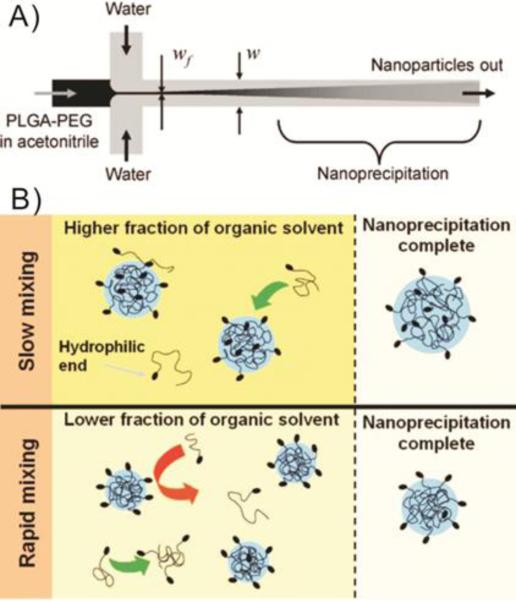
Abstract
Coating the surface of nanoparticles with polyethylene glycol (PEG), or “PEGylation”, is a commonly used approach for improving the efficiency of drug and gene delivery to target cells and tissues. Building from the success of PEGylating proteins to improve systemic circulation time and decrease immunogenicity, the impact of PEG coatings on the fate of systemically administered nanoparticle formulations has, and continues to be, widely studied. PEG coatings on nanoparticles shield the surface from aggregation, opsonization, and phagocytosis, prolonging systemic circulation time. Here, we briefly describe the history of the development of PEGylated nanoparticle formulations for systemic administration, including how factors such as PEG molecular weight, PEG surface density, nanoparticle core properties, and repeated administration impact circulation time. A less frequently discussed topic, we then describe how PEG coatings on nanoparticles have also been utilized for overcoming various biological barriers to efficient drug and gene delivery associated with other modes of administration, ranging from gastrointestinal to ocular. Finally, we describe both methods for PEGylating nanoparticles and methods for characterizing PEG surface density, a key factor in the effectiveness of the PEG surface coating for improving drug and gene delivery.
Keywords: Mucosal delivery, liposomes, stealth coatings, mononuclear phagocyte system (MPS), enhanced permeability and retention (EPR) effect
Graphical abstract
1. Introduction
In order to deliver adequate concentrations of systemically administered therapeutics to target tissues, these materials must circulate in the blood stream for as long as possible. However, proteins and peptides are rapidly degraded and cleared from the blood stream, necessitating approaches for increasing circulation time. One such approach is to coat the surface of the therapeutic with an inert polymer that resists interactions with components of the blood stream, imparting “stealth” properties. Polyethylene glycol (PEG) is the most widely used “stealth” polymer in the drug delivery field, due to its long history of safety in humans and classification as Generally Regarded as Safe (GRAS) by the FDA. Considered the first reports of PEGylation for drug delivery, Davis and Abuchowski described in 1977 the covalent attachment of PEG to bovine serum albumin and liver catalase proteins [1]. They found that by optimizing the PEGylation chemistry and the extent of PEGylation, they could increase the systemic circulation time and decrease the immunogenicity of the proteins without significantly compromising activity. In 1990, the FDA approved the first PEGylated protein product, Adagen®, a PEGylated adenosine deaminase enzyme for severe combined immunodeficiency disease [2]. Since then, 8 other PEGylated protein therapeutics have been FDA approved for treatment of diseases ranging from rheumatoid arthritis to age-related macular degeneration [2].
The success of protein PEGylation as a method for producing longer circulating, and thus, more efficacious intravenous therapies led to investigations of nanoparticle (NP) PEGylation for systemic applications in the early 80’s and 90’s [3-5]. Recognized as foreign objects, NPs are readily cleared from systemic circulation by the cells of the mononuclear phagocyte system (MPS), precluding accumulation in target cells and tissues. However, similar to what was observed with PEGylated proteins, PEG coatings on NPs shield the surface from aggregation, opsonization, and phagocytosis, thereby prolonging circulation time. The first FDA approval of a PEGylated nanoparticle (NP) product, Doxil®, came in 1995. Doxil “Stealth®” liposomes increased doxorubicin bioavailability nearly 90-fold at 1 week from injection versus free drug, with a drug half-life of 72 h and circulation half-life of 36 h [6-8]. In the years since, PEGylation has become a mainstay in NP formulation. Although much of the initial development of PEGylated NPs focused on systemic administration, in this review we also highlight the benefits of NP PEGylation for overcoming biological barriers to effective delivery associated with numerous modes of delivery, ranging from injection into the eye to topical mucosal applications. Special emphasis is given to studies that directly compare PEGylated to non-PEGylated formulations to specifically demonstrate the benefits of NP PEGylation. Further, we discuss common methods for PEGylating NPs, as well as quantifying a critical parameter that influences the efficiency of delivery, the surface PEG density.
2. Nanoparticle PEGylation for improved systemic delivery
2.1 The potential fates of systemically administered nanoparticles
Systemically administered NPs can potentially reach and deliver therapeutic payloads to every vascularized organ/tissue in our body. Prolonging the retention time in the blood has been accepted as the frontline strategy, since it provides higher probability of circulating NPs to encounter, and partition into, the targets of interest. However, this task has been challenging primarily due to the presence of the MPS. The MPS consists of dendritic cells, blood monocytes, granulocytes, and tissue-resident macrophages in the liver, spleen, and lymph nodes that are responsible for clearing, processing, and degrading exogenous materials in the blood stream [9]. Unlike other organs, endothelia in organs associated with the MPS are often fenestrated, which facilitates screening of circulating entities. NPs as large as 100 nm can passage through the endothelial fenestrae in the liver and spleen, and also through permeable vascular endothelia in lymph nodes [10, 11]. Thus, the MPS provides a critical defense mechanism that protects against foreign pathogens, but at the same time, rapidly eliminates therapeutic NPs from the blood stream. NPs circulating in the blood are readily recognized by serum proteins called opsonins, including complement compounds, immunoglobulins, fibronectin and apolipoproteins [12]. Adsorption of opsonins onto NP surfaces (opsonization) renders NPs more susceptible to phagocytosis by cells in the MPS. Opsonized NPs are taken up by MPS cells via numerous types of opsonin-recognizing receptors abundant on the cell surface, including complement, Fc and fibronectin receptors [13, 14]. Although opsonin absorption to NPs occurs preferentially via hydrophobic interactions [15, 16], electrostatic interactions and hydrogen bonding interactions have also been shown to mediate opsonization [17]. Of note, NPs can also be directly captured by macrophages by opsonin-independent scavenger receptors [18-20] that often recognize repeating patterns [12]. It has been reported that several tens to hundreds of types of serum proteins readily interact with circulating NPs, thereby forming a protein corona on the NP surface [21, 22]. The corona formation non-specifically facilitates uptake of NPs by cells encountered during their circulation, including endothelial cells [21, 22], similar to the opsonin-dependent MPS cell uptake. Thus, protein absorption not only reduces the circulation time, but also weakens the targeting capabilities of NPs functionalized for targeting specific cells [23].
Aggregation of circulating NPs can also undermine their circulation time, regardless of uptake by MPS or other non-target cells. Uncharged, hydrophobic NPs rapidly aggregate via van der Waals and/or hydrophobic forces in aqueous conditions. In contrast, positively or negatively charged NPs, due to the repulsive forces, generally retain their colloidal stability in aqueous solutions with low ionic strength. However, under high ionic strength, such as in the blood, electrostatic interactions between NPs with counterions neutralize the particle surface charge, thereby rendering the NP surface amenable to aggregation. Prolonged exposure to circulating serum proteins also elevates the chance of NP aggregation [22]. Large aggregates formed by NP-NP interactions and/or protein adsorption are prone to physically block pulmonary capillary beds, providing another mechanism by which NPs are eliminated from the blood circulation. For example, DNA NPs based on numerous cationic polymers or lipids were found destined to the lung rather than the liver [24-26], presumably due to the entrapment of aggregates in narrow capillary beds. Lastly, NPs can be also cleared by renal excretion, but typical NPs designed for drug and gene delivery applications are likely to avoid glomerular filtration due to their relatively large sizes (> 10 nm). Overall, conventional NPs are generally cleared from the blood circulation within 10 min following systemic administration, irrespective of the NP composition [9, 27].
2.2 The impact of PEGylation on systemically administered nanoparticles
Due to its hydrophilic nature, PEG chains grafted on NPs generate a hydrated cloud with a large excluded volume that sterically precludes NPs from interacting with neighboring NPs and/or blood components [28]. In addition, the large conformational freedom provided by the flexibility of PEG renders interpenetration of foreign matters into the PEG corona thermodynamically unfavorable [12]. Klibanov and coworkers demonstrated that PEGylation increased the blood circulation half-life of systemically administered liposomes from <30 min to up to 5 h [5]. Subsequently, Gref and coworkers introduced the first PEGylated polymeric NPs, based on poly(lactic-co-glycolic acid) (PLGA) [29]. They discovered that PEGylation significantly increased the circulation time of NPs while reducing liver uptake compared to otherwise identical non-PEGylated PLGA NPs. These pioneering studies led to the future clinical development of PEGylated NP formulations such as Doxil® (approved by the US FDA in 1995) and Genexol-PM® (approved by Korean FDA and currently in clinical trials in the US) [30]. In addition to synthetic NPs, PEGylation has been applied to reduce the immunogenicity and/or prolong the circulation of viral gene vectors, including adenovirus [31] and adeno-associated virus [32].
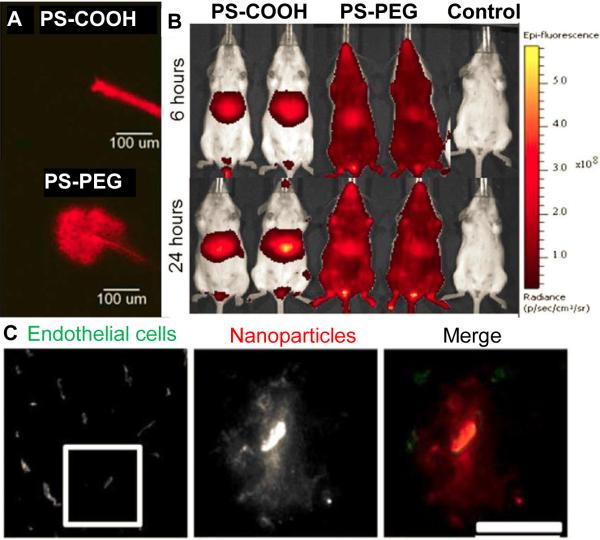
The enhanced permeability and retention (EPR) effect provides long-circulating PEGylated NPs with a unique opportunity to target tumors following systemic administration. Matsumura and coworkers characterized the poorly developed leaky vasculature and impaired lymphatic system of tumors, first proposing the EPR effect [33]. The openings present throughout the tumor vasculature (> 400 nm) are even larger than those of endothelial fenestrae in the liver, and thus circulating NPs can preferentially accumulate in tumors [34]. Amongst the numerous examples, Doxil® was shown to provide long circulation half-life (3 – 4 days in human) and passive accumulation in tumors [35]. Similarly, PEGylated NPs capable of stably circulating in the blood can be potentially utilized to target other diseases characterized by abnormal neovascularization, including several ocular disorders, diabetes, obesity, asthma and multiple sclerosis [11]. Increasing circulation time also increases the likelihood of interactions between ligands displayed on the NP surface and the respective receptors on cell surfaces. However, incorporation of ligands adds a layer of complexity in the formulation process and can undermine the “stealth” properties provided by surface PEG coatings [9, 36]. Several PEGylated NPs decorated with active targeting agents are currently under clinical investigation [37]. Recently, Nance and coworkers demonstrated that densely PEG-coated, tissue penetrating (Fig. 1A), long-circulating (Fig. 1B) NPs can be effectively delivered to the brain following the application of focused ultrasound (FUS) to reversibly open the blood brain barrier (BBB) in vivo [38]. FUS provided non-invasive, transient opening of blood vessels with a submillimeter spatial precision [38, 39], thereby enabling delivery of therapeutic NPs to target tissues without targeting ligands (Fig. 1C). It should be noted that only NPs capable of resisting protein adsorption and/or aggregation and circulating long-term in the blood can fully exploit the benefit of a physical targeting mechanism like FUS.
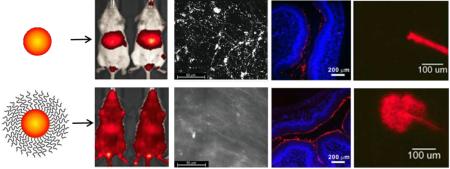
Figure 1.
Dense PEG coatings provide improved brain tissue penetration and extended circulation time, leading to improved delivery to the brain with focused ultrasound (FUS). (A) Densely PEG-coated 60 nm PS-PEG NPs spread widely throughout brain tissue when administered locally to the brain, which is necessary for distributing throughout the tissue after crossing the BBB. (B) PS-PEG NPs remained in systemic circulation, whereas uncoated PS-COOH NPs accumulate in the liver. (C) Long circulating PS-PEG NPs were delivered across the BBB using reversible opening by FUS, leading to NP penetration and widespread distribution throughout brain tissue. Adapted with permission from [35].
2.3 Factors that affect the circulation time of PEGylated nanoparticles
There are many factors that influence the interactions and circulation of PEGylated NP in the blood. Numerous reports have attempted to investigate the effects of individual parameters on the behavior of PEGylated NP in blood in vitro and in vivo. However, it is often difficult to carefully control for unintended changes to other physiochemical properties; for example, increasing PEG molecular weight (MW) often increases the NP size [40-42], or may affect the PEG surface density. In the case of liposomes formulated with PEGylated lipids, increasing PEG MW or PEG surface density may impact stability. Additionally, variations in experimental methods as well as methods for quantification and analysis can make directly comparisons of separate studies challenging. Nevertheless, here we summarize and discuss what has been reported for some key factors, including PEG MW, PEG surface density, and NP physicochemical properties that impact circulation of NPs in the blood. This topic has been extensively reviewed previously [11, 12, 43, 44], and here we add additional examples of more recent works.
2.3.1 PEG molecular weight (MW)
To evade interactions with serum proteins and MPS cells, the PEG corona must provide a sufficiently thick layer to sterically shield the NP surface. Since the MW of grafted PEG chains is proportional to the polymer chain length, PEG MW is considered to be an important determinant of effective surface shielding. Increasing the PEG MW incorporated into polymer-based PEGylated micelles (from 2 to 20 kDa) was shown to prevent aggregation and adsorption to blood components, leading to increased circulation time in vivo [41]. The blood circulation half-lives were 4.6, 7.5 and 17.7 min for micelles coated with 5, 10 and 20 kDa PEG, respectively. Likewise, while PEGylated liposomes coated with 750 Da PEG were comparable to non-PEGylated liposomes, prolonged blood circulation and reduced MPS uptake was observed when the PEG MW was increased to 5 kDa [42]. In contrast, while PEGylated liposomes exhibited prolonged circulation times compared to non-PEGylated liposomes, the differences in circulation time between formulations with increasing PEG MW (range: 350 Da - 2 kDa) were negligible [45]. Thus, both studies demonstrated an improvement in circulation time for PEGylated liposomes, but one study did not find additional improvements by increasing PEG MW; this may be related to physiochemical differences between the liposome formulations, including core material and particle diameter. Another study evaluated the adsorption of plasma proteins onto the surfaces of PEGylated poly(lactic acid) (PLA) (PLA-PEG) NPs with varying PEG MW [40]. They found that the total amount of protein adsorbed onto the NP surfaces significantly decreased as PEG MW increased up to 5 kDa, but no further decrease in protein adsorption was observed as PEG MW was further increased to 10, 15 and 20 kDa; all PEG MW ≥ 5 kDa tested provided ~75% decrease in protein adsorption to the PLA-PEG NP surface compared to PLA NPs [40].
It has generally been demonstrated that PEG MW of 2 kDa or higher is required to shield NP surfaces from protein adsorption and reduce recognition by the MPS [46]. Cui and coworkers found that increasing PEG MW from 10 to 40 kDa, while maintaining constant particle size, led to reduced phagocytic blood cell association of PEGylated mesoporous silica NPs (MSN) [47]. In another study exploring size-controlled MSN, PEG MW at least as large as 10 kDa was required to effectively shield NPs from protein adsorption and association with human monocytic leukemia cell line-derived macrophages (THP-1) [48]. Likewise, the circulation half-life of systemically administered PEGylated gold NPs increased with increasing PEG MW between 2 – 10 kDa [49]. Although NP size was carefully controlled in these studies, it was unclear whether the surface PEG grafting densities remained constant as PEG MW was increased. In a study where PLA-PEG NPs with similar sizes (180 – 200 nm) but with different PEG MW were compared, 20 kDa PEG resulted in reduced NP associated with macrophages in vitro compared to 5 kDa PEG [50]. In a subsequent study, NPs with 20 kDa PEG exhibited decreased liver uptake in vivo, and thus, increased circulation time compare to NPs coated with 5 kDa PEG [51]. Bazile and coworkers also showed that the half-life of ~150 nm PLA-PEG NPs increased as PEG MW increased [52]. Of note, the PEG surface densities were estimated to be similar regardless of the PEG MW, though it was assumed that all PEG incorporated into the particle was displayed on the NP surface. Increasing the PEG MW coating poly(hexadecyl cyanoacrylate) NPs from 2 to 5 to 10 kDa decreased protein absorption, phagocytic uptake, and liver uptake, leading to increased circulation time [53]. However, the NPs coated with higher MW PEG were also coated at higher PEG density, so it is difficult to separate the two effects. Recently, Yang and coworkers reported that PEG with a MW as low as 559 Da can effectively shield surfaces of 100 nm polystyrene (PS) NPs when the grafting density is “high” [54]. Thus, grafting lower MW PEG at a high surface density may be similarly effective in prolonging circulation time as grafting higher MW PEG. Complement activation was found to be comparable for PLA and lipid-based NPs coated with 2 kDa and 660 Da PEG, respectively, incubated in normal human serum, likely due the highly dense PEG coatings achieved [12].
2.3.2 PEG content, surface density and conformation
In addition to PEG MW, the surface density of the grafted PEG layer is a critical factor that affects resistance to protein adsorption and clearance following systemic administration [12]. Braeckmans and coworkers reported that 10 mol % PEGylated lipids prevented liposome aggregation in whole blood, whereas liposomes formulated with lower PEG contents (5 and 3 mol %), agglomerated over time [55]. Small, well-shielded liposomes would be less likely to become trapped in capillaries in the lung and/or taken up by MPS cells. In another study, stealth lipid NPs formulated with increasing amounts of PEG exhibited more near-neutral ζ-potential, suggesting that inclusion of more PEG led to improved shielding of the anionic NP surface [56]. Accordingly, increased circulation time in the blood was observed for liposomes with greater PEG content following intravenous injection into rabbits. Another study found that although PEGylation increased the circulation time of lipid nanocapsules (LNC) compared to uncoated LNCs, the differences in circulation time for liposomes containing 6 - 15 mol% PEGylated lipids were insignificant [57]. However, the surface charge and PEG density were not reported. Similarly, although a significant increase in blood circulation time was observed for PEGylated polycyanoacrylate NPs, doubling the amount of PEG in the formulation did not further increase the circulation time [58]. These results suggest that increasing PEG content during the NP formulation may not ensure greater surface coverage of PEG. In addition, there is a threshold for maximum achievable PEG surface density that may vary depending on the type of NPs and formulation methods. It is thus essential to accurately quantify the surface PEG density when interpreting the impact of PEG density on NP circulation (for methods, see section 4.3).
The average distance between neighboring PEG chains on a NP surface (D) determines the structural conformation of PEG molecules on the surface, and thus, its effectiveness for shielding the NP surface. If D is greater than the Flory radius (RF ~ aN3/5, where N is the degree of polymerization, and thus is proportional to PEG MW, and a is the effective monomer length = 0.35 nm) of the PEG chain (RF/D ≤ 1), neighboring PEG chains will not overlap and are said to be in a “mushroom” conformational regime. As the surface PEG density increases such that adjacent PEG chains overlap (RF/D > 1), the PEG must stretch away from the NP surface [59] and forms a “brush” layer. It is generally believed that surface PEG densities in the mushroom-to-brush transition are required to resist adsorption of serum proteins and avoid uptake of NPs by MPS cells [44, 46]. Further, it has been reported that higher RF/D values may be required for longer PEG chains (≥ 10 kDa), presumably due to their tendency to entangle with neighboring chains [54]. In the subsequent discussion of the effect of PEG surface density on NP behavior, RF/D values were either given or calculated from the measured or estimated surface PEG density. Several research groups have investigated the effect of PEG coverage on NP stability in serum and/or circulation time in the blood using PLA-PEG NPs. PLA-PEG NPs with higher content of 2 kDa PEG (RF/D > 1.73) efficiently resisted adsorption of complement compounds, but similar NPs with slightly lower PEG content (RF/D ~1.5) were unable to do so [60]. Likewise, PLA-PEG NPs formulated with 30% w/w 20 kDa PEG content exhibited increased circulation times compared to NPs with 10% w/w PEG [51]. Based on the previously estimated D values for these particles [50], RF/D values for 10% and 30% PEG NP were ~1.8 and ~3.0. In another study, PLA-PEG NPs formulated with varying content of 5 kDa PEG (0.5 to 20% w/w) were tested for protein adsorption [40]. A drastic reduction in protein adsorption was observed when the PEG content was increased from 2% to 5% w/w, but no significant change was observed as PEG content was further increased up to 20% w/w. Similarly, a marked reduction of NP uptake by human MPS cells was observed at 5% PEG; PEG surface density was estimated assuming that all PEG chains from PLA-PEG copolymers migrated to the NP surface, yielding RF/D ~4.3 [40]. Although the MW of PEG used in these described studies varied, it appears that effective surface shielding was consistently achieved at PEG densities far beyond the mushroom/brush transition (RF/D >> 1).
