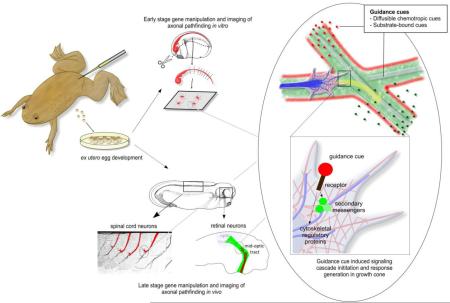
Abstract
The intricate and precise establishment of neuronal connections in the developing nervous system relies on accurate navigation of growing axons. Since Ramón y Cajal's discovery of the growth cone, the phenomenon of axon guidance has been revealed as a coordinated operation of guidance molecules, receptors, secondary messengers, and responses driven by the dynamic cytoskeleton within the growth cone. With the advent of new and accelerating techniques, Xenopus laevis emerged as a robust model to investigate neuronal circuit formation during development. We present here the advantages of the Xenopus nervous system to our growing understanding of axon guidance.
Keywords: Xenopus laevis, retinal ganglion neurons, spinal neurons, axon guidance, growth cone
Graphical Abstract
1. Advantages of Xenopus laevis as a model organism for axon guidance
The complexity of neuronal networks has been a long-standing puzzle that has challenged scientists for centuries. Unveiling how this complex wiring is established in the mammalian brain has, in large part, relied on examination of simpler organisms with comparatively less intricate networks. For example, Ramón y Cajal's work on the chick brain produced the first description of the growth cone [1], [2], and Harrison's work with frogs established the first neuronal culture system [3]. Furthermore, Sperry's pivotal experiment on frog retinal neuron regeneration [4] explained the chemospecificity of connections [5], which has been refined by further studies in systems such as Xenopus [6].
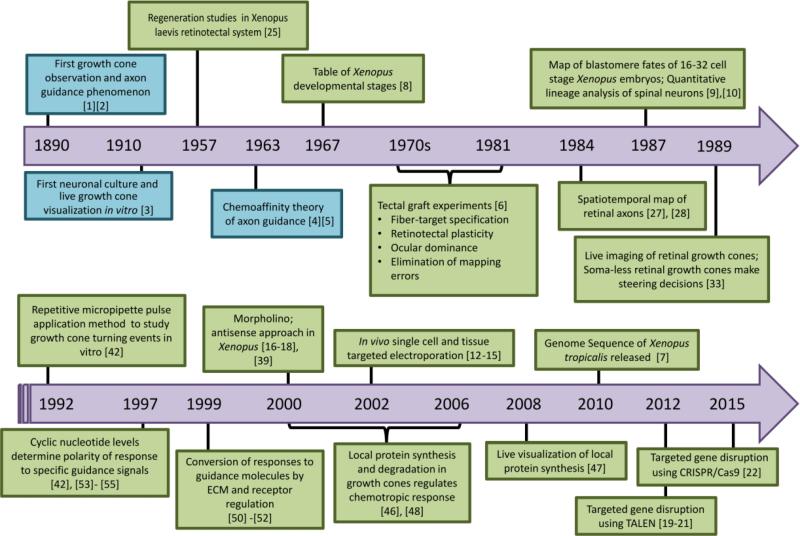
Xenopus, as a whole, offers an advantageous complementary vertebrate model, with a multitude of benefits. First of all, recently sequenced genomic data from Xenopus shows high similarity with the human genome [7]. There are several species of the Xenopus genus, but two have become increasingly popular in research. The diploid western-clawed Xenopus tropicalis offers advantages in genomic studies due to its smaller genome. On the other hand, despite its large allotetraploid genome and longer maturation time, the African clawed frog Xenopus laevis provides numerous advantages which make it a gold standard for studying axon guidance in development (Figure 1).
Figure 1. Timeline of Xenopus laevis in axon guidance.
Key findings and important advances in Xenopus laevis (shown in green boxes) as a model organism during the development of the axon guidance field.
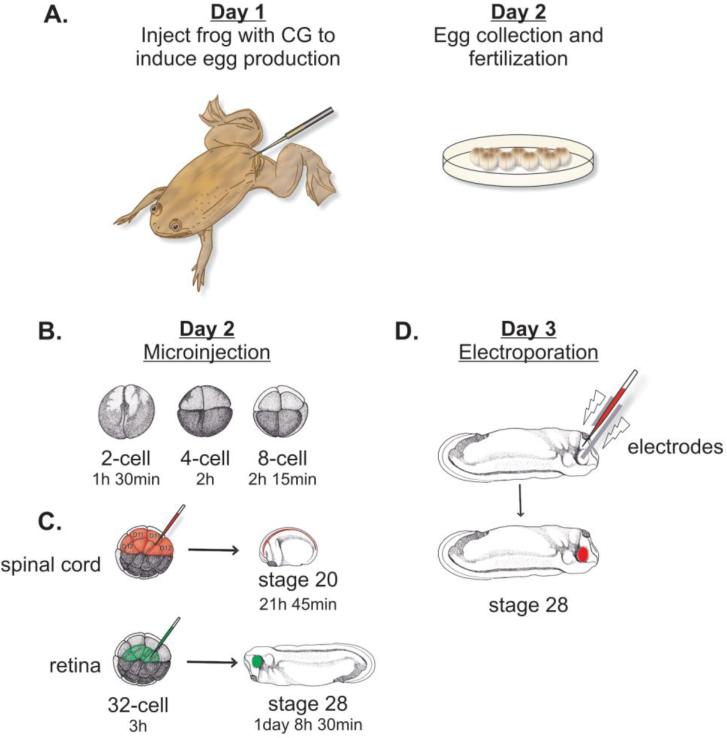
The use of Xenopus laevis in axon guidance research is advantageous for multiple reasons. Frog husbandry is relatively straightforward, and female frogs can be easily stimulated to produce eggs by simply injecting chorionic gonadotropin hormone. Eggs are comparatively large in diameter, 1-2 mm, and are produced in large quantities. Fertilization occurs ex utero and provides the opportunity to track and manipulate embryonic development at desired stages (Figure 2A). Furthermore, embryos can tolerate extensive surgical manipulations varying from microinjection to cell and tissue transplantation. Based upon the given developmental stage and the known fate map of Xenopus laevis, it is possible to target specific cell types. For instance, microinjecting mRNA at stages as early as the 1-4 cell stage results in global alteration of gene levels (Figure 2B). Alternatively, injecting embryos at later stages, for example 16-64 cell, allows the restriction of gene manipulations to a more specific tissue [9], [10] (Figure 2C).
Figure 2. Gene manipulations at various stages of Xenopus embryonic development.
