Abstract
Rationale
We previously reported that VEGF-induced binding of VEGFR2 to epsins 1 and 2 triggers VEGFR2 degradation and attenuates VEGF signaling. The epsin ubiquitin interacting motif (UIM) was shown to be required for the interaction with VEGFR2, however the molecular determinants that govern how epsin specifically interacts with and regulates VEGFR2 were unknown.
Objective
The goals for the present study were (1) to identify critical molecular determinants that drive the specificity of the epsin and VEGFR2 interaction and (2) to ascertain if such determinants were critical for physiological angiogenesis in vivo.
Methods and Results
Structural modeling uncovered two novel binding surfaces within VEGFR2 that mediate specific interactions with epsin UIM. Three glutamic acid residues in epsin UIM were found to interact with residues in VEGFR2. Further, we found that the VEGF-induced VEGFR2-epsin interaction promoted c-Cbl-mediated ubiquitination of epsin, and uncovered a previously unappreciated ubiquitin-binding surface within VEGFR2. Mutational analysis revealed that the VEGFR2-epsin interaction is supported by VEGFR2 interacting specifically with the UIM and with ubiquitinated epsin. An epsin UIM peptide, but not a mutant UIM peptide, potentiated endothelial cell proliferation, migration and angiogenic properties in vitro, increased postnatal retinal angiogenesis, and enhanced VEGF-induced physiological angiogenesis and wound healing.
Conclusions
Distinct residues in the epsin UIM and VEGFR2 mediate specific interactions between epsin and VEGFR2, in addition to UIM recognition of ubiquitin moieties on VEGFR2. These novel interactions are critical for pathophysiological angiogenesis, suggesting that these sites could be selectively targeted by therapeutics to modulate angiogenesis.
Keywords: Epsin, UIM, VEGFR2, ubiquitination, angiogenesis, ubiquitin
INTRODUCTION
Vascular endothelial growth factor receptor 2 (VEGFR2) is the master regulator controlling both physiological and pathological angiogenesis1-6. Vascular endothelial growth factor A (VEGF-A), secreted in response to a variety of different pro-angiogenic stimuli, binds to and induces the autophosphorylation of dimerized VEGFR21, 5, 7. Downstream VEGFR2 signaling promotes angiogenic responses including endothelial cell proliferation, migration and network formation1, 7. Accordingly, VEGFR2 signaling should be tightly regulated in order to promote neovascularization where beneficial vascular regeneration is diminished and to attenuate angiogenesis where adverse vascular growth is excessive7. Such tight regulation is in part mediated by the control of cell surface abundance of activated VEGFR28-11. Activated VEGFR2 is ubiquitinated shortly after phosphorylation, and this ubiquitination is critical for its endocytosis and degradation in lysosomes, leading to the termination of VEGF siganling3, 12, 13.
Epsins are a family of multivalent adaptor proteins that facilitate clathrin-dependent endocytosis of a subset of ubiquitinated cell surface proteins9, 10, 14-24. Selectivity towards ubiquitinated proteins is mediated by two evolutionarily conserved ubiquitin interacting motifs (UIMs) located between the membrane-localizing epsin NH2-terminal homology (ENTH) domain and carboxyl-terminal clathrin- and AP-2 binding motifs9, 16, 17, 19, 23, 25, 26. Mammals express three epsins: epsins 1 and 2, which are ubiquitously expressed and redundant in function, and epsin 3, which is expressed primarily in the stomach and epidermis9, 10, 14, 19, 27-30. Germ line epsins 1 and 2 double knockout (DKO) mice exhibit severe vascular defects resulting in embryonic lethality10, 27. In contrast, we previously discovered that inducible deletion of epsins 1 and 2 in endothelial cells in adult mice (EC-iDKO) has minimal effects on quiescent vascular beds but significantly alters tumor angiogenesis resulting in hyperplastic, dilated and dysfunctional tumor vascular networks resulting in retarded tumor growth9, 10.
We previously reported that in endothelial cells, epsins 1 and 2 preferentially bind ubiquitinated VEGFR2 to mediate its lysosomal degradation and subsequent signaling attenuation9, 10. Loss of epsins impairs VEGFR2 downregulation, resulting in elevated VEGFR2 level and heightened VEGF signaling9. Interestingly, epsin loss does not affect other angiogenic receptors and their downstream signaling, including PDGFR, FGFR, EGFR and TGFβR9, suggesting that epsins are unique adaptor proteins for VEGFR2 endocytosis and degradation in endothelial cells. In further support, our recent study showed that genetic reduction of VEGFR2 in endothelial cells decreases elevated VEGF signaling and rescues aberrant angiogenesis caused by epsins 1 and 2 deficiencies10. Intriguingly, epsins 1, 2 and 3 have been implicated in the generation of membrane curvature that is critical for clathrin-mediated endocytosis31. Deficiency of all three epsins impairs endocytosis in general by stalling the actin-dependent invagination of endocytic clathrin-coated pits31. However, our previous study clearly demonstrated that prototypical clathrin-mediated endocytosis of transferin receptor and EGFR still occurs in both global knockout and endothelial-specific knockout of epsins 1 and 2 mice9, 27, thus deficiency of epsins 1 and 2 in either primary endothelial cells or mouse embryonic fibroblasts does not impair housekeeping clathrin-mediated endocytosis.
Despite a specific role for epsins 1 and 2 in regulating VEGFR2 endocytosis and degradation, how the binding specificity between VEGFR2 and epsins is achieved was poorly understood. Although we previously determined that the epsin-VEGFR2 interaction is dependent on the epsin UIM and that deletion of UIM completely abolishes the binding of epsin to VEGFR29, 10, the molecular determinants that govern this specific interaction remain unknown. A detailed understanding of the molecular mechanisms driving this unique specificity will provide potential new therapeutic strategies to spatially and temporally promote favorable physiological angiogenesis, while also restraining undesirable pathological angiogenesis. Herein, we uncovered novel binding mechanisms that drive the specific interaction between epsin and VEGFR2. Using structural modeling, we have identified unique residues in the epsin UIM and VEGFR2 that reciprocally determine the specific interaction between epsin and VEGFR2. Guided by these discoveries, we designed an epsin inhibitory UIM peptide that effectively blocked the interaction between epsin and VEGFR2, thereby promoting VEGFR2 signaling and angiogenesis in vitro. Consistently, administration of the inhibitory peptide into wild type mice potentiated postnatal retina angiogenesis and VEGF-mediated angiogenesis in subcutaneous Matrigel plugs. Similar pro-angiogenic effects were also observed after dermal wound healing, where UIM peptide administration enhanced physiological angiogenesis and wound healing relative to control peptide. These responses were not observed with a mutant UIM peptide containing substitutions at the critical VEGFR2-binding residues. Collectively, our studies have revealed previously unappreciated molecular mechanisms underlying the specific epsin-VEGFR2 interaction, and identified unique molecular determinants of epsin-VEGFR2 complex assembly that are critical to regulate physiological angiogenic processes.
METHODS
See online data supplement for complete methods.
