochim Biophys Acta. 1995;1272:154–158. doi: 10.1016/0925-4439(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 115.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, Ruggiero FM. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic Biol Med. 1999;27:42–50. doi: 10.1016/s0891-5849(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 116.Turrens JF, Beconi M, Barilla J, Chavez UB, McCord JM. Mitochondrial generation of oxygen radicals during reoxygenation of ischemic tissues. Free Radic Res Commun. 1991;12–13(Pt 2):681–689. doi: 10.3109/10715769109145847. [DOI] [PubMed] [Google Scholar]
- 117.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 119.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. BBA-Mol Basis Dis. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 120.Kay L, Daneshrad Z, Saks VA, Rossi A. Alteration in the control of mitochondrial respiration by outer mitochondrial membrane and creatine during heart preservation. Cardiovasc Res. 1997;34:547–556. doi: 10.1016/s0008-6363(97)00058-8. [DOI] [PubMed] [Google Scholar]
- 121.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 122.Chen L, Knowlton A. Mitochondria and heart failure: new insights into an energetic problem. Minerva Cardioangiol. 2010;58:213. [PMC free article] [PubMed] [Google Scholar]
- 123.Luo M, Anderson ME. Mechanisms of altered Ca2+ handling in heart failure. Circ Res. 2013;113:690–708. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther. 2001;89:29–46. doi: 10.1016/s0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 125.Granville DJ, Gottlieb RA. The mitochondrial voltage-dependent anion channel (VDAC) as a therapeutic target for initiating cell death. Curr Med Chem. 2003;10:1527–1533. doi: 10.2174/0929867033457214. [DOI] [PubMed] [Google Scholar]
- 126.Empel VPv, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67:21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 127.Gellerich F, Trumbeckaite S, Opalka J, Seppet E, Rasmussen HN, Neuhoff C, et al. Function of the mitochondrial outer membrane as a diffusion barrier in health and diseases. Biochem Soc T. 2000;28:164–169. doi: 10.1042/bst0280164. [DOI] [PubMed] [Google Scholar]
- 128.Salnikov V, Lukyanenko Y, Frederick C, Lederer W, Lukyanenko V. Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles. Biophys J. 2007;92:1058–1071. doi: 10.1529/biophysj.106.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ono M, Sawa Y, Ryugo M, Alechine AN, Shimizu S, Sugioka R, et al. BH4 peptide derivative from Bcl-xL attenuates ischemia/reperfusion injury thorough anti-apoptotic mechanism in rat hearts. Eur J Cardiothorac Surg. 2005;27:117–121. doi: 10.1016/j.ejcts.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 131.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Correa F, Soto V, Zazueta C. Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. Int J Biochem Cell Biol. 2007;39:787–798. doi: 10.1016/j.biocel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 133.Nahon E, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005;579:5105–5110. doi: 10.1016/j.febslet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 134.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein–protein interactions and implications in disease states. BBA-Mol Cell Biol L. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. BBA-Mol Cell Biol L. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 137.Ren S, Hylemon P, Marques D, Hall E, Redford K, Gil G, et al. Effect of increasing the expression of cholesterol transporters (StAR, MLN64, and SCP-2) on bile acid synthesis. J Lipid Res. 2004;45:2123–2131. doi: 10.1194/jlr.M400233-JLR200. [DOI] [PubMed] [Google Scholar]