(A) Female frogs are injected with chorionic gonadotropin (CG) 12-18 h before egg collection. Eggs are collected and maintained in a salt solution and fertilized with minced testes. Owing to its ex utero development, Nieuwkoop and Faber (NF) based developmental stages can be tracked as the development proceeds at room temperature and manipulations can be performed at desired stages. (B) Injection at early stages (2-8 cell) will target larger tissues, however targeting lighter blastomeres which form the dorsal tissues will target neural plate or notochord. (C) Injection at later stages will target more specific tissues. Based on the Xenopus fate map, retinal cells derive primarily from blastomeres D1.1.1 and D1.2.1 in a 32-cell stage embryo. The neural tube receives its major contribution from D1.1 and D1.2. (D) Electroporation for spatiotemporal targeting of the nervous system is possible particularly between stages 21-40, when retinal axonal tracts are first forming.
Compared to other systems, Xenopus laevis neurons can be simply isolated and maintained at room temperature, permitting easy manipulation of live neurons as high resolution images are acquired, forgoing the need for strict incubation conditions such as those provided by CO2 imaging chambers [11]. The primary benefit of Xenopus laevis for these studies, however, is its large growth cones, which can be up to 10 to 30 microns in diameter and are perfect for clear and detailed analysis of subcellular cytoskeletal structures and dynamics. There may be no other vertebrate model system with growth cones as large and as easy to culture, manipulate, and image as Xenopus laevis.
2. Manipulation of the Xenopus laevis molecular arsenal
Delivery of molecules such as DNA, mRNA, antibodies, or fluorescent dextrans to modify expression of a particular gene or label a specific tissue is available via approaches such as microinjection or electroporation [12]–[15]. Genetic knockdown can be achieved via a variety of methods, the most common of which has been microinjection of antisense morpholino oligonucleotides (MOs). With the use of standard controls [16], MOs are advantageous tools to manipulate gene products [17], and they have been widely used for Xenopus gene knockdown since 2000 [18]. While the effects of MOs last for only a few days, however, the ability to achieve prolonged and heritable gene modifications is now possible with recently developed gene-editing nuclease systems. Transcription activator-like effector nucleases (TALENs), able to deliver high efficiency genetic knockout, have been used in laevis for multiple genes [19]–[21]. CRISPR-Cas9, for which it is much easier to produce guide RNA, and which displays even less off-target effects than TALENs [22], has been shown to be effective at disrupting pancreatic genes and pigment genes in laevis [22], and this technique will likely useful for investigations into neuronal genes as well. Together, as long as the proper control experiments are conducted, traditional MO approaches and/or newer CRISPR-Cas9 and TALEN systems of genetic manipulation provide complementary tools for efficient alteration of Xenopus laevis proteins for axon guidance studies.
In addition to microinjection, electroporation allows manipulation of genes in later stage tissue and can be advantageous over other delivery methods. For instance, if the molecule of interest takes part in neuronal development as well as earlier stages of embryonic development, manipulation of its levels at blastomeric stages may result in lethality or embryonic abnormalities. Therefore, the cell autonomous role of a particular protein during axon guidance is better examined if its function or level is manipulated at stages 20-40 when axonogenesis and brain wiring are still in progress [24] (Figure 2D).
With respect to axon guidance studies, the developing Xenopus nervous system provides a valuable working space. In particular, retinal and spinal neurons have been extensively used to decipher aspects of axon guidance machinery. Modern approaches have been developed for both systems to monitor mechanisms of axon guidance in vitro and in vivo, and this review aims to characterize some of these important examples.
3. Use of the Xenopus laevis retinotectal pathway to study axon guidance in vivo and in vitro
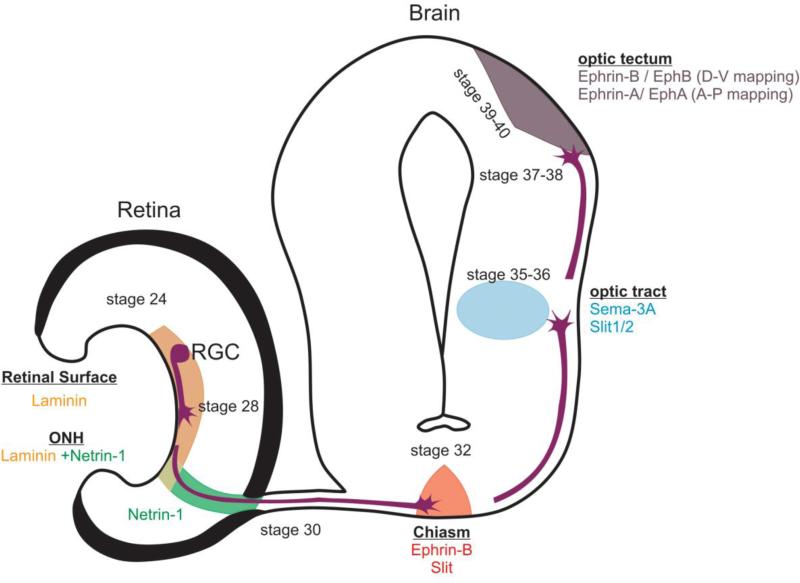
Since studies beginning in 1959 with Gaze [25] and others [6], the visual pathway formation of Xenopus has been used as a model for axon pathfinding. The retinotectal neural pathway lies close to the surface, allowing for easy accessibility to neurons for gene manipulation and imaging. Moreover, the pathway is solely formed by the projection of retinal ganglion cells (RGCs), providing a homogenous population of axons that protrude in a stereotypical manner. Most importantly, the basic structure of the pathway as well as the molecules that sculpt this structure are shared between Xenopus and mammals [26] (Figure 3).
Figure 3. Diagram of embryonic Xenopus retinal pathway map.
The spatiotemporal position of axons and guidance signals effective along the visual pathway during embryonic development. RGC retinal ganglion cell, ONH optic nerve head.
Christine Holt and colleagues have made significant contributions to the field by developing key techniques to manipulate and image live retinal axons at the cellular level. Seminal studies from the Holt lab have documented the spatiotemporal map and morphology of RGC axons along the visual pathway [27], [28]. Following up the initial observations of growth cones in fixed tissue, it has become possible to trace living growth cones in action. In addition to the Holt Lab, the Cohen-Cory and Cline labs have also made significant contributions by characterizing the dynamics of retinal axon innervation and synapse formation in the tectum of Xenopus tadpoles in vivo [29]-[32]. Harris et al recorded the motility of growth cones along the optic tract in the living brain and characterized initial and final growth cone advance rates, branching, pausing and retracting behaviors [33]. One of their most striking observations was that the growth cones of retinal axons isolated from their cell bodies are still able to navigate correctly in the tectum. This suggested that the growth cone has the necessary machinery to recognize the guidance cues and determine whether or not to advance, pause, or retract, as it navigates retinal axons.