RESULTS
The Epsin UIM mediates VEGFR2 signaling and interacts with the VEGFR2 kinase domain
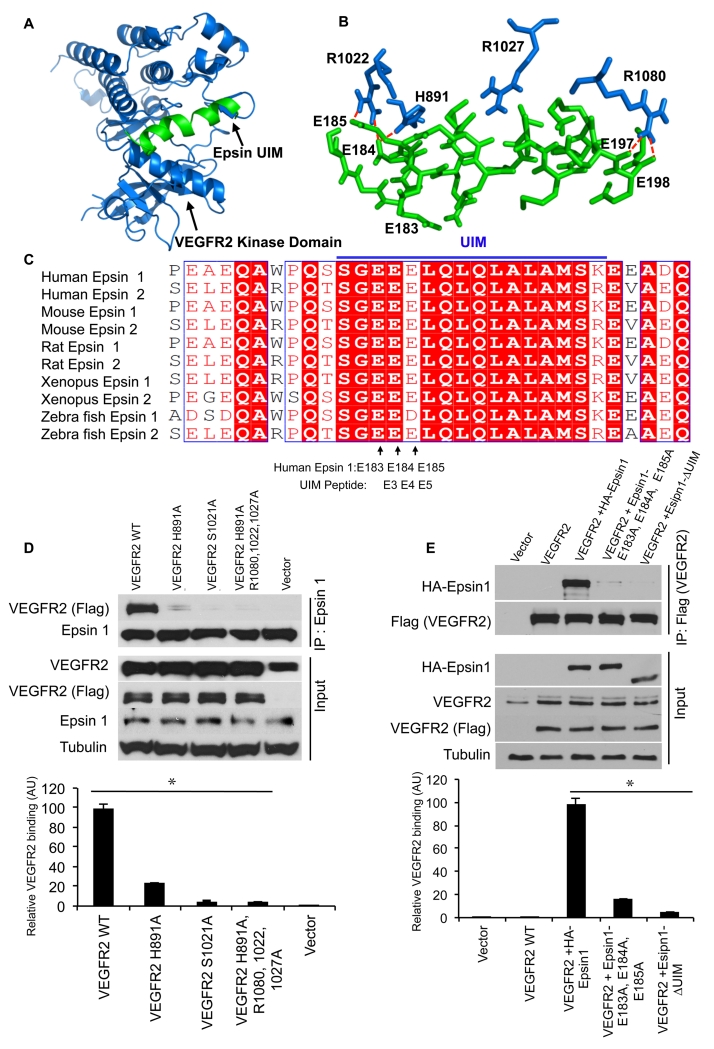
We previously showed that epsins facilitate the internalization and degradation of activated VEGFR2, but not other angiogenic modulators, via interactions between epsin UIM and VEGFR2 kinase domain (KD)9. We created an inducible endothelial cell-specific epsin deficient mouse line by crossing Epn1fl/fl; Epn2−/− mice (EC-iDKO, Online Figure IA) with iCDH5 Cre deleter mice that express Cre recombinase specifically in endothelial cells after tamoxifen administration (Online Figure IB)9. Using primary mouse endothelial cells isolated from wild type or EC-iDKO mice, we demonstrated that loss of epsin promoted VEGF-dependent VEGFR2 phosphorylation (Online Figure IIA), consistent with impaired internalization and degradation of activated VEGFR2. Moreover, expression of wild type epsin 1 (HA-Epsin 1), but not UIM deficient epsin 1 (HA-Epsin 1-ΔUIM) in DKO mouse endothelial cells restored VEGFR2 signaling (Online Figure IIA), further indicating the importance of the epsin UIM to VEGF-dependent VEGFR2 internalization. The UIM interacts with ubiquitin moieties with low affinity and minimal specificity12, 16, 23, 25; however, we have revealed that it regulates VEGFR2 specifically. Thus, how the epsin UIM achieves this specific interaction VEGFR2 is intriguing. To explore the molecular mechanism underlying this binding specificity, we used structural modeling with PEP-FOLD and ClusPro softwares32. We uncovered a novel putative epsin UIM binding cleft within the VEGFR2 KD (Figure 1A,B). Detailed examination identified several binding elements predicted to facilitate this interaction, including EpsinE184:VEGFR2H891,R1022, EpsinE185:VEGFR2R1022, and EpsinE197,E198:VEGFR2R1080 (Figure 1B; Online Figure IIC; Online Table I). Indeed, E184 and E185 within the predicted epsin UIM-VEGFR2 binding domain are highly conserved between mouse, zebrafish, Xenopus and human epsins 1 and 2 (Figure 1C). Our findings suggest that, in addition to the traditional binding between the epsin UIM and the ubiquitin moiety on VEGFR2, epsins may also selectivity interact with VEGFR2 via specific protein-protein interactions.
Figure 1. The Epsin UIM mediates VEGFR2 signaling and interacts with the VEGFR2 kinase domain.
(A) Ribbon representation and (B) stick representation of epsin UIM (green) docked into the putative hairpin-shaped binding pocket of VEGFR2 KD (blue). VEGFR2 KD crystal structure (3U6J) was obtained from the Protein Data Bank. Ribbon diagram for epsin UIM was predicted using PEP-FOLD. ClusPro and PyMol software were used for the docking; highest scoring model with good topologies is shown. (C) Alignment of epsin UIM sequences from mouse, rat and human epsins 1 and epsin 2. Note: E183 (corresponding to E3 in UIM peptide), E184 (corresponding to E4 in UIM peptide) and E185 (corresponding to E5 in UIM peptide) are highly conserved. (D) Western blot analysis of VEGFR2 co-immunoprecipitation by epsin 1 in HUVEC cells overexpressing wild type VEGFR2, or VEGFR2 with the indicated substitutions, and stimulated with VEGF (50 ng/mL) for 2 min. (E) Western blot analysis of VEGFR2 co-immunoprecipitation by epsin 1 in HUVEC cells overexpressing wild type VEGFR2 and either HA-tagged full-length epsin 1 (HA-Epsin 1), HA-Epsin 1E183A, E184A, E185A or HA-Epsin 1-ΔUIM, and stimulated with 50 ng/mL VEGF for 2 min. All representative Western blots were selected from n=3. Error bars indicate the mean ± s.e.m. *P<0.05.
To investigate the molecular binding mechanism described above, we substituted specific residues within the putative binding clefts in VEGFR2 KD and epsin UIM and evaluated their effects on the VEGFR2-epsin interaction. We used site-directed mutagenesis to create expression constructs with mutated versions of VEGFR2, and expressed these or wild-VEGFR2 in HUVEC cells as indicated (Figure 1D). Cells were subsequently stimulated with VEGF to induce VEGFR2 activation and ubiquitination, and processed for immunoprecipitation using epsin 1-specific antibody as previously described (Figure 1D)9, 33. We found that WT VEGFR2 co-immunoprecipitated with epsin1; however all of the VEGFR2 mutants impeded binding to epsin1 (Figure 1D). Likewise, substitutions at the corresponding residues in HA-tagged full-length epsin1, E183A, E184A and E185A, also abolished VEGFR2 immunoprecipitation by epsin 1 (Figure 1E). Notably, impaired VEGFR2 binding by HA-Epsin 1E183,184,185A was comparable to UIM-deficient Epsin 1. However, the mutation of other epsin UIM residues predicted to interact with VEGFR2R1080, EpsinE197, 198G (Figure 1B), did not disrupt VEGFR2 immunoprecipitation (Online Figure IID). These findings emphasize the critical importance of the epsin UIM for binding to this novel VEGFR2 interface, and identify key residues that achieve specificity of this interaction, in particular H891 of VEGFR2 and E183, E184 and E185 of the epsin UIM.
c-Cbl-dependent ubiquitination of epsin promotes the interaction with VEGFR2
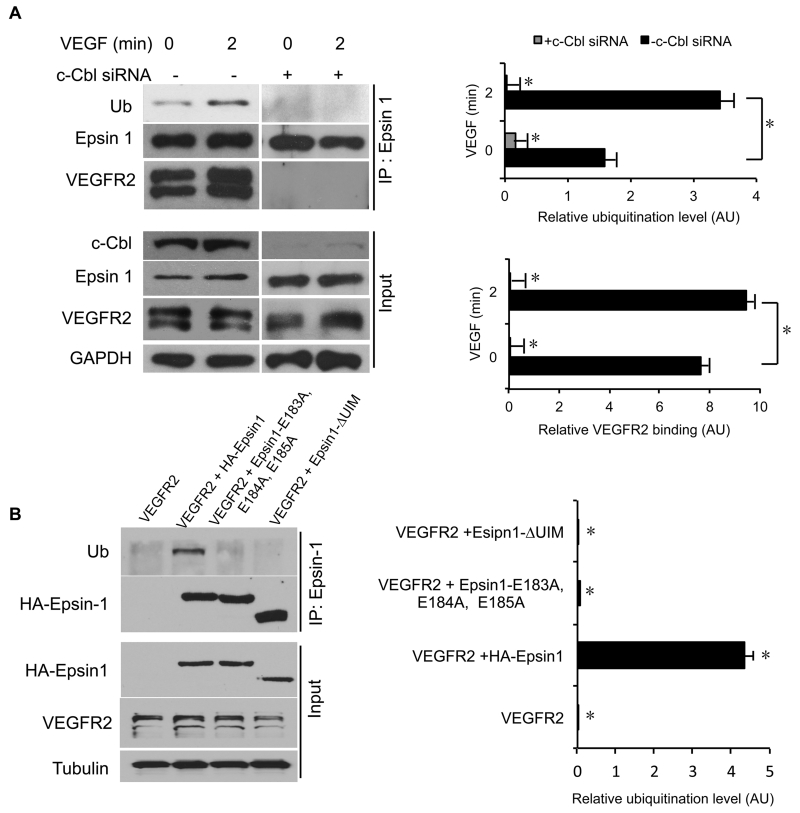
VEGF-stimulation reportedly induces VEGFR2 ubiquitination via the recruitment of Casitas B-lineage Lymphoma E3 ubiquitin-protein ligase (c-Cbl)3. Interestingly, epsins are also reportedly ubiquitinated under some conditions22, 34, including VEGF stimulation (Figure 2A). To determine whether epsin ubiquitination is mediated by c-Cbl, we used RNAi to deplete c-Cbl in HUVECs prior to serum starvation and VEGF stimulation. We found that VEGF-dependent epsin 1 ubiquitination was abolished in c-Cbl depleted HUVECs compared to control siRNA treated cells (Figure 2A). Further, we observed that epsin-VEGFR2 interaction is increased by c-Cbl-mediated ubiquitination of epsin (Figure 2A). Conversely, epsin-VEGFR2 interaction is completely inhibited by c-Cbl knockdown (Figure 2A). Given that c-Cbl-dependent ubiquitination of epsin is increased in response to VEGF stimulation (Figure 2A), we next tested whether ubiquitination of epsin is facilitated by its interaction with VEGFR2. We co-transfected VEGFR2 with either full-length HA-Epsin 1, HA-Epsin 1-ΔUIM, or HA-Epsin 1E183,184,185A into HUVEC cells and evaluated epsin ubiquitination by immunoprecipitation/Western blot. We found that both HA-Epsin 1-ΔUIM and HA-Epsin 1E183,184,185A, which do not interact with VEGFR2 (Figure 1E), show reduced epsin ubiquitination compared to HA-Epsin 1 (Figure 2B). Collectively, our data suggest that VEGF enhances c-Cbl-mediated epsin ubiquitination, which in turn reinforces the interaction between epsin and VEGFR2.