- 138.Korytowski W, Wawak K, Pabisz P, Schmitt JC, Girotti AW. Macrophage mitochondrial damage from StAR transport of 7-hydroperoxycholesterol: Implications for oxidative stress-impaired reverse cholesterol transport. FEBS Lett. 2014;588:65–70. doi: 10.1016/j.febslet.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 139.Kim CK, Kim T, Choi IY, Soh M, Kim D, Kim YJ, et al. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl. 2012;124:11201–11205. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- 140.Pramanik D, Campbell NR, Das S, Gupta S, Chenna V, Bisht S, et al. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget. 2012;3:640. doi: 10.18632/oncotarget.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park S, Yoon J, Bae S, Park M, Kang C, Ke Q, et al. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials. 2014;35:5944–5953. doi: 10.1016/j.biomaterials.2014.03.084. [DOI] [PubMed] [Google Scholar]
- 142.Schubert D, Dargusch R, Raitano J, Chan S-W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Bioph Res Co. 2006;342:86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 143.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Houtkooper R, Vaz F. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 146.Petrosillo G, Di Venosa N, Pistolese M, Casanova G, Tiravanti E, Colantuono G, et al. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. FASEB J. 2006;20:269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- 147.Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Philippe P, Joanne EP, Elinor JG, Andrew PH. The role of oxidized cytochrome c in regulating mitochondrial reactive oxygen species production and its perturbation in ischaemia. Biochem J. 2011;436:493–505. doi: 10.1042/BJ20101957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Binder CJ. Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv Exp Med Biol. 2012;750:2–13. doi: 10.1007/978-1-4614-3461-0_1. [DOI] [PubMed] [Google Scholar]
- 150.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grover GJ, Atwal KS, Sleph PG, Wang F-L, Monshizadegan H, Monticello T, et al. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol. 2004;287:H1747–H1755. doi: 10.1152/ajpheart.01019.2003. [DOI] [PubMed] [Google Scholar]
- 152.Grover GJ, Malm J. Pharmacological profile of the selective mitochondrial F1F0 ATP hydrolase inhibitor BMS-199264 in myocardial ischemia. Cardiovasc Ther. 2008;26:287–296. doi: 10.1111/j.1755-5922.2008.00065.x. [DOI] [PubMed] [Google Scholar]
- 153.Hausenloy DJ, Boston-Griffiths E, Yellon D. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol. 2012;165:1235–1245. doi: 10.1111/j.1476-5381.2011.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ikeda G. Nanoparticle-mediated targeting of cyclosporine A to mitochondria in reperfused myocardium enhances cardioprotection from ischemia-reperfusion injury. Circulation. 2013;128:A15712. doi: 10.1038/srep20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lim SY, Hausenloy DJ, Arjun S, Price AN, Davidson SM, Lythgoe MF, et al. Mitochondrial cyclophilin-D as a potential therapeutic target for post-myocardial infarction heart failure. J Cell Mol Med. 2011;15:2443–2451. doi: 10.1111/j.1582-4934.2010.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fancelli D, Abate A, Amici R, Bernardi P, Ballarini M, Cappa A, et al. Cinnamic anilides as new mitochondrial permeability transition pore inhibitors endowed with ischemia-reperfusion injury protective effect in vivo. J Med Chem. 2014;57:5333–5347. doi: 10.1021/jm500547c. [DOI] [PubMed] [Google Scholar]
- 157.Yin Q, Pei Z, Wang H, Zhao Y. Cyclosporine A-nanoparticles enhance the therapeutic benefit of adipose tissue-derived stem cell transplantation in a swine myocardial infarction model. Int J Nanomedicine. 2014;9:17–26. doi: 10.2147/IJN.S52005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ayer A, Macdonald P, Stocker R. CoQ10 in Heart Failure and Ischemic Heart Disease. Annu Rev Nutr. 2015;35 doi: 10.1146/annurev-nutr-071714-034258. [DOI] [PubMed] [Google Scholar]
- 159.Gvozdjákova A, Takahashi T, Singh R, De Meester F, Wilson DW, Crane FL. New roles of coenzyme Q10 in cardiovascular diseases, discovered by a single group. World Heart J. 2013;5:159. [Google Scholar]