3.1 Exposed brain preparations for live imaging of retinal growth cones in vivo
The technique that Harris et al introduced to examine living growth cones was called the “exposed brain preparation,” in which the developing optic tract is exposed in an intact Xenopus embryo [33]. With this technique, bath-application of reagents can easily penetrate through the exposed tissue, allowing pharmacological manipulations. The paths of living or fixed growth cones labeled with dyes such as DiI or Horseradish Peroxidase (HRP), respectively, can then be easily traced. By employing these advantages, Chien et al tested whether or not filopodia (finger-like, actin-rich protrusions that act as sensory and motor structures) are necessary for growth cone navigation [34], [35], [36]. They demonstrated that bath-applied cytochalasin, a drug that can disrupt actin networks, reduces the number of filopodia and results in slower growth cone advance rates. Moreover, fixed and HRP-filled optic-tracts of Xenopus embryos show aberrant pathfinding and retinal axons fail to make caudal turns at the mid-diencephalon [34]. One limitation of this study is that bath-application of a drug might have a global effect on both the retinal axons addressed and the surrounding neuroepithelial tissue along which axons travel. Targeting solely filopodia may give rise to different results; as shown in later reports, abolishing filopodia through depletion of Xena/XVASP does not cause navigational errors [37]. This example demonstrates how cell-autonomous strategies may be necessary to mitigate certain non-cell-autonomous interferences.
3.2 Electroporation of retinal neurons for targeted gene delivery
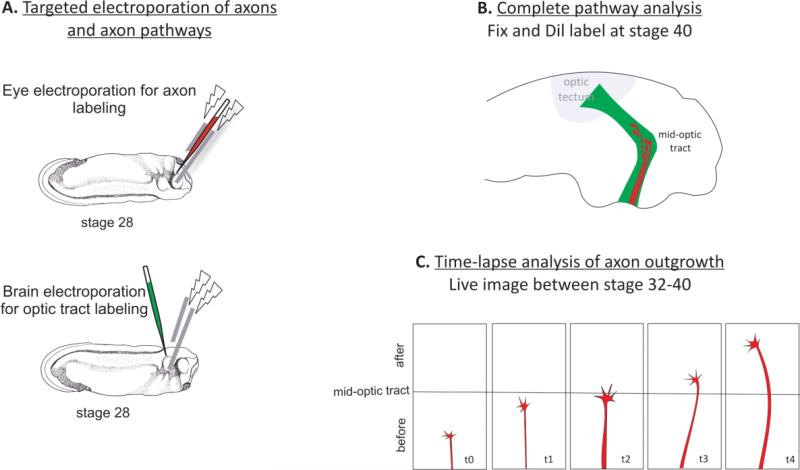
Current techniques in Xenopus offer ways to overcome non-cell-autonomous impacts by enabling tissue-targeted manipulations via microinjection approaches or electroporation. Electroporation emerged as a successful approach to mediate gene transfer into the intact Xenopus nervous system at the single cell level or in bulk during their development (Figure 4A) [13], [14], [15]. While microinjection of embryos sometimes causes early death or developmental abnormalities, electroporation is more efficient and can produce cell or tissue cultures that are in many ways more reliable [24]. Falk et al describe a detailed electroporation protocol to specifically study the wiring of Xenopus embryonic brains between stages 21-40. Depending upon the region of interest, commonly either brain or eye, embryos are positioned in electroporation chambers tailored to fit their developmental stage. For eye-targeted delivery, a solution containing the molecule of interest is injected into the lumen of the eye of embryos ranging from stage 22 to stage 35/36. Injection is immediately followed by a voltage application with the appropriate frequency and duration. GFP expression can be readily observed 12h after electroporation. Importantly, electroporation of different regions of the same embryo is possible [24]. This dual-electroporation technique is highly exciting because it allows, for example, simultaneous manipulation of receptors on the growth cone and guidance cues on its terrain [38], making it possible to study axon-pathway interactions during pathfinding.
Figure 4. Xenopus visual pathway as a model of axon pathfinding.
Manipulation of genes in retinal axons and axonal pathways in the same embryo is possible via eye and brain targeted electroporation respectively. (A) Retinal axons and the neuroepithelium of the optic tract can be electroporated between stage 27 after axon initiation begins and stage 32 when the first retinal axons pass the optic chiasm and enter the optic tract in the brain. (B) For complete pathway analysis, embryos can be fixed at stage 40 and labeled with DiI or HRP. Labeled retinal pathway can be exposed via open brain preparation and pathfinding behaviors of axons can be analyzed. (C) Open brain preparations prepared in living embryos after stage 32 allows live imaging of retinal axons as they navigate through the optic tract in the brain. Time-lapse movies of axons can be recorded for 24h with 3min intervals.
In addition to DNA electroporation, MO electroporation is described to knockdown gene expression in Xenopus embryos [24] and tadpoles [39]. At early-stages (2 to 64 cell), MO can be directly injected into the cell; however, beyond these cell-stages, the neutral MO is confronted by the negatively charged cell membrane. This is why conventional MOs cannot be used for electroporation; instead, tagging MOs with a negatively charged fluorescent reporter (such as Lissamine™) ensures both tracing and delivery. MO can interfere with protein expression as soon as 1h after electroporation and can persist up to 48h after electroporation. Co-electroporation coupling DNA/DNA or DNA/MO also yields efficient uptake with this technique [24], [39]. For example, Bestman et al used this approach and co-electroporated MO along with a plasmid that drives GFP expression under the control of cell specific promoter to identify genes that contribute to neurogenesis of the optic tectum in Xenopus tadpoles [40].
Coupling the aforementioned gene manipulation approaches with in vivo imaging techniques allows for the deciphering of guidance machinery components while the growth cone continuously makes decisions along its terrain [41]. A study by Leung et al beautifully exemplifies this. The group spatially modulated the function of NF-protocadherin (NFPC) via targeted electroporation of either dominant negative NFPCΔE-myc or NFPC-MO in retinal axons or along their substrate along the optic tract in brain. DiI staining and live imaged then revealed navigational errors along the mid-optic tract, suggesting a requirement for homophilic interactions between the axon and its substrate at this region [38] (Figure 4A-C).
3.3 Whole pathway explant preparation to assess retinal growth cone response in vitro
While these studies enable us to understand the topography of axon pathfinding in an intact organism, it still remains to be understood how an individual growth cone is modulated during guidance decisions. Thus, some current investigations have shifted toward understanding the intracellular machinery. In this regard, Lohof et al developed an approach to assess the behavior of individual cultured Xenopus laevis growth cones in response to a gradient of signals repetitively released by micropipette [42]. Taking advantage of this technique, de la Torre et al demonstrated that the growth cone turning of RGCs induced by netrin-1 involves the DCC receptor to mediate the chemotropic action of netrin-1, as antibodies to DCC interfere with the chemotropic response to netrin-1 [43]. This experimental system has also been extensively used with Xenopus spinal neurons (discussed in section 4.1).