Figure 2. c-Cbl-dependent ubiquitination of epsin promotes the interaction with VEGFR2.
(A) Western blot analysis of epsin 1 ubiquitination and VEGFR2 co-immunoprecipitation with epsin 1 in HUVECs transfected with either control or c-Cbl-targeted siRNA. Cells were serum starved overnight and stimulated with or without 50 ng/mL VEGF for 2 min prior to lysis. (B) Western blot analysis of epsin 1 ubiquitination in HUVECs overexpressing wild type VEGFR2 and either HA-Epsin 1, HA-Epsin 1E183A, E184A, E185A or HA-Epsin 1-ΔUIM and stimulated with 50 ng/mL VEGF for 2 min. All representative Western blots were selected from n=3. Error bars indicate the mean ± s.e.m. *P<0.05.
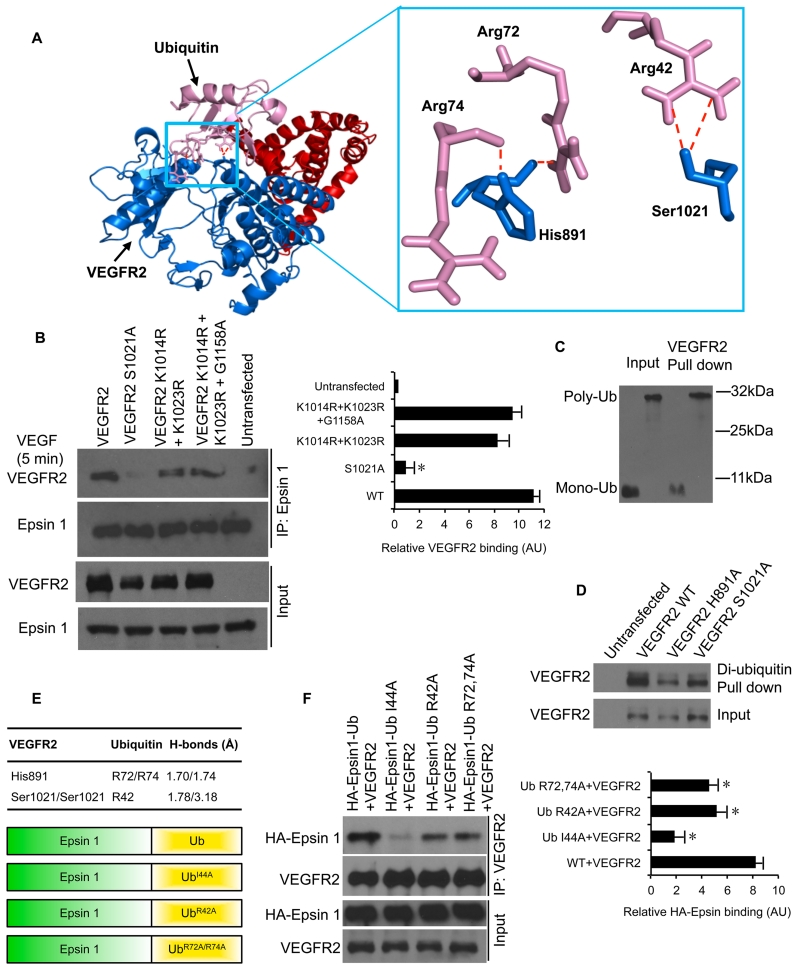
Interestingly, our structural modeling also identified a novel interface in the VEGFR2 kinase domain that is predicted to interact with an ubiquitin moiety, such as the ubiquitin on epsin (Figure 3A). Among the residues within VEGFR2 that could potentially interact with ubiquitin, we found that VEGFR2H891 and VEGFR2S1021 form two critical interacting surfaces with ubiquitin, VEGFR2H891:UbR72,74 and VEGFR2S1021:UbR42 (Figure 3A). More importantly, we found that both VEGFR2H891 and VEGFR2S1021 interfaces are critical for its interaction with epsin (Figure ID, 3B). We also found that purified VEGFR2 specifically immunoprecipitated both mono- and poly-ubiquitin chains, further supporting this novel ubiquitin-interacting interface (Figure 3C). In addition, pull-down by di-ubiquitin was significantly impaired by disruption of the putative ubiquitin-binding motif in VEGFR2H981A or VEGFR2S1021A (Figure 3D). To determine if this interface contributes to the interaction with ubiquitinated epsin, we conjugated HA-tagged full-length Epsin 1-fusion proteins to wild type or mutant ubiquitin moieties (Figure 3C)35, 36. Biochemical and structural studies have revealed the importance of isoleucine 44 within Ub for binding to ubiquitin interaction domains (Chen and Camilli 2005); thus, the UbI44A mutation serves as a positive control that disrupts its interaction with ubiquitin-interacting proteins37-39. We evaluated the contribution of the residues R42, R72 and R74 in ubiquitin that are predicted to interact with VEGFR2 by co-expressing VEGFR2 with HA-Epsin 1-UbWT, HA-Epsin 1-UbI44A, HA-Epsin 1-UbR42A or HA-Epsin 1-UbR72A, R74A (Figure 3E), followed by VEGF stimulation. We found that VEGFR2 co-immunoprecipitated HA-Epsin 1-UbWT, but not HA-Epsin 1-UbI44A (Figure 3F), suggesting that VEGFR2 interacts with the ubiquitin moiety conjugated to epsin. Importantly, both HA-Epsin 1-UbR72A, R74A and HA-Epsin 1-UbR42A also displayed significantly impaired VEGFR2 co-immunoprecipitation (Figure 3F). These findings suggest that c-Cbl-dependent ubiquitination of epsin creates a binding site for the novel ubiquitin-interaction interface of VEGFR2, and thus may contribute to the specificity of epsin for VEGFR2.
Figure 3. Identification of a novel ubiquitin-binding interface within VEGFR2 that promotes an interaction with ubiquitinated epsin.
(A) Ribbon representation of the predicted supercomplex between epsin ENTH (red), VEGFR2 KD (blue) and ubiquitin (pink). Ubiquitin was docked into the epsin ENTH:VEGFR2 KD structure using ClusPro and PyMol software; highest scoring model with good topologies is shown. Enlarged stick representation to the right highlights the interacting residues between ubiquitin and VEGFR2 KD. (B) Western blot analysis of VEGFR2 co-immunoprecipitation with epsin 1 in HEK 293T cells overexpressing wild type VEGFR2, VEGFR2S1021A, VEGFR2S1021AK1014R, K1023R or VEGFR2K1014R, K1023R, G1158A. Cells were serum starved overnight and stimulated with 50 ng/mL VEGF for 2 min. (C) Western blot analysis of mono- or poly-ubiquitin pulldown by wild type VEGFR2 overexpressed in HEK 293T cells. (D) Western blot analysis of VEGFR2 pulldown by di-ubiquitin in HEK 293T cells overexpressing wild type VEGFR2, VEGFR2H891A or VEGFR2S1021A. (E) Putative interacting residues of VEGFR2 and ubiquitin with their respective hydrogen bond distances, and schematic representation of epsin 1 conjugated to wild type ubiquitin or ubiquitin with I44A, R42A or R72A and R74A substitutions. (F) Western blot analysis of epsin 1 co-immunoprecipitation with VEGFR2 in HEK 293T cells overexpressing wild type VEGFR2 and either HA-Epsin1 conjugated to wild type ubiquitin (HA-Epsin 1-Ub) or ubiquitin with I44A, R42A, R72A or R74A point substitutions. Cells were serum starved overnight and stimulated with 50 ng/mL VEGF for 2 min. All representative Western blots were selected from n=3. Error bars indicate the mean ± s.e.m. *P<0.05.
