Abstract
The digestive system comprises numerous cells, tissues and organs that are essential for the proper assimilation of nutrients and energy. Many aspects of digestive organ function are highly conserved among vertebrates, yet the final anatomical configuration of the gut varies widely between species, especially those with different diets. Improved understanding of the complex molecular and cellular events that orchestrate digestive organ development is pertinent to many areas of biology and medicine, including the regeneration or replacement of diseased organs, the etiology of digestive organ birth defects, and the evolution of specialized features of digestive anatomy. In this review, we highlight specific examples of how investigations using Xenopus laevis frog embryos have revealed insight into the molecular and cellular dynamics of digestive organ patterning and morphogenesis that would have been difficult to obtain in other animal models. Additionally, we discuss recent studies of gut development in non-model frog species with unique feeding strategies, such as Lepidobatrachus laev is and Eleutherodactylouscoqui, which are beginning to provide glimpses of the evolutionary mechanisms that may generate morphological variation in the digestive tract. The unparalleled experimental versatility of frog embryos make them excellent, integrative models for studying digestive organ development across multiple disciplines.
Keywords: embryo, Xenopus, Lepidobatrachus, Eleutherodactylous coqui, gut, specification, morphogenesis, evolution
1. Introduction
The anatomical and physiological complexity of the vertebrate digestive system develops from a simple primitive gut tube (PGT). This PGT undergoes intricate patterning and differentiation events to enable the epithelial lining of the tube to assume the absorptive and secretory functions required of a gastrointestinal (GI) tract, while discrete segments bud off of the original structure to form accessory organs, including the pancreas and liver. Concomitantly, the tube lengthens and rotates, as it transforms from a short, occluded cylinder to a long, hollow conduit arranged in a three dimensional configuration of loops and coils.
Elucidating the mechanisms of digestive organ development has broad implications for many areas of biology and medicine. Some of the most common human birth defects affect the digestive tract, yet the genetic and/or environmental factors that contribute to the etiology of these malformations remain to be discovered. In addition, diseases of the digestive system affect millions worldwide, generating substantial demand for therapeutic interventions; full knowledge of the developmental events that pattern and shape the PGT is likely to be vital for successful regeneration or engineering of human digestive tissues. Finally, although many features of digestive anatomy are highly conserved among vertebrates, the length, compartmentalization and topological orientation of the GI tract can vary tremendously among and between species, especially those with different diets, yet the evolutionary origins of this ecologically-relevant variation are largely unknown.
1.1. The advantages of the frog embryo
Amphibians have long been used as model organisms for studying embryonic development, and have played instrumental roles in unraveling the intricate events that guide germ layer formation, gastrulation and neurulation [1–3]. Beyond early development, frog embryos also boast several advantages for the study of organ specification and morphogenesis [4]. Unlike amniote embryos that are confined to a uterus or shell during development, frog embryos are externally fertilized and can be easily cultured in vitro, making them amenable to a wide variety of experimental manipulations. For example, the rate of development of frog embryos can be accelerated or slowed by adjusting temperature, facilitating convenient analyses of any stage of organogenesis [5]. Moreover, precise fate maps have been generated for the early blastomeres (32-cell stage), allowing loss- and/or gain-of-function (LOF/GOF) reagents and lineage tracers to be targeted to specific organs by standard microinjection technology, enabling gene function to be queried in a tissue-specific manner [6, 7]. Furthermore, because frog embryos are relatively large and harbor an innate, intracellular yolk supply, tissue explants can be dissected, recombined and transplanted, or cultured in simple saline, at almost any stage of development, facilitating expedient, inexpensive specification and trans-differentiation studies [8–14]. Finally, the frog embryo’s accessibility to chemical agonists/antagonists allows the role of specific signaling pathways to be interrogated during critical windows of organogenesis (i.e., subsequent to earlier developmental events that may also depend on such pathways). In fact, thanks to large clutch sizes, frog embryos provide a powerful platform for high-throughput “chemical genetic” or toxin screening using organ morphology as a phenotypic readout [15–18]. This experimental amenability makes the frog embryo an ideal model in which to interrogate the mechanisms of organ development.
1.2. More than one frog in the water
Amphibian models (mainly urodeles) have been employed in developmental biology research for over a century, but the convenience of in vitro fertilization methods made Xenopus species the most popular frogs in the laboratory [19]. Nonetheless, many non-model frog species are equally amenable to experimentation as Xenopus. Comparative “evo-devo” studies utilizing frogs with different reproductive strategies and/or developmental rates [20] are beginning to provide fascinating insight into the molecular and cellular mechanisms that shape different embryos, while species that fill unique ecological niches or possess intriguing specializations are shedding light on the developmental origins of novel morphologies [21].
In this review, we provide a broad perspective on the ways in which Xenopus and emerging frog models have yielded new insight into digestive organ patterning, morphogenesis, and evolution.
2. What can the frog tell us about foregut organ specification?
The developing digestive tract may be divided into foregut (esophagus, stomach, duodenum, liver, pancreas, gall bladder) and midgut/hindgut (intestine) domains. The foregut-derived organs play critical roles in processes such as digestion, glucose homeostasis, and detoxification. Therefore, congenital defects or disease in these organs (e.g., diabetes, pancreatitis, fatty liver disease, biliary atresia, gall stones and gastric/pancreatic cancer) are the cause of substantial morbidity and mortality worldwide [22–25]. To ameliorate such afflictions, translational researchers seek to develop regenerative therapies and engineer replacement tissues in vitro. Progress in these areas has been profoundly influenced by models of the normal process of foregut organ specification and morphogenesis in the embryo [26, 27].
In all vertebrates, the PGT is comprised of an inner endoderm layer, which differentiates into the epithelial lining of the GI tract, surrounded by an outer layer of mesoderm, which will give rise to the visceral muscle and connective tissue. Early in gut development, reciprocal signaling between the endoderm and mesoderm layers gradually distinguishes anterior foregut and posterior hindgut domains [28]. In addition to digestive tissues, numerous structures with diverse physiological functions are derived from the anterior region of the PGT (including organs of the respiratory and endocrine systems), all of which must undergo morphogenesis in close proximity. The expression of foregut organ-specific genes must therefore be tightly coordinated in time and space to allow individual organs to differentiate appropriately. Moreover, many of the signaling factors involved in foregut organogenesis are re-deployed during different stages of development, and/or evoke contradictory responses depending on their concentration [27].
Studying such spatially intricate and seemingly paradoxical signaling dynamics is not trivial in mouse models due to the functional redundancy of the factors involved, and the challenges of achieving tissue-specific or conditional perturbation of gene function to overcome early lethality and pleiotropy. In contrast, the experimental amenability of the Xenopus embryo— which enables the pattern, timing and dosage of gene expression to be manipulated in a tissue-specific manner—has provided key insights into the spatiotemporal signaling dynamics that specify region and organ identities in the PGT. We highlight a few salient examples in sections 2.1–2.4 below.
2.1. Complex control of Wnt signaling is required for foregut specification
Wnt signaling pathways are highly conserved and involved in many fundamental developmental events, including body axis patterning, cell fate specification, cell proliferation, and cell migration. Multiple Wnt ligands stimulate canonical (Wnt/β-catenin) and/or non-canonical (e.g., Wnt/JNK) pathways [29, 30]. Early in Xenopus development, the establishment of the dorso-anterior axis of the embryo is accompanied by high levels of nuclear β-catenin in the anterior endoderm, a readout of canonical Wnt signaling. However, soon afterwards, this same tissue exhibits low levels of nuclear β-catenin, suggesting that Wnt signaling must become restricted from the prospective foregut during gut patterning [31]. This idea was confirmed by experiments in which the Wnt/β-catenin pathway was ectopically activated in the prospective foregut of the Xenopus embryo, resulting in ablation of liver- and pancreas-specific gene expression; in the converse experiment, inhibition of Wnt/β-catenin signaling in the prospective hindgut region expanded liver and pancreas domains at the expense of intestinal tissue [31].
The expression of Wnt antagonists in the anterior endoderm also suggests that Wnt signaling is actively suppressed in this region. In Xenopus, foregut-specific knockdown of Sfrp5, a secreted antagonist that sequesters Wnt ligands in the extracellular space to prevent their binding to Wnt receptors, reduced liver and pancreas gene expression [32]. In contrast, ectopic Sfrp5 activity, achieved via targeted injection of synthetic mRNA, expanded the foregut region, inducing massive liver and pancreatic buds[32]. Co-immunoprecipitation assays confirm that Sfrp5 binds and antagonizes Wnt 11 and 5, demonstrating a direct inhibition of the posteriorizing Wnt pathway in the anterior foregut endoderm [32].
Interestingly, Sfrp molecules have been shown to have biphasic potential, i.e., acting to inhibit Wnt ligands at high concentrations, but improving diffusion and signaling at low concentrations [33, 34]. The ability to manipulate the effective concentrations of LOF/GOF reagents targeted to specific tissues in Xenopus revealed that this biphasic functionality of Sfrp5 is also deployed during foregut specification—high levels of the Wnt inhibitor Sfrp5 decreased levels of the liver-specific transcription factor, hhex, but moderate levels increased hhex expression [35]. These results suggest that, although Wnt/β-catenin signaling must be suppressed in the prospective foregut region of the PGT, relative to the midgut/hindgut region, low levels of Wnt/β-catenin signaling actually potentiate foregut organ development. Indeed, foregut-targeted knockdown of the Wnt receptor Fzd7 elicits foregut organ hypoplasia [35]. Interestingly, in addition to canonical Wnt/β-catenin signaling, non-canonical Wnt/Jnk mediated cellular morphogenetic pathways were also implicated in this process; Fzd7 knockdown caused the endoderm cells of the developing foregut to be enlarged and loosely adherent with reduced C-cadherin, β-integrin, cortical β-catenin and F-actin levels and disorganized microtubules [35].
Importantly, the frog model of Wnt-mediated foregut specification is nicely corroborated by results obtained in mammalian studies. For example, transgenic mice with foregut-specific Wnt overexpression exhibit pancreas agenesis [36] and knock out [37] or downregulation [38] of mouse Sfrps leads to hypoplastic stomach development. Thus, tissue-targeted LOF/GOF assays in frog embryos can provide detailed mechanistic insights into the complex spatiotemporal roles of Wnt signaling in foregut specification (see Figure 1) that are directly relevant to higher vertebrates but would have been difficult to ascertain in such models.