RGC axons first leave the retina at stage 28 [24]; therefore, any attempt to culture the retina beyond that stage will sever axons and represent a regeneration study rather than a study of de novo axon outgrowth. Shewan et al, however, developed an approach they term “whole pathway explant preparation,” used for testing the responsiveness of de novo growing RGC axons to a netrin-1 signal at stages ranging from 24 to 40 during visual pathway formation [44]. Based upon the visual pathway map [27], cuts can be made ahead of the growth cones at various stages along the visual pathway. Cultured retinal axons extending from the cuts onto the substrate can then be tested for their response to a specific chemotropic gradient. The purpose of whole pathway explant testing is to allow axons to experience their native environment up until the point where their response is examined in vitro. Using this method, Shewan et al found that the response of RGCs to netrin-1 gradients depends on the developmental stage, as young retinal axons (pre-optic nerve head) show attraction toward gradients of netrin-1, whereas older axons (post-chiasm) are repelled [44]. This study revealed that growth cones aged in culture without experiencing a native pathway also switch their response in parallel with the response of axons in vivo, suggesting the presence of an intrinsic molecular program within the growth cone for response generation. The intrinsic molecular program proposed by Shewan et al supports the findings of Harris et al on growth cones’ ability to navigate through the optic tectum in vivo even when separated from their cell bodies [45].
3.4 Photoconvertible protein recovery to investigate local mRNA translation
Campbell and Holt, using the aforementioned chemotropic assay, demonstrated for the first time that the attractive and repulsive responses of cultured Xenopus retinal growth cones to netrin-1 and Sema3A are abolished in the growth cone upon inhibition of local protein synthesis with anisomycin and cyclohexamide [46], suggesting a translation-dependent chemotropic response in growth cones. The role of translation also explains the reduction in immunoreactivity to DCC in cultures of old axons compared to young axons concomitant with reduced attraction response to netrin-1 that Shewan et al observed [41].
To further investigate the dynamics of protein translation in response to gradients of guidance signals, a photoconvertible protein called Kaede can be linked to a protein of interest [46]. Neuronal cultures can be established from Xenopus embryos injected with mRNA for this construct. The initial green fluorescence of Kaede can then be converted into red light upon UV irradiation. With de novo protein synthesis of the injected mRNA, green signal is recovered, allowing for an assay that can help determine the rate at which the protein of interest is translated under certain conditions [47]. Using this technique, Leung et al showed that Xenopus retinal growth cones introduced to gradients of netrin-1 initiate a recovery of green signal near the netrin-1 applied site when Kaede is linked to beta-actin, implying netrin-1-induced asymmetric beta-actin translation. This recovery occurs in isolated growth cones as well, suggesting an increase in translation controlled by local machinery [48]. The advent of photoconvertible molecules shows promise for furthering our understanding of protein turnover in cells, especially in the area of the tightly regulated local translation of the growth cone.
4. Xenopus laevis spinal neurons to study axon guidance in vivo and in vitro
Another well-studied neuronal system in regard to axon guidance is that of Xenopus spinal neurons. Previously described techniques used for manipulation of gene expression in RGC neurons are applicable to spinal neurons as well [49]. Xenopus spinal neurons have been central to the examination of the interaction between extracellular guidance cues [50], [51], their receptors [51], [52] and cytoplasmic secondary signals [53]-[55] in growth cone turning events. Axon guidance mechanisms that operate during development can be studied in vitro with cultured spinal neurons prepared at the time (stage 20) that the neural tube closes and axon outgrowth just begins. Isolation or manipulation of neurons beyond this stage can be used to study the dynamics of axon regeneration [56], [57]. In this section, we explore some of the ways in which Xenopus spinal neuron techniques have been used to help develop a further understanding of axon guidance.
4.1 Modulation of intracellular signals in spinal neuron growth cones
Asymmetric application of molecular gradients to cultured Xenopus spinal growth cones and examination of subsequent growth cone turning response elucidate the link between the extracellular cues cytoplasmic signals in growth cone turning events [42] (Figure 5). As well as testing the impact of a single type of molecule on growth cone turning, homologous and heterologous modulations can be tested by bath applying molecules and simultaneously administering them in gradients. This approach allows for characterization of adaptation phenomena in growth cones that undergo consecutive desensitization and re-sensitization cycles, such as in response to gradients of netrin-1 when netrin-1 is also bath applied in culture. This adaptation effect can also be observed heterologously between two guidance cues that share common cytosolic transduction pathways, such as with netrin-1 and BDNF [50].
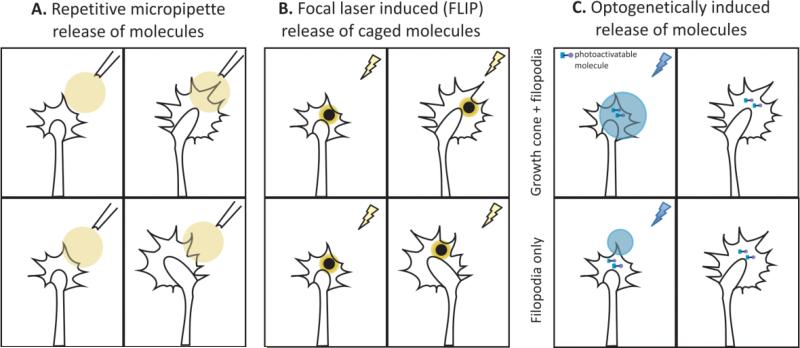
Figure 5. Assessing the response of cultured growth cones to guidance signals and secondary messengers.
(A) Repetitive pulse application method to assess growth cone response to chemical gradients released via micropipette positioned 100 microns away from the center of the growth cone at an angle of 45°. (B-C) Growth cone response to asymmetrical alterations of intracellular secondary molecules Ca++ and cAMP. (B) Focal laser induced photolysis of caged Ca++ loaded (NP-EGTA) growth cones [60]. (C) Optogenetically generated cAMP transients in growth cone expressing photoactivated adenyl cyclase (PAC) [55].
In addition to guidance signals, secondary messengers like cAMP, cGMP or Ca++ and their inhibitors, can also be applied in gradients and/or via bath application. Studies have identified that when cAMP is uniformly applied to Xenopus spinal neuron cultures, the attractant turning response toward BDNF [53] and netrin-1 [54] was inhibited and instead induced repulsive turning. Similarly, a repulsive response to Sema3D was found to be converted to attraction when a cGMP agonist or cAMP antagonist was applied to the culture [55].
