Abstract
A phase 1 study with carfilzomib and vorinostat was conducted in 20 B-cell lymphoma patients. Vorinostat was given orally twice daily on days 1, 2, 3, 8, 9, 10, 15, 16, and 17 followed by carfilzomib (given as a 30 min infusion) on days 1, 2, 8, 9, 15, and 16. A treatment cycle was 28 days. Dose escalation initially followed a standard 3+3 design, but adapted a more conservative accrual rule following dose de-escalation. The maximum tolerated dose was 20 mg/m2 carfilzomib and 100 mg vorinostat (twice daily). The dose-limiting toxicities were grade 3 pneumonitis, hyponatremia, and febrile neutropenia. One patient had a partial response and 2 patients had stable disease. Correlative studies showed a decrease in NF-κB activation and an increase in Bim levels in some patients, but these changes did not correlate with clinical response.
Keywords: Carfilzomib, lymphoma, phase 1 clinical trial, vorinostat
INTRODUCTION
Non-Hodgkin lymphomas (NHLs) comprise a heterogeneous group of hematopoietic cancers, with about 90% arising from B lymphocytes [1]. Diffuse large B-cell lymphoma (DLBCL; 22%) and follicular lymphoma (12%) represent the most common types of NHL [2]. Chemotherapy, either with or without radiation therapy, is the primary therapy for two-thirds of NHL patients [2]. Although primary therapy can often be curative, patients with relapsed or refractory NHL often die of their disease despite the therapies currently available, thus indicating a need for novel approaches [3].
The molecular characterization of NHL has paved the way for targeted therapy in this disease [1,3-6]. The proteasome and histone deacetylases (HDAC) represent two promising targets in NHL. The proteasome, a multi-subunit protease complex that is responsible for protein degradation, regulates the disposition of proteins involved in apoptosis and cell cycle progression. Bortezomib, the first proteasome inhibitor approved by the U.S. Food and Drug Administration (FDA), is currently approved to treat patients with mantle cell lymphoma (MCL) or multiple myeloma. Bortezomib is also approved to retreat multiple myeloma patients who had previously responded to treatment with this agent and who have relapsed at least 6 months after completing prior bortezomib treatment. Carfilzomib is a second-generation proteasome inhibitor that, in contrast to bortezomib, acts in an irreversible manner. It is FDA-approved for multiple myeloma patients who have received at least 2 prior therapies. In vitro studies in multiple myeloma cell lines demonstrated that in comparison to bortezomib, carfilzomib is more active with respect to inhibition of proliferation, increased phospho-JNK levels, and increased caspase activity [7]. Carfilzomib was also active against bortezomib-resistant cell lines and samples obtained from NHL patients with bortezomib-resistant disease [7]. More recently, in vitro treatment with carfilzomib has been shown to induce growth inhibition and apoptosis in both MCL cell lines and primary cells [8]. Finally, in vivo studies involving severe combined immune deficiency (SCID) mice injected with MCL cell lines, or humanized-SCID mice injected with primary MCL cells, demonstrated that treatment with carfilzomib delayed tumor growth and prolonged survival [8].
HDACs play a central role in regulating both transcription-dependent and transcription-independent cellular functions [9]. HDACs function by removing an acetyl group from both histone and non-histone proteins. The deacetylation of histones is associated with a condensed chromatin structure and a decrease in DNA transcription. Because HDAC substrates are involved in many cellular pathways that regulate proliferation and cell death, the antitumor effects of HDAC inhibitors (HDACIs) may be mediated through multiple mechanisms. Vorinostat, an HDACI, has been approved by the FDA for the treatment of cutaneous T-cell lymphoma in patients with progressive, persistent, or relapsed disease on or following 2 systemic therapies. Vorinostat, as a single agent or in combination therapy, continues to be the focus of ongoing clinical trials in B- and T-cell lymphomas [4,10,11].
Previous studies have described synergism between proteasome inhibitors such as bortezomib and HDACIs in malignant hematopoietic cells such as multiple myeloma and NHL through a variety of mechanisms, including disruption of NF-κB signaling and aggresome function [12-20]. Moreover, pre-clinical studies have described synergism between carfilzomib and vorinostat in multiple myeloma cells [19], and more recently in DLBCL cells [17]. Coadministration of individually sublethal doses of the 2 agents to DLBCL cell lines resulted in increased mitochondrial injury, caspase activation, and apoptosis. Synergistic induction of apoptosis was also observed in primary DLBCL cells and bortezomib-resistant cell lines. Studies involving DLBCL xenografts demonstrated that carfilzomib and vorinostat synergized in vivo, reflected by a reduction in tumor growth and the induction of apoptosis.