To isolate the effect of PEG surface density, Yang and coworkers conjugated 5 kDa PEG chains to carboxyl groups on the surface of 100 nm polystyrene (PS) NPs at varying PEG grafting concentrations [54]. The advantage of this approach is that all of the incorporated PEG must be on the surface of the NPs, as opposed to leaving the potential for interpenetration of PEG into the polymer core during formation. The surface PEG densities were assessed by quantifying fluorescently labeled PEG chains and unreacted carboxyl groups. They found that although NPs generally exhibited increasingly near-neutral ζ-potential with increasing surface PEG coverage, near-neutral surface charge could be achieved at relatively low grafting densities, suggesting that ζ-potential may be limited in accurately assessing the extent of NP surface shielding. It should also be noted that ζ-potential measurements significantly vary depending on the buffer conditions, including ionic concentration and pH. Consistent with studies described earlier, they discovered that a “dense brush” conformation (RF/D > 2.8) was required for evading uptake by human monocytic THP-1 cells in vitro (i.e. ~20-fold reduced uptake compared to uncoated control) (Fig. 2A). In a similar study, a dense brush layer (RF/D ~3.7) was required to significantly reduce uptake of PEGylated gold NPs by J774A.1 murine macrophage-like cells in vitro; the amount of PEG on the NP surface was measured by quantifying unreacted thiol groups of PEG chains [20]. In comparison, PS NPs grafted with 3.4 kDa PEG chains at RF/D ~ 2.5 demonstrated 90% reduced adsorption of proteins in human plasma compared to uncoated PS NPs [61]. Yang and coworkers found that further increases in PEG surface density were required for increased circulation time in the blood in vivo; NPs with RF/D ≤ 4.2 were completely cleared from the blood (Fig. 2B) and accumulated in the liver (Fig 2C) after 2 h, but only <20% of NPs possessing denser PEG coverage (RF/D > 6.6) were removed from the circulation at 2 h [54]. The authors suggested that the flexible nature of PEG in combination with high concentrations of proteins and biomacromolecules in the blood necessitated extremely dense PEG surface coverage to effectively reduce the chance of transient gap openings that allow access to the NP surface [54]. In contrast to the aforementioned studies, Perry and coworkers demonstrated that 5 kDa PEGylated PRINT® hydrogel NPs coated with PEG in the mushroom conformation (RF/D ~0.9) exhibited reduced uptake by MH-S macrophages to an extent similar to NPs coated with PEG in a brush conformation (RF/D ~1.5) [62]. The half-life in circulation increased from 0.89 h for uncoated NPs to 15.5 and 19.5 h for NPs with mushroom PEG and brush PEG, respectively, suggesting relatively low PEG surface coverage was sufficient to increase the blood circulation time of PRINT NPs. The discrepancy is likely due to the deformable nature of PRINT hydrogel NPs; it has been previously demonstrated that flexible PRINT NPs were eliminated from blood circulation significantly slower than their rigid counterparts [63].
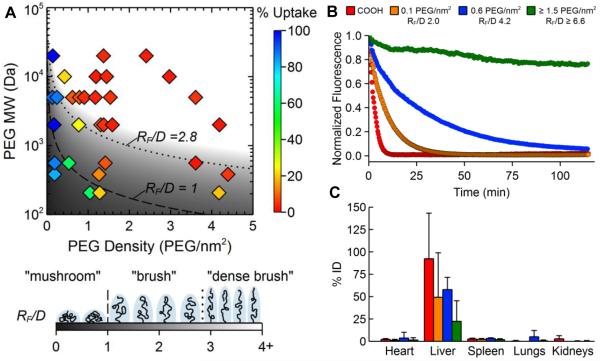
Figure 2.
The effect of surface PEG surface density on the uptake of polystyrene NPs by macrophages in vitro and circulation time in the blood in mice in vivo. (A) Phase diagram mapping NP uptake by differentiated human THP-1 cells as a function of PEG MW and coating density. A “dense brush” was most effective at reducing macrophage uptake in vitro. (B) The amount of NPs and NPs coated with PEG at various grafting densities circulating in the blood of mice over time, as observed using intravital microscopy. The RF/D values required for extended circulation time in vivo were increased compared to the PEG density required for reduced cell uptake in vitro. (C) Biodistribution of the various NP formulations 2 h after intravenous injection. Increased PEG surface density led to decreased accumulation in the liver. Adapted with permission from [51].
Despite achieving highly dense surface coatings of PEG, no NP formulation has been developed that can completely resist interactions with blood components [20, 54]. Thus, PEGylation likely improved the circulation time of NP formulations reducing rather than eliminating protein adsorption. Interestingly, the composition of the adsorbed protein layer on PEGylated NPs appeared to be a function of surface PEG density [20, 40]. For example, among 10 different serum proteins tested, adsorption of apoprotein (apo)C-III on PLA-PEG NPs was completely eliminated when NPs were formulated with 5% PEG by weight [40]. Walkey and coworkers demonstrated that high MW serum proteins were preferentially blocked at low PEG densities, whereas blockage of smaller proteins (50 – 80 kDa) was favored as PEG densities increases [20]. At RF/D < 2.1, macrophage uptake appeared to be dependent upon adsorption of serum proteins, such as complement component C3, which opsonizes foreign materials and facilitates macrophage uptake [51, 64]. Low levels of protein adsorption on NPs with higher PEG densities did not promote their uptake by macrophages, suggesting potential serum-independent macrophage uptake mechanisms.
2.3.3 Physiochemical properties of the nanoparticle core
The core physicochemical and mechanical properties of NPs can also influence protein adsorption and circulation time [27]. Larger NPs at a given PEG surface density may adhere to MPS cells more effectively than smaller NPs due to weak, multivalent interactions between the PEG chains and/or terminal groups with cell surfaces [65]. Smaller PEGylated polymeric and gold NPs provided more resistance against murine macrophage uptake in vitro and/or improved blood PK in vivo compared to otherwise identical larger NPs [49, 53, 66]. Phagocytosis of PEGylated LNCs by a murine monocyte/macrophage cell line also increased with increasing NP diameter [67]. On the other hand, higher PEG density may be required to effectively shield smaller NPs due to the increased surface curvature [68]. Decreasing NP size from 90 to 15 nm increased the total protein absorption at a fixed PEG grafting density, since the flexible PEG chains could spread out more [20]. Nevertheless, larger PEGylated NPs with similar PEG densities exhibited increased macrophage uptake compared to smaller NPs, presumably due to a serum-independent mechanism that is more influenced by the collective avidity of interactions with larger NPs [20]. Of note, NPs less than 10 nm in particle size are readily eliminated from the blood circulation by renal clearance [11], which sets a minimum size cutoff for achieving increased circulation time.
Jiang and coworkers demonstrated that the in vivo gene transfer efficiency of systemically administered PEGylated polymer-based gene vectors was dependent upon the shape of the NPs [69]. They observed significantly higher transgene expression in the liver parenchyma with rod- and worm-shaped PEGylated NPs compared to spherical PEGylated NPs, speculating reduced uptake of elongated NPs by MPS cells. Likewise, PEGylated rod-shaped gold NPs exhibited decreased macrophage uptake in vitro and increased circulation time in vivo compared to otherwise identical spherical PEGylated NPs [18]. These findings are in good agreement with a previous finding that elongated worm-like non-PEGylated NPs were less likely to be taken up by macrophages compared to spherical NPs [70]. Assuming identical PEG surface density, spherical PEGylated particles may be more susceptible to opsonization and/or direct interactions with MPS cells due to their higher surface curvature. The chemical properties of the core materials may also impact the behavior of PEGylated NPs in the blood, given that complete shielding of NP surfaces is extremely challenging [20, 54]. Indeed, it has been shown that the composition and the extent of protein absorption were distinct among similarly sized PEGylated NPs formulated with three different core biodegradable polymers, including PLA, PLGA and polycaprolactone (PCL), and similar coatings with 5 kDa PEG [40]. Additionally, the integrity of the surface PEG layer on biodegradable NPs is influenced by rate of hydrolytic degradation of the core material. NPs composed of more rapidly degrading polymers are likely to lose their stealth properties more rapidly. The retention of the surface PEG layer on liposomes is also largely dependent on the property of core lipids to which PEG chains are anchored. PEGylated liposomes formulated with an identical amount of PEG anchored to ceramide were more prone to aggregation in the blood compared to liposomes composed of PEG anchored to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) [55]; of note, PEGylated DSPE is a component of Doxil® [35]. It is likely that ceramide molecules gradually diffuse out of the liposomes [71]. More recently, Anselmo and coworkers demonstrated that softer PEGylated NPs exhibited reduced uptake by J774 macrophages and increased circulation time compared to harder particles; NPs with an 8-fold lower elastic modulus exhibited greater than 30-fold increase in the elimination half-life [72]. This finding suggests that the circulation time of densely PEGylated NPs can be further increased by modulating NP elasticity.
2.4 PEGylation for reduced systemic toxicity
The systemic toxicity of NPs is also an important issue, as systemically administered NPs can theoretically reach any vascularized tissues in the body. Also, NPs can directly interact with circulating erythrocytes, leading to erythrocyte aggregation and/or hemolysis that is accompanied by hemoglobin release [73]. Since erythrocytes constitute a markedly greater volume compared to MPS cells in the blood circulation, NPs are more likely to encounter erythrocytes in circulation. Although the mechanism has not been fully elucidated, NP surface properties are known to play a critical role in NP-erythrocyte interactions. Specifically, disruption of erythrocytes is often observed with cationic NPs, due to their tendency to interact with negatively charged cell surfaces via electrostatic interactions. For example, cationic polymers widely utilized to formulate synthetic DNA NPs, including polyethylenimine (PEI), poly-L-lysine and poly (amido amine) (PAMAM) dendrimers, were shown to interact with and damage the membranes of erythrocytes [74, 75]. Likewise, cationic lipoplexes were shown to mediate erythrocyte aggregation [76] and/or hemolysis [77].
It has been demonstrated in several cases that PEGylation can reduce the hemotoxic properties of NPs. PEGylation was shown to decrease hemolysis and/or the aggregation of erythrocytes induced by PEI-based NPs [78] and lipoplexes [76]. Kurosaki and coworkers also reported that PEG significantly reduced hemolysis by PAMAM-based DNA NPs, especially as PEG content was increased [79]. In contrast, insufficient shielding of the cationic surface charge by 550 Da PEG led to hemolysis and erythrocyte aggregation [80]. Similarly, NP-induced hemolysis was significantly reduced by PEGylation of silica NPs, but only at increased PEG content; hemolysis percentage was less than 1% for MSNs with 0.75 wt% PEG in comparison to 13.2% for MSNs with 0.075% PEG [48]. These findings indicate that dense surface PEG coatings may be required to minimize interactions between NPs and erythrocytes, similar to what has been observed for interactions with serum proteins and MPS cells. Of note, hemoglobin and/or cell debris released by hemolysis may adsorb onto NP surfaces, facilitating the phagocytosis of NPs by macrophages [73]. Thus, reduction of hemolysis may be an additional mechanism by which PEGylation increases the circulation time of NPs.
2.5 Immunogenicity and alternatives to nanoparticle PEGylation for systemic applications
The widespread use of PEGylation in drug and gene delivery has led to many promising developments, as well as brought attention to some potential drawbacks to the widespread use of PEG. Here, we briefly discuss the potential immunogenicity of PEG and the development of PEG alternatives, which has been thoroughly discussed elsewhere [28, 81-84]. The potential immunogenicity of PEG has gained more attention as an increasing number of investigators have observed rapid clearance of PEGylated therapeutics upon repeated administrations [28, 85]. Of note, much of the work on systemic immunogenicity has focused on liposomes, and thus, additional study of PEG immunogenicity with polymeric NPs is needed. Indeed, the physicochemical properties of polymeric NPs are different from those of liposomal NPs, which as previously discussed, influences NP behavior in systemic circulation, and potentially immunogenicity. Numerous reports have found that PEG-specific antibodies were formed after a single intravenous injection of PEGylated liposomes in various animal models and humans [86-88]. Following the first injection, a significant reduction in the circulation time of subsequently administered liposomes has been observed, a phenomenon known as accelerated blood clearance (ABC). Laverman and Dams demonstrated complement activation and a dramatic reduction in half-life after the second injection of PEGylated liposomes, which they later showed involved hepatosplenic macrophages [89, 90]. Kiwada and coworkers published a series of reports regarding the initiation of antibody production and the immunological events that follow. An early report highlighted IgM anti-PEG antibodies as the main serum factor responsible for ABC [88]. In agreement with the prior implication of hepatosplenic macrophages, they found that IgM antibodies were produced by the spleen shortly after one injection of PEGylated liposomes [87, 91]. Strikingly, it was found that splenectomized mice did not produce IgM antibodies or exhibit ABC phenomenon with the second injection of PEGylated liposomes [92]. They went on to demonstrate that IgM antibodies produced by the spleen bind to liposomes, activating the complement system and enhancing uptake by Kupffer cells [93]. Furthermore, T-cell deficient Balb/c mice were able to produce anti-PEG IgM antibodies, indicating that IgM antibodies were produced by B cells in a T-cell independent manner [87, 94].
Another factor that has been shown to influence the extent of ABC is the cargo inside the NPs [95-97]. For example, IgM production against PEG was decreased when doxorubicin-loaded liposomes were administered compared to empty PEGylated liposomes [90, 98]. It was speculated that the cytotoxic effect of doxorubicin led to apoptosis or anti-proliferation of splenic B cells, therefore hindering the production of antibodies. Similarly, IgM production was shown to be dependent on the sequence of siRNA loaded into PEGylated liposomes [96, 99]. Increased IgM production was observed when siRNA with an immune-stimulatory sequence was encapsulated within the liposome. Moreover, loading CpG-free or methylated nucleic acids into NPs helped reduce induction of inflammatory cytokines [99-101]. Additionally, anti-PEG antibody production has been shown to be inversely correlated with the amount of PEGylated liposomes injected in the first dose [102, 103]. Increasing the dose may overwhelm the splenic B cells with antigen, leading to immune tolerance or anergy [82, 103]. Ultimately, injecting a higher initial dose of liposomes could be a strategy to overcome subsequent immunological responses and accelerated clearance.
The physiochemical properties of the NPs can also influence the immunogenicity. Xu and coworkers observed that the extent of the ABC response varied with different phospholipid core materials in PEGylated liposomes [104]. Shiraishi and coworkers suggested that the antigenic determinant for anti-PEG antibody production was the chemical linkage between the PEG chains and the core lipids [105]. They found that hydrophilic PEGylated polymeric micelles did not appear to induce production of anti-PEG antibodies and rapid clearance with subsequent doses, whereas hydrophobic PEGylated polymeric micelles experienced rapid clearance from the systemic circulation [105]. It was also reported that the physicochemical properties of both the first and subsequent dose of liposomes influence the immune response [86, 106, 107]. Ishida and coworkers demonstrated that increasing the PEG surface density reduced the extent of clearance of a subsequent dose of PEGylated liposomes [86]. Wang and coworkers observed that the presence of PEG on the first liposome dose was not a prerequisite for observing ABC with a second PEG-coated liposome dose. Further the size and the surface charge of the first liposome dose had a significant effect on the clearance rate of the second dose [106]. Similarly, Koide and coworkers observed that smaller polymeric micelles (9.7 – 31.5 nm) did not initiate ABC phenomenon in BALB/c mice, whereas larger PEGylated liposomes or micelles triggered rapid blood clearance and hepatic uptake with the second injection [107]. The PEG functionality may also play a role; Sherman and Saifer recently performed competitive analysis of PEG and protein-PEG conjugates to elucidate the role of methoxy end groups in antibody generation, and reported that the antibody binding affinity depended on the backbone lengths of the PEG polymers and the hydrophobicities of the methoxy containing end groups. Antibodies preferentially bound to the PEG backbone when the end functional group was a hydroxyl, and with lesser affinity than binding to methoxy functionalized PEG conjugates [108, 109]. Further, factors such as the injection interval may also influence the observed immune response. Multiple reports indicated that the systemic IgM levels were remarkably reduced 14 days after the first injection, then became similar to the pre-injection levels after 28 days [94, 101, 110]. Thus, careful adjustment of the dosing frequency can potentially offset some of the effects of ABC.
Concern is also growing over pre-existing anti-PEG antibodies; 22-25% of individuals who were never exposed to PEGylated therapeutics were found to have anti-PEG antibodies, which is a significant increase from 0.2% reported two decades ago [111]. The driving force behind this increase is unclear, though it is thought to be related to improved detection methods and the ubiquitous use of PEG in cosmetic, pharmaceutical, and food products [85, 112]. In clinical settings, pre-existing anti-PEG antibody levels in each patient should be carefully considered. Measuring anti-PEG antibody levels in an individual could be used to determine whether a particular therapeutic is a suitable treatment option. As the impact of dose on immune response becomes more clear, patients with low anti-PEG antibody levels prior to treatment could be exposed to an adequate amount of placebo PEGylated NPs to reduce or prevent ABC with subsequent injections.
In addition to the previously described strategies under investigation for reducing ABC of PEGylated therapeutics, there is also significant effort toward identifying potential PEG alternatives, including polymers such as poly(amino acids), poly(glycerol) (PG), poly(N-(2-hydroxypropyl) methacrylamide) (HPMA), and others [43, 81, 113]. PGA, a biodegradable poly(amino acid), is perhaps one of the most thoroughly studied PEG alternatives. PGA-conjugated paclitaxel (PGA-paclitaxel) was the first poly(amino acid) coated drug to reach phase III clinical trials for treatment of ovarian cancer [113]. A phase I/II study of PGA-paclitaxel and Cetuximab is also in progress for advanced head and neck cancer. Some PGA derived therapeutics have exhibited complement activation, however it only caused moderate hypersensitivity reactions [81]. Other poly(amino acid)-based coatings, including 3 kDa poly(hydroxyethyl-L-asparagine) and 4 kDa poly(hydroxyethyl-L-glutamine), have facilitated prolonged liposome circulation in the blood, but ABC comparable to 5 kDa PEGylated liposomes was also observed [81]. PG is a non-biodegradable, biocompatible hydrophilic polymer that has a similar chemical structure to PEG. PG is often used in its linear or hyperbranched form, and the latter has exhibited prolonged plasma half-life in some reports (33 h for 106 kDa and 57 h for 540 kDa) [114, 115]. In contrast to the MW dependent blood circulation characteristics of PEG, no difference in biocompatibility and complement activation were observed for hyperbranched PG of 106 kDa and 870 kDa; however, these characteristics are not the only factors that affect the blood circulation (e.g. complement-independent MPS uptake) [114]. Liposomes and lipoplexes modified with PG elicited reduced IgM production with repeated injections, compared to their PEGylated counterparts [116, 117]. Also to be considered, hyperbranched PG has been observed to slightly increase blood viscosity in vivo, which has been shown to cause a variety of physiological side effects [118]. To compare the potential of several different PEG alternatives as surface modifiers to increase circulation time and reduce the ABC effect, Kiersted and coworkers synthesized a panel of polymer diacyl chain lipids of similar MW and polydispersity to incorporate into liposomes [119]. They evaluated liposomes ~100 nm in size containing lipids modified with PEG, HPMA, poly(vinylpyrrolidone) (PVP), poly(2-methyl-2-oxazoline) (PMOX), poly(N,N-dimethyl acrylamide) (PDMA), and poly(N-acryloyl morpholine) (PAcM), and compared the circulation time in mice and rats. They confirmed that HPMA-, PVP-, PMOX-, PDMA-, and PAcM-modified liposomes had increased circulation time in rodents, and that PVP-, PDMA-, and PAcM-modification did not induce an ABC effect in rats with a second dose administered one week later. They were also able to demonstrate for the first time that HPMA-modification did not cause an ABC effect, whereas PMOX-modified liposomes were rapidly cleared with the second dose in rats. They confirmed that PMOX-modified liposomes induced an IgM response in rats with one dose, similar to PEG-modified liposomes [119]. This work suggests that alternatives such as HPMA or PVP deserve further consideration as polymer coatings to improve the circulation time of liposomes. However, widespread adoption of any PEG alternative would ultimately lead to similar concerns about immunogenicity in the population, but simply having access to a wider array of options for therapeutic formulations is certainly advantageous.
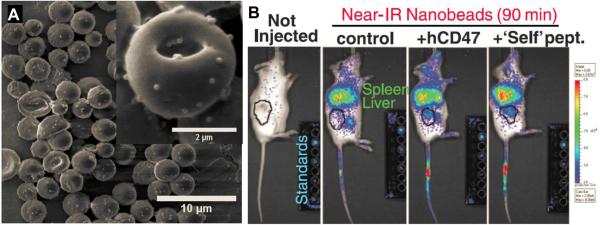
In addition to polymeric alternatives to PEG, other approaches have been suggested for increasing NP circulation time. Mitragotri and coworkers suggested a ‘hitchhike’ method, in which polystyrene NPs were attached to red blood cells for prolonged circulation (Fig. 3A) [120]. NP binding was mediated by electrostatic and hydrophobic interactions, and thus reversible under physiological shear stresses experienced by red blood cells in circulation. Functionalizing the NPs with anti-ICAM-1 antibodies led to increased accumulation of NPs in the lungs [120]. Rodriguez and coworkers computationally designed minimal peptide sequences that mimic CD47, a reported “marker of self” that impedes phagocytosis. When these “self” peptides were attached to PS NPs, the NPs experienced delayed macrophage-mediated clearance, increased circulation time, and enhanced tumor accumulation (Fig. 3B) compared to control PS NPs [121].