- 160.Langsjoen PH, Langsjoen PH, Folkers K. Long-term efficacy and safety of coenzyme Q 10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1990;65:521–523. doi: 10.1016/0002-9149(90)90824-k. [DOI] [PubMed] [Google Scholar]
- 161.Rosenfeldt F, Marasco S, Lyon W, Wowk M, Sheeran F, Bailey M, et al. Coenzyme Q 10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J Thorac Cardiovasc Surg. 2005;129:25–32. doi: 10.1016/j.jtcvs.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 162.Swarnakar NK, Jain AK, Singh RP, Godugu C, Das M, Jain S. Oral bioavailability, therapeutic efficacy and reactive oxygen species scavenging properties of coenzyme Q10-loaded polymeric nanoparticles. Biomaterials. 2011;32:6860–6874. doi: 10.1016/j.biomaterials.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 163.Yamada Y, Nakamura K, Abe J, Hyodo M, Haga S, Ozaki M, et al. Mitochondrial delivery of Coenzyme Q 10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J Control Release. 2015;213:86–95. doi: 10.1016/j.jconrel.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 164.Amorim Cde M, Couto AG, Netz DJ, de Freitas RA, Bresolin TM. Antioxidant idebenone-loaded nanoparticles based on chitosan and N-carboxymethylchitosan. Nanomedicine. 2010;6:745–752. doi: 10.1016/j.nano.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 165.Ghule A, Mandpe L, Pokharkar V, Jadhav S, Bodhankar S. Cardioprotective effect of idebenone nanoparticles in doxorubicin induced cardiomyopathy in rats: assessment of myocardial performance, haemapoietic parameters and immunological changes. J Pharm Sci Pharmacol. 2014;1:26–39. [Google Scholar]
- 166.Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide–peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 167.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 168.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 169.Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 2010;48:161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 170.Chacko B, Reily C, Srivastava A, Johnson M, Ye Y, Ulasova E, et al. Prevention of diabetic nephropathy in Ins2+/Minus SignAkitaJ mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, et al. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163–168. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mercer JR, Yu E, Figg N, Cheng K-K, Prime TA, Griffin JL, et al. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/−mice. Free Radic Biol Med. 2012;52:841–849. doi: 10.1016/j.freeradbiomed.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 174.Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128:702–712. doi: 10.1161/CIRCULATIONAHA.113.002271. [DOI] [PubMed] [Google Scholar]
- 175.Jia Z, Wang X, Qin Y, Xue L, Jiang P, Meng Y, et al. Coronary heart disease is associated with a mutation in mitochondrial tRNA. Hum Mol Genet. 2013;22:4064–4073. doi: 10.1093/hmg/ddt256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ghezzi D, Baruffini E, Haack TB, Invernizzi F, Melchionda L, Dallabona C, et al. Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis. Am J Hum Genet. 2012;90:1079–1087. doi: 10.1016/j.ajhg.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zaragoza MV, Brandon MC, Diegoli M, Arbustini E, Wallace DC. Mitochondrial cardiomyopathies: how to identify candidate pathogenic mutations by mitochondrial DNA sequencing, MITOMASTER and phylogeny. Eur J Hum Genet. 2011;19:200–207. doi: 10.1038/ejhg.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dai D-F, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nair N, Gongora E. MicroRNAs as therapeutic targets in cardiomyopathies: myth or reality? Biomol Concepts. 2014;5:439–448. doi: 10.1515/bmc-2014-0026. [DOI] [PubMed] [Google Scholar]