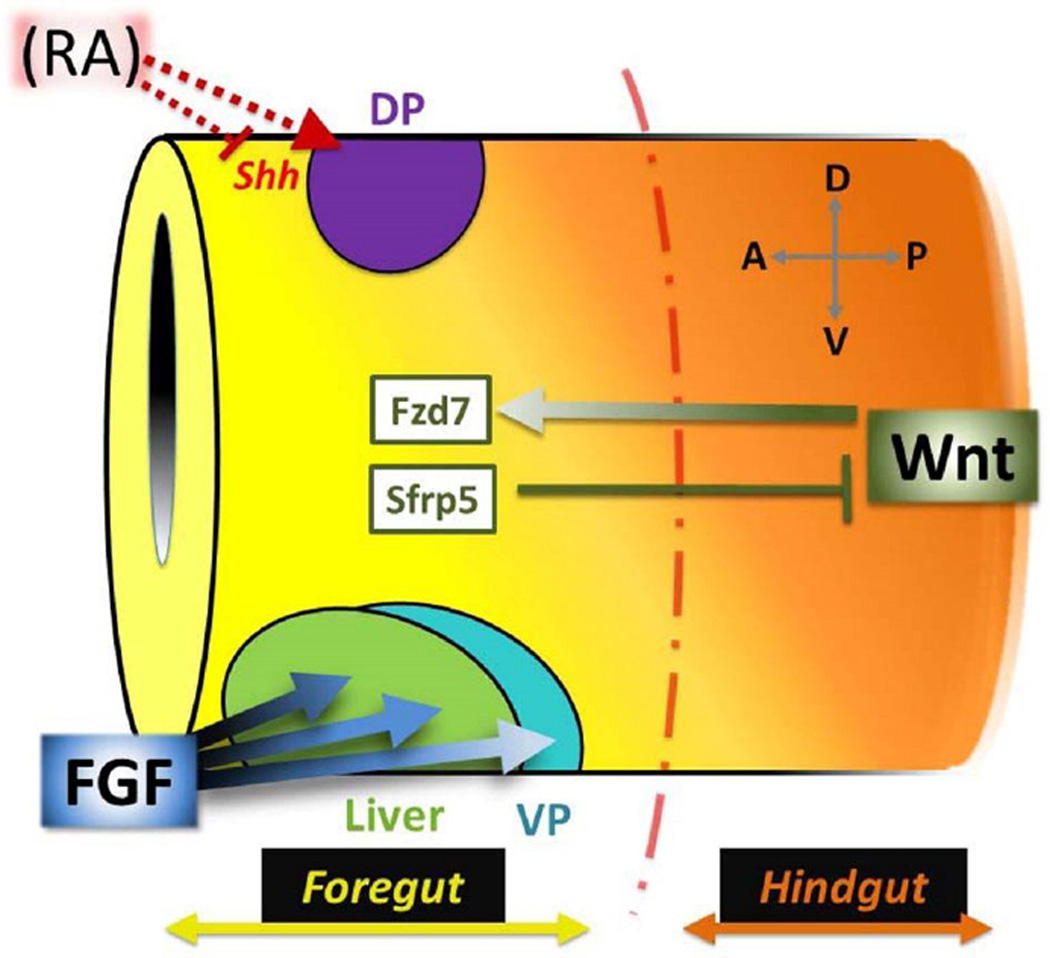
Figure 1. Wnt, RA, and FGF pattern the foregut.
The primitive gut tube is regionalized along both A–P and D–V axes. During gastrulation, RA (red) is required for dorsal pancreas (DP; purple) specification, likely by inhibiting Shh expression. Slightly later, the foregut is distinguished from the hindgut by a gradient of Wnt signaling (orange). High posterior Wnt specifies the hindgut domain, while low anterior Wnt (yellow; limited by Sfrp5) signals through the Fzd7 receptor to promote foregut fates and initiate cellular morphogenesis. Finally, a gradient of FGF signaling from the neighboring cardiac/lateral plate mesoderm segregates ventral foregut organs; prolonged, higher levels of FGF are needed to specify liver (green) versus ventral pancreas (VP; blue).
2.2. Concentration and time-dependent FGF signals segregate foregut organs
Fibroblast Growth Factors (FGFs) are required for multiple developmental processes including mesoderm induction, limb bud development, neural patterning, myogenesis, and organ morphogenesis [39, 40]. During gastrulation, FGF signaling specifies posterior fates in the PGT but, shortly thereafter, FGF secreted from the anterior lateral plate and cardiac mesoderm is required to specify anterior foregut organs (pancreas, liver and lung) in the ventral endoderm [27]. In in vitro studies, the induction of different organs by FGF appears to be concentration-dependent, suggesting that FGF signaling must be tightly regulated during foregut specification. However, it is not known whether the segregation of organ fates in vivo is determined by proximity to, or duration of contact with, the neighboring FGF-secreting mesoderm.
The experimental amenability of Xenopus, which permits straightforward explant culture of isolated endoderm and mesoderm layers of the PGT [41], has facilitated a deeper appreciation of the in vivo context-dependent regulation of FGF dosage during foregut organ specification. Consistent with higher vertebrates, gut-targeted hyper-activation of FGF signaling in Xenopus embryos results in the expansion of liver gene expression and repression of pancreas genes, while inhibition of FGF signaling causes a loss of liver gene expression and expansion of pancreatic markers [42]. Mechanistic insight was obtained from ex vivo cultures in which removal of the lateral plate mesoderm from explants of ventral endoderm at successive stages of development showed that the pancreas and liver require different time periods of interaction with the neighboring FGF-expressing mesoderm; the liver requires a more prolonged period of incubation to form correctly [42]. Chemical inhibitors of both the PI3K and MEK branches of the FGF pathway are capable of eliciting a reduction in liver gene expression, suggesting that both branches are likely required [42]. However, in the absence of mesoderm, addition of FGF to cultured foregut endoderm explants was not sufficient to induce liver, suggesting that other signaling pathways must also be involved in ventral foregut organ specification.
This example illustrates how the unique ability to isolate and culture Xenopus explants in different tissue-layer and chemical reagent combinations can enable elegant analyses of the concentration and time-dependent nature of conserved growth factor signaling in specifying vertebrate foregut organs (see Figure 1).
2.3. Early retinoic acid signaling is required for dorsal pancreas specification
The pancreas is formed from three different progenitor populations, one in the dorsal region of the foregut and two (left and right) in the ventral domain. Endocrine cells are initially specified in the dorsal pancreas to make hormones such as Insulin, while the ventral pancreas produces mainly exocrine cells and digestive enzymes. As development proceeds, the dorsal and ventral buds fuse together to form one organ [4, 43–45], with endocrine and exocrine cells distributed throughout the fused structure. At somite stages, the development of the dorsal (endocrine) pancreas is regulated by signaling molecules secreted from neighboring tissues (e.g., the notochord), including TGF-β and FGF signals, which repress sonic hedgehog (shh) expression in the dorsal foregut endoderm, a prerequisite for pancreatic fate [46].
The ability to culture early embryonic tissues from Xenopus embryos provided some of the first evidence of the key role of another signaling molecule, retinoic acid (RA), in vertebrate pancreas specification. RA is a small, diffusible lipophilic molecule (synthesized from a vitamin A precursor) that acts as a morphogen to exert concentration-dependent effects on embryonic patterning. RA signals through Retinoic Acid Receptors (RARs), converting them from transcriptional repressors to activators [47]. Utilizing the ability to culture tissue explants from early Xenopus embryos, Moriya et al showed that treatment of naïve ectoderm tissue from blastulae with a combination of both Activin and RA induces the differentiation of morphological and functional pancreatic tissue [48]. In a related study, slightly later explants from early gastrulae were also induced to form pancreas after exposure to RA [49]. In both cases, the RA-treated explants expressed endocrine hormones, including Insulin, and developed pancreas-like tissue architecture.
The above studies indicate that RA influences pancreas specification very early, during or soon after gastrulation. To determine whether RA signaling is also required at this time for endogenous pancreas specification, Xenopus embryos were exposed to RA, chemical RAR inhibitors or injected with mRNA encoding dominant-negative mutant versions of RARs. In all cases, inhibition of RA signaling ablated both exocrine and endocrine gene expression in the dorsal pancreas, while the ventral pancreas was unaffected [50, 51]. This result correlated with ectopic shh expression in the dorsal pancreatic endoderm [50], suggesting that RA signaling contributes to the exclusion of Shh in the dorsal pancreas field. In the reciprocal experiment, exposing Xenopus gastrulae to exogenous RA resulted in enlargement of the pancreas domain. This perturbation expanded the dorsal endocrine population at the expense of the exocrine population, as indicated by a dose-dependent decrease in exocrine cell markers and an increase in insulin expression [50].
Consistent with the role of RA in the frog, mice deficient in an enzyme required to synthesize RA (Retinaldehyde dehydrogenase; RALDH) exhibit dorsal pancreas hypoplasia, but retain pancreas markers in the ventral endoderm [52, 53]. Thus, Xenopus explant and pharmacological assays have facilitated clarification of the critical roles of a specific signaling pathway (RA) in pancreas specification and differentiation that are highly relevant to mammalian systems (see Figure 1).
2.4. Genome-wide microarray screens identify new foregut genes
While sections 2.1–2.3 highlight a few of the key signaling pathways known to be involved in foregut specification and patterning, there is still a paucity of knowledge of all the molecular players and effectors involved in integrating and implementing these signals. Fortunately, microarray analyses of Xenopus embryos, explants and developing organs are now being used to identify, on a genome-wide scale, hundreds of new factors likely to play important roles in foregut patterning and organogenesis in all vertebrates. These examples illustrate the power of frog embryos as a platform for unbiased gene discovery.
2.4.1. Microarray analyses of chemically-treated Xenopus embryos
The ability to culture frog embryos in the presence of compounds that modulate specific signaling pathways facilitates straightforward profiling of genes downstream of these pathways. For example, recent microarray profiling of Xenopus embryos exposed to an RA inhibitor, identified Ndrg1α as a new RA-responsive factor [54]. Ndrg1α has diverse functions in development and tumorigenesis, but has not previously been associated with digestive organ development [55]. Interestingly, Ndrg1α was found to repress the Wnt/β-catenin pathway allowing specification of foregut progenitor cells [54]. This study thereby revealed novel crosstalk between RA and Wnt signaling in foregut development. Given the central role of RA and Wnt signaling in foregut specification, it will be important to ascertain the role of Ndrg1α in the development of the digestive organs of higher vertebrates.
2.4.2. Microarray analyses of Xenopus explants
It is well established that reciprocal signaling between the mesoderm and endoderm layers of the vertebrate PGT is crucial for its regional patterning [41]. To identify new molecules involved in mesoderm-endoderm signaling, Kenny et al cultured isolated endoderm explants versus endoderm/mesoderm explants from the Xenopus PGT and conducted microarray analyses to interrogate resultant differences in gene expression [56]. One endoderm gene upregulated in response to mesoderm was the Sfrp-related protein Sizzled (Szl) [56]. Szl was found to be required for foregut organ specification downstream of mesodermal BMP signaling, a highly conserved developmental pathway critical for axial patterning and the development of multiple organs [57] . Like Wnt, BMP also plays dynamic roles in foregut organ specification as BMP signaling initially promotes hindgut development and inhibits foregut fates [58], but is later required to specify foregut lineages [59, 60]. Interestingly, Szl maintains BMP signaling by regulating Fibronectin deposition between the endoderm and mesoderm layers of the PGT. In this example, the experimental amenability of Xenopus revealed a novel extracellular feedback mechanism that mediates reciprocal Wnt/BMP crosstalk between the endoderm and mesoderm during foregut patterning.
2.4.3. Microarray analyses of Xenopus embryonic organs
It is relatively easy to isolate individual prospective organs within the large and accessible PGT of the frog embryo. This approach has been exploited to discover a trove of new factors and cellular processes involved in the morphogenesis of the pancreas. For example, microarray profiling of ptf1α–positive pancreatic endoderm isolated from Xenopus embryos revealed putative target genes of this important pancreas-specific transcription factor [61]. The genes identified in this analysis contribute to a surprising variety of cellular functions with, as yet, unexplored roles in pancreas morphogenesis, such as intracellular vesicle docking and fusion, metabolism, cell adhesion, and extracellular matrix stabilization [61]. In another study, Jarikji et al used microarray technology to identify genes differentially expressed between isolated dorsal and ventral pancreatic buds of the Xenopus embryo [43]. This study identified Tetraspanin (Tn4sf3), a transmembrane scaffolding protein that is up-regulated in ventral pancreatic tissue and, intriguingly, required for dorsal and ventral pancreatic fusion [43].
The above examples suggest that investigation of the molecules and pathways identified by microarray (or RNAseq) analyses in frog embryos could yield profound new insight into the regulatory networks and cellular processes required for the specification and morphogenesis of vertebrate foregut organs. Additional studies are necessary to determine the degree to which the new genes identified in these and other frog studies are conserved in higher vertebrates.
3. What can the frog tell us about intestinal lengthening?
In contrast to the multiple organs specified in the foregut region of the PGT, the posterior (midgut/hindgut) zone is destined to become intestine. This segment of the PGT must undergo dramatic morphogenetic changes, including lumen formation, extensive elongation and counterclockwise rotation while, concomitantly, the visceral mesoderm and epithelial lining of the tract undergo lineage restriction and cellular differentiation. These concurrent events shape and integrate multiple levels of biological organization, from cellular architecture to intricate three-dimensional anatomy. The mechanisms underlying the complex morphogenesis of the intestine are just beginning to be understood.