Based on the encouraging results of these pre-clinical studies, we conducted a phase 1 clinical trial to determine the maximum tolerated dose (MTD) of the combination of carfilzomib and vorinostat in patients with relapsed or refractory NHL. Correlative studies on patient samples were also conducted to determine the feasibility of assessing pharmacodynamic measures of biochemical and apoptotic activity.
MATERIALS AND METHODS
Drug Supply
Carfilzomib (PR-171, Kyprolis; Onyx Pharmaceuticals, Inc., South San Francisco, CA) and vorinostat (SAHA, ZOLINZA; Merck & Co., Inc., Whitehouse Station, NJ) were obtained from the manufacturers.
Patient Eligibility
Patients had to have a histologically confirmed B-cell lymphoma (excluding chronic lymphocytic leukemia) that was relapsed or refractory and for which no other potentially curative therapy was available.
Additional inclusion criteria included the following: at least 18 years of age, Eastern Cooperative Oncology Group performance status score ≤ 2, absolute lymphocyte count < 5,000/μL (if small lymphocytic lymphoma), absolute neutrophil count ≥ 1,000/μL, platelet count of ≥ 75,000/μL, creatinine ≤ 1.5x the upper limit of normal (ULN) or calculated creatinine clearance of > 40 mL/min (Cockcroft and Gault formula was used), AST and ALT ≤ 2.5x ULN, bilirubin ≤ 2.0 mg/mL, serum potassium and serum magnesium within normal institutional limits, PT < 1.5x ULN, and PTT < 1.2x ULN. Patients were excluded based upon brain or leptomeningeal involvement, prior HDACI use, inability to take oral medication, uncontrolled hypertension, chemotherapy or radiotherapy within 3 weeks prior to entering the study, or prior allogeneic stem cell transplant (SCT).
Treatment Plan
This study was designed as a multi-institutional (University of Rochester and Virginia Commonwealth University), phase 1, non-randomized, dose-escalation study to determine the MTD for the combination of carfilzomib and vorinostat. The combination dose-escalation schedule used in this trial closely approximated the one employed in the pre-clinical studies upon which the trial was based [17]. The vorinostat dosing also approximates that used in other trials [21-23] in which this agent was administered 3 days/week, and in which a dose of 300 mg bid was found to be tolerable [22]. Given potential overlapping toxicities, it was felt that a starting vorinostat dose of 200 mg for this combination trial would be safe and appropriate. Based on these considerations, the following dosing schedule was utilized. A treatment cycle was 28 days. Vorinostat (200 mg) was given orally twice daily on days 1, 2, 3, 8, 9, 10, 15, 16, and 17. The first daily dose of vorinostat was given prior to carfilzomib on days 1, 2, 8, 9, 15, and 16. Carfilzomib was given as a 30 min infusion. Carfilzomib was given at a dose of 20 mg/m2 for days 1 and 2 of cycle 1 only, and then escalated to 27 mg/m2 on day 8 of cycle 1 and thereafter. Disease status was assessed every 3 cycles and at the end of treatment. Adverse events were assessed during study participation. Patients were allowed to continue receiving treatment for up to one year or at the discretion of the investigator.
Study Design, Definition of Dose-Limiting Toxicity, and Identification of the MTD
Patients were initially enrolled to dose levels based on a standard 3+3 dose-escalation design, with a dose level expanded to include 6 patients if a dose-limiting toxicity (DLT) was noted. The MTD was defined as the highest dose level at which fewer than 2 of 6 patients experienced a DLT. After completion of dose level −1, the coordinating center DSMB recommended addition of two dose levels (−1a and −1b) intermediate between dose levels −1 and 1. Patients were alternately enrolled to dose levels −1a and −1b so that no more than 2 patients at a given dose level could experience a DLT.
A DLT was defined as: (1) a grade 3 or 4 non-hematological toxicity occurring during the first cycle of treatment (except nausea or vomiting responding to symptomatic therapy or fatigue responding to maximal management); (2) any grade 4 hematologic toxicity occurring during the first cycle of treatment except neutropenia or thrombocytopenia lasting less than 5 days (febrile neutropenia and grade 3 or 4 thrombocytopenia associated with bleeding were considered DLTs); and (3) failure of ANC to recover to ≥ 1,000/μL or platelets to recover to ≥ 50,000/μL within 14 days of the last treatment during cycle 1.
Toxicity Evaluation
Adverse events were characterized in terms of attribution and severity and are reported according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Response Evaluation
Patients were evaluated using the NCI-sponsored Working Group Lymphoma Response Criteria [24].