- 180.Damiano MG, Mutharasan RK, Tripathy S, McMahon KM, Thaxton CS. Templated high density lipoprotein nanoparticles as potential therapies and for molecular delivery. Adv Drug Deliv Rev. 2013;65:649–662. doi: 10.1016/j.addr.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 181.Marrache S, Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Natl Acad Sci U S A. 2013;110:9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 183.Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990;46:428–433. [PMC free article] [PubMed] [Google Scholar]
- 184.Schon EA, Bonilla E, DiMauro S. Mitochondrial DNA mutations and pathogenesis. J Bioenerg Biomembr. 1997;29:131–149. doi: 10.1023/a:1022685929755. [DOI] [PubMed] [Google Scholar]
- 185.Coleman M, Blass JP. Autism and lactic acidosis. J Autism Dev Disord. 1985;15:1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- 186.Lombard J. Autism: a mitochondrial disorder? Med Hypotheses. 1998;50:497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- 187.Giulivi C, Zhang Y, Omanska-Klusek A, et al. MItochondrial dysfunction in autism. JAMA. 2010;304:2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tang G, Gutierrez Rios P, Kuo SH, Akman HO, Rosoklija G, Tanji K, et al. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis. 2013;54:349–361. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem. 2011;117:209–220. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rahimov F, Kunkel LM. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol. 2013;201:499–510. doi: 10.1083/jcb.201212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kelly-Worden M, Thomas E. Mitochondrial dysfunction in duchenne muscular dystrophy. Open J Endocr Metab Dis. 2014;04(08):8. [Google Scholar]
- 193.Akopova OV, Kolchynskayia LY, Nosar VY, Smyrnov AN, Malisheva MK, Man'kovskaia YN, et al. The effect of permeability transition pore opening on reactive oxygen species production in rat brain mitochondria. Ukr Biokhim Zh (1999) 2011;83:46–55. [PubMed] [Google Scholar]
- 194.Onopiuk M, Brutkowski W, Wierzbicka K, Wojciechowska S, Szczepanowska J, Fronk J, et al. Mutation in dystrophin-encoding gene affects energy metabolism in mouse myoblasts. Biochem Biophys Res Commun. 2009;386:463–466. doi: 10.1016/j.bbrc.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 195.Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Falzarano MS, Passarelli C, Ferlini A. Nanoparticle delivery of antisense oligonucleotides and their application in the exon skipping strategy for Duchenne muscular dystrophy. Nucleic Acid Ther. 2014;24:87–100. doi: 10.1089/nat.2013.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jarver P, Coursindel T, Andaloussi SEL, Godfrey C, Wood MJA, Gait MJ. Peptide-mediated cell and in vivo delivery of antisense oligonucleotides and siRNA. Mol Ther Nucleic Acids. 2012;1:e27. doi: 10.1038/mtna.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bibee KP, Cheng YJ, Ching JK, Marsh JN, Li AJ, Keeling RM, et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014;28:2047–2061. doi: 10.1096/fj.13-237388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Folbergrova J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12:35–40. doi: 10.1016/j.mito.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 200.Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88:23–45. doi: 10.1016/j.eplepsyres.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kunz WS, Kudin AP, Vielhaber S, Blumcke I, Zuschratter W, Schramm J, et al. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann Neurol. 2000;48:766–773. [PubMed] [Google Scholar]
- 202.Waldbaum S, Liang LP, Patel M. Persistent impairment of mitochondrial and tissue redox status during lithium-pilocarpine-induced epileptogenesis. J Neurochem. 2010;115:1172–1182. doi: 10.1111/j.1471-4159.2010.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jabir NR, Tabrez S, Firoz CK, Zaidi SK, Baeesa SS, Gan SH, et al. A synopsis of nano-technological approaches toward anti-epilepsy therapy: present and future research implications. Curr Drug Metab. 2015;16:336–345. doi: 10.2174/1389200215666141125142605. [DOI] [PubMed] [Google Scholar]