Intestinal development is pertinent to a variety of human afflictions. Congenital anomalies of intestinal morphogenesis include narrowing (stenosis) or occlusion (atresia) of the GI tract [62–66], deficits in the normal length of the intestine (congenital short bowel syndrome [67–73]), and intestinal rotation and fixation abnormalities, which occur in as many as 1 in 500 infants [67, 69, 74–79]. While malrotation itself is not always symptomatic, it predisposes affected individuals to volvulus, a life-threatening strangulation of the gut tube [74]. In addition to birth defects, inflammatory bowel diseases (e.g., Crohn’s, ulcerative colitis) are increasingly common chronic conditions in both pediatric and adult populations; progressive complications from these disorders often require surgical resection of the damaged regions of the gut, leading to a shortened GI tract and attendant nutritional issues [80–82]. Understanding the events that control normal intestinal development is therefore critical, not only for preventing the causes of common birth defects, but also for devising strategies to restore normal gut length in congenital or acquired short bowel syndromes.
The concentrically coiled anatomy of the pre-metamorphic Xenopus tadpole intestine is relatively simple compared to the visceral anatomy of higher vertebrates, yet it undergoes analogous elongation and rotation events, which occur over the course of only a few days [5, 83]. Because of its internal location, the gut can be challenging to visualize in amniotes, but tadpoles are transparent throughout organogenesis, allowing the cells of the PGT to be labeled and tracked during morphogenesis. Combined with the ability to target LOF/GOF reagents to the gut by microinjection, such studies have led to a deeper understanding of the cellular and molecular events that drive intestine development.
3.1. Endoderm cell rearrangements drive gut lengthening
Despite early differences in the initial formation of the PGT in amphibian and amniote embryos [84–86], there are remarkable similarities in the process of gut elongation. In both frogs and mammals, the early gut tube narrows and elongates coincident with an apparent remodeling of the endoderm (i.e., future epithelial) layer, suggesting that a cell rearrangement process may be involved in vertebrate gut elongation [87, 88]. In the frog, very little cell division is observed during the early stages of gut elongation, supporting the idea that (at least initially), this event is driven almost exclusively by cell rearrangement [89, 90]. In the Xenopus embryo, small groups of gut cells can be easily labeled with vital dyes, and their behavior visualized during gut elongation. Such studies revealed that the most central endoderm cells of the PGT radially intercalate during gut morphogenesis [85]. As the gut lengthens, the number of endoderm cell layers is reduced from 4–5 cells deep to a single epithelial layer, suggesting that the intercalation of the central cells and concomitant thinning of the epithelium provides the increased surface area necessary to generate length [85, 90]. Moreover, clusters of labeled endoderm cells become aligned in longitudinal tracts along the A-P axis of the lengthening intestine [85, 90]. This indicates that radial intercalation is biased to preferentially occur between A-P neighbors, or is closely coordinated with a convergent extension process, increasing intestinal length, rather than girth [85, 90, 91] (Figure 2).
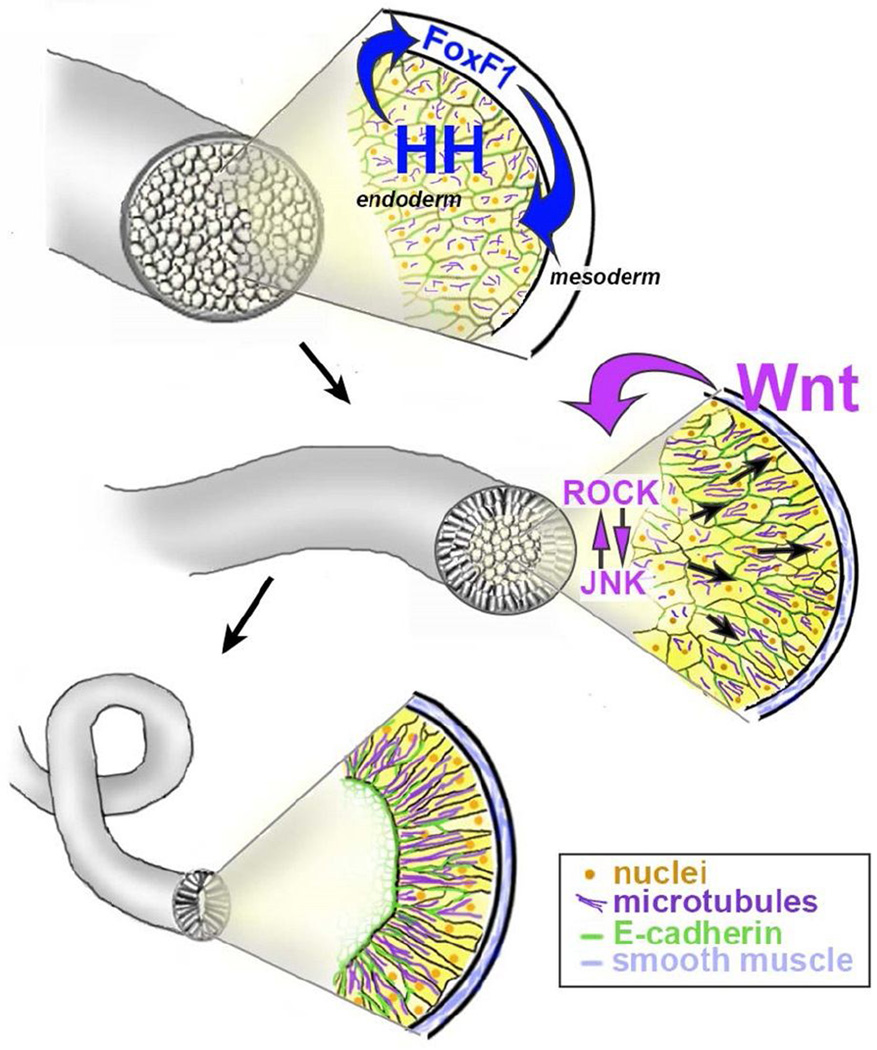
Figure 2. Intestine lengthening involves Hedghog- and Wnt/PCP-mediated endoderm cell polarization, rearrangement and epithelial differentiation.
Initially, the endoderm cells of the prospective intestine are rounded, unpolarized and disorganized. Signaling via Hedgehogs (HH; from the endoderm) induces foxF1 expression in the surrounding mesoderm layer of the gut tube. This facilitates reciprocal signaling from the now differentiating visceral mesoderm, which regulates the rate of epithelial differentiation in the underlying endoderm. Concomitant non-canonical Wnt signals (presumably from the mesoderm) are required for the endoderm cells to become polarized, starting with the outermost (most basal) layers and progressing towards the center of the gut tube. Both actomyosin contractile forces, regulated by ROCK, and microtubule organization, regulated by JNK, are required to dynamically remodel adhesive contacts between the polarized endoderm cells. This enables productive radial intercalation of the most central cells into the outermost layer, resulting in tissue lengthening and the morphogenesis of a single layer intestinal epithelium.
In amphibian embryos, the PGT begins as a solid cylinder full of concentrically stratified endoderm cells, but in amniotes, the endoderm lining of the PGT is already a single-layer, albeit pseudostratified, epithelium[92]; thus, multilayer radial intercalation per se is not likely to drive gut elongation, but cell rearrangements are likely still involved. It has been hypothesized that the elongation of the mammalian gut tube results from the reorganization of the pseudostratified endoderm layer into a columnar epithelium, but how this results in anisotropic tissue lengthening is not known [92]. Nonetheless, short gut phenotypes in both frogs and mice are associated with disorganization and stratification of the intestinal epithelium [37, 88, 92, 93], and the genes and pathways found to direct endoderm rearrangement in frogs (by regulating cell shape/polarity/adhesion—discussed in Sections 3.2–3.4 below) also control gut elongation and epithelial morphogenesis in mammalian models. Therefore, despite differences in endoderm tissue architecture in the initial PGT, the processes of gut elongation appear to be conserved at many levels, making the frog embryo a highly relevant model for investigating intestine lengthening.
3.2. Shroom3 mediates endoderm cell shape changes
The PDZ-containing protein Shroom3 is required for directing cell shape changes, including apical constriction and apicobasal cell elongation during neural tube closure [94–96]. In Xenopus, intestine-targeted microinjection of mRNA encoding a dominant-negative mutant form of Shroom3 (DN-Shroom3) results in severely shortened intestinal tracts, demonstrating a requirement for this protein during gut development [97]. At the cellular level, DN-Shroom3 expressing cells display reduced apical constriction, or remain rounded and do not intercalate, resulting in a stratified disorganized epithelium; thus, Shroom3 must normally direct cell shape changes in the endoderm that are necessary for both epithelial morphogenesis and gut elongation [97]. Interestingly, Pitx transcription factors, which are also required for intestinal elongation [98, 99], were found to directly regulate shroom3 expression, suggesting that Shroom3 directs intestinal morphogenesis downstream of Pitx factors [97]. These results underscore the relationship between endoderm morphogenesis and gut elongation. Notably, Shroom3 activity has also been correlated with endodermal shape changes and epithelial architecture in the mouse intestine [92, 100].
3.3. Non-canonical Wnt/PCP signaling controls intestine lengthening and endoderm rearrangements
In addition to foregut specification, Wnt signaling is also essential for intestinal morphogenesis as knock-out/down of Wnt signaling components results in short, malformed intestinal tracts in all species studied [37, 88, 90, 93, 101]. However, while canonical Wnt/β-catenin signaling is necessary for cell specification and maintenance of stem cell niches in the intestine, the elongation of the gut tube depends primarily on the non-canonical Planar Cell Polarity (Wnt/PCP) pathway, which involves distinct downstream effectors such as JNK and Rho family GTPases [102]. Isolating the mechanisms by which Wnt/PCP signaling coordinates cell movements in the gut is non-trivial in murine models due to the redundancy of Wnt signaling components, and the need for stage- and tissue-specific promoters and/or combinations of mutant alleles [37]. However, the use of small molecules and gut-specific targeting of LOF reagents in Xenopus has contributed a mechanistic understanding of the molecular players and cellular mechanisms involved in gut elongation.
For example, both pharmacological inhibition of RhoA and gut-targeted microinjection of mRNA encoding a dominant negative mutant version of RhoA (DN-RhoA) implicate Rho activity in Xenopus gut elongation, as both perturbations abrogate intestinal elongation [90]. As they prepare to undergo intercalation, endoderm cells normally become polarized and radially oriented, progressing from the most basally located cells toward the center of the PGT [90]. However, DN-RhoA-expressing cells remain round in shape, unpolarized and do not intercalate. Rho-inhibited cells also exhibit aberrant Myosin II organization and increased expression of the adherens junction protein, E-cadherin, suggesting that, without Rho activity, endoderm cells are unable to remodel adhesive contacts and, as a result, cannot rearrange or intercalate [90]. Chemical inhibitors of downstream effectors of Rho kinase (ROCK) and Myosin II phenocopy Rho-deficient intestinal malformations at both the gross and cellular level, suggesting that actomyosin contractility regulated by the Rho/ROCK/Myosin II branch of the Wnt/PCP network is required for the endoderm cell rearrangements that generate gut length [90]. Interestingly, embryos with less severe gut elongation phenotypes display abnormal intestinal coiling, revealing a potential mechanistic link between the processes of gut lengthening and rotation.