Figure 3.
Novel alternative approaches to PEGylation for increasing the systemic circulation time of NPs. (A) PS NPs were attached to red blood cells via reversible electrostatic and hydrophobic interactions, prolonging systemic circulation time and increasing accumulation in the lungs. Adapted with permission from [115]. (B) Attaching minimal “self” peptides designed to mimic the “marker of self”, CD47, to the surface of PS NPs led to increased circulation time and enhanced tumor accumulation compared to control NPs. Adapted with permission from [116].
3. Non-systemic applications for improved delivery with PEGylated nanoparticles
For many applications, local rather than systemic delivery can improve efficacy while minimizing off-target side effects, but every mode of administration has associated barriers to effective delivery. Although PEGylation was first employed to increase circulation time, improve stability in circulation, and reduce interactions with serum components, benefits of coating NPs with PEG have also be observed with various other non-systemic modes of administration. Discussed in this section, PEG coatings can improve the penetration of “biological barriers”, including reducing interactions with tissue extracellular matrix, cellular barriers, and biological fluids such as mucus, leading to improved delivery. Here, we discuss only studies that directly compared NPs with and without PEG coatings and/or variations in PEG molecular weight and surface density to demonstrate the effect that the PEG coating had on delivery.
3.1 PEGylation for improved vaginal nanoparticle delivery
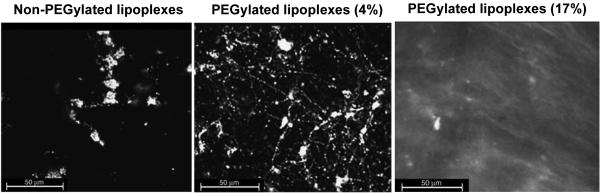
Local delivery to the vaginal tract may be advantageous for treating and preventing various conditions that affect the female reproductive tract, such as cervical cancer, bacterial vaginosis, and sexually transmitted infections. However, the viscoelastic and rapidly cleared mucus layers coating the cervicovaginal tract pose a barrier to NP-based drug and gene delivery [122-124]. It was demonstrated that polystyrene (PS) NPs 59-1000 nm in size were completely immobilized in human cervical mucus [125], leading Lai and coworkers to investigate methods for improving NPs transport across the cervicovaginal mucus (CVM) barrier [68]. They confirmed using multiple particle tracking [126] that carboxylate-modified PS NPs 100-500 nm in size were completely immobilized in human CVM, whereas the same particles coated with PEG (PS-PEG) diffused only a few times slower than their theoretical diffusion rates in pure water. This result suggested that (i) mucus is a mesh of proteins with low viscosity fluid-filled pores large enough for 500 nm NPs (if non-adhesive) to diffuse through, and (ii) NPs without PEG coatings become trapped in mucus by adhesive interactions [68]. However, it has also been reported extensively in the literature that PEG can be strongly mucoadhesive via interpenetration effects and hydrogen bonding [127-129], leading Wang and coworkers to explore the role of PEG molecular weight surface density on nanoparticle mucoadhesion [130]. They found that 200 nm PS-PEG particles diffused rapidly in human CVM when coated with low molecular weight (2 or 5 kDa) PEG, but PS-PEG NPs coated with 10 kDa PEG were immobilized. Using a fluorophore conjugation method, they then determined that 200 nm PS-PEG with 40% less 2 kDa PEG coverage, compared to the densely coated and rapidly diffusing NPs, were adhesively immobilized in CVM. Thus, they concluded that a dense coating of low MW PEG was necessary to effectively shield the NP core from interactions with mucin components, while also preventing interactions between the PEG and the mucus [130].
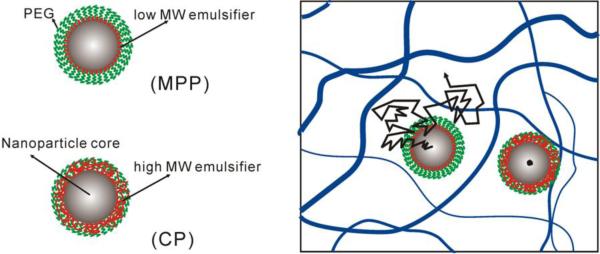
It was later confirmed that penetration of NPs through mucus provided improved delivery in the cervicovaginal tract. Cu and coworkers devised a PEG-coated poly(lactic-co-glycolic) (PLGA) NP (PEG-NP) formulation using avidin-biotin chemistry that diffused more rapidly in human cervical mucus than uncoated PLGA NPs [131]. They then went on to demonstrate that the PEG-NP were retained at significantly higher levels in the cervicovaginal tracts of Depo-Provera-treated mice for up to 6 h [132]. They further suggested that because PEG-NP were able to penetrate the mucus barrier, they were also able to penetrate into the vaginal tissue [132]. Ensign and coworkers went on to demonstrate that by penetrating through CVM, both PS-PEG NPs and PLGA NPs coated with a physically-adsorbed Pluronic F127-based PEG coating [133] (PLGA/F127) provided greatly improved and more uniform coverage of the cervical and vaginal surfaces in mice [134]. They hypothesized that the uniform coating of the tissue with PEG-coated NPs, including penetration into the more slowly cleared mucus layers in the vaginal folds (rugae), led to the prolonged retention observed (60% of PS-PEG NPs compared to 10% of PS NPs 6 h after dosing) [134]. Yang and coworkers then went on to demonstrate that PEG-coated NPs with non-mucoadhesive surfaces provided improved suppression of cervical tumor growth after vaginal administration in vivo [135]. They formulated paclitaxel-loaded, mucus-penetrating PLGA/F127 NPs (PTX/MPP) and paclitaxel-loaded, mucoadhesive PLGA NPs (PTX/CP) with similar size and drug loading/release properties. Presumably, the only difference between the formulations was that the PTX/MPP rapidly penetrated human CVM, whereas the PTX/CP were mucoadhesive. Thus, the PTX/MPP distributed more uniformly in the mouse cervicovaginal tract, including up against the epithelial tumor, whereas the PTX/CP aggregated in the mucus far from the epithelial surface. The vaginally-administered PTX/MPP suppressed tumor growth compared to PTX/CP and free Taxol, leading to enhanced median survival time (19, 11, and 9 days, respectively). Importantly, numerous methods for producing biodegradable NPs with sufficiently dense PEG coatings for rapid penetration through human CVM have been described [133, 136-138], providing an array of options for developing NP-based therapies for more efficacious vaginal drug delivery.
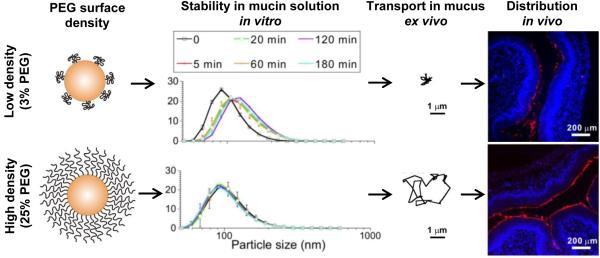
The effect of PEG density on vaginal nanoparticle distribution and delivery has also been studied. Yu and coworkers prepared liposomes composed of standard neutrally charged lipids (DSPC), cholesterol, and different amounts of lipids conjugated to 2 kDa PEG (DSPE-PEG2k) to produce liposomes with a range of PEG surface densities [139]. They found that the non-PEGylated liposome formulation was relatively polydisperse and showed some signs of aggregation, whereas the PEGylated liposomes were relatively uniform in size, highlighting the added stability provided by PEG coatings. They then used an nuclear magnetic resonance (NMR)-based method (see section 4.3) to measure the molar fraction of PEGylated lipids incorporated into the liposomes, which allowed them to estimate the ratio of the theoretical surface area that would be covered by unconstrained 2 kDa PEG chains to the actual surface area of the liposome (Γ/SA). They concluded that their liposomes containing 7, 10, and 12 mol% PEG were coated by dense, brush-like PEG coatings, whereas their liposomes containing 3 and 5 mol% PEG were covered with mushroom-like PEG chains that would provide a less effective surface shielding effect. Upon observation of transport behavior in human CVM, it was evident that the contrast between PEGylated and non-PEGylated liposomes was not as drastic as what was observed previously with polymeric NPs, though the PEGylated liposomes uniformly diffused more rapidly. The authors concluded that the zwitterionic DSPC headgroups may have reduced interactions with mucus compared to hydrophobic polymer NPs like PS, but nonetheless, adhesion and aggregation of the liposomes in mucus was reduced by PEG coatings [139]. They then went on to investigate the impact of the PEG coatings on vaginal distribution in vivo, and found that the non-PEGylated liposomes distributed very poorly. The 3 mol% liposomes provided some improvement in vaginal tissue coverage and uniformity, while the 7 mol% liposomes provided much more uniform vaginal coverage. Although it might be assumed that the 12 mol% liposomes would also distribute much more uniformly in the vagina, the uniformity of coverage was decreased compared to 7 mol%. The authors concluded that the further increase in PEG content may have created some instability of the 12 mol% liposomes in the complex vaginal environment in vivo. They then went on to demonstrate that the 7 mol% liposomes containing a novel diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI) contrast agent provided increased retention of the contrast agent for non-invasive monitoring of vaginally administered liposomes [139]. In similar studies using biodegradable NPs, Xu and coworkers formulated PEG-coated PLGA NPs with various PEG surface densities by blending PLGA and PLGA-PEG (5 kDa PEG) block copolymers [140]. Using an NMR-based method, they determined that a dense, brush-like PEG coating was achieved for particles containing 5 wt% PEG (PLGA-PEG5%) and above (up to 25 wt%, PLGA-PEG25%). Indeed, these formulations were stable in mucin solution in vitro and rapidly penetrated human CVM ex vivo (Fig. 4). In contrast, formulations containing 3 wt% PEG (PLGA-PEG3%) and less, were relatively unstable in mucin solution in vitro and were hindered and/or immobilized in human CVM ex vivo (Fig. 4). The uncoated PLGA NPs were the most unstable in vitro and were completely immobilized in CVM. They then investigated whether the trends of improved stability with increasing PEG coverage in vitro and more rapid transport with increasing PEG coverage ex vivo would translate to improved distribution in the mouse vagina in vivo. Indeed, they observed that uncoated PLGA NPs aggregated and distributed poorly over the tissue surface in the mouse vagina, whereas some improvement was observed for PLGA-PEG3%. Indeed, the tissue surface coverage was increased even further with PLGA-PEG10%, with the highest and most uniform coverage provided by PLGA-PEG25% (Fig. 4). In contrast to the liposomal work, increasing the PEG content in these polymeric NPs to 25 wt% did not introduce instability. They went on to describe several formulations with sufficient PEG surface density for improved vaginal mucosal delivery containing paclitaxel at various loadings and with various release rates, providing a framework for tailoring formulations with improved mucosal delivery [140].
Figure 4.
The impact of PEG surface density on interactions with mucus in vitro, ex vivo, and in vivo. A polymer blending approach was used to produce biodegradable PLGA NPs of similar size with increasing PEG surface densities. Above a certain threshold (5% target PEG content for this formulation), NPs were more stable in mucin solution in vitro, diffused rapidly in human cervicovaginal mucus ex vivo, and distributed more uniformly over the vaginal surface of mice in vivo. A dense PEG coating protected the NP surface from interactions with mucus, leading to more uniform delivery to the mucosal surface. Adapted with permission from [135].
In addition to providing delivery benefits in the vagina, PEG-coated NPs have also been used as probes to characterize CVM structure using particle tracking microrheology (PTM), which can provide important insights into how particles and pathogens can penetrate mucus [141]. PTM assumes that the motions of particles within a complex environment are directed by the local properties of that material. If particles are larger than the pores within the material or adhesive to the material, then the motions of the particles reflect the bulk viscoelastic properties of the material, which is the most common way that PTM is used to characterize materials. In contrast, if the particles are sufficiently smaller than the pores in the material and are non-adhesive to the material, such as densely PEGylated NPs 500 nm or less in size in human CVM, then the particles experience the low viscosity of the interstitial fluid within the pores. For example, Lai and coworkers demonstrated that CVM transitions from an impermeable elastic barrier to PEGylated particles 1 µm and larger in size to a highly permeable viscoelastic liquid to PEGylated NPs 500 nm and less in diameter [141]. They also demonstrated that pretreatment of mucus with a detergent caused this transition to occur on a much shorter length scale (200 nm PEGylated NPs experienced an impermeable elastic barrier), though the macroscopic viscoelastic properties were unchanged. This observation gave additional insight into the microscopic structure of mucus as a mesh of bundled mucin proteins that could be disrupted by the addition of a detergent, but increased protein entanglements could offset the decreased hydrophobic bundling on the macroscale [141]. Similarly, PEG-coated NPs revealed microscopic structural changes in CVM that occurred after the addition of a large amount of mucoadhesive NPs, changes which also could not be detected using traditional characterization of macroscopic rheology [142]. In this case, the increased mucin bundling caused by the mucoadhesive particles enlarged the effective pore size of the mucus mesh on the microscopic scale, which has implications both for therapeutic delivery and the potential for environmental NPs to disrupt mucus barriers [142]. The diffusional trajectories of various sized non-adhesive PEG-coated probe NPs can also be used to estimate the pore size distribution within a biopolymer matrix such as mucus or tissue. For example, it was estimated that the average pore size in human CVM was 340 ± 70 nm with a range of 50-1800 nm [143]. Such a large range was not predicted based on estimations if mucus was a mesh of randomly arrayed individual mucin fibers (15-100 nm pores), and further suggested that trapping of pathogens like viruses in the 100-200 nm size range must be based on adhesive interactions rather than steric obstruction [143]. Such characterization of changes in the microscopic structure of mucus that cannot be detected on the macroscopic scale would not be possible without densely PEG-coated, non-adhesive NPs.
3.2 PEGylation for improved nanoparticle delivery to the airways
Pulmonary administration also presents an opportunity for more efficacious local treatment and prevention of conditions that affect the airways. However, the airways are also covered with a layer of mucus that is rapidly cleared and regenerated via mucociliary clearance (MCC) mechanisms. Mucus is certainly an effective barrier in the normal airways, but in many inflammatory and obstructive disease states, such as cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD), airway mucus is a particularly viscoelastic and difficult to penetrate barrier. Suk and coworkers investigated the effect of PEG coatings on NP penetration through freshly expectorated CF sputum [144]. They found that 200 nm amine-modified PS particles were immobilized, whereas PS-PEG particles 100 and 200 nm in size were able to penetrate. PS-PEG particles 500 nm in size were sterically trapped in CF sputum, highlighting that the CF sputum mesh contains smaller pores on average than what was observed in human CVM (estimated average pore size was 140 ± 50 nm, range 60-300 nm). Importantly, the local microviscosity experienced by 100 and 200 nm PS-PEG was fluid-like (5x higher viscosity than water), whereas the macroscopic properties of the CF sputum was indicative of an elastic solid (10,000x higher bulk viscosity than water) [144]. Based on the bulk properties, CF sputum would be expected to be impenetrable, highlighting the importance of densely PEG-coated NPs for both characterizing CF sputum microstructure and designing more effective therapeutic strategies for delivery to the CF airways. Forier and coworkers also thoroughly characterized the transport of amine-modified, carboxylate-modified, and PEG-coated (both 2 kDa and 5 kDa) PS NPs in expectorated CF sputum [145]. They also confirmed that PEG coatings on 100 and 200 nm PS NPs led to improved penetration through CF sputum, though they noted marked intra-patient variability in the NP transport, highlighting the effect that disease severity can have on the efficacy of therapeutic delivery. Also, importantly, they investigated the penetration of NPs through biofilms, which is another significant barrier that may lie under the CF sputum barrier in the lungs of CF patients. They generally observed that PS-PEG particles diffused through two species of biofilm cultures similarly to their theoretical diffusion through pure water, whereas both amine-modified and carboxylate-modified PS NPs were hindered. However, interactions between the PEGylated NPs and the bacteria were reduced compared to charged PS NPs, and the authors suggest that the binding observed between charged NPs and biofilm components would be preferable for antibiotic delivery. However, the charged NPs were not able to penetrate CF sputum, so they would not be able to reach the biofilm to deliver the antibiotic payload. The authors concluded that a drug-loaded nanocarrier with a sheddable PEG coating or an active targeting moiety on the end of the PEG may be beneficial for first penetrating the CF sputum barrier and then directly interacting with an underlying biofilm [145].
It has also been discovered that NP PEG surface density plays an important role in the penetration of CF sputum. In particular for gene therapy strategies, NPs must be able to penetrate the CF sputum barrier in order to reach target cells for transfection. Boylan and coworkers investigated the diffusion of a clinically-tested gene vector system [146] composed of poly-L-lysine conjugated with a 10 kDa PEG segment (CK30PEG10k) in CF sputum [147]. In addition, they formulated CK30PEG5k and CK30PEG2k polymers, and all three polymers produced small, colloidally stable, nuclease resistant DNA NPs. All three DNA NP formulations provided increased gene transfer in the lung airways in mice in vivo compared to naked DNA, yet all were also immobilized in CF sputum freshly expectorated from patients. To explain this phenomenon, they approximated the Γ/SA values and the PEG surface density, and concluded that the density of the PEG coatings were insufficient to allow for penetration of CF sputum, which would limit their effectiveness as gene delivery vectors for CF therapy [147]. Suk and coworkers then went on to explore the possibility of pretreating CF sputum with mucolytics, including a reducing agent (N-acetylcysteine, NAC) and recombinant human DNase (rhDNase), to attempt to partially degrade the CF sputum barrier and enhance the penetration of CK30PEG10k [148]. They found that pretreatment with NAC and NAC+rhDNase improved the DNA NP mobility in CF sputum, but a significant portion of the particles were still hindered. However, even this small improvement in CK30PEG10k penetration due to pretreatment with NAC led to a significant increase in in vivo gene transfer in a mouse model of mucus hypersecretion (mice challenged with intranasal P. aeruginosa lipopolysaccharide) when the mice were pretreated with inhalation of 0.5 M NAC solution prior to DNA NPs [148].
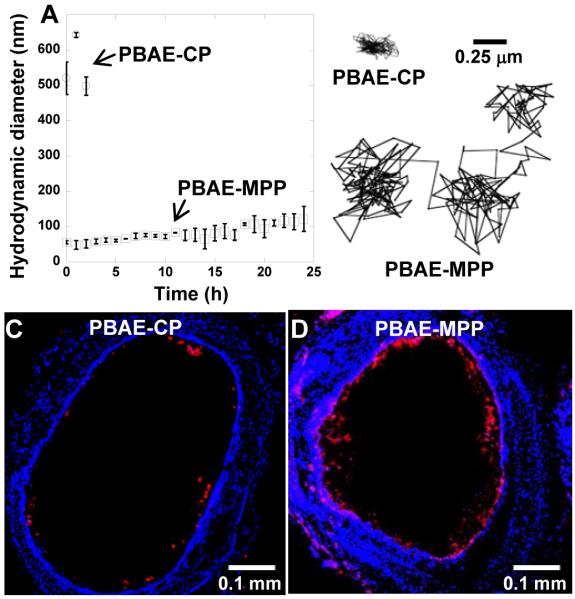
However, it would be preferable to design DNA NP platforms that can penetrate CF sputum without the need for pretreatments. Kim and coworkers utilized cystamine core poly(amido amine) (PAMAM S-S) dendrimers conjugated to cationic polymers, since the branched dendrimer structure provided a means for conjugating many molecules of PEG to each dendron to increase the PEG surface density upon complexation with DNA [149]. Using both PAMAM G4 dendrimers and polyethyleneimine (PEI) as the cationic polymer, they were able to produce PEG-dendron-based DNA NPs (dPEG-PAMAM/DNA and dPEG-PEI/DNA) that were colloidally stable, resistant against nuclease, and rapidly penetrated CF sputum. Importantly, the PEGylation resulted in minimal reduction in gene transfer efficiency in vitro compared to the non-PEGylated DNA NPs. In addition, both dPEG-PAMAM/DNA and dPEG-PEI/DNA NPs were able to transfect cystic fibrosis bronchial epithelial cells grown in conventional cell culture conditions to produce wild-type cystic fibrosis transmembrane conductance regulator (CFTR) protein, which is the main target for CF therapy [149]. Suk and coworkers went on to describe highly compacted DNA NPs composed of PEGylated PEI (PEI-MPP) and PLL (PLL-MPP) capable of penetrating through CF sputum [150]. In contrast, non-PEGylated formulations and formulations with “conventional” PEGylation (meaning lower PEG surface density) were largely hindered or immobilized in CF sputum. They then went on to demonstrate that PEI-MPP distributed uniformly in the airways of mice, whereas the non-PEGylated formulation (PEI-UCP) largely aggregated and distributed poorly in the airways. The improved mucus penetration and distribution of PEI-MPP also led to prolonged retention in the airways, whereas the PEI-UCP were rapidly cleared by MCC and accumulated in the stomach. Lastly, they confirmed that the uniform distribution of PEI-MPP over the epithelial surface in the airways also led to increased and more uniform transgene expression in the mouse airways, and that the improved level of transgene expression achieved with PEI-MPP did not decrease after multiple doses [150]. However, potential concerns over the non-degradability and immunogenicity of PEI, even though PEGylated versions do not seem to cause the same toxicity as naked PEI, led Mastorakos and coworkers to develop PEGylated DNA NPs based on biodegradable poly(β-amino ester) (PBAE) [151]. They condensed plasmid DNA with a blend of PBAE and PEG-conjugated PBAE polymers to form small, colloidally stable DNA-NP (PBAE-MPP); conventional non-PEGylated PBAE DNA NPs (PBAE-CP) aggregated rapidly in bronchoalveolar lavage fluid (Fig. 5A). Further, PBAE-MPP rapidly penetrated human CF sputum, whereas PBAE-CP were hindered or immobilized (Fig. 5B). As expected due to the rapid penetration of PBAE-MPP in CF sputum, PBAE-MPP distributed throughout the mouse large airways and lung parenchyma, whereas the mucoadhesive PBAE-CP distributed poorly in vivo (Fig. 5C, D). Accordingly, the distribution of gene expression was similar to the distribution of the DNA NP formulations, with expression mediated by PBAE-MPP being widespread and highly uniform. The densely PEGylated PBAE-MPP also provided similar high levels of transgene expression after multiple administrations, and did not cause significant inflammation in the mouse airways compared to saline treatment [151]. Thus, in the case of pulmonary administration of DNA NPs, dense PEG coatings have been demonstrated to provide colloidal stability, protection of DNA cargo, improved penetration of mucus leading to more uniform distribution, reduced toxicity, and the ability to administer multiple doses without decreasing the efficiency of delivery. The latter may be similar to what was observed by O’Riordan and coworkers previously with adenovirus; PEGylation could protect the viral vectors from neutralizing antibody binding in vitro and in the mouse lungs in vivo, leading to maintained infectivity after multiple administrations [152].