Quantitative Immunofluorescence
For patients with easily accessible tissue, core biopsies were obtained before (pre) and 48 ± 6 hours after (post) the first dose of carfilzomib. The tissue was processed according to institutional standards for paraffin embedded tissue blocks, sections, and slides. Quantitative immunofluorescence was performed as detailed in the Supplementary Information.
IL-10 and TNF ELISA
Optional plasma samples from consenting patients were obtained at baseline (C1D1), 48 ± 6 hours after the first dose of carfilzomib (C1D3), prior to treatment on day 8 of cycle 1 (C1D8), and at the end of treatment (EoT). The samples were then stored at −80°C until assayed. IL-10 was detected using a human IL-10 ELISA set (BD Pharmingen) according to the manufacturer’s protocol. TNF was detected using a Human TNF ELISA set (BD Pharmingen) according to the manufacturer’s protocol. Samples were measured in triplicate and samples with concentrations approaching the upper limit of detection were diluted 10-fold and reanalyzed.
Statistical Analysis
Patient characteristics, adverse events, DLTs, dose levels, and best clinical responses were summarized by descriptive statistics such as frequency, proportion, mean, median, and/or range. Immunohistochemical analyses, including all biomarkers and IL-10 and TNF ELISA analyses, were exploratory and similarly limited to descriptive statistics.
Human Investigation Studies
The study was conducted under separate FDA Investigational New Drug applications held by each institution and in accordance with assurances filed with and approved by the Department of Health and Human Services. Both the University of Rochester and Virginia Commonwealth University Institutional Review Boards approved the study, and informed consent was obtained from each patient for clinical participation and optional correlative studies.
RESULTS
Patient Characteristics
The characteristics of the 20 patients enrolled between January 2011 and March 2013 are reported in Table I.
Table I.
Patient enrollment and characteristics
| Gender | No. of patients |
| Female | 6 |
| Male | 14 |
| Total | 20 |
| Race | No. of patients |
| Black or African American | 1 |
| White | 19 |
| Ethnicity | No. of patients |
| Hispanic or Latino | 0 |
| Non-Hispanic | 20 |
| Age Range | Years |
| Mean | 64.6 |
| Median | 66.5 |
| Range | 36-79 |
| Age Group | No. of patients |
| 20-29 | 0 |
| 30-39 | 1 |
| 40-49 | 0 |
| 50-59 | 4 |
| 60-69 | 9 |
| 70-79 | 6 |
| 80-89 | 0 |
| Performance Status | No. of patients |
| 0 | 4 |
| 1 | 14 |
| 2 | 2 |
| Diagnosis | No. of patients |
| Diffuse large B-cell lymphoma* | 8 |
| Follicular lymphoma | 2 |
| Mantle cell lymphoma | 10 |
| Prior Treatment | No. of regimens |
| Mean | 4.4 |
| Median | 3.5 |
| Range | 1-13 |
| Prior Treatment | No. of patients |
| Autologous stem cell transplantation | 11 |
| Radiation | 9 |
| Study Treatment | No. of courses initiated |
| Mean | 2.6 |
| Median | 2.5 |
| Range | 1-6 |
Includes 2 patients noted to have transformed lymphoma (Follicular Lymphoma to DLBCL)
Safety and Tolerability
A total of 28 different grade 2 toxicities were reported with the most frequent being anemia (25%), fatigue (20%), and thrombocytopenia (20%). The most common grade 3 and 4 hematologic toxicities included anemia (15%), neutropenia (25%), and leukopenia (15%) (Table II). Grade 3 non-hematologic toxicities were uncommon and no grade 4 non-hematologic toxicities were reported (Table II). Two grade 5 events were reported, neither attributed to study treatment, with one death attributed to disease progression (DLBCL) and one to lung infection (pneumonia).
Table II.
Grade 3 and 4 hematologic and non-hematologic toxicities occurring during any treatment course and deemed possibly, probably, or definitely related to the treatment
| # of events, # of patients (% of patients) | ||
|---|---|---|
| Hematologic toxicities | Grade 3 | Grade 4 |
| Anemia | 3, 3 (15) | 0, 0 (0) |
| Lymphocyte count decreased | 2, 1 (5) | 0, 0 (0) |
| Neutrophil count decreased | 6, 5 (25) | 1, 1 (5) |
| Platelet count decreased | 2, 2 (10) | 0, 0 (0) |
| White blood cell decreased | 5, 3 (15) | 2, 2 (10) |
|
| ||
| # of events, # of patients (% of patients) | ||
| Non-hematologic toxicities | Grade 3 | Grade 4 |
|
| ||
| Dyspnea | 1, 1 (5)* | 0, 0 (0) |
| Febrile neutropenia | 1, 1 (5) | 0, 0 (0) |
| Hypokalemia | 1, 1 (5) | 0, 0 (0) |
| Hyponatremia | 2, 2 (10) | 0, 0 (0) |
| Pneumonitis | 1, 1 (5)* | 0, 0 (0) |
Dyspnea and pneumonitis were observed in the same patient.