Non-canonical Wnt/PCP signaling is also mediated by activation of Jun N-terminal kinase (JNK) [30, 103]. Chemical inhibition or gut-targeted knockdown of JNK activity results in shortened intestines, similar to perturbation of Rho/ROCK/Myosin II activity. However, the JNK-deficient phenotype differs at the cellular level, as endoderm cell adhesion is lost in guts lacking JNK activity, in contrast to the increased adhesion observed in ROCK-deficient guts [101]. Abrogation of microtubule polymerization phenocopies loss of JNK activity, and suggests that this arm of the Wnt/PCP signaling cascade mediates cell rearrangement by promoting microtubule polymerization, and maintaining cell adhesion [101]. The amenability of Xenopus explants to ex vivo cell assays further confirmed that adhesive remodeling is likely to be involved in gut elongation. PGT cells were isolated and dissociated, and their ability to reaggregate was used as an assay for changes in cell adhesion that may be caused by perturbing different branches of the Wnt/PCP cascade. The results confirmed that Rho kinase promotes decreased cell-cell adhesion, while the JNK pathway increases adhesion, suggesting that these two arms of the Wnt/PCP signaling network act in complementary ways to regulate intestinal cell intercalation [101]. Thus, the accessibility of the frog embryo was instrumental in clarifying Wnt/PCP mediated cellular and molecular dynamics underlying the cell rearrangements that lengthen the gut.
3.4. Hedgehog signals mediate reciprocal mesoderm-endoderm signaling
Hedgehogs (Hhs) are secreted proteins that elicit concentration dependent responses via multi-pass transmembrane receptors [104]. Hh signaling plays crucial patterning and morphogenetic roles in ectoderm, mesoderm and endoderm-derived tissues throughout development [104]. In mouse models, loss of Hh signaling results in shortened, malrotated gastrointestinal tracts, with disrupted architecture in all three tissue layers [105, 106]. Xenopus have been instrumental in refining our understanding of how Hh-mediated communication between tissue layers functions in gut development. Hhs are expressed in the endoderm layer of the gut tube but, surprisingly, microinjection of mRNA encoding a constitutively active version of the Smoothened receptor (to induce excessive Hh signaling) does not affect gut development when targeted to the endoderm layer [107]. In contrast, ectopic Hh signaling severely disrupts gut elongation and coiling when targeted to the mesoderm layer, indicating that Hh ligands from the endoderm act by binding to receptors in the mesoderm [107]. Of significance, this study also showed that Hh-mediated signaling within the mesoderm is essential for the endoderm epithelial rearrangements that elongate the intestine, revealing insight into the molecular nature of the reciprocal signaling known to be required between the layers of the developing gut [107].
Xenopus experiments also shed light on the role of downstream components of Hh signaling in gut morphogenesis. The transcription factor foxf1 is upregulated in the gut mesoderm in response to Hh signaling [108, 109]. However, knockout of foxf1 is lethal in mice prior to gastrointestinal elongation and looping, precluding use of the murine model for understanding the function of FoxF1 in intestinal development [110]. In contrast, FoxF1 can be directly knocked down in Xenopus using targeted microinjection of morpholino oligonucleotides. Morpholino-mediated loss of FoxF1 activity resulted in disruption of mesodermal differentiation and severely reduced the elongation and rotation of the Xenopus intestine, confirming that FoxF1 is essential for normal gastrointestinal morphogenesis [111]. Subsequent to this work, foxf2 knockouts and compound foxf1+/−/foxf2+/− mice were generated, which survive to birth [112]. Similar to the observations in Xenopus, the intestines of these mice were deformed and showed severe disruption in mesoderm-derived tissues [112]. These examples underscore how the frog embryo can be used to discern the tissue-specific functions of highly conserved genes in directing crucial aspects of gut morphogenesis.
4. Insights from chemical screening
One of the greatest advantages of externally-fertilized, aquatic embryos, like those of the frog, is that they can be exposed to exogenous chemicals to reveal potential roles for the cellular target of the compound in development. For studies of organ morphogenesis, these are particularly useful reagents because they allow earlier events that might be dependent on the same signaling pathway, to proceed unperturbed. The effects of chemical reagents targeting RA, Wnt, and FGF signaling pathways have been described above, but a few studies using additional small molecules are also worth highlighting (section 4.1). Although the potential mechanisms of action of these compounds in disrupting gut specification or morphogenesis is not yet well understood, these reports nonetheless implicate interesting pathways and processes in digestive organogenesis. Finally, it is important to mention that numerous chemical toxicants, many of which have unknown mechanisms of action, also elicit gut phenotypes (section 4.2); these chemicals could provide interesting avenues for future research on both normal and abnormal gut development.
4.1.1 Calcineurin implicates Wnt/Ca++ signaling in gut elongation and rotation
Calcineurin is a calcium/calmodulin-dependent serine/threonine phosphatase which is a component of the non-canonical Wnt/Calcium signaling pathway [113]. Exposure of Xenopus tadpoles to the Calcineurin inhibitors cyclosporine A, FK506, or FK520 for six hour windows beginning at Nieuwkoop and Faber (NF) stage 18, 29/30, 37/38, or 41 resulted in shortened gut tubes often displaying a reversed coiling direction [114]. Given the roles of non-canonical Wnt signaling in gut morphogenesis, it seems possible that Calcineurin regulates endoderm cell properties, such as adhesive or cytoskeletal dynamics, and/or cell polarity. Injection of these inhibitors into dorsal blastomeres at the four cell stage similarly disrupted gut coiling, supporting the idea that Calcineurin plays a specific role in gut development [114]. However, other dorsally derived organs (heart, liver, etc.) were also affected by this injection, and further experimentation is required to determine the importance of Calcineurin in gut morphogenesis per se. It will be interesting to determine how Wnt/Calcium signaling is integrated with canonical and/or non-canonical Wnt signaling in this context, using more specific reagents to target Calcineurin activity within the developing intestine.
4.1.2. Lysyl Oxidase and a role for the extracellular matrix in gut elongation
Lysyl oxidase (Lox) is a copper-dependent enzyme that catalyzes cross-linkage of Collagen and Elastin in the extracellular matrix (ECM). lox knockout mice die at birth and have a number of deformities including cleft palate, spinal, cardiovascular and respiratory defects, indicating that Lox is required for normal development in a number of systems [115–117]. In Xenopus, exposure to β-aminoproprionitrile (β-APN), a specific inhibitor of the Lox catalytic domain, from NF 6–45 affects many developmental processes, including proper cross-linking of connective tissue fibers in the notochord and somites [118]. Of particular interest, β-APN exposure also results in short, straight gut tubes, suggesting that Lox may also function in gut morphogenesis, but its role in intestinal development has not been specifically investigated. In mice, Lox regulates ECM organization in muscle connective tissue, suggesting that Lox could be required for proper ECM assembly between the mesoderm and endoderm layers of the PGT [119]. Additional studies that investigate the role of Lox specifically within the gut are necessary to evaluate the potential importance of this protein in intestinal development.
4.1.3. mTOR signaling is implicated in gut elongation
The mTORs are serine/threonine kinases which form complexes with FKBP12 and Raptor [120–122]. In Xenopus, inhibition of mTOR with rapamycin treatment from NF 2 – 45 results in shorter, fatter gut tubes as compared with controls [123]. Other organs form relatively normally, suggesting that mTORs may be specifically required in gut elongation [123]. Consistent with this idea, injection of a dominant-negative Rheb (an upstream positive regulator of mTOR) at the 2 cell stage also decreases intestinal elongation and coiling [123]. As zebrafish exposed to rapamycin also have GI defects, this pathway may play a conserved role in vertebrate intestinal organogenesis [124].
Interestingly, Sirtuin deacetylases (Sirts) are also implicated in regulation of the TOR pathway [125]. Inhibition of Sirt-1 with the specific inhibitor, Ex-527, from the 2 cell stage on also disrupts intestinal elongation and coiling, although Sirt-1 deficient tadpoles exhibit more general defects than those treated with rapamycin, including decreased lengthening along the anterior-posterior body axis, edema, and abnormal eye development [126]. As Sirt-1 inactivates p53, and p53 activity is essential for normal embryonic development through regulation of TGF-β signaling [127, 128], Sirt-1 could be involved in regulating multiple pathways during gut development. It is important to note that none of these experiments were conducted in a way that specifically evaluates the function(s) of Sirt-1/mTOR in GI morphogenesis, and it is unclear exactly when these reagents may be acting to impact gut development. Thus, additional studies that specifically address the functions of Sirts and TORs in the gut are necessary to determine how this pathway may contribute to intestinal morphogenesis.
4.2 Numerous toxicants perturb gut morphogenesis
The etiology of intestinal malrotation is largely unknown but it is believed to have a multifactorial origin, implicating both genetic and environmental causes. Frog embryos have proven to be excellent models for screening toxicants that impact development, and many studies have implicated exogenous chemicals, including insecticides, nanoparticles and explosives, in digestive tract malformations (Table 1). Unfortunately, few of these reports attempt to identify the underlying molecular or cellular developmental mechanism(s) disrupted by toxicant exposure. Future research on the mechanism(s) of action of these compounds in Xenopus could provide invaluable insight into the pathways required for gut development and potentially reveal environmental factors that contribute to the relatively high incidence of intestinal malrotation and other gut defects in the human population.
Table 1.
Anthropogenic toxicants found to disrupt gut development in Xenopus laevis.
| Chemical Class | Chemicals tested | Uses | References |
|---|---|---|---|
| Azoles | Triadimefon, n-butyl isocaynate, carbendazim |
Fungicide | [129, 130] |
| Bipyridyliums | Paraquat | Herbicide | [131, 132] |
| Carbamates | Carbaryl | Insecticide | [133] |
| Carboxylic Acids | Valproic acid, pentanoic acid, butyric acid, 2-ethylhexanoic acid |
Various: Plasticizer, lubricant |
[134] |
| Chlorophenoxy Acids | 2,4-D | Herbicide | [129] |
| Estrogen | 17β - estradiol | Drug | [135] |
| Nanoparticles | CuO, ZnO, polystyrene | Various: semiconductors; drugs, skin care |
[136–138] |
| Nitroaromatic compounds |
TNT, 2ADNT, 4ADNT | Explosive | [139] |
| Organochlorines | Chlorothalonil, DDT, DDD | Insecticide | [139, 140] |
| Organophosphates | Malathion, Malaoxon, Parathion, Paraoxon, Dicrotophos, Monocrotophos, Chlorpyrifos, Diazinon |
Insecticide | [141–144] |
| Phenols | Bisphenol A, nonylphenol | Various: Plastics, resins, adjuvant |
[135] |
| Phosphonoglycines | Glyphosate | Herbicide | [129] |
| Triazines | Atrazine | Herbicide | [129, 145, 146] |
| Polymer Mixtures | Tire Debris Organic Extract | Tire product | [147] |
| Chemical Mixtures | Corexit 9500 | Dispersant | [148] |
5. From Frogs to Humans
Because the molecules, pathways and processes important for endoderm specification and digestive morphogenesis are conserved across vertebrates, amphibian studies are highly relevant to human gut development. Indeed, frog studies have already informed translational research strategies. For example, knowledge of the hierarchical relationship of factors such as Wnt, Fgf, RA, Bmp, and Hh in Xenopus endoderm specification was instrumental in the successful development of protocols to direct human pluripotent stem cells to digestive organ fates and generate digestive “organoids” from human stem cells [26]. Likewise, one of the most important genes in pancreatic development, pdx1, was discovered in the Xenopus model [149]. Activation of this pancreatic master regulator is now used in human transdifferentiation protocols to elicit endocrine pancreas fates from extra-pancreatic tissues, such as liver cells, a critical step towards successfully reprogramming adult cells as a cell replacement therapy for diabetes [150].
Genes for human congenital GI malformations are just beginning to be identified [151]. To complement these efforts, frog embryos not only provide excellent models for the discovery of genes critical for normal digestive organ morphogenesis (see section 2.4 above), but facilitate rapid in vivo validation of candidate birth defect gene function. For example, the importance of FoxF1 in gut morphogenesis was first demonstrated in Xenopus, as discussed above (section 3.4;[111]). Mutations in this gene have recently been detected in human patients with similar malformations, including intestinal malrotation and congenital short bowel [152–154]. Moreover, trisomy of chromosome 16, which contains the human foxf1 gene, is also associated with intestinal maladies [153] . Finally, mutations in zic3, a transcription factor involved in directing organ laterality in animal models [155], have recently been detected in humans with congenital GI defects [152]. In Xenopus, overexpression of zic3, or injection of a mutant zic3 mRNA that acts in a dominant-negative manner, disrupt the direction of intestinal looping, providing in vivo confirmation of the suspected role of this molecule in organogenesis [155]. These examples illustrate the immense potential and relevance of the Xenopus model for human biomedical research.