Figure 5.
The effect of PEG surface coatings on the behavior of biodegradable PBAE-based DNA-NPs in vitro, ex vivo and in vivo. (A) PEG-coated PBAE-MPP remained stable in BALF at 37ºC, whereas uncoated PBAE-CP rapidly aggregated. (B) PBAE-MPP diffused rapidly in freshly expectorated CF sputum, whereas PBAE-CP were largely hindered or immobilized. Immobilization in CF sputum correlated with poor distribution of (C) PBAE-CPs in the airways of mice in vivo, whereas (D) PBAE-MPPs distributed more uniformly in the mouse trachea. Adapted with permission from [146].
In disease states like CF, sputum can be spontaneously expectorated for studies ex vivo, whereas it is difficult to obtain undiluted mucus from the healthy airways to study the barrier properties to NP formulations. Yang and coworkers studied the barrier properties of porcine tracheal mucus to NPs and pseudorabies virus (PRV) [153]. They found that 200 nm amine-modified and carboxylate-modified PS NPs were immobilized in porcine tracheal mucus, whereas PEG-coated NPs rapidly diffused. Although PRV is of similar size to the NPs (246 nm), it was also immobilized in porcine tracheal mucus, indicating that it must interact adhesively with mucins similarly to uncoated PS NPs. Thus, they then found that PEGylating the surface of the PRV virus resulted in increased mobility in the tracheal mucus, further highlighting the role of adhesive interactions with mucus in host defense [153]. NPs that stick to mucus will also be trapped and removed, whereas dense PEGylation results in improved penetration. Schuster and coworkers characterized the transport of NPs in human respiratory mucus collected from the endotracheal tubes of surgical patients with no pulmonary comorbidities [154]. They found that aside from a subpopulation of 100 nm carboxylate-modified PS NPs that may have become coated in airway surfactants, the non-PEGylated NPs were immobilized in normal human respiratory mucus. In contrast, densely PEGylated NPs as large as 200 nm were capable of penetrating normal human respiratory mucus as if it were primarily a viscous liquid [154]. As previously discussed, such a property could be beneficial for NP-mediated delivery to the human airways. Further, using various sized non-adhesive PEGylated NPs to characterize the structure of normal human respiratory mucus can provide additional insight into transport mechanisms of respiratory viruses and environmental NPs.
PEGylation has also been explored for improving NP delivery to the nasal mucosa. Nasal mucus is also a barrier to NP-based delivery. In particular, highly viscoelastic mucus accumulates in the sinuses of chronic rhinosinusitis (CRS) patients. Lai and coworkers observed that uncoated PS NPs were immobilized in CRS mucus (CRSM) [155], similar to what was previously observed for CF sputum. In addition, PEGylated NPs as large as 200 nm were able to rapidly penetrate CRSM, including samples from more than half of patients with nasal polyps. They were also able to demonstrate similar penetration for biodegradable PEG-coated NPs (PLGA/F127), suggesting the possibility of developing new nanomedicine-based therapies for improved treatment of CRS [155]. In addition to nasal mucus, the nasal mucosa stands as a significant barrier to absorption. Brooking and coworkers investigating the absorption of NPs across the rat nasal mucosa and into the systemic circulation [156]. They coated sulfate-modified PS NPs with a poloxamine 908, a PEG-containing diblock copolymer, which was previously shown to increase the circulation time of NPs after intravenous administration. They found that the coating reduced intranasal NP uptake, which they attributed to reduced interactions between the PEG-coated NPs and the nasal associated lymphoid tissue (NALT) [156]. However, others have found PEG coatings to be beneficial for NP absorption across the nasal mucosa. Tobio and coworkers described improved absorption of tetanus toxoid-loaded PLA-PEG NPs compared to uncoated PLA NPs across the nasal mucosa [157]. They found that 24 h after intranasal administration, the percentage of radioactive tetanus toxoid in the lymph nodes, lungs, liver, and spleen was between 3-6 fold higher for PLA-PEG NPs than for PLA NPs. They proposed that the PLA-PEG NPs could be partially taken up by the NALT, but that they could also be transported by transcellular or paracellular pathways to be drained to the lymphatics and blood. They also do not rule out the effect of uptake in the lower respiratory tract and gastrointestinal tract, considering that intranasal administration does not only target the nasal passages [157]. Vila and coworkers went on to study the effect of PEG surface density on the absorption of PLA-PEG NPs across the nasal mucosa [158]. They used NMR to characterize the PEG coating density, measured as a mass of PEG per 100 mg of particles. The generally found that smaller, more densely PEG-coated PLA-PEG NPs appeared to be absorbed more readily by the rat nasal mucosa. However, the absorption was inferred using confocal image stacks of the mucosa, rather than uptake into the circulation or any particular compartment. They suggested that increased stability in the presence of mucus and transcytosis may contribute to the improved absorption of PEG-coated NPs across the nasal mucosa [158].
3.3 PEGylation for improved gastrointestinal nanoparticle delivery
The gastrointestinal (GI) tract is a common target site for drug and gene delivery, as oral administration is a simple and often preferred mode of delivery. However, there are numerous barriers to effective GI delivery, such as the harsh GI environment. The known stability enhancing properties of PEG coatings have also been studied for improved NP delivery to the GI tract. Tobio and coworkers demonstrated that PEG improved the stability of PLA-PEG NPs in digestive fluids in vitro, which led to enhanced oral tetanus toxoid delivery in rats compared to uncoated PLA NPs [159]. They observed 5-times higher radioactive tetanus toxoid levels in the blood after administration PLA-PEG particles compared to PLA particles for up to 24 h, despite the belief that hydrophobic NPs are more favorably absorbed across the GI mucosa [159]. Feeney and coworkers also utilized PEG coatings to improve the stability of lipid-based formulations (LBFs) containing medium chain triglycerides (MCT) [160]. They incorporated non-ionic PEG-containing surfactants with various PEG MWs into the MCT LBFs to attempt to reduce digestion in the GI tract. They found a parabolic relationship between the surfactant PEG MW and the resistance to digestion; increasing PEG MW to 2644 Da provided enhanced resistance to digestion, but the surfactant with PEG MW 4407 Da caused an increase in digestibility. It is possible that similar to other lipid-based formulations, incorporating too much hydrophilic PEG can introduce some instability to the formulation. They went on to demonstrate that oral administration of the “stealth” PEGylated formulations to rats led to increased absorption of a poorly water soluble drug, danazol [160]. Cheng and coworkers PEGylated adenoviral vectors to improve their stability for oral administration as gene delivery vectors for colorectal cancer [161]. They covalently attached one of three different types of functionalized 5 kDa PEG to the free lysine groups on the protein capsids, and found that PEGylation with all three types of PEG actually increased the efficiency of transduction efficiency in differentiated enterocytes. They then confirmed that the PEGylation improved the in vitro stability of the adenoviral vectors in gastric fluid and pancreatic enzymes to varying degrees depending on the type of PEG used. They selected the vectors PEGylated with succinimidyl succinate functionalized PEG (SSPEG) for in vivo oral administration studies, finding significantly increased reporter protein expression throughout the GI tract (40-times higher expression in the colon) with the SSPEG vector compared to the unmodified adenoviral vector. Additional characterization of the internalization of SSPEG vectors by Caco-2 cells suggested that although they PEGylated 70-80% of the available attachment sites on the viral capsid, the PEGylation appeared to actually change the type of receptor interaction that led to internalization, which may have also led to increased gene transduction in vivo [161].
In addition to the harsh GI environment, NPs administered to the GI tract encounter rapidly cleared layers of viscoelastic and adhesive mucus [162]. If the goal is systemic absorption of NPs or cargo released from the NPs, penetrating through the mucus barrier would provide closer proximity to the absorptive epithelium. Yuan and coworkers evaluated the potential for PEGylation to improve the oral delivery of solid lipid NPs (SLN) [163]. They hypothesized that the PEG coating would increase the hydrophilicity of the SLN and minimize mucoadhesion. Doxorubicin-loaded, PEGylated SLN (pSLN) were formulated by incorporating PEG monostearate (2 kDa PEG) into the formulation, though both the SLN and pSLN were suspended in poloxamer 188 solution (a PEG-containing triblock copolymer). They found that increasing the amount of PEG in the pSLN decreased the in vitro cytotoxicity. Further, they found increased drug permeation across Caco-2 cell layers (non-mucus secreting) for the SLN formulation with increasing amounts of PEG in the pSLN further decreasing the drug permeability. However, when mucus-secreting HT29 cells were incorporated into the Caco-2 cell layers, the pSLN formulation with 10% PEG (pSLN-10%) provided the highest levels of drug permeation across the cell layers. Accordingly, they found that the pSLN-10% formulation provided higher plasma drug levels after oral administration to rats, compared to the SLN (~2-fold higher bioavailability) and oral drug solution (~7.5-fold higher bioavailability) [163]. Chen and coworkers also explored the effect of coating cyclosporine A (CyA)-loaded liposomes with either PEG or chitosan, a mucoadhesive polymer, for oral delivery [163]. Unmodified liposomes (Lip) were made by membrane extrusion, and PEG-coated liposomes (PF127-Lip) were formulated by incorporating Pluronic F127. Unmodified liposomes were coated in chitosan to make mucoadhesive liposomes (CS-Lip). All three liposome formulations were relatively stable in simulated gastric fluid, whereas the CS-lip aggregated extensively in simulated intestinal fluid. Two hours after oral administration to rats, they found that the PF127-Lip appeared to penetrate through the mucus and into the GI tissues throughout the small intestine. In contrast, the CS-lip appeared to be aggregated and associated with the mucus layers. As confirmation of the importance of the mucus barrier, they found that hydrolyzing the mucus prior to oral administration resulted in higher tissue-associated fluorescence for CS-Lip compared to PF127-Lip (and much higher delivery to the tissue overall), whereas the PF127-Lip provided higher fluorescent signals when the mucus layers were intact. Lastly, they confirmed that the PF127-Lip provided increased CyA bioavailability upon oral administration compared to Lip (1.3-fold higher AUC) and CS-Lip (1.8-fold higher AUC) [163].
Inchaurraga and coworkers investigated the effect of PEG molecular weight on orally administered NPs prepared from methyl vinyl ether-maleic anhydride copolymer (Gantrez® AN) [164]. In this study, the PEG coating was formed by incubation of the two polymers and desolvation, rather than covalent conjugation. Interestingly, the ζ-potential of the NPs was still highly negative for the PEG-coated particles; the uncoated NPs (NP) were −52 mV while NPs with 10 kDa PEG (NP10) were −43 mV. They observed that the NP formulations with PEG had a faster transit time through the GI tract of rats after oral administration, whereas the uncoated NP were retained longer in the stomach. Fluorescent images of the ileum suggested that the PEG containing particles were able to reach the villus cell surfaces, whereas the uncoated NP remained in the luminal mucus away from the tissue. They further suggested that the effect was more pronounced for NPs containing 2 kDa and 6 kDa PEG than 10 kDa PEG [164]. Maisel and coworkers investigated the effect of dense PEG coatings and NP size on both orally and rectally administered NPs [165]. In prior work, the group had confirmed that uncoated PS NPs (mucoadhesive particle; MAP) were adhesively immobilized in mouse mucus coating colorectal and small intestine tissues ex vivo, whereas densely PEG-coated PS NPs (mucus-penetrating particle; MPP) below a certain location-specific size limit could rapidly penetrate [166]. Maisel and coworkers first demonstrated that diffusion of 200 nm MPP in mouse small intestine mucus led to more uniform surface coverage in the small intestine compared to the adhesively immobilized MAP. Due to natural fluid absorption in the intestines, the MPP were able to distribute throughout the small intestine lumen, penetrating between villi, whereas MAP largely aggregated in the center of the lumen away from the epithelium. The striking difference in distribution was even more pronounced when the particles were dosed orally to fed mice, as the non-adhesive MPP did not stick to digesta. Further, they demonstrated that using large gavage volumes or using intestinal loop models artificially distributed adhesive MAP throughout the intestine, due to high fluid pressures that distended the epithelium and overwhelmed the mucus barrier, effects that would not normally be present when taking a pill. They then went on to demonstrate that rectally administered MPP distributed throughout the colorectum, including into the tissue folds, whereas MAP did not. The benefits of the NP PEG coating on MPP distribution orally and rectally were also seen in two mouse models of inflammatory bowel disease (IBD). Furthermore, the PEG-coated MPP were able to penetrate into ulcerated tissue regions in the colorectum of mice with 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS)-induced colitis, which may provide additional delivery benefits. It has yet to be demonstrated whether delivering PEG-coated MPP to more of the absorptive tissue surface can provide improved drug absorption, as expected [154]. Other work has also demonstrated potential benefits of PEGylated nano- and microparticles in IBD. Lautenschlager and coworkers compared the uptake of uncoated PLGA, chitosan coated PLGA, and PEG-coated PLGA nano- and microparticles into healthy and IBD human tissue biopsies [167]. Microparticles generally demonstrated low penetration into healthy tissue biopsies, and only the PEG-coated microparticles (PEG-PLGA-MP) appeared to penetrate into the inflamed tissue biopsies. The chitosan coated NPs (chito-PLGA-NP) largely adhered to the healthy tissue surface, and showed limited penetration into the inflamed tissue. In contrast, the PEG-coated PLGA NPs (PEG-PLGA-NP) appeared to penetrate extensively into healthy and inflamed human colorectal biopsy samples. The authors further concluded that the PEG-PLGA-MP demonstrated improved “targeting” to inflamed tissue over healthy tissue (i.e. penetration of inflamed tissue was observed, but minimal penetration of healthy tissue was observed) compared to PEG-PLGA-NP [167].
3.4 PEGylation for improved nanoparticle delivery to the brain
As previously described in section 2, PEG coatings on NPs can increase time in systemic circulation, which can be exploited to increase delivery to the brain. However, once a NP crosses the blood brain barrier, or if NPs are administered directly to the brain by bolus injection or convection enhanced delivery (CED), the tissue extracellular matrix (ECM) presents an additional barrier to reaching target cells. Using intravital imaging in the rat brain to assess the diffusional spread of commercially available PEGylated quantum dots (QDs), it was previously determined that the brain ECM contained pores with diameters ranging from 38-64 nm [168]. However, further highlighting the importance of PEG density, Nance, Woodworth and coworkers demonstrated that exceptionally densely coated PS-PEG NPs as large as 114 nm could rapidly penetrate through ex vivo human and rat brain tissue [169]. Their observations were further corroborated in the mouse brain in vivo using intravital particle tracking and imaging of particle spread after injection in the mouse brain. Uncoated PS NPs of any size were adhesively immobilized in the various types of brain tissues, and they confirmed that the commercially available PEG-coated QDs were also hindered in the brain ECM. However, upon subsequent PEG modification of the QD surface using the optimized reaction conditions, they were able to produce even more densely PEGylated QDs that could rapidly penetrate rat brain tissue, confirming that insufficient PEG coatings led to an underestimate of the pore size of the brain ECM. Using the individual PEG-coated NP trajectories (see section 3.1 for more details on this technique), they were able to estimate that 25% of all pores in the brain ECM were actually ≥ 100 nm in size [169]. Nance and coworkers then went on to demonstrate that the increased nanoparticle spread and distribution achieved with densely PEG-coated NPs in the brain would also provide enhanced drug delivery and efficacy in a rat model of malignant glioma [170]. They first demonstrated that dense brain tumor tissue presented an even more significant diffusional barrier to NP transport, but that densely PEG-coated PS (PS-PEG) 70 nm in size could rapidly penetrate the tumor tissue ex vivo. Thus, similarly sized PLGA-PEG NPs (69 nm) rapidly diffused in brain tumor tissue, but uncoated PLGA NPs (88 nm) were adhesively immobilized. Upon direct injection intracranially into the tumor core, densely PEG-coated and paclitaxel-loaded PLGA-PEG NPs (PTX-PLGA-PEG) spread much farther throughout the brain than paclitaxel-loaded PLGA NPs (PTX-PLGA) within 24 h. They then confirmed that the increased distribution of the PTX-PLGA-PEG NPs provided improved tumor suppression (only 8% of the bioluminescent signal of the control group tumors at day 15) with a single intracranial dose compared to PTX-PLGA (45%) and free PTX (85%), likely by reaching and delivering paclitaxel to more of the diffuse tumor cells [170]. Mastorakos and coworkers then went on to explore the effect of PEG coatings on DNA NP delivery in the brain [171]. Using a PEI/PEI-PEG blending strategy to increase PEG surface density without loss of particle stability, they formulated PEI-based DNA NPs with either highly dense PEG coatings (brain-penetrating, DNA-BPN), “conventional” lower density PEG coatings (DNA-CPN), or no PEG coating (uncoated, DNA-UPN). They first confirmed that the PEG coatings improved the stability of the NPs in artificial cerebral spinal fluid and reduced the cell toxicity in vitro and in vivo, without significantly compromising the in vitro cellular uptake. They then demonstrated that densely PEG-coated DNA-BPN diffused more uniformly and rapidly than the DNA-CPN, and DNA-UPN were largely adhesively immobilized. Thus, the DNA-BPN spread to a larger area of the rat brain than DNA-CPN after direct injection. To further take advantage of the non-adhesive nature of densely PEG-coated DNA-BPN, they administered the particles by CED; the overall volume of distribution for DNA-BPN was 3.1-fold higher than the volume of distribution achieved with DNA-CPN. The increased spread of DNA-BPN also led to a 2.4- and 3.2-fold higher volume of transfection compared to DNA-CPN and DNA-UPN, respectively [171]. Similarly, Mastorakos and coworkers demonstrated that a densely PEGylated, biodegradable PBAE-based DNA-BPN provided 11-fold greater volume of transgene expression in the rat brain striatum compared to non-PEGylated PBAE-based DNA-NP [172].
3.5 PEGylation for improved ocular nanoparticle delivery
Although the eyes are relatively accessible, numerous barriers to efficient drug delivery preclude effective treatment of the various blinding diseases that afflict the eye. The most common mode of administration to the eye is topical drops, but it is often cited that because of rapid clearance and poor absorption, less than 5% of the applied dose reaches intraocular tissues [173]. NPs have been explored as a way to increase the residence time and/or penetration of drugs administered to the ocular surface. Giannavola and coworkers explored the use of PEG as a mucoadhesive to promote interactions between acyclovir-loaded PLA NPs and the surface of the eye [174]. Although largely described thus far as a mucoinert surface coating, PEG has a long history of use to promote mucoadhesion by interpenetration and/or hydrogen bonding with mucus [175]. Their PEGylation approach was to incorporate PEGylated lipids (DSPE-MPEG) with the polymer prior to a single step nanoprecipitation process, though both the PLA and PLA-PEG NPs were formulated using various PEG-containing nonionic surfactants (Tween 80 was used for further development). They demonstrated that the PLA-PEG NPs provided increased levels of acyclovir in the aqueous humor of rabbits after instillation into the conjunctival sac compared to PLA NPs or free drug. They further demonstrated that if the eye was pretreated with NAC to break up the mucins in the conjunctival sac/tear film, the amount of acyclovir in the aqueous humor after treatment with PLA-PEG NPs decreased to levels similar to that observed after treatment with PLA NPs. They attributed this decrease to the reduction of mucoadhesive forces between the PLA-PEG NPs and the surface of the eye [174]. However, other more recent work by Schopf and coworkers suggested that nanoparticles coated with a dense, mucoinert PEG surface coating provided increased loteprednol etabonate (LE) delivery to the cornea and retina when applied topically to the surface of the rabbit eye compared to nanoparticles without a mucoinert PEG surface coating [176]. However, the mucoinert surface coating only led to improved LE delivery for nanoparticles (240 nm) and not microparticles (> 1 µm), suggesting that the nanoparticles (LE-MPP) were able to penetrate into the membrane-bound mucus layer of the eye, providing increased residence time and improved drug delivery. They then demonstrated that topical LE-MPP significantly prevented vascular leakage in a pigmented rabbit model of induced retinal vascular permeability [176]. Mun and coworkers aimed to better characterize the barrier properties of the cornea to fluorescently labeled NPs [177]. They compared silica NPs (thiol-functionalized) and silica particles functionalized with 750 or 5000 Da PEG, administered to bovine eyes obtained from a butcher the day before use. The eyes were placed in a beaker with a Franz cell donor compartment on top, and the various NP solutions were exposed to the central cornea. They found that the thiolated silica NPs (21-45 nm) adhered to the superficial surface of the cornea, even if the epithelium was removed prior to exposure. Similar observations were reported for the silica particles functionalized with 750 Da PEG (27-54 nm). The silica NPs functionalized with 5000 Da PEG (43-69 nm) did not penetrate the intact cornea, but did penetrate into the stroma when the epithelium was removed prior to treatment. They attributed the difference in penetration between the corneal epithelium and the stroma to the difference in the barrier properties of the tissue layers: the cornea is the main barrier restricting drug absorption into the eye, whereas the stroma is more of an aqueous layer that provides little resistance to transcorneal permeation [177].