DLT and MTD
Two of 6 patients experienced DLT events at dose level 1 (Table III). Because dose level 1 exceeded the MTD, the next cohort of patients was treated at dose level −1, with none of the 6 DLT-evaluable patients experiencing a DLT. Subsequently, 2 limited cohorts of patients were treated at dose levels at which the dose of either vorinostat (−1a) or carfilzomib (−1b) was increased relative to dose level −1. Due to competing clinical trials, dose levels −1a and −1b were closed to accrual after each had accrued 4 evaluable patients. No DLTs were experienced at dose levels −1a and −1b. Dose level −1 was declared to be the MTD.
Table III.
Dose Levels and DLTs
| Dose Level |
Carfilzomib (mg/m2) Days 1 & 2 of Cycle 1 Only |
Carfilzomib (mg/m2) All Subsequent Doses |
Vorinostat (mg) Twice daily |
Patients Treated / # Patients with DLT |
DLT Event(s) |
|---|---|---|---|---|---|
| −1 | 20 | 20 | 100 | 7/0*,† | |
| −1a | 20 | 20 | 200 | 4/0 | |
| −1b | 20 | 27 | 100 | 5/0* | |
| 1 | 20 | 27 | 200 | 4/2‡ | Grade 3 pneumonitis (1 patient), grade 3 febrile neutropenia (1 patient), grade 3 hyponatremia (both patients) |
One patient could not be evaluated for DLT
Maximum tolerated dose (MTD)
Exceeded MTD
Disease Response
Stable disease (SD) was observed in 2 patients and a partial response (PR) was observed in one patient with DLBCL who had previously relapsed after autologous SCT (Table IV). Treatment was stopped after 5 treatment cycles in the patient with the PR, and the patient proceeded to allogeneic SCT.
Table IV.
Response Data
| Best Response | No. of patients |
|---|---|
| Complete response (CR) | 0 |
| Partial response (PR) | 1a |
| Stable disease (SD) | 2b |
| Progressive disease (PD) | 17 |
| Not Evaluable (NE) | 0 |
DLBCL; prior autologous SCT
1 MCL and 1 DLBCL
Immunohistochemical Analysis
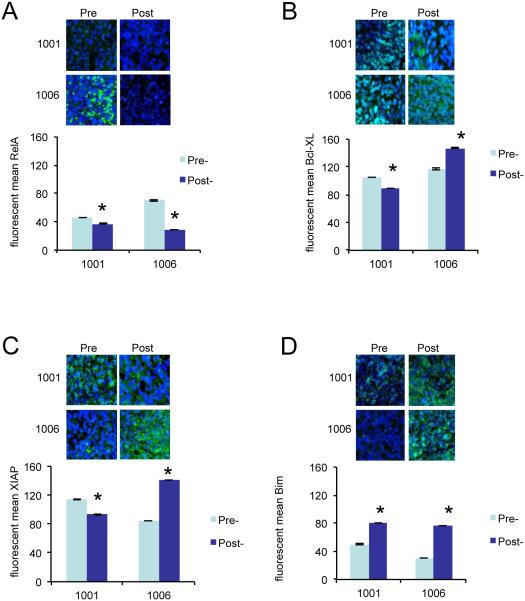
Pre- and post-treatment (48 ± 6 hours after the first doses of drugs) core biopsies from 2 patients (1001 and 1006) were obtained for biological correlates (Figure 1). Nuclear RelA was down-regulated in both biopsies after treatment (Figure 1A), although it only declined 18% in the case of patient 1001. The biopsy from patient 1006 exhibited a 59% down-regulation of RelA. The expression of total Bcl-XL (Figure 1B) and XIAP (Figure 1C) declined 16% and 19%, respectively in post-treatment samples for patient 1001. In contrast, the post-treatment sample from patient 1006 showed a 26% increase in Bcl-XL and a 65% increase of XIAP expression. The expression of Bim increased in the post-treatment samples of both patients with a 62% increase for patient 1001 and a 145% increase for patient 1006 (Figure 1D).
Figure 1.