6. What can frogs tell us about digestive organ evolution?
6.1. The diversity of the tadpole gut
The morphology of the digestive tract determines an organism’s ability to assimilate the energy necessary to grow, survive and reproduce--and thus has a profound effect on fitness. For example, the dimensions of the gut tube itself, including its diameter, length and compartmentalization, impact the capacity to digest different food resources. Although there is remarkable disparity between these parameters in the GI tracts of different vertebrates, the underlying mechanism by which different topologies of the digestive tract evolve is unknown.
Frogs inhabit most of the planet, including every continent except Antarctica. This success is facilitated by a wide array of reproductive strategies which allows them to breed in diverse environments, including terrestrial niches [156]. Because the tadpoles of many terrestrial breeding frogs are derived from eggs laid in environments with limited water and food supplies (e.g., bromeliads, leaves), these species may exhibit unusual larval feeding strategies. For example, in contrast to typical herbivorous tadpoles, terrestrial tadpoles may be carnivorous and feed on unfertilized eggs, invertebrates or even other tadpoles. Alternatively, they may delay or omit the feeding stage entirely by becoming more dependent on maternal yolk stores, as observed in direct-developing species [157]. Not surprisingly, these novel feeding (or non-feeding) strategies are complemented by specialized larval gut morphologies. Evo-devo investigations of gut development in two emerging frog models, Lepidobatrachus laevis and Eleutherodactylous coqui, are providing novel insight into the potential sources of variation that lead to diverse gut morphologies during evolution.
6.2. The evolution of a carnivorous foregut in Lepidobatrachus
The Budgett’s frog, Lepidobatrachus laevis, lives in the semi-arid regions of South America [158]. As adults, Lepidobatrachus are aggressive, and often cannibalistic, predators, while their tadpole larvae are obligate carnivores that routinely consume other tadpoles, including siblings [159–161]. Unlike tadpoles that have a long, un-compartmentalized tract adapted for their nutrient-poor herbivorous diet, Lepidobatrachus tadpoles have a large, distendable stomach compartment [161, 162]. Analysis of foregut morphogenesis in Lepidobatrachus has revealed that the development of this unusual anatomy is preceded by a disparity in the proportion of the PGT that is ascribed to foregut versus hindgut, as compared to that observed in Xenopus (which is used as a point of comparison to represent the ancestral state [163]). This ultimately results in dramatic differences in stomach morphogenesis and the final anatomical orientation of the gastroduodenal (GD) loop (Figure 3).
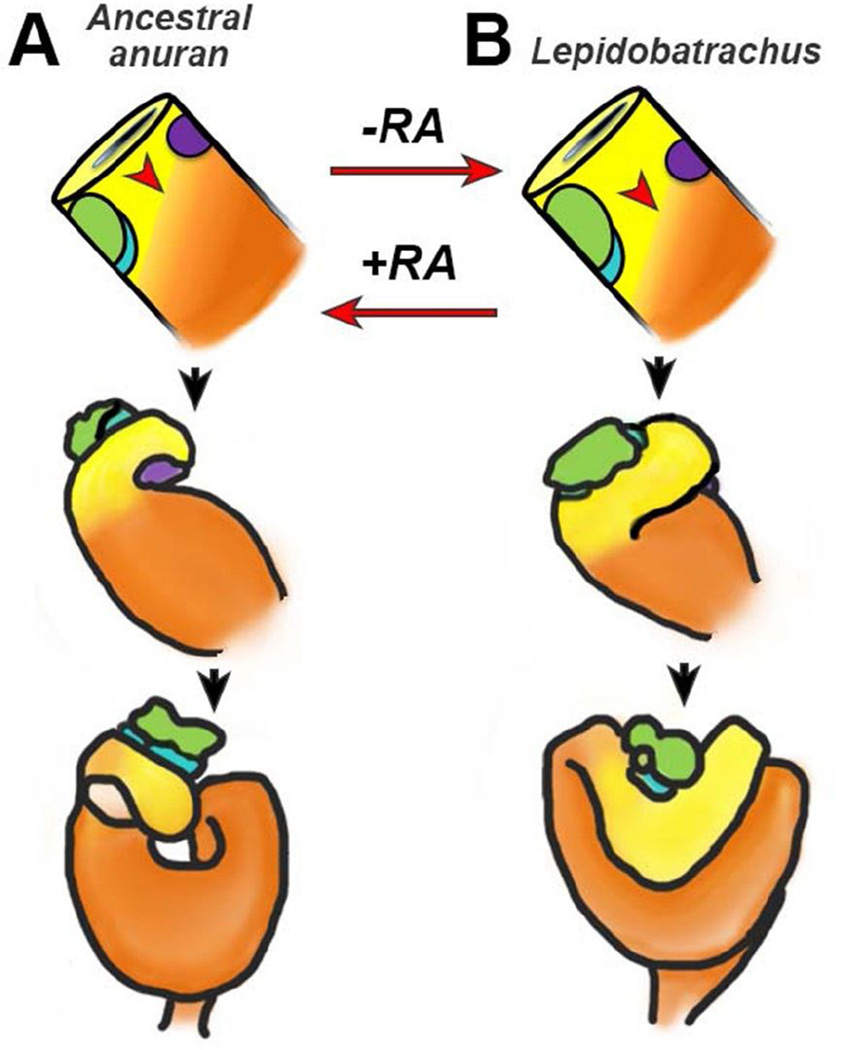
Figure 3. Altered RA signaling may have led to a novel foregut morphology.
A) In the hypothetical ancestral anuran (represented by Xenopus), the herbivorous tadpole requires only a rudimentary stomach. The foregut domain of the primitive gut tube is small relative to the hindgut domain, causing the gastroduodenal (GD) loop to form in a relatively anterior position and acquire an acute curvature during later foregut morphogenesis. B) In contrast, in the carnivorous Lepidobatrachus tadpole, which requires a capacious stomach, the ratio of foregut to hindgut is greater, and the GD loop forms in a more posterior position. Consequently, the larger carnivore stomach becomes more transversely oriented. This anatomical change may have been dependent on a decrease in RA signaling during foregut development in the Lepidobatrachus lineage, since inhibiting RA in Xenopus (representative of the ancestral condition) transforms the GD loop to resemble that observed in Lepidobatrachus. Conversely, exposing Lepidobatrachus embryos to excess RA elicits a more typical foregut configuration.
To identify potential signaling pathways that may have been involved in the evolution of this novel carnivore morphology, a small molecule screen was conducted in Xenopus embryos [163]. Compounds targeting known morphogenetic pathways were screened for the ability to transform the more typical herbivore GD loop found in Xenopus to resemble that found in the carnivorous Lepidobatrachus tadpole. Remarkably, five compounds produced this change, two of which inhibit RA signaling [163]. RA plays an early role in dorsal pancreas specification (described in section 2.3 above), but RALDH expression also persists throughout the development of the stomach and duodenum, and perturbations of RA signaling in tailbud stage Xenopus embryos implicate RA patterning in GD looping [164]. Thus decreased RA signaling in the Lepidobatrachus lineage may have led to the unusual carnivore foregut morphology, an idea supported by the formation of a smaller stomach and shallower GD loop (anatomically similar to an ancestral tadpole like Xenopus) in Lepidobatrachus embryos exposed to exogenous RA [163]. This study demonstrated that subtle changes in the levels of a specific foregut signaling factor can lead to anatomical variants that closely mimic extant interspecific variation.
6.3. The evolution of nutritional endoderm in a direct-developing frog
The epithelial lining of the vertebrate digestive tract arises from the endoderm germ layer of the early embryo. In the frog, the endoderm is derived from the cellularization of the yolky vegetal pole of the egg. In typical tadpoles, all of the endoderm-derived cells rearrange in the gut tube to become the gut epithelium and contribute to intestinal lengthening (see section 3.1 above). In most species, the inherent yolk in the embryonic cells supports development only through the initial tadpole stages, after which the animal needs a functional digestive system to acquire the energy necessary to continue to grow and, ultimately, metamorphose, when the long gut is remodeled into the shorter gut of an adult frog. In contrast, endotrophic (non-feeding, yolk-dependent) species, such as direct developers, often delay or completely skip the formation of a long, coiled gut, since they do not need to feed [157]. Instead, these embryos directly form a short adult-like gut by the time they become a froglet.
The mechanisms by which the processes of yolk utilization and gut development are altered in direct-developing species may provide insight into the origin of novel gut morphologies and feeding strategies during evolution. Indeed, in contrast to species like Xenopus that produce feeding tadpoles, in the direct-developing embryo of Eleutherodactylous coqui, much of the yolky endoderm does not contribute to the final epithelial lining of the gut tube [165, 166]. Instead, it becomes nutritional endoderm (NE) that is utilized solely as a source of energy—the yolk platelets in these cells are metabolized, extruded and, eventually, eliminated as waste from the body. Some of the yolky vegetal cells are specifically set aside for this function during blastula stages, as indicated by the existence of a population of endoderm with reduced signaling/responsiveness in such species [167]. This alternate fate of the NE is likely related to the modified germ layer patterning often seen in larger eggs [168]. The use of endoderm to provide energy to sustain growth, rather than form a longer gut, has interesting implications for the ancient origins of definitive vs. extra-embryonic endoderm, as well as the acellular yolk sac of higher vertebrates [165].
7. Conclusion/ Future directions
Amphibian embryos have a rich history as developmental models and have been instrumental in understanding fundamental embryological events, such as gastrulation and neurulation. Here, we argue that the experimental versatility of frog embryos—e.g., the ability to isolate tissue explants, target LOF/GOF reagents, and/or use pharmacological agents to investigate the underlying mechanisms of development—also makes them ideal models in which to examine many facets of digestive organogenesis. Their amenability to these experimental manipulations has enhanced our understanding of the spatiotemporal dynamics of conserved signaling pathways, such as Wnt, FGF, BMP, RA and Hh, in foregut specification and intestinal elongation. In addition, microarray profiling and small molecule/toxicant screening in frog models have revealed novel proteins and pathways likely to play critical roles in normal and abnormal gut morphogenesis. Such information enhances our understanding of gut patterning and morphogenesis in all vertebrates, including humans, making the frog a powerful model for translational embryology.
While many signaling pathways are conserved in gut organogenesis, differences do exist in the size and shapes of digestive organs among species, which is exemplified by the gastrointestinal tracts of tadpoles with different feeding strategies. Studies designed to elucidate differences in gut development in frog species with unique feeding ecologies are beginning to provide intriguing insight into the variety of molecular and cellular mechanisms underlying morphological evolution. Such knowledge has the potential to illuminate specific environmental or ecological parameters that affect gut development and, therefore, impact survival and fitness. Since frog species are continuing to disappear at an alarming rate, this line of evo-devo research may provide critical information for conservation efforts [169].
Rapid advances in de novo transcriptome assembly, proteomics, and genome editing (CRISPR-Cas) continue to make functional genetic studies even more accessible for Xenopus, and nearly any frog species [170–175]. New techniques continue to arise for refining tissue-specific gene manipulation at late stages of organogenesis in the frog, including lipofection [176] and electroporation [177]. Utilizing these cutting-edge technologies to investigate amphibian digestive organ development—integrating aspects of organ specification, morphogenesis, toxicology, and/or evolution—has the potential to advance multiple scientific disciplines.