In order to circumvent the various barriers to delivery to the front of the eye, particularly for drug delivery to the back of the eye, injections into the eye are commonly used. Intravitreal injections are often used for delivery to the retina, yet the vitreous gel still poses a significant barrier to effective retinal delivery. Sanders and coworkers studied the impact of PEGylation on DNA NPs for retinal gene delivery [178]. They hypothesized that PEG coatings would improve mobility and penetration through the vitreous, which would increase their proximity to target cells for transfection, but they did not want to negatively impact the transfection efficiency of the DNA NPs. They prepared DNA NPs by coating DNA onto cationic liposomes composed of DOTAP and DOPE lipids. The “pre-PEGylated” lipoplexes contained DSPE-PEG lipids, and were coated with DNA after formation. The “post-PEGylated” lipoplexes were prepared by preparing non-PEGylated lipoplexes that were then incubated with ceramide-C8-PEG lipids. They isolated the vitreous from bovine eyes that were obtained within half an hour of slaughter and added the various lipoplexes. They found that the non-PEGylated lipoplexes aggregated extensively upon injection into the vitreous. In contrast, incorporation of 4 mol% PEG in pre-PEGylated lipoplexes provided some stability, while the 17 mol% PEG pre-PEGylated lipoplexes appeared to be stable and well distributed after injection into the isolated vitreous (Fig. 6). Fluorescence recovery after photobleaching (FRAP) revealed that non-PEGylated and 4 mol% pre-PEGylated lipoplexes were immobilized in the vitreous, whereas the 17 mol% pre-PEGylated lipoplexes were mobile. However, they went on to find that the pre-PEGylation (using DSPE-PEG) drastically decreased in vitro transfection of retinal pigment epithelium (RPE) cells. They then demonstrated that the post-PEGylated lipoplexes (using PEG-ceramides) were internalized by RPE cells similarly to the non-PEGylated lipoplexes, but maintained stability in the vitreous. Thus, they demonstrated that it was possible to produce PEG-coated DNA lipoplexes to improve stability in the vitreous without compromising in vitro RPE cell transfection [178]. Martens and coworkers then went on to more broadly characterize the barrier properties of bovine vitreous to NPs [179]. Rather than excise the fragile vitreous gel, they removed the cornea and lens to create a viewing window for microscopy, injected the NPs into the vitreous through the sclera, and incubated the eyes for 24 h. Cationic PS NPs in the size range of 100-1000 nm aggregated in the vitreous and did not distribute far from the needle track. In contrast, anionic and PEGylated (2 kDa) PS NPs up to 1000 nm in size were able to diffuse away from the injection site, though the anionic NPs diffused more slowly than similarly sized PEG-coated NPs. Having confirmed that PEGylation increased the mobility of NPs in the vitreous, they then tested non-PEGylated and PEGylated DNA polyplexes. As expected, the non-PEGylated, cationic polyplexes formed large, immobile aggregates in the vitreous, whereas the majority of the PEGylated polyplexes were stable and mobile in the vitreous gel [179]. Xu and coworkers also performed broad characterization of the barrier properties of the bovine vitreous, including using the diffusion of PEG-coated NPs to characterize the pore structure of the vitreous gel and the local length-scale dependent viscoelastic properties (see section 3.1 for more details on these techniques) [180]. For these experiments, the bovine eyes were prepared within 3 h of slaughter by removing the anterior portion of the eyeball and directly injecting NPs into the vitreous. They also found that 200 nm cationic PS NPs were adhesively immobilized in the vitreous. Anionic PS NPs 100 and 200 nm in size diffused rapidly, whereas 500 nm and 1000 nm anionic PS NPs were significantly hindered and too extensively aggregated to track, respectively. In contrast, PS-PEG NPs 100, 200, 500, and 750 nm in size uniformly diffused rapidly, and steric hindrance (no aggregation) was observed for 1000 nm PS-PEG. This diffusional data for PS-PEG NPs was used to determine that the average pore size in the bovine vitreous was 550 ± 50 nm, with less than 2% of total pores as large as 1000 nm in size. They then went on to demonstrate that conventional cationic PEI-based DNA NPs aggregated and were immobilized in the vitreous, whereas highly compacted, PEG-coated CK30PEG10k DNA NPs rapidly diffused through the vitreous gel [180].
Figure 6.
The impact of PEG surface coatings on the distribution of lipoplexes injected into bovine vitreous. Non-PEGylated lipoplexes aggregated extensively upon injection into bovine vitreous. Some improvement in distribution was observed with PEGylated lipoplexes containing 4% PEGylated lipids, but aggregation throughout the vitreous was still observed. In contrast, lipoplexes containing 17% PEGylated lipids spread diffusely upon injection into bovine vitreous. Adapted with permission from [172].
3.6 PEGylation for improved nanoparticle-based vaccine delivery
As previously discussed, surface PEGylation is routinely used to reduce interactions between NPs and cells, proteins, etc. in circulation to delay clearance. Thus, it is generally assumed that surface PEGylation will also decrease desirable cell uptake, such as in the case of NP-based vaccine delivery to antigen-presenting cells (APCs). Weaver and coworkers explored the effects of surface PEGylation of human adenovirus serotype 5 (Ad5) on intranasal immunization as a way to reduce neutralizing antibody inactivation of viral vectors [181]. Amine-reactive PEG (5, 20, and 35 kDa) was used to PEGylate Ad5 vectors, and the extent of surface PEGylation was determined by quantifying the amount of free amines remaining on the viral surface. They found that 65-75% of the free amines on the viral surface were conjugated to PEG, resulting in an increased in viral diameter. Viral transduction, as measured by luciferase expression, was seen in the nasal cavity of naïve mice after intranasal administration; virus modified with 5 and 20 kDa PEG produced approximately 50% of the expression of unmodified Ad5, whereas the transduction signal achieved by virus modified with 35 kDa PEG was comparable to unmodified Ad5. Similarly, the anti-luciferase antibody levels in serum after intranasal administration were similar for virus modified with 35 kDa PEG and unmodified virus, but reduced for virus modified with 5 and 20 kDa PEG. It was not clear why virus modified with 35 kDa PEG provided similar efficiency as unmodified virus in naïve animals. A key finding was that in mice with pre-existing anti-Ad5 immunity, the viruses modified with 5 and 35 kDa PEG were more effective than unmodified Ad5 for generating T cell responses when administered intranasally as a prime/boost, suggesting that the PEG coating protected the vectors from existing neutralizing antibodies against the virus [181]. Van den Berg and coworkers explored the use of PEG to improve the efficiency of cationic DNA nanoparticle-mediated dermal vaccination [182]. The high density of APCs in the skin makes it an attractive site for vaccine delivery, though the skin tissue ECM presents a steric and adhesive barrier to NPs. Cationic non-PEGylated liposomes were prepared using DOTAP-DOPE, and DSPE-PEG was incorporated to form PEGylated liposomes prior to complexation with DNA to form lipoplexes. Polyplexes were formed between DNA and cationic non-PEGylated and PEGylated poly(amido amine) (PAA) polymers with protonable amino groups and bioreducible disulfide linkages in the main chain and hydroxybutyl groups in the side chains. Using isolated human skin cells, they found that in vitro cell transfection mediated by the non-PEGylated lipo- and polyplexes was increased compared to naked DNA, yet naked DNA mediated far higher transfection than lipo- and polyplexes after DNA tattoo application directly to ex vivo human skin. The authors hypothesized that the interactions between the cationic NPs and the skin ECM led to the dramatic decrease in transfection ex vivo compared to in vitro. In contrast, luciferase expression mediated by lipoplexes in ex vivo human skin increased as the mol% of incorporated PEG increased. Lipoplexes containing 17.5 mol% PEG achieved significantly higher luciferase expression in human ex vivo skin over at least 18 h compared to naked DNA or non-PEGylated lipoplexes. Similar results were observed for the PEGylated polyplexes compared to naked DNA and non-PEGylated polyplexes. They then demonstrated in mice in vivo that mice tattooed with the PEGylated lipoplexes and polyplexes had similar antigen-specific T cell responses compared to mice tattooed with naked DNA, whereas the T cell response for non-PEGylated lipo- and polyplexes was negligible. The authors speculated that further targeting modifications to the PEGylated NP formulations may increase the immunogenicity compared to naked DNA [182]. Qiao and coworkers explored PEGylation to reduce the cytotoxicity of a cationic polymer, poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) as a gene delivery vector for vaccination [183]. In vitro experiments demonstrated that PEGylation dramatically reduced the cytotoxicity of the PDMAEMA polyplexes, but reduced the transfection efficiency compared to PDMAEMA and PEI polyplexes. However, in an in vivo prime/boost experiment where the polyplexes were used for intranasal priming followed by an intramuscular boost with vaccinia virus carrying a vector for the same antigen, the PEGylated PDMAEMA polyplexes provided increased cellular and humoral responses than conventional PEI polyplexes or naked DNA. Interestingly, they found that the PEG-PDMAEMA polymer itself stimulated murine macrophages in vitro, even compared to the polyplexes and the PDMAEMA and PEI polymers. The authors speculate that the PEG-PDMAEMA polymer also had some adjuvant properties that contributed to the immune response in vivo [183]. Zhan and coworkers explored the effect of PEG coatings on NP drainage to the lymph nodes and access and uptake of NPs by APCs after subcutaneous administration [184]. They used 200 nm carboxylate-modified PS NPs as fluorescent probes and conjugated 5 kDa PEG at varying amounts to the NP surface; the size and ζ-potential of the NPs increased as the PEG surface density increased. When NPs were injected into the hind footpad or the lateral torso of mice, there was a non-significant trend toward increasing particle drainage to the lymph nodes over the 7 days following injection with increasing PEG density. They then characterized the cells in the draining lymph nodes that were able to take up NPs as a function of the surface PEG content. They found that increasing PEGylation increased the relative ratio of dendritic cells to B cells that contained NPs. Interestingly, PEGylation also increased the amount of NPs internalized by all four dendritic cell subsets examined. The authors speculated that although PEGylation of NPs reduces cellular uptake by phagocytic cells in vitro, the relative increase in the amount of PEGylated NPs that reached the draining lymph nodes may have resulted in the relative increase in uptake [184]. Xie and coworkers explored PEG modification of recombinant adenoviral (rAd) vectors to overcome the vaginal mucus barrier for improved vaginal vaccination [185]. They synthesized two cationic PEG derivatives to coat the negatively charged rAd with 2-5 kDa MW PEG. They demonstrated that rAd with a 5 kDa PEG coating (5k-APC-rAd) provided a modest increase in mucus penetration in an Ussing chamber set up and increased vaginal gene expression in Depo-Provera-treated mice in vivo compared to non-PEGylated rAd. Using HIVgag as a model antigen, they went on to demonstrate that intravaginally administered 5k-APC-rAd provided increased antigen specific cellular responses, increased levels of IgA in vaginal lavage fluid, and increased antigen-specific serum IgG compared to non-PEGylated rAd, suggesting promise as an intravaginal gene delivery system for vaccination and other therapeutic purposes [185].
3.7 Achieving efficient intracellular delivery while retaining dense PEGylation
Hydrophobic surfaces readily interact with lipophilic cell membranes, facilitating cellular uptake of NPs. Amongst hydrophilic NPs, positively charged NPs are generally taken up well by cells in vitro, due to the ample presence of negatively charged macromolecules, such as anionic glycoproteins, on cellular surfaces. However, as described previously, hydrophobic or cationic NPs are unable to retain colloidal stability in physiological conditions and/or overcome a variety of extracellular barriers in vivo, and thus are unlikely to efficiently reach target cells. The “stealth” property endowed by NP PEGylation that minimizes undesired phagocytic clearance can also interfere with the ability of NP to interact with and be internalized by target cells [186, 187]. Although as described in previous sections, PEGylation can be optimized to provide increased delivery to target cells by overcoming extracellular barriers, another approach to increasing intracellular delivery is to incorporate targeting ligands to the terminal ends of the PEG chains [37]. In order to balance the increased distribution of PEG-coated NPs in the brain with specificity to cancer cells, Schneider and coworkers developed fibroblast growth factor-inducible 14 (Fn14)-targeted NPs that were densely PEG-coated to avoid adhesion to the brain ECM [188]. Thus, the antibody functionalized NPs would spread throughout the brain, but target Fn14-positive glioblastoma cells to minimize delivery to healthy cells. They first confirmed that the antibody functionalization did not affect the stealth properties of the PEG-coated NPs in the brain. They then demonstrated that although PEG-coated NPs (CNP) and PEG-coated particles with the antibody functionalization (CNP-ITEM4) spread throughout the mouse, the CNP-ITEM4 NPs showed more overlap with luciferase-expressing glioblastoma tumor cells after direct injection to the brain [188]. Likewise, PEGylated cyclodextrin-containing polymer (CDP)-based NPs decorated with transferrin (Tf) for targeting mediated enhanced blood circulation and gene transfer to cancer cells in vivo compared to non-PEGylated CDP NPs and non-targeted, PEGylated CDP NPs, respectively [189]. The blood circulation times were comparable between non-targeted and Tf-targeted PEGylated CDP NPs, implying that the incorporation of Tf did not compromise the stealth properties of the PEG coating. Further, these Tf-targeted, PEGylated CDP NPs (i.e. CALAA-01) have been shown to be well-tolerated in a phase I clinical trial [190]. PLA-PEG NPs decorated with S,S-2-[3-[5-amino-1-carboxypentyl]-ureido]-pentanedioic acid to target prostate-specific membrane antigen (PSMA) (i.e. BIND-014), also currently under clinical investigation, were shown to provide prolonged blood circulation, as well as enhanced cancer cell uptake [191]. However, it should be noted that coupling of targeting ligands, particularly at high ligand densities, can potentially reintroduce non-specific interactions that PEG coatings are meant to reduce, and potentially elicit immune responses [187]. Alternatively, endocytosis of PEGylated NPs can be increased simply by modulating NP size; smaller PEGylated NPs have shown increased cellular uptake compared to larger but similarly PEG-coated NPs [192, 193]. In vitro cellular uptake of small (≤100 nm) PEGylated polymeric gene vectors was found to be indistinguishable from that of non-PEGylated cationic polymeric gene vectors [90, 171]. The findings here suggest that PEGylation did not inhibit cellular interactions and subsequent endocytosis of these NPs. Importantly, smaller PEGylated NPs have exhibited superior resistance against phagocytosis compared to otherwise identical larger NP [49, 53, 66, 67]. Of note, it has also been observed that PEGylated NPs may be internalized by cells by alternative mechanisms; clinically tested DNA NPs based on CK30PEG10k have been shown to access the nucleolin pathway for cell entry [194].
NPs taken up by cells encounter a molecularly crowded cytoplasm. Cytoplasmic transport of NPs can be largely hindered by cytoskeletal elements and cellular organelles by adhesive interactions and/or steric obstruction. PEGylated NPs directly injected into the cytoplasm demonstrated increased diffusion rates compared to non-PEGylated NPs, likely by reducing adhesive interactions between NPs and intracellular components [195]. However, PEGylation often compromises the ability of NPs to escape endosomes, which is a required step prior to entering the cell cytoplasm [186]. PEGylation has been shown to sterically interfere with membrane fusion of lipid-based NPs, resulting in the poor endosome escape [90, 196]. Likewise, reducing PEG content enhanced escape of polymeric micelles from acidic intracellular vesicles (i.e. endo- and lysosomes) [41]. This is a critical challenge that must be addressed, since many NP formulations are intended to be internalized by target cells. Although incorporation of endosomolytic moieties may promote endosome escape of PEGylated NPs, this approach may add complexity to the formulation and delivery processes. Alternatively, NPs can be coated with sheddable PEG coatings with environmentally-sensitive, cleavable linkages. Following endocytosis, PEG chains conjugated via acid-labile, reducible and/or proteolytic linkers, can be liberated from the NP surface in intracellular vesicles; this approach has been extensively reviewed elsewhere [187].
4. Methods for nanoparticle PEGylation and quantification of PEG surface density
In this section, we highlight the various techniques that can be employed for formulating PEGylated NPs with a primary focus on polymeric formulations followed by a brief discussion of lipid-based and micelle type NPs. In general, the methods either involve self-assembly of PEG-containing molecules, or surface modification of preformed NPs with PEG. As described in previous sections, the surface density of PEG on NPs is a key factor that influences delivery, so here we also describe methods for quantification of PEG surface density.
4.1. Self-assembly from PEG-containing molecules
PEG is hydrophilic, so when linked to hydrophobic molecules such as polymers and lipids, the PEG-containing molecules are amphiphilic in nature and will self-assemble. There are two basic approaches used to formulate PEGylated NPs by self-assembly: nanoprecipitation (also called solvent diffusion) and emulsification (also called solvent evaporation or nanoemulsion). Nanoprecipitation has been widely used to prepare long-circulating PEGylated biodegradable NPs for controlled delivery of therapeutics through intravenous injection [197-200]. It is a one-step formulation procedure without the need of high energy or high shear. In the nanoprecipitation method, PEG-containing molecules are dissolved together with drugs in a water-miscible organic solvent or solvent mixture (such as acetone, acetonitrile and tetrahydrofuran). The organic phase is then slowly dispersed into an aqueous phase with or without surfactants. The hydrophilic PEG chains will partition towards the aqueous phase at the nanoparticle surface, and the hydrophobic segments will separate from the aqueous phase to form the NP core. Particle size and PEG density can be controlled through the change of the composition and concentration of the organic solution. In a targeted cancer therapy study, the optimal drug release profile, range for surface aptamer content, and maximal stealth properties were precisely controlled through the blending of PLGA-PEG and PLGA-PEG-aptamer using the nanoprecitation method [198].
One limitation of the nanoprecipitation method is that the drug and PEG-containing molecules must be dissolved in the same water-soluble organic solvent mixture. Certain biologics that are poorly soluble or unstable in water-miscible organic solvents can be challenging to encapsulate into PEGylated NPs with high active drug loading. Furthermore, the nanoprecipitation method typically provides decreased drug loading compared to other encapsulation methods; only 1 wt% docetaxel loading into PLGA-PEG NPs could be achieved using nanoprecipitation [191]. Nanoprecipitation also requires the dropwise addition of the water-miscible organic phase into a larger quantity of aqueous phase, resulting in slow and uncontrolled mixing. Uncontrolled mixing can result in incomplete self-assembly and the potential for PEG chains to remain buried within the NP core rather than fully partitioning to the NP surface [201]. Xu and coworkers measured the PEG surface density of PLGA-PEG NPs prepared by nanoprecipitation and found that ~89% of the incorporated PEG was located on the NP surface [140]. One potential approach for improving the PEG partitioning, as well as reducing polydispersity of NPs formed by nanoprecipitation is to use microfluidics (Fig. 7A) [201]. The hydrodynamic flow focusing provided by microfluidics can result in complete, rapid mixing of the organic phase with the aqueous phase, providing more complete PEG partitioning and smaller, more uniform NP size (Fig. 7B) [201]. Furthermore, many microfluidic devices can be parallelized to make it possible for continuous synthesis and scale-up for clinical scale production [202].
Figure 7.
(A) A schematic showing the hydrodynamic flow focusing to form nanoparticles by nanoprecipitation in a microfluidic device. (B) A schematic illustrating slow mixing in traditional nanoprecipitation and rapid mixing during microfluidic nanoprecipitation. Adapted with permission from [194].
The emulsification method involves dispersing PEG-containing molecules dissolved in a water immiscible organic phase into an aqueous phase, with the help of emulsifiers, under homogenization or sonication. As the organic solvent is gradually evaporated, the hydrophilic PEG chains will partition towards the oil/water interface in the emulsion droplets. The relatively slow solidification of the emulsion droplets allows the PEG chains to more fully partition to the NP surface; Xu and coworkers observed that nearly 100% of the PEG in PLGA-PEG NPs prepared by the emulsification method was located at the NP surface, resulting in a more dense surface PEG coating than that achieved using nanoprecipitation [140]. Additionally, the emulsification method typically provides increased drug loading in comparison to the nanoprecipitation method. For example, up to 20 wt% docetaxel loading was achieved in PLGA-PEG NPs [203]. Further, the emulsion method can be implemented on the industrial scale using homogenization, sonication, mixing, and microfluidics. There are also a large variety of complex emulsion structures that can be used for NP formulation, including water/oil/water (w/o/w), solid/oil/water (s/o/w), o/o/w, etc. [201]. Using these complex emulsion structures, NPs have been formulated loaded with water-soluble drugs (proteins, peptide, nuclide acids, and small molecules) and water-insoluble drugs. Drawbacks to the emulsification method include high shear and high energy requirements, which may potentially denature sensitive drugs and biologics. Diffusion and evaporation of the organic solvent from the emulsion droplets can cause drugs to accumulate at the particle-water interface, leading to burst release [201].