Analysis of expression of biomarkers by immunofluorescence. Immunofluorescence staining of RelA/p65 (A), Bcl-XL (B), XIAP (C), and Bim (D) for biopsies obtained before (pre) and 48 ± 6 hours after the first dose of carfilzomib (post) from 2 patients (1001 and 1006; both with a response of PD). Biopsies where stained with appropriate primary antibody, Alexa Fluor 488 secondary antibody (green) and with DAPI (blue). Below the confocal images showing a representative picture of each staining is a graphical display of the quantified mean pixel intensity (mean ± SE). Expression of nuclear p65/RelA (A) and total Bcl-XL, XIAP and Bim (B-D) was calculated. * = P < 0.001.
IL-10 and TNF Expression in Plasma
The concentration of IL-10 and TNF was determined in plasma samples from 15 patients (14 with progressive disease [PD]; 1 with SD [patient 1028]; Table V). Because the sampling was optional, a complete set of samples was obtained from only for 4 patients (1002, 1004, 1005 and 1006). Generally, plasma IL-10 levels varied greatly in all of the samples without any clear pattern. Similar results were obtained for TNF, with no obvious pattern of expression.
Table V.
IL-10 and TNF Expression Levels in Patient Plasma Samplesa
| Sample | Dose Level |
Response | Timepoint | Plasma IL-10 (pg/ml) |
Plasma TNF (pg/ml) |
|---|---|---|---|---|---|
| 1001 | 1 | PD | C1D1 | 92.89 ± 7.57 | 0 |
| C1D3 | 39.94 ± 7.97 | 16.31 ± 1.89 | |||
| C1D8 | 2.35 ± 0.23 | 0 | |||
| 1002 | 1 | PD | C1D1 | 163.49 ± 2.12 | 25.51 ± 3.38 |
| C1D3 | 94.38 ± 4.54 | 7.19 ± 1.00 | |||
| C1D8 | 156.78 ± 4.60 | 32.43 ± 2.80 | |||
| EoT | 219.67 ± 2.40 | 73.35 ± 7.57 | |||
| 1004 | 1 | PD | C1D1 | 380.51 ± 4.25 | 1.33 ± 0.26 |
| C1D3 | 292.75 ± 3.36 | 0 | |||
| C1D8 | 262.67 ± 11.76 | 0 | |||
| EoT | 470.50 ± 8.85 | 20.73 ± 1.47 | |||
| 1005 | −1 | PD | C1D1 | 35.71 ± 2.60 | 0.30 ± 0.06 |
| C1D3 | 27.26 ± 3.95 | 18.04 ± 9.47 | |||
| C1D8 | 37.20 ± 2.63 | 4.04 ± 0.71 | |||
| EoT | 15.33 ± 1.55 | 0 | |||
| 1006 | −1 | PD | C1D1 | 647.65 ± 6.47 | 4.78 ± 0.23 |
| C1D3 | 282.21 ± 2.25 | 0 | |||
| C1D8 | 656.78 ± 6.82 | 0 | |||
| EoT | 726.79 ± 15.63 | 0 | |||
| 1008 | −1 | PD | C1D1 | 45.41 ± 3.66 | 0 |
| C1D8 | 52.19 ± 6.37 | 0.67 ± 0.34 | |||
| EoT | 69.94 ± 0.51 | 0 | |||
| 1012 | −1 | PD | C1D2 | 211.96 ± 1.78 | 7.63 ± 2.28 |
| 1013 | −1 | PD | C1D1 | 14.83 ± 0.25 | 0 |
| EoT | 20.80 ± 1.08 | 0 | |||
| 1015 | −1a | PD | EoT | 13.59 ± 1.51 | 21.68 ± 7.69 |
| 1016 | −1b | PD | C1D1 | 76.12 ± 3.55 | 2.12 ± 0.70 |
| 1020 | −1a | PD | C1D1 | 180.57 ± 4.83 | 0 |
| EoT | 98.66 ± 7.22 | 0 | |||
| 1022 | −1b | PD | C1D1 | 140.74 ± 8.24 | 0 |
| 1026 | −1b | PD | C1D1 | 18.47 ± 1.38 | 10.80 ± 2.22 |
| 1027 | −1a | PD | C1D1 | 30.28 ± 1.99 | 0.21 ± 0.12 |
| 1028 | −1b | SD | C1D1 | 15.50 ± 2.44 | 0.70 ± 0.23 |
Determination of IL-10 and TNF in plasma samples of patients obtained at baseline (C1D1), 48 ± 6 hours after first dose of carfilzomib (C1D3), prior to treatment on day 8 of cycle 1 (C1D8) and at the end of the treatment (EoT) by ELISA. Samples were measured in triplicate and mean values with SE are presented.