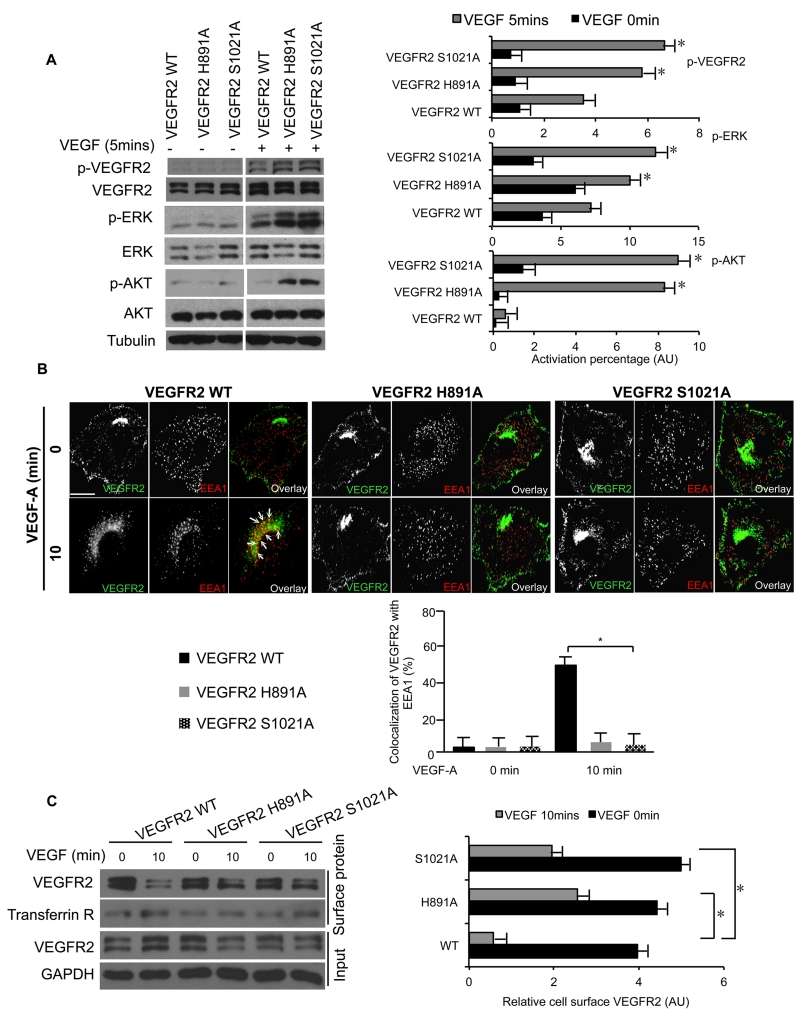
We next determined the importance of our newly identified ubiquitin- and epsin UIM-interacting interfaces in VEGFR2 for epsin-mediated VEGFR2 internalization and degradation, and VEGF signaling. MECs isolated from WT or Flk1fl/fl; iCDH5 Cre (VEGFR2 fl/fl/iCDH5-ERT2 Cre) mice were treated with tamoxifen to knock out endogenous VEGFR2 in MECs, and then expression was restored with either wild type VEGFR2 (VEGFR2WT), or VEGFR2H891A VEGFR2S1021A mutants that disrupt critical interactions with residues in epsin and ubiquitin. Expression of either VEGFR2H891A or VEGFR2S1021A significantly enhanced VEGF-induced phosphorylation of VEGFR2 (p-VEGFR2) and downstream signaling components, including phosphorylation of AKT (p-AKT) and ERK (p-ERK) relative to VEGFR2WT (Figure 4A). Further, compared to VEGFR2WT, both VEGFR2H891A and VEGFR2S1021A mutants exhibited impaired VEGF-induced endocytosis and decreased colocalization with EEA1, an endosomal marker (Figure 4B). The reduced endocytosis of VEGFR2H891A and VEGFR2S1021A mutants was further examined by surface biotinylation. Following VEGF stimulation, we observed more VEGFR2H891A and VEGFR2S1021A at the cell surface compared to VEGFR2WT (Figure 4C). Collectively, these findings suggest that the specificity with which epsins preferentially bind and modulate VEGFR2 internalization is guided by multiple mechanisms, including epsin UIM binding to ubiquitinated VEGFR29, 10, ubiquitin-independent VEGFR2 binding to epsin UIM (Figure 1), and VEGFR2 binding to ubiquitinated epsin (Figure 3), and that these interactions are all support VEGF-dependent VEGFR2 internalization and downregulation of signaling.
Figure 4. Internalization of VEGFR2 upon VEGF stimulation is critical for productive downstream signaling.
(A) Western blot analysis of VEGF-induced phosphorylation of VEGFR2 and downstream AKT and ERK in mouse primary endothelial cells. Tamoxifen-induced the knockout of endogenous VEGFR2, expression was restored with wild type VEGFR2, VEGFR2H891A or VEGFR2S1021A. Cells were serum starved overnight and stimulated with 50 ng/mL VEGF for 5 min. (B) Representative immunofluorescence images of VEGF-dependent changes in the subcellular localization of VEGFR2 in HUVECs overexpressing wild type VEGFR2, VEGFR2H891A or VEGFR2S1021A. Cells were serum starved overnight and stimulated with 50 ng/mL VEGF for 10 min. Arrows indicate the colocaliztion of wild type VEGFR2 with EEA1. (C) Western blot analysis of VEGF-induced VEGFR2 internalization, relative to Transferrin Receptor, in mouse primary endothelial cells overexpressing wild type VEGFR2, VEGFR2H891A or VEGFR2S1021A using a cleavable surface biotinylation and internalization assay. Cells were serum starved overnight and stimulated with 50 ng/mL VEGF for 10 min. All representative Western blots and immunofluorescence images were selected from n=3. Error bars indicate the mean ± s.e.m. *P<0.05. Scale bar in B: 10 μm.
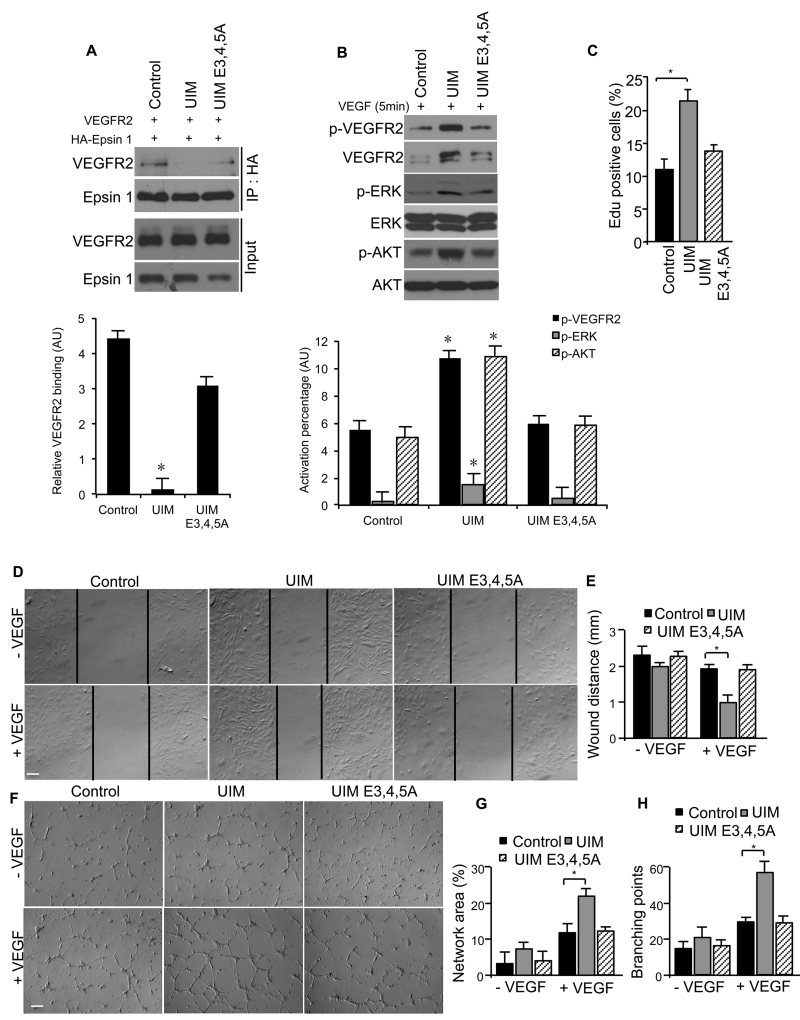
UIM peptide, but not UIME3,4,5A mutant peptide, promotes VEGF-dependent VEGFR2 signaling and in vitro angiogenesis
Our discovery that E183A, E184A and E185A substitutions within the epsin UIM significantly abolished its VEGF-dependent interaction with VEGFR2 (Figure 1E), suggests that these residues contribute to the specificity and affinity of the interaction. To further establish the importance of epsin UIM E183, E184 and E185, and to investigate the feasibility of targeted epsin inhibition, we created a synthetic UIM-containing peptide designed to competitively inhibit UIM-dependent epsin binding to VEGFR2. Importantly, we synthesized this UIM peptide with a plasma membrane permeable peptide, Antennapedia (AP; also known as penetratin), to ensure intracellular delivery40. We also created a mutant UIM peptide (UIME3,4,5A) by incorporating three glutamic acid-to-alanine substitutions (E3A, E4A and E5A) within the UIM peptide sequence that correspond to E183, E184 and E185 of the epsin UIM. Based on our findings, the mutant UIM peptide should not compete with epsin binding to VEGFR2. We treated HEK 293T cells transfected with VEGFR2 and HA-Epsin 1 with either UIM or UIME3,4,5A peptide for 16 hrs prior to VEGF stimulation, then processed the lysates for immunoprecipitation using HA-specific antibody (Figure 5A). The UIM peptide, but not the mutant UIME3,4,5A peptide, competitively inhibited HA-Epsin 1 binding to VEGFR2 (Figure 5A). Consistent with impaired epsin binding to VEGFR2, the UIM peptide enhanced VEGFR2 protein levels and downstream signaling in wild type mouse endothelial cells pre-treated for 16 hrs with peptide prior to serum-starvation and VEGF stimulation (Figure 5B). Importantly, the results obtained using the novel UIM peptide closely mimicked the effects of epsin depletion on VEGFR2 protein levels and downstream signaling9, 10. These findings provide further support for the critical importance of the molecular determinants we identified herein, and suggest the therapeutic potential of this innovative UIM peptide to effectively alter angiogenesis.