Moreover, interaction between receptors and their associated guidance signals can be tested by bath applying antibodies that occupy receptors and interfere with signal transduction. When an antibody against the extracellular domain of DCC, a netrin-1 receptor, was introduced to the Xenopus spinal neuron cultures, netrin-1-induced attractive turning was abolished [54]. Alternatively, spinal neurons expressing wild-type or chimeric receptors isolated from Xenopus embryos microinjected with mRNA encoding receptors can be tested for receptor engagements. Using this approach, Hong et al identified that interaction between the cytoplasmic domains of the UNC-5 and DCC receptors is required to generate a repulsive response to netrin-1 gradients [52]. Interactions between receptors of opposing signals, like netrin-1 and slit, have also been reported in Xenopus spinal neurons, underlying the hierarchical response to attractive and repellent signals [53] during midline crossing.
Techniques used in vitro to investigate signal transduction mechanisms within the growth cone can be adapted to be used in vivo, as well. Bath application of guidance molecules, pharmacological agents, antibodies, or fluorescent dyes can be taken up by an exposed Xenopus spinal cord. Moreover, asymmetric elevation or inhibition of molecules can be achieved by photoactivatable caged molecules or optogenetic approaches [58] (Figure 5B-C). For example, Gomez and Spitzer used these approaches to investigate Ca++ dynamics, which have been shown to be responsible for growth cone turning events downstream of neurotransmitters and guidance signals [59] – [62]. They exposed the anterior half of the Xenopus spinal cord and labeled neurons with the fluorescent calcium indicator Fluo-3 AM. Subsequently, exposing the posterior half of the spinal cord enabled them to trace labeled growth cones and measure the frequency of endogenous Ca++ transients as axons elongated into the unlabeled region. Additionally, by loading neurons with photoactivatable caged diazo-2 AM or NP-EGTA AM, the group performed targeted reduction or elevation of Ca++ levels, respectively. Their results demonstrated that Ca++ transients are regulated in a region-specific manner, and that higher frequencies of Ca++ transients reduce the axon elongation rate and increase growth cone stalling [63].
4.2 Extracellular matrix and adhesion dynamics in Xenopus laevis spinal neuron guidance in vivo
In addition to guidance signals, growth cone pathfinding is influenced by components of the ECM [64]. Xenopus spinal axons travel through environments presenting different ECM makeups, and engage with them via integrin receptors at adhesion sites [64]. Adhesions formed between the growth cone and the ECM are referred to as “point contacts” [65], [66]. Formation, maintenance, and turnover of these contact sites determined by intracellular signaling events are important determinants of growth cone morphology and motility, and hence pathfinding behavior [67], [68].
Examination of these sites mostly relies on immunofluorescence labeling of cell adhesion signaling proteins in fixed and living growth cones, followed by high resolution microscopy imaging [69]. Labeling or modulation of protein levels can be achieved by microinjecting mRNA of fluorescently tagged phosphotyrosine reporter constructs [69], adhesion markers, or their corresponding MOs. Antibodies are also available for targeting Xenopus scaffolding proteins involved in focal adhesion [69], allowing for assessment of their spatial distributions within lamellipodia or filopodia in fixed growth cones [70] via immunocytochemistry analysis. Live cell imaging is also possible: this permits monitoring of the changes in protein localization, modification, or turnover as the growth cone moves along different ECM substrates and makes navigational decisions in response to cues. Examples of such investigations come from the work of the Gomez Lab; Robles and Gomez characterized focal adhesion kinase (FAK) activity in Xenopus spinal growth cones and showed that tyrosine-phosphorylated protein accumulation depends on the formation of integrin-dependent adhesive contacts with the ECM, which ultimately impact growth cone motility and outgrowth [71]. Another work by the Gomez group examines adhesion dynamics upon guidance cue stimulation. They show that BDNF and netrin-1 gradients increase phospho-tyrosine-positive filopodial tips through activation of FAK, and hence Src, coinciding with enhanced filopodial motility and suggesting an asymmetric distribution of motile filopodia in growth cone steering [70], [72].
4.3 Xenopus laevis growth cones to assess cytoskeletal rearrangements during guidance
The above-mentioned Xenopus studies are examples of how interactions with guidance signals and ECM components give instructions through signaling molecules to affect growth cone protrusion and steering during pathfinding. Downstream of these, the reorganization of the cytoskeletal polymers, actin filaments and microtubules modulate growth cone steering [73], [74]. Although neurofilaments are less well-studied, this third member of the axonal cytoskeletal polymer family also plays a crucial role, and Xenopus has been a particularly useful model to examine the role of neurofilaments as a modulator of axon growth during development and regeneration [75] - [78].
Examination of the growth cone in detail is difficult in an intact tissue, as it travels in a 3D environment, and this makes it challenging to track the moving growth cone without losing focus of its cytoskeletal features. Although recently developed imaging approaches have begun to make in vivo growth cone cytoskeleton examination possible [79], most of our knowledge on the structural organization of the growth cone comes from in vitro studies [80], [81]. In this regard, with their large growth cones, Xenopus laevis neurons provide an ideal system to study cytoskeletal dynamics. With the advance of protein labeling and high-resolution imaging techniques, it is possible to track fluorescently tagged proteins to gain insights into the motility dynamics of the growth cone during axon growth and guidance.
The leading edge of the growth cone, where initial contacts with the adhesive substrate are made, is assembled by a filamentous actin network. Dynamic remodeling of the actin network in this region allows the growth cone to explore and interact with the molecules in the extracellular milieu [82]. Tracing both actin and its interacting partners in living growth cones allows for a better understanding of the mechanism by which actin is remodeled downstream of guidance signals [83], [84]. Actin labeling is mostly achieved by expressing fluorescently tagged actin or actin binding peptide markers such as F-lifeact- GFP [85] or utrophin [86], which bind along the F-actin lattice, or by loading growth cone cultures with cell-permeable kabiramide C conjugated to tetramethylrhodamine (TMR-KabC) [87], which binds the growing ends of F-actin and allows for visualization of actin dynamics such as growth, depolymerization, or retrograde flow. Alternatively, immunolabeling against phalloidin, an F-actin binding protein, can be used to monitor actin localization in fixed growth cones [79].