When preparing NPs by emulsification, the emulsifying agent plays a key role in stabilizing the emulsion during the solidification process. Without a suitable emulsifier, aggregation, high polydispersity, and reduced drug encapsulation can occur. Polyvinyl alcohol (PVA) is the most commonly used emulsifier for making both PEGylated and non-PEGylated NPs. It has been proposed that the interpenetration of PVA into the hydrophobic emulsion droplets contributes to the strong stabilization effect, resulting in entrapment into the core matrix of the NPs [204]. Thus, it is very difficult to wash residual PVA from the NP surface [204-206]. The amount of PVA associated with NPs depended on the type of NP, the PVA structure, the PVA concentration, the NP size, and the formulation procedure. As much as 13 wt% of PVA residue was reported for PLA NPs [205]. Considering that numerous reports studying NP PEGylation used NPs prepared by the emulsification method using PVA of various MW and degrees of hydrolysis, it is possible that the outcomes could have been influenced by the residual PVA [207-211], which may partially mask the stealth properties of these PEGylated NPs. Xu, Yang and coworkers studied the effect of PVA on the interactions of PEGylated NPs with human mucus, and found that the existence of residual PVA on the NP surface rendered even densely PEG-coated NPs mucoadhesive [137, 212]. They then went on to explore the use of various small molecule emulsifiers, such as cholic acid (CHA), sucrose esters and dioctyl sulfosuccinate sodium, to prepare the PLGA-PEG NPs [137]. The small molecular emulsifiers could produce stable emulsions without affecting the PEG surface coating (Fig. 8) [137]. In particular, CHA was used to produce PLGA-PEG NPs as small as 80 nm a PEG surface density of ~13.9 PEG5k molecules per 100 nm2 [137]. In addition to rapidly penetrating mucus [137], these PEGylated NPs could penetrate the ECM of brain and brain tumor tissue [170]. Of note, the BIND-014 in clinical trials utilizes CHA as the emulsifying agent [203].
Figure 8.
The impact of emulsifiers on the PEG surface layer of NPs formulated using an emulsification method. The use of low MW emulsifiers resulted in dense PEG coatings on NPs, facilitating rapid penetration through mucus (mucus penetrating particles, MPP); whereas, conventional high MW emulsifiers disrupted the PEG coating, resulting in adhesive interactions and immobilization in mucus (conventional particles, CP). Adapted with permission from [132].
Polyplexes are core-shell structures formed by the condensation of PEG-polycation or PEG-lipid with plasmid DNA, and the driving force for self-assembly is the electrostatic interactions between the phosphate groups of the DNA and the cationic groups of the PEG-containing molecules. PEG can be covalently linked to one side of the cationic segment to form block copolymers, or PEG molecules can be grafted to the sides of cationic segments to form a comb-like structure. Copolymer structures showed strong influence on the physiochemical properties and the biological activities of polyplexes. In the latter case of PEG-grafted polycations, the amount of PEG grafting must be optimized to not interfere with the condensation and NP formation process. When the amount of 5 kDa PEG grafted onto PEI was increased, complexation of DNA was impeded and a less compact polyplex was formed [80]. In contrast, when 20 kDa PEG was grafted to form a PEG-PEI block copolymer, it formed small and compact nanostructures when complexed with DNA [80]. However, the block copolymer approach may not provide sufficient NP PEG surface density. As discussed in section 3.2, Boylan and coworkers found that DNA polyplexes formed from CK30-PEG10k block copolymers did not have dense enough PEG coatings to shield interactions with human CF sputum [147]. To overcome this limitation, Suk and coworkers prepared densely PEG-coated DNA polyplexes by blending PEG-grafted-PEI with unmodified PEI at a controlled ratio. These PEGylated polyplexes were sufficiently coated with PEG to allow for rapid penetration of human CF sputum, whereas polyplexes formed entirely from PEG-grafted-PEI were large and unstable [150].
PEGylated liposomes or polymersomes are also often formed by self-assembly processes, also called the pre-insertion approach. Liposomes can be prepared by thin film hydration with an aqueous medium with the help of sonication, extrusion or freeze-thaw processing [213]. PEG-containing lipids added during the self-assembly process will insert into the bilayer with the hydrophilic PEG chains extending toward the aqueous phase. For liposomes, this means that PEG can partition to the inner and outer sides of the lipid bilayer. PEG on the liposome interior does not contribute to the PEG surface coating, and may interfere with drug loading. To overcome this limitation, PEG can also be incorporated by post-insertion or post-conjugation methods (see next section). PEG density on the liposome surface can be controlled by changing the ratio of PEG-lipids to unmodified lipids, the type of PEG-lipid conjugate, and the molecular weight of PEG used for self-assembly. The presence of PEG on the liposome surface can prevent aggregation of liposomes, though incorporating too much PEG can compromise liposome formation and stability. Yu and coworkers reported that when the PEG-lipid conjugate content was increased to 12 mol%, both the drug loading and in vivo vaginal distribution was reduced [139].
4.2. Surface modification of nanoparticles with PEG chains
Modifying preformed NPs with PEG can guarantee that all of the grafted PEG molecules will be present on the NP surface. In the physical adsorption strategy, PEG-containing molecules are dissolved in an aqueous phase and interactions with the NP surface are driven by hydrophobic interactions, electrostatic interactions, or ligand-binding. Pluronics are a class of polyethylene oxide-b-polypropylene oxide-b-polyethylene oxide (PEO-PPO-PEO) triblock copolymer, where the hydrophobic PPO segment is flanked by two hydrophilic PEO (here PEO is equivalent to PEG) chains. Pluronic molecules can adsorb on hydrophobic NP surfaces by hydrophobic interactions between the PPO segment and NP surface [133, 135]. Yang and coworkers observed that the PPO chain length played an important role in binding to the NP surface [133]. A dense PEG surface coating sufficient for NP diffusion in human mucus was only achieved with Pluronics with a PPO chain ~3 kDa in MW [133]. Additionally Vitamin-E-TPGS-PEG [214-218], fatty-acid-PEG esters [219], phospholipid-PEG [220] and others can be absorbed on polymeric nanoparticles and inorganic nanoparticles to form a PEG corona. NPs with charged surfaces can be coated with oppositely-charged PEG-containing molecules through electrostatic interactions. For example, PEG-cationic block polymers such as PEG-PLL and PEG-PEI can bind to negatively charged PLGA NPs [221]. Although these physioadsorption procedures are a simple way to prepare PEGylated nanoparticles, the binding of PEG molecules on the NP surface is a weak non-covalent linkage, so the PEG can get desorbed from the NP surface. Furthermore, it is difficult to separate and remove the non-adsorbed PEG from the adsorbed PEG. Biotinylated PEG can bind to avidin-functionalized NPs to form a more stable PEG coating via protein-ligand interactions [131, 222]. Biotin-avidin interactions can also be used to attach targeting ligands or drugs to the NP surface [222]. However, the unintended consequences on delivery and translational limitations arising from having avidin/biotin on the NP surface must also be considered.
PEGylated NPs can also be produced by chemical conjugation of PEG molecules to preformed NPs (also called grafting). This approach has been taken to produce PEGylated PS NPs [130, 223], PEGylated dendrimers [149, 224-226], and PEGylated gold NPs [20, 227]. However, steric limitations can limit the density of PEG that can be grafted to the NP surface. Nance and coworkers determined optimized reaction conditions for obtaining sufficiently dense methoxy-PEG-amine coatings on carboxyl-modified PS NPs for penetration of brain tissue ECM, which required higher PEG density than that required for penetration through human mucus [169]. Chemical conjugation approaches are also limited by potential drug leakage from the pre-formed NPs, and batch-to-batch variation.
PEG-lipid conjugates can be inserted into pre-formed liposomes, as the hydrophobic lipid tail preferentially inserts into the lipid bilayer. The concentration of PEG-lipids added should be below the critical micellar concentration to avoid the formation of micelles, and the PEG-lipid solutions should be slowly added at temperatures close to the melting temperature of the lipids [228]. The post-insertion approach allows for modification of the outer surface of liposome bilayer, rather than allowing random insertion of PEG into the liposome interior as may occur with self-assembly. Thus, the post-insertion method has been demonstrated to require less PEG-lipid conjugate to achieve similar surface PEGylation to the pre-insertion method and provided longer blood circulation half-life [229]. The post-conjugation method can also be used to prepare PEGylated liposomes, especially when modifying the surface with targeting ligands. The fragile nature of liposomes requires selective and efficient reactions that are suitable for mild reaction conditions, such as reaction between activated carboxyl groups and amino groups, reaction between pyridyldithiols and thiols, and reaction between maleimide and thiols [230]. “Click” chemistry as also been used for PEG post-conjugation, such as reactions between alkynyl groups on the liposome surface and azide-functionalized PEG [231, 232].
4.3 Quantification of PEG surface density
Many methods have been used to either qualitatively determine the presence of PEG on the NP surface or quantitatively measure the PEG surface density. After PEGylation, nanoparticles will show changes in physiochemical properties, such as surface charge [133], hydrodynamic diameter [221], hydrophilicity [233] and protein binding capability [20]. By monitoring changes in these physiochemical properties, surface PEGylation can be confirmed. For example, grafting PEG on PS NPs resulted in a slight increase of the NP diameter (from 217 nm to 231 nm), and a dramatic decrease in the surface charge (from −59 mV to −2 mV) [130]. These indirect methods only provide qualitative information about the relative degree of PEGylation, but fail to quantify PEG density. The advantage of these indirect methods is that these physiochemical properties can be routinely measured in laboratory.
Direct methods have been developed and optimized in order to get the quantitative information about the extent of NP surface PEGylation. Thermogravimetric analysis (TGA) is able to measure PEG content as the difference in mass before and after PEGylation due to thermal decomposition, but it is limited to inorganic materials and requires a large sample amount [234]. The reaction of functionalized PEG (with end groups such as -SH, -NH2, etc) labeled with molecules or dyes that can be detected by absorbance or fluorescence, can be used to measure the amount of un-reacted PEG in the supernatant and indirectly calculate the amount of PEG grafted on the NP surface [20, 235]. A similar method was used to quantify surface PEG density on PRINT (particle replication in non-wetting templates) NPs by measuring un-reacted fluorescein-PEG in the supernatant [236]. Measuring the amount of ligand conjugated to PEG on the NP surface can provide information about the PEG density and the ligand density [237]. Alternatively, nuclear magnetic resonance (NMR) was applied to assess the surface PEG density of polymeric NPs both qualitatively and quantitatively (PEG peak typically observed ~3.65 ppm) [52, 137, 238, 239]. When NPs were dispersed within the NMR solvent D2O, only the surface PEG, not the PEG buried within the core, could be detected by NMR [158, 239, 240]. Xu and coworkers also described a modified NMR method for determining the surface PEG density in which an internal standard (3-(trimethylsilyl)-1-propanesulfonic acid) was used to generate a calibration curve for PEG [137]. The NPs were directly prepared in the D2O solution containing the internal standard to avoid the potential of NP aggregation caused by the standard approach of lyophilizing NPs and reconstituting in the NMR solvent [137]. In contrast, when PEGylated nanoparticles were prepared by the surface modification method, the surface PEG density could be quantified by directly dissolving the freeze-dried NPs in a deuterated organic solvent containing an internal standard, because all PEG in the formulation is assumed to be on the NP surface [169]. The NMR quantification method is fairly easy, fast, cheap, and suitable for routine laboratory quality control.
Other methods have also been applied to measure the surface PEG on PEGylated NPs, such as x-ray photoelectron spectroscopy (XPS) [241], anti-PEG antibody binding [242-245], and chromatographic measurements [246] [247]. XPS provides elemental information about the upper layer of the dried particles (~8-10 nm depth from the surface) with a significant error margin (~10 to 20%), and the PEG chains are in the collapsed status during XPS measurements. Therefore, XPS can provide relative surface chemical composition, rather than a surface PEG density quantification. Anti-PEG antibodies can specifically bind to PEG or PEG-containing molecules with high sensitivity (picomolar range), and can potentially be used to quantify PEG density in complex biological conditions [243-245]. However, reaction of PEG antibodies can potentially be affected by steric interactions between densely packed neighboring PEG chains. Free/unreacted PEG can be detected by a refractive index (RI) detector [248] or evaporative light scattering detector [246], which both allow for quantification of the content of free PEG after chromatographic separation, such as HPLC. Chromatographic methods can be used to quantify PEG content without modification or conjugation to a dye, though they are limited by sensitivity to contaminants, unstable baselines, and high detection limits when the RI detectors are used. The combination of evaporative light scattering detection and LC-MS was shown to have better sensitivity with less interference than an RI detector alone [247].
5. Conclusion
Building on the success of protein PEGylation for improved systemic delivery of therapeutic proteins, PEGylation of NPs has also proven to be a highly effective approach for improving systemic delivery of therapeutic cargo. Furthermore, we highlighted here how NP PEGylation has also been used as an approach to overcome various extracellular barriers associated with other modes of administration, ranging from mucosal delivery to delivery to brain tissue. Undoubtedly, PEG coatings will continue to be a mainstay in the design of NP for drug and gene delivery applications, which will facilitate continued studies into how the properties of PEG coatings impact NP biodistribution and clearance from the body. It is unclear what role the emerging PEG alternatives will play as more NP-based products are translated to the clinical setting, but further investigation of the potential immunogenic properties of PEG coatings as a function of molecular weight, functional group, surface density, NP core properties, dosing frequency, etc. will certainly lead to more efficacious products. Importantly, much more investigation into the impact of these properties on the systemic administration of polymeric NPs to complement the extensive literature on lipid-based NPs is needed.
Acknowledgments
This work was supported by National Institutes of Health grants U19AI133127 (J.H., L.M.E.), R01HL127413 (J.H., J.S.S.), R33AI094519 (J.H., L.M.E.), and R01EB020147 (J.H.); the Johns Hopkins University Center for AIDS Research P30AI094189 (L.M.E.); the 2015 Burroughs Wellcome Fund Preterm Birth Initiative (L.M.E); and the Cystic Fibrosis Foundation (J.H., J.S.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The mucus-penetrating particle technology described in this publication is being developed by Kala Pharmaceuticals. Justin Hanes is co-founder of Kala. He owns company stock, which is subject to certain restrictions under Johns Hopkins University policy. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
References
- [1].Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. The Journal of biological chemistry. 252(1977):3582–3586. [PubMed] [Google Scholar]
- [2].Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. International journal of nanomedicine. 9(2014):4357–4373. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arturson P, Laakso T, Edman P. Acrylic microspheres in vivo IX: Blood elimination kinetics and organ distribution of microparticles with different surface characteristics. Journal of pharmaceutical sciences. 72(1983):1415–1420. doi: 10.1002/jps.2600721213. [DOI] [PubMed] [Google Scholar]
- [4].Tan JS, Butterfield DE, Voycheck CL, Caldwell KD, Li JT. Surface modification of nanoparticles by PEO/PPO block copolymers to minimize interactions with blood components and prolong blood circulation in rats. Biomaterials. 14(1993):823–833. doi: 10.1016/0142-9612(93)90004-l. [DOI] [PubMed] [Google Scholar]
- [5].Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS letters. 268(1990):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- [6].Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: effect of chemotherapeutic agent, nanoparticle size, and circulation time. Journal of vascular and interventional radiology : JVIR. 16(2005):1365–1371. doi: 10.1097/01.RVI.0000175324.63304.25. [DOI] [PubMed] [Google Scholar]
- [7].Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clinical pharmacokinetics. 42(2003):419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- [8].Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 11(2005):6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- [9].Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews. 53(2001):283–318. [PubMed] [Google Scholar]
- [10].Braet F, Wisse E, Bomans P, Frederik P, Geerts W, Koster A, Soon L, Ringer S. Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three-dimensions. Microscopy research and technique. 70(2007):230–242. doi: 10.1002/jemt.20408. [DOI] [PubMed] [Google Scholar]
- [11].Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular pharmaceutics. 5(2008):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vonarbourg A, Passirani C, Saulnier P, Benoit JP. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 27(2006):4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- [13].Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 17(1999):593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- [14].Patel HM. Serum opsonins and liposomes: their interaction and opsonophagocytosis. Critical reviews in therapeutic drug carrier systems. 9(1992):39–90. [PubMed] [Google Scholar]
- [15].Gessner A, Waicz R, Lieske A, Paulke B, Mader K, Muller RH. Nanoparticles with decreasing surface hydrophobicities: influence on plasma protein adsorption. International journal of pharmaceutics. 196(2000):245–249. doi: 10.1016/s0378-5173(99)00432-9. [DOI] [PubMed] [Google Scholar]
- [16].Roser M, Fischer D, Kissel T. Surface-modified biodegradable albumin nano- and microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 46(1998):255–263. doi: 10.1016/s0939-6411(98)00038-1. [DOI] [PubMed] [Google Scholar]
- [17].Yoon JY, Kim JH, Kim WS. Interpretation of protein adsorption phenomena onto functional microspheres. Colloid Surface B. 12(1998):15–22. [Google Scholar]
- [18].Arnida, Janat-Amsbury MM, Ray A, Peterson CM, Ghandehari H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 77(2011):417–423. doi: 10.1016/j.ejpb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu F, Liu DX. Serum independent liposome uptake by mouse liver. Bba-Biomembranes. 1278(1996):5–11. doi: 10.1016/0005-2736(95)00196-4. [DOI] [PubMed] [Google Scholar]
- [20].Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. Journal of the American Chemical Society. 134(2012):2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- [21].Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nature nanotechnology. 7(2012):779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- [22].Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Landfester K, Schild H, Maskos M, Knauer SK, Stauber RH. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature nanotechnology. 8(2013):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- [23].Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nature nanotechnology. 8(2013):137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- [24].Li S, Rizzo MA, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene therapy. 5(1998):930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- [25].Bragonzi A, Boletta A, Biffi A, Muggia A, Sersale G, Cheng SH, Bordignon C, Assael BM, Conese M. Comparison between cationic polymers and lipids in mediating systemic gene delivery to the lungs. Gene therapy. 6(1999):1995–2004. doi: 10.1038/sj.gt.3301039. [DOI] [PubMed] [Google Scholar]
- [26].Liu Y, Mounkes LC, Liggitt HD, Brown CS, Solodin I, Heath TD, Debs RJ. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nature biotechnology. 15(1997):167–173. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- [27].Yoo JW, Chambers E, Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Current pharmaceutical design. 16(2010):2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- [28].Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2015 doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 263(1994):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- [30].Stirland DL, Nichols JW, Miura S, Bae YH. Mind the gap: a survey of how cancer drug carriers are susceptible to the gap between research and practice. Journal of controlled release : official journal of the Controlled Release Society. 172(2013):1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim J, Kim PH, Kim SW, Yun CO. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials. 33(2012):1838–1850. doi: 10.1016/j.biomaterials.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee GK, Maheshri N, Kaspar B, Schaffer DV. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnology and bioengineering. 92(2005):24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- [33].Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 46(1986):6387–6392. [PubMed] [Google Scholar]
- [34].Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 95(1998):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dawidczyk CM, Kim C, Park JH, Russell LM, Lee KH, Pomper MG, Searson PC. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. Journal of controlled release : official journal of the Controlled Release Society. 187(2014):133–144. doi: 10.1016/j.jconrel.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer research. 69(2009):3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Advanced drug delivery reviews. 65(2013):1284–1298. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- [38].Nance E, Timbie K, Miller GW, Song J, Louttit C, Klibanov AL, Shih TY, Swaminathan G, Tamargo RJ, Woodworth GF, Hanes J, Price RJ. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. Journal of controlled release : official journal of the Controlled Release Society. 189(2014):123–132. doi: 10.1016/j.jconrel.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, Cranston D. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 42(2004):931–935. doi: 10.1016/j.ultras.2004.01.089. [DOI] [PubMed] [Google Scholar]