Figure 5. UIM peptide, but not UIME3,4,5A mutant peptide promotes VEGF-dependent VEGFR2 signaling and in vitro angiogenesis.
(A) Western blot analysis of VEGFR2 co-immunoprecipitation with epsin 1 in HEK 293T cells overexpressing wild type VEGFR2 and HA-Epsin 1 and treated with 12.5 μM control, full length UIM or E3A, E4A and E5A UIM mutant (UIME3,4,5A) peptide for 16 hrs. Cells were stimulated with 50 ng/mL VEGF for 2 min prior to lysis. (B) Western blot analysis of VEGF-VEGFR2 signaling in WT primary MEC treated with 12.5 μM control, UIM or UIME3,4,5A peptide for 16 hrs. Cells were stimulated with 50 ng/mL VEGF for 5 min prior to lysis. (C) Quantification of EdU labeling for MEC cell proliferation, (D,E) scratch “wound” analysis of cell migration in MEC and (G-I) Matrigel culture analysis of tube formation in HUVECs treated with 12 μM control, UIM or UIME3,4,5A peptide for 16 hrs in the presence of 50 ng/mL VEGF. Representative images selected from n=5 in Online Figure VIII, D, F. Quantification of scratch wound and tube formation are shown in E,G and H, respectively. All representative Western blots were selected from n=3. Scale bar in D and F: 50 μm. Error bars indicate the mean ± s.e.m. *P<0.05.
Next, we wanted to determine whether the UIM peptide can sufficiently inhibit epsin function to alter VEGF-dependent angiogenesis. We examined the effects of UIM peptide treatment on mouse endothelial cell proliferation, migration and network formation using EdU incorporation10, 41, scratch “wound”42 and Matrigel network formation assays42, respectively. In all of these approaches, wild type mouse endothelial cells were pre-treated with UIM wild type or mutant peptides for 16 hrs followed by VEGF stimulation. We found that treatment with the UIM peptide significantly enhanced the in vitro angiogenic characteristics of mouse endothelial cells relative to the control peptide, including increased proliferation (Figure 5C, Online Figure VIII), accelerated migration (Figure 5D,E) and more prominent network formation (Figure 5F to H), consistent with prolonged VEGFR2 signaling (Figure 5B). In stark contrast, treatment with the mutant UIME3,4,5A peptide did not alter in vitro angiogenesis and phenotypically mimicked the effects of control peptide (Figure 5C to H and Online Figure VIII ). In summary, our in vitro functional angiogenesis assays support the critical importance of the three identified glutamic acid residues within the epsin UIM for epsin function as an endocytic adaptor protein involved in VEGFR2 internalization, signaling attenuation and angiogenesis.
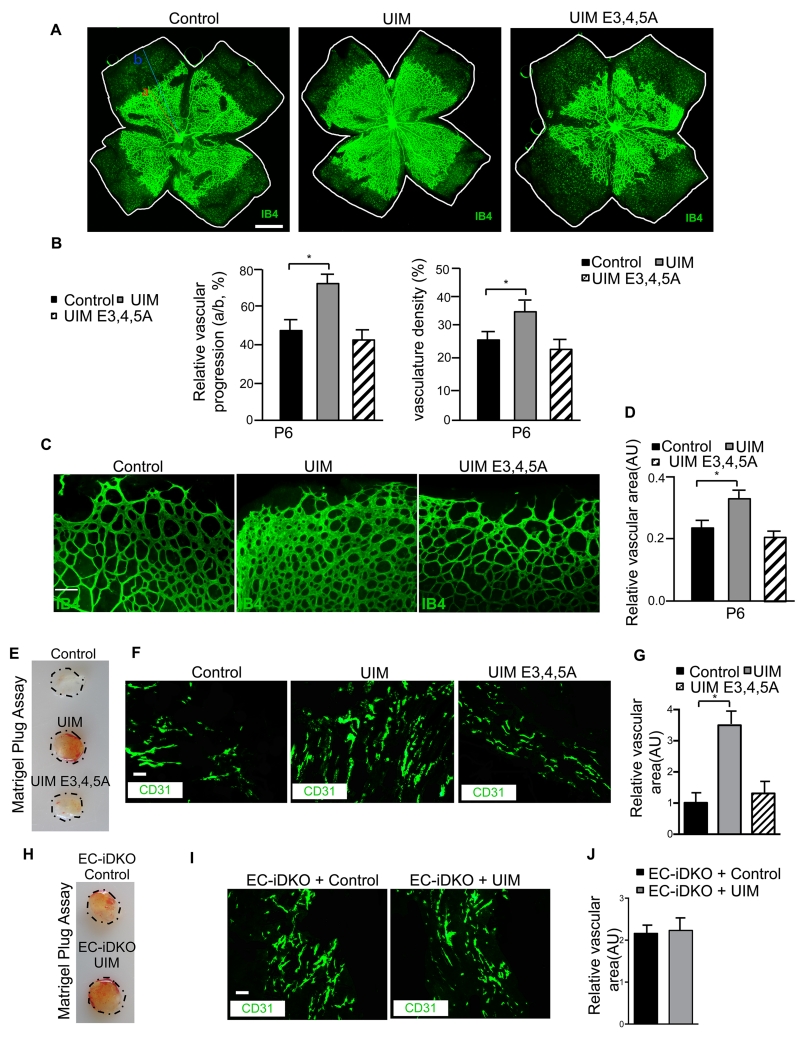
Physiological angiogenesis is increased by administration of UIM, but not UIME3,4,5A peptides
The development of the innovative UIM peptide provides a unique opportunity to examine the therapeutic potential of exogenously inhibiting epsin function in vivo. Given that retinal angiogenesis occurs primarily during postnatal development43, 44, we examined the effects of control, UIM or UIME3,4,5A peptide administration on early postnatal angiogenesis and vascular network maturation in wild type mouse pups. Peptides were administered via intraperitoneal injection at postnatal day 2 (P2), P3, P4 and P5, and retinas were harvested at P6 for whole-mount immunofluorescence staining using the endothelial cell marker, isolectin B4. Consistent with our in vitro angiogenesis experiments, the UIM peptide enhanced angiogenesis, characterized by increased vessel diameter in the P6 retina (Figure 6A,B; Online Figure III), relative to control or UIME3,4,5A peptide. These findings further establish epsins, specifically the epsin UIM, as a critical determinant of physiological angiogenesis. To confirm that the epsins are the sole target of the UIM, UIM peptides were administered to EC-iDKO mouse pups in which epsins are depleted specifically in endothelial cells. Abnormal and elevated angiogenesis in retina was observed in control peptide treated EC-iDKO pups, providing further support that epsin is required for proper angiogenesis (Figure 6C,D). Administration of the UIM peptide did not exacerbate the retinal angiogenesis abnormality observed in EC-iDKO pups treated with control peptide (Figure 6C,D), suggesting that the UIM peptide is specific for epsin and does not cause off-target effects on other pro-angiogenic regulators.
Figure 6. Physiological angiogenesis is increased by administration of UIM, but not UIME3,4,5A peptides.