Expressing rhodamine-labeled tubulin emerged as a technique to track singular microtubules in cultured living Xenopus growth cones [85]. Tracking labeled microtubules revealed, for example, that spatial stabilization of microtubules via FLIP-released taxol can mediate growth cone turning [88]. Thus, mechanisms that promote microtubule growth have gained further attention. Microtubule binding proteins, particularly plus-end tracking proteins (+TIPs), have become a focus of study as they directly regulate microtubule plus-end dynamics [89], and tracking and quantitative analysis of fluorescently-tagged +TIPs in cultured Xenopus growth cones has provided new insights into how microtubule dynamics are regulated in the growth cone [90], [91]. Lee et al unveiled a mechanism for the +TIP CLASP and proposed that growth cones exposed to asymmetrically distributed repellent signal Slit triggers spatially restricted Abl kinase activation in the growth cone, which then impedes microtubule growth by targeting CLASP phosphorylation [92], [93]. In addition to CLASP, multiple other +TIPs have been shown to be potentially involved in the regulation of microtubule dynamics during axon outgrowth and steering events [94].
Furthermore, acquisition of high-resolution live images of fluorescently-labeled known +TIPs, such as EB1, in cultured Xenopus growth cones can be used to measure parameters of microtubule growth dynamics ex vivo [95]. There is open-source software, such as plusTipTracker, available for automated detection and analysis of these tagged +TIPs [96], [97]. Additionally, tools such as quantitative fluorescent speckle microscopy (QFSM) or kymography are also commonly used to extract quantitative information regarding cytoskeletal dynamics from high-resolution microscopy images of growth cones [90].
5. Conclusions
Since the first speculations on the phenomenon of axon guidance, studies using the Xenopus laevis nervous system have made significant contributions toward our understanding of this crucial developmental orchestration. Developing Xenopus neurons have been a key subject from the characterization of nerve patterns of the visual system to the identification and creation of novel mechanisms and techniques.
Although many of the techniques described in this review can be applied to higher vertebrate models such as mouse [98]–[101], rat [102], or chick [103], [104], the affordability and practicality of Xenopus, coupled with its large and easy-to-culture growth cones, makes it a highly attractive model system for deciphering the molecular mechanisms of axon guidance. Its phylogenetic position also makes it a preferable organism in comparison to other experimentally-utilized lower vertebrates or invertebrates.
In conclusion, Xenopus laevis, with existing and newly emerging techniques, will persist as an excellent model to study elusive aspects of the axon guidance mechanism both in vivo and in vitro, from network organization, to the single neuron or growth cone, and all the way down to the single molecule level.
Highlights.
X. laevis is an affordable and practical vertebrate model for in vitro and in vivo studies of axon guidance.
Gene manipulation techniques are well established and can be applied at any developmental stage.
Retinal and spinal axonal tracts are accessible and can be live imaged for guidance decisions in vivo.
Neurons from X. laevis retina and spinal cord are easy to isolate, maintain and manipulate in vitro.
X. laevis growth cones are large enough for high-resolution live imaging.
Acknowledgments
We thank members of the Lowery Lab, particularly Leslie Carandang and Erin Rutherford, for helpful discussions and suggestions. LAL is funded by the National Institutes of Health R00 MH095768.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cajal S, Ramón y. A quelle epoque apparaissent les expansions des cellules nerveuses de la moëlle épinière du poulet? Anat. Anz. 1890;5(Nr. 21 and 22):609–613. 631–639. [Google Scholar]
- 2.Cajal S, Ramón y. La rétine des vertébrés. La Cellule. 1892;9:121–133. [Google Scholar]
- 3.Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 1910;9:787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- 4.Sperry RW. Optic Nerve Regeneration with Return of Vision in Anurans. Journal of Neurophysiology. 1944;7:57–69. [Google Scholar]
- 5.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser SE, Hunt RK. Retinotectal Specifity: Models and Experiments in Search of a Mapping Function. Ann. Rev. Neurosci. 1980;3:319–352. doi: 10.1146/annurev.ne.03.030180.001535. [DOI] [PubMed] [Google Scholar]
- 7.Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, et al. The Genome of the Western Clawed Frog Xenopus tropicalis. Science. 2010;328(5978):633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. North Holland Publishing Co; Amsterdam, The Netherlands: 1967. [Google Scholar]
- 9.Moody S. Fates of the Blastomeres of the 16-Cell Stage Xenopus Embryo. Developmental Biology. 1987;578:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- 10.Moody S. Fates of the Blastomeres of the 32-Cell-Stage Xenopus Embryo. Developmental Biology. 1987:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 11.Lowery LA, Faris AER, Stout A, Van Vactor D. Neural Explant Cultures from Xenopus laevis. J. Vis. Exp. 2012;(68):2–5. doi: 10.3791/4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eide FF, Eisenberg SR, Sanders T. a. Electroporation-mediated gene transfer in free-swimming embryonic Xenopus laevis. FEBS Lett. 2000;486(1):29–32. doi: 10.1016/s0014-5793(00)02124-4. [DOI] [PubMed] [Google Scholar]
- 13.Bestman JE, Ewald RC, Chiu S-L, Cline HT. In vivo single-cell electroporation for transfer of DNA and macromolecules. Nat. Protoc. 2006;1(3):1267–1272. doi: 10.1038/nprot.2006.186. [DOI] [PubMed] [Google Scholar]
- 14.Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K. Improved mRNA electroporation method for Xenopus neurula embryos. Genesis. 2002;33(2):81–85. doi: 10.1002/gene.10094. [DOI] [PubMed] [Google Scholar]
- 15.Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo -from single cells to the entire brain. Differentiation. 2002;70(4–5):148–154. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- 16.Eisen JS, Smith JC. Controlling morpholino experiments: don't stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- 17.Blum M, De Robertis EM, Wallingford JB, Niehrs C. Morpholinos: Antisense and Sensibility. Developmental Cell Perspective. 2015;35:145–149. doi: 10.1016/j.devcel.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 2000;222(1):124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K. -i. T., Isoyama Y, Kashiwagi K, Sakuma T, Ochiai H, Sakamoto N, Furuno N, Kashiwagi a., Yamamoto T. High efficiency TALENs enable F0 functional analysis by targeted gene disruption in Xenopus laevis embryos. Biol. Open. 2013;2(5):448–452. doi: 10.1242/bio.20133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakane Y, Sakuma T, Kashiwagi K, Kashiwagi A, Yamamoto T, Suzuki KIT. Targeted mutagenesis of multiple and paralogous genes in Xenopus laevis using two pairs of transcription activator-like effector nucleases. Dev. Growth Differ. 2014;56(1):108–114. doi: 10.1111/dgd.12105. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima K, Yaoita Y. Highly efficient gene knockout by injection of TALEN mRNAs into oocytes and host transfer in Xenopus laevis. Biol. Open. 2015;4(2):180–185. doi: 10.1242/bio.201410009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt SM, Gull M, Brändli AW. Engineering Xenopus embryos for phenotypic drug discovery screening. Adv. Drug Deliv. Rev. 2014;69–70:225–246. doi: 10.1016/j.addr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Shi Z, Cui Y, Guo X, Shi Y-B, Chen Y. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 2015;5(1):1–5. doi: 10.1186/s13578-015-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falk J, Drinjakovic J, Leung KM, Dwivedy A, Regan AG, Piper M, Holt CE. Electroporation of cDNA/Morpholinos to targeted areas of embryonic CNS in Xenopus. BMC Dev. Biol. 2007;7:107. doi: 10.1186/1471-213X-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaze RM. Regeneration of the optic nerve in Xenopus laevis. Q J Exp Physiol Cogn Med Sci. 1959;44(3):290–308. doi: 10.1113/expphysiol.1959.sp001402. [DOI] [PubMed] [Google Scholar]