- 204.Lee J-H, Jung S-W, Kim I-S, Jeong Y-I, Kim Y-H, Kim S-H. Polymeric nanoparticle composed of fatty acids and poly(ethylene glycol) as a drug carrier. Int J Pharm. 2003;251:23–32. doi: 10.1016/s0378-5173(02)00582-3. [DOI] [PubMed] [Google Scholar]
- 205.Fresta M, Cavallaro G, Giammona G, Wehrli E, Puglisi G. Preparation and characterization of polyethyl-2-cyanoacrylate nanocapsules containing antiepileptic drugs. Biomaterials. 1996;17:751–758. doi: 10.1016/0142-9612(96)81411-6. [DOI] [PubMed] [Google Scholar]
- 206.Hamidi M, Azadi A, Mohamadi-Samani S, Rafiei P, Ashrafi H. Valproate-Loaded hydrogel nanoparticles: Preparation and characterization. J Appl Poly Sci. 2012;124:4686–4693. [Google Scholar]
- 207.Ueda M, Kreuter J. Optimization of the preparation of loperamide-loaded poly (L-lactide) nanoparticles by high pressure emulsification-solvent evaporation. J Microencapsul. 1997;14:593–605. doi: 10.3109/02652049709006812. [DOI] [PubMed] [Google Scholar]
- 208.Ying X, Wang Y, Liang J, Yue J, Xu C, Lu L, et al. Angiopep-conjugated electro-responsive hydrogel nanoparticles: therapeutic potential for epilepsy. Angew Chem Int Ed Engl. 2014;53:12436–12440. doi: 10.1002/anie.201403846. [DOI] [PubMed] [Google Scholar]
- 209.Yokoyama H, Mori N, Osonoe K, Ishida S, Kumashiro H. Anticonvulsant effect of liposome-entrapped superoxide dismutase in amygdaloid-kindled rats. Brain Res. 1992;572:273–275. doi: 10.1016/0006-8993(92)90483-p. [DOI] [PubMed] [Google Scholar]
- 210.Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:20–25. doi: 10.1016/j.jneuroim.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 211.Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochimica et biophysica acta. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bunton-Stasyshyn RKA, Saccon RA, Fratta P, Fisher EMC. SOD1 function and its implications for amyotrophic lateral sclerosis pathology: new and renascent themes. Neuroscientist. 2015;21:519–529. doi: 10.1177/1073858414561795. [DOI] [PubMed] [Google Scholar]
- 213.Eisen A. Amyotrophic lateral sclerosis: A 40-year personal perspective. J Clin Neurosci. 2009;16:505–512. doi: 10.1016/j.jocn.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 214.Reddy M, Wu L, Kou W, Ghorpade A, Labhasetwar V. Superoxide dismutase-loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl Biochem Biotechnol. 2008;151:565–577. doi: 10.1007/s12010-008-8232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ali SS, Hardt JI, Dugan LL. SOD Activity of carboxyfullerenes predicts their neuroprotective efficacy: a structure-activity study. Nanomedicine. 2008;4:283–294. doi: 10.1016/j.nano.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cho Y, Shi R, Borgens RB, Ivanisevic A. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine. 2008;3:507–519. doi: 10.2217/17435889.3.4.507. [DOI] [PubMed] [Google Scholar]
- 217.Hong S, Choi I, Lee S, Yang YI, Kang T, Yi J. Sensitive and colorimetric detection of the structural evolution of superoxide dismutase with gold nanoparticles. Anal Chem. 2009;81:1378–1382. doi: 10.1021/ac802099c. [DOI] [PubMed] [Google Scholar]
- 218.McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010;9:829–840. doi: 10.1016/S1474-4422(10)70116-2. [DOI] [PubMed] [Google Scholar]
- 219.Holmes D. New IVF techniques put mitochondrial diseases in focus. Lancet Neurol. 13:28–29. doi: 10.1016/S1474-4422(13)70190-X. [DOI] [PubMed] [Google Scholar]
- 220.Su B, Wang X, Zheng L, Perry G, Smith MA, Zhu X. Abnormal mitochondrial dynamics and neurodegenerative diseases. BBA-Mol Basis Dis. 2010;1802:135–142. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Venna N. Mitochondrial neurological diseases: a clinician's perspective. Neurol India. 2004;52:305–306. [PubMed] [Google Scholar]
- 222.Zhu J, Wang KZQ, Chu CT. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9:1663–1676. doi: 10.4161/auto.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]