(A, B) Representative montage images with entire retina review of whole-mount retinas isolated from P6 pups after intraperitoneal injection with control, UIM or UIME3,4,5A mutant peptide and immunofluorescently labeled with biotinylated isolectin B4. Respective quantifications for A are shown in B, including vascular progression length and vasculature density. (C, D) Representative images of whole-mount retinas isolated from P6 pups after intraperitoneal injection with control, UIM or UIME3,4,5A mutant peptide and immunofluorescently labeled with biotinylated isolectin B4. Respective quantification for C is shown in D. (E) Representative images of subcutaneous Matrigel plugs supplemented with 200 ng/mL VEGF and either control, UIM or UIME3,4,5A peptide isolated 7 days post-implantation from WT mice. (F,G) Representative image (F) and quantification (G) of CD31-positive vessels in cryopreserved, sectioned and immunofluorescently stained Matrigel plugs from (E). (H) Representative images of subcutaneous Matrigel plugs supplemented with 200 ng/mL VEGF and either control or UIM peptide isolated 7 days post-implantation from EC-iDKO mice. (I, J) Representative image (I) and quantification (J) of CD31-positive vessels in cryopreserved, sectioned and immunofluorescently stained Matrigel plugs supplemented with either control or UIM peptide and isolated from (H). Representative images selected from n=6. Scale bar in A:1000 μm; C, F and I: 50 μm. ON, a and b in A represented optic nerve center, distance from ON to retina vasculature edge and distance from ON to retina edge. Error bars indicate the mean ± s.e.m. *P<0.05.
To test whether the UIM inhibitory potential is VEGF-dependent, we subcutaneously implanted Matrigel plugs containing VEGF and either control, UIM or UIME3,4,5A peptide into wild type adult mice to directly examine the effects of UIM peptide treatment on endothelial cell migration and network formation in vivo. As predicted, the UIM peptide caused significant pro-angiogenic effects on the Matrigel, resulting in enhanced vascularization (Figure 6E). Mutating the three glutamic acids within the UIM peptide significantly impaired Matrigel vascularization, relative to UIM peptide (Figure 6E), further establishing that the epsin UIM is the critical domain responsible for epsin-mediated angiogenic regulation. Immunofluorescent staining of cryosections from the Matrigel plugs with CD31-specific antibody further confirmed the enhanced vascularization and revealed increased vessel dilation in the UIM peptide-containing Matrigel plugs (Figure 6F,G). In contrast, this effect was not observed if the Matrigel plugs contained control or UIME3,4,5A peptide. Of note, UIM peptide administration to Matrigel plugs implanted in EC-iDKO mice did not cause additional vascular abnormalities compared to control peptide treatment (Figure 6H,I).
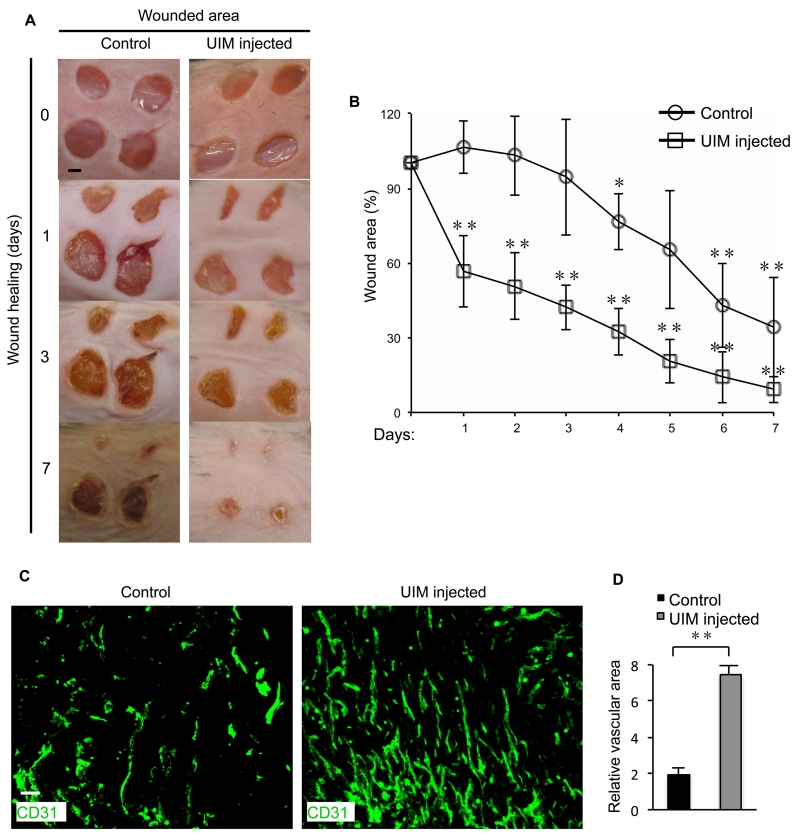
Lastly, to determine if the pro-angiogenic effects of our UIM peptide can provide therapeutic benefits, we examined the effects of UIM peptide administration on wound healing; a physiologic process dependent in part on enhanced angiogenesis. We used a previously established5 dermal biopsy procedure to create four circular wounds on the shaved back of wild-type mice and administered control or UIM peptide via peritoneal injection daily for seven days while monitoring wound healing (Figure 7A)5. UIM peptide administration significantly accelerated wound healing, relative to control peptide (Figure 7A,B), suggesting that the UIM peptide does effectively enhance wound-healing angiogenesis. Consistently, CD31-specific immunofluorescence staining of wounds isolated from UIM-peptide-treated wild type mice at day 7 revealed significantly more vascularization of the wound area (Figure 7C,D). Thus, three critical glutamic acid residues within the epsin UIM mediate the specificity of binding to VEGFR2 in endothelial cells, likely through interactions with H891 of VEGFR2. Further, our study demonstrates the therapeutic potential of inhibiting this interaction with the innovative use of designer peptides to promote physiological angiogenesis.
Figure 7. UIM peptide administration increases physiological angiogenesis in wild type mice.
(A) Representative images of dermal wound healing 0, 1, 3 and 7 days after dermal biopsy of wild type mice receiving control or UIM peptide by intraperitoneal injection. (B) Quantification of wound area shown in (A) and reported as a wound-healing curve. (C) Representative images of CD31-positive blood vessels in cryopreserved, sectioned and immunfluorescently stained dermal wounds from control or UIM peptide treated wild type mice isolated 7 days after dermal biopsy. (D) Quantification of CD31-positive vessel area relative to total vessel area of immunofluorescently stained dermal wounds from (C) using SlideBook software. All representative dermal wound and immunofluorescence images were selected from n=3 mice. Scale bars: A, 2 mm; C, 50 μm. Error bars indicate the mean ± s.e.m. *P<0.05; **P<0.01.
DISCUSSION
Angiogenesis is essential to achieve and maintain physiological homeostasis. Disruptions in the regulatory signaling pathways such as VEGF-VEGFR2 can have devastating physiological and pathological consequences and have therefore been a major research focus. In fact, the development of anti-VEGF antibody therapy has been extremely successful in the clinical treatment of cancers with elevated VEGF secretion and hyper-angiogenic phenotypes44, 45. Development of angiogenic promoters for clinical use in wound healing, ischemia and other conditions in which impaired angiogenesis are common have been more restricted due to the complex regulatory events controlling VEGF-VEGFR2 signaling activation and propagation.
Although receptor internalization is a recognized mechanism of signal modification, and that there have been conflicting reports that VEGFR2 internalization is necessary for signal propagation, we have demonstrated in several publications that, at least in conditions in which epsins are deficient or inhibited, VEGFR2 is capable of signaling downstream to ERK and AKT from the cell surface9, 10, 46. We hypothesize that interactions between different adaptor proteins may facilitate VEGFR2 internalization and incorporation into recycling signaling endosomes thereby amplifying VEGFR2 signaling cascades. In contrast, VEGFR2 activation results in VEGFR2 ubiquitination and subsequent epsin-mediated VEGFR2 internalization and degradation. Loss of epsins may shift the fate of activated cell surface VEGFR2 from degradation to recycling, thus providing a feasible explanation for heightened VEGFR2 signaling and increased angiogenesis. More importantly, consistent with what we have reported, using matrix immobilized VEGF, multiple independently published papers have provided compelling evidence that immobilized VEGF can activate VEGFR2 and stimulate downstream signaling without VEGFR2 internalization47-49. Furthermore, increased cell surface VEGFR2 levels, and heightened VEGF signaling as demonstrated by enhanced phosphorylation of VEGFR2, PLCγ, ERK and AKT were observed when VEGFR2 endocytosis was block by loss of dynamin 250.
We previously reported that genetic ablation of epsins 1 and 2 in vascular endothelial cells significantly enhances angiogenesis9, 10. Further examination determined that epsins modulate angiogenesis by binding to and facilitating the internalization and downregulation of activated VEGFR2. Given that epsins were previously implicated as regulators of Notch-mediated vasculogenesis during embryonic development27, we previously confirmed that epsin depletion specifically modulated VEGFR2 signaling in postnatal angiogenesis by overexpressing the active form of Notch, NICD, in epsin-deficient endothelial cells. NICD did not rescue angiogenesis suggesting that, at least in postnatal conditions, epsin modulates angiogenesis independent of Notch signaling9. In contrast, our study further demonstrated that genetic VEGFR2 haploinsufficiency does rescue the hyper-angiogenic phenotype of EC-iDKO mice10, thus firmly establishing epsins as a specific regulator of VEGFR2 signaling.