- 26.Mann F, Harris WA, Holt CE. New views on retinal axon development : a navigation guide. Int J Dev Biol. 2013;48(0):957–964. doi: 10.1387/ijdb.041899fm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt E. Does Timing Of Axon Outgrowth Influence Topography in Xenopus. J. Neurosci. 1984;4(4):1130–1152. doi: 10.1523/JNEUROSCI.04-04-01130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt CE. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J. Neurosci. 1989;9(9):3123–3145. doi: 10.1523/JNEUROSCI.09-09-03123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu GY, Cline HT. Time Lapse in vivo Imaging of the Morphological Development of Xenopus Optci Tectal Interneurons. The Journal of Comparative Neurology. 2003;459:392–406. doi: 10.1002/cne.10618. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010;70(5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat. Neurosci. 2001;4(11):1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 32.Shirkey NJ, Manitt C, Zuniga L, Cohen-Cory S. Dynamic responses of Xenopus retinal ganglion cell axon growth cones to netrin-1 as they innervate their in vivo target. Dev. Neurobiol. 2012;72(4):628–648. doi: 10.1002/dneu.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos : a time-lapse video study of single fibres in vivo. Development. 1987;133:123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 34.Chien CB, Rosenthal DE, Harris W. a, Holt CE. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11(2):237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- 35.Kater SB, Rehder V. The sensory-motor role of growth cone filopodia. Curr. Opin. Neurobiol. 1995;5(1):68–74. doi: 10.1016/0959-4388(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 36.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J. Neurosci. 1996;16(3):1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134(11):2137–2146. doi: 10.1242/dev.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung LC, Urban V, Baudet M, Dwivedy A, Timothy G, Lee AC, Harris WA, Holt CE. Coupling of NF-protocadherin signalling to axon guidance by cue-induced translation. Nature Neuroscience. 2013;16(2):166–173. doi: 10.1038/nn.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bestman JE, Cline HT. Morpholino studies in Xenopus brain development. Methods Mol Biol. 2014;1082:155–171. doi: 10.1007/978-1-62703-655-9_11. [DOI] [PubMed] [Google Scholar]
- 40.Bestman JE, Huang LC, Lee-Osbourne J, Cheung P, Cline HT. An in vivo screen to identify candidate neurogenic genes in the developing Xenopus visual system. Dev. Biol. 2015 doi: 10.1016/j.ydbio.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung L, Holt CE. Imaging axon pathfinding in Xenopus in vivo. Cold Spring Harb. Protoc. 2012;2012(9):984–91. doi: 10.1101/pdb.prot070003. [DOI] [PubMed] [Google Scholar]
- 42.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12(4):1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de la Torre JR, Höpker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19(6):1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 44.Shewan D, Dwivedy a, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat. Neurosci. 2002;5(10):955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- 45.Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101(1):123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 46.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32(6):1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 47.Leung K-M, Holt CE. Live visualization of protein synthesis in axonal growth cones by microinjection of photoconvertible Kaede into Xenopus embryos. Nat. Protoc. 2008 Jan.3(8):1318–27. doi: 10.1038/nprot.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung K-M, van Horck FPG, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006;9(10):1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez TM, Harrigan D, Henley J, Robles E. Working with Xenopus spinal neurons in live cell culture. Methods Cell Biol. 2003;71:129–156. doi: 10.1016/s0091-679x(03)01008-2. [DOI] [PubMed] [Google Scholar]
- 50.Ming G, Wong ST, Henley J, Yuan X, Songk H, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 51.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291(5510):1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 52.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97(7):927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 53.Song H, Ming G, Poo M, M. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 54.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19(6):1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 55.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281(5382):1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 56.Gibbs KM, Chittur SV, Szaro BG. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. European Journal of Neuriscience. 2011;33(1):9–25. doi: 10.1111/j.1460-9568.2010.07477.x. [DOI] [PubMed] [Google Scholar]
- 57.Gibbs KM, Szaro BG. Regeneration of descending projections in tadpole spinal cord demonstrated by retrograde Xenopus laevis double labeling. Brain Research. 2006;1088(1):68–72. doi: 10.1016/j.brainres.2006.02.126. [DOI] [PubMed] [Google Scholar]
- 58.Nicol X, Hong KP, Spitzer NC. Spatial and temporal second messenger codes for growth cone turning. Proc. Natl. Acad. Sci. U. S. A. 2011;108(33):13776–13781. doi: 10.1073/pnas.1100247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14(6):320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403(6765):93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 61.Ming GL, Henley J, Tessier-Lavigne M, Song HJ, Poo MM. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29(2):441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 62.Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: New dynamics to earlier perspectives. J. Neurobiol. 2000;44(2):174–183. [PubMed] [Google Scholar]