DISCUSSION
The results of this study indicate that carfilzomib and vorinostat can be safely and tolerably administered to patients with B-cell lymphomas (Table II). Overall, the most common hematologic toxicities among the grade 3 and 4 adverse events were anemia, leukopenia, and neutropenia. With the exception of hyponatremia reported in 2 patients, no other grade 3 or 4 non-hematologic toxicities were reported in more than a single patient. These toxicities are similar to those observed for carfilzomib [25] and vorinostat [26-29] when used as single agents for the treatment of hematologic malignancies, with anemia and thrombocytopenia representing the most commonly observed hematologic toxicities. Although neutropenia is not particularly common for single-agent vorinostat or carfilzomib, it has been observed in vorinostat combination treatment trials [30-33]. We hypothesize that this might be related to bone marrow suppression from prior therapies, particularly cytotoxic therapies. The number of prior therapies in our trial ranged from 1-13 (including autologous stem cell transplant) (Table I), indicating a heavily pretreated patient population.
Dose level 1 exceeded the MTD with 2 of 6 patients experiencing DLTs (Table III). Dose level −1 was evaluated next. Because none of the 6 DLT-evaluable patients on dose level −1 experienced a DLT, two intermediate dose levels (−1a and −1b) were added to the dosing schedule. Dose level −1a maintained the carfilzomib dose at dose level −1 and raised the vorinostat dose to that of dose level 1; conversely, dose level −1b raised the carfilzomib dose to that of dose level 1 and held the vorinostat dose to that of dose level −1. Although no DLTs were experienced at either dose levels ‐1a or −1b, neither dose level was expanded to 6 patients and thus dose level −1 was declared to be the MTD.
Of the 20 patients enrolled on this study, there was a single objective response, which was characterized as a PR (Table IV). This response rate compares to that obtained in a phase 1 trial of carfilzomib in which there were no objective responses among 15 NHL patients [25]. Likewise, a limited number of complete and partial responses have been observed in phase 1 [26,28] and phase 2 [29] trials using vorinostat for the treatment of NHL. An additional phase 1 trial did not observe any objective responses among the 3 enrolled NHL patients [27]. It should be noted that patients in this phase 1 trial were very heavily pre-treated, with over 50% having progressed after autologous SCT. Of the 10 patients enrolled with MCL, none had an objective response, although one patient had stable disease. In this context, the response rate to single-agent bortezomib has been reported to be 33% [34], and results for a phase 2 trial of carfilzomib in MCL are pending (ClinicalTrials.Gov #: NCT02042950).
The primary goal of the correlative studies was to test the feasibility of obtaining candidate pharmacodynamic biomarkers for future phase 2 trials by determining if adequate samples could be obtained and if the techniques were sufficiently robust to be employed in a cooperative group setting. Cytokines are deregulated in several cancers, particularly in lymphomas. Cytokines such as tumor necrosis factor alpha (TNF-α), IL-10, and IL-6 promote cancer cell survival and growth through key pathways that include NF-κB. High levels of TNF and IL-10 have been associated with poor outcome in DLBCL [35] and NHL [36]. In general, histone acetylation leads to an increase in expression of the pro-apoptotic proteins Bim, Bax, and Bak, and a decrease in the anti-apoptotic proteins XIAP, Bcl-2, Bcl-XL, Mcl-1, and c-FLIP. While HDACIs induce NF-κB activation, the combined use of an HDACI and proteasome inhibitor can block NF-κB activation [17]. These findings raise the possibility that NF-κB activation, expression levels of XIAP, Bim, and Bcl-XL, and plasma levels of IL-10 and TNF-α might represent surrogate markers for carfilzomib/vorinostat activity.
We first sought to determine whether in vivo exposure of lymphoma cells to carfilzomib and vorinostat can induce pharmacodynamic effects previously observed in vitro: (1) down-regulation of NF-κB as indicated by diminished nuclear RelA; (2) down-regulation of the NF-κB-dependent proteins XIAP and Bcl-XL; and (3) up-regulation of Bim. Because pre- and post-treatment (48 ± 6 hours after the first doses of drugs) core biopsy samples were collected only from patients 1001 and 1006 (both with PD), it is clear that sample collection in this study was very limited and definite conclusions cannot be drawn. In the biopsy samples taken from both patients, nuclear RelA was down-regulated after treatment in both patients, although for patient 1001, down-regulation was marginal (18% versus 59%; Figure 1A). These results are, however, compatible with previously observed in vitro effects [17]. Total Bcl-XL and XIAP expression was slightly down-regulated in the post-treatment sample of one patient (1001) and up-regulated in the post-treatment sample of the other patient (1006) (Figure 1B,C). Bim expression increased in the post-treatment sample of both patients (Figure 1D), consistent with previous in vitro studies. Given the small sample size, these results are clearly not definitive, but they do suggest that the methodology may be adequate for a broader group of samples in the setting of a successor phase 2 study.