Interestingly, generation of membrane curvature that is critical for clathrin-mediated endocytosis has reportedly to be assisted by epsin 1, 2 and 331. Lack of all three epsins can impair endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits31. However, our study clearly demonstrated that lack of epsins 1 and 2 specifically blocks VEGFR2 endocytosis but not prototypical clathrin-mediated endocytosis, such as transferin receptor and EGFR endocytosis in endothelial cells, suggesting that epsin 3 in these cells may compensate to support the housekeeping clathrin-mediated endocytosis. In support, we have detected expression of epsin 3 in endothelial cells including HUVECs (Online Figure VII).
In this study, we delved into the molecular details determining the specificity between epsin and VEGFR2. In doing so, we identified two novel binding interfaces that function independently of the previously established interaction between epsin UIM and the ubiquitin moiety conjugated to activated VEGFR29, 10. Our findings suggest that epsin and VEGFR2 binding is guided by a complex and multifaceted binding mechanism that involves both ubiquitin-independent and –dependent interactions. Specifically, our complementary structural modeling and site-directed mutagenesis approaches determined that epsin UIM interacts with the VEGFR2 kinase domain prior to or independent of VEGFR2 ubiquitination (Figure 1). VEGF-dependent activation of VEGFR2, and subsequent recruitment of c-Cbl, results in the ubiquitination of both VEGFR2 and epsin (Figure 2A). Epsin then binds ubiquitinated VEGFR2 via the classical epsin UIM function as an ubiquitin interacting motif, while the novel ubiquitin interacting interface of VEGFR2 kinase domain binds ubiquitinated epsin. Whether these interactions occur in a specific sequential order remains to be determined, but given that disrupting the ubiquitin-independent interface between epsin UIM and VEGFR2 impaired epsin ubiquitination (Figure 2B) and abolished VEGFR2 binding (Figure 1E), we speculate that this is the limiting event required for epsin and VEGFR2 to interact. Further, disrupting this interface may provide a highly selective therapeutic targeting strategy.
Toward this end, we developed an innovative UIM peptide designed to competitively inhibit epsin UIM binding to VEGFR2. Foresight to include the cell-penetrating AP motif significantly enhanced UIM peptide delivery and efficacy, such that UIM peptide treatment significantly promoted angiogenesis in wild type, but not EC-iDKO mice (Figure 6A-D, Online Figure IV). Also as further testament to peptide delivery and the importance of the critical ubiquitin-independent epsin UIM binding to VEGFR2, replacing the three critical glutamic acids with alanine in the UIM peptide abolished its pro-angiogenic effects (Figure 5, 6 and Online Figure VIII). We believe that this peptide will become an essential and much needed tool for future investigations into the multivalent roles of epsin UIM in other cellular processes in which epsins have been implicated. Further, we believe that this study provides an important proof-of-concept for the use of similar peptide-targeting strategies to disrupt epsin function and promote angiogenesis in clinical conditions such as ischemia, surgical recovery, and secondary diabetic peripheral ulcerations where VEGF secretion or VEGFR2 expression is limited due to alternative mechanisms51-54. For such future clinical purposes, the UIM peptide must be further refined to ensure specific endothelial cell targeting and additional experimentations need to be completed to ensure limited off target effects. Although preliminary studies in which the UIM peptide was conjugated with FITC (FITC-UIM) suggests that it selectively targets vascular endothelial cells (Online Figure IIIB), an alternative future approach should include the incorporation of an endothelial-specific targeting sequence to ensure such selectively. Novel studies on effective UIM peptide designs and their implications in clinically relevant models of altered angiogenesis are a major focus and future direction of our research.
In summary, our study identified critical molecular determinants responsible for epsin-mediated VEGFR2 downregulation and angiogenic attenuation, including two novel VEGFR2 interfaces predicted to engage epsin prior to and/or in addition to epsin UIM binding to ubiquitinated VEGFR2. We postulate that multiple layers of presumably weak interactions, including those between epsin UIM and the VEGFR2 kinase domain binding cleft, between ubiquitinated epsin and the VEGFR2 kinase domain ubiquitin-interacting interface, and between epsin UIM and ubiquitinated VEGFR2, ultimately establish a specific and stable interaction between epsin and VEGFR2 in response to VEGF stimulation (Online Figure IX). Our data further suggest that epsins play a more critical role in VEGFR2 turnover than previously thought. Rather than simply mediating the internalization of ubiquitinated VEGFR2, our unpublished results suggest that epsins are critically involved in the ubiquitination of VEGFR2 by recruiting and interacting with c-Cbl in response to VEGF stimulation. While the molecular determinants regulating the interaction between epsin and c-Cbl, and the c-Cbl-dependent ubiquitination sites within epsin have yet to be identified, our data strongly suggest that these events are critical prerequisites for epsin and VEGFR2 complex assembly and c-Cbl-dependent VEGFR2 and epsin ubiquitination. Collectively, our findings highlight the multivalent nature of epsins and the continued potential for new functional discoveries regarding how these unique and versatile proteins modulate specific cell functions. Importantly, these discoveries resulted in the development of an innovative and highly specific epsin inhibitory peptide can be used in the future to investigate the epsin-dependent regulation of other cardiovascular disease processes.
Supplementary Material
Novelty and Significance.
What Is Known?
Vascular endothelial growth factor receptor 2 (VEGFR2) is a key regulator of physiological angiogenesis.
Epsin is a family of adaptor proteins that aid in the clathrin-mediated endocytosis of VEGFR2, rendering its degradation.
What New Information Does This Article Contribute?
Identified novel interactions governing epsin-VEGFR2 binding.
Designed peptide inhibitor that specifically disrupts these interactions.
Targeting these interactions may provide an effective means to enhance angiogenesis in cases where it is inadequate.
A thorough understanding of VEGF-dependent vascular remodeling is critical for the effective design of therapies that can promote angiogenesis in cases where it is inadequate or diminish it where it is excessive. While many advances have been made regarding intracellular trafficking that regulates VEGF signaling, several aspects are still poorly understood. In particular, the thorough analysis of the contribution of endocytosis to the regulation of the duration and magnitude of VEGF signaling pathways has not been fully explored and is the focus of this study. Epsin mediates VEGFR2 internalization and degradation to tightly regulate angiogenesis. However, the specific determinants of epsin-VEGFR2 interaction were unknown. Here, we have identified two novel binding surfaces on the VEGFR2. When mutated, these sites disrupt the binding of the epsin UIM with ubiquitinated VEGFR2, a key step in the classical epsin-dependent internalization and degradation of VEGFR2, and subsequent attenuation of angiogenesis. Further, we have designed a novel UIM peptide, which competitively inhibits the epsin UIM binding to VEGFR2, consequently enhancing angiogenesis. We believe that our studies on the therapeutic potential of the epsin UIM peptide in angiogenesis will pave the way for further application of this unique peptide to treat a range of cardiovascular diseases.
ACKNOWLEDGMENTS
We want to thank Dr. Xiaolei Liu and Scott Hahn for their technical assistance and helpful discussion. We also want to thank the Boston Children’s Hospital Vascular Biology Imaging Core for help with the sample preparations.
SOURCES OF FUNDING
Work was supported by NIH Grants R01HL-093242, R01HL-118676, R01HL-130845, P20 RR018758, Established Investigator Award from the American Heart Association (AHA) and Department of Defense Grant W81XWH-11-1-00226 to H. Chen; OCAST Grant AR11-043, HR14-056 and AHA SDG grant 12SDG8760002 to Y. Dong; AHA fellowships 13POST16940008 to K.L. Tessneer, 13POST17270006 to S. Pasula and 15PRE21400010 to M.L. Brophy; and NIH fellowship 1F32HL121954-01 to K.L. Tessneer and 1F31HL127982-01 to M.L. Brophy.