- 63.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 64.Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ecm interactions. Dev. Neurobiol. 2011;71(11):901–923. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez TM, Roche FK, Letourneau PC. Chick Sensory Neuronal Growth Cones Distinguish Fibronectin from Laminin by Making Substratum Contacts that Resemble Focal Contacts. J Neurobiol. 1996;29(1):18–34. doi: 10.1002/(SICI)1097-4695(199601)29:1<18::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 66.Renaudin A, Lehmann M, Girault J-A, McKerracher L. Organization of Point Contacts in Neuronal Growth Cones. J Neuroscience Resaerch. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 67.Woo S, Gomez TM. Rac1 and RhoA Promote Neurite Outgrowth through Formation and Stabilization of Growth Cone Point Contacts. J Neurosci. 2006;26(5):1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woo S, Rowan DJ, Gomez TM. Retinotopic Mapping Requires Focal Adhesion Kinase-Mediated Regulation of Growth Cone Adhesion. J Neurosci. 2009;29(44):13981–13991. doi: 10.1523/JNEUROSCI.4028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santiago-medina M, Myers JP, Gomez TM. Imaging Adhesion and Signaling Dynamics in Xenopus laevis Growth Cones. Dev. Biol. 2011:585–599. doi: 10.1002/dneu.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robles E, Woo S, Gomez TM. Src-dependent tyrosine phosphorylation at the tips of growth cone filopodia promotes extension. J. Neurosci. 2005;25(33):7669–7681. doi: 10.1523/JNEUROSCI.2680-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat. Neurosci. 2006 Oct.9(10):1274–83. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 72.Myers JP, Gomez TM. Focal Adhesion Kinase Promotes Integrin Adhesion Dynamics Necessary for Chemotropic Turning of Nerve Growth Cones. J. Neurosci. 2011;31(38):13585–13595. doi: 10.1523/JNEUROSCI.2381-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suter DM, Forscher P. Substrate – Cytoskeletal Coupling as a Mechanism for the Regulation of Growth Cone Motility and Guidance. J Neurobiol. 2000;44:97–113. [PubMed] [Google Scholar]
- 74.Lowery LA, Vactor DV. The trip of the tip: understanding the growth. Nature Reviews. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin W, Szaro BG. Neurofilaments help maintain normal morphologies and support elongation of neurites in Xenopus laevis cultured embryonic spinal cord neurons. J Neurosci. 1995;15(12):8331–8344. doi: 10.1523/JNEUROSCI.15-12-08331.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker KL, Yoo HK, Undamatla J, Szaro BG. Loss of Neurofilaments Alters Axonal Growth Dynamics. J Neurosci. 2001;21(24):9655–9666. doi: 10.1523/JNEUROSCI.21-24-09655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y, Szaro BG. The Optic Tract and Tectal Ablation Influence the Composition Neurofilaments in Regenerating Optic Axons of Xenopus laevis. J Neurosci. 1995;15(6):4229–4240. doi: 10.1523/JNEUROSCI.15-06-04629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ananthakrishnan L, Szaro BG. Transcriptional and translational dynamics of light neurofilament subunit RNAs during Xenopus laevis optic nerve regeneration. Brain Research. 2009;1250:27–40. doi: 10.1016/j.brainres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Kleele T, Marinković P, Williams PR, Stern S, Weigand EE, Engerer P, Naumann R, Hartmann J, Karl RM, Bradke F, Bishop D, Herms J, Konnerth A, Kerschensteiner M, Godinho L, Misgeld T. An assay to image neuronal microtubule dynamics in mice. Nat. Commun. 2014 Jan.5:4827. doi: 10.1038/ncomms5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 2002;158(1):139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka EM, Kirschner MW. Microtubule Behavior in the Growth Cones of Living Neurons during Axon Elongation. 1991;115(2) doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez TM, Letourneau PC. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014;129(2):221–234. doi: 10.1111/jnc.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dent EW, Gertler FB. Cytoskeletal Dynamics and Transport in Growth Cone Motility and Axon Guidance. Neuron. 2003;40(2):209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 84.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J. Cell Sci. 2009;122(Pt 20):3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak T. a, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat. Methods. 2008;5(7):605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burkel BM, Bement WM, Labs FH. HHS Public Access. Cell Motil Cytoskelet. 2007;64(11):822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Petchprayoon C, Suwanborirux K, Tanaka J, Yan Sakata T, Marriott G. Fluorescent kabiramides: New probes to quantify actin in vitro and in vivo. Bioconjug Chem. 2005;16:1382–1389. doi: 10.1021/bc050006j. [DOI] [PubMed] [Google Scholar]
- 88.Buck KB, Zheng JQ. Growth Cone Turning Induced by Direct Local Modification of Microtubule Dynamics. 2002;22(21):9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 2008;9(4):309–22. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 90.Lowery LA, Stout A, Faris AE, Ding L, Baird MA, Davidson MW, Danuser G, Vactor DV. Growth cone-specific functions of XMAP215 in restricting microtubule dynamics and promoting axonal outgrowth. Neural Dev. 2013;8(1) doi: 10.1186/1749-8104-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nwagbara BU, Faris AE, Bearce EA, Erdogan B, Ebbert PT, Evans MF, Rutherford EL, Enzenbacher TB, Lowery LA. TACC3 is a microtubule plus end–tracking protein that promotes axon elongation and also regulates microtubule plus end dynamics in multiple. MBoC. 2014;25 doi: 10.1091/mbc.E14-06-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee H, Engel U, Rusch J, Scherrer S, Sheard K, Van Vactor D. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 2004;42(6):913–26. doi: 10.1016/j.neuron.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 93.Engel U, Zhan Y, Long JB, Boyle SN, Ballif B. a, Dorey K, Gygi SP, Koleske AJ, Vanvactor D. Abelson phosphorylation of CLASP2 modulates its association with microtubules and actin. Cytoskeleton. 2014;71(3):195–209. doi: 10.1002/cm.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bearce EA, Erdogan B, Lowery LA. TIPsy tour guides: how microtubule plus-end tracking proteins (+TIPs) facilitate axon guidance. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 2003;23(7):2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Applegate KT, Besson S, Matov A, Bagonis MH, Jaqaman K, Danuser G. PlusTipTracker: Quantitative image analysis software for the measurement of microtubule dynamics. J. Struct. Biol. 2011;176(2):168–184. doi: 10.1016/j.jsb.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stout A, D'Amico S, Enzenbacher T, Ebbert P, Lowery LA. Using plusTipTracker software to measure microtubule dynamics in Xenopus laevis growth cones. J. Vis. Exp. 2008;7(91) doi: 10.3791/52138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmidt H, Rathjen FG. DiI-Labeling of DRG Neurons to Study Axonal Branching in a Whole Mount Preparation of Mouse Embryonic Spinal Cord. J. Vis. Exp. 2011;(58):1–6. doi: 10.3791/3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-Induced Axon Growth Is Mediated by Localized Inactivation of GSK-3β and Functions of the Microtubule Plus End Binding Protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 100.Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA. Retinal Ganglion Cell Axon Guidance in the Mouse Optic Chiasm: Expression and Function of Robos and Slits. J. Neurosci. 2000;20(13):4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petros TJ, Rebsam A, Mason CA. In utero and ex vivo Electroporation for Gene Expression in Mouse Retinal Ganglion Cells. J. Vis. Exp. 2009;(31) doi: 10.3791/1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen-Ba-Charvet KT, Brose K, Marillat V, Sotelo C, Tessier-Lavigne M, Chédotal A. Sensory Axon Response to Substrate-Bound Slit2 Is Modulated by Laminin and Cyclic GMP. Mol. Cell. Neurosci. 2001;17:1048–1058. doi: 10.1006/mcne.2001.0994. [DOI] [PubMed] [Google Scholar]
- 103.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 Is Required for Commissural Axon Guidance in the Developing Vertebrate Nervous System. Cell. 1996;87(6):1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 104.Matsunaga E, Nakamura H, Chédotal A. Repulsive Guidance Molecule Plays Multiple Roles in Neuronal Differentiation and Axon Guidance. J. Neurosci. 2006;26(22):6082–6088. doi: 10.1523/JNEUROSCI.4556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]