It is well recognized that cytokines play roles in the pathogenesis of various cancers via paracrine and/or autocrine mechanisms [37]. Several studies have shown evidence for the role of human IL-10 in the pathogenesis of malignant B-cell neoplasms [35,38,39]. In patients with untreated CLL and DLBCL, increasing serum IL-10 levels were correlated with inferior survival [40]. Also, high levels of TNF in pre-treatment samples of NHL patients were correlated with poor outcome [36], and there was a significant decrease in serum TNF reported in low-grade NHL patients who achieved a complete response to standard chemotherapy [41]. In the current phase 1 study with a limited number of patients and samples, we found no correlation between levels of TNF or IL-10 and response to treatment. These results should be interpreted with caution as complete sample sets were obtained from only 4 of the 20 treated patients and because the 15 patients from whom samples were obtained were heavily skewed in their response to treatment (14 PD, 1 SD). Furthermore, phase 1 trials involving a limited number of patients treated at non-uniform drug doses are not optimally designed to identify response correlations. Whether such correlative studies can predict activity for successor proteasome/HDACI regimens in NHL awaits further evaluation in the setting of more highly powered phase 2 trials.
In summary, an MTD was identified for the combination of carfilzomib and vorinostat in the treatment of patients with B-cell lymphomas. Among 20 heavily pre-treated patients, there was 1 PR, 2 SDs, and 17 PDs. As noted in the pre-clinical setting, NF-kB activation was diminished and Bim levels were increased post-treatment in a very limited number of specimens, but these changes did not correlate with clinical response. Overall, the combination of carfilzomib and vorinostat in relapsed B-cell lymphoma patients refractory to multiple lines of therapy resulted in very modest responses. Although the present results do not clearly support a phase 2 clinical trial in relapsed/refractory NHL, at least with the current schedule, the possibility remains of exploring alternative schemata and potentially newer generation agents, or different NHL sub-types such as PTCL or CTCL, which are known to be HDACI-responsive. In this context, ongoing clinical trials in patients with relapsed and/or refractory multiple myeloma with carfilzomib and the HDACI, panobinostat, have yielded promising results [42].
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by grants from the National Institutes of Health (NCI R01 CA93738, NCI R01 CA100866, NCI R21 CA110953, NCI R01 CA167708, NCI P50 CA130805 [Lymphoma SPORE], NCI P30 CA016059 [Cancer Center Support Grant to the Virginia Commonwealth (VCU) Massey Cancer Center (MCC) supported, in part, the use of the VCU MCC Biostatistics Shared Resource], and UL1TR000058 [CTSA award to VCU from NCATS/NIH]) and an award from the Multiple Myeloma Research Foundation.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
Trial Registration ID: NCT01276717.
REFERENCES
- 1.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Sawas A, Diefenbach C, O'Connor OA. New therapeutic targets and drugs in non-Hodgkin's lymphoma. Current Opinion in Hematology. 2011;18:280–287. doi: 10.1097/MOH.0b013e328347786d. [DOI] [PubMed] [Google Scholar]
- 4.Younes A. Beyond chemotherapy: new agents for targeted treatment of lymphoma. Nature Reviews: Clinical Oncology. 2011;8:85–96. doi: 10.1038/nrclinonc.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunning MA, Vose JM. Management of indolent lymphoma: where are we now and where are we going. Blood Reviews. 2012;26:279–288. doi: 10.1016/j.blre.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mato AR, Feldman T, Goy A. Proteasome inhibition and combination therapy for non-Hodgkin's lymphoma: from bench to bedside. Oncologist. 2012;17:694–707. doi: 10.1634/theoncologist.2011-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Pham LV, Newberry KJ, et al. In vitro and in vivo therapeutic efficacy of carfilzomib in mantle cell lymphoma: targeting the immunoproteasome. Molecular Cancer Therapeutics. 2013 doi: 10.1158/1535-7163.MCT-13-0156. [DOI] [PubMed] [Google Scholar]
- 9.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 10.Siegel D, Hussein M, Belani C, et al. Vorinostat in solid and hematologic malignancies. Journal of Hematology & Oncology. 2009;2:31. doi: 10.1186/1756-8722-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant S, Dai Y. Histone deacetylase inhibitors and rational combination therapies. Advances in Cancer Research. 2012;116:199–237. doi: 10.1016/B978-0-12-394387-3.00006-9. [DOI] [PubMed] [Google Scholar]