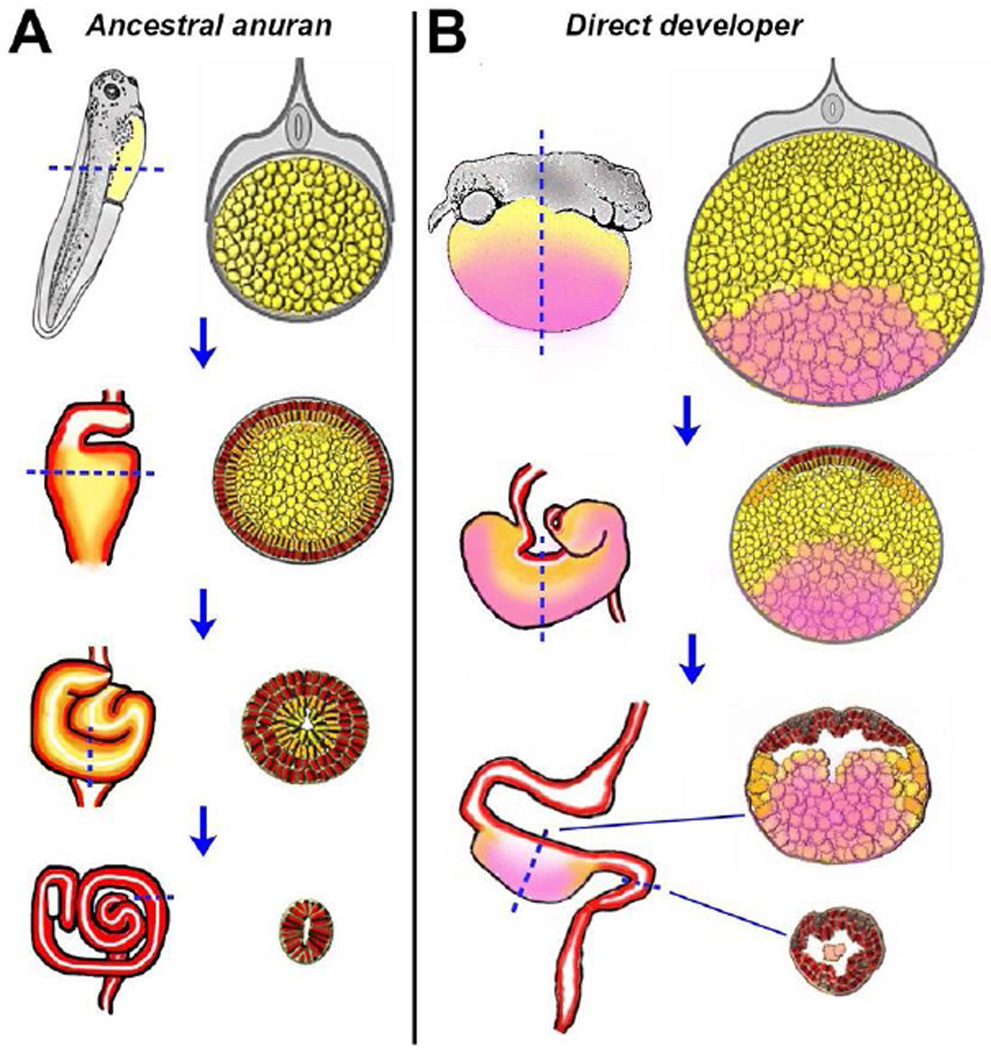
Figure 4. Endoderm morphogenesis in ancestral versus direct-developing frog species.
A) In ancestral frogs that produce feeding (exotrophic) tadpoles, all of the yolky vegetal endoderm cells (yellow) in the primitive gut tube (PGT) are used to generate the lining of the tadpole gut. As these cells radially rearrange (see Figure 2) and differentiate into the final digestive epithelium (orange/red), a central lumen is formed and the intestine is lengthened to form a long, coiled tract. The extensive gut is eventually remodeled to a shorter adult tract during metamorphosis (not shown). B) In contrast, in the direct-developing (endotrophic) frog embryo, a subset of the vegetal endoderm cells are fated to become nutritional endoderm (NE; pink), a cell type that does not rearrange nor contribute to the gut epithelium. Instead, these cells are gradually depleted of their yolk, extruded and eliminated as waste. Consequently, the PGT does not generate a long tract, and the developing froglet directly forms a short adult-length intestine.
Acknowledgments
We thank members of the Nascone-Yoder lab for helpful comments on the manuscript. The authors’ work on Xenopus gut morphogenesis has been funded by NIH R01DK085300, and research on Lepidobatrachus has been funded by NIH R21OD017963.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keller R. Cell migration during gastrulation. Current opinion in cell biology. 2005;17:533–541. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 3.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- 4.Blitz IL, Andelfinger G, Horb ME. Germ layers to organs: using Xenopus to study “later” development. Seminars in cell & developmental biology. 2006;17:133–145. doi: 10.1016/j.semcdb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwkoop PaF J. Normal Table of Xenopus Laevis (Daudin) New York, NY: Garland Publishing Inc; 1994. [Google Scholar]
- 6.Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Developmental biology. 1987;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 7.Moody SA, Kline MJ. Segregation of fate during cleavage of frog (Xenopus laevis) blastomeres. Anat Embryol (Berl) 1990;182:347–362. doi: 10.1007/BF02433495. [DOI] [PubMed] [Google Scholar]
- 8.Logan M, Mohun T. Induction of cardiac muscle differentiation in isolated animal pole explants of Xenopus laevis embryos. Development. 1993;118:865–875. doi: 10.1242/dev.118.3.865. [DOI] [PubMed] [Google Scholar]
- 9.Milet C, Monsoro-Burq AH. Dissection of Xenopus laevis neural crest for in vitro explant culture or in vivo transplantation. J Vis Exp. 2014 doi: 10.3791/51118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saint-Jeannet JP, Karavanov AA, Dawid IB. Expression of mesoderm markers in Xenopus laevis Keller explants. Int J Dev Biol. 1994;38:605–611. [PubMed] [Google Scholar]
- 11.Sater AK, Jacobson AG. The specification of heart mesoderm occurs during gastrulation in Xenopus laevis. Development. 1989;105:821–830. doi: 10.1242/dev.105.4.821. [DOI] [PubMed] [Google Scholar]
- 12.Sater AK, Jacobson AG. The restriction of the heart morphogenetic field in Xenopus laevis. Developmental biology. 1990;140:328–336. doi: 10.1016/0012-1606(90)90083-u. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PA, Oster G, Keller R. Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants. Development. 1989;105:155–166. doi: 10.1242/dev.105.1.155. [DOI] [PubMed] [Google Scholar]
- 14.Smith JC, Symes K, Hynes RO, DeSimone D. Mesoderm induction and the control of gastrulation in Xenopus laevis: the roles of fibronectin and integrins. Development. 1990;108:229–238. doi: 10.1242/dev.108.2.229. [DOI] [PubMed] [Google Scholar]
- 15.Dush MK, McIver AL, Parr MA, Young DD, Fisher J, Newman DR, et al. Heterotaxin: a TGF-beta signaling inhibitor identified in a multi-phenotype profiling screen in Xenopus embryos. Chem Biol. 2011;18:252–263. doi: 10.1016/j.chembiol.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomlinson ML, Field RA, Wheeler GN. Xenopus as a model organism in developmental chemical genetic screens. Mol Biosyst. 2005;1:223–228. doi: 10.1039/b506103b. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson ML, Guan P, Morris RJ, Fidock MD, Rejzek M, Garcia-Morales C, et al. A chemical genomic approach identifies matrix metalloproteinases as playing an essential and specific role in Xenopus melanophore migration. Chem Biol. 2009;16:93–104. doi: 10.1016/j.chembiol.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler GN, Brandli AW. Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:1287–1308. doi: 10.1002/dvdy.21967. [DOI] [PubMed] [Google Scholar]
- 19.Callery EM. There’s more than one frog in the pond: A survey of the Amphibia and their contributions to developmental biology. Seminars in cell & developmental biology. 2006;17:80–92. doi: 10.1016/j.semcdb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Benitez MS, Del Pino EM. Expression of Brachyury during development of the dendrobatid frog Colostethus machalilla. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;225:592–596. doi: 10.1002/dvdy.10190. [DOI] [PubMed] [Google Scholar]
- 21.Elinson RP, del Pino EM. Developmental diversity of amphibians. Wiley Interdiscip Rev Dev Biol. 2012;1:345–369. doi: 10.1002/wdev.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 23.Desmet VJ. Cystic diseases of the liver. From embryology to malformations. Gastroenterol Clin Biol. 2005;29:858–860. doi: 10.1016/s0399-8320(05)86360-2. [DOI] [PubMed] [Google Scholar]
- 24.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 25.Nair RJ, Lawler L, Miller MR. Chronic pancreatitis. Am Fam Physician. 2007;76:1679–1688. [PubMed] [Google Scholar]
- 26.Wells JM, Spence JR. How to make an intestine. Development. 2014;141:752–760. doi: 10.1242/dev.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arterbery AS, Bogue CW. Endodermal and mesenchymal cross talk: a crossroad for the maturation of foregut organs. Pediatr Res. 2014;75:120–126. doi: 10.1038/pr.2013.201. [DOI] [PubMed] [Google Scholar]
- 29.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 30.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 31.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- 34.Mii Y, Taira M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev Growth Differ. 2011;53:911–923. doi: 10.1111/j.1440-169X.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Rankin SA, Zorn AM. Different thresholds of Wnt-Frizzled 7 signaling coordinate proliferation, morphogenesis and fate of endoderm progenitor cells. Developmental biology. 2013;378:1–12. doi: 10.1016/j.ydbio.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, Madsen OD, et al. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;225:260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- 37.Matsuyama M, Aizawa S, Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS genetics. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pownall ME, Isaacs HV. FGF Signalling in Vertebrate Development. San Rafael (CA) 2010 [PubMed] [Google Scholar]
- 41.Horb ME, Slack JM. Endoderm specification and differentiation in Xenopus embryos. Developmental biology. 2001;236:330–343. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- 42.Shifley ET, Kenny AP, Rankin SA, Zorn AM. Prolonged FGF signaling is necessary for lung and liver induction in Xenopus. BMC Dev Biol. 2012;12:27. doi: 10.1186/1471-213X-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarikji Z, Horb LD, Shariff F, Mandato CA, Cho KW, Horb ME. The tetraspanin Tm4sf3 is localized to the ventral pancreas and regulates fusion of the dorsal and ventral pancreatic buds. Development. 2009;136:1791–1800. doi: 10.1242/dev.032235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly OG, Melton DA. Development of the pancreas in Xenopus laevis. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;218:615–627. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1027>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Pearl EJ, Bilogan CK, Mukhi S, Brown DD, Horb ME. Xenopus pancreas development. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:1271–1286. doi: 10.1002/dvdy.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebrok M. Hedgehog signaling in pancreas development. Mech Dev. 2003;120:45–57. doi: 10.1016/s0925-4773(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 47.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 48.Moriya N, Komazaki S, Takahashi S, Yokota C, Asashima M. In vitro pancreas formation from Xenopus ectoderm treated with activin and retinoic acid. Dev Growth Differ. 2000;42:593–602. doi: 10.1046/j.1440-169x.2000.00542.x. [DOI] [PubMed] [Google Scholar]
- 49.Moriya N, Komazaki S, Asashima M. In vitro organogenesis of pancreas in Xenopus laevis dorsal lips treated with retinoic acid. Dev Growth Differ. 2000;42:175–185. doi: 10.1046/j.1440-169x.2000.00498.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Developmental biology. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Stafford D, Hornbruch A, Mueller PR, Prince VE. A conserved role for retinoid signaling in vertebrate pancreas development. Dev Genes Evol. 2004;214:432–441. doi: 10.1007/s00427-004-0420-6. [DOI] [PubMed] [Google Scholar]
- 52.Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, et al. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Developmental biology. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 53.Ostrom M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, et al. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3:e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang T, Guo X, Chen Y. Retinoic acid-activated Ndrg1a represses Wnt/beta-catenin signaling to allow Xenopus pancreas, oesophagus, stomach, and duodenum specification. PLoS One. 2013;8:e65058. doi: 10.1371/journal.pone.0065058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruine AP, Baldwin HS, et al. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153–4166. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 56.Kenny AP, Rankin SA, Allbee AW, Prewitt AR, Zhang Z, Tabangin ME, et al. Sizzled-tolloid interactions maintain foregut progenitors by regulating fibronectin-dependent BMP signaling. Dev Cell. 2012;23:292–304. doi: 10.1016/j.devcel.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 58.Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 59.Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev Cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wills A, Dickinson K, Khokha M, Baker JC. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:2177–2186. doi: 10.1002/dvdy.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilogan CK, Horb ME. Microarray analysis of Xenopus endoderm expressing Ptf1a. Genesis. 2012;50:853–870. doi: 10.1002/dvg.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyden EA, Cope JG, Bill AH., Jr Anatomy and embryology of congenital intrinsic obstruction of the duodenum. Am J Surg. 1967;114:190–202. doi: 10.1016/0002-9610(67)90372-8. [DOI] [PubMed] [Google Scholar]