- [40].Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption, Colloids and surfaces. B, Biointerfaces. 18(2000):301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- [41].Miteva M, Kirkbride KC, Kilchrist KV, Werfel TA, Li H, Nelson CE, Gupta MK, Giorgio TD, Duvall CL. Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials. 38(2015):97–107. doi: 10.1016/j.biomaterials.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mori A, Klibanov AL, Torchilin VP, Huang L. Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS letters. 284(1991):263–266. doi: 10.1016/0014-5793(91)80699-4. [DOI] [PubMed] [Google Scholar]
- [43].Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Advanced drug delivery reviews. 16(1995):215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 6(2011):715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dos Santos N, Allen C, Doppen AM, Anantha M, Cox KA, Gallagher RC, Karlsson G, Edwards K, Kenner G, Samuels L, Webb MS, Bally MB. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochimica et biophysica acta. 1768(2007):1367–1377. doi: 10.1016/j.bbamem.2006.12.013. [DOI] [PubMed] [Google Scholar]
- [46].Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International journal of pharmaceutics. 307(2006):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [47].Cui J, De Rose R, Alt K, Alcantara S, Paterson BM, Liang K, Hu M, Richardson JJ, Yan Y, Jeffery CM, Price RI, Peter K, Hagemeyer CE, Donnelly PS, Kent SJ, Caruso F. Engineering Poly(ethylene glycol) Particles for Improved Biodistribution. ACS nano. 9(2015):1571–1580. doi: 10.1021/nn5061578. [DOI] [PubMed] [Google Scholar]
- [48].He Q, Zhang J, Shi J, Zhu Z, Zhang L, Bu W, Guo L, Chen Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 31(2010):1085–1092. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- [49].Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano letters. 9(2009):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- [50].Mosqueira VC, Legrand P, Gref R, Heurtault B, Appel M, Barratt G. Interactions between a macrophage cell line (J774A1) and surface-modified poly (D,L-lactide) nanocapsules bearing poly(ethylene glycol) Journal of drug targeting. 7(1999):65–78. doi: 10.3109/10611869909085493. [DOI] [PubMed] [Google Scholar]
- [51].Mosqueira VC, Legrand P, Morgat JL, Vert M, Mysiakine E, Gref R, Devissaguet JP, Barratt G. Biodistribution of long-circulating PEG-grafted nanocapsules in mice: effects of PEG chain length and density. Pharmaceutical research. 18(2001):1411–1419. doi: 10.1023/a:1012248721523. [DOI] [PubMed] [Google Scholar]
- [52].Bazile D, Prudhomme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. Journal of pharmaceutical sciences. 84(1995):493–498. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- [53].Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 27(2006):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [54].Yang Q, Jones SW, Parker CL, Zamboni WC, Bear JE, Lai SK. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Molecular pharmaceutics. 11(2014):1250–1258. doi: 10.1021/mp400703d. [DOI] [PubMed] [Google Scholar]
- [55].Braeckmans K, Buyens K, Bouquet W, Vervaet C, Joye P, De Vos F, Plawinski L, Doeuvre L, Angles-Cano E, Sanders NN, Demeester J, De Smedt SC. Sizing nanomatter in biological fluids by fluorescence single particle tracking. Nano letters. 10(2010):4435–4442. doi: 10.1021/nl103264u. [DOI] [PubMed] [Google Scholar]
- [56].Zara GP, Cavalli R, Bargoni A, Fundaro A, Vighetto D, Gasco MR. Intravenous administration to rabbits of non-stealth and stealth doxorubicin-loaded solid lipid nanoparticles at increasing concentrations of stealth agent: pharmacokinetics and distribution of doxorubicin in brain and other tissues. Journal of drug targeting. 10(2002):327–335. doi: 10.1080/10611860290031868. [DOI] [PubMed] [Google Scholar]
- [57].Khalid MN, Simard P, Hoarau D, Dragomir A, Leroux JC. Long circulating poly(ethylene glycol)-decorated lipid nanocapsules deliver docetaxel to solid tumors. Pharmaceutical research. 23(2006):752–758. doi: 10.1007/s11095-006-9662-5. [DOI] [PubMed] [Google Scholar]
- [58].Peracchia MT, Fattal E, Desmaele D, Besnard M, Noel JP, Gomis JM, Appel M, d'Angelo J, Couvreur P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. Journal of controlled release : official journal of the Controlled Release Society. 60(1999):121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- [59].DeRouchey J, Walker GF, Wagner E, Radler JO. Decorated rods: a "bottom-up" self-assembly of monomolecular DNA complexes. The journal of physical chemistry. 110(2006):4548–4554. doi: 10.1021/jp053760a. B. [DOI] [PubMed] [Google Scholar]
- [60].Vittaz M, Bazile D, Spenlehauer G, Verrecchia T, Veillard M, Puisieux F, Labarre D. Effect of PEO surface density on long-circulating PLA-PEO nanoparticles which are very low complement activators. Biomaterials. 17(1996):1575–1581. doi: 10.1016/0142-9612(95)00322-3. [DOI] [PubMed] [Google Scholar]
- [61].Meng F, Engbers GH, Feijen J. Polyethylene glycol-grafted polystyrene particles. Journal of biomedical materials research. 70(2004):49–58. doi: 10.1002/jbm.a.30056. Part A. [DOI] [PubMed] [Google Scholar]
- [62].Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, Napier M, Bear JE, DeSimone JM. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano letters. 12(2012):5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu HL, Zamboni WC, Wang AZ, Bear JE, DeSimone JM. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proceedings of the National Academy of Sciences of the United States of America. 108(2011):586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cellular microbiology. 9(2007):2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- [65].Unsworth LD, Sheardown H, Brash JL. Protein-resistant poly(ethylene oxide)-grafted surfaces: chain density-dependent multiple mechanisms of action. Langmuir : the ACS journal of surfaces and colloids. 24(2008):1924–1929. doi: 10.1021/la702310t. [DOI] [PubMed] [Google Scholar]
- [66].Choi CH, Zuckerman JE, Webster P, Davis ME. Targeting kidney mesangium by nanoparticles of defined size. Proceedings of the National Academy of Sciences of the United States of America. 108(2011):6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vonarbourg A, Passirani C, Saulnier P, Simard P, Leroux JC, Benoit JP. Evaluation of pegylated lipid nanocapsules versus complement system activation and macrophage uptake. Journal of biomedical materials research. 78(2006):620–628. doi: 10.1002/jbm.a.30711. Part A. [DOI] [PubMed] [Google Scholar]
- [68].Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proceedings of the National Academy of Sciences of the United States of America. 104(2007):1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jiang X, Qu W, Pan D, Ren Y, Williford JM, Cui H, Luijten E, Mao HQ. Plasmid-templated shape control of condensed DNA-block copolymer nanoparticles. Adv Mater. 25(2013):227–232. doi: 10.1002/adma.201202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences of the United States of America. 103(2006):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Shi F, Wasungu L, Nomden A, Stuart MC, Polushkin E, Engberts JB, Hoekstra D. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. The Biochemical journal. 366(2002):333–341. doi: 10.1042/BJ20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS nano. 9(2015):3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- [73].Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular pharmaceutics. 5(2008):487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ziemba B, Matuszko G, Bryszewska M, Klajnert B. Influence of dendrimers on red blood cells. Cellular & molecular biology letters. 17(2012):21–35. doi: 10.2478/s11658-011-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 24(2003):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- [76].Eliyahu H, Servel N, Domb AJ, Barenholz Y. Lipoplex-induced hemagglutination: potential involvement in intravenous gene delivery. Gene therapy. 9(2002):850–858. doi: 10.1038/sj.gt.3301705. [DOI] [PubMed] [Google Scholar]
- [77].Kurosaki T, Kitahara T, Fumoto S, Nishida K, Yamamoto K, Nakagawa H, Kodama Y, Higuchi N, Nakamura T, Sasaki H. Chondroitin sulfate capsule system for efficient and secure gene delivery. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 13(2010):351–361. doi: 10.18433/j3gk52. [DOI] [PubMed] [Google Scholar]
- [78].Petersen H, Fechner PM, Fischer D, Kissel T. Synthesis, characterization, and biocompatibility of polyethylenimine-graft-poly(ethylene glycol) block copolymers. Macromolecules. 35(2002):6867–6874. [Google Scholar]
- [79].Qi R, Gao Y, Tang Y, He RR, Liu TL, He Y, Sun S, Li BY, Li YB, Liu G. PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. The AAPS journal. 11(2009):395–405. doi: 10.1208/s12248-009-9116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ, Fischer D, Davies MC, Kissel T. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjugate chemistry. 13(2002):845–854. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- [81].Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 49(2010):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- [82].Verhoef JJ, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug discovery today. 19(2014):1945–1952. doi: 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- [83].Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. Journal of controlled release : official journal of the Controlled Release Society. 172(2013):38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- [84].Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 13(2014):655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Richter AW, Akerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. International archives of allergy and applied immunology. 74(1984):36–39. doi: 10.1159/000233512. [DOI] [PubMed] [Google Scholar]
- [86].Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. Journal of controlled release : official journal of the Controlled Release Society. 105(2005):305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [87].Ichihara M, Shimizu T, Imoto A, Hashiguchi Y, Uehara Y, Ishida T, Kiwada H. Anti-PEG IgM Response against PEGylated Liposomes in Mice and Rats. Pharmaceutics. 3(2010):1–11. doi: 10.3390/pharmaceutics3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. Journal of controlled release : official journal of the Controlled Release Society. 112(2006):15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [89].Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. The Journal of pharmacology and experimental therapeutics. 292(2000):1071–1079. [PubMed] [Google Scholar]
- [90].Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. European journal of cell biology. 83(2004):97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- [91].Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 218(2013):725–732. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- [92].Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. Journal of controlled release : official journal of the Controlled Release Society. 115(2006):243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [93].Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. Journal of controlled release : official journal of the Controlled Release Society. 126(2008):162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- [94].Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. Journal of controlled release : official journal of the Controlled Release Society. 122(2007):349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- [95].Saadati R, Dadashzadeh S, Abbasian Z, Soleimanjahi H. Accelerated blood clearance of PEGylated PLGA nanoparticles following repeated injections: effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharmaceutical research. 30(2013):985–995. doi: 10.1007/s11095-012-0934-y. [DOI] [PubMed] [Google Scholar]
- [96].Tagami T, Uehara Y, Moriyoshi N, Ishida T, Kiwada H. Anti-PEG IgM production by siRNA encapsulated in a PEGylated lipid nanocarrier is dependent on the sequence of the siRNA. Journal of controlled release : official journal of the Controlled Release Society. 151(2011):149–154. doi: 10.1016/j.jconrel.2010.12.013. [DOI] [PubMed] [Google Scholar]
- [97].Abu Lila AS, Eldin NE, Ichihara M, Ishida T, Kiwada H. Multiple administration of PEG-coated liposomal oxaliplatin enhances its therapeutic efficacy: a possible mechanism and the potential for clinical application. International journal of pharmaceutics. 438(2012):176–183. doi: 10.1016/j.ijpharm.2012.08.030. [DOI] [PubMed] [Google Scholar]
- [98].Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. Journal of controlled release : official journal of the Controlled Release Society. 115(2006):251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- [99].Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H. CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes. Journal of controlled release : official journal of the Controlled Release Society. 142(2010):160–166. doi: 10.1016/j.jconrel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [100].Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature biotechnology. 23(2005):457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- [101].Judge A, McClintock K, Phelps JR, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Molecular therapy : the journal of the American Society of Gene Therapy. 13(2006):328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- [102].Tagami T, Nakamura K, Shimizu T, Ishida T, Kiwada H. Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. Journal of controlled release : official journal of the Controlled Release Society. 137(2009):234–240. doi: 10.1016/j.jconrel.2009.04.006. [DOI] [PubMed] [Google Scholar]
- [103].Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, Kiwada H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biological & pharmaceutical bulletin. 35(2012):1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- [104].Xu H, Ye F, Hu M, Yin P, Zhang W, Li Y, Yu X, Deng Y. Influence of phospholipid types and animal models on the accelerated blood clearance phenomenon of PEGylated liposomes upon repeated injection. Drug delivery. 2014 doi: 10.3109/10717544.2014.885998. [DOI] [PubMed] [Google Scholar]
- [105].Shiraishi K, Hamano M, Ma H, Kawano K, Maitani Y, Aoshi T, Ishii KJ, Yokoyama M. Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. Journal of controlled release : official journal of the Controlled Release Society. 165(2013):183–190. doi: 10.1016/j.jconrel.2012.11.016. [DOI] [PubMed] [Google Scholar]
- [106].Wang XY, Ishida T, Ichihara M, Kiwada H. Influence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in rats. Journal of controlled release : official journal of the Controlled Release Society. 104(2005):91–102. doi: 10.1016/j.jconrel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [107].Koide H, Asai T, Hatanaka K, Urakami T, Ishii T, Kenjo E, Nishihara M, Yokoyama M, Ishida T, Kiwada H, Oku N. Particle size-dependent triggering of accelerated blood clearance phenomenon. International journal of pharmaceutics. 362(2008):197–200. doi: 10.1016/j.ijpharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- [108].Saifer MG, Williams LD, Sobczyk MA, Michaels SJ, Sherman MR. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Molecular immunology. 57(2014):236–246. doi: 10.1016/j.molimm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- [109].Sherman MR, Williams LD, Sobczyk MA, Michaels SJ, Saifer MG. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjugate chemistry. 23(2012):485–499. doi: 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, Yuki K, Tanaka K, Takenaga M, Igarashi R, Maeda T, Yamakawa N, Okamoto Y, Otsuka M, Ishida T, Kiwada H, Mizushima Y, Mizushima T. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharmaceutical research. 26(2009):2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- [111].Armstrong J. Veronese F, editor. The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol) PEGylated Protein Drugs: Basic Science and Clinical Applications. 2009:147–168. Birkhäuser Basel. [Google Scholar]
- [112].Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert opinion on drug delivery. 9(2012):1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- [113].Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Advanced drug delivery reviews. 60(2008):886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kainthan RK, Brooks DE. In vivo biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials. 28(2007):4779–4787. doi: 10.1016/j.biomaterials.2007.07.046. [DOI] [PubMed] [Google Scholar]
- [115].Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 4(2012):219–233. doi: 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Abu Lila AS, Nawata K, Shimizu T, Ishida T, Kiwada H. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. International journal of pharmaceutics. 456(2013):235–242. doi: 10.1016/j.ijpharm.2013.07.059. [DOI] [PubMed] [Google Scholar]
- [117].Abu Lila AS, Uehara Y, Ishida T, Kiwada H. Application of polyglycerol coating to plasmid DNA lipoplex for the evasion of the accelerated blood clearance phenomenon in nucleic acid delivery. Journal of pharmaceutical sciences. 103(2014):557–566. doi: 10.1002/jps.23823. [DOI] [PubMed] [Google Scholar]
- [118].Kainthan RK, Brooks DE. Unimolecular micelles based on hydrophobically derivatized hyperbranched polyglycerols: biodistribution studies. Bioconjugate chemistry. 19(2008):2231–2238. doi: 10.1021/bc800090v. [DOI] [PubMed] [Google Scholar]
- [119].Kierstead PH, Okochi H, Venditto VJ, Chuong TC, Kivimae S, Frechet JM, Szoka FC. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. Journal of controlled release : official journal of the Controlled Release Society. 213(2015):1–9. doi: 10.1016/j.jconrel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Anselmo AC, Gupta V, Zern BJ, Pan D, Zakrewsky M, Muzykantov V, Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS nano. 7(2013):11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal "Self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 339(2013):971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Cone RA. Barrier properties of mucus. Advanced drug delivery reviews. 61(2009):75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [123].Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater. 24(2012):3887–3894. doi: 10.1002/adma.201201800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced drug delivery reviews. 61(2009):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophysical Journal. 81(2001):1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Advanced drug delivery reviews. 57(2005):63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [127].Huang YB, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: Tethered structures and site-specific surfaces. J. Control. Release. 65(2000):63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- [128].Sahlin JJ, Peppas NA. Enhanced hydrogel adhesion by polymer interdiffusion: Use of linear poly(ethylene glycol) as an adhesion promoter. J Biomat Sci-Polym E. 8(1997):421–436. doi: 10.1163/156856297x00362. [DOI] [PubMed] [Google Scholar]
- [129].Serra L, Domenech J, Peppas NA. Design of poly(ethylene glycol)-tethered copolymers as novel mucoadhesive drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. 63(2006):11–18. doi: 10.1016/j.ejpb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [130].Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that "slip" through the human mucus barrier. Angew. Chem. Int. Ed. 47(2008):9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cu Y, Saltzman WM. Controlled surface modification with poly(ethylene)glycol enhances diffusion of PLGA nanoparticles in human cervical mucus. Molecular pharmaceutics. 6(2009):173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. Journal of controlled release : official journal of the Controlled Release Society. 156(2011):258–264. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yang M, Lai SK, Wang YY, Zhong WX, Happe C, Zhang M, Fu J, Hanes J. Biodegradable Nanoparticles Composed Entirely of Safe Materials that Rapidly Penetrate Human Mucus. Angew. Chem. Int. Ed. 50(2011):2597–2600. doi: 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3003453. 138ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Yang M, Yu T, Wang AZ, Lai SK, Zeng Q, Miao B, Tang BC, Simons BW, Ensign L, Liu G, Chan KWY, Juang C-Y, Mert O, Wood J, Fu J, McMahon MT, Wu T-C, Hung C-F, Hanes J. Vaginal delivery of paclitaxel via nanoparticles with non-mucoadhesive surfaces suppresses cervical tumor growth. Adv. Healthcare Mater. 2013;3:1044–1052. doi: 10.1002/adhm.201300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proceedings of the National Academy of Sciences of the United States of America. 106(2009):19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xu Q, Boylan NJ, Cai S, Miao B, Patel H, Hanes J. Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J. Control. Release. 170(2013):279–286. doi: 10.1016/j.jconrel.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yu T, Wang YY, Yang M, Schneider C, Zhong W, Pulicare S, Choi WJ, Mert O, Fu J, Lai SK, Hanes J. Biodegradable mucus-penetrating nanoparticles composed of diblock copolymers of polyethylene glycol and poly(lactic--glycolic acid) Drug delivery and translational research. 2012;2 doi: 10.1007/s13346-011-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Yu T, Chan KW, Anonuevo A, Song X, Schuster BS, Chattopadhyay S, Xu Q, Oskolkov N, Patel H, Ensign LM, van Zjil PC, McMahon MT, Hanes J. Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI) Nanomedicine : nanotechnology, biology, and medicine. 11(2015):401–405. doi: 10.1016/j.nano.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Xu Q, Ensign LM, Boylan NJ, Schon A, Gong X, Yang J-C, Cai S, Yu T, Freire E, Hanes J. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS nano. doi: 10.1021/acsnano.5b03876. L. N.W. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Advanced drug delivery reviews. 61(2009):86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wang YY, Lai SK, So C, Schneider C, Cone R, Hanes J. Mucoadhesive Nanoparticles May Disrupt the Protective Human Mucus Barrier by Altering Its Microstructure. PloS one. 2011;6 doi: 10.1371/journal.pone.0021547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proceedings of the National Academy of Sciences of the United States of America. 107(2010):598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP, Hanes J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 30(2009):2591–2597. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Forier K, Messiaen AS, Raemdonck K, Deschout H, Rejman J, De Baets F, Nelis H, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Transport of nanoparticles in cystic fibrosis sputum and bacterial biofilms by single-particle tracking microscopy. Nanomedicine (Lond) 8(2013):935–949. doi: 10.2217/nnm.12.129. [DOI] [PubMed] [Google Scholar]