Nonstandard Abbreviations and Acronyms
- AP
Antennapedia
- c-Cbl
casitas B-lineage lymphoma
- DKO
epsins 1 and 2 double knockout mouse endothelial cells
- EC-iDKO
tamoxifen-inducible vascular endothelial epsins deletion mice
- EdU
5-ethynyl-2′-deoxyuridine
- ENTH
epsin NH2-terminal homology
- Epn
epsin
- Fl
foxed
- HA
hemagglutinin
- HUVEC
human umbilical vein endothelial cells
- IB4
isolectin B4
- KD
kinase domain
- MEC
Mouse endothelial cells
- P
postnatal
- SEM
standard error of the mean
- Ub
ubiquitin
- UIM
ubiquitin interacting motif
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- WT
wild type mice.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Tille JC, Wang X, Lipson KE, McMahon G, Ferrara N, Zhu Z, Hicklin DJ, Sleeman JP, Eriksson U, Alitalo K, Pepper MS. Vascular endothelial growth factor (VEGF) receptor-2 signaling mediates VEGF-C(deltaNdeltaC)- and VEGF-A-induced angiogenesis in vitro. Experimental cell research. 2003;285:286–98. doi: 10.1016/s0014-4827(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Duval M, Bedard-Goulet S, Delisle C, Gratton JP. Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. Consequences on nitric oxide production from endothelial cells. The Journal of biological chemistry. 2003;278:20091–7. doi: 10.1074/jbc.M301410200. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein BM, Lawson ND. Arteries, veins, Notch, and VEGF. Cold Spring Harbor symposia on quantitative biology. 2002;67:155–62. doi: 10.1101/sqb.2002.67.155. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circulation research. 2006;98:743–56. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- 9.Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, Hahn S, Keeling J, Saunders D, Ko G, Knight J, Newton G, Luscinskas F, Sun X, Towner R, Lupu F, Xia L, Cremona O, De Camilli P, Min W, Chen H. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. The Journal of clinical investigation. 2012;122:4424–38. doi: 10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tessneer KL, Pasula S, Cai X, Dong Y, McManus J, Liu X, Yu L, Hahn S, Chang B, Chen Y, Griffin C, Xia L, Adams RH, Chen H. Genetic reduction of vascular endothelial growth factor receptor 2 rescues aberrant angiogenesis caused by epsin deficiency. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:331–7. doi: 10.1161/ATVBAHA.113.302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Developmental cell. 2010;18:713–24. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicke L. Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–12. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 13.Bruns AF, Herbert SP, Odell AF, Jopling HM, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic. 2010;11:161–74. doi: 10.1111/j.1600-0854.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–7. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal JA, Chen H, Slepnev VI, Pellegrini L, Salcini AE, Di Fiore PP, De Camilli P. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. The Journal of biological chemistry. 1999;274:33959–65. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2766–71. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nature cell biology. 2002;4:389–93. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 18.Tessneer KL, Cai X, Pasula S, Dong Y, Liu X, Chang B, McManus J, Hahn S, Yu L, Chen H. Epsin Family of Endocytic Adaptor Proteins as Oncogenic Regulators of Cancer Progression. J Can Res Updates. 2013;2:144–150. doi: 10.6000/1929-2279.2013.02.03.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Pasula S, Song H, Tessneer KL, Dong Y, Hahn S, Yago T, Brophy ML, Chang B, Cai X, Wu H, McManus J, Ichise H, Georgescu C, Wren JD, Griffin C, Xia L, Srinivasan RS, Chen H. Temporal and spatial regulation of epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function. Sci Signal. 2014;7:ra97. doi: 10.1126/scisignal.2005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–51. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 21.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–6. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 22.De Camilli P, Chen H, Hyman J, Panepucci E, Bateman A, Brunger AT. The ENTH domain. FEBS letters. 2002;513:11–8. doi: 10.1016/s0014-5793(01)03306-3. [DOI] [PubMed] [Google Scholar]
- 23.Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–5. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 24.Kazazic M, Bertelsen V, Pedersen KW, Vuong TT, Grandal MV, Rodland MS, Traub LM, Stang E, Madshus IH. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–45. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends in biochemical sciences. 2001;26:347–50. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 26.Wendland B. Epsins: adaptors in endocytosis? Nat Rev Mol Cell Biol. 2002;3:971–7. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, De Camilli P, Cremona O. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13838–43. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko G, Paradise S, Chen H, Graham M, Vecchi M, Bianchi F, Cremona O, Di Fiore PP, De Camilli P. Selective high-level expression of epsin 3 in gastric parietal cells, where it is localized at endocytic sites of apical canaliculi. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21511–6. doi: 10.1073/pnas.1016390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. The Journal of biological chemistry. 2001;276:29257–67. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 30.Tessneer KL, Pasula S, Cai X, Dong Y, Liu X, Yu L, Hahn S, McManus J, Chen Y, Chang B, Chen H. Endocytic adaptor protein epsin is elevated in prostate cancer and required for cancer progression. ISRN Oncol. 2013;2013:420597. doi: 10.1155/2013/420597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messa M, Fernandez-Busnadiego R, Sun EW, Chen H, Czapla H, Wrasman K, Wu Y, Ko G, Ross T, Wendland B, De Camilli P. Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits. Elife. 2014;3:e03311. doi: 10.7554/eLife.03311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic acids research. 2004;32:W96–9. doi: 10.1093/nar/gkh354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang B, Tessneer KL, McManus J, Liu X, Hahn S, Pasula S, Wu H, Song H, Chen Y, Cai X, Dong Y, Brophy ML, Rahman R, Ma JX, Xia L, Chen H. Epsin is required for Dishevelled stability and Wnt signalling activation in colon cancer development. Nat Commun. 2015;6:6380. doi: 10.1038/ncomms7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Polo S, Di Fiore PP, De Camilli PV. Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14908–13. doi: 10.1073/pnas.2136625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S, Kowanetz K, Breitling R, Mann M, Stenmark H, Dikic I. Regulation of ubiquitin-binding proteins by monoubiquitination. Nature cell biology. 2006;8:163–9. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- 36.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends in biochemical sciences. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Nakatsu F, Sakuma M, Matsuo Y, Arase H, Yamasaki S, Nakamura N, Saito T, Ohno H. A Di-leucine signal in the ubiquitin moiety. Possible involvement in ubiquitination-mediated endocytosis. The Journal of biological chemistry. 2000;275:26213–9. doi: 10.1074/jbc.M907720199. [DOI] [PubMed] [Google Scholar]
- 38.Shih SC, Sloper-Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. The EMBO journal. 2000;19:187–98. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sloper-Mould KE, Jemc JC, Pickart CM, Hicke L. Distinct functional surface regions on ubiquitin. The Journal of biological chemistry. 2001;276:30483–9. doi: 10.1074/jbc.M103248200. [DOI] [PubMed] [Google Scholar]
- 40.Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nature cell biology. 2004;6:189–96. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- 41.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, Luo D, Jones D, Tang S, Chen H, Sessa WC, Min W. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. The Journal of clinical investigation. 2008;118:3904–16. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhrt H, Gryga M, Wolburg H, Joffe B, Grosche J, Reichenbach A, Noori HR. Postnatal mammalian retinal development: quantitative data and general rules. Progress in retinal and eye research. 2012;31:605–21. doi: 10.1016/j.preteyeres.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Tual-Chalot S, Allinson KR, Fruttiger M, Arthur HM. Whole mount immunofluorescent staining of the neonatal mouse retina to investigate angiogenesis in vivo. Journal of visualized experiments : JoVE. 2013:e50546. doi: 10.3791/50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mordenti J, Thomsen K, Licko V, Chen H, Meng YG, Ferrara N. Efficacy and concentration-response of murine anti-VEGF monoclonal antibody in tumor-bearing mice and extrapolation to humans. Toxicologic pathology. 1999;27:14–21. doi: 10.1177/019262339902700104. [DOI] [PubMed] [Google Scholar]
- 46.Dong Y, Wu H, Rahman HN, Liu Y, Pasula S, Tessneer KL, Cai X, Liu X, Chang B, McManus J, Hahn S, Dong J, Brophy ML, Yu L, Song K, Silasi-Mansat R, Saunders D, Njoku C, Song H, Mehta-D’Souza P, Towner R, Lupu F, McEver RP, Xia L, Boerboom D, Srinivasan RS, Chen H. Motif mimetic of epsin perturbs tumor growth and metastasis. The Journal of clinical investigation. 2015;125:4349–64. doi: 10.1172/JCI80349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Backer MV, Patel V, Jehning BT, Claffey KP, Backer JM. Surface immobilization of active vascular endothelial growth factor via a cysteine-containing tag. Biomaterials. 2006;27:5452–8. doi: 10.1016/j.biomaterials.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Clegg LW, Mac Gabhann F. Site-Specific Phosphorylation of VEGFR2 Is Mediated by Receptor Trafficking: Insights from a Computational Model. PLoS Comput Biol. 2015;11:e1004158. doi: 10.1371/journal.pcbi.1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan WH, Popel AS, Mac Gabhann F. Computational model of VEGFR2 pathway to ERK activation and modulation through receptor trafficking. Cellular signalling. 2013;25:2496–510. doi: 10.1016/j.cellsig.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MY, Skoura A, Park EJ, Landskroner-Eiger S, Jozsef L, Luciano AK, Murata T, Pasula S, Dong Y, Bouaouina M, Calderwood DA, Ferguson SM, De Camilli P, Sessa WC. Dynamin 2 regulation of integrin endocytosis, but not VEGF signaling, is crucial for developmental angiogenesis. Development. 2014;141:1465–72. doi: 10.1242/dev.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine reviews. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 52.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, Scotney P, Nyqvist D, Samen E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjoholm A, Nash A, Eriksson U. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012 doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 53.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. Journal of the American College of Cardiology. 2005;46:827–34. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell metabolism. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.