- 12.Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clinical Cancer Research. 2004;10:3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 14.Campbell RA, Sanchez E, Steinberg J, et al. Vorinostat enhances the antimyeloma effects of melphalan and bortezomib. European Journal of Haematology. 2010;84:201–211. doi: 10.1111/j.1600-0609.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 15.Heider U, von Metzler I, Kaiser M, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. European Journal of Haematology. 2008;80:133–142. doi: 10.1111/j.1600-0609.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 16.Paoluzzi L, Scotto L, Marchi E, Zain J, Seshan VE, O'Connor OA. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clinical Cancer Research. 2010;16:554–565. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 17.Dasmahapatra G, Lembersky D, Kramer L, et al. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood. 2010;115:4478–4487. doi: 10.1182/blood-2009-12-257261. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Batalo M, Bose P, Holkova B, Grant S. Targeting Mantle Cell Lymphoma with a Strategy of Combined Proteasome and Histone Deacetylase Inhibition. In: Dou QP, editor. Resistance to Proteasome Inhibitors in Cancer: Molecular Mechanisms and Strategies to Overcome Resistance. Springer International Publishing; New York: 2014. pp. 149–179. [Google Scholar]
- 19.Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Molecular Cancer Therapeutics. 2011;10:2034–2042. doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncology. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 21.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crump M, Coiffier B, Jacobsen ED, et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Annals of Oncology. 2008;19:964–969. doi: 10.1093/annonc/mdn031. [DOI] [PubMed] [Google Scholar]
- 23.Schelman WR, Traynor AM, Holen KD, et al. A phase I study of vorinostat in combination with bortezomib in patients with advanced malignancies. Investigational New Drugs. 2013;31:1539–1546. doi: 10.1007/s10637-013-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 25.Alsina M, Trudel S, Furman RR, et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clinical Cancer Research. 2012;18:4830–4840. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum M, Frankel P, Popplewell L, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. Journal of Clinical Oncology. 2011;29:1198–1203. doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly WK, Richon VM, O'Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clinical Cancer Research. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 28.Kelly WK, O'Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. Journal of Clinical Oncology. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe T, Kato H, Kobayashi Y, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Science. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson MA, Rathkopf DE, Carvajal RD, et al. A phase I pharmacokinetic study of pulse-dose vorinostat with flavopiridol in solid tumors. Investigational New Drugs. 2011;29:1004–1012. doi: 10.1007/s10637-010-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke MJ, Lamba JK, Pounds S, et al. A therapeutic trial of decitabine and vorinostat in combination with chemotherapy for relapsed/refractory acute lymphoblastic leukemia. American Journal of Hematology. 2014;89:889–895. doi: 10.1002/ajh.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopfinger G, Nosslinger T, Lang A, et al. Lenalidomide in combination with vorinostat and dexamethasone for the treatment of relapsed/refractory peripheral T cell lymphoma (PTCL): report of a phase I/II trial. Annals of Hematology. 2014;93:459–462. doi: 10.1007/s00277-014-2009-0. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, Frankel P, Popplewell L, et al. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica. 2015;100:357–362. doi: 10.3324/haematol.2014.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 35.Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119:2844–2853. doi: 10.1182/blood-2011-10-388538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warzocha K, Salles G, Bienvenu J, et al. Tumor necrosis factor ligand-receptor system can predict treatment outcome in lymphoma patients. Journal of Clinical Oncology. 1997;15:499–508. doi: 10.1200/JCO.1997.15.2.499. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nacinovic-Duletic A, Stifter S, Dvornik S, Skunca Z, Jonjic N. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. International Journal of Laboratory Hematology. 2008;30:230–239. doi: 10.1111/j.1751-553X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 39.Lech-Maranda E, Baseggio L, Bienvenu J, et al. Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood. 2004;103:3529–3534. doi: 10.1182/blood-2003-06-1850. [DOI] [PubMed] [Google Scholar]
- 40.Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97:256–263. doi: 10.1182/blood.v97.1.256. [DOI] [PubMed] [Google Scholar]
- 41.Zinzani PL, Baccini C, Zaccaria A, et al. Clinical implications of serum levels of soluble CD23 and tumor necrosis factor alpha in low-grade non-Hodgkin's lymphoma. European Journal of Haematology. 1996;57:335–340. doi: 10.1111/j.1600-0609.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 42.Berdeja J, Savona M, Mace JR, et al. A Single-Arm, Open-Label, Multi-Center Phase I/II Study Of The Combination Of Panobinostat and Carfilzomib In Patients (pts) With Relapsed Or Relapse/Refractory Multiple Myeloma (MM) Blood. 2013;122:1937. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.