- 63.Carpenter HM. Pathogenesis of congenital jejunal atresia. Arch Pathol. 1962;73:390–396. [PubMed] [Google Scholar]
- 64.Dalla Vecchia LK, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, Engum SA. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133:490–496. doi: 10.1001/archsurg.133.5.490. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 65.DeLorimier AA, Fonkalsrud EW, Hays DM. Congenital atresia and stenosis of the jejunum and ileum. Surgery. 1969;65:819–827. [PubMed] [Google Scholar]
- 66.Seashore JH, Collins FS, Markowitz RI, Seashore MR. Familial apple peel jejunal atresia: surgical, genetic, and radiographic aspects. Pediatrics. 1987;80:540–544. [PubMed] [Google Scholar]
- 67.Chu SM, Luo CC, Chou YH, Yen JB. Congenital short bowel syndrome with malrotation. Chang Gung Med J. 2004;27:548–550. [PubMed] [Google Scholar]
- 68.Hasosah M, Lemberg DA, Skarsgard E, Schreiber R. Congenital short bowel syndrome: a case report and review of the literature. Can J Gastroenterol. 2008;22:71–74. doi: 10.1155/2008/590143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kern IB, Leece A, Bohane T. Congenital short gut, malrotation, and dysmotility of the small bowel. J Pediatr Gastroenterol Nutr. 1990;11:411–415. doi: 10.1097/00005176-199010000-00023. [DOI] [PubMed] [Google Scholar]
- 70.van der Werf CS, Halim D, Verheij JB, Alves MM, Hofstra RM. Congenital Short Bowel Syndrome: from clinical and genetic diagnosis to the molecular mechanisms involved in intestinal elongation. Biochim Biophys Acta. 2015;1852:2352–2361. doi: 10.1016/j.bbadis.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Palle L, Reddy B. Case report: Congenital short bowel syndrome. Indian J Radiol Imaging. 2010;20:227–229. doi: 10.4103/0971-3026.69366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabharwal G, Strouse PJ, Islam S, Zoubi N. Congenital short-gut syndrome. Pediatr Radiol. 2004;34:424–427. doi: 10.1007/s00247-003-1087-2. [DOI] [PubMed] [Google Scholar]
- 73.Schalamon J, Schober PH, Gallippi P, Matthyssens L, Hollwarth ME. Congenital short-bowel; a case study and review of the literature. Eur J Pediatr Surg. 1999;9:248–250. doi: 10.1055/s-2008-1072255. [DOI] [PubMed] [Google Scholar]
- 74.Aslanabadi S, Ghalehgolab-Behbahan A, Jamshidi M, Veisi P, Zarrintan S. Intestinal malrotations: a review and report of thirty cases. Folia Morphol (Warsz) 2007;66:277–282. [PubMed] [Google Scholar]
- 75.Filston HC, Kirks DR. Malrotation - the ubiquitous anomaly. J Pediatr Surg. 1981;16:614–620. doi: 10.1016/0022-3468(81)90015-4. [DOI] [PubMed] [Google Scholar]
- 76.Ford EG, Senac MO, Jr, Srikanth MS, Weitzman JJ. Malrotation of the intestine in children. Ann Surg. 1992;215:172–178. doi: 10.1097/00000658-199202000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamal IM. Defusing the intra-abdominal ticking bomb: intestinal malrotation in children. CMAJ. 2000;162:1315–1317. [PMC free article] [PubMed] [Google Scholar]
- 78.Stewart DR, Colodny AL, Daggett WC. Malrotation of the bowel in infants and children: a 15 year review. Surgery. 1976;79:716–720. [PubMed] [Google Scholar]
- 79.Torres AM, Ziegler MM. Malrotation of the intestine. World J Surg. 1993;17:326–331. doi: 10.1007/BF01658699. [DOI] [PubMed] [Google Scholar]
- 80.O’Sullivan M, O’Morain C. Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2006;20:561–573. doi: 10.1016/j.bpg.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106–1113. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 82.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 83.Chalmers AD, Slack JM. Development of the gut in Xenopus laevis. Developmental dynamics : an official publication of the American Association of Anatomists. 1998;212:509–521. doi: 10.1002/(SICI)1097-0177(199808)212:4<509::AID-AJA4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 84.Cervantes S. Cellular and molecular mechanisms of intestinal elongation in mammals: the long and short of it. Histol Histopathol. 2013;28:427–436. doi: 10.14670/HH-28.427. [DOI] [PubMed] [Google Scholar]
- 85.Chalmers AD, Slack JM. The Xenopus tadpole gut: fate maps and morphogenetic movements. Development. 2000;127:381–392. doi: 10.1242/dev.127.2.381. [DOI] [PubMed] [Google Scholar]
- 86.Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;219:109–120. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 87.Matsumoto A, Hashimoto K, Yoshioka T, Otani H. Occlusion and subsequent re-canalization in early duodenal development of human embryos: integrated organogenesis and histogenesis through a possible epithelial-mesenchymal interaction. Anat Embryol (Berl) 2002;205:53–65. doi: 10.1007/s00429-001-0226-5. [DOI] [PubMed] [Google Scholar]
- 88.Yamada M, Udagawa J, Matsumoto A, Hashimoto R, Hatta T, Nishita M, et al. Ror2 is required for midgut elongation during mouse development. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:941–953. doi: 10.1002/dvdy.22212. [DOI] [PubMed] [Google Scholar]
- 89.Muller JK, Prather DR, Nascone-Yoder NM. Left-right asymmetric morphogenesis in the Xenopus digestive system. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;228:672–682. doi: 10.1002/dvdy.10415. [DOI] [PubMed] [Google Scholar]
- 90.Reed RA, Womble MA, Dush MK, Tull RR, Bloom SK, Morckel AR, et al. Morphogenesis of the primitive gut tube is generated by Rho/ROCK/myosin II-mediated endoderm rearrangements. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:3111–3125. doi: 10.1002/dvdy.22157. [DOI] [PubMed] [Google Scholar]
- 91.Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, et al. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, Hildebrand JD, et al. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Developmental biology. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 95.Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- 96.Lee C, Scherr HM, Wallingford JB. Shroom family proteins regulate gamma-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134:1431–1441. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- 97.Chung MI, Nascone-Yoder NM, Grover SA, Drysdale TA, Wallingford JB. Direct activation of Shroom3 transcription by Pitx proteins drives epithelial morphogenesis in the developing gut. Development. 2010;137:1339–1349. doi: 10.1242/dev.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, et al. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126:1225–1234. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- 99.Al Alam D, Sala FG, Baptista S, Galzote R, Danopoulos S, Tiozzo C, et al. FGF9-Pitx2-FGF10 signaling controls cecal formation in mice. Developmental biology. 2012;369:340–348. doi: 10.1016/j.ydbio.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plageman TF, Jr, Zacharias AL, Gage PJ, Lang RA. Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis. Developmental biology. 2011;357:227–234. doi: 10.1016/j.ydbio.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dush MK, Nascone-Yoder NM. Jun N-terminal kinase maintains tissue integrity during cell rearrangement in the gut. Development. 2013;140:1457–1466. doi: 10.1242/dev.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 104.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 105.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 106.Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- 107.Zhang J, Rosenthal A, de Sauvage FJ, Shivdasani RA. Downregulation of Hedgehog signaling is required for organogenesis of the small intestine in Xenopus. Developmental biology. 2001;229:188–202. doi: 10.1006/dbio.2000.9953. [DOI] [PubMed] [Google Scholar]
- 108.Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 109.Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahlapuu M, Ormestad M, Enerback S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 111.Tseng HT, Shah R, Jamrich M. Function and regulation of FoxF1 during Xenopus gut development. Development. 2004;131:3637–3647. doi: 10.1242/dev.01234. [DOI] [PubMed] [Google Scholar]
- 112.Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, et al. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 113.Kuhl M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci. 2004;9:967–974. doi: 10.2741/1307. [DOI] [PubMed] [Google Scholar]
- 114.Yoshida Y, Kim S, Chiba K, Kawai S, Tachikawa H, Takahashi N. Calcineurin inhibitors block dorsal-side signaling that affect late-stage development of the heart, kidney, liver, gut and somitic tissue during Xenopus embryogenesis. Development, growth & differentiation. 2004;46:139–152. doi: 10.1111/j.1440-169X.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 115.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 116.Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J, Yang R, Liu Z, Hou C, Zong W, Zhang A, et al. Loss of lysyl oxidase-like 3 causes cleft palate and spinal deformity in mice. Hum Mol Genet. 2015;24:6174–6185. doi: 10.1093/hmg/ddv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geach TJ, Dale L. Members of the lysyl oxidase family are expressed during the development of the frog Xenopus laevis. Differentiation; research in biological diversity. 2005;73:414–424. doi: 10.1111/j.1432-0436.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- 119.Kutchuk L, Laitala A, Soueid-Bomgarten S, Shentzer P, Rosendahl AH, Eilot S, et al. Muscle composition is regulated by a Lox-TGFbeta feedback loop. Development. 2015;142:983–993. doi: 10.1242/dev.113449. [DOI] [PubMed] [Google Scholar]
- 120.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 121.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 122.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 123.Moriyama Y, Ohata Y, Mori S, Matsukawa S, Michiue T, Asashima M, et al. Rapamycin treatment causes developmental delay, pigmentation defects, and gastrointestinal malformation on Xenopus embryogenesis. Biochemical and biophysical research communications. 2011;404:974–978. doi: 10.1016/j.bbrc.2010.12.093. [DOI] [PubMed] [Google Scholar]
- 124.Makky K, Tekiela J, Mayer AN. Target of rapamycin (TOR) signaling controls epithelial morphogenesis in the vertebrate intestine. Developmental biology. 2007;303:501–513. doi: 10.1016/j.ydbio.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohata Y, Matsukawa S, Moriyama Y, Michiue T, Morimoto K, Sato Y, et al. Sirtuin inhibitor Ex-527 causes neural tube defects, ventral edema formations, and gastrointestinal malformations in Xenopus laevis embryos. Development, growth & differentiation. 2014;56:460–468. doi: 10.1111/dgd.12145. [DOI] [PubMed] [Google Scholar]
- 127.Takebayashi-Suzuki K, Funami J, Tokumori D, Saito A, Watabe T, Miyazono K, et al. Interplay between the tumor suppressor p53 and TGF beta signaling shapes embryonic body axes in Xenopus. Development. 2003;130:3929–3939. doi: 10.1242/dev.00615. [DOI] [PubMed] [Google Scholar]
- 128.Wallingford JB, Seufert DW, Virta VC, Vize PD. p53 activity is essential for normal development in Xenopus. Current biology : CB. 1997;7:747–757. doi: 10.1016/s0960-9822(06)00333-2. [DOI] [PubMed] [Google Scholar]