- [146].Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, Oette SM, Payne JM, Muhammad O, Ziady AG, Moen RC, Cooper MJ. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Human gene therapy. 15(2004):1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- [147].Boylan NJ, Suk JS, Lai SK, Jelinek R, Boyle MP, Cooper MJ, Hanes J. Highly compacted DNA nanoparticles with low MW PEG coatings: In vitro, ex vivo and in vivo evaluation. Journal of Controlled Release. 157(2012):72–79. doi: 10.1016/j.jconrel.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Suk JS, Boylan NJ, Trehan K, Tang BC, Schneider CS, Lin JMG, Boyle MP, Zeitlin PL, Lai SK, Cooper MJ, Hanes J. N-acetylcysteine Enhances Cystic Fibrosis Sputum Penetration and Airway Gene Transfer by Highly Compacted DNA Nanoparticles. Molecular Therapy. 19(2011):1981–1989. doi: 10.1038/mt.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kim AJ, Boylan NJ, Suk JS, Hwangbo M, Yu T, Schuster BS, Cebotaru L, Lesniak WG, Oh JS, Adstamongkonkul P, Choi AY, Kannan RM, Hanes J. Use of Single-Site Functionalized PEG-Dendrons to Prepare Gene Vectors that Penetrate Human Mucus Barriers. Angewandte Chemie (International ed. in English) 52(2013):3985–3988. doi: 10.1002/anie.201208556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Suk JS, Kim AJ, Trehan K, Schneider CS, Cebotaru L, Woodward OM, Boylan NJ, Boyle MP, Lai SK, Guggino WB, Hanes J. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Control. Release. 178(2014):8–17. doi: 10.1016/j.jconrel.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Mastorakos P, da Silva AL, Chisholm J, Song E, Choi WK, Boyle MP, Morales MM, Hanes J, Suk JS. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.1502281112. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Human gene therapy. 10(1999):1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- [153].Yang XY, Forier K, Steukers L, Van Vlierberghe S, Dubruel P, Braeckmans K, Glorieux S, Nauwynck HJ. Immobilization of Pseudorabies Virus in Porcine Tracheal Respiratory Mucus Revealed by Single Particle Tracking. PloS one. 2012;7 doi: 10.1371/journal.pone.0051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Schuster BS, Suk JS, Woodworth GF, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 34(2013):3439–3446. doi: 10.1016/j.biomaterials.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, Chen J, Kim J, Hanes J. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials. 32(2011):6285–6290. doi: 10.1016/j.biomaterials.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Brooking J, Davis SS, Illum L. Transport of nanoparticles across the rat nasal mucosa. Journal of drug targeting. 9(2001):267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- [157].Tobio M, Gref R, Sanchez A, Langer R, Alonso MJ. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharmaceutical research. 15(1998):270–275. doi: 10.1023/a:1011922819926. [DOI] [PubMed] [Google Scholar]
- [158].Vila A, Gill H, McCallion O, Alonso MJ. Transport of PLA-PEG particles across the nasal mucosa: effect of particle size and PEG coating density. J. Control. Release. 98(2004):231–244. doi: 10.1016/j.jconrel.2004.04.026. [DOI] [PubMed] [Google Scholar]
- [159].Tobio M, Sanchez A, Vila A, Soriano II, Evora C, Vila-Jato JL, Alonso MJ. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration, Colloids and surfaces. B, Biointerfaces. 18(2000):315–323. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- [160].Feeney OM, Williams HD, Pouton CW, Porter CJ. 'Stealth' lipid-based formulations: poly(ethylene glycol)-mediated digestion inhibition improves oral bioavailability of a model poorly water soluble drug. Journal of controlled release : official journal of the Controlled Release Society. 192(2014):219–227. doi: 10.1016/j.jconrel.2014.07.037. [DOI] [PubMed] [Google Scholar]
- [161].Cheng X, Ming X, Croyle MA. PEGylated adenoviruses for gene delivery to the intestinal epithelium by the oral route. Pharmaceutical research. 20(2003):1444–1451. doi: 10.1023/a:1025714412337. [DOI] [PubMed] [Google Scholar]
- [162].Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Advanced drug delivery reviews. 64(2012):557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yuan H, Chen CY, Chai GH, Du YZ, Hu FQ. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Molecular pharmaceutics. 10(2013):1865–1873. doi: 10.1021/mp300649z. [DOI] [PubMed] [Google Scholar]
- [164].Inchaurraga L, Martin-Arbella N, Zabaleta V, Quincoces G, Penuelas I, Irache JM. In vivo study of the mucus-permeating properties of PEG-coated nanoparticles following oral administration. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014 doi: 10.1016/j.ejpb.2014.12.021. [DOI] [PubMed] [Google Scholar]
- [165].Maisel K, Ensign L, Reddy M, Cone R, Hanes J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. Journal of controlled release : official journal of the Controlled Release Society. 197(2015):48–57. doi: 10.1016/j.jconrel.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ensign LM, Henning A, Schneider CS, Maisel K, Wang YY, Porosoff MD, Cone R, Hanes J. Ex vivo characterization of particle transport in mucus secretions coating freshly excised mucosal tissues. Molecular pharmaceutics. 10(2013):2176–2182. doi: 10.1021/mp400087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Lautenschlager C, Schmidt C, Lehr CM, Fischer D, Stallmach A. PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 85(2013):578–586. doi: 10.1016/j.ejpb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- [168].Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proceedings of the National Academy of Sciences of the United States of America. 103(2006):5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3003594. 149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Nance E, Zhang C, Shih TY, Xu Q, Schuster BS, Hanes J. Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS nano. 8(2014):10655–10664. doi: 10.1021/nn504210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mastorakos P, Zhang C, Berry S, Oh Y, Lee S, Eberhart CG, Woodworth GF, Suk JS, Hanes J. Highly PEGylated DNA nanoparticles provide uniform and widespread gene transfer in the brain. Adv Healthc Mater. 2015 doi: 10.1002/adhm.201400800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Mastorakos P, Song E, Zhang C, Berry S, Park HW, Kim YE, Park JS, Lee S, Suk JS, Hanes J. Biodegradable DNA nanoparticles that provide widespread gene therapy in the brain. doi: 10.1002/smll.201502554. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Jarvinen K, Jarvinen T, Urtti A. Ocular Absorption Following Topical Delivery. Advanced drug delivery reviews. 16(1995):3–19. [Google Scholar]
- [174].Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, Lee VH, Fresta M. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharmaceutical research. 20(2003):584–590. doi: 10.1023/a:1023290514575. [DOI] [PubMed] [Google Scholar]
- [175].Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that "slip" through the human mucus barrier. Angew Chem Int Ed Engl. 47(2008):9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Schopf LR, Popov AM, Enlow EM, Bourassa JL, Ong WZ, Nowak P, Chen H. Topical Ocular Drug Delivery to the Back of the Eye by Mucus-Penetrating Particles. Translational vision science & technology. 2015;4:11. doi: 10.1167/tvst.4.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mun EA, Morrison PW, Williams AC, Khutoryanskiy VV. On the barrier properties of the cornea: a microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Molecular pharmaceutics. 11(2014):3556–3564. doi: 10.1021/mp500332m. [DOI] [PubMed] [Google Scholar]
- [178].Sanders NN, Peeters L, Lentacker I, Demeester J, De Smedt SC. Wanted and unwanted properties of surface PEGylated nucleic acid nanoparticles in ocular gene transfer. Journal of controlled release : official journal of the Controlled Release Society. 122(2007):226–235. doi: 10.1016/j.jconrel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- [179].Martens TF, Vercauteren D, Forier K, Deschout H, Remaut K, Paesen R, Ameloot M, Engbersen JF, Demeester J, De Smedt SC, Braeckmans K. Measuring the intravitreal mobility of nanomedicines with single-particle tracking microscopy. Nanomedicine (Lond) 8(2013):1955–1968. doi: 10.2217/nnm.12.202. [DOI] [PubMed] [Google Scholar]
- [180].Xu Q, Boylan NJ, Suk JS, Wang YY, Nance EA, Yang JC, McDonnell PJ, Cone RA, Duh EJ, Hanes J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. Journal of controlled release : official journal of the Controlled Release Society. 167(2013):76–84. doi: 10.1016/j.jconrel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Weaver EA, Barry MA. Effects of shielding adenoviral vectors with polyethylene glycol on vector-specific and vaccine-mediated immune responses. Human gene therapy. 19(2008):1369–1382. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].van den Berg JH, Oosterhuis K, Hennink WE, Storm G, van der Aa LJ, Engbersen JF, Haanen JB, Beijnen JH, Schumacher TN, Nuijen B. Shielding the cationic charge of nanoparticle-formulated dermal DNA vaccines is essential for antigen expression and immunogenicity. Journal of controlled release : official journal of the Controlled Release Society. 141(2010):234–240. doi: 10.1016/j.jconrel.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [183].Qiao Y, Huang Y, Qiu C, Yue X, Deng L, Wan Y, Xing J, Zhang C, Yuan S, Dong A, Xu J. The use of PEGylated poly [2-(N,N-dimethylamino) ethyl methacrylate] as a mucosal DNA delivery vector and the activation of innate immunity and improvement of HIV-1-specific immune responses. Biomaterials. 31(2010):115–123. doi: 10.1016/j.biomaterials.2009.09.032. [DOI] [PubMed] [Google Scholar]
- [184].Zhan X, Tran KK, Shen H. Effect of the poly(ethylene glycol) (PEG) density on the access and uptake of particles by antigen-presenting cells (APCs) after subcutaneous administration. Molecular pharmaceutics. 9(2012):3442–3451. doi: 10.1021/mp300190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Xie Z, Ji Z, Zhang Z, Gong T, Sun X. Adenoviral vectors coated with cationic PEG derivatives for intravaginal vaccination against HIV-1. Biomaterials. 35(2014):7896–7908. doi: 10.1016/j.biomaterials.2014.05.056. [DOI] [PubMed] [Google Scholar]
- [186].Hatakeyama H, Akita H, Harashima H. The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biological & pharmaceutical bulletin. 36(2013):892–899. doi: 10.1248/bpb.b13-00059. [DOI] [PubMed] [Google Scholar]
- [187].Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharmaceutical research. 25(2008):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Schneider CS, Perez JG, Cheng E, Zhang C, Mastorakos P, Hanes J, Winkles JA, Woodworth GF, Kim AJ. Minimizing the non-specific binding of nanoparticles to the brain enables active targeting of Fn14-positive glioblastoma cells. Biomaterials. 42(2015):42–51. doi: 10.1016/j.biomaterials.2014.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Molecular pharmaceutics. 6(2009):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- [190].Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME, Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proceedings of the National Academy of Sciences of the United States of America. 111(2014):11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3003651. 128ra139. [DOI] [PubMed] [Google Scholar]
- [192].Pamujula S, Hazari S, Bolden G, Graves RA, Chinta DD, Dash S, Kishore V, Mandal TK. Cellular delivery of PEGylated PLGA nanoparticles. The Journal of pharmacy and pharmacology. 64(2012):61–67. doi: 10.1111/j.2042-7158.2011.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Hu Y, Xie J, Tong YW, Wang CH. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. Journal of controlled release : official journal of the Controlled Release Society. 118(2007):7–17. doi: 10.1016/j.jconrel.2006.11.028. [DOI] [PubMed] [Google Scholar]
- [194].Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Molecular therapy : the journal of the American Society of Gene Therapy. 16(2008):333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- [195].Suh J, Choy KL, Lai SK, Suk JS, Tang BC, Prabhu S, Hanes J. PEGylation of nanoparticles improves their cytoplasmic transport. International journal of nanomedicine. 2(2007):735–741. [PMC free article] [PubMed] [Google Scholar]
- [196].Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Advanced drug delivery reviews. 63(2011):152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- [197].Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proceedings of the National Academy of Sciences of the United States of America. 107(2010):17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA. 105(2008):2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proceedings of the National Academy of Sciences of the United States of America. 108(2011):1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. USA. 105(2008):17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles. Nano letters. 8(2008):2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- [202].Valencia PM, Farokhzad OC, Karnik R, Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 7(2012):623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Hoven TVG, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- [204].Boury F, Ivanova T, Panaotov I, Proust JE, Bois A, Richou J. Dynamic Properties of Poly(DL-lactide) and Polyvinyl Alcohol Monolayers at the Air/Water and Dichloromethane/Water Interfaces. J. Colloid. Interf. Sci. 169(1995):380–392. [Google Scholar]
- [205].Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E, Alonso MJ, Labrude P, Vigneron C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Release. 50(1998):31–40. doi: 10.1016/s0168-3659(97)00106-5. [DOI] [PubMed] [Google Scholar]
- [206].Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release. 82(2002):105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- [207].Li Y-P, Pei Y-Y, Zhang X-Y, Gu Z-H, Zhou Z-H, Yuan W-F, Zhou J-J, Zhu J-H, Gao X-J. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J. Control. Release. 71(2001):203–211. doi: 10.1016/s0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- [208].Park J, Fong PM, Lu J, Russell KS, Booth CJ, Saltzman WM, Fahmy TM. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine: Nanotechnology, Biology and Medicine. 5(2009):410–418. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Khalil NM, Nascimento T.C.F.d., Casa DM, Dalmolin LF, Mattos A.C.d., Hoss I, Romano MA, Mainardes RM. Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids and Surfaces B: Biointerfaces. 101(2013):353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- [210].Esmaeili F, Ghahremani MH, Esmaeili B, Khoshayand MR, Atyabi F, Dinarvand R. PLGA nanoparticles of different surface properties: Preparation and evaluation of their body distribution. International journal of pharmaceutics. 349(2008):249–255. doi: 10.1016/j.ijpharm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- [211].Sheng Y, Yuan Y, Liu C, Tao X, Shan X, Xu F. In vitro macrophage uptake and in vivo biodistribution of PLA–PEG nanoparticles loaded with hemoglobin as blood substitutes: effect of PEG content. J Mater Sci: Mater Med. 20(2009):1881–1891. doi: 10.1007/s10856-009-3746-9. [DOI] [PubMed] [Google Scholar]
- [212].Yang M, Lai SK, Yu T, Wang Y-Y, Happe C, Zhong W, Zhang M, Anonuevo A, Fridley C, Hung A, Fu J, Hanes J. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Control. Release. 192(2014):202–208. doi: 10.1016/j.jconrel.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Torchilin Vladimir, Weissig V. Liposomes : a practical approach. 2nd Oxford University Press; Oxford: 2003. [Google Scholar]
- [214].Lee SH, Zhang Z, Feng S-S. Nanoparticles of poly(lactide)--tocopheryl polyethylene glycol succinate (PLA-TPGS) copolymers for protein drug delivery. Biomaterials. 28(2007):2041–2050. doi: 10.1016/j.biomaterials.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [215].Mert O, Lai SK, Ensign L, Yang M, Wang Y-Y, Wood J, Hanes J. A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. J. Control. Release. 157(2012):455–460. doi: 10.1016/j.jconrel.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Mi Y, Liu Y, Feng S-S. Formulation of Docetaxel by folic acid-conjugated D-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials. 32(2011):4058–4066. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- [217].Mu L, Feng SS. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol (R)) J. Control. Release. 80(2002):129–144. doi: 10.1016/s0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- [218].Zhang Z, Feng S-S. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: Synthesis, formulation, characterization and in vitro drug release. Biomaterials. 27(2006):262–270. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- [219].Lin J-J, Chen J-S, Huang S-J, Ko J-H, Wang Y-M, Chen T-L, Wang L-F. Folic acid-Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 30(2009):5114–5124. doi: 10.1016/j.biomaterials.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [220].Chan JM, Zhang L, Yuet KP, Liao G, Rhee J-W, Langer R, Farokhzad OC. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 30(2009):1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [221].Wang H, Zhao P, Su W, Wang S, Liao Z, Niu R, Chang J. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials. 31(2010):8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- [222].Pulkkinen M, Pikkarainen J, Wirth T, Tarvainen T, Haapa-aho V, Korhonen H, Seppälä J, Järvinen K. Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin–biotin technology: Formulation development and in vitro anticancer activity. European Journal of Pharmaceutics and Biopharmaceutics. 70(2008):66–74. doi: 10.1016/j.ejpb.2008.04.018. [DOI] [PubMed] [Google Scholar]
- [223].Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proceedings of the National Academy of Sciences of the United States of America. 104(2007):1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Kim Y, Klutz AM, Jacobson KA. Systematic Investigation of Polyamidoamine Dendrimers Surface-Modified with Poly(ethylene glycol) for Drug Delivery Applications: Synthesis, Characterization, and Evaluation of Cytotoxicity. Bioconjugate chemistry. 19(2008):1660–1672. doi: 10.1021/bc700483s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sweet DM, Kolhatkar RB, Ray A, Swaan P, Ghandehari H. Transepithelial Transport of PEGylated Anionic Poly(amidoamine) Dendrimers: Implications for Oral Drug Delivery. Journal of controlled release : official journal of the Controlled Release Society. 2009;138:78. doi: 10.1016/j.jconrel.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Kim Y, Hechler B, Gao Z-G, Gachet C, Jacobson KA. PEGylated Dendritic Unimolecular Micelles as Versatile Carriers for Ligands of G Protein-Coupled Receptors. Bioconjugate chemistry. 20(2009):1888–1898. doi: 10.1021/bc9001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Perrault SD, Chan WCW. Synthesis and Surface Modification of Highly Monodispersed, Spherical Gold Nanoparticles of 50−200 nm. Journal of the American Chemical Society. 131(2009):17042–17043. doi: 10.1021/ja907069u. [DOI] [PubMed] [Google Scholar]
- [228].Uster PS, Allen TM, Daniel BE, Mendez CJ, Newman MS, Zhu GZ. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS letters. 386(1996):243–246. doi: 10.1016/0014-5793(96)00452-8. [DOI] [PubMed] [Google Scholar]
- [229].Nakamura K, Yamashita K, Itoh Y, Yoshino K, Nozawa S, Kasukawa H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1818(2012):2801–2807. doi: 10.1016/j.bbamem.2012.06.019. [DOI] [PubMed] [Google Scholar]
- [230].Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 4(2005):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- [231].Cavalli S, Tipton AR, Overhand M, Kros A. The chemical modification of liposome surfaces via a copper-mediated [3 + 2] azide-alkyne cycloaddition monitored by a colorimetric assay. Chemical Communications. 2006:3193–3195. doi: 10.1039/b606930d. [DOI] [PubMed] [Google Scholar]
- [232].Kumar A, Erasquin UJ, Qin G, Li K, Cai C. "Clickable", polymerized liposomes as a versatile and stable platform for rapid optimization of their peripheral compositions. Chemical Communications. 46(2010):5746–5748. doi: 10.1039/c0cc00784f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Sheng Y, Liu CS, Yuan Y, Tao XY, Yang F, Shan XQ, Zhou HJ, Xu F. Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials. 30(2009):2340–2348. doi: 10.1016/j.biomaterials.2008.12.070. [DOI] [PubMed] [Google Scholar]
- [234].Wuelfing WP, Gross SM, Miles DT, Murray RW. Nanometer gold clusters protected by surface-bound monolayers of thiolated poly(ethylene glycol) polymer electrolyte. Journal of the American Chemical Society. 120(1998):12696–12697. [Google Scholar]
- [235].Xia X, Yang M, Wang Y, Zheng Y, Li Q, Chen J, Xia Y. Quantifying the coverage density of poly(ethylene glycol) chains on the surface of gold nanostructures. ACS nano. 6(2012):512–522. doi: 10.1021/nn2038516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, Napier M, Bear JE, DeSimone JM. PEGylated PRINT Nanoparticles: The Impact of PEG Density on Protein Binding, Macrophage Association, Biodistribution, and Pharmacokinetics. Nano Letters. 12(2012):5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Valencia PM, Hanewich-Hollatz MH, Gao W, Karim F, Langer R, Karnik R, Farokhzad OC. Effects of ligands with different water solubilities on self-assembly and properties of targeted nanoparticles. Biomaterials. 32(2011):6226–6233. doi: 10.1016/j.biomaterials.2011.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Livaniou E, Evangelatos G, Ithakissios DS. Effect of copolymer composition on the physicochemical characteristics, in vitro stability, and biodistribution of PLGA-mPEG nanoparticles. International journal of pharmaceutics. 259(2003):115–127. doi: 10.1016/s0378-5173(03)00224-2. [DOI] [PubMed] [Google Scholar]
- [239].Heald CR, Stolnik S, Kujawinski KS, De Matteis C, Garnett MC, Illum L, Davis SS, Purkiss SC, Barlow RJ, Gellert PR. Poly(lactic acid)-poly(ethylene oxide) (PLA-PEG) nanoparticles: NMR studies of the central solidlike PLA core and the liquid PEG corona. Langmuir : the ACS journal of surfaces and colloids. 18(2002):3669–3675. [Google Scholar]
- [240].Garcia-Fuentes M, Torres D, Martin-Pastor M, Alonso MJ. Application of NMR spectroscopy to the characterization of PEG-stabilized lipid nanoparticles. Langmuir : the ACS journal of surfaces and colloids. 20(2004):8839–8845. doi: 10.1021/la049505j. [DOI] [PubMed] [Google Scholar]
- [241].Sun C, Du K, Fang C, Bhattarai N, Veiseh O, Kivit F, Stephen Z, Lee D, Ellenbogen RG, Ratner B, Zhang M. PEG-Mediated Synthesis of Highly Dispersive Multifunctional Superparamagnetic Nanoparticles: Their Physicochemical Properties and Function In Vivo. ACS nano. 4(2010):2402–2410. doi: 10.1021/nn100190v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Xu Y, Mehl JT, Bakhtiar R, Woolf EJ. Immunoaffinity Purification Using Anti-PEG Antibody Followed by Two-Dimensional Liquid Chromatography/Tandem Mass Spectrometry for the Quantification of a PEGylated Therapeutic Peptide in Human Plasma. Anal. Chem. 82(2010):6877–6886. doi: 10.1021/ac1009832. [DOI] [PubMed] [Google Scholar]
- [243].Chuang KH, Tzou SC, Cheng TC, Kao CH, Tseng WL, Shiea J, Liao KW, Wang YM, Chang YC, Huango BJ, Wu CJ, Chu PY, Roffler SR, Cheng TL. Measurement of Poly(ethylene glycol) by Cell-Based Anti-poly(ethylene glycol) ELISA. Anal. Chem. 82(2010):2355–2362. doi: 10.1021/ac902548m. [DOI] [PubMed] [Google Scholar]
- [244].Su Y-C, Chen B-M, Chuang K-H, Cheng T-L, Roffler SR. Sensitive Quantification of PEGylated Compounds by Second-Generation Anti-Poly(ethylene glycol) Monoclonal Antibodies. Bioconjugate chemistry. 21(2010):1264–1270. doi: 10.1021/bc100067t. [DOI] [PubMed] [Google Scholar]
- [245].Cheng T-L, Cheng C-M, Chen B-M, Tsao D-A, Chuang K-H, Hsiao S-W, Lin Y-H, Roffler SR. Monoclonal Antibody-Based Quantitation of Poly(ethylene glycol)-Derivatized Proteins, Liposomes, and Nanoparticles. Bioconjugate chemistry. 16(2005):1225–1231. doi: 10.1021/bc050133f. [DOI] [PubMed] [Google Scholar]
- [246].Zabaleta V, Campanero MA, Irache JM. An HPLC with evaporative light scattering detection method for the quantification of PEGs and Gantrez in PEGylated nanoparticles. Journal of Pharmaceutical and Biomedical Analysis. 44(2007):1072–1078. doi: 10.1016/j.jpba.2007.05.006. [DOI] [PubMed] [Google Scholar]
- [247].Nair LM, Konkel J, Thomas M, Koberda M. Comparison of electrospray ionization mass spectrometry and evaporative light scattering detections for the determination of Poloxamer 188 in itraconazole injectable formulation. Journal of Pharmaceutical and Biomedical Analysis. 41(2006):725–730. doi: 10.1016/j.jpba.2005.12.028. [DOI] [PubMed] [Google Scholar]
- [248].Zillies JC, Zwiorek K, Winter G, Coester C. Method for Quantifying the PEGylation of Gelatin Nanoparticle Drug Carrier Systems Using Asymmetrical Flow Field-Flow Fractionation and Refractive Index Detection. Anal. Chem. 79(2007):4574–4580. doi: 10.1021/ac062135e. [DOI] [PubMed] [Google Scholar]