- 129.Lenkowski JR, Sanchez-Bravo G, McLaughlin KA. Low concentrations of atrazine, glyphosate, 2,4-dichlorophenoxyacetic acid, and triadimefon exposures have diverse effects on Xenopus laevis organ morphogenesis. J Environ Sci (China) 2010;22:1305–1308. doi: 10.1016/s1001-0742(09)60254-0. [DOI] [PubMed] [Google Scholar]
- 130.Yoon CS, Jin JH, Park JH, Yeo CY, Kim SJ, Hwang YG, et al. Toxic effects of carbendazim and n-butyl isocyanate, metabolites of the fungicide benomyl, on early development in the African clawed frog, Xenopus laevis. Environ Toxicol. 2008;23:131–144. doi: 10.1002/tox.20338. [DOI] [PubMed] [Google Scholar]
- 131.Vismara C, Bacchetta R, Cacciatore B, Vailati G, Fascio U. Paraquat embryotoxicity in the Xenopus laevis cleavage phase. Aquat Toxicol. 2001;55:85–93. doi: 10.1016/s0166-445x(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 132.Vismara C, Battista VV, Vailati G, Bacchetta R. Paraquat induced embryotoxicity on Xenopus laevis development. Aquat Toxicol. 2000;49:171–179. doi: 10.1016/s0166-445x(99)00080-6. [DOI] [PubMed] [Google Scholar]
- 133.Bacchetta R, Mantecca P, Andrioletti M, Vismara C, Vailati G. Axial-skeletal defects caused by Carbaryl in Xenopus laevis embryos. The Science of the total environment. 2008;392:110–118. doi: 10.1016/j.scitotenv.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 134.Dawson DA. Joint actions of carboxylic acid binary mixtures on Xenopus embryo development: comparison of joint actions for malformation types. Archives of environmental contamination and toxicology. 1994;27:243–249. doi: 10.1007/BF00214269. [DOI] [PubMed] [Google Scholar]
- 135.Sone K, Hinago M, Kitayama A, Morokuma J, Ueno N, Watanabe H, et al. Effects of 17beta-estradiol, nonylphenol, and bisphenol-A on developing Xenopus laevis embryos. Gen Comp Endocrinol. 2004;138:228–236. doi: 10.1016/j.ygcen.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 136.Bacchetta R, Moschini E, Santo N, Fascio U, Del Giacco L, Freddi S, et al. Evidence and uptake routes for Zinc oxide nanoparticles through the gastrointestinal barrier in Xenopus laevis. Nanotoxicology. 2014;8:728–744. doi: 10.3109/17435390.2013.824128. [DOI] [PubMed] [Google Scholar]
- 137.Nations S, Wages M, Canas JE, Maul J, Theodorakis C, Cobb GP. Acute effects of Fe(2)O(3), TiO(2), ZnO and CuO nanomaterials on Xenopus laevis. Chemosphere. 2011;83:1053–1061. doi: 10.1016/j.chemosphere.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 138.Tussellino M, Ronca R, Formiggini F, De Marco N, Fusco S, Netti PA, et al. Polystyrene nanoparticles affect Xenopus laevis development. Journal of Nanoparticle Research. 2015:17. [Google Scholar]
- 139.Saka M. Developmental toxicity of p,p’-dichlorodiphenyltrichloroethane, 2,4,6-trinitrotoluene, their metabolites, and benzo[a]pyrene in Xenopus laevis embryos. Environmental toxicology and chemistry / SETAC. 2004;23:1065–1073. doi: 10.1897/03-272. [DOI] [PubMed] [Google Scholar]
- 140.Yu S, Wages MR, Cobb GP, Maul JD. Effects of chlorothalonil on development and growth of amphibian embryos and larvae. Environ Pollut. 2013;181:329–334. doi: 10.1016/j.envpol.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 141.Bonfanti P, Colombo A, Orsi F, Nizzetto I, Andrioletti M, Bacchetta R, et al. Comparative teratogenicity of chlorpyrifos and malathion on Xenopus laevis development. Aquat Toxicol. 2004;70:189–200. doi: 10.1016/j.aquatox.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 142.Modra H, Vrskova D, Macova S, Kohoutkova J, Hajslova J, Haluzova I, et al. Comparison of diazinon toxicity to embryos of Xenopus laevis and Danio rerio; degradation of diazinon in water. Bull Environ Contam Toxicol. 2011;86:601–604. doi: 10.1007/s00128-011-0273-4. [DOI] [PubMed] [Google Scholar]
- 143.Snawder JE, Chambers JE. Toxic and developmental effects of organophosphorus insecticides in embryos of the South African clawed frog. Journal of environmental science and health Part B, Pesticides, food contaminants, and agricultural wastes. 1989;24:205–218. doi: 10.1080/03601238909372644. [DOI] [PubMed] [Google Scholar]
- 144.Snawder JE, Chambers JE. Critical time periods and the effect of tryptophan in malathion-induced developmental defects in Xenopus embryos. Life sciences. 1990;46:1635–1642. doi: 10.1016/0024-3205(90)90377-4. [DOI] [PubMed] [Google Scholar]
- 145.Lenkowski JR, McLaughlin KA. Acute atrazine exposure disrupts matrix metalloproteinases and retinoid signaling during organ morphogenesis in Xenopus laevis. J Appl Toxicol. 2010;30:582–859. doi: 10.1002/jat.1529. [DOI] [PubMed] [Google Scholar]
- 146.Lenkowski JR, Reed JM, Deininger L, McLaughlin KA. Perturbation of organogenesis by the herbicide atrazine in the amphibian Xenopus laevis. Environmental health perspectives. 2008;116:223–230. doi: 10.1289/ehp.10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mantecca P, Gualtieri M, Andrioletti M, Bacchetta R, Vismara C, Vailati G, et al. Tire debris organic extract affects Xenopus development. Environ Int. 2007;33:642–648. doi: 10.1016/j.envint.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 148.Smith EE, Carr JA, Wages M, Wang JF, Murali S, Kendall R. Response of larval frogs to Corexit 9500. Toxicol Environ Chem. 2012;94:1199–1210. [Google Scholar]
- 149.Wright CV, Schnegelsberg P, De Robertis EM. XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development. 1989;105:787–794. doi: 10.1242/dev.105.4.787. [DOI] [PubMed] [Google Scholar]
- 150.Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 151.Dauve V, McLin VA. Recent advances in the molecular and genetic understanding of congenital gastrointestinal malformations. J Pediatr Gastroenterol Nutr. 2013;57:4–13. doi: 10.1097/MPG.0b013e3182922b49. [DOI] [PubMed] [Google Scholar]
- 152.Hilger AC, Halbritter J, Pennimpede T, van der Ven A, Sarma G, Braun DA, et al. Targeted Resequencing of 29 Candidate Genes and Mouse Expression Studies Implicate ZIC3 and FOXF1 in Human VATER/VACTERL Association. Hum Mutat. 2015;36:1150–1154. doi: 10.1002/humu.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin V, Shaw-Smith C. Review of genetic factors in intestinal malrotation. Pediatric surgery international. 2010;26:769–781. doi: 10.1007/s00383-010-2622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kitaguchi T, Nagai T, Nakata K, Aruga J, Mikoshiba K. Zic3 is involved in the left-right specification of the Xenopus embryo. Development. 2000;127:4787–4795. doi: 10.1242/dev.127.22.4787. [DOI] [PubMed] [Google Scholar]
- 156.McDiarmid RW, Altig R. Tadpoles : the biology of anuran larvae. Chicago: University of Chicago Press; 1999. [Google Scholar]
- 157.Altig RJG. Guilds of Anuran Larvae: Relationships among Developmental Modes, Morphologies, and Habitats. Herpetological Monographs. 1989;3:81–109. [Google Scholar]
- 158.Budgett JS. Notes on the batrachians of the Paraguayan Chaco, with observations upon their breeding habits and development, especially with regard to Phyllomedusa hy pochondrialis, Cope. Also a description of a new genus. QJ Microsc Sci. 1899:305. [Google Scholar]
- 159.Fabrezi JS. Morphological evolution of Ceratophyrinae (Anura, Neobatrachia) Journal of Zoological Systematics and Evolutionary Research. 2006:44. [Google Scholar]
- 160.Hanken J. Model Systems Versus Outgroups - Alternative Approaches to the Study of Head Development and Evolution. American Zoologist. 1993:33. [Google Scholar]
- 161.Ruibal RTE. The obligate carnivorous larvae of the frog Lepidobatrachus laevis (Leptodactylidae) Copeia. 1988:3. [Google Scholar]
- 162.Carroll EJSAM, Ruibal R. Gastric pepsin in an anuran larva. Development Growth & Differentiation. 1991;33:499. doi: 10.1111/j.1440-169X.1991.00499.x. [DOI] [PubMed] [Google Scholar]
- 163.Bloom S, Ledon-Rettig C, Infante C, Everly A, Hanken J, Nascone-Yoder N. Developmental origins of a novel gut morphology in frogs. Evolution & development. 2013;15:213–223. doi: 10.1111/ede.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lipscomb K, Schmitt C, Sablyak A, Yoder JA, Nascone-Yoder N. Role for retinoid signaling in left-right asymmetric digestive organ morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:2266–2275. doi: 10.1002/dvdy.20879. [DOI] [PubMed] [Google Scholar]
- 165.Buchholz DR, Singamsetty S, Karadge U, Williamson S, Langer CE, Elinson RP. Nutritional endoderm in a direct developing frog: a potential parallel to the evolution of the amniote egg. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:1259–1272. doi: 10.1002/dvdy.21153. [DOI] [PubMed] [Google Scholar]
- 166.Karadge U, Elinson RP. Characterization of the nutritional endoderm in the direct developing frog Eleutherodactylus coqui. Dev Genes Evol. 2013;223:351–362. doi: 10.1007/s00427-013-0451-y. [DOI] [PubMed] [Google Scholar]
- 167.Chatterjee S, Elinson RP. Commitment to nutritional endoderm in Eleutherodactylus coqui involves altered nodal signaling and global transcriptional repression. J Exp Zool B Mol Dev Evol. 2014;322:27–44. doi: 10.1002/jez.b.22543. [DOI] [PubMed] [Google Scholar]
- 168.Elinson RP, Beckham Y. Development in frogs with large eggs and the origin of amniotes. Zoology (Jena) 2002;105:105–117. doi: 10.1078/0944-2006-00060. [DOI] [PubMed] [Google Scholar]
- 169.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 170.Amin NM, Greco TM, Kuchenbrod LM, Rigney MM, Chung MI, Wallingford JB, et al. Proteomic profiling of cardiac tissue by isolation of nuclei tagged in specific cell types (INTACT) Development. 2014;141:962–973. doi: 10.1242/dev.098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Blitz IL, Biesinger J, Xie X, Cho KW. Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis. 2013;51:827–834. doi: 10.1002/dvg.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guo X, Zhang T, Hu Z, Zhang Y, Shi Z, Wang Q, et al. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development. 2014;141:707–714. doi: 10.1242/dev.099853. [DOI] [PubMed] [Google Scholar]
- 173.Wuhr M, Freeman RM, Jr, Presler M, Horb ME, Peshkin L, Gygi SP, et al. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol. 2014;24:1467–1475. doi: 10.1016/j.cub.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rao N, Song F, Jhamb D, Wang M, Milner DJ, Price NM, et al. Proteomic analysis of fibroblastema formation in regenerating hind limbs of Xenopus laevis froglets and comparison to axolotl. BMC Dev Biol. 2014;14:32. doi: 10.1186/1471-213X-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun L, Bertke MM, Champion MM, Zhu G, Huber PW, Dovichi NJ. Quantitative proteomics of Xenopus laevis embryos: expression kinetics of nearly 4000 proteins during early development. Sci Rep. 2014;4:4365. doi: 10.1038/srep04365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ohnuma S, Mann F, Boy S, Perron M, Harris WA. Lipofection strategy for the study of Xenopus retinal development. Methods. 2002;28:411–419. doi: 10.1016/s1046-2023(02)00260-8. [DOI] [PubMed] [Google Scholar]
- 177.Chernet BT, Levin M. A versatile protocol for mRNA electroporation of Xenopus laevis embryos. Cold Spring Harb Protoc. 2012;2012:447–452. doi: 10.1101/pdb.prot067694. [DOI] [PMC free article] [PubMed] [Google